- GBT Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
-
ETFs
- Insider
- Institutional
- Shorts
-
DEF 14A Filing
Global Blood Therapeutics (GBT) DEF 14ADefinitive proxy
Filed: 28 Apr 21, 4:01pm
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
(Amendment No. )
Filed by the Registrant ☒ Filed by a Party other than the Registrant ☐
Check the appropriate box:
| ☐ | Preliminary Proxy Statement | |
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | |
| ☒ | Definitive Proxy Statement | |
| ☐ | Definitive Additional Materials | |
| ☐ | Soliciting Material under §240.14a-12 | |
Global Blood Therapeutics, Inc.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ☒ | No fee required. | |||
| ☐ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | |||
| (1) | Title of each class of securities to which transaction applies:
| |||
| (2) | Aggregate number of securities to which transaction applies:
| |||
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
| |||
| (4) | Proposed maximum aggregate value of transaction:
| |||
| (5) | Total fee paid:
| |||
| ☐ | Fee paid previously with preliminary materials. | |||
| ☐ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing | |||
| (1) | Amount Previously Paid:
| |||
| (2) | Form, Schedule or Registration Statement No.:
| |||
| (3) | Filing Party:
| |||
| (4) | Date Filed:
| |||

GLOBAL BLOOD THERAPEUTICS, INC.
181 Oyster Point Boulevard
South San Francisco, CA 94080
NOTICE OF ANNUAL MEETING
OF STOCKHOLDERS
TO BE HELD ON JUNE 17, 2021
Dear Stockholder:
You are cordially invited to attend the 2021 Annual Meeting of Stockholders (the “Annual Meeting”) of Global Blood Therapeutics, Inc., a Delaware corporation (the “Company”), to be held on Thursday, June 17, 2021, at 8:00 a.m. local time. Our Board of Directors has determined, in the interests of public health and safety in light of the ongoing COVID-19 pandemic, that this year’s Annual Meeting will be held virtually via a live interactive audio webcast on the Internet. You will be able to vote and to ask questions of, and engage in dialogue with, members of our Board of Directors and senior management at www.virtualshareholdermeeting.com/GBT2021 during the meeting. Our Board of Directors currently intends to hold future stockholder meetings in person or using a “hybrid” in-person and virtual format as soon as practicable once it is safe to do so.
The Annual Meeting will be held for the following purposes:
| 1. | to elect the three Class III directors, as nominated by our Board of Directors, to hold office until the 2024 Annual Meeting of Stockholders or until their successors are duly elected and qualified; |
| 2. | to approve, on a non-binding, advisory basis, the compensation of our named executive officers, as disclosed in the proxy statement accompanying this notice; |
| 3. | to ratify the appointment of KPMG LLP as the independent registered public accounting firm of the Company for its fiscal year ending December 31, 2021; and |
| 4. | to transact such other business as may properly come before the Annual Meeting or any adjournment or postponement thereof. |
These items of business are more fully described in the proxy statement accompanying this notice.
Our Board of Directors has fixed the close of business on Thursday, April 22, 2021, as the record date for the determination of stockholders entitled to notice of, and to vote at, the Annual Meeting, or at any adjournments of the Annual Meeting.
Your vote is important. Whether or not you plan to attend the Annual Meeting, you are urged to vote as soon as possible as instructed in the Important Notice Regarding the Availability of Proxy Materials that you will receive in the mail. You may vote via the Internet or telephone or via mail, by requesting, at any time on or before Thursday, June 3, 2021, a printed copy of the proxy card. Voting promptly will help us avoid the additional expense of further solicitation to assure a quorum at the Annual Meeting. If you attend the Annual Meeting and file with the Secretary of the Company an instrument revoking your proxy or a duly executed proxy bearing a later date, your proxy will not be used.
By Order of the Board of Directors
| Global | Blood Therapeutics, Inc. |
Ted W. Love, M.D. |
Ted W. Love, M.D.
President and Chief Executive Officer
South San Francisco, California
April 28, 2021
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement i

GLOBAL BLOOD THERAPEUTICS, INC.
181 Oyster Point Boulevard
South San Francisco, CA 94080
PROXY STATEMENT
FOR THE 2021 ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON JUNE 17, 2021
INFORMATION CONCERNING SOLICITATION AND VOTING
General
This proxy statement (“Proxy Statement”) is furnished in connection with the solicitation of proxies for use prior to or at the 2021 Annual Meeting of Stockholders (the “Annual Meeting”) of Global Blood Therapeutics, Inc. (the “Company,” “we,” “us” and “our”), a Delaware corporation, to be held virtually at 8:00 a.m., local time, on Thursday, June 17, 2021, and at any adjournments or postponements thereof for the following purposes:
| 1. | to elect the three Class III directors, as nominated by the Board of Directors, to hold office until the 2024 Annual Meeting of Stockholders or until their successors are duly elected and qualified; |
| 2. | to approve, on a non-binding, advisory basis, the compensation of our named executive officers; |
| 3. | to ratify the appointment of KPMG LLP as the independent registered public accounting firm of the Company for its fiscal year ending December 31, 2021; and |
| 4. | to transact such other business as may properly come before the Annual Meeting or any adjournment or postponement thereof. |
Our Board of Directors has determined, in the interests of public health and safety in light of the ongoing COVID-19 pandemic, that this year’s Annual Meeting will be held virtually via a live interactive audio webcast on the Internet at www.virtualshareholdermeeting.com/GBT2021. You will be able to vote and to ask questions of, and engage in dialogue with, members of our Board of Directors and senior management during the meeting. Our Board of Directors currently intends to hold future stockholder meetings in person or using a “hybrid” in-person and virtual format as soon as practicable once it is safe to do so.
On or about April 28, 2021, we will mail to all stockholders entitled to vote at the Annual Meeting a Notice of Internet Availability of Proxy Materials, or Notice, containing instructions on how to access this Proxy Statement and our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, or Annual Report.
Solicitation
This solicitation is made on behalf of our Board of Directors. We will bear the costs of preparing, mailing, online processing and other costs of the proxy solicitation made by our Board of Directors. Certain of our officers and employees may solicit the submission of proxies authorizing the voting of shares in accordance with our Board of Directors’ recommendations. Such solicitations may be made by email, telephone or personal solicitation. No additional compensation will be paid to our officers, directors or regular employees for such services. We will reimburse banks, brokerage firms and other custodians, nominees and fiduciaries for reasonable out-of-pocket expenses incurred by them in sending proxy materials to stockholders.
Important Notice Regarding the Availability of Proxy Materials
In accordance with rules and regulations of the Securities and Exchange Commission, or the SEC, instead of mailing a printed copy of our proxy materials to each stockholder of record, we may furnish proxy materials via the Internet. Accordingly, all of our stockholders will receive a Notice, to be mailed on or about April 28, 2021.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 1
On the date of mailing the Notice, stockholders will be able to access all of the proxy materials on the website at www.proxyvote.com. The proxy materials will be available free of charge. The Notice will instruct you as to how you may access and review all of the important information contained in the proxy materials (including the Annual Report) over the Internet or through other methods specified on the website. The website contains instructions as to how to vote by Internet or over the telephone. The Notice also instructs you as to how you may request a paper or email copy of the proxy card. If you received a Notice and would like to receive printed copies of the proxy materials, you should follow the instructions included in the Notice for requesting such materials.
Voting Rights and Outstanding Shares
Only holders of record of our common stock as of the close of business on April 22, 2021, are entitled to receive notice of, and to vote at, the Annual Meeting. Each holder of common stock will be entitled to one vote for each share held on all matters to be voted upon at the Annual Meeting. At the close of business on April 22, 2021, there were 62,266,753 shares of common stock issued and outstanding.
A quorum of stockholders is necessary to take action at the Annual Meeting. Stockholders representing a majority of the outstanding shares of our common stock as of the record date (present virtually at the Annual Meeting or represented by proxy) will constitute a quorum. We will appoint an inspector of elections for the meeting to determine whether or not a quorum is present and to tabulate votes cast by proxy or virtually at the Annual Meeting. Abstentions, withheld votes and broker non-votes (which occur when a broker, bank or other nominee holding shares for a beneficial owner does not vote on a particular matter because such broker, bank or other nominee does not have discretionary authority to vote on that matter and has not received voting instructions from the beneficial owner) are counted as present for purposes of determining the presence of a quorum for the transaction of business at the Annual Meeting.
Vote Required for Each Proposal
The vote required, and the method of calculation, for each proposal at our Annual Meeting is described below.
Proposal | Vote Required | Discretionary Voting Permitted? | ||||||
Election of Directors | Plurality | No | ||||||
Approval, on a Non-Binding, Advisory Basis, of the Compensation of our Named Executive Officers |
| Majority |
|
| No |
| ||
Approval of the Ratification of KPMG LLP | Majority | Yes | ||||||
“Discretionary Voting Permitted” means that brokers will have discretionary voting authority with respect to shares held in street name for their clients, even if the broker does not receive voting instructions from their client.
“Majority” means a majority of the votes properly cast for and against such matter.
“Plurality” means a plurality of the votes properly cast on such matter. For the election of directors, the three nominees receiving the highest number of votes, submitted virtually during the Annual Meeting or by proxy, will be elected as directors.
Proposal One—Election of Directors
The three Class III director nominees receiving the highest number of votes, submitted virtually during the Annual Meeting or by proxy, will be elected. You may vote “FOR” all nominees, “WITHHOLD” for all nominees, or “WITHHOLD” for any individual nominee by specifying the name of the nominee on your proxy card. This proposal is not considered to be a discretionary item, so if you do not instruct your broker how to vote with respect to this proposal, your broker may not vote on this proposal, and those votes will be counted as broker “non-votes.” Withheld votes and broker non-votes will have no effect on the outcome of the election of the directors.
Proposal Two—Approval, on a Non-Binding, Advisory Basis, of the Compensation of our Named Executive Officers
Approval of this proposal requires the affirmative vote of a majority of the votes properly cast for and against this proposal. You may vote “FOR,” “AGAINST” or “ABSTAIN” from voting on this proposal. If you abstain from voting on this proposal, your shares will not be counted as “votes cast” with respect to this proposal, and the abstention will have no effect on this proposal. This proposal is not considered to be a routine item, so if you do not instruct your broker how to vote with respect to this proposal, your broker may not vote on this proposal, and those votes will be counted as broker “non-votes.” Broker non-votes will have no effect on the outcome of this proposal.
2 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Proposal Three—Approval of the Ratification of KPMG LLP as Independent Registered Public Accounting Firm
Approval of this proposal requires the affirmative vote of a majority of the votes properly cast for and against this proposal. You may vote “FOR,” “AGAINST” or “ABSTAIN” from voting on this proposal. If you abstain from voting on this proposal, your shares will not be counted as “votes cast” with respect to this proposal, and the abstention will have no effect on the proposal. This proposal is considered to be a discretionary item, and your broker will be able to vote on this proposal even if it does not receive instructions from you. Accordingly, we do not anticipate that there will be any broker non-votes on this proposal; however, any broker non-votes will not be counted as “votes cast” and will, therefore, have no effect on this proposal.
Voting Methods
We request that you vote your shares by proxy as instructed in the Notice by one of the following methods: over the Internet, by telephone or by mail. If you choose to vote by mail, your shares will be voted in accordance with your voting instructions if the proxy card is received prior to or at the Annual Meeting. If you sign and return your proxy card but do not give voting instructions, your shares will be voted FOR (i) the election of each of the three nominees as Class III directors; (ii) the approval, on a non-binding, advisory basis, of the compensation of our named executive officers; (iii) the ratification of the appointment of KPMG LLP as the independent registered public accounting firm for the Company for the fiscal year ending December 31, 2021; and (iv) as the proxy holders deem advisable, in their discretion, on other matters that may properly come before the Annual Meeting.
Voting by Proxy Over the Internet or by Telephone
Stockholders whose shares are registered in their own names may vote by proxy by mail, over the Internet or by telephone. Instructions for voting by proxy over the Internet or by telephone are set forth on the Notice. The Internet and telephone voting facilities will close at 11:59 p.m. (Eastern Time) on June 16, 2021. The Notice will also provide instructions on how you can elect to receive future proxy materials electronically or in printed form by mail. If you choose to receive future proxy materials electronically, you will receive an email next year with instructions containing a link to the proxy materials and a link to the proxy voting site. Your election to receive proxy materials electronically or in printed form by mail will remain in effect until you terminate such election.
If your shares are held in street name, the voting instruction form sent to you by your broker, bank or other nominee should indicate whether the institution has a process for beneficial holders to provide voting instructions over the Internet or by telephone. A number of banks and brokerage firms participate in a program that also permits stockholders whose shares are held in street name to direct their vote over the Internet or by telephone. If your bank or brokerage firm gives you this opportunity, the voting instructions from the bank or brokerage firm that accompany this Proxy Statement will tell you how to use the Internet or telephone to direct the vote of shares held in your account. If your voting instruction form does not include Internet or telephone information, please complete and return the voting instruction form in the self-addressed, postage-paid envelope provided by your broker. Stockholders who vote by proxy over the Internet or by telephone need not return a proxy card or voting instruction form by mail, but may incur costs, such as usage charges, from telephone companies or Internet service providers.
Revocability of Proxies
Any proxy may be revoked at any time before it is exercised by filing an instrument revoking it with our Secretary or by submitting a duly executed proxy bearing a later date prior to the time of the Annual Meeting. Stockholders who have voted by proxy over the Internet or by telephone or have executed and returned a proxy and who then virtually attend the Annual Meeting and desire to vote during the meeting are requested to notify our Secretary in writing prior to the time of the Annual Meeting. We request that all such written notices of revocation be addressed to Tricia Suvari, Secretary, c/o Global Blood Therapeutics, Inc., at the address of our principal executive offices at 181 Oyster Point Boulevard, South San Francisco, CA 94080. Our telephone number is 650.741.7700. Stockholders may also revoke their proxy by entering a new vote over the Internet or by telephone before these voting facilities close at 11:59 p.m. (Eastern Time) on June 16, 2021.
Stockholder Proposals to be Presented at the Next Annual Meeting
Any stockholder who meets the requirements of the proxy rules under the Securities Exchange Act of 1934, as amended, or the Exchange Act, may submit proposals to the Board of Directors to be presented at the 2022 Annual Meeting of Stockholders. Such proposals must comply with the requirements of Rule 14a-8 under the Exchange Act and be submitted in writing by notice delivered or mailed by first-class United States mail, postage prepaid, to our Secretary at our principal executive offices at the address set forth above no later than December 29, 2021, to be considered for inclusion in the proxy
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 3
materials to be disseminated by the Board of Directors for such annual meeting. If the date of the 2022 Annual Meeting of Stockholders is moved by more than 30 days from the date contemplated at the time of the previous year’s proxy statement, then notice must be received within a reasonable time before we begin to print and send proxy materials. If that happens, we will publicly announce the deadline for submitting a proposal in a press release or in a document filed with the U.S. Securities and Exchange Commission, or SEC. A proposal submitted outside the requirements of Rule 14a-8 under the Exchange Act will be considered untimely if received after March 14, 2022.
Our Amended and Restated Bylaws, or Bylaws, also provide for separate notice procedures to recommend a person for nomination as a director or to propose business to be considered by stockholders at a meeting. To be considered timely under these provisions, the stockholder’s notice must be received by our Secretary at our principal executive offices at the address set forth above no earlier than February 17, 2022, and no later than March 19, 2022. Our Bylaws also specify requirements as to the form and content of a stockholder’s notice.
The Board of Directors, a designated committee thereof or the chair of the meeting may refuse to acknowledge the introduction of any stockholder proposal if it is not made in compliance with the applicable notice provisions.
4 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
ELECTION OF DIRECTORS
Our certificate of incorporation provides for a Board of Directors that is divided into three classes. The term for each class is three years, staggered over time. In 2021, the term of the directors in Class III expires, and all three of our Class III directors—Messrs. Scott W. Morrison, Deval L. Patrick and Mark L. Perry—will stand for re-election at the Annual Meeting.
Our Board of Directors is currently comprised of ten members. If the Class III director nominees who are standing for re-election are elected at the Annual Meeting, our Board of Directors will be as follows:
| • | Class I—Drs. Ted Love, Glenn F. Pierce and Alexis A. Thompson and Ms. Dawn A. Svoronos; |
| • | Class II—Mr. Willie L. Brown, Jr., Dr. Philip A. Pizzo and Ms. Wendy L. Yarno; and |
| • | Class III—Messrs. Morrison, Patrick and Perry. |
In the absence of instructions to the contrary, the persons named as proxy holders in the accompanying proxy intend to vote in favor of the election of the three Class III nominees designated below to serve until the 2024 Annual Meeting of Stockholders and until their successors shall have been duly elected and qualified. Each nominee is currently a director. The Board of Directors expects that each nominee will be available to serve as a director, but if any such nominee should become unavailable or unwilling to stand for election, it is intended that the shares represented by the proxy will be voted for such substitute nominee as may be designated by the Board of Directors.
The biographies of our director nominees and each director whose term will continue after the Annual Meeting and their ages as of March 31, 2021, are set forth below.
Name | Position | Year First Elected or Appointed Director | Age | |||||||
Ted W. Love, M.D. | President, Chief Executive Officer and Director | 2013 | 62 | |||||||
Willie L. Brown, Jr. | Director | 2015 | 87 | |||||||
Scott W. Morrison | Director | 2016 | 63 | |||||||
Deval L. Patrick | Director | 2015 | 64 | |||||||
Mark L. Perry | Director | 2015 | 65 | |||||||
Glenn F. Pierce, M.D., Ph.D. | Director | 2016 | 65 | |||||||
Philip A. Pizzo, M.D. | Director | 2015 | 76 | |||||||
Dawn A. Svoronos | Director | 2018 | 67 | |||||||
Alexis A. Thompson, M.D., M.P.H. | Director | 2021 | 62 | |||||||
Wendy L. Yarno | Director | 2017 | 66 | |||||||
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 5
The persons listed below are nominated for election to Class III of the Board of Directors to serve a three-year term ending at the 2024 Annual Meeting of Stockholders and until their successors are elected and qualified.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR”
|
Scott W. Morrison
Age 63
Director since January 2016
Committees: • Audit (Chair) • Compensation • Commercial | �� | Mr. Morrison has served as a member of our Board of Directors since January 2016. From 1996 to December 2015, Mr. Morrison was a partner at Ernst & Young LLP, a public accounting firm, and served as its U.S. Life Sciences Leader since 2002. He currently serves on the board of directors of Corvus Pharmaceuticals, Inc., a biopharmaceutical company, and Ideaya Biosciences, Inc., a biotechnology company. Mr. Morrison has served on numerous life sciences industry boards, including the Biotechnology Innovation Organization (BIO) ECS Board, the Bay Area Bioscience Center Board (now California Life Sciences Association), the California Life Sciences Foundation and the Biotechnology Institute. Within the past five years, Mr. Morrison previously served on the board of directors of Audentes Therapeutics, Inc., a biotechnology company. Mr. Morrison holds a B.S. in Accounting and Finance from the University of California, Berkeley and is a Certified Public Accountant (inactive).
Qualifications: Mr. Morrison’s qualifications to serve on our Board of Directors include significant accounting expertise and knowledge of the life sciences industry through his 35-year career in public accounting serving public and private companies in the life sciences sector, as well as his experience serving on the board of directors (and certain key standing committees) of other biotechnology companies since 2015.
| ||||
Deval L. Patrick
Age 64
Director since 2020; previously April 2015
Committees: • Audit • Nominating and Corporate Governance | Mr. Patrick has served as a member of our Board of Directors since May 2020 and previously served as a member of our Board of Directors from April 2015 to November 2019. Since March 2021, Mr. Patrick has served as a Senior Advisor at Bain Capital, LP, an investment firm, and he previously served as managing director of Bain Capital from April 2015 to December 2019. Since May 2020, Mr. Patrick has served as co-chair of American Bridge 21st Century, a political action committee and an extension of his work to elevate the values of generational responsibility and servant leadership in politics and public service. Mr. Patrick also serves on the board of directors of AmWell Corp, a telehealth company, Twilio Inc., a cloud communications platform company, Cerevel Therapeutics, a biotechnology company, and Environmental Impact Acquisition Corp, a special purpose acquisition company focused on sustainability. From January 2007 to January 2015, Mr. Patrick served as the governor of Massachusetts. Before his tenure in government, Mr. Patrick served as the executive vice president and general counsel at The Coca-Cola Company, and as vice president and general counsel at Texaco Inc. Mr. Patrick received an A.B. in English and American literature from Harvard College and a J.D. from Harvard Law School.
Qualifications: Mr. Patrick’s qualifications to serve on our Board of Directors include his significant experience as a business and government leader with a record of success in solving complex problems, making strategic investments, managing crises and building teams locally, nationally and internationally.
|
6 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Mark L. Perry
Age 65
Director since April 2015
Committees: • Audit • Compensation • Commercial | Mr. Perry has served as a member of our Board of Directors since April 2015. From October 2012 to October 2013, Mr. Perry served as an entrepreneur-in-residence at Third Rock Ventures. In October 2004, Mr. Perry joined Aerovance, Inc., a biopharmaceutical company, as a director, and he served as president and chief executive officer of Aerovance from February 2007 to October 2011. Prior to that, Mr. Perry served as the senior business adviser of Gilead Sciences, Inc., a biopharmaceutical company, from April 2004 to February 2007 and as an executive officer from May 1994 to April 2004, during which time he served in a variety of capacities, including general counsel, chief financial officer and executive vice president of operations. Earlier in his career, from 1981 to 1994, Mr. Perry served as an attorney at Cooley LLP, and was a partner of the firm from 1987 to 1994. Since August 2011, Mr. Perry has served on various boards of companies and non-profit organizations. Mr. Perry currently serves on the board of directors of Nvidia Corporation, a visual computing company, and, within the past five years, he previously served on the board of directors of MyoKardia, Inc., a biopharmaceutical company. Mr. Perry received a B.A. in history from the University of California, Berkeley and a J.D. from the University of California, Davis.
Qualifications: Mr. Perry’s qualifications to serve on our Board of Directors include more than 30 years of experience serving in professional and management positions in the biotechnology industry, as well as his experience serving on the board of directors (and certain key standing committees) of other biopharmaceutical companies.
|
Class I: Directors Currently Serving Until the 2022 Annual Meeting of Stockholders
Ted W. Love, M.D.
Age 62
Director since September 2013
Committees: • N/A | Dr. Love has served as our Chief Executive Officer and President since June 2014, and as a member of our Board of Directors since September 2013. From February 2010 to August 2012, Dr. Love served as executive vice president, research and development and technical operations of Onyx Pharmaceuticals, Inc. Prior to that, from 2001 to January 2009, he served as president, chief executive officer and chairman of Nuvelo, Inc., and previously served as senior vice president, development of Theravance, Inc., from 1998 to 2001. Previously, he spent six years at Genentech, Inc., where he held a number of senior management positions in medical affairs and product development and served as chairman of Genentech’s Product Development Committee. Dr. Love currently serves on the board of directors of Seagen Inc., a biotechnology company, and Royalty Pharma plc, a biopharmaceutical company. Dr. Love also currently serves on the board of directors of the Biotechnology Innovation Organization (BIO), a non-profit biotechnology trade organization. Within the past five years, Dr. Love previously served on the board of directors of Amicus Therapeutics, Inc., a biotechnology company, and Cascadian Therapeutics, Inc., a biopharmaceutical company. Dr. Love holds a B.A. from Haverford College and an M.D. from Yale Medical School. He completed a residency in internal medicine and fellowship in cardiology at the Massachusetts General Hospital.
Qualifications: Dr. Love’s qualifications to serve on our Board of Directors include his role as our principal executive officer and more than 25 years of broad leadership and management experience in the pharmaceutical industry.
|
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 7
Glenn F. Pierce, M.D., Ph.D.
