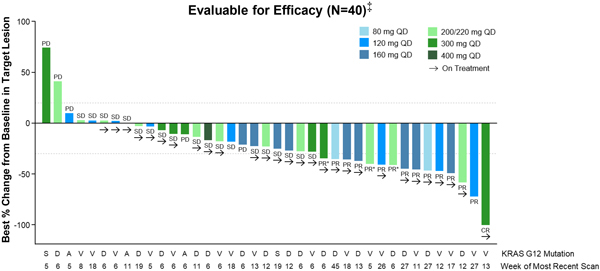
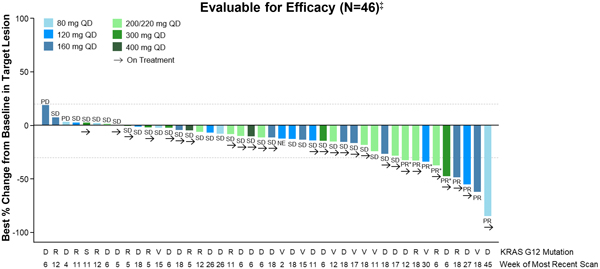
Table 3. RMC-6236-001: Tumor Response per RECIST for efficacy-evaluable KRASG12X PDAC patients
| | | | |
Tumor Response (per RECIST 1.1) | |
Best Overall Response, n (%) | | | | |
PR SD PD NE† | |
| 9(20)
31(67) 3(7) 3(7) |
|
ORR, n (%) | | | 9(20) | |
Confirmed, n | | | 5 | |
DCR (CR+PR+SD), n (%) | | | 40(87) | |
PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, objective response rate; DCR, disease control rate.
† Two patients died prior to first post-baseline scan; one patient had scan after 11 days of treatment and subsequently died due to PD.
The Company is planning a global randomized phase 3 trial comparing RMC-6236 against docetaxel in patients with previously treated RAS-mutated NSCLC who have been treated with immunotherapy and platinum-containing chemotherapy. The study design for this planned trial is subject to change based on regulatory authority feedback. The Company is aiming to initiate this study in 2024.
The Company may potentially plan a global randomized phase 3 trial comparing RMC-6236 against a physician’s choice of chemotherapy regimens in patients with previously treated RAS-mutated PDAC. The study design for this potential trial is subject to change based on regulatory authority feedback. The Company expects to make a decision regarding plans for this study after additional patient follow-up regarding durability of response and dose optimization, but the Company believes the study could potentially be initiated in 2024.
Planning is underway for one or more combination pivotal clinical trials for RMC-6236 with standard of care therapies.
RMC-6291
Active recruitment of patients is underway for the Phase 1/1b clinical trial to evaluate the combination of RMC-6236 and RMC-6291.
RMC-4630
Based on the Company’s review of the complete data set from the RMC-4630-03 study, the Company concluded that the combination of RMC-4630 (200 mg intermittent dosing D1D2) with sotorasib (960 mg daily dosing) showed additive side effects compared to either agent alone and that tolerability was insufficient to confer a clinical benefit (objective response rate or durability) due to dose interruptions and discontinuations. The Company has no immediate plans for further development of RMC-4630, but believes this compound remains an option for potential evaluation in other combinations.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this report that are not historical facts may be considered “forward-looking statements,” including, without limitation, statements regarding the scope, progress and results of developing the Company’s product candidates, and conducting clinical trials. Forward-looking statements are typically, but not always, identified by the use of words such as “may,” “will,” “would,” “believe,” “intend,” “plan,” “anticipate,” “estimate,” “expect” and other similar terminology indicating future results. Such forward-looking statements are subject to substantial risks and uncertainties that could cause the Company’s development programs, future results, performance or achievements to differ materially from those anticipated in the forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties inherent in the drug development process, including the Company’s programs’ early stage of development, the process of designing and conducting preclinical and clinical trials, the