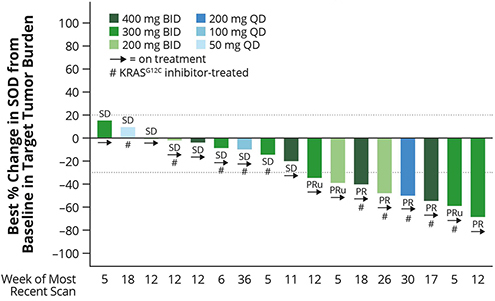
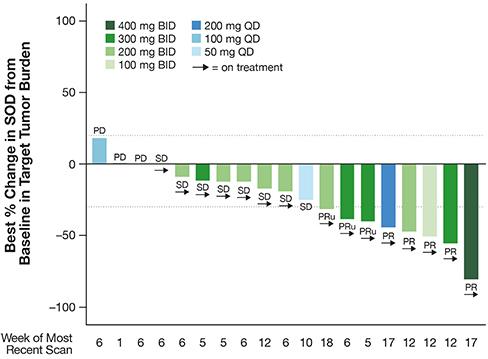
Figure 2. RMC-6291-001: Change in tumor burden from efficacy-evaluable patients with CRC that were naïve to treatment with a
KRASG12C(OFF) inhibitor
Evaluable for Efficacy* (N=19)
Data Extracted October 5, 2023.
| | * | All treated patients who received first dose of RMC-6291 at least eight weeks prior to the data extract date. | |
Tumor response per RECIST 1.1 for the patients reflected in Figure 2 is summarized below (Table 4).
Table 4. RMC-6291-001: Tumor Response per RECIST for efficacy-evaluable patients with CRC that were naïve to treatment with a
KRASG12C(OFF) inhibitor
| | | | |
Tumor Response (per RECIST 1.1) | |
Best Overall Response, n (%) | | | N=20‡ | |
Partial response† | | | 8 (40) | |
Stable disease | | | 8 (40) | |
Progressive disease | | | 4 (20) | |
ORR, n (%) | | | 8 (40) | |
DCR (CR+PR+SD), n (%) | | | 16 (80) | |
| | † | Partial Response includes five confirmed and three unconfirmed. | |
| | ‡ | One patient had progressive disease due to a new lesion; target lesion measurements were not available. | |
The Company believes the reported data provide preliminary evidence of differentiation of RMC-6291 from KRASG12C(OFF) inhibitors.
RMC-5127
The Company has selected RMC-5127, which is designed as an oral covalent tri-complex inhibitor of KRASG12V(ON), as a development candidate.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this report that are not historical facts may be considered “forward-looking statements,” including, without limitation, statements regarding the scope, progress and results of developing the Company’s product candidates, and conducting clinical trials. Forward-looking statements are typically, but not always, identified by the use of words such as “may,” “will,” “would,” “believe,” “intend,” “plan,” “anticipate,” “estimate,” “expect” and other similar terminology indicating future results. Such forward-looking statements are subject to substantial risks and uncertainties that could cause the Company’s development programs, future results, performance or achievements to differ materially from those anticipated in the forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties inherent in the drug development process, including the Company’s programs’ early stage of development, the process of designing and conducting preclinical and clinical trials, the regulatory approval processes, the timing of regulatory filings, the challenges associated with manufacturing drug products, the Company’s ability to successfully establish, protect and defend its intellectual property, other matters that could affect the sufficiency of the Company’s capital resources to fund operations, reliance on third parties for manufacturing and development efforts, changes in the competitive landscape and the effects on the Company’s business of global events and other macroeconomic conditions. For a further description of the risks and uncertainties that could cause actual results to differ from those anticipated in these forward-looking statements, as well as risks relating to the business of the Company in general, see the Company’s Quarterly Report on Form 10-Q filed with the SEC on August 8, 2023, and its future periodic reports to be filed with the SEC. Except as required by law, the Company undertakes no obligation to update any forward-looking statements to reflect new information, events or circumstances, or to reflect the occurrence of unanticipated events.