Age 65
Director since February 2016
Committees: • Nominating and Corporate Governance • Research and Development (Chair)
| Dr. Pierce has served as a member of our Board of Directors since February 2016. In February 2016, Dr. Pierce joined Third Rock Ventures, a venture capital firm, as an independent consultant and entrepreneur-in-residence. In 2018, Dr. Pierce co-founded Ambys Medicines, a biopharmaceutical company, and currently serves as interim Chief Medical Officer. He also serves on the World Federation of Hemophilia (WFH) Board and the National Hemophilia Foundation (NHF) (US) Medical and Scientific Advisory Council. Dr. Pierce is also a director of Voyager Therapeutics, a biopharmaceutical company. Dr. Pierce retired from Biogen in 2014 as senior vice president of Hematology, Cell and Gene Therapies. He had overall R&D responsibility for hemophilia and hemoglobinopathies and served as Chief Medical Officer since joining the company in 2009. Dr. Pierce was also responsible for global medical affairs for Biogen’s portfolio from 2012 to 2014. Dr. Pierce has 30 years’ experience in biotechnology research and development, beginning at Amgen, is the co-author of more than 150 scientific papers and is a named inventor in over 15 patents. Dr. Pierce also served on the Blood Products Advisory Committee at the United States Food and Drug Administration and the Committee on Blood Safety and Availability at the United States Department of Health and Human Services. He received an M.D. and a Ph.D. in Immunology, both from Case Western Reserve University in Cleveland, Ohio and did his postgraduate training in pathology and hematology research at Washington University in St. Louis, Missouri.
Qualifications: Dr. Pierce’s qualifications to serve on our Board of Directors include more than 30 years of experience in leading biotechnology research and development in small, large, public and private biotechnology and biopharmaceutical firms.
| |||||
Dawn A. Svoronos
Age 67
Director since December 2018
Committees: • Commercial (Chair) | Ms. Svoronos has served as a member of our Board of Directors since December 2018. Ms. Svoronos has more than 30 years of experience in the biopharmaceutical industry, including extensive commercial work with the multinational pharmaceutical company Merck & Co. Inc., where she held roles of increasing seniority over nearly 25 years of service. Prior to her retirement from Merck in 2011, Ms. Svoronos most recently served as President of Merck in Europe/Canada from 2009 to 2011, President of Merck in Canada from 2006 to 2009 and Vice-President of Merck for Asia Pacific from 2005 to 2006. Ms. Svoronos currently serves on the boards of directors of the following public biotechnology companies: Adverum Biotechnologies, Inc., PTC Therapeutics, Inc., Theratechnologies Inc., and Xenon Pharmaceuticals, Inc. Within the past five years, Ms. Svoronos previously served on the board of directors of Medivation Inc., a biopharmaceutical company, and Endocyte, Inc., a biopharmaceutical company. Ms. Svoronos holds a B.A. from Carleton University in Ottawa, Canada.
Qualifications: Ms. Svoronos’ qualifications to serve on our Board of Directors include her extensive experience in the global commercialization of pharmaceutical products, including her substantial international commercial experience as well as her leadership experience and her service on the boards of directors of other public companies.
| |||||
Alexis A. Thompson, M.D., M.P.H.
Age 62
Director since March 2021
Committees: • Research and Development | Dr. Thompson has served as a member of our Board of Directors since March 2021. Since April 2001, she has served as an attending physician at the Ann and Robert H. Lurie Children’s Hospital of Chicago, where she currently serves as the head of the Hematology Section and director of the Comprehensive Thalassemia Program and as the A. Watson and Sarah Armour Endowed Chair for Childhood Cancer and Blood Disorders. Since 2008, Dr. Thompson has served as the associate director for equity and minority health at the Robert H. Lurie Cancer Center and Northwestern University Feinberg School of Medicine. She has served on national advisory committees for governmental agencies as well as non-profit organizations focused on improving healthcare access, increasing workforce diversity and reducing health disparities. In 2018, Dr. Thompson served as president of the American Society of Hematology (ASH) and continues to serve on ASH’s Sickle Cell Disease Task Force. She has been a leader in multicenter collaborations, such as the National Heart, Lung, and Blood Institute’s Sickle Cell Disease Implementation Consortium, and has received numerous awards recognizing her expertise in teaching and clinical care. Dr. Thompson received an M.D. from Tulane University, received her M.P.H. from University of California, Los Angeles, and completed her postgraduate training at Children’s Hospital Los Angeles and the Children’s Hospital of Philadelphia.
Qualifications: Dr. Thompson’s qualifications to serve on our Board of Directors include more than 20 years of experience as a world-renowned hematologist and sickle cell disease expert, her leadership in academic medicine, and expertise in science, education and healthcare.
|
8 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Class II: Directors Currently Serving Until the 2023 Annual Meeting of Stockholders
Willie L. Brown, Jr.
Age 87
Director since January 2015
Committees: • Nominating and Corporate Governance (Chair) | Mr. Brown has served as a member of our Board of Directors since January 2015. Since January 2004, Mr. Brown has served as an attorney at law representing clients before state and local governments. Prior to that, from January 1996 to January 2004, Mr. Brown served as the 41st mayor of San Francisco. Mr. Brown is a practicing attorney, community leader and well-respected public official who served over 31 years in the California State Assembly, spending more than 14 years as its Speaker, from 1980 to 1995. He currently serves as chairman and chief executive officer of The Willie L. Brown, Jr. Institute on Politics and Public Service, an independent, non-profit organization providing a forum for non-partisan education, debate and discussion of public policy issues. Mr. Brown holds a degree in political science from San Francisco State University and a J.D. from University of California, Hastings College of the Law. He has received over 17 honorary degrees from prestigious institutions throughout his life.
Qualifications: Mr. Brown’s qualifications to serve on our Board of Directors include more than 50 years of political, business and non-profit experience.
| |||||
Philip A. Pizzo, M.D.
Age 76
Director since September 2015
Committees: • Nominating and Corporate Governance • Research and Development | Dr. Pizzo has served as a member of our Board of Directors since September 2015. Dr. Pizzo currently serves as the David and Susan Heckerman Professor of Pediatrics and of Microbiology and Immunology at Stanford School of Medicine and is a founding director of the Stanford Distinguished Careers Institute. He previously served as Dean of the Stanford School of Medicine from 2001 to 2012, where he was also the Carl and Elizabeth Naumann Professor of Pediatrics and of Microbiology and Immunology. Before joining Stanford, he was the physician-in-chief of Children’s Hospital in Boston and chair of the Department of Pediatrics at Harvard Medical School from 1996 to 2001. Prior to that, Dr. Pizzo was at the National Cancer Institute, eventually serving as chief of pediatrics and acting scientific director in the Division of Clinical Sciences. Dr. Pizzo is the author of more than 630 scientific articles and 16 books and monographs, has received numerous awards and honors, is a member of the National Academy of Medicine, and serves on several international boards. Dr. Pizzo holds a B.A. from Fordham University and an M.D. from the University of Rochester, School of Medicine.
Qualifications: Dr. Pizzo’s qualifications to serve on our Board of Directors include his leadership in academic medicine, and his work in the fields of pediatric medicine, science, education and healthcare.
| |||||
Wendy L. Yarno
Age 66
Director since December 2017
Committees: • Compensation (Chair) • Commercial | Ms. Yarno has served as a member of our Board of Directors since December 2017. Ms. Yarno retired in September 2008 from Merck & Co, Inc., where she served in commercial and human resource positions of increasing seniority, most recently as Chief Marketing Officer, before she retired. Prior to this position, Ms. Yarno served as General Manager for Merck’s Cardiovascular/Metabolic United States Business Unit and as Senior Vice President, Human Resources. From 2010 to 2011, Ms. Yarno was the Chief Marketing Officer of HemoShear LLC, a biotechnology research company. Ms. Yarno currently serves on the boards of directors of the following public biopharmaceutical companies: Ideaya Biosciences, Inc., Inovio Pharmaceuticals, Inc. and Tarsus Pharmaceuticals, Inc. Within the past five years, Ms. Yarno previously served as a member of the board of directors of St. Jude Medical, Inc., a medical device company, Medivation, Inc., a biopharmaceutical company, Alder Biopharmaceuticals, Inc., a biopharmaceutical company, Aratana Therapeutics, Inc., a therapeutics company, and MyoKardia, Inc., a biopharmaceutical company. Ms. Yarno received an M.B.A. from Temple University, Fox School of Business, and a B.S. in business administration from Portland State University.
Qualifications: Ms. Yarno’s qualifications to serve on our Board of Directors include her extensive experience commercializing pharmaceutical products, and her extensive operational and senior management experience, in the biopharmaceutical industry, as well as her experience serving on the board of directors (and certain key standing committees) of other biopharmaceutical companies.
|
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 9
Board Skills, Experience and Diversity
The below table provides information regarding our Board of Directors, including certain attributes possessed by our directors, which we and our Board of Directors believe are relevant to our business. The below table does not include all of the attributes of our directors, and the fact that a director may not have a particular attribute does not mean such director is unable to contribute to the decision-making process in that area.
| Knowledge, Skills and Experience | ||||||||||||||||||||||||||||||||||||||||||||
| 6 of 10 | 8 of 10 | |||||||||||||||||||||||||||||||||||||||||||
| Executive leadership | Healthcare/life sciences | |||||||||||||||||||||||||||||||||||||||||||
| 7 of 10 | 7 of 10 | |||||||||||||||||||||||||||||||||||||||||||
| Business strategy/operations | Public company/other governance role | |||||||||||||||||||||||||||||||||||||||||||
| 6 of 10 | 6 of 10 | |||||||||||||||||||||||||||||||||||||||||||
| Commercial experience | Government/regulatory | |||||||||||||||||||||||||||||||||||||||||||
| 7 of 10 | 4 of 10 | |||||||||||||||||||||||||||||||||||||||||||
| International operating experience | Scientific expertise | |||||||||||||||||||||||||||||||||||||||||||
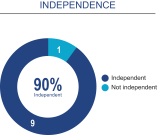
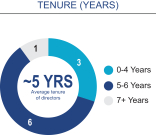
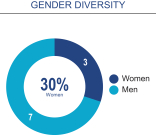
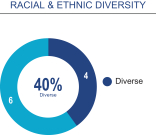
Board composition overview
 |  |  |  |
10 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Board of Directors’ Role in Risk Management
Risk is inherent with every business, and how well a business manages risk can ultimately determine its success. We face a number of risks, including but not limited to risks relating to our financial condition, commercialization activities, research and development activities, clinical and regulatory matters, operations and intellectual property. Management is responsible for the day-to-day management of risks we face, while our Board of Directors, as a whole and through its committees, has responsibility for the oversight of risk management. In its risk oversight role, our Board of Directors has the responsibility to satisfy itself that the risk management processes designed and implemented by management are adequate and functioning as designed.
The role of our Board of Directors in overseeing the management of our risks is conducted primarily through committees of the Board of Directors, as disclosed in the descriptions of each of the committees below and in the charters of each of the committees. For example, the Compensation Committee assesses risks created by the incentives inherent in our compensation programs, policies and practices. The full Board of Directors (or the appropriate board committee in the case of risks that are under the purview of a particular committee) discusses with management our major risk exposures, their potential impact on our company, and the steps we take to manage them. When a board committee is responsible for evaluating and overseeing the management of a particular risk or risks, the chair of the relevant committee reports on the discussion to the full Board of Directors during the committee reports portion of the next board meeting. This enables our Board of Directors and its committees to coordinate the risk oversight role, particularly with respect to risk interrelationships.
Board of Directors and Committees of the Board
During 2020, the Board of Directors held a total of four meetings. All directors attended at least 75% of the aggregate of the number of Board meetings and the number of meetings of Board committees on which each such director served during the time each such director served on the Board or such committees.
Our Board of Directors has determined that all of our directors, except for Dr. Love, are independent, as determined in accordance with the rules of The Nasdaq Stock Market LLC, or NASDAQ, and the SEC. In making such independence determination, the Board of Directors considered the relationships that each non-employee director has with us and all other facts and circumstances that the Board of Directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director. In considering the independence of the directors listed above, our Board of Directors also considers the association, if any, of our directors with the holders of more than 5% of our common stock. There are no family relationships among any of our directors or executive officers.
The Board of Directors has a standing Audit Committee, Compensation Committee, and Nominating and Corporate Governance Committee. Each of our Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee is composed entirely of independent directors in accordance with current NASDAQ listing standards. Furthermore, our Audit Committee meets the enhanced independence standards established by the Sarbanes-Oxley Act of 2002 and related rulemaking of the SEC. The authority and responsibility of each of these committees are summarized below, and more detailed descriptions of their functions are included in their written charters, which are available along with our corporate governance guidelines on our website at www.gbt.com.
Pursuant to their charters, each of the Audit Committee, Compensation Committee, and Nominating and Corporate Governance Committee is authorized to access, at our expense, such internal and external resources as the particular committee deems necessary or appropriate to fulfill its defined responsibilities. Each committee has sole authority to approve fees, costs and other terms of engagement of such outside resources.
The Board of Directors also has a standing Research and Development Committee and Commercial Committee, which are advisory committees.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 11
The following chart provides membership as of April 28, 2021, for each of the Board of Directors’ standing committees:
|
| Audit Committee | Compensation Committee | Nominating and Corporate Governance Committee | Research and Development Committee (advisory) | Commercial Committee (advisory) | |||||
Willie L. Brown, Jr. |
|
| C |
|
| |||||
Scott W. Morrison | C | ✔ |
|
| ✔ | |||||
Deval L. Patrick | ✔ |
| ✔ |
|
| |||||
Mark L. Perry | ✔ | ✔ |
|
| ✔ | |||||
Glenn F. Pierce, M.D., Ph.D. |
|
| ✔ | C |
| |||||
Philip A. Pizzo, M.D. |
|
| ✔ | ✔ |
| |||||
Dawn A. Svoronos |
|
|
|
| C | |||||
Alexis A. Thompson, M.D., M.P.H. |
|
|
| ✔ |
| |||||
Wendy L. Yarno |
| C |
|
| ✔ | |||||
✔ - Committee member C - Committee chair
Audit Committee | The Audit Committee’s responsibilities include: | |
Chair: Mr. Morrison
Members: Mr. Patrick Mr. Perry
| • appointing, approving the compensation of, and assessing the independence of our independent registered public accounting firm;
• pre-approving auditing and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm;
• reviewing the audit plan with our independent registered public accounting firm and members of management responsible for preparing our financial statements;
• reviewing and discussing with management and our independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us;
• coordinating the oversight and reviewing the adequacy of our internal control over financial reporting and performance of our internal audit function, if any;
• establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns;
• recommending, based on the Audit Committee’s review and discussions with management and our independent registered public accounting firm, whether our audited financial statements shall be included in our Annual Report on Form 10-K;
• monitoring the integrity of our financial statements and our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters;
• preparing the Audit Committee report required by SEC rules to be included in our annual proxy statement;
• reviewing all related person transactions for potential conflict of interest situations and approving all such transactions;
• reviewing quarterly earnings releases;
• overseeing our compliance with legal and regulatory requirements;
• discussing guidelines and policies governing risk assessment matters, and monitoring and overseeing matters related to cybersecurity risks affecting the Company; and
• periodically conducting a performance evaluation of the Audit Committee and reporting to the Board on the results of such evaluation.
| |
12 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
During 2020, Messrs. Morrison, Patrick and Perry and Ms. Svoronos served on the Audit Committee, with Mr. Patrick being appointed as a member of the Audit Committee in June 2020, and the Committee held four meetings. Our Board of Directors has determined that each of Messrs. Morrison, Patrick and Perry is an “Audit Committee financial expert,” as defined under the applicable rules of the SEC. In April 2021, Ms. Svoronos stepped down as a member of the Audit Committee, subsequent to approving the Audit Committee Report included below.
Compensation Committee | The Compensation Committee’s responsibilities include: | |
Chair: Ms. Yarno
Members: Mr. Morrison Mr. Perry | • reviewing and approving corporate goals relevant to the compensation of our Chief Executive Officer;
• evaluating the performance of our Chief Executive Officer in light of such corporate goals and determining the compensation of our Chief Executive Officer;
• reviewing and approving any peer group of companies used to inform the Company’s evaluation of compensation for its employees and directors;
• reviewing and approving the compensation of our other executive officers;
• reviewing and establishing our overall management compensation, philosophy, policy and practices;
• reviewing, overseeing, and administering our compensation and similar plans;
• evaluating and assessing potential and current compensation advisors in accordance with the independence standards identified in the applicable NASDAQ Stock Market rules;
• retaining and approving the compensation of any compensation advisors;
• reviewing and recommending to our Board of Directors our policies and procedures for the grant of equity-based awards;
• reviewing and making recommendations to our Board of Directors with respect to non-employee director compensation;
• reviewing and discussing with the Board of Directors the corporate succession plans for the Chief Executive Officer and other executive officers;
• preparing the compensation committee report required by the SEC rules to be included in our annual proxy statement and Annual Report on Form 10-K;
• reviewing and discussing with management the compensation discussion and analysis to be included in our annual proxy statement or Annual Report on Form 10-K; and
• periodically conducting a performance evaluation of the Compensation Committee and reporting to the Board on the results of such evaluation.
| |
Pursuant to its charter, the Compensation Committee has the authority to engage compensation consultants to assist in its evaluation of executive and non-employee director compensation. For 2020, the Compensation Committee engaged Compensia, Inc., a national compensation consulting firm, or Compensia, as a compensation consultant to, among other things, develop a peer group of companies to assess the competitiveness of our executive, equity and non-employee director compensation programs, advise on our non-employee director compensation and to review our equity compensation program and broader equity practices. Our Compensation Committee plans to engage Compensia or one or more other third-party compensation advisors to provide similar information and advice in future years for consideration in establishing overall compensation for our executives and non-employee directors. We do not believe the retention of, and the work performed by, Compensia creates any conflict of interest. See “Executive Compensation-Compensation Discussion and Analysis-Governance of Executive Compensation Program—Role of Compensation Consultant” below for more information.
During 2020, Messrs. Perry and Morrison and Ms. Yarno served on the Compensation Committee, and the Committee held five meetings.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 13
Nominating and Corporate Governance Committee | The Nominating and Corporate Governance Committee’s responsibilities include: | |
Chair: Mr. Brown
Members: Mr. Patrick Dr. Pierce Dr. Pizzo | • developing and recommending to the Board of Directors criteria for board and committee membership;
• establishing procedures for identifying and evaluating Board of Director candidates, including nominees recommended by stockholders;
• reviewing the size and composition of the Board of Directors to ensure that it is composed of members containing the appropriate skills and expertise to advise us;
• identifying individuals qualified to become members of the Board of Directors;
• recommending to the Board of Directors the persons to be nominated for election as directors and to each of the Board’s committees;
• developing and recommending to the Board of Directors a code of business conduct and ethics and a set of corporate governance guidelines;
• developing a mechanism by which violations of the code of business conduct and ethics can be reported in a confidential manner;
• overseeing the evaluation of the Board of Directors and its committees;
• reviewing and discussing with the Board of Directors the corporate succession plans for directors; and
• periodically conducting a performance evaluation of the Nominating and Corporate Governance Committee and reporting to the Board on the results of such evaluation.
| |
During 2020, Messrs. Brown and Patrick, Drs. Pierce and Pizzo and Ms. Yarno served on the Nominating and Corporate Governance Committee, and the Committee held two meetings. In June 2020, Mr. Patrick and Dr. Pierce were appointed as members of the Nominating and Corporate Governance Committee, and Ms. Yarno was removed as a member of such committee.
Research and Development Committee (Advisory) | ||
Chair: Dr. Pierce
Members: Dr. Pizzo Dr. Thompson
| Our Board of Directors formed a Research and Development Committee in March 2016. The Research and Development Committee’s responsibilities include providing advice and support related to our research and development programs, strategy and goals. Drs. Pierce, Pizzo and Thompson serve on the Research and Development Committee, which is chaired by Dr. Pierce.
| |
Commercial Committee (Advisory) | ||
Chair: Ms. Svoronos
Members: Mr. Morrison Mr. Perry Ms. Yarno
| Our Board of Directors formed a Commercial Committee in December 2018. The Commercial Committee’s responsibilities include providing advice and support related to our potential and actual commercialization of any products, including our strategy and goals from pre-launch planning through any approval, launch and later phases of the product lifecycle. Messrs. Morrison and Perry and Mses. Svoronos and Yarno serve on the Commercial Committee, which is chaired by Ms. Svoronos.
| |
We do not currently have a Chair of the Board, but we have appointed Mr. Perry to serve as our lead independent director. We believe that the appointment of a lead independent director allows our Chief Executive Officer to focus on our day-to-day business, while allowing the lead independent director to lead our Board of Directors in its fundamental role of providing advice to and independent oversight of management. Our Board of Directors recognizes the time, effort and energy that the Chief Executive Officer is required to devote to his position in the current business environment, as well as the commitment required to serve as our lead independent director, particularly as our Board of Directors’ oversight responsibilities continue to grow.
14 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
While our Bylaws and corporate governance guidelines do not require that we appoint a separate Chair of the Board or lead independent director and Chief Executive Officer, our Board of Directors believes that having a Chief Executive Officer and a separate designated lead independent director is the appropriate leadership structure for us at this time and demonstrates our commitment to good corporate governance. Our separated lead independent director and Chief Executive Officer positions are augmented by the independence of nine of our 10 directors, and our entirely independent Board committees that provide appropriate oversight in the areas described above. At executive sessions of independent directors, these directors speak candidly on any matter of interest, without the Chief Executive Officer or other executives present. The independent directors met four times in 2020 without management present. We believe this structure provides consistent and effective oversight of our management and the Company.
Annual Performance Evaluations; Assessment of Charters; Director Education
Our Board of Directors, as well as each of its standing committees, conducts an annual self-evaluation, which includes a review of its performance and, in the case of each of the committees, an assessment of the adequacy and appropriateness of its charter. The Nominating and Corporate Governance Committee is responsible for overseeing this evaluation process, evaluating all standing committees and their charters and recommending to our Board of Directors any changes to our Board and the authority, charters, compositions and chairs of such committees.
Each director is expected to maintain the necessary level of expertise to perform his or her responsibilities as a director. Our Board of Directors regularly discusses recent developments related to corporate governance, legal and regulatory compliance, disclosure obligations or industry-specific matters. In addition, we encourage our directors to participate in outside continuing education programs to assist them in maintaining such expertise and may, from time to time and depending on the circumstances, pay for all or a portion of such outside continuing education programs.
The director qualifications developed to date focus on what our Board believes to be essential competencies to effectively serve on the Board of Directors. The Nominating and Corporate Governance Committee reassesses such criteria from time to time and submits any proposed changes to the Board of Directors for approval. Presently, at a minimum, the Nominating and Corporate Governance Committee must be satisfied that each nominee it recommends (i) has experience at a strategic or policymaking level in a business, government, non-profit or academic organization of high standing, (ii) is highly accomplished in his or her respective field, with superior credentials and recognition, (iii) is well regarded in the community and has a long-term reputation for the highest personal and professional integrity, (iv) has sufficient time and availability to devote to the affairs of the Company, particularly in light of the number of boards of directors on which such nominee may serve, (v) to the extent such nominee serves or has previously served on other boards, the nominee has a demonstrated history of actively contributing at board meetings, and (vi) will be effective, collectively with other members of and/or candidates for our Board of Directors, in serving the long-term interests of our stockholders.
In addition to those minimum qualifications, the Nominating and Corporate Governance Committee recommends that our Board of Directors select persons for nomination to help ensure that:
| • | a majority of our Board is “independent” in accordance with NASDAQ standards; |
| • | each of the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee be comprised entirely of independent directors; and |
| • | at least one member of the Audit Committee shall have the experience, education and other qualifications necessary to qualify as an “Audit Committee financial expert” as defined by the rules of the SEC. |
In addition to other standards the Nominating and Corporate Governance Committee may deem appropriate from time to time for the overall structure and compensation of the Board of Directors, the Nominating and Corporate Governance Committee may consider the following factors when recommending that our Board select persons for nomination:
| • | whether a nominee has direct experience in the biotechnology or pharmaceuticals industry or in other fields relevant to our operations; and |
| • | whether the nominee, if elected, assists in achieving a mix of Board members that represents a diversity of background and experience. |
Although we have no formal policy regarding board diversity, we and our Board of Directors believe that corporate board diversity, including gender, racial and ethnic diversity, can provide a more effective and dynamic Board of Directors that can better mitigate risk and enhance long-term performance for stockholders. Accordingly, the Nominating and Corporate Governance Committee considers whether nominees assist in achieving a mix of Board members that represents a diversity of background and experience.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 15
The Nominating and Corporate Governance Committee adheres to the following process for identifying and evaluating nominees for the Board of Directors. First, it solicits recommendations for nominees from non-management directors, our Chief Executive Officer, other executive officers, third-party search firms or any other source it deems appropriate. The Nominating and Corporate Governance Committee then reviews and evaluates the qualifications of proposed nominees and conducts inquiries it deems appropriate; all proposed nominees are evaluated in the same manner, regardless of who initially recommended such nominee. In reviewing and evaluating proposed nominees, the Nominating and Corporate Governance Committee may consider, in addition to the minimum qualifications and other criteria for Board membership approved by our Board from time to time, all facts and circumstances that it deems appropriate or advisable, including, among other things, the skills of the proposed nominee, his or her depth and breadth of business experience or other background characteristics, his or her independence and the needs of the Board.
If the Nominating and Corporate Governance Committee decides to retain a third-party search firm to identify proposed nominees, it has sole authority to retain and terminate such firm and to approve any such firm’s fees and other retention terms.
Each nominee for election as director at the Annual Meeting is recommended by the Nominating and Corporate Governance Committee and is presently a director and stands for re-election by the stockholders. From time to time, we may pay fees to third-party search firms to assist in identifying and evaluating potential nominees, although no such fees have been paid in connection with nominations to be acted upon at the Annual Meeting.
Pursuant to our Bylaws, stockholders who wish to nominate persons for election to the Board of Directors at an annual meeting must be a stockholder of record at the time of giving the notice, entitled to vote at the meeting, present (in person or by proxy) at the meeting and must comply with the notice procedures in our Bylaws. A stockholder’s notice of nomination to be made at an annual meeting must be delivered to our principal executive offices not less than 90 days nor more than 120 days before the anniversary date of the immediately preceding annual meeting. However, if an annual meeting is more than 30 days before or more than 60 days after such anniversary date, the notice must be delivered no later than the later of the 90th day prior to such annual meeting or the 10th day following the day on which the first public announcement of the date of such annual meeting was made. A stockholder’s notice of nomination may not be made at a special meeting unless such special meeting is held in lieu of an annual meeting. The stockholder’s notice must include the following information for the person making the nomination:
| • | name and address; |
| • | the class and number of shares of the Company owned beneficially or of record; |
| • | disclosure regarding any derivative, swap or other transactions which give the nominating person economic risk similar to ownership of shares of the Company or provide the opportunity to profit from an increase in the price of value of shares of the Company; |
| • | any proxy (other than a revocable proxy given in response to a public proxy solicitation made pursuant to, and in accordance with, the Exchange Act), agreement, arrangement, understanding or relationship that confers a right to vote any shares of the Company; |
| • | any agreement, arrangement, understanding or relationship engaged in for the purpose of acquiring, holding, disposing or voting of any shares of any class or series of capital stock of the Company; |
| • | any rights to dividends or other distributions on the shares that are separate from the underlying shares; |
| • | any performance-related fees that the nominating person is entitled to based on any increase or decrease in the value of any shares of the Company; |
| • | a description of all agreements, arrangements or understandings by and between the proposing stockholder and another person relating to the proposed business (including an identification of each party to such agreement, arrangement or understanding and the names, addresses and class and number of shares owned beneficially or of record of other stockholders known by the proposing stockholder support such proposed business); |
| • | a statement whether or not the proposing stockholder will deliver a proxy statement and form of proxy to holders of, in the case of a business proposal, at least the percentage of voting power of all shares of capital stock required to approve the proposal or, in the case of director nominations, at least the percentage of voting power of all of the shares of capital stock reasonably believed by the proposing stockholder to be sufficient to elect the nominee; and |
| • | any other information relating to the nominating person that would be required to be disclosed in a proxy statement filed with the SEC. |
With respect to proposed director nominees, the stockholder’s notice must include all information required to be disclosed in a proxy statement in connection with a contested election of directors or otherwise required pursuant to Regulation 14A under the Exchange Act (including such person’s written consent to being named in the proxy statement as a nominee and to serving as a director if elected).
16 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
For matters other than the election of directors, the stockholder’s notice must also include a brief description of the business desired to be brought before the meeting, the reasons for conducting such business at the meeting and any material interest in such business of the stockholder(s) proposing the business.
The stockholder’s notice must be updated and supplemented, if necessary, so that the information required to be provided in the notice is true and correct as of the record date for the meeting and as of the date that is 10 business days prior to the meeting.
The Board of Directors, a designated committee thereof or the chairman of the meeting will determine if the procedures in our Bylaws have been followed, and if not, declare that the proposal or nomination be disregarded. The nominee must be willing to provide any other information reasonably requested by the Nominating and Corporate Governance Committee in connection with its evaluation of the nominee’s independence. There have been no material changes to the process by which stockholders may recommend nominees to our Board of Directors.
Stockholder Communications with the Board of Directors
Stockholders may send correspondence to the Board of Directors c/o the lead independent director of the Board at our principal executive offices at the address set forth above. We will forward all correspondence addressed to the Board or any individual Board member. Stockholders may also communicate online with our Board of Directors as a group by accessing our website at www.gbt.com and selecting “IR Contact” under the Investors tab.
Director Attendance at Annual Meetings
Directors are encouraged to attend our annual meetings of stockholders, and eight of our then nine directors attended the 2020 Annual Meeting of Stockholders.
Compensation Committee Interlocks and Insider Participation
None of the members of the Compensation Committee is, or has at any time during the past fiscal year been, an officer or employee of the Company or had any relationship requiring disclosure under Item 404 of Regulation S-K. None of the members of the Compensation Committee has formerly been an officer of the Company. None of our executive officers serve, or in the past fiscal year, have served as a member of the Board of Directors or Compensation Committee of any other entity that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
Environmental, Social and Governance Practices
Our mission is to discover, develop and deliver life-changing treatments for people living with grievous blood-based disorders, starting with sickle cell disease. We view our environmental, social and governance practices as important pillars that will help us achieve our mission and contribute to sustainable long-term performance, including recruiting and retaining the best talent.
Environmental
We seek to build a sustainable, vibrant company to develop medicines with optimized efficacy and safety for patients globally. We recognize that the sustainability of our company is linked to our ability to understand and engage all our stakeholders in a consistent and in meaningful manner. Starting with our Board of Directors and our leadership team, we are committed to long-term value driven by the pillars of governance, social responsibility, and integrity across all we do, including employee engagement, research and development, operations, commercialization and access to medicines for patients. Our efforts to help preserve the environment include, among other things, moving to our new headquarters location with pending LEED Silver certification (with efforts underway to achieve LEED Gold status); utilizing technology to reduce energy and water consumption; reducing, recycling and reusing our resources when practicable; promoting employee commuting programs; and partnering with third-party organizations for the ethical treatment of animals in research.
Social—Diversity, Equity & Inclusion
We have a values-driven culture that starts with our senior management team. Since our founding, we have recognized that having a more diverse, equitable and inclusive environment leads to increased performance, better decision making, increased productivity, and greater motivation. As we believe our efforts in this area are important to our success, we are pleased to be one of only 380 companies, including 22 in healthcare, included in the 2021 Bloomberg Gender Equality Index, which measures gender equality across five pillars: female leadership and talent pipeline, equal pay and gender pay parity, inclusive culture, sexual harassment policies, and pro-women brand.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 17
Our team represents a broad range of cultural and professional backgrounds that enrich our culture and drive our future growth and success:
| GBT Employee Population as of December 31, 2020 | Female | People of Color* | ||
Overall Population | 58% | 59% | ||
Senior Management Team | 33% | 22% | ||
Executive Director and Above (excluding Senior Management Team) | 40% | 60% | ||
Associate Director—Senior Director | 54% | 53% | ||
Senior Manager & Below | 66% | 65% | ||
| * | All U.S. Equal Employment Opportunity Commission’s categories of race other than white (not Hispanic or Latino). |
We are committed to fostering workplace development, diversity and inclusion at our company and more broadly across the biotechnology industry. We are also dedicated to being at the forefront of efforts to develop a diverse and talented global workforce, and doing our part to foster diversity and inclusion among our employees and vendors. As part of these efforts, we have established employee-resource groups, or ERGs, to build awareness, grow personally and professionally, and give back to communities, with our first two ERGs focused on social justice issues and advancement for women.
We are actively involved in supporting our local and patient communities, which have been underserved, including through volunteering and charitable donation initiatives. Due in part to the COVID-19 pandemic, 2020 was an extraordinarily difficult year for the sickle cell disease, or SCD, patient community, and we are proud to have helped in a variety of ways. This included our total funding to SCD organizations in 2020 of more than $2 million, including more than $350,000 in contributions from GBT, our board of directors and our employees. In addition, we also continue to support non-profit organizations through our ongoing Access to Excellent Care for Sickle Cell Patients (ACCEL) Grant Program, which we founded in February 2019 to provide grant funding to accelerate the development of sustainable access to care programs for people with SCD.
We believe that a critical component of workplace diversity is our commitment to pay equity. To that end, in 2020, we engaged Radford, part of the rewards practice at Aon plc, to conduct a detailed independent assessment of the impact of gender and ethnic diversity on our pay practices for the first time. This assessment revealed no evidence of discrepancies in our pay practices based on gender and ethnicity. Further, the results of the assessment showed that differences in pay among our employees were attributed to factors such as performance, job level and experience, which are considered “bona fide” factors. We intend to repeat this assessment on a regular basis.
Governance
One of our core values is accountability, and we commit to operating with truthfulness and transparency in accordance with the highest ethical and corporate governance standards. To this end, we maintain a Code of Business Conduct and Ethics and hold ourselves accountable to it in all we do. All of our employees across our operations are provided with training and reference materials to reinforce this commitment to integrity and ethics in our business. Our policies are clearly defined and include guidance on topics including, but not limited to, conflicts of interest; compliance with laws, rules and regulations; confidentiality; fair dealing; antitrust compliance; accuracy of records; corporate disclosure; improper payments; and interactions with healthcare professionals and government employees.
Our Board of Directors, along with the Audit, Compensation and Nominating and Corporate Governance committees, conducts an annual governance review, which includes review, benchmarking and revision of our significant corporate and compensation policies. In addition to our Code of Business Conduct and Ethics mentioned above, these policies include, among others, our Anti-Bribery and Anti-Corruption Policy, our Corporate Governance Guidelines, and our Insider Trading Policy. This periodic review also encompasses the structure and governance of our Board of Directors, voting standards for directors and other matters, and review of the Company’s defensive profile. The Nominating and Corporate Governance Committee is responsible for overseeing this governance review process, and each committee evaluates policies within its purview and recommends changes to our Board of Directors.
18 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
In March 2020, we adopted a stock ownership policy for our non-employee directors, which requires each director to acquire and hold a number of shares of our common stock equal in value to at least three times his or her applicable annual cash retainer for regular service on our Board of Directors, excluding any annual cash retainers paid for committee service, until such director’s service on the Board of Directors ceases. We only count directly and beneficially owned shares, including, with respect to non-employee directors, 50% of shares underlying vested and unexercised in-the-money stock options. Each non-employee director has until the later of April 1, 2025, or the Initial Determination Date, or the April 1st in the year that is the fifth anniversary of his or her initial election to our Board of Directors, to attain the required ownership level. Once a director satisfies his or her stock ownership requirement, the director must continue to satisfy such stock ownership requirement as assessed on each April 1st thereafter, or Determination Date. If a director fails to satisfy such stock ownership requirement as of any Determination Date (including the Initial Determination Date), then such director shall be required to come into compliance with his or her applicable stock ownership requirement within two years following the Determination Date on which he or she failed to satisfy such stock ownership requirement. As of April 1, 2021, eight of our 10 non-employee directors met their stock ownership requirement, and the remaining non-employee directors have until the later of the Initial Determination Date or the April 1st in the year that is the fifth anniversary of his or her initial election to our Board of Directors to attain the required ownership level.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 19
NON-EMPLOYEE DIRECTOR COMPENSATION
The Non-Employee Director Compensation Policy adopted by our Board of Directors is designed to provide a total compensation package, including cash, stock options and restricted stock units, or RSUs, that is competitive in the market to enable us to attract and retain, on a long-term basis, high-caliber non-employee directors.
The Compensation Committee and our Board of Directors review non-employee director compensation and the Non-Employee Director Compensation Policy, including an assessment compared to peer companies, on an annual basis. In March 2020, our Board approved certain adjustments to our Director Compensation Policy upon the recommendation of our Compensation Committee in consultation with the Compensation Committee’s compensation consultant, Compensia. As a result, the annual cash retainer for Board membership increased from $40,000 to $45,000 and the additional annual cash retainer for lead independent director or non-executive Chairperson was increased from $20,000 to $25,000 in order to align cash compensation with the 50th percentile of our peers. The Non-Employee Director Compensation Policy, as amended, provides that all non-employee directors would receive cash compensation for service on our Board of Directors and committees of the Board of Directors as set forth below, prorated based on days of service during a calendar year.
Board of Directors: | Annual Retainer | ||||
All non-employee members | $45,000 | ||||
| Additional Annual Retainer | |||||
Lead Independent Director or Non-Employee Chairperson of the Board of Directors: | $25,000 | ||||
Audit Committee: |
| ||||
Chairperson | $20,000 | ||||
Non-Chairperson members | $10,000 | ||||
Compensation Committee: |
| ||||
Chairperson | $15,000 | ||||
Non-Chairperson members | $ 7,500 | ||||
Nominating and Corporate Governance Committee: |
| ||||
Chairperson | $10,000 | ||||
Non-Chairperson members | $ 5,000 | ||||
Research and Development Committee: |
| ||||
Chairperson | $15,000 | ||||
Non-Chairperson members | $ 7,500 | ||||
Commercial Committee: |
| ||||
Chairperson | $15,000 | ||||
Non-Chairperson members | $ 7,500 | ||||
In addition to the cash retainer amounts described above, the Non-Employee Director Compensation Policy also provides that each new non-employee director who is initially appointed or elected to our Board of Directors will receive a stock option and RSU grant. Prior to the March 2020 amendment, the Non-Employee Director Compensation Policy provided for an option to purchase 15,000 shares of our common stock and an RSU grant for 9,600 RSUs. As amended in March 2020, the Non-Employee Director Compensation Policy now provides for an option to purchase shares of our common stock with an aggregate value of $415,000, subject to a maximum of 11,200 shares, and an RSU grant for an aggregate value of $415,000, subject to a maximum of 7,200 RSUs, in order to align equity compensation with the 75th percentile of our peers but also to include a maximum share limit in the event of extraordinary market fluctuations. The initial grant of stock options will vest in equal monthly installments during the 36 months following the grant date, subject to the director’s continued service on our Board of Directors. The initial grant of RSUs will vest in equal annual installments during the three years following the grant date, subject to the director’s continued service on our Board of Directors.
Thereafter, on the date of each annual meeting of stockholders, each continuing non-employee director will be eligible to receive an annual stock option and RSU grant. Prior to the March 2020 amendment, the Non-Employee Director
20 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Compensation Policy provided for an option to purchase 7,500 shares of our common stock and an RSU grant for 4,800 RSUs. As amended in March 2020, the Non-Employee Director Compensation Policy now provides for an option to purchase shares of our common stock with an aggregate value of $207,500, subject to a maximum of 5,600 shares, and an RSU grant for an aggregate value of $207,500, subject to a maximum of 3,600 RSUs, in order to align equity compensation with the 75th percentile of our peers but also to include a maximum share limit in the event of extraordinary market fluctuations. The annual grant of stock options will vest 1/12th on each month following the grant date for 11 months and the remaining 1/12th on the earlier of (i) the one-year anniversary of the grant date or (ii) our next annual meeting of stockholders, and such annual grant of RSUs will vest on the earlier of (A) the one-year anniversary of the grant date or (B) our next annual meeting of stockholders, in each case subject to the director’s continued service on our Board of Directors through each applicable vesting date. In addition, as amended in March 2020, the Non-Employee Director Compensation Policy now provides that if a non-employee director joins our Board of Directors on a date other than our annual meeting of stockholders, then such non-employee director will be granted a pro-rata portion of the annual stock option and RSU grant based on the time between the non-employee director’s appointment and our next annual meeting of stockholders in order to align with market practice.
For awards of restricted stock or RSUs is denominated in dollars, the number of shares subject to the award will be determined by dividing the dollar value by the average closing market price on the NASDAQ Global Market of a share of our common stock over the trailing 20-trading day period ending on the trading day immediately preceding the grant date. For awards of stock options denominated by reference to a fair value calculated under FASB ASC Topic 718, the number of shares to be subject to such stock option will be determined based on such fair value. In addition, all of the foregoing stock options will be granted with a per share exercise price equal to the fair market value of a share of our common stock on the date of grant, and will be exercisable (to the extent vested) for up to one year following cessation of the director’s service on our Board of Directors, so long as the director is not removed for cause.
All stock options and RSUs granted to our non-employee directors pursuant to the Non-Employee Director Compensation Policy are subject to full acceleration of vesting upon the consummation of a “Sale Event,” as defined in the 2015 Stock Option and Incentive Plan, as amended from time to time, or 2015 Plan. Our non-employee directors could also be granted such additional stock options and RSUs in such amounts and on such dates as our Board of Directors recommended.
We have also agreed to reimburse all reasonable out-of-pocket expenses incurred by non-employee directors in attending Board and committee meetings.
2020 Director Compensation Table
The table below sets forth information with respect to the compensation received by our non-employee directors during the fiscal year ended December 31, 2020. Dr. Love does not receive compensation for service on the Board of Directors and the compensation paid to Dr. Love as an employee of the Company is set forth under the heading “Executive Compensation—2020 Summary Compensation Table” below.
Name | Fees Earned or Paid in Cash ($) | Stock Awards ($)(1) | Option Awards ($)(2) | Total ($) | ||||||||||||||||
Willie L. Brown, Jr.(3) | $53,847 | $196,646 | $207,478 | $457,971 | ||||||||||||||||
Scott W. Morrison(4) | $78,847 | $196,646 | $207,478 | $482,971 | ||||||||||||||||
Deval L. Patrick(5) | $34,767 | $361,646 | $232,432 | $628,845 | ||||||||||||||||
Mark L. Perry(6) | $92,597 | $196,646 | $207,478 | $496,721 | ||||||||||||||||
Glenn F. Pierce, M.D., Ph.D.(7) | $61,539 | $196,646 | $207,478 | $465,663 | ||||||||||||||||
Philip A. Pizzo, M.D.(8) | $56,347 | $196,646 | $207,478 | $460,471 | ||||||||||||||||
Dawn A Svoronos(9) | $68,847 | $196,646 | $207,478 | $472,971 | ||||||||||||||||
Wendy L Yarno(10) | $68,847 | $196,646 | $207,478 | $472,971 | ||||||||||||||||
| (1) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the restricted stock and/or RSUs granted during 2020, computed in accordance with Financial Accounting Standard Board Accounting Standards Codification Topic 718, or FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 10 to our financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020. These amounts do not reflect the actual economic value that may be realized by the directors upon the vesting or settlement of the restricted stock or RSUs, as applicable, or the sale of the common stock underlying such awards. |
| (2) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the stock option awards granted during 2020, computed in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 10 to our financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020. These amounts do not reflect the actual economic value that may be realized by the directors upon the exercise of the stock options or the sale of the common stock underlying such stock options. |
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 21
| (3) | Mr. Brown held stock options to purchase an aggregate of 81,347 shares of common stock and 3,037 RSUs as of December 31, 2020. |
| (4) | Mr. Morrison held stock options to purchase an aggregate of 87,747 shares of common stock and 3,037 RSUs as of December 31, 2020. |
| (5) | Mr. Patrick resigned from the Board of Directors in November 2019, and rejoined the Board of Directors in May 2020. In connection with rejoining the Board of Directors, Mr. Patrick received a pro-rata portion of the annual stock option and RSU grant based on the time between when he rejoined the Board of Directors and the Company’s 2020 annual meeting of stockholders. Mr. Patrick received the regular annual stock option and RSU grant, as described above, at the 2020 annual meeting of stockholders. Mr. Patrick held stock options to purchase an aggregate of 5,872 shares of common stock and 3,037 RSUs as of December 31, 2020. |
| (6) | Mr. Perry held stock options to purchase an aggregate of 27,747 shares of common stock and 3,037 RSUs as of December 31, 2020. |
| (7) | Dr. Pierce held stock options to purchase an aggregate of 72,747 shares of common stock and 3,037 RSUs as of December 31, 2020. |
| (8) | Dr. Pizzo held stock options to purchase an aggregate of 57,747 shares of common stock and 3,037 RSUs as of December 31, 2020. |
| (9) | Ms. Svoronos held stock options to purchase an aggregate of 42,747 shares of common stock and 3,037 RSUs as of December 31, 2020. |
| (10) | Ms. Yarno held stock options to purchase an aggregate of 57,747 shares of common stock and 3,037 RSUs as of December 31, 2020. |
22 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
NON-BINDING, ADVISORY VOTE ON THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS
The Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, or Dodd-Frank Act, added Section 14A to the Securities Exchange Act of 1934, as amended, which requires that we provide our stockholders with the opportunity to vote to approve, on a non-binding, advisory basis, the compensation of our named executive officers as disclosed in this Proxy Statement, commonly known as a “Say-on-Pay” vote. Stockholders may also abstain from voting. This Say-on-Pay vote is not intended to address any specific element of the compensation of our named executive officers, but rather the overall executive compensation of our named executive officers and our overall executive compensation program, philosophy and practices as described in this Proxy Statement.
As described in this Proxy Statement, we believe the compensation of our named executive officers and our executive compensation program, philosophy and practices are appropriate, and enable us to attract, motivate and retain top-performing executive officers, including our named executive officers, while aligning the long-term interests of our executive officers with the long-term interests of our stockholders. Accordingly, we ask our stockholders to approve, on a non-binding, advisory basis, the following resolution at the Annual Meeting:
RESOLVED, that the compensation paid to the Company’s named executive officers as disclosed pursuant to Item 402 of Regulation S-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion is hereby APPROVED.
The approval of this advisory non-binding proposal requires the affirmative vote of a majority of the voting power of the shares of our common stock present virtually or by proxy at the Annual Meeting and entitled to vote thereon. Abstentions and broker non-votes will have no effect on this proposal.
This Say-on-Pay vote is advisory; therefore, it is not binding on the Company, our Board of Directors or our Compensation Committee. However, our Board of Directors and our Compensation Committee will consider the result of this year’s vote in reviewing and determining the compensation of our named executive officers in the future because we value the opinions of our stockholders.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE, ON A NON-BINDING, ADVISORY BASIS, “FOR” THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS.
|
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 23
RATIFICATION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Audit Committee has appointed KPMG LLP as our independent registered public accounting firm for 2021. Representatives of KPMG LLP will attend the Annual Meeting and will have the opportunity to make a statement if they desire to do so. They will also be available to respond to appropriate questions.
Our organizational documents do not require that the stockholders ratify the selection of KPMG LLP as our independent registered public accounting firm, and stockholder ratification is not binding on the Company, the Board or the Audit Committee. We request such ratification, however, as a matter of good corporate practice. The ratification of the selection of KPMG LLP requires the affirmative vote of a majority of the votes cast on the proposal at the Annual Meeting. Our Board, including our Audit Committee, values the opinions of our stockholders and, to the extent there is any significant vote against the ratification of the selection of KPMG LLP as disclosed in this Proxy Statement, we will consider our stockholders’ concerns and evaluate what actions may be appropriate to address those concerns, although the Audit Committee, in its discretion, may still retain KPMG LLP.
The following table shows information about fees billed to the Company by KPMG LLP for the fiscal years ended December 31, 2020, and 2019:
Fees billed by KPMG LLP | 2020 | 2019 | ||||||
Audit Fees(1) | $1,285,190 | $935,000 | ||||||
Audit Related Fees | — | — | ||||||
Tax Fees(2) | 287,533 | 38,676 | ||||||
All Other Fees | — | — | ||||||
Total | $1,572,723 | $973,676 | ||||||
| (1) | Audit fees of KPMG LLP for the years ended December 31, 2020, and 2019 were for professional services rendered for the audits of our financial statements, including accounting consultation, reviews of quarterly financial statements and professional services rendered in connection with our registration statements. The fees for 2020 include services associated with our automatic shelf registration statement filed in August 2020. The fees for 2019 include services associated with our follow-on offering of common stock in June 2019. |
| (2) | Tax Fees consist of fees billed for permissible tax services in connection with tax compliance, tax advice and tax planning. The fees for 2020 include services associated with our European subsidiaries. |
Audit Committee Pre-Approval Policies
The Audit Committee is directly responsible for the appointment, retention and termination, and for determining the compensation, of our independent registered public accounting firm. The Audit Committee shall pre-approve all auditing services and the terms thereof and non-audit services (other than non-audit services prohibited under Section 10A(g) of the Exchange Act or the applicable rules of the SEC or the Public Company Accounting Oversight Board), except that pre-approval is not required for the provision of non-audit services if the “de minimus” provisions of Section 10A(i)(1)(B) of the Exchange Act are satisfied. The Audit Committee may delegate to the chairperson of the Audit Committee the authority to grant pre-approvals for audit and non-audit services, provided such approvals are presented to the Audit Committee at its next scheduled meeting. All services provided by KPMG LLP during fiscal years 2020 and 2019 were pre-approved by the Audit Committee in accordance with the pre-approval policy described above.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” THE RATIFICATION OF THE APPOINTMENT OF KPMG LLP AS THE INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM OF THE COMPANY FOR ITS FISCAL YEAR ENDING DECEMBER 31, 2021.
|
24 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
The following Audit Committee Report is not considered proxy solicitation material and is not deemed filed with the Securities and Exchange Commission. Notwithstanding anything to the contrary set forth in any of our filings made under the Securities Act of 1933 or the Exchange Act that might incorporate our filings under those statutes, the Audit Committee Report shall not be incorporated by reference into any of our prior filings or into any of our future filings under those statutes.
The Audit Committee of the Board of Directors, or Audit Committee, has furnished this report concerning the independent audit of the Company’s financial statements. Each member of the Audit Committee meets the enhanced independence standards established by the Sarbanes-Oxley Act of 2002 and rulemaking of the Securities and Exchange Commission, or SEC, and the NASDAQ Stock Market regulations. A copy of the Audit Committee Charter is available on the Company’s website at www.gbt.com.
The Audit Committee’s responsibilities include assisting the Board of Directors regarding the oversight of the integrity of the Company’s financial statements, the Company’s compliance with legal and regulatory requirements, the independent registered public accounting firm’s qualifications and independence, and the performance of the independent registered public accounting firm.
In fulfilling its oversight responsibilities, the Audit Committee reviewed and discussed the Company’s financial statements for the fiscal year ended December 31, 2020, with the Company’s management and KPMG LLP. In addition, the Audit Committee has discussed with management, and with KPMG LLP, with and without management present, their evaluation of the Company’s internal controls over financial reporting and overall quality of the Company’s financial reporting. The Audit Committee also discussed with KPMG LLP the matters required to be discussed by the Public Company Accounting Oversight Board and the SEC. The Audit Committee also received the written disclosures and the letter from KPMG LLP required by the Public Company Accounting Oversight Board Rule 3526 and the Audit Committee discussed the independence of KPMG LLP with that firm.
Based on the Audit Committee’s review and discussions noted above, the Audit Committee recommended to the Board of Directors, and the Board of Directors approved, that the audited financial statements be included in the Company’s Annual Report for the fiscal year ended December 31, 2020.
The Audit Committee and the Board of Directors have recommended the selection of KPMG LLP as the Company’s independent registered public accounting firm for the year ending December 31, 2021.
AUDIT COMMITTEE
SCOTT W. MORRISON, CHAIR
DEVAL L. PATRICK
MARK L. PERRY
DAWN A. SVORONOS
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 25
The table below sets forth certain information regarding our executive officers as of March 31, 2021.
Name | Age | Position | ||
Ted W. Love, M.D. | 62 | President, Chief Executive Officer and Director | ||
Jeffrey Farrow | 59 | Chief Financial Officer | ||
Brian Cathers, Ph.D. | 51 | Chief Scientific Officer | ||
Jung E. Choi | 51 | Chief Business and Strategy Officer | ||
Eric Fink | 44 | Chief Human Resources Officer | ||
David L. Johnson | 52 | Chief Commercial Officer | ||
Tricia Suvari, Esq. | 60 | Chief Legal Officer | ||
The biographies of our executive officers, other than Dr. Love, whose biography is set forth above, appear below.
Jeffrey Farrow has served as our Chief Financial Officer since April 2016. Mr. Farrow previously served as chief financial officer of ZS Pharma, Inc., a biopharmaceutical company, which was acquired by AstraZeneca in December 2015. Prior to ZS Pharma, he served as the chief financial officer at Hyperion Therapeutics, Inc., a commercial pharmaceutical company, from July 2010 until May 2015 where he was part of the team responsible for the successful regulatory approval and commercial launch of RAVICTI® for the treatment of urea cycle disorders. He previously served as vice president of finance at Evotec AG, a drug discovery and development company. Prior to Evotec, Mr. Farrow served as vice president of finance and chief accounting officer at Renovis, Inc., a drug discovery and development company, which was acquired by Evotec AG. Earlier in his career, Mr. Farrow spent seven years working in the audit practice of KPMG LLP. Mr. Farrow holds a B.A. in business administration with a concentration in corporate finance from California State University at Fullerton and is a certified public accountant (inactive).
Brian Cathers, Ph.D., has served as our Chief Scientific Officer since February 2019. Dr. Cathers previously served as executive director and head of drug discovery at Celgene Corporation, or Celgene, from November 2015 to February 2019, and as senior director of biochemistry and structural biology at Celgene from October 2013 to November 2015. At Celegene, Dr. Cathers’ teams produced eight new development candidates and advanced five investigational drugs into clinical testing. Prior to joining Celgene, Dr. Cathers served as senior group leader and director at NewBiotics, Inc. from August 2000 to February 2004, where he oversaw enzymology and biophysical chemistry research, and was part of small team that developed a colorectal cancer drug from basic research to clinical testing. Prior to joining NewBiotics, he served as an enzymologist at Axys Pharmaceuticals. Dr. Cathers holds a B.S. in chemistry from Emporia State University and an M.S. and Ph.D. in medicinal chemistry from the University of Kansas.
Jung E. Choi has served as our Chief Business and Strategy Officer since April 2015. From April 2014 to March 2015, Ms. Choi served as senior vice president, corporate development for InterMune, Inc., a biotechnology company (acquired by Roche Holding AG in 2014), and served as an adviser on strategy and business development to InterMune from March 2013 to April 2014. Prior to joining InterMune, from February 2011 to March 2013, Ms. Choi led corporate and business development for Chimerix, Inc., a biopharmaceutical company, as a consultant and senior vice president, corporate development. Prior to that, from August 2001 to August 2010, Ms. Choi held various management positions at Gilead Sciences, Inc., a biopharmaceutical company, including leadership of business development, licensing, and mergers and acquisition activities. During her tenure at Gilead Sciences, Ms. Choi built and oversaw the corporate development group, and led the U.S. commercial launch of Hepsera® for the treatment of the hepatitis B virus. Ms. Choi holds a B.A. in human biology and an M.B.A. from Stanford University.
Eric Fink has served as our Chief Human Resources Officer since August 2019. Prior to joining us, from 2010 until July 2019, he most recently served as Vice President, Human Resources, at Jazz Pharmaceuticals, a global biopharmaceutical company. While there, he held a variety of human resource senior leadership positions to develop human capital and organizational development strategies and scale the global HR operating model to better serve the expanding business. From 2008 to 2009, he held a leadership position in the sales training organization at Bayer Healthcare, a multinational pharmaceutical and life sciences company. From 1999 to 2008, he held various roles at GlaxoSmithKline, a global healthcare company, across a wide spectrum of the U.S. commercial function, including Sales, Sales Training, Commercial Analytics, and Sales Management. He received a B.S. in Biology from Pennsylvania State University and an M.S. in Organizational Leadership from Mercyhurst University.
26 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
David L. Johnson has served as our Chief Commercial Officer since March 2018. From October 2003 until February 2018, Mr. Johnson served in roles of increasing responsibility in the commercial organization at Gilead Sciences, Inc., a biopharmaceutical company, ultimately as vice president, sales and marketing, for Gilead’s Liver Disease Business Unit. At Gilead, Mr. Johnson was responsible for the commercial launch of Gilead’s hepatitis C treatments Sovaldi®, Harvoni®, Epclusa® and Vosevi®, hepatitis B treatment Vemlidy®, and HIV treatments Complera® and Stribild®. Prior to Gilead, from April 1992 to September 2003, Mr. Johnson served in various roles in sales, product marketing, business development, global commercial strategy and portfolio development at GlaxoSmithKline PLC, a British pharmaceutical company. Mr. Johnson holds a B.A. in business marketing from the University of Puget Sound and an M.B.A. from the Kenan-Flagler Business School at the University of North Carolina.
Tricia Suvari, Esq., has served as our Chief Legal Officer since October 2016. From 2000 until 2009, Ms. Suvari served in several senior roles at CV Therapeutics, Inc., a biopharmaceutical company (acquired by Gilead Sciences, Inc. in 2009), ultimately as senior vice president, general counsel and chief compliance officer. Prior to CV Therapeutics, from 1991 until 2000, she served as corporate counsel at Genentech, Inc., in increasingly senior roles. From February 2012 until July 2016, Ms. Suvari served as a vice president and general counsel at the non-profit Peninsula Open Space Trust, and from early 2011 to February 2012 she served as an independent consultant to biopharmaceutical companies. Ms. Suvari earned her Bachelor of Sciences degree in Geology and Geophysics from Yale University and her J.D. degree from Harvard Law School.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 27
Compensation Discussion and Analysis
This Compensation Discussion and Analysis, or CD&A, describes our executive compensation program and the 2020 compensation for: (i) each individual who served as our principal executive officer during 2020; (ii) each individual who served as our principal financial officer during 2020 and (iii) our three most highly compensated executive officers during 2020 other than the individuals set forth above in clauses (i) and (ii), all of whom we refer to collectively as our named executive officers, or NEOs. This CD&A should be read with the compensation tables and related disclosures for our NEOs.
Our NEOs for 2020 were as follows:
| • | Ted W. Love, our President and Chief Executive Officer, or CEO; |
| • | Jeffrey Farrow, our Chief Financial Officer; |
| • | Jung Choi, our Chief Business and Strategy Officer; |
| • | David L. Johnson, our Chief Commercial Officer; and |
| • | Tricia Suvari, our Chief Legal Officer. |
Overview
We are a biopharmaceutical company dedicated to the discovery, development and delivery of life-changing treatments that provide hope to underserved patient communities. Founded in 2011, our goal is to transform the treatment and care of sickle cell disease, or SCD, a lifelong, devastating inherited blood disorder that is marked by red blood cell destruction and occluded blood flow and hypoxia, which leads to anemia, stroke, multi-organ failure, severe pain crises, and shortened patient life span. Since 2019, we have grown to become a commercial-stage company with 389 employees globally as of December 31, 2020, a marketed drug in the United States with the approval of Oxbryta® (voxelotor) tablets, and a marketing approval application under review in the European Union for Oxbryta.
In November 2019, the U.S. Food and Drug Administration, or FDA, granted accelerated approval for our first medicine, Oxbryta for the treatment of SCD in adults and children 12 years of age and older. Oxbryta, an oral therapy taken once daily, is the first FDA-approved treatment that directly inhibits sickle hemoglobin polymerization, the root cause of SCD. This FDA approval was three months ahead of the FDA’s Prescription Drug User Fee Act, or PDUFA, target action date of February 26, 2020, and we began to make Oxbryta available to patients through our specialty pharmacy partner network in early December 2019.
We are conducting and plan to conduct additional studies of Oxbryta. Such ongoing studies include, as a condition of accelerated approval, our Phase 3 HOPE-KIDS 2 Study, a post-approval confirmatory study we initiated in December 2019 that is using transcranial Doppler, or TCD, flow velocity to seek to demonstrate a decrease in stroke risk in children two to 15 years of age.
In January 2021, the European Medicines Agency, or EMA, accepted for review our Marketing Authorization Application, or MAA, seeking full marketing authorization of Oxbryta to treat hemolytic anemia (which is low hemoglobin due to red blood cell destruction) in SCD patients ages 12 years and older. In addition, our goals include expanding the current Oxbryta label in the United States to include treatment of SCD in children ages 4 to 11 years. In addition, we entered into an exclusive agreement in 2020 with Biopharma-Middle East and Africa, or Biopharma-MEA, to distribute Oxbryta in the six countries that make up the Gulf Cooperation Council, or GCC, region (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates), where the U.S. approval of Oxbryta can be referenced to allow for access to the medicine while health authorities conduct their reviews.
Beyond Oxbryta, we are engaged in other research and development activities, including working on new targets to potentially develop next generation treatments for SCD, including inclacumab, a P-selectin inhibitor, which is a clinically validated target in SCD, known to reduce the incidence of vaso-occlusive crises, or VOCs, and our next generation hemoglobin polymerization inhibitor, GBT021601, or GBT601. In addition, our drug discovery team is working on new targets to develop the next generation of treatments for SCD.
As part of those efforts, we regularly evaluate opportunities to in-license, acquire or invest in new business, technology or assets or engage in related discussions with other business entities. In December 2019, we entered into a license and collaboration agreement with Syros Pharmaceuticals, Inc., to discover, develop and commercialize novel therapies for SCD and beta thalassemia, and, in March 2021, we entered into a license agreement with Sanofi, under which we received an
28 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
exclusive license under certain intellectual property controlled by Sanofi to use, develop, manufacture, commercialize and otherwise exploit certain compounds for the treatment of human diseases.
Corporate Performance Highlights
Our executive compensation program seeks to incentivize and reward strong corporate performance. Highlights of our 2020 corporate performance are set forth below.
| • | Regulatory and Commercial |
| ✔ | Throughout 2020, we executed the commercial launch of Oxbryta in the United States, leveraging our field team and GBT Source Solutions® to educate healthcare professionals, or HCPs, payors and other stakeholders on Oxbryta. Over the course of the year, we secured FDA approval for patient and healthcare provider marketing materials in support of the Oxbryta launch. |
| ✔ | Starting in March 2020, we worked to adapt our commercial launch of Oxbryta to the challenging environment created by the COVID-19 pandemic through proactive measures to reduce the risk of spreading the COVID-19 virus among our employees, customers, business partners and local communities. |
| ✔ | In June 2020, we announced plans to seek the potential expansion of the use of Oxbryta for the treatment of SCD in children ages 4 to 11 years. |
| ✔ | In June 2020, we announced plans to submit an MAA to the EMA for Oxbryta to treat hemolytic anemia in SCD patients ages 12 years and older by mid-2021, and, in January 2021, we were notified that the EMA has completed the validation of the MAA we submitted and has started its standard review process. |
| ✔ | In September 2020, we announced our entry into an exclusive agreement with Biopharma-MEA to distribute Oxbryta in the GCC region. |
| ✔ | As of the end of September 2020 (a quarter ahead of our goal), we achieved broad payer coverage for Oxbryta in the United States, defined as 90% of lives covered by payers either through published policies or verified patient adjudication. |
| ✔ | In December 2020, we initiated two early access programs for Oxbryta. One is in Europe and other regions outside the United States, for the treatment of hemolytic anemia in eligible SCD patients ages 12 years and older, and the other is a multi-center expanded access protocol in the United States for eligible pediatric SCD patients to provide access prior to potential market authorization for children ages 4 to 11 who have no alternative treatment options. |
| • | Corporate |
| ✔ | We announced the appointment of a head of research and development who is expected to join us in May 2021, and, in August 2020, we added a chief medical officer to lead our development organization. |
| ✔ | In October 2020, we received the 2020 Rare Impact Award® for Industry Innovation for Oxbryta from the National Organization for Rare Disorders (NORD); in addition, Oxbryta was selected as Breakthrough Drug of the Year by the 2020 National Xconomy Awards. |
Impact of COVID-19
In March 2020, the Centers for Disease Control and Prevention, or CDC, declared a global pandemic related to SARS-CoV-2, the virus that causes coronavirus disease 2019, or COVID-19, and the pandemic has impacted our business, including our commercialization of Oxbryta and our research and development activities. For example, we implemented a temporary work from home policy; temporarily suspended our field team from most in-person interactions, including visits to physician offices, clinics and hospitals as well as in-person meetings with payors; and temporarily delayed or paused certain research and development activities, including screening and enrollment in all clinical studies sponsored by us.
Despite the impact of COVID-19, which we believe negatively impacted new patient prescriptions for Oxbryta after March 2020, we successfully executed the launch of Oxbryta, with nearly 5,000 new prescriptions written for Oxbryta between launch and the end of 2020 and net sales of $123.8 million in 2020, Oxbryta’s first full year of launch.
While the COVID-19 pandemic has had a significant impact on our company, including throughout most of 2020, we did not make any changes to our 2020 corporate goals, including our 2020 revenue goal. As discussed below, we considered the impact of COVID-19 in granting our executive officers performance-based RSUs, or PSUs, effective June 1, 2020, for retention purposes and to further focus them on our long-term performance.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 29
Overview of Executive Compensation Program
Executive Compensation Philosophy
Our executive compensation program is guided by our overarching philosophy of paying for demonstrable performance. Consistent with this philosophy, we have designed our executive compensation program to achieve the following primary goals:
| • | attract, motivate and retain top-performing senior executives; |
| • | establish compensation opportunities that are competitive and reward performance; and |
| • | align the interests of our senior executives with the interests of our stockholders to drive the creation of sustainable long-term value. |
Executive Compensation Program Design
Our executive compensation program is designed to be reasonable and competitive, and balance our goal of attracting, motivating, rewarding and retaining top-performing senior executives with our goal of aligning their interests with those of our stockholders. The Compensation Committee annually evaluates our executive compensation program to ensure that it is consistent with our short- and long-term goals and the dynamic nature of our business.
Our executive compensation program consists of a mix of compensation elements that balance achievement of our short-term goals with our long-term performance. We provide short-term incentive compensation opportunities in the form of annual cash bonuses, which focus on our achievement of annual corporate goals. We also provide long-term incentive compensation opportunities in the form of equity awards, including stock options, time-based RSUs and periodic PSUs.
We believe that stock options provide a strong reward for growth in the market price of our common stock because their entire value depends on future stock price appreciation. We believe RSUs and PSUs, which are subject to vesting conditions tied to our stock price also reward growth in the market price of our common stock because they derive additional value from future stock price appreciation, and they are less dilutive to our stockholders because they require fewer shares than stock options. In addition, we believe that the multi-year vesting requirements applicable to stock options and RSUs, as well as the additional time-based vesting requirements applicable to our PSUs, encourage retention because our senior executives are incentivized to remain employed through the applicable vesting or performance periods. We also have executive share ownership guidelines to further support our effort to align the interests of executive officers and shareholders.
Our executive compensation program is also designed to incorporate sound practices for compensation governance. Below we summarize such practices.
30 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
What We Do: | What We Don’t Do: | |||
✔ Maintain an Independent Compensation Committee. The Compensation Committee consists solely of independent directors.
✔ Retain an Independent Compensation Advisor. The Compensation Committee engages its own compensation advisor to provide information and analysis related to annual executive compensation decisions, including the 2020 executive compensation decisions, and other advice on executive compensation independent of management.
✔ Review Executive Compensation Annually and Related Risk Assessment. The Compensation Committee annually reviews our compensation strategy, including a review and determination of our compensation peer group used for comparative purposes. The Compensation Committee also reviews, on an annual basis, our compensation-related risk profile.
✔ Emphasize At-Risk Compensation. Our executive compensation program is designed so that a significant portion of our executive officers’ compensation is “at risk” based on our corporate performance, as well as equity-based, to align the interests of our executive officers and stockholders.
✔ Use a Pay-for-Performance Philosophy. The majority of our executive officers’ compensation is directly linked to corporate performance and includes a significant long-term equity component, thereby making a substantial portion of each executive officer’s total compensation dependent upon our stock price and/or total stockholder return.
✔ Stock Ownership Policy to Align Executives and Board with Shareholder Interests. We maintain a stock ownership policy for our executive officers (and non-executive members of our senior management team), as well as the non-employee members of our Board of Directors, which requires each of them to maintain ownership of a predetermined amount of company stock.
✔ Clawback Policy. In March 2021, we adopted a clawback policy under which cash- and equity-based incentive compensation of our CEO and other executive officers may be recovered by us under certain circumstances in the event of a financial restatement.
✔ Ongoing Shareholder Outreach and Engagement. We proactively engage with shareholders to solicit and consider shareholder feedback regarding our board governance and executive compensation programs and policies to better understand investor viewpoints and inform discussions in the boardroom.
✔ Use Double Trigger Change-in-Control Protection. Change-in-control payments and benefits to our executive officers occur only upon a qualifying termination of employment in connection with a change in control of the Company, not merely upon a change in control.
| ✘ No Executive Retirement Plans. We do not offer pension arrangements or retirement plans or arrangements to our executive officers that are different from or in addition to those offered to our other employees.
✘ Limited Perquisites. We do not view perquisites as a significant component of our executive compensation program. Accordingly, we do not provide significant perquisites to our executive officers, including our NEOs, except for limited travel stipends or limited housing and travel reimbursements for recruitment and retention purposes.
✘ No Special Health and Welfare Benefits. Our executive officers participate in our health and welfare benefits programs on the same basis as our other employees.
✘ No Post-Employment Tax Payment Reimbursement. We do not provide any tax reimbursement payments (including “gross-ups”) on any change-in-control or severance payments or benefits.
✘ No Hedging or Pledging Our Equity Securities. We prohibit our executive officers, the members of our Board of Directors and certain other employees from hedging or pledging our securities without the prior approval of the Audit Committee of our Board of Directors, or Audit Committee.
✘ No Stock Option Re-Pricing. Our equity plans do not permit stock options to be repriced to a lower exercise or strike price without the approval of our stockholders. | |||
“Say-on-Pay” Vote on Executive Compensation
At our 2020 Annual Meeting of Stockholders, we held a non-binding, advisory vote on the compensation of our NEOs (a “Say-on-Pay” vote), which received the support of approximately 78% of the votes cast. This was significantly lower than the greater than 97% support received on the Say-on-Pay proposal in each of the previous two years. In response to the relatively lower say-on-pay vote in 2020, we reached out to multiple stockholders and key investors, with aggregate holdings of over 75% of our outstanding shares (as of September 30, 2020), to discuss our executive compensation program and practices as well as our environmental, social and governance (ESG) policies and practices, and solicit feedback on these topics. While not all stockholders accepted our invitation to engage at this time, as of April 1, 2021, we have held these calls with stockholders with aggregate holdings of over 35% of our outstanding shares (as of September 30, 2020). Our calls were led by our Chief Financial Officer, Chief Human Resources Officer and Chief Legal Officer, with the chair of our Compensation Committee also participating on a smaller number of calls. During these
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 31
discussions, the majority of our stockholders that we spoke with expressed support of our peer group selection and process, support for our equity programs (including utilization of options and RSUs for senior personnel and support for options as an appropriate performance-based component at this stage of our development), and appreciation of the evolution of our compensation program and the inclusion of stock ownership and clawback policies. In addition, the majority of these stockholders encouraged further disclosure on our peer group (which we have sought to address in this proxy statement), a continued focus on the role of performance-based equity in our compensation program, and continued active engagement with investors.
As a result of these stockholder discussions, as well as through the Compensation Committee’s regular annual review process, the Compensation Committee determined to take certain actions to further enhance the pay-for-performance alignment of our executive compensation program for 2021 and beyond. Specifically, the actions taken by the Compensation Committee (and the full Board of Directors, in the case of the two new policies noted below), included:
| • | adjusting the peer group in December 2020 following a significant shift in our market capitalization that moved us significantly below the median of the peer group originally approved by the Compensation Committee in June 2020; |
| • | adding PSUs to our equity mix for executive officers to further incentivize long-term corporate performance and better align their interests with those of our stockholders; and |
| • | strengthening our corporate governance by adopting a clawback policy in March 2021, in addition to the stock ownership policy we adopted in March 2020. |
We are committed to continuing our ongoing engagement with our stockholders on matters of executive compensation and corporate governance. As our stockholders’ views and market practices on executive compensation evolve, the Compensation Committee will continue to evaluate and, when needed, make changes to our executive compensation program, ensuring that the program continues to reflect our pay-for-performance compensation philosophy and objectives.
As we value the opinions of our stockholders, our Board of Directors and the Compensation Committee will continue to consider the feedback received throughout the year, including when making compensation decisions for our executive officers in the future. In addition, consistent with the recommendation of our Board of Directors and the preference of our stockholders as reflected in the non-binding, advisory vote on the frequency of future Say-on-Pay votes held at our 2018 Annual Meeting of Stockholders, we intend to continue holding an annual Say-on-Pay vote. Our next Say-on-Pay vote will be held at the 2022 Annual Meeting.
Governance of Executive Compensation Program
Role of the Compensation Committee and the Board of Directors
The Compensation Committee discharges many of the responsibilities of our Board of Directors relating to the compensation of our executive officers, including our NEOs. The Compensation Committee oversees and evaluates our compensation and benefits policies generally, and the compensation plans, policies and practices applicable to our CEO and other executive officers. As described below, the Compensation Committee retains a compensation consultant to provide support in its review and assessment of our executive compensation program.
In addition, during 2020, pursuant to our Amended and Restated Equity Award Grant Policy, or Equity Award Grant Policy, the Compensation Committee delegated to a committee comprised of our CEO and another executive (which in practice has been our Chief Human Resources Officer), the authority to approve grants of equity awards to certain non-executive employees, subject to certain parameters, under the 2015 Plan and any other equity compensation plan that the Compensation Committee or the Board may determine to be subject to the policy, excluding our 2017 Inducement Equity Plan, or 2017 Inducement Plan. See “Other Compensation Policies and Practices—Equity Award Grant Policy.”
At the beginning of the year, the Compensation Committee reviews and approves the primary elements of compensation—base salary increases, annual cash bonuses, and annual equity awards—for our CEO, and for all individuals at or above the level of Vice President who report directly to our CEO, which includes our other NEOs. In addition, the Compensation Committee may deem it advisable to review and approve subsequent compensation opportunities for our CEO and such other individuals.
Compensation-Setting Factors
When reviewing and approving the amount of each compensation element and the target total compensation opportunity for all individuals at or above the level of Vice President who report directly to our CEO, which includes our other NEOs, the Compensation Committee considers the following factors:
| • | our performance against the annual corporate goals established by the Compensation Committee in consultation with management; |
32 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
| • | each executive officer’s skills, experience and qualifications relative to other similarly-situated executives at the companies in our compensation peer group and/or selected broad-based compensation surveys; |
| • | the scope of each executive officer’s role compared to other similarly-situated executives at the companies in our compensation peer group and/or selected broad-based compensation surveys; |
| • | the performance of each individual executive officer, based on an assessment of his or her contributions to our overall performance, ability to lead his or her department and work as part of a team, all of which reflect our core values; |
| • | compensation parity among our executive officers, and our employee population in general, that aligns with our commitment to diversity, equity and inclusion; |
| • | our retention goals; |
| • | our financial performance relative to our peers; |
| • | the compensation practices of our compensation peer group and selected broad-based compensation surveys and the positioning of each executive officer’s compensation in a ranking of peer company compensation levels based on competitive market data; and |
| • | the recommendations provided by our CEO with respect to the compensation of our other executive officers. |
These factors provide the framework for compensation decisions for each of our executive officers, including our NEOs. The Compensation Committee does not assign relative weights or rankings to these factors, and does not consider any single factor as determinative in the compensation of our executive officers. Rather, the Compensation Committee relies on its own knowledge and judgment in assessing these factors and making compensation decisions.
Role of Management
In discharging its responsibilities, the Compensation Committee works with management, including our CEO. Our management assists the Compensation Committee by providing information on corporate and individual performance, market compensation data and management’s perspective on compensation matters.
In addition, at the beginning of each year, our CEO reviews the performance of our other executive officers, including our other NEOs, based on our achievement of our annual corporate goals and each executive officer’s achievement of his or her departmental and individual goals established for the prior year and his or her overall performance during that year. The Compensation Committee solicits and reviews our CEO’s recommendations for base salary increases, annual cash bonuses, annual equity awards and any other compensation opportunities for our other executive officers, including our other NEOs, and considers our CEO’s recommendations in determining such compensation.
Role of Compensation Consultant
The Compensation Committee engages an external compensation consultant to assist it by providing information, analysis and other advice relating to our executive compensation program. For 2020, the Compensation Committee engaged Compensia as its compensation consultant to advise on executive compensation matters, including:
| • | review and analysis of the compensation for our executive officers, including our NEOs; |
| • | a review and analysis of the compensation for the non-employee members of the Board of Directors, including an analysis of a proposed equity award for a returning non-employee director; |
| • | review and input on the Compensation Discussion and Analysis section of our proxy statement for our 2020 Annual Meeting of Stockholders; |
| • | a qualitative and quantitative assessment of our Say-on-Pay proposal; |
| • | an analysis of the company original and year-to-date equity usage; |
| • | review, research, and updating of our compensation peer group; |
| • | an evaluation of performance-based equity alternatives; |
| • | a review of market practice with respect to compensation recovery policies; |
| • | a review of market practice with respect to stock ownership guidelines; |
| • | an analysis of competitive market compensation practices for the chief medical officer and chief operating officer positions; |
| • | a compensation-related risk assessment; |
| • | an analysis of a total equity budget and equity grant guidelines for the coming year; |
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 33
| • | an assessment of executive compensation trends within our industry, and updating on corporate governance and regulatory issues and developments; and |
| • | support on other compensation matters as requested throughout the year. |
Compensia reports directly to the Compensation Committee and to the chair of the Compensation Committee. Compensia also coordinates with our management for data collection and job matching for our executive officers. Compensia did not provide any other services to us in 2020. The Compensation Committee has evaluated Compensia’s independence pursuant to the listing standards of the relevant NASDAQ and SEC rules and has determined that no conflict of interest has arisen as a result of the work performed by Compensia.
Role of Market Data
For purposes of comparing our executive compensation against the competitive market, the Compensation Committee reviews and considers the compensation levels and practices of a group of peer companies. This compensation peer group consists of public biotechnology companies that are similar to us in terms of market capitalization, stage of development, geographical location and number of employees. The Compensation Committee reviews our compensation peer group at least annually and makes adjustments to our peer group if necessary, taking into account changes in both our business and our peer companies’ businesses.
In July 2019, the Compensation Committee, with the assistance of Compensia, reviewed our compensation peer group to determine our peer group for the remainder of 2019 and for 2020. The Compensation Committee considered our potential estimated revenues in 2020, the increase in our market capitalization and headcount and the near-commercial stage of our lead product candidate, as reflected in the following criteria:
| • | publicly traded companies headquartered in the United States; |
| • | companies in the biotechnology and pharmaceutical sector; |
| • | similar estimated revenues in 2020—up to 4.0x our projected 2020 revenue of approximately $150 million (resulting in a range of $46 million to $600 million)—new criteria for 2019/2020; |
| • | similar market capitalization—within a range of approximately 0.33x to approximately 3.0x our then-current market capitalization of approximately $3.3 billion (approximately $1.1 billion to approximately $9.9 billion); |
| • | the stage of development of each company’s lead candidate (with a preference for companies with candidates pending approval, approved or commercialized, and excluding companies whose lead candidates were in Phase 2 or Phase 3 development); |
| • | companies developing either orphan drugs or with a rare disease focus; and |
| • | similar headcount—within a range of approximately 0.33x to approximately 3.0x our then-current headcount of 171 employees (approximately 50 to 500 employees). |
Based on a review of the analysis prepared by Compensia, the Compensation Committee approved the updated compensation peer group below for the remainder of 2019 and for 2020.
2019—2020 Compensation Peer Group | ||||||||
ACADIA Pharmaceuticals Acceleron Pharma Agios Pharmaceuticals Aimmune Therapeutics Alnylam Pharmaceuticals Amicus Therapeutics bluebird bio
| Coherus Biosciences Epizyme FibroGen Insmed Intercept Pharmaceuticals Nektar Therapeutics | Portola Pharmaceuticals Regenxbio Sage Therapeutics Sarepta Therapeutics Spark Therapeutics Ultragenyx Pharmaceuticals | ||||||
34 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
The following chart illustrates that our market capitalization was at about the median against this peer group as of the time Compensia prepared its final recommendation, using the median for our company and the median for the peer group:
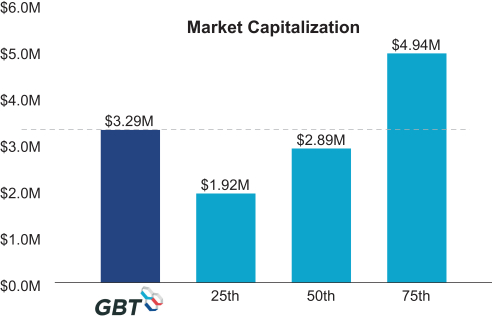
The Compensation Committee uses market data—from our compensation peer group and from the Radford Global Life Sciences Compensation survey—as one factor in evaluating whether the compensation for our executive officers is competitive in the market. The Compensation Committee also relies on its own knowledge and judgment in evaluating market data and making compensation decisions.
In June 2020, the Compensation Committee, with the assistance of Compensia, reviewed our compensation peer group to determine its continuing relevancy in the current economic environment. For this purpose, the Compensation Committee considered our then estimated revenues in 2021, the increase in our market capitalization and headcount and the status of Oxbryta, as reflected in the following criteria:
| • | publicly traded companies headquartered in the United States; |
| • | companies in the biotechnology and pharmaceutical sectors; |
| • | similar estimated revenues in 2021 of less than $1.1 billion, approximately 2.5x (down from 4.0x in 2019/2020) our projected 2021 revenue; |
| • | similar market capitalization—within a range of approximately 0.33x to approximately 3.0x our then-current market capitalization of approximately $4.8 billion (approximately $1.6 billion to approximately $14.5 billion); |
| • | the stage of development of each company’s lead candidate as pending approval, approved, or commercialized, and excluding candidates in the diagnostics and animal health fields; |
| • | companies with a focus on orphan drugs or rare disease indications; and |
| • | similar headcount to our headcount of approximately 0.33x to approximately 3.0x our then-current headcount of 356 employees (approximately 120 to approximately 1,100 employees). |
Based on a review of the analysis prepared by Compensia, the Compensation Committee approved the updated compensation peer group below for 2021, which was not used to make any compensation decisions.
2021 Compensation Peer Group | ||||||||
ACADIA Pharmaceuticals Acceleron Pharma Agios Pharmaceuticals Amarin Amicus Therapeutics Bluebird bio
| Blueprint Medicines Epizyme Exelixis FibroGen Insmed Immunomedics | Intercept Pharmaceuticals Nektar Therapeutics REGENXBIO Sage Therapeutics Sarepta Therapeutics Ultragenyx Pharmaceuticals | ||||||
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 35
In December 2020, in light of the sharp decline in our stock price starting in November 2020 (which had an impact on our market capitalization), the Compensation Committee, with the assistance of Compensia, reviewed the above compensation peer group again to determine a more suitable group of peer companies for 2021. The Compensation Committee used the same criteria that it had considered in updating the peer group in June 2020 within a range of 0.33x to approximately 3.0x our then-current market capitalization of approximately $2.7 billion (approximately $89 million to approximately $8.1 billion). Based on its review of the analysis prepared by Compensia, the Compensation Committee approved the updated compensation peer group below for 2021, which has only been used in evaluating the 2021 annual base salary, target annual bonus opportunities and equity awards for our NEOs, and was not used for setting any 2020 compensation elements.
2021 Compensation Peer Group | ||||||||
Acceleron Pharma Agios Pharmaceuticals Amarin Amicus Therapeutics bluebird bio Blueprint Medicines
| Epizyme Exelixis FibroGen Insmed Intercept Pharmaceuticals Intra-Cellular Therapies | Ironwood Pharmaceuticals Nektar Therapeutics Pacira BioSciences REGENXBIO Sage Therapeutics Sorrento Therapeutics | ||||||
Primary Elements of Executive Compensation Program
The primary elements of our executive compensation program are:
| • | base salary; |
| • | short-term incentive compensation in the form of annual cash bonuses; and |
| • | long-term incentive compensation in the form of annual equity awards. |
We do not have a specific policy regarding the percentage allocation between short- and long-term, or fixed and variable, compensation elements. The balance between these components may change from year to year based on corporate strategy, company performance, market forces and company objectives, among other considerations, but consistent with our philosophy of paying for demonstrable performance, our executive compensation program emphasizes at-risk pay over fixed pay. For example, in 2020, our CEO and other NEOs had the following target pay mix:
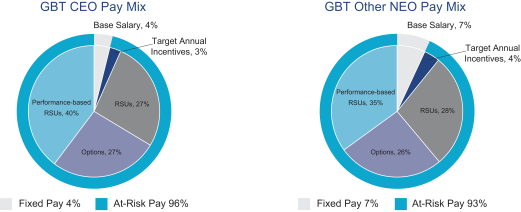
Our executive officers, including our NEOs, are also eligible to participate in our standard employee benefit plans, such as our health and welfare benefits plans, our 2015 Employee Stock Purchase Plan, or ESPP, and our 401(k) Plan on the same basis as our other employees. In addition, as described below, our executive officers, including our NEOs, are entitled to certain change-in-control severance payments and benefits and certain termination payments and benefits not in connection with a change in control pursuant to our Amended and Restated Severance and Change in Control Policy.
Base Salary
We pay base salaries to our executive officers, including our NEOs, as the fixed portion of their compensation to provide them with a reasonable degree of financial certainty, and to attract and retain top-performing individuals. At the time of hire, base salaries are determined for our executive officers, including our NEOs, based on the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above. Typically, at the beginning of each year, the
36 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Compensation Committee reviews base salaries for our executive officers, including our NEOs, based on such factors to determine if an increase is appropriate. In addition, base salaries may be adjusted in the event of a promotion or significant change in responsibilities.
2020 Annual Base Salary
In January 2020, the Compensation Committee reviewed the base salaries of our executive officers, including our NEOs. The Compensation Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above. In particular, the Compensation Committee increased Dr. Love’s base salary to better align with the median of the competitive market based on the individuals holding the chief executive officer position at the companies in our compensation peer group. Effective in February 2020, the Compensation Committee approved the base salaries of our NEOs below.
NEO | 2019 Annual Base Salary | 2020 Annual Base Salary | Percentage Increase | |||
Dr. Love | $600,000 | $670,000 | 12% | |||
Mr. Farrow | $442,500 | $460,000 | 4% | |||
Mr. Johnson | $455,000 | $465,000 | 2% | |||
Ms. Choi | $420,000 | $435,000 | 4% | |||
Ms. Suvari | $405,000 | $425,000 | 5% | |||
The actual base salaries paid to our NEOs in 2020 are set forth in the “Summary Compensation Table” below.
Short-Term Incentive Compensation
Annual Cash Bonuses
We provide short-term incentive compensation opportunities to our executive officers, including our NEOs, in the form of annual cash bonuses to drive our short-term success. Our annual cash bonuses for 2020 were tied to the achievement of annual corporate and individual performance goals pursuant to our Cash Incentive Bonus Plan, as amended from time to time, or Cash Incentive Plan, and no adjustments were made in light of the COVID-19 pandemic.
Corporate and Individual Performance Goals
At the beginning of each year, the Compensation Committee, after reviewing management’s proposal, establishes the annual corporate performance goals that it believes will be the most significant drivers of our short- and long-term success. The corporate performance goals include target achievement dates based on calendar quarters. Each corporate performance goal has a percentage weighting, and may include an additional percentage weighting for overachievement, based on the Compensation Committee’s assessment of the goal’s relative significance.
Each executive officer is responsible for contributing to the corporate objectives, individually, and as part of the leadership team. In approving individual bonus awards, the compensation committee considers the individual contribution towards the company’s achievement of the corporate objectives by each executive officer (other than the CEO), with a significant weighting towards corporate as compared to individual achievement as described in greater detail below. This weighting is the same for each of our executive officers, including our NEOs, who are at the same management level. Our CEO does not have individual performance goals. Rather, his annual cash bonus is based 100% on achievement of our corporate performance goals in recognition of his overall responsibility for our corporate performance.
At the beginning of the year after the corporate performance goals are established, the Compensation Committee, after reviewing management’s self-assessment, evaluates our achievement of the prior year’s corporate performance goals, and our overall success in the prior year, and determines our total percentage achievement level. Our CEO evaluates the other executive officers, including the other NEOs, in terms of their individual performance in the prior year, and makes recommendations for a total percentage achievement level for each executive officer. The Compensation Committee considers our CEO’s recommendations, and independently reviews and approves the total percentage achievement level for each of the other executive officers, including our other NEOs.
Target Annual Bonuses
The target annual bonus is determined for each of our executive officers, including our NEOs, at the beginning of each year, by reference to then applicable Cash Incentive Plan, which sets out the target annual bonuses for employees by position level. In approving the Cash Incentive Plan, the Compensation Committee considers the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, with an emphasis on market data from our
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 37
compensation peer group for comparable positions. Target annual bonuses are the same for executive officers, including our NEOs, who are at the same level, and represent a specific percentage of annual base salary.
In January 2020, the Compensation Committee reviewed the target annual bonuses of our executive officers, including our NEOs. The Compensation Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, particularly market data from the companies in our compensation peer group, and approved the 2020 target annual bonuses of our NEOs.
| 2019 | 2020 | ||||||||||||||||||||||||
NEO | Target Annual Bonus | Goal Weighting Corporate/Individual | Target Annual Bonus | Goal Weighting Corporate/Individual | |||||||||||||||||||||
Dr. Love | 60% | 100%/0% | 70% | 100%/0% | |||||||||||||||||||||
Mr. Farrow | 40% | 75%/25% | 50% | 80%/20% | |||||||||||||||||||||
Mr. Johnson | 40% | 75%/25% | 50% | 80%/20% | |||||||||||||||||||||
Ms. Choi | 40% | 75%/25% | 50% | 80%/20% | |||||||||||||||||||||
Ms. Suvari | 40% | 75%/25% | 50% | 80%/20% | |||||||||||||||||||||
Annual Cash Bonus Formula
The Compensation Committee uses the following formula to calculate annual cash bonuses for each of our executive officers, including our NEOs:

38 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
2020 Corporate Performance Goals
In January 2020, our Board of Directors approved our 2020 annual corporate performance goals and weightings as set forth below.
Category | Corporate Goal | Stretch Corporate Goal | Weighting | |||
Oxbryta | Commercial • Net sales in 2020 exceed $158 million | Commercial • Net sales in 2020 exceed $178 million | 50% (subject to increase to 75% upon achievement of the stretch goal, and includes linear interpolation for determining payout) | |||
Clinical • First dose in clinical study of Oxbryta at doses outside of approved labeling by third quarter | 5% | |||||
Regulatory • Gain FDA agreement on regulatory path to support label expansion in pediatric patients and secure scientific advice to inform EU strategy | 5% | |||||
Geographic Expansion • Execute Saudi Arabia partnering/distributor agreement and advance plan for certain other geographies | Geographic Expansion • Saudi Arabia by third quarter, or another specified territory | 10% (subject to increase to 20% upon achievement of the stretch goal) | ||||
Pipeline | • File investigational new drug, or IND, application for inclacumab
• File IND for GBT601 | • File inclacumab IND by third quarter | 20% (subject to increase to 40% upon achievement of the stretch goal) | |||
Corporate | • Achieve certain business development goals
• Complete year with a cash reserve of at least a certain minimum
• Maintain voluntary turnover below industry standard | 10% | ||||
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 39
2020 Annual Cash Bonuses
In January 2021, the Compensation Committee evaluated our achievement of the 2020 corporate performance goals. The Compensation Committee considered whether we had achieved each goal, the weighting established for each goal, including the weighting for overachievement, management’s self-assessment, and our overall corporate performance in 2020.
Goal | Weighting | Actual Results | Bonus Pool Funding | |||
Oxbryta Net Sales of $158 million in 2020 (stretch of $178 million in 2020) | 50% (75% with stretch) | $123.6 million in 2020 | 39.6% | |||
First dose in clinical study of Oxbryta at doses outside of approved labeling by third quarter | 5% | Achieved in September | 5% | |||
Regulatory—Gain alignment with FDA on label expansion in ages less than 12 years old and secure scientific advice to inform EU strategy | 5% | FDA Type B meeting held in May EU MAA submitted in December | 5% | |||
Geographic Expansion—Execute Saudi Arabi partnering/distributor agreement (stretch goal by third quarter) and advance plan for certain other geographies | 10% (20% with stretch) | Distribution Agreement for Gulf Cooperation Council region signed in August, achieving stretch goal of third quarter timing | 20% | |||
Pipeline—File IND for inclacumab (stretch goal of filing such IND by third quarter) and IND for GBT601 | 20% (40% with stretch) | Both INDs filed in November | 20% | |||
Corporate • Achieve certain business development goals
• Complete year with a cash reserve of at least a certain minimum
• Maintain voluntary turnover below the industry standard | 10% | • Confidential business development goals achieved by year end
• Ended 2020 with approximately $580 million in cash, which significantly exceeded the specified minimum
• GBT 2020 voluntary turnover at 10.3% compared to life science market at 13.2% | 10% | |||
Total | 99.6% | |||||
The Compensation Committee also reviewed the 2020 individual performance of each of our executive officers, other than our CEO, based on an evaluation conducted by our CEO of their performance against their 2020 individual performance and contribution to the achievement of the corporate objectives above. The Compensation Committee approved an achievement level of 105% of the 2020 individual performance goals for each of our NEOs based upon their scope of responsibilities and significant contributions to us meeting our corporate objectives in 2020.
The table below sets forth the target annual cash bonuses, the relative weighting of corporate and individual performance, the actual achievement level for corporate and individual performance and the 2020 annual cash bonuses earned by our NEOs.
NEO | 2020 Annual Base Salary ($) | Target Annual Cash Bonus (% of annual base salary) | Weighting (corporate/ individual performance) (%) | Corporate Performance (%) | Individual Performance (%) | Annual Cash Bonus ($) | ||||||||||||||||||||||||
Dr. Love | $ | 670,000 | 70 | % | 100 | %/0 | 99.6 | % | N/A | $ | 467,000 | |||||||||||||||||||
Mr. Farrow | $ | 460,000 | 50 | % | 80 | %/20% | 99.6 | % | 105 | % | $ | 231,500 | ||||||||||||||||||
Mr. Johnson | $ | 465,000 | 50 | % | 80 | %/20% | 99.6 | % | 105 | % | $ | 234,000 | ||||||||||||||||||
Ms. Choi | $ | 435,000 | 50 | % | 80 | %/20% | 99.6 | % | 105 | % | $ | 219,000 | ||||||||||||||||||
Ms. Suvari | $ | 425,000 | 50 | % | 80 | %/20% | 99.6 | % | 105 | % | $ | 214,000 | ||||||||||||||||||
40 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
The annual cash bonuses earned by each of our NEOs for 2020 are set forth in the “Summary Compensation Table” below.
Long-Term Incentive Compensation
We view long-term incentive compensation in the form of equity awards as an important element of our executive compensation program. The value of equity awards is directly related to stock price appreciation over time, which incentivizes our executive officers to achieve long-term corporate goals and create long-term value for our stockholders. Equity awards also help us attract and retain top-performing executive officers in a competitive market.
Typically, at the beginning of each year, the Compensation Committee reviews the equity awards for our executive officers, including our NEOs, and determines the size and relative weighting of the annual equity awards it deems reasonable and appropriate based on the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above. The size and relative weighting is the same for each of our executive officers, including our NEOs, who are at the same level. In addition, the Compensation Committee may deem it advisable to grant subsequent equity awards to our executive officers, including our NEOs, and may adjust their equity awards in the event of a promotion or significant change in responsibilities.
2020 Equity Awards
In January 2020, the Compensation Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, particularly market data from the companies in our compensation peer group and selected broad-based compensation surveys, and approved the 2020 annual equity awards for our NEOs below. Mr. Johnson received a larger grant of time-based RSUs than the other non-CEO NEOs, as 5,000 of such RSUs were granted in recognition of his contribution toward us achieving a $4 billion market capitalization milestone.
NEO | Stock Options (Number of Shares) | Time-Based RSUs (Number of Shares) | ||
Dr. Love | 114,190 | 71,640 | ||
Mr. Farrow | 38,500 | 24,160 | ||
Mr. Johnson | 38,500 | 29,160 | ||
Ms. Choi | 38,500 | 24,160 | ||
Ms. Suvari | 38,500 | 24,160 | ||
The stock options vest, and become exercisable, over a four-year period, with 1/16th of the underlying shares vesting on a quarterly basis (every three months) after the vesting commencement date of February 1, 2020, so that all of the underlying shares will be vested on the date four years after the vesting commencement date, so long as the NEO remains an employee or other service provider (including a consultant) of the Company on each such vesting date.
The time-based RSUs vest over a four-year period, with 1/8th of the underlying shares vesting on a semi-annual basis (every six months) after the vesting commencement date of February 1, 2020, so that all of the underlying shares will be vested on the date four years after the vesting commencement date, so long as the NEO remains an employee or other service provider (including a consultant) of the Company on each such vesting date.
Shortly after the World Health Organization declared COVID-19 a pandemic in March 2020, the Compensation Committee began to review whether the above annual equity awards approved in January 2020 would be insufficient in light of the pandemic and our reduced stock price to serve as an appropriate motivational and retention vehicle for our executive officers, particularly as we were entering our first full year of the commercial launch of our first medicine, Oxbryta. To address these concerns, the Compensation Committee decided to grant an additional performance-based equity award to our executive officers, including our NEOs, to provide an opportunity for them to earn equity compensation in line with the 75th percentile of our peer group’s equity compensation for similarly situated executives.
In determining the form of additional equity to grant the NEOs, the Compensation Committee revisited the positive impact of the PSUs the Committee granted our executive officers in August 2017, or 2017 PSUs. The 2017 PSUs were scheduled to vest, if ever, upon the first instance of us achieving a market capitalization of certain specified amounts of up to $4 billion on or before December 31, 2019, which the Compensation Committee considered as a significant threshold at the time such PSUs were granted. The Compensation Committee concluded that the 2017 PSUs had significant motivation and retention value, including in our achievement of the highest market capitalization threshold of $4 billion, which resulted in the vesting of the final tranche of the 2017 PSUs in December 2019.
Effective June 1, 2020, the Compensation Committee granted PSUs to certain of our senior management, including our NEOs, which are eligible to be earned and vest contingent upon the achievement of three escalating and challenging stock
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 41
price targets ($109.20, $145.60 and $182.00 per share), based on average closing market price on the NASDAQ Global Select Market over a 20 consecutive trading day period, with the percentage of shares allotted to the three tranches increasing with each tranche. Upon the achievement of each respective stock price target, 50% of the PSUs allotted to that tranche will vest, while the remaining 50% will vest on the first anniversary of the date the stock price target was achieved, subject to the executive officer’s continued employment or other service relationship with us through such vesting date. The Compensation Committee included such delayed vesting to further foster retention and alignment with stockholder interest. Under the terms of the awards, if the stock price targets are not achieved for all or some of the tranches on or before June 30, 2024, the unvested awards will be automatically terminate and be forfeited.
The following illustrates the market capitalization we would need to achieve to meet each of the stock price targets and the relative shareholder value created:
Stock Price | % Increase | Approx. Market Cap* | NEOs Shares Earned | NEOs Value Earned | Shareholder Value Creation | Payouts as a % of Shareholder Value | ||||||||
| NEOs | Shareholders | |||||||||||||
$109.20 | +50% | $6.7B | 62,200 | $6.8M | +$1.9B | 0.3% | 99.7% | |||||||
$145.60 | +100% | $9.0B | 171,050 | $24.9M | +$4.2B | 0.6% | 99.4% | |||||||
$182.00 | +150% | $11.2B | 311,000 | $56.6M | +$6.4B | 0.9% | 99.1% | |||||||
| * | Approximate market cap is calculated from ~61.7M total common shares outstanding. |
Shareholder Value Created at Maximum Payout
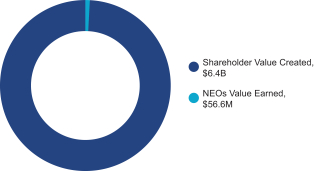
The Compensation Committee granted these performance-based awards as a way to further align the incentives of our senior management, including our NEOs, with our stockholders and to further our pay-for-performance compensation philosophy, as the PSUs will only be earned if our stock price appreciates significantly over a four-year period. Moreover, the PSUs serve to further retain our executive officers, including our NEOs, as they have a time-based vesting component in addition to the stock price target, which requires the applicable executive officer to remain employed for one year following the achievement of the applicable stock price target in order to fully receive the number of PSUs allotted for that tranche. In addition, the Compensation Committee determined to tie achievement of the PSUs to our stock price instead of market capitalization (in contrast to the 2017 PSUs) as it viewed increase in stock price as a better gauge of the return to stockholders than market capitalization, which may simply reflect an increase in the number of shares outstanding rather than an indicator of an increase in equity value.
The PSUs granted to our NEOs are as follows:
NEO | Performance-Based RSUs (Number of Shares) | ||||
Dr. Love | 141,400 | ||||
Mr. Farrow | 42,400 | ||||
Mr. Johnson | 42,400 | ||||
Ms. Choi | 42,400 | ||||
Ms. Suvari | 42,400 | ||||
The equity awards granted to our NEOs in 2020 are set forth in the “Summary Compensation Table” and the “Grants of Plan-Based Awards for Fiscal Year 2020” table below.
42 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Health and Welfare Benefits
Our executive officers, including our NEOs, are eligible to participate in the same employee benefit plans that are generally available to all of our employees, subject to the satisfaction of certain eligibility requirements, such as medical, dental, and life and disability insurance plans. We pay, on behalf of our employees, all or a portion of the premiums for health, life and disability insurance.
2015 Employee Stock Purchase Plan
Our executive officers, including our NEOs, are eligible to participate in our ESPP on the same basis as our other full-time employees. The ESPP permits eligible employees to set aside a portion of their compensation during offering periods that are generally two years long, with purchase periods generally every six months during each offering period, and use such contributions to purchase shares of our common stock at a purchase price equal to 85% of the lower of the fair market value of the shares on the first business day of the offering period or the last business day of the purchase period.
401(k) Savings Plan
Our U.S. executive officers, including our NEOs, are eligible to participate in a tax-qualified retirement plan, or 401(k) Plan, on the same basis as our other employees. The 401(k) Plan provides eligible U.S. employees with an opportunity to save for retirement on a tax advantaged basis. Eligible employees are able to defer eligible compensation subject to applicable annual limits of the Internal Revenue Code of 1986, as amended, or the Code. Employees’ pre-tax contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participants’ directions. Employees are immediately and fully vested in their contributions. Our 401(k) Plan is intended to be qualified under Section 401(a) of the Code with our 401(k) Plan’s related trust intended to be tax exempt under Section 501(a) of the Code. As a tax-qualified retirement plan, contributions to our 401(k) Plan and earnings and matching amounts on those contributions are not taxable to the employees until distributed from our 401(k) Plan.
Since December 2015, the Company has approved various matching contributions under the 401(k) Plan. Under our current matching policy, effective January 1, 2018, we match in cash 100% of an employee’s 401(k) contributions, subject to an annual cap of $6,000 per employee.
Perquisites
Perquisites or other personal benefits are not a significant component of our executive compensation program. Accordingly, we do not provide significant perquisites or other personal benefits to our executive officers, including our NEOs.
Employment Arrangements with our NEOs
Post-Employment Compensation
We consider it essential to the best interests of our stockholders to foster the continuous employment of our key management personnel. Accordingly, we believe that reasonable and competitive post-employment compensation arrangements are an important part of an executive compensation program to attract and retain highly-qualified senior executives. While the Compensation Committee does not consider the specific amounts payable under these post-employment compensation arrangements when determining the annual compensation of our NEOs, we believe that providing our executive officers with post-employment payments and benefits if they lose their position in connection with a change in control are in the best interests of our stockholders because the possibility of a change in control and the related uncertainty may lead to the departure or distraction of senior executives to the detriment of our company and stockholders.
In July 2015, our Board of Directors adopted a change in control policy, which has been subsequently amended and restated (the “Amended and Restated Severance and Change in Control Policy”). The Amended and Restated Severance and Change in Control Policy provides our NEOs with certain payments and benefits upon a qualified termination event outside of a Change in Control Period (as defined below). Based on its review of our pre-amended and restated change in control policy compared to the post-employment compensation arrangements of the companies in our compensation peer group, the Compensation Committee determined that these changes were necessary to recruit and retain top talent and to align with market norms. The Amended and Restated Severance and Change in Control Policy applies to our executive officers, including our NEOs, and reinforces and encourages the continued attention and dedication of certain key senior executives in the event of a change in control by providing these executive officers with certain cash payments, equity acceleration and other benefits upon a qualifying termination of employment event in connection with a change in control.
Pursuant to our Amended and Restated Severance and Change in Control Policy, in the event the employment of any of our NEOs is terminated by us or our acquirer or successor without Cause or an NEO resigns for Good Reason (as such
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 43
terms are defined in our change in control policy), in either case, within one year after the consummation of a Sale Event (as defined in the 2015 Plan) (such one-year period, the “Change in Control Period”), he or she will be entitled to receive the following payments and benefits, or CIC Benefits, subject to his or her execution and non-revocation of a severance agreement within 60 days following the date of such termination, including a general release of claims in our favor:
| • | a lump sum cash payment equal to 12 months (or 18 months in the case of our CEO) of the NEO’s “base salary” (i.e., the greater of the NEO’s base salary in effect immediately prior to the termination or the base salary in effect immediately prior to the Sale Event, as applicable); |
| • | a lump sum cash payment equal to 100% of the NEO’s annual “target incentive bonus” (i.e., the greater of the NEO’s target bonus in effect immediately prior to the termination or the target bonus in effect immediately prior to the Sale Event, as applicable) for the year in which the closing of the Sale Event occurred, which, under our Amended and Restated Severance and Change in Control Policy, was increased from 100% to 150% in the case of our CEO; |
| • | a lump sum cash payment equal to the prorated annual cash bonus payout of the NEO for the portion of the year in which the closing of the Sale Event occurred, based on the NEO’s target annual bonus and the date of termination of his or her employment or other service relationship with us; |
| • | if the NEO elects to continue his or her group healthcare benefits, a cash payment of an amount equal to the monthly employer contribution we would have made to provide the NEO with health insurance if he or she had remained employed by us until the earlier of (i) 12 months (or 18 months in the case of our CEO) following the date of termination, or (ii) the end of the NEO’s COBRA health continuation period; and |
| • | full acceleration of vesting of all outstanding equity awards under the 2015 Plan, the 2017 Inducement Plan and such additional equity incentive plans and arrangements as may be applicable from time to time, including all performance-based equity awards, which will accelerate and vest based on the deemed achievement of 100% of the target performance levels as of the date of the NEO’s termination. |
As described above, the CIC Benefits are “double trigger” because the change in control alone does not trigger such payments and benefits. Rather, the CIC Benefits are triggered only if there is a qualifying termination of an NEO’s employment within the Change in Control Period. In the case of the acceleration of vesting of outstanding equity awards, we use this double-trigger arrangement to protect against the loss of retention value following a change in control of the Company and to avoid windfalls, both of which could occur if vesting of either equity or cash-based awards accelerated automatically as a result of the transaction.
In addition, upon a Sale Event, to the extent Section 280G of the Code, or Section 280G, is applicable, each NEO who is then employed with us will be entitled to receive the better treatment of: (i) payment of the full amounts set forth above to which the NEO is entitled or (ii) payment of such lesser amount that does not trigger excise taxes under Section 280G. None of our NEOs are entitled to excise tax payments (or “gross-ups”) relating to a change in control of the Company.
The payments and benefits provided under our Amended and Restated Severance and Change in Control Policy are designed to be competitive in the market. Pursuant to the Amended and Restated Severance and Change in Control Policy, in the event the employment of any of our NEOs is terminated by us or our acquirer or successor without Cause or an NEO resigns for Good Reason outside of the Change in Control Period, he or she will be entitled to receive the following payments and benefits, subject to his or her execution and non-revocation of a severance agreement within 60 days following the date of such termination, including a general release of claims in our favor:
| • | a lump sum cash payment equal to 12 months of the NEO’s then-current base salary; |
| • | in the case of our CEO only, a lump sum cash payment equal to (i) 100% of the CEO’s target incentive bonus for the year in which the termination of his employment or other service relationship with the company occurred, plus (ii) a prorated annual cash bonus payout for the portion of the year in which the termination of his employment or other service relationship with the company occurred, based on the CEO’s annual target bonus and the date of termination of his employment or other service relationship with the company; and |
| • | if the NEO elects to continue his or her group healthcare benefits, a cash payment of an amount equal to the monthly employer contribution we would have made to provide the NEO with health insurance if he or she had remained employed by us until the earlier of (i) 12 months following the date of termination, or (ii) the end of the NEO’s COBRA health continuation period. |
For an estimate of the potential payments and benefits that our NEOs would have been eligible to receive under the Amended and Restated Severance and Change in Control Policy if a hypothetical change in control or other trigger event had occurred on December 31, 2020, see “Potential Payments on Termination or Change in Control” below.
44 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Other Compensation Policies and Practices
Equity Award Grant Policy
We adopted an Amended and Restated Equity Award Grant Policy in January 2020, as amended in November 2020, that sets forth the process and timing for us to follow when we grant equity awards for shares of our common stock to our employees, including our executive officers, or advisors or consultants to us pursuant to any of our equity compensation plans. Pursuant to the policy, all grants of equity awards must be approved in advance by our Board of Directors, the Compensation Committee or, subject to the delegation requirements in the policy, our CEO or a committee comprised of the CEO and at least one other executive officer of the Company, or Equity Grant Committee. The Equity Grant Committee is currently comprised of our CEO and another executive (which in practice has been our Chief Human Resources Officer). The equity award granting authority delegated to the Equity Grant Committee applies to non-executive employees and covers awards of stock options and RSUs within specific ranges set forth in the policy, which will be updated annually by the Compensation Committee.
The Amended and Restated Equity Award Grant Policy sets forth that equity awards are generally granted on the following regularly scheduled basis:
| • | Equity awards granted in connection with the hiring of a new employee or the engagement of a new consultant are effective on the first trading day of the month following the later of the date on which such individual’s employment or consulting term begins or the date on which such award is approved by the Board, the Compensation Committee or the Equity Grant Committee. |
| • | Equity awards granted in connection with the promotion of an existing employee are effective on the first trading day of the month following the later of the date on which such individual’s promotion occurs or the date on which such award is approved by the Board of Directors, the Compensation Committee or the Equity Grant Committee; provided, that in the case of any promotion effective on the first trading day of a particular month, the award will be effective on the effective date of such promotion so long as the Board of Directors, the Compensation Committee or the Equity Grant Committee approves the award on or before such date. |
| • | Equity awards granted to existing employees (other than in connection with a promotion) will generally be granted, if at all, on an annual basis effective on the first trading day of the month following the later of the date on which we complete the focal review process with respect to such individual or the date on which such award is approved by the Board of Directors, the Compensation Committee or the Equity Grant Committee. |
Our Board of Directors and the Compensation Committee retain the discretion to grant equity awards at other times to the extent appropriate in light of the circumstances of such awards.
In addition, the policy sets forth the manner in which our equity awards will be priced. If an award of restricted stock or restricted stock units is denominated in dollars, the number of shares subject to the award will be determined by dividing the dollar value by the average closing market price on the NASDAQ Global Market of a share of our common stock over the trailing 20-trading day period ending on the trading day immediately preceding the grant date. The exercise price of all stock options will be at least equal to the closing market price on the NASDAQ Global Market of a share of our common stock on the effective date of grant or if no closing price is reported for such date, the closing price on the last date preceding such date for which a closing price is reported. If the amount of a stock option award is to be determined by reference to a fair value calculated under Financial Accounting Standards Board Accounting Standard Codification Topic 718, Stock Compensation , or FASB ASC Topic 718, then the number of shares to be subject to such stock option will be determined based on such fair value, and the exercise price determined in accordance with the preceding sentence and the approved valuation assumptions, subject to any other limits on the number of shares that may be subject to such stock option.
Policy Prohibiting Hedging and Pledging
Our Insider Trading Policy prohibits our executive officers, the non-employee members of our Board of Directors and certain other employees from engaging in the following transactions:
| • | selling any of our securities that they do not own at the time of the sale (referred to as a “short sale”); |
| • | buying or selling puts, calls, other derivative securities of the Company or any derivative securities that provide the economic equivalent of ownership of any of our securities or an opportunity, direct or indirect, to profit from any change in the value of our securities or engaging in any other hedging transaction with respect to our securities without the prior approval of the Audit Committee; |
| • | using our securities as collateral in a margin account; and |
| • | pledging our securities as collateral for a loan (or modifying an existing pledge) unless the pledge has been approved by the Audit Committee. |
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 45
As of the date of this Proxy Statement, none of our NEOs or non-employee directors had previously sought or obtained approval from the Audit Committee to engage in any hedging or pledging transaction involving our securities.
Stock Ownership Policy
In March 2020, we adopted a stock ownership policy for our senior executive officers (i.e., our CEO and each member of our senior management team, which includes our NEOs), which requires (i) our CEO to acquire and hold a number of shares of our common stock equal in value to at least six times his or her annual base salary and (ii) each senior management team member to acquire and hold a number of shares of our common stock equal in value to at least two times his or her applicable annual base salary, in each case until such executive’s service as our CEO or senior management team member, respectively, ceases. We only count directly and beneficially owned shares, including shares purchased through our ESPP or 401(k) plan, and 50% of shares underlying vested and unexercised in-the-money stock options. Each executive has until the later of April 1, 2025, the Initial Determination Date, or the April 1st in the year that is the fifth anniversary of his or her initial appointment in the capacity of an executive to attain the required ownership level. Once an executive satisfies his or her stock ownership requirement, the executive must continue to satisfy such stock ownership requirement as assessed on each Determination Date. If an executive fails to satisfy such stock ownership requirement as of any Determination Date (including the Initial Determination Date), then such executive shall be required to come into compliance with his or her applicable stock ownership requirement within two years following the Determination Date on which he or she failed to satisfy such stock ownership requirement. As of April 1, 2021, all of our NEOs met their respective stock ownership requirement:
Name | Ownership Requirement | Actual Ownership | ||||||||||||
Dr. Love | 6.0x | 67.7x | ||||||||||||
Mr. Farrow | 2.0x | 8.2x | ||||||||||||
Mr. Johnson | 2.0x | 2.4x | ||||||||||||
Ms. Choi | 2.0x | 24.4x | ||||||||||||
Ms. Suvari | 2.0x | 6.4x | ||||||||||||
Clawback Policy
In March 2021, we adopted a policy for recoupment of executive incentive compensation, or Clawback Policy, which provides that if our financial statements are materially restated, whether in part or in their entirety, due to misconduct by one or more covered officer (meaning any Section 16 officer(s)), then our Board of Directors or Compensation Committee will have the discretion to recoup a portion of any performance-based compensation that has been paid or distributed to the covered officer during the clawback period (i.e., the three-year period preceding the publication of the restated financials), to the extent such compensation paid or distributed was in excess of what would have been paid under the restated financials. Our Board of Directors or the Compensation Committee, in its sole discretion, may also reduce the amount of future compensation, including, without limitation, any bonus or severance, or the future grant or vesting of any equity award, payable to any covered officer by an amount equal to such excess proceeds from performance-based compensation received by the covered officer during the clawback period. The Clawback Policy is applicable to all cash and equity-based compensation predicated on the achievement of financial performance goals or financial metrics (excluding any such compensation based on TSR or similar stock price-based metrics).
Tax and Accounting Considerations
Deductibility of Executive Compensation
Generally, Section 162(m) of the Code, or Section 162(m), disallows a federal income tax deduction for public corporations of remuneration in excess of $1 million paid in any fiscal year to certain specified former or current executive officers.
Other than for remuneration provided pursuant to written binding contracts which were in effect on November 2, 2017 (and which are not subsequently modified in any material respect), for taxable years beginning after December 31, 2017, all remuneration in excess of $1 million paid to a specified executive will not be deductible.
To maintain flexibility to compensate our executive officers in a manner designed to promote our short- and long-term corporate goals, the Compensation Committee has not adopted a policy that all compensation must be deductible. The Compensation Committee believes that our stockholders’ interests are best served if its discretion and flexibility in awarding compensation is not restricted in order to allow such compensation to be consistent with the goals of our executive compensation program, even though some compensation awards may result in non-deductible compensation expense. Consequently, the Compensation Committee will not necessarily limit executive compensation to that which is or may be deductible under Section 162(m).
46 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Accounting for Stock-Based Compensation
We follow FASB ASC Topic 718 for our stock-based compensation awards. FASB ASC Topic 718 requires us to measure the compensation expense for all share-based payment awards made to our employees and non-employee members of our Board of Directors, including stock options to purchase shares of our common stock and other stock awards, based on the grant date “fair value” of these awards. This calculation is performed for accounting purposes and reported in the executive compensation tables required by the federal securities laws, even though the recipient of the awards may never realize any value from their awards.
Taxation of “Parachute” Payments
Sections 280G and 4999 of the Code provide that executive officers and directors who hold significant equity interests and certain other service providers may be subject to significant additional taxes if they receive payments or benefits in connection with a change in control of the company that exceeds certain prescribed limits, and that the company (or a successor) may forfeit a deduction on the amounts subject to this additional tax. We have not agreed to provide any executive officer, including any NEO, with a “gross-up” or other reimbursement payment for any tax liability that the executive officer might owe as a result of the application of Sections 280G or 4999 of the Code.
Section 409A of the Internal Revenue Code
Section 409A of the Code imposes additional significant taxes in the event that an executive officer, director or service provider receives “deferred compensation” that does not satisfy the requirements of Section 409A of the Code. Although we do not maintain a traditional nonqualified deferred compensation plan, Section 409A of the Code does apply to certain severance arrangements, bonus arrangements and equity awards. We structure all our severance arrangements, bonus arrangements and equity awards in a manner to either avoid the application of Section 409A or, to the extent doing so is not possible, to comply with the applicable requirements of Section 409A of the Code.
Compensation Risk Assessment
We structure our pay to consist of both fixed and variable compensation to motivate our employees, including our NEOs, to produce superior short- and long-term results that are in the best interests of our company and stockholders to attain our ultimate objective of increasing stockholder value. In addition, we have established, and the Compensation Committee endorses, several controls to address and mitigate compensation-related risk, such as maintaining an anti-hedging and anti-pledging policy and stock ownership guidelines for our executive officers (including our NEOs) and directors.
The Compensation Committee, in consultation with its compensation consultant, Compensia, evaluates whether our policies and practices create excessive risk in our compensation programs. In 2020, this risk assessment included, among other things, a review of our cash and equity incentive-based compensation plans to ensure that they are aligned with our corporate performance goals and overall target total direct compensation to ensure an appropriate balance between fixed and variable pay components. Based on this assessment, the Compensation Committee concluded that our compensation policies and practices are not reasonably likely to have a material adverse effect on our company.
The Compensation Committee intends to continue to evaluate on an annual basis the potential risks associated with our compensation policies and practices, and has engaged Compensia to conduct an updated assessment of our compensation policies and practices during 2021. As a result of the approval and launch of our first commercial product, Oxbryta, in late 2019, we expect this evaluation will include the potential risks associated with field-based incentive compensation and commercial-related goals and targets.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 47
2020 Summary Compensation Table
The following table sets forth information regarding total compensation awarded to, earned by and paid to each of our NEOs during the fiscal years ended December 31, 2020, 2019 and 2018.
Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($)(1) | Option Awards ($)(2) | Non-equity incentive plan compensation ($)(3) | All other Compensation ($) | Total ($) | ||||||||||||||||||||||||||||||||
Ted Love, M.D. President, Chief Executive Officer and Director | 2020 | 661,250 | (4) | — | 11,778,840 | (5) | 4,693,666 | 467,000 | 5,000 | (6) | 17,605,756 | |||||||||||||||||||||||||||||
| 2019 | 596,876 | — | 4,359,600 | 4,595,717 | 540,000 | 5,000 | 10,097,193 | |||||||||||||||||||||||||||||||||
| 2018 | 568,750 | — | 4,648,800 | 4,623,690 | 414,000 | 5,000 | 10,260,240 | |||||||||||||||||||||||||||||||||
David Johnson Chief Commercial Officer | 2020 | 463,750 | (7) | — | 4,037,361 | (8) | 1,582,504 | 234,000 | 5,000 | (6) | 6,322,615 | |||||||||||||||||||||||||||||
| 2019 | 453,125 | — | 1,211,000 | 1,267,784 | 250,000 | 5,000 | 3,186,909 | |||||||||||||||||||||||||||||||||
| 2018 | 355,000 | 75,000 | 1,897,750 | 1,898,534 | 169,000 | 5,000 | 4,394,284 | |||||||||||||||||||||||||||||||||
Jeffrey Farrow Chief Financial Officer | 2020 | 457,813 | (9) | — | 3,708,261 | (10) | 1,582,504 | 231,500 | 5,000 | (6) | 5,985,078 | |||||||||||||||||||||||||||||
| 2019 | 440,625 | — | 1,211,000 | 1,267,784 | 244,000 | 5,000 | 3,168,409 | |||||||||||||||||||||||||||||||||
| 2018 | 425,938 | — | 1,370,800 | 1,326,469 | 201,000 | 5,000 | 3,329,207 | |||||||||||||||||||||||||||||||||
Jung Choi Chief Business and Strategy Officer | 2020 | 433,125 | (11) | — | 3,708,261 | (12) | 1,582,504 | 219,000 | 5,000 | (6) | 5,947,890 | |||||||||||||||||||||||||||||
| 2019 | 416,875 | — | 1,211,000 | 1,267,784 | 231,000 | 5,000 | 3,131,659 | |||||||||||||||||||||||||||||||||
| 2018 | 392,063 | — | 1,370,800 | 1,326,469 | 185,000 | 5,000 | 3,279,332 | |||||||||||||||||||||||||||||||||
Tricia Suvari Chief Legal Officer | 2020 | 422,499 | (13) | — | 3,708,261 | (14) | 1,582,504 | 214,000 | 5,000 | (6) | 5,932,264 | |||||||||||||||||||||||||||||
| 2019 | 403,125 | — | 1,211,000 | 1,267,784 | 223,000 | 5,000 | 3,109,909 | |||||||||||||||||||||||||||||||||
| 2018 | 386,875 | — | 1,549,600 | 1,515,964 | 183,000 | 5,000 | 3,640,439 | |||||||||||||||||||||||||||||||||
| (1) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the RSUs granted during 2018, 2019 and 2020, as applicable, computed in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 10 to our financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020. These amounts do not reflect the actual economic value that may be realized by the NEOs upon the vesting or settlement of RSUs or the sale of the common stock underlying such awards. For performance-based RSUs, or PSUs, this table assumes the maximum achievement as of December 31, 2020, of all three escalating stock price targets under such awards over a four-year period ending June 30, 2024; based on this assumption, the value of the awards made to our NEOs at the date of grant would be as follows: Dr. Love - $7,063,496; Mr. Johnson - $2,118,050; Mr. Farrow - $2,118,050; Ms. Choi - $2,118,050; and Ms. Suvari - $2,118,050. |
| (2) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the stock option awards granted during 2018, 2019 and 2020, as applicable, computed in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 10 to our financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020. These amounts do not reflect the actual economic value that may be realized by the NEOs upon the exercise of the stock options or the sale of the common stock underlying such stock options. |
| (3) | The amounts reported reflect the annual cash incentive compensation earned by the NEOs under our Cash Incentive Plan, based on our achievement of certain corporate performance goals and each NEO’s (except for the CEO) achievement of his or her individual performance goals. |
| (4) | From January 1, 2020, Dr. Love’s annual base salary was $600,000, which increased to $670,000, effective February 16, 2020. |
| (5) | Includes (a) $7,063,496, representing the PSUs granted to Dr. Love in June 2020, and (b) $4,715,344, representing the time-based RSUs granted to Dr. Love in January 2020. |
| (6) | The amounts reported consist of employer matching contributions under our 401(k) of $5,000 each for each NEO. |
| (7) | From January 1, 2020, Mr. Johnson’s annual base salary was $455,000, which increased to $465,000, effective February 16, 2020. |
| (8) | Includes (a) $2,118,050, representing the PSUs granted to Mr. Johnson in June 2020, and (b) $1,919,311, representing the time-based RSUs granted to Mr. Johnson in January 2020. |
| (9) | From January 1, 2020, Mr. Farrow’s annual base salary was $442,500, which increased to $460,000, effective February 16, 2020. |
| (10) | Includes (a) $2,118,050, representing the PSUs granted to Mr. Farrow in June 2020, and (b) $1,590,211, representing the time-based RSUs granted to Mr. Farrow in January 2020. |
| (11) | From January 1, 2020, Ms. Choi’s annual base salary was $420,000, which increased to $435,000, effective February 16, 2020. |
| (12) | Includes (a) $2,118,050, representing the PSUs granted to Ms. Choi in June 2020, and (b) $1,590,211, representing the time-based RSUs granted to Ms. Choi in January 2020. |
| (13) | From January 1, 2020, Ms. Suvari’s annual base salary was $405,000, which increased to $425,000, effective February 16, 2020. |
| (14) | Includes (a) $2,118,050, representing the PSUs granted to Ms. Suvari in June 2020, and (b) $1,590,211, representing the time-based RSUs granted to Ms. Suvari in January 2020. |
48 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Grants of Plan-Based Awards for Fiscal Year 2020
The following table sets forth the individual awards made to each of our NEOs during 2020. For a description of the types of awards indicated below, please see our “Compensation Discussion and Analysis” above.
Name | Grant date | Estimated Target ($) | Estimated Future Payouts Under Equity Incentive Pan Awards(2) | All Other Stock Awards: Number of Shares of Stock or Units (#)(3) | All Other Option Awards: Number Of Underlying Options (#)(4) | Exercise or Base Price of Option Awards ($/sh.) | Grant Date Fair Market Value of Awards ($)(5) | |||||||||||||||||||||||||||||||||||
| Threshold (#) | Maximum (#) | |||||||||||||||||||||||||||||||||||||||||
Ted Love, M.D. | Time-based RSUs | 2/3/2020 | — |
|
|
|
|
|
| 71,640 | — | — | 4,715,345 | |||||||||||||||||||||||||||||
| Time-based Stock Options | 2/3/2020 | — |
|
|
|
|
|
| — | 114,190 | 65.82 | 4,693,666 | |||||||||||||||||||||||||||||
| Performance-based RSUs | 6/1/2020 | — | 28,280 | 141,400 |
|
|
| — | — | 7,063,496 | |||||||||||||||||||||||||||||||
| Annual Bonus Opportunity |
|
|
| 469,000 |
|
|
|
|
|
| — | — | — | — | |||||||||||||||||||||||||||
David Johnson | Time-based RSUs | 2/3/2020 | — |
|
|
|
|
|
| 29,160 | — | — | 1,919,311 | |||||||||||||||||||||||||||||
| Time-based Stock Options | 2/3/2020 | — |
|
|
|
|
|
| — | 38,500 | 65.82 | 1,582,504 | |||||||||||||||||||||||||||||
| Performance-based RSUs | 6/1/2020 | — | 8,480 | 42,400 |
|
|
| — | — | 2,118,050 | |||||||||||||||||||||||||||||||
| Annual Bonus Opportunity |
|
|
| 232,500 |
|
|
|
|
|
| — | — | — | — | |||||||||||||||||||||||||||
Jeffrey Farrow | Time-based RSUs | 2/3/2020 | — |
|
|
|
|
|
| 24,160 | — | — | 1,590,211 | |||||||||||||||||||||||||||||
| Time-based Stock Options | 2/3/2020 | — |
|
|
|
|
|
| — | 38,500 | 65.82 | 1,582,504 | |||||||||||||||||||||||||||||
| Performance-based RSUs | 6/1/2020 | — | 8,480 | 42,400 |
|
|
| — | — | 2,118,050 | |||||||||||||||||||||||||||||||
| Annual Bonus Opportunity |
|
|
| 230,000 |
|
|
|
|
|
| — | — | — | — | |||||||||||||||||||||||||||
Jung Choi | Time-based RSUs | 2/3/2020 | — |
|
|
|
|
|
| 24,160 | — | — | 1,590,211 | |||||||||||||||||||||||||||||
| Time-based Stock Options | 2/3/2020 | — |
|
|
|
|
|
| — | 38,500 | 65.82 | 1,582,504 | |||||||||||||||||||||||||||||
| Performance-based RSUs | 6/1/2020 | — | 8,480 | 42,400 |
|
|
| — | — | 2,118,050 | |||||||||||||||||||||||||||||||
| Annual Bonus Opportunity |
|
|
| 217,500 |
|
|
|
|
|
| — | — | — | — | |||||||||||||||||||||||||||
Tricia Suvari | Time-based RSUs | 2/3/2020 | — |
|
|
|
|
|
| 24,160 | — | — | 1,590,211 | |||||||||||||||||||||||||||||
| Time-based Stock Options | 2/3/2020 | — |
|
|
|
|
|
| — | 38,500 | 65.82 | 1,582,504 | |||||||||||||||||||||||||||||
| Performance-based RSUs | 6/1/2020 | — | 8,480 | 42,400 |
|
|
| — | — | 2,118,050 | |||||||||||||||||||||||||||||||
|
| Annual Bonus Opportunity |
|
|
| 212,500 |
|
|
|
|
|
| — | — | — | — | |||||||||||||||||||||||||||
| (1) | The amounts shown reflect the target cash incentive compensation for our NEOs, which are disclosed in the “2020 Target Annual Bonus” section of the above “Compensation Discussion and Analysis.” The actual amounts paid for 2020 are disclosed in the “Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table. There were no threshold or maximum payout levels for the cash incentive compensation (however, the portion based on corporate performance goals had a maximum of 155% of target for 2020). |
| (2) | The amounts shown reflect the threshold and maximum payout levels associated with PSUs granted pursuant to our 2015 Plan, which amounts will be payable in shares of our common stock if the stock price targets for such PSUs are met, with each such share price based on the average closing market price on the NASDAQ Global Select Market over a 20 consecutive trading day period. The target payout for the PSUs is the maximum payout. 20% of the maximum number of PSUs will be earned if a stock price of $109.20 per share is achieved on or before June 30, 2024; an additional 35% (for a total of 55%) of the maximum number of PSUs will be earned if a stock price of $145.60 per share is achieved on or before June 30, 2024; and an additional 45% (for a total of 100%) of the maximum number of PSUs will be earned if a stock price of $182.00 per share is achieved on or before June 30, 2024. Upon the achievement of each respective stock price target, 50% of the PSUs allotted to that tranche will vest, while the remaining 50% will vest on the first anniversary of the date the stock price target was achieved, subject to the executive officer’s continued employment or other service relationship with us through such vesting date. |
| (3) | The amounts shown represent time-based RSUs granted pursuant to our 2015 Plan, which amounts will be payable in shares of our common stock if the service-based conditions for such time-based RSUs are met. The time-based RSUs vest semi-annually over four years, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (4) | The amounts shown represent time-based stock options granted pursuant to our 2015 Plan. The time-based stock options vest quarterly over four years, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (5) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the equity awards granted during 2020, computed in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 10 to our financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020. These amounts do not reflect the actual economic value that may be realized by the NEOs upon the vesting or settlement of PSUs or RSUs or the exercise of the stock options, as applicable, or the sale of the common stock underlying such awards. For PSUs, this table assumes the maximum achievement as of December 31, 2020, of all three escalating stock price targets under such awards over a four-year period ending June 30, 2024; based on this assumption, the value of the awards made to our NEOs at the date of grant would be as follows: Dr. Love - $7,063,496; Mr. Johnson - $2,118,050; Mr. Farrow - $2,118,050; Ms. Choi - $2,118,050; and Ms. Suvari - $2,118,050. |
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 49
2020 Outstanding Equity Awards at Fiscal Year End
The following table sets forth certain information with respect to outstanding equity awards held by each of our NEOs as of December 31, 2020.
| Option Awards(1) | Stock Awards(1) | |||||||||||||||||||||||||||||||||||||||||||||||||
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of shares or units of that have not vested (#)(1) | Market value of shares or units of stock that have not vested ($)(2) | Equity Incentive Plan Awards: Number of unearned shares, units or other that have not vested (#) | Equity Incentive Plan Awards: Market value or payout value of unearned shares, units or other rights that have not vested ($)(2) | |||||||||||||||||||||||||||||||||||||||||
(a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||||||||||||||||||||||||||||||
Ted Love, M.D. | — | — | — | — |
|
|
| — | 12,063 | (3) | 522,449 | — | — | |||||||||||||||||||||||||||||||||||||
| 123,743 | (4) | 9,063 | (4) | — | 16.40 |
|
|
| 1/17/2027 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| 83,875 | (5) | 38,125 | (5) | — | 59.60 |
|
|
| 2/1/2028 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 29,250 | (6) | 1,266,818 | — | — | |||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 56,250 | (7) | 2,436,188 | — | — | |||||||||||||||||||||||||||||||||||||
| 63,437 | (8) | 81,563 | (8) | — | 48.44 |
|
|
| 2/1/2029 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 62,685 | (9) | 2,714,887 | — | — | |||||||||||||||||||||||||||||||||||||
| 21,410 | (10) | 92,780 | (10) | — | 65.82 |
|
|
| 2/3/2030 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | — | — | 141,400 | (11) | 6,124,034 | |||||||||||||||||||||||||||||||||||||
David Johnson | — | — | — | — |
|
|
| — | 13,125 | (12) | 568,444 | — | — | |||||||||||||||||||||||||||||||||||||
| 37,812 | (13) | 17,188 | (13) | — | 54.05 |
|
|
| 3/12/2028 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 15,625 | (7) | 676,719 | — | — | |||||||||||||||||||||||||||||||||||||
| 17,500 | (8) | 22,500 | (8) | — | 48.44 |
|
|
| 2/1/2029 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 25,515 | (9) | 1,105,055 | — | — | |||||||||||||||||||||||||||||||||||||
| 7,218 | (10) | 31,282 | (10) | — | 65.82 |
|
|
| 2/3/2030 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | — | — | 42,400 | (11) | 1,836,344 | |||||||||||||||||||||||||||||||||||||
Jeffrey Farrow | 120,000 | (14) | — | — | 14.96 |
|
|
| 2/24/2026 | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 3,063 | (3) | 132,659 | — | — | |||||||||||||||||||||||||||||||||||||
| 31,639 | (4) | 2,313 | (4) | — | 16.40 |
|
|
| 1/17/2027 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| 24,062 | (5) | 10,938 | (5) | — | 59.60 |
|
|
| 2/1/2028 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 8,625 | (6) | 373,549 | — | — | |||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 15,625 | (7) | 676,719 | — | — | |||||||||||||||||||||||||||||||||||||
| 17,500 | (8) | 22,500 | (8) | — | 48.44 |
|
|
| 2/1/2029 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 21,140 | (9) | 915,573 | — | — | |||||||||||||||||||||||||||||||||||||
| 7,218 | (10) | 31,282 | (10) | — | 65.82 |
|
|
| 2/3/2030 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | — | — | 42,400 | (11) | 1,836,344 | |||||||||||||||||||||||||||||||||||||
50 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
| Option Awards(1) | Stock Awards(1) | |||||||||||||||||||||||||||||||||||||||||||||||||
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of shares or units of that have not vested (#)(1) | Market value of shares or units of stock that have not vested ($)(2) | Equity Incentive Plan Awards: Number of unearned shares, units or other that have not vested (#) | Equity Incentive Plan Awards: Market value or payout value of unearned shares, units or other rights that have not vested ($)(2) | |||||||||||||||||||||||||||||||||||||||||
(a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||||||||||||||||||||||||||||||
Jung Choi | 74,964 | (14) | — | — | 3.40 |
|
|
| 4/9/2025 | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| 5,350 | (14) | — | — | 3.40 |
|
|
| 4/9/2025 | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 4,063 | (3) | 175,969 | — | — | |||||||||||||||||||||||||||||||||||||
| 45,937 | (4) | 3,063 | (4) | — | 16.40 |
|
|
| 1/17/2027 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| 24,062 | (5) | 10,938 | (5) | — | 59.60 |
|
|
| 2/1/2028 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 8,625 | (6) | 373,549 | — | — | |||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 15,625 | (7) | 676,719 | — | — | |||||||||||||||||||||||||||||||||||||
| 17,500 | (8) | 22,500 | (8) | — | 48.44 |
|
|
| 2/1/2029 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 21,140 | (9) | 915,573 | — | — | |||||||||||||||||||||||||||||||||||||
| 7,218 | (10) | 31,282 | (10) | — | 65.82 |
|
|
| 2/3/2030 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | — | — | 42,400 | (11) | 1,836,344 | |||||||||||||||||||||||||||||||||||||
Tricia Suvari | 100,000 | (14) | — | — | 18.25 |
|
|
| 10/11/2026 | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| 27,500 | (5) | 12,500 | (5) | — | 59.60 |
|
|
| 2/1/2028 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 9,750 | (6) | 422,273 | — | — | |||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 15,625 | (7) | 676,719 | — | — | |||||||||||||||||||||||||||||||||||||
| 17,500 | (8) | 22,500 | (8) | — | 48.44 |
|
|
| 2/1/2029 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | 21,140 | (9) | 915,573 | — | — | |||||||||||||||||||||||||||||||||||||
| 7,218 | (10) | 31,282 | (10) | — | 65.82 |
|
|
| 2/3/2030 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| — | — | — | — |
|
|
| — | — | — | 42,400 | (11) | 1,836,344 | |||||||||||||||||||||||||||||||||||||
| (1) | All of the outstanding equity awards held by our NEOs will become fully vested and exercisable or non-forfeitable, as applicable, if the NEO is terminated without Cause or resigns for Good Reason, in either case, within 12 months following a Sale Event. The vesting acceleration of the outstanding equity awards held by our NEOs is described in greater detail in “Employment Arrangements with Our Named Executive Officers—Change in Control Policy”. |
| (2) | Computed in accordance with SEC rules as the number of unvested shares or units multiplied by the closing market price of a share of our common stock on December 31, 2020, which was $43.31. The actual value (if any) to be realized by the NEO depends on whether the shares or units vest and the future performance of our common stock. |
| (3) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date of January 17, 2017, such that all of the RSUs were fully vested on January 17, 2021, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (4) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of January 17, 2017, such that all of the shares were fully vested on January 17, 2021, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (5) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of February 1, 2018, such that all of the shares will be fully vested on February 1, 2022 subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (6) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date of February 1, 2018, such that all of the RSUs will be fully vested on February 1, 2022, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (7) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date of February 1, 2019, such that all of the RSUs will be fully vested on February 1, 2023, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 51
| (8) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of February 1, 2019, such that all of the shares will be fully vested on February 1, 2023, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (9) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date of February 3, 2020, such that all of the RSUs will be fully vested on February 3, 2024, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (10) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of February 3, 2020, such that all of the shares will be fully vested on February 3, 2024, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (11) | Represents the maximum number of PSUs that may be earned. The vesting of the PSUs is contingent upon the achievement of three escalating stock price targets, with each such share price based on the average closing market price on the NASDAQ Global Select Market over a 20 consecutive trading day period. 20% of the maximum number of PSUs will be earned if a stock price of $109.20 per share is achieved on or before June 30, 2024; an additional 35% (for a total of 55%) of the maximum number of PSUs will be earned if a stock price of $145.60 per share is achieved on or before June 30, 2024; and an additional 45% (for a total of 100%) of the maximum number of PSUs will be earned if a stock price of $182.00 per share is achieved on or before June 30, 2024. Upon the achievement of the respective stock price targets, 50% of the PSUs allotted to that tranche will vest, while the remaining 50% will vest on the first anniversary of the date the stock price target was achieved, subject to the NEO’s continued employment or other service relationship with us through such vesting date. |
| (12) | 25% of the RSUs vested on April 1, 2019 and 1/8th of the RSUs vest on a semi-annual basis from such date, such that all of the RSUs will be fully vested on April 1, 2022, subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (13) | 25% of the shares subject to the stock option vested on March 12, 2019 and 1/16th of the shares subject to the stock option vest on a quarterly basis from such date, such that all of the shares will be fully vested on March 12, 2022 subject to the NEO’s continued employment or other service relationship with us through each applicable vesting date. |
| (14) | Shares subject to the stock option have fully vested. |
Option Exercises and Stock Vested in Fiscal Year 2020
The following table sets forth the number of shares acquired and the value realized upon exercises of stock options and vesting of RSUs during the fiscal year ended December 31, 2020, by each of our NEOs.
|
|
| Option Awards |
| Stock Awards | |||||||||||||||||||||||
Name |
| Number of Shares Acquired on Exercise (#) | Value Realized ($)(1) |
| Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($)(2) | |||||||||||||||||||||
Ted Love, M.D. |
|
|
| — | — |
| 75,080 | 4,992,999 | |||||||||||||||||||
David Johnson |
|
|
| — | — |
| 18,645 | 1,127,546 | |||||||||||||||||||
Jeffrey Farrow |
|
|
| — | — |
| 21,145 | 1,406,745 | |||||||||||||||||||
Jung Choi |
|
|
| — | — |
| 23,145 | 1,539,485 | |||||||||||||||||||
Tricia Suvari |
|
|
| — | — |
| 15,770 | 1,050,007 | |||||||||||||||||||
| (1) | The value realized upon the exercise of stock options is calculated by (a) subtracting the stock option exercise price from the market price on the date of exercise to get the realized value per share, and (b) multiplying the realized value per share by the number of shares underlying the stock options exercised. |
| (2) | The value realized upon vesting of RSUs is calculated by multiplying the number of shares of RSUs vested by the market price on the vest date. |
52 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Potential Payments on Termination or Change in Control
Our Amended and Restated Severance and Change in Control Policy provides for certain payments and benefits to each of our NEOs if the NEO is terminated by us or our acquirer or successor without Cause or resigns for Good Reason (as such terms are defined in such policy), both during and outside of the Change in Control Period (assuming that all outstanding equity awards were assumed, continued or substituted by an acquirer or successor in such change in control), subject to the NEO’s execution and non-revocation of a severance agreement, including a general release of claims. The table below quantifies the potential payments and benefits that would have become due to our NEOs assuming that Amended and Restated Severance and Change in Control Policy had been in effect as of December 31, 2020, and that one of the triggering events above occurred as of December 31, 2020. The market price of a share of our common stock on December 31, 2020, was $43.31.
Name | Qualifying termination Not in connection with a Change in Control ($) | Qualifying Termination in Connection with a Change in Control ($) | ||||||||
Ted Love, M.D. |
|
|
|
|
|
| ||||
Cash Severance Payment | 670,000 | (1) | 1,005,000 | (2) | ||||||
Cash Incentive Bonus Payment | 469,000 | (3) | 703,500 | (4) | ||||||
COBRA Premiums | 21,103 | (5) | 31,655 | (6) | ||||||
Accelerated Equity Vesting (Time-Based) | — | 7,184,226 | (7) | |||||||
Accelerated Equity Vesting (Performance-Based) | — | — | ||||||||
David Johnson |
|
|
|
|
|
| ||||
Cash Severance Payment | 465,000 | (1) | 465,000 | (1) | ||||||
Cash Incentive Bonus Payment | — | 232,500 | (3) | |||||||
COBRA Premiums | 21,783 | (5) | 21,783 | (5) | ||||||
Accelerated Equity Vesting (Time-Based) | — | 2,350,217 | (7) | |||||||
Accelerated Equity Vesting (Performance-Based) | — | — | ||||||||
Jeffrey Farrow |
|
|
|
|
|
| ||||
Cash Severance Payment | 460,000 | (1) | 460,000 | (1) | ||||||
Cash Incentive Bonus Payment | — | 230,000 | (3) | |||||||
COBRA Premiums | 21,103 | (5) | 21,103 | (5) | ||||||
Accelerated Equity Vesting (Time-Based) | — | 2,160,742 | (7) | |||||||
Accelerated Equity Vesting (Performance-Based) | — | — | ||||||||
Jung Choi |
|
|
|
|
|
| ||||
Cash Severance Payment | 435,000 | (1) | 435,000 | (1) | ||||||
Cash Incentive Bonus Payment | — | 217,500 | (3) | |||||||
COBRA Premiums | — | (5) | — | (5) | ||||||
Accelerated Equity Vesting (Time-Based) | — | 2,224,235 | (7) | |||||||
Accelerated Equity Vesting (Performance-Based) | — | — | ||||||||
Tricia Suvari |
|
|
|
|
|
| ||||
Cash Severance Payment | 425,000 | (1) | 425,000 | (1) | ||||||
Cash Incentive Bonus Payment | — | 212,500 | (3) | |||||||
COBRA Premiums | 17,740 | (5) | 17,740 | (5) | ||||||
Accelerated Equity Vesting (Time-Based) | — | 2,014,565 | (7) | |||||||
Accelerated Equity Vesting (Performance-Based) | — | — | ||||||||
| (1) | Represents 12 months of the NEO’s base salary. |
| (2) | Represents 18 months of Dr. Love’s base salary. |
| (3) | Represents 100% of the NEO’s target annual bonus opportunity. The table does not include the prorated annual bonus opportunity that the NEO would be entitled to since, on December 31, 2020, the full annual bonus, which is shown in the “Summary Compensation Table,” was earned. |
| (4) | Represents 150% of Dr. Love’s target annual bonus opportunity. The table does not include the prorated annual bonus opportunity that Dr. Love would be entitled to since, on December 31, 2020, the full annual bonus, which is shown in the “Summary Compensation Table,” was earned. |
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 53
| (5) | Represents 12 months of our contribution towards health insurance, based on our actual costs to provide health insurance to the NEO as of the date of termination. Since Ms. Choi did not participate in the Company’s health insurance plan, she will receive zero months of payment of COBRA premiums. |
| (6) | Represents 18 months of our contribution towards health insurance, based on our actual costs to provide health insurance to Dr. Love as of the date of termination. |
| (7) | Represents the value of acceleration of vesting of 100% of the NEO’s unvested and outstanding equity awards, other than PSUs, based on the market price of a share of our common stock on December 31, 2020, which was $43.31. For the PSUs, represents the value of acceleration of vesting of 0% of the NEO’s unvested and outstanding PSUs, based on an assumed sale price of a share of our common stock in the change in control of $43.31. For stock options with a per share exercise price greater than $43.31, no amount was included with respect to such stock options. |
Securities Authorized for Issuance under Equity Compensation Plans
Equity Compensation Plans
The following table sets forth information as of December 31, 2020, regarding shares of common stock that may be issued under our equity compensation plans, consisting of our 2012 Stock Option and Grant Plan (or 2012 Plan), our 2015 Plan, our 2017 Inducement Plan and our ESPP.
Plan Category | Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights (#) (a) | Weighted Average Exercise Price of Outstanding Options, Warrants and Rights (b)(1) | Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) (c) | ||||||||||||
Equity compensation plans approved by security holders(2) | 4,408,439 | (3) | $40.38 | (3) | 4,995,682 | (4) | |||||||||
Equity compensation plans not approved by security holders(5) | 1,543,947 | $50.44 | 1,277,475 | ||||||||||||
Total | 5,952,386 | $42.07 | 6,233,157 | ||||||||||||
| (1) | The weighted average exercise price is calculated based solely on outstanding stock options. |
| (2) | Includes the 2012 Plan, the 2015 Plan and the ESPP. Our 2015 Plan provides that the number of shares reserved and available for issuance under the plan will automatically increase each January 1, beginning on January 1, 2016 until January 1, 2025, by 4% of the outstanding number of shares of common stock on the immediately preceding December 31 or such lesser number of shares as determined by the compensation committee of our Board of Directors. Our ESPP provides that the number of shares reserved and available for issuance will automatically increase each January 1, from January 1, 2016 until January 1, 2025, by the lesser of (i) 3,000,000 shares of common stock, (ii) 1% of the outstanding number of shares of common stock on the immediately preceding December 31 or (iii) such lesser number of shares as determined by the compensation committee of our Board of Directors. On January 1, 2020, the number of shares available for issuance under our 2015 Plan and our ESPP increased by 2,425,694 shares and 100,000 shares, respectively, pursuant to these provisions. These increases are not reflected in the table above. We no longer grant new awards under the 2012 Plan, and any awards previously granted under such plan prior to our initial public offering that are forfeited, canceled, reacquired by us prior to vesting satisfied without the issuance of stock or otherwise terminated (other than by exercise) are added to shares available for issuance under the 2015 Plan. |
| (3) | Does not include purchase rights accruing under the ESPP as of December 31, 2020, because the purchase rights (and, therefore, the number of shares to be purchased) were not determined until the end of the purchase periods on January 31, 2021, and February 28, 2021. |
| (4) | As of December 31, 2020, there were 4,741,092 shares of common stock available for issuance under the 2015 Plan and 254,590 shares of common stock available for issuance under the ESPP (which number includes shares subject to purchase during the current purchase periods which commenced on February 1, 2021 and March 1, 2021, since the exact number of which will not be known until the end of the purchase periods on August 31, 2021. |
| (5) | Consists solely of the 2017 Inducement Plan. |
54 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
As required by Section 953(b) of the Dodd-Frank Act, and Item 402(u) of Regulation S-K, we are disclosing the ratio of the annual total compensation of our CEO to the median of the annual total compensation of all our employees (other than our CEO).
For 2020:
| • | the annual total compensation of our CEO was $17,605,756 (as disclosed in the Summary Compensation Table above); |
| • | the median of the annual total compensation of all our employees was $373,366; and |
| • | the ratio of the annual total compensation of our CEO to the annual total compensation of our median employee was 47 to 1. |
In 2020, there was no change in our employee population or employee compensation arrangements that we reasonably believe would result in a significant change to our pay ratio disclosure. However, in 2020 there was a change in the circumstances of the employee identified as the median employee for 2019 as that individual was promoted and received a special promotional award. Accordingly, it is no longer appropriate to use that median employee because we reasonably believe that the change in such employee’s circumstances would result in a significant change in our pay ratio disclosure. As permitted by SEC rules, we have elected to use another employee, whose 2019 compensation was substantially similar to the original median employee’s 2019 compensation based on the same consistently applied compensation measure used to select the original median employee, as our median employee for 2020. After identifying our median employee and ensuring this employee did not have anomalous compensation in 2020, we then calculated the annual total compensation of our median employee using the same methodology we used for our CEO, and our other NEOs, in the 2020 Summary Compensation Table above.
In addition, in 2020, our CEO was granted an award of performance-based RSUs with a grant date fair value of $7,063,496 to be earned and vest contingent upon the achievement of three escalating stock price targets over a four-year period ending June 30, 2024. This one-time award that the Compensation Committee granted to our CEO (as well as other members of our senior management) as a special motivational and retention vehicle had the effect of increasing our CEO’s annual total compensation by nearly 75% year-over-year (based on the calculations and assumptions described above), resulting in a significantly higher pay ratio than in 2019.
In 2019, we identified our median employee as of December 31, 2019, by: (i) calculating for each full-time employee, except our CEO, and part-time employee on that date (a) actual annual base salary in 2019 (annualized for new hires who were not employed for the entire year and for permanent employees on an unpaid leave of absence for a portion of the year), (b) actual annual bonus earned in 2019 (annualized for new hires who were not employed for the entire year and for permanent employees on an unpaid leave of absence for a portion of the year), and (c) the grant date fair value of all equity awards granted in 2019; and (ii) ranking this compensation from lowest to highest to identify our median employee. For purposes of this disclosure, earnings of any employee outside the U.S. were converted to U.S. dollars using the monthly average Swiss franc to U.S. dollar exchange rate for each month in 2019, based on exchange rates used by us for various purposes. We used this compensation as our consistently applied compensation measure because we believe it was representative of our employee compensation.
The pay ratio above is a reasonable estimate calculated in a manner consistent with the SEC rules. The SEC rules provide companies with significant flexibility in identifying the median employee and calculating the pay ratio, including flexibility to adopt a variety of methodologies, to apply certain exclusions and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices. Accordingly, the pay ratio above may not be comparable with the pay ratios of other companies, even companies within our industry.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 55
The following Compensation Committee Report is not considered proxy solicitation material and is not deemed filed with the Securities and Exchange Commission. Notwithstanding anything to the contrary set forth in any of our filings made under the Securities Act of 1933 or the Exchange Act that might incorporate our filings under those statutes, the Compensation Committee Report shall not be incorporated by reference into any of our prior filings or into any of our future filings under those statutes.
The Compensation Committee of the Board of Directors has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K with the Company’s management. Based on this review and discussion, the Compensation Committee recommended to the Board of Directors, and the Board of Directors approved, that the Compensation Discussion and Analysis be included in this Proxy Statement for the Annual Meeting and incorporated by reference in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020.
COMPENSATION COMMITTEE
WENDY L. YARNO, CHAIR
SCOTT W. MORRISON
MARK L. PERRY
56 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than the compensation agreements and other arrangements with our directors and NEOs described above, and the transactions described below, since January 1, 2020, there has not been and there is not currently proposed, any transaction or series of similar transactions to which we were, or will be, a party in which the amount involved exceeded, or will exceed, $120,000 and in which any director, executive officer, holder of five percent or more of any class of our capital stock or any member of the immediate family of, or entities affiliated with, any of the foregoing persons, had, or will have, a direct or indirect material interest.
We have entered into agreements to indemnify our directors and executive officers. These agreements will, among other things, require us to indemnify these individuals for certain expenses (including attorneys’ fees), judgments, fines and settlement amounts reasonably incurred by such person in any action or proceeding, including any action by or in our right, on account of any services undertaken by such person on behalf of our company or that person’s status as a member of our board of directors to the maximum extent allowed under Delaware law.
Procedures for Approval of Related Person Transactions
The Audit Committee conducts an appropriate review of all related party transactions for potential conflict of interest situations on an ongoing basis, and the approval of the Audit Committee is required for all such transactions. The Audit Committee follows the policies and procedures set forth in our Related Person Transaction Policy in order to facilitate such review. The Related Person Transaction Policy is written.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 57
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth the beneficial ownership information of our common stock by:
| • | each person known to us to be the beneficial owner of more than 5% of our common stock as of March 31, 2021; |
| • | each NEO; |
| • | each current director; and |
| • | all current executive officers and directors as a group. |
We have based our calculation of the percentage of beneficial ownership of 62,240,050 shares of common stock outstanding on March 31, 2021.
Each individual or entity shown in the table has furnished information with respect to beneficial ownership. The information with respect to each individual or entity is as of March 31, 2021, unless otherwise noted. The information with respect to certain significant stockholders is based on filings by the beneficial owners with the SEC pursuant to Sections 13(d) and 13(g) of the Exchange Act. We have determined beneficial ownership in accordance with the SEC’s rules. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options that are either immediately exercisable or exercisable on or before May 30, 2021, which is 60 days after March 31, 2021. These shares are deemed to be outstanding and beneficially owned by the person holding those stock options for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Beneficial Owner(1) | Number of Shares Beneficially Owned | Percentage of Shares Beneficially Owned | ||||||||
5% or Greater Stockholders: |
|
|
|
|
|
| ||||
Entities affiliated with Janus Henderson Group plc(2) 201 Bishopsgate EC2M 3AE, United Kingdom | 5,966,862 | 9.6 | % | |||||||
Entities affiliated with Bank of America Corporation(3) 100 N Tryon Street Charlotte, NC 28255 | 5,883,578 | 9.5 | % | |||||||
Entities affiliated with Perceptive(4) 51 Astor Place, 10th Floor New York, NY 10003 | 5,674,711 | 9.1 | % | |||||||
Entities affiliated with The Vanguard Group(5) 100 Vanguard Blvd. Malvern, PA 19355 | 5,460,482 | 8.8 | % | |||||||
Entities affiliated with Fidelity(6) 245 Summer Street Boston, MA 02210 | 4,407,065 | 7.1 | % | |||||||
Entities affiliated with T. Rowe Price Associates, Inc.(7) 100 E. Pratt Street Baltimore, MD 21202 | 4,063,123 | 6.5 | % | |||||||
Entities affiliated with BlackRock(8) 55 East 52nd Street New York, NY 10055 | 3,683,260 | 5.9 | % | |||||||
58 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
Beneficial Owner(1) | Number of Shares Beneficially Owned | Percentage of Shares Beneficially Owned | ||||||||
Named Executive Officers and Directors: |
|
|
|
|
|
| ||||
Ted W. Love, M.D.(9) | 1,434,488 | 2.3 | % | |||||||
Jeffrey Farrow(10) | 232,322 | * | * | |||||||
Jung E. Choi(11) | 394,372 | * | * | |||||||
David L. Johnson(12) | 103,188 | * | * | |||||||
Tricia Suvari, Esq.(13) | 186,596 | * | * | |||||||
Willie L. Brown, Jr.(14) | 117,137 | * | * | |||||||
Scott W. Morrison(15) | 92,109 | * | * | |||||||
Deval L. Patrick(16) | 7,834 | * | * | |||||||
Mark L. Perry(17) | 58,537 | * | * | |||||||
Glenn F. Pierce, M.D., Ph.D.(18) | 92,109 | * | * | |||||||
Philip A. Pizzo, M.D.(19) | 62,109 | * | * | |||||||
Dawn A. Svoronos(20) | 38,731 | * | * | |||||||
Alexis A. Thompson, M.D., M.P.H.(21) | 622 | * | * | |||||||
Wendy L. Yarno(22) | 62,109 | * | * | |||||||
All directors and executive officers as a group (16 persons)(23) | 2,964,928 | 4.7 | % | |||||||
| * | Represents beneficial ownership of less than 1% of the shares of common stock. |
| (1) | Unless otherwise indicated, the address for each beneficial owner is c/o Global Blood Therapeutics, Inc., 181 Oyster Point Boulevard, South San Francisco, CA 94080. |
| (2) | Based solely on a report on Schedule 13G filed with the SEC on February 12, 2021, which indicates that Janus Henderson Group plc (“Janus Henderson”) had shared voting power and shared dispositive power over 5,966,862 shares of common stock. Janus Henderson has an indirect 97% ownership stake in Intech Investment Management LLC (“Intech”) and a 100% ownership stake in Janus Capital Management LLC (“JCM”), Perkins Investment Management LLC, Henderson Global Investors Limited and Janus Henderson Investors Australia Institutional Funds Management Limited (each an “Asset Manager”). Each Asset Manager is an investment adviser registered or authorized in its relevant jurisdiction and each furnishing investment advice to various fund, individual and/or institutional clients (collectively referred to herein as “Managed Portfolios”). As a result of its role as investment adviser or sub-adviser to the Managed Portfolios, JCM may be deemed to be the beneficial owner of 5,897,668 shares of common stock, and as a result of its role as investment adviser or sub-adviser to the Managed Portfolios, Intech may be deemed to be the beneficial owner of 69,194 shares of common stock. |
| (3) | Based solely on a report on Schedule 13G/A filed with the SEC on February 8, 2021, which indicates that Bank of America Corporation had shared voting power with respect to 5,778,958 shares of common stock and had shared dispositive power over 5,883,578 shares of common stock. |
| (4) | Based solely on a report on Schedule 13G/A filed with the SEC on February 16, 2021, which indicates that Perceptive Advisors LLC and Joseph Edelman had shared voting power and shared dispositive power over 5,674,711 shares of common stock, all of which are held by Perceptive Life Sciences Master Fund Ltd. (the “Fund”). Perceptive Advisors LLC serves as the investment manager to the Fund. Joseph Edelman is the managing member of Perceptive Advisors LLC. |
| (5) | Based solely on a report on Schedule 13G/A filed with the SEC on February 10, 2021, which indicates that The Vanguard Group had (i) shared voting power with respect to 46,133 shares of common stock, (ii) sole dispositive power over 5,368,569 shares of common stock and (iii) shared dispositive power over 91,913 shares of common stock. |
| (6) | Based solely on a report on Schedule 13G/A filed with the SEC on February 8, 2021, which indicates that FMR LLC had sole voting power with respect to 463,185 shares of common stock and had sole dispositive power over 4,407,065 shares of common stock. Abigail P. Johnson is a Director, the Chairman, and the Chief Executive Officer of FMR LLC. Members of the Johnson family, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act (“Fidelity Funds”) advised by Fidelity Management & Research Company (“FMR Co”), a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds’ Boards of Trustees. Fidelity Management & Research Company carries out the voting of the shares under written guidelines established by the Fidelity Funds’ Boards of Trustees. |
| (7) | Based solely on a report on Schedule 13G/A filed with the SEC on February 16, 2021, which indicates that T. Rowe Price Associates, Inc. had sole voting power with respect to 837,735 shares of common stock and had sole dispositive power over 4,063,123 shares of common stock. |
| (8) | Based solely on a report on Schedule 13G/A filed with the SEC on January 29, 2021, which indicates that BlackRock, Inc. had sole voting power with respect to 3,470,400 shares of common stock and had sole dispositive power over 3,683,260 shares of common stock. |
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 59
| (9) | Includes (i) 774,311 shares of common stock held by Dr. Love; (ii) 5,000 shares of common stock held by Dr. Love’s two daughters, as to which Dr. Love disclaims beneficial ownership; (iii) 306,000 shares of common stock held by three trusts for the benefit of Dr. Love’s daughters; and (iv) 349,177 shares of common stock that Dr. Love has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (10) | Includes (i) 15,402 shares of common stock held by Mr. Farrow; and (ii) 216,920 shares of common stock that Mr. Farrow has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (11) | Includes (i) 177,090 shares of common stock held by Ms. Choi; (ii) 25,000 shares of common stock held in The 2005 William Park and Jung Choi Family Trust; and (iii) 192,282 shares of common stock that Ms. Choi has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (12) | Includes (i) 27,407 shares of common stock held by Mr. Johnson; and (ii) 75,781 shares of common stock that Mr. Johnson has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (13) | Includes (i) 19,565 shares of common stock held by Ms. Suvari; and (ii) 167,031 shares of common stock that Ms. Suvari has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (14) | Includes (i) 36,228 shares of common stock held by Mr. Brown; and (ii) 80,909 shares of common stock that Mr. Brown has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (15) | Includes (i) 4,800 shares of common stock held by Mr. Morrison; and (ii) 87,309 shares of common stock that Mr. Morrison has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (16) | Includes (i) 2,400 shares of common stock held by Mr. Patrick; and (ii) 5,434 shares of common stock that Mr. Patrick has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (17) | Includes (i) 31,228 shares of common stock held by Mr. Perry; and (ii) 27,309 shares of common stock that Mr. Perry has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (18) | Includes (i) 19,800 shares of common stock held by Dr. Pierce; and (ii) 72,309 shares of common stock that Dr. Pierce has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (19) | Includes (i) 4,800 shares of common stock held by Dr. Pizzo; and (ii) 57,309 shares of common stock that Dr. Pizzo has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (20) | Includes (i) 2,256 shares of common stock held by Ms. Svoronos; and (ii) 36,475 shares of common stock that Ms. Svoronos has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (21) | Includes 622 shares of common stock that Dr. Thompson has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (22) | Includes (i) 4,800 shares of common stock held by Ms. Yarno; and (ii) 57,309 shares of common stock that Ms. Yarno has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
| (23) | Includes the number of shares beneficially owned by the named executive officers and directors listed in the table above, as well as: (i) 12,005 shares of common stock held by Brian Cathers, Ph.D. and 37,343 shares of common stock that Dr. Cathers has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options; and (ii) 4,661 shares of common stock held by Eric Fink and 28,656 shares of common stock that Mr. Fink has the right to acquire from us within 60 days of March 31, 2021 pursuant to the exercise of stock options. |
60 2021 Proxy Statement ï Global Blood Therapeutics, Inc.
HOUSEHOLDING OF PROXY MATERIALS
We have made available a procedure approved by the SEC known as “householding.” This procedure allows multiple stockholders residing at the same address the convenience of receiving a single copy of our Notice, Annual Report and proxy materials, as applicable. This allows us to save money by reducing the number of documents we must print and mail, and helps protect the environment as well.
Householding is available to both registered stockholders (i.e., those stockholders with certificates registered in their name) and street name holders (i.e., those stockholders who hold their shares through a brokerage).
If you are a registered stockholder and would like to consent to a mailing of proxy materials and other stockholder information only to one account in your household, as identified by you, we will deliver or mail a single copy of our Annual Report and proxy materials for all registered stockholders residing at the same address. Your consent will be perpetual unless you revoke it, which you may do at any time by contacting the Proxy Department of Continental Stock Transfer & Trust Company, LLC, or Continental, at 1 State Street, 30th Floor, New York, NY 10004.
Registered stockholders who have not consented to householding will continue to receive copies of Annual Reports and proxy materials for each registered stockholder residing at the same address. As a registered stockholder, you may elect to participate in householding and receive only a single copy of Annual Reports or proxy statements for all registered stockholders residing at the same address by contacting Continental as outlined above.
Stockholders who hold their shares through a brokerage may elect to participate in householding or revoke their consent to participate in householding by contacting their respective brokers.
Global Blood Therapeutics, Inc. ï 2021 Proxy Statement 61
We are not aware of any matters that may come before the meeting other than those referred to in the Notice. If any other matter shall properly come before the Annual Meeting, however, the persons named in the accompanying proxy intend to vote all proxies in accordance with their best judgment.
Accompanying this Proxy Statement is our Annual Report. Copies of our Annual Report are available free of charge on our website at ir.gbt.com/financial-information/sec-filings or you can request a copy free of charge by calling Investor Relations at 650.741.7730 or sending an e-mail request to investor@gbt.com. Please include your contact information with the request.
By Order of the Board of Directors,
| Global | Blood Therapeutics, Inc. |
Ted W. Love, M.D. |
Ted W. Love, M.D.
President and Chief Executive Officer
April 28, 2021
62 2021 Proxy Statement ï Global Blood Therapeutics, Inc.

GLOBAL BLOOD THERAPEUTICS, INC.
181 OYSTER POINT BOULEVARD
SOUTH SAN FRANCISCO, CA 94080
VOTE BY INTERNET
Before The Meeting - Go to www.proxyvote.com
Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 p.m. Eastern Time on June 16, 2021. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form.
During The Meeting - Go to www.virtualshareholdermeeting.com/GBT2021
You may attend the meeting via the Internet and vote during the meeting. Have the information that is printed in the box marked by the arrow available and follow the instructions.
VOTE BY PHONE - 1-800-690-6903
Use any touch-tone telephone to transmit your voting instructions up until 11:59 p.m. Eastern Time on June 16, 2021. Have your proxy card in hand when you call and then follow the instructions.
VOTE BY MAIL
Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717.
| TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: | ||||
D52148-P49635 | KEEP THIS PORTION FOR YOUR RECORDS | |||
| — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — —— — —— — —— — —— — —— — — — | ||||
| DETACH AND RETURN THIS PORTION ONLY | ||
| THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. | ||
| GLOBAL BLOOD THERAPEUTICS, INC. | For All | Withhold All | For All Except | To withhold authority to vote for any individual nominee(s), mark “For All Except” and write the number(s) of the nominee(s) on the line below. | ||||||||||||||||||||||||||||
| The Board of Directors recommends you vote FOR the following Class III Directors in Proposal 1: | ☐ | ☐ | ☐ | |||||||||||||||||||||||||||||
| 1. | Election of Directors. |
| ||||||||||||||||||||||||||||||
Nominees: | ||||||||||||||||||||||||||||||||
| 01) | Scott W. Morrison | |||||||||||||||||||||||||||||||
| 02) | Deval L. Patrick | |||||||||||||||||||||||||||||||
| 03) | Mark L. Perry | |||||||||||||||||||||||||||||||
| The Board of Directors recommends you vote FOR Proposals 2 and 3: | For | Against | Abstain | |||||||||||||||||||||||||||||
| 2. | Approval, on a non-binding, advisory basis, of the compensation of the Company’s named executive officers as disclosed in the poxy statement. | ☐ | ☐ | ☐ | ||||||||||||||||||||||||||||
| 3. | Ratification of the appointment of KPMG LLP as the independent registered public accounting firm of the Company for its fiscal year ending December 31, 2021. | ☐ | ☐ | ☐ | ||||||||||||||||||||||||||||
| 4. | Transaction of such other business as may properly come before the meeting or any adjournment or postponement thereof. | |||||||||||||||||||||||||||||||
| NOTE: This proxy, when properly executed, will be voted as directed herein by the undersigned Stockholder. If no direction is made, this proxy will be voted “FOR ALL” in Proposal 1 and “FOR” Proposals 2 and 3. | ||||||||||||||||||||||||||||||||
| Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized office. |
| Signature [PLEASE SIGN WITHIN BOX] | Date | |||||
| Signature (Joint Owners) | Date |
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting:
The Notice and Proxy Statement and Annual Report are available at www.proxyvote.com.
| — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — |
D52149-P49635
GLOBAL BLOOD THERAPEUTICS, INC. Annual Meeting of Stockholders June 17, 2021 8:00 AM This proxy is solicited by the Board of Directors
The stockholder(s) hereby appoint(s) Ted Love, M.D. and Jeffrey Farrow, or either of them, as proxies, each with the power to appoint his substitute, and hereby authorize(s) them to represent and to vote, as designated on the reverse side of this ballot, all of the shares of Common Stock of GLOBAL BLOOD THERAPEUTICS, INC. that the stockholder(s) is/are entitled to vote at the Annual Meeting of Stockholders to be held at 8:00 AM PDT on June 17, 2021, held virtually at www.virtualshareholdermeeting.com/GBT2021, and any adjournment or postponement thereof.
This proxy, when properly executed, will be voted in the manner directed herein. If no such direction is made, this proxy will be voted in accordance with the Board of Directors’ recommendations.
Continued and to be signed on reverse side
|