
Nestlé Health Science and Aimmune Therapeutics 31st August 2020 Exhibit (a)(17)

Aimmune and NHSc - A global leader in solutions and treatment for food allergies Strong strategic fit Aligned Purpose: enhancing the quality of life for patients and consumers Extension of NHSc food allergy portfolio Complementing expertise in allergy and prevention Strengthen science-based innovative product development & global reach Growing the business and building additional capabilities + Transform the lives of millions of people who live with serious food allergies Empowering healthier lives through nutrition
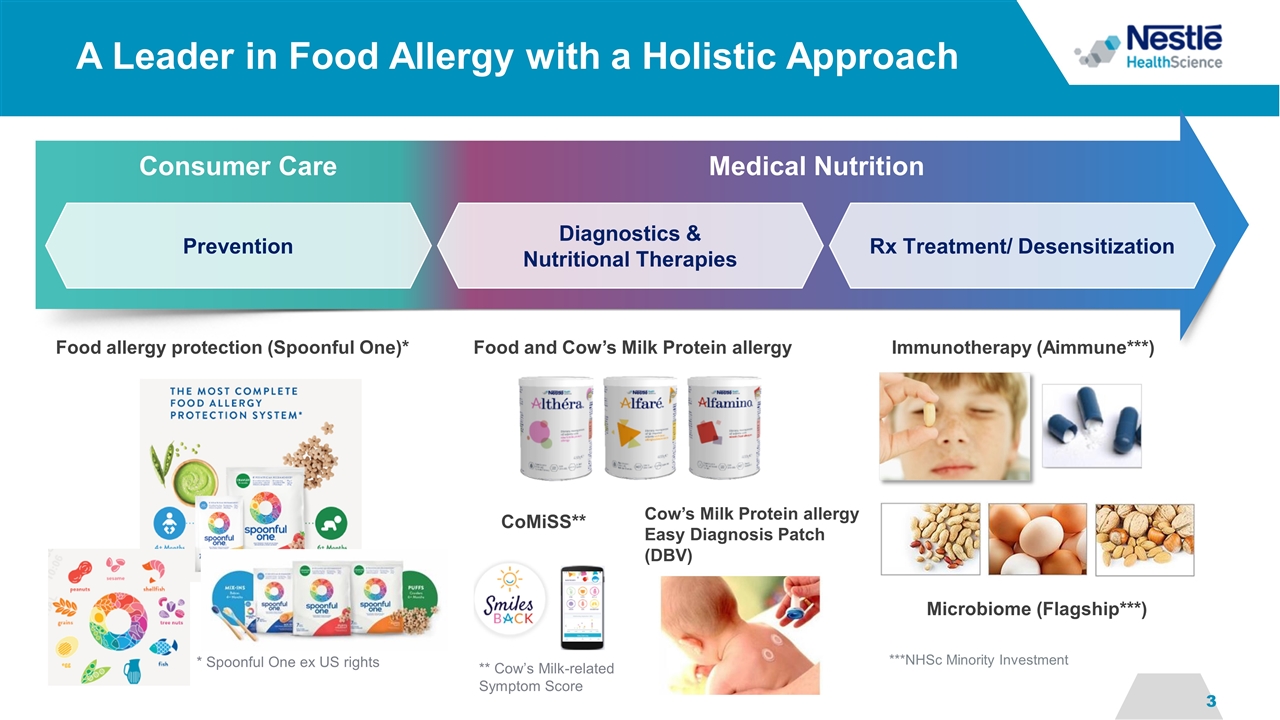
A Leader in Food Allergy with a Holistic Approach Diagnostics & Nutritional Therapies Prevention Rx Treatment/ Desensitization Medical Nutrition Consumer Care ** Cow’s Milk-related Symptom Score CoMiSS** Food and Cow’s Milk Protein allergy Immunotherapy (Aimmune***) Microbiome (Flagship***) ***NHSc Minority Investment * Spoonful One ex US rights Food allergy protection (Spoonful One)* Cow’s Milk Protein allergy Easy Diagnosis Patch (DBV)

Presentation to NHSc August 31st, 2020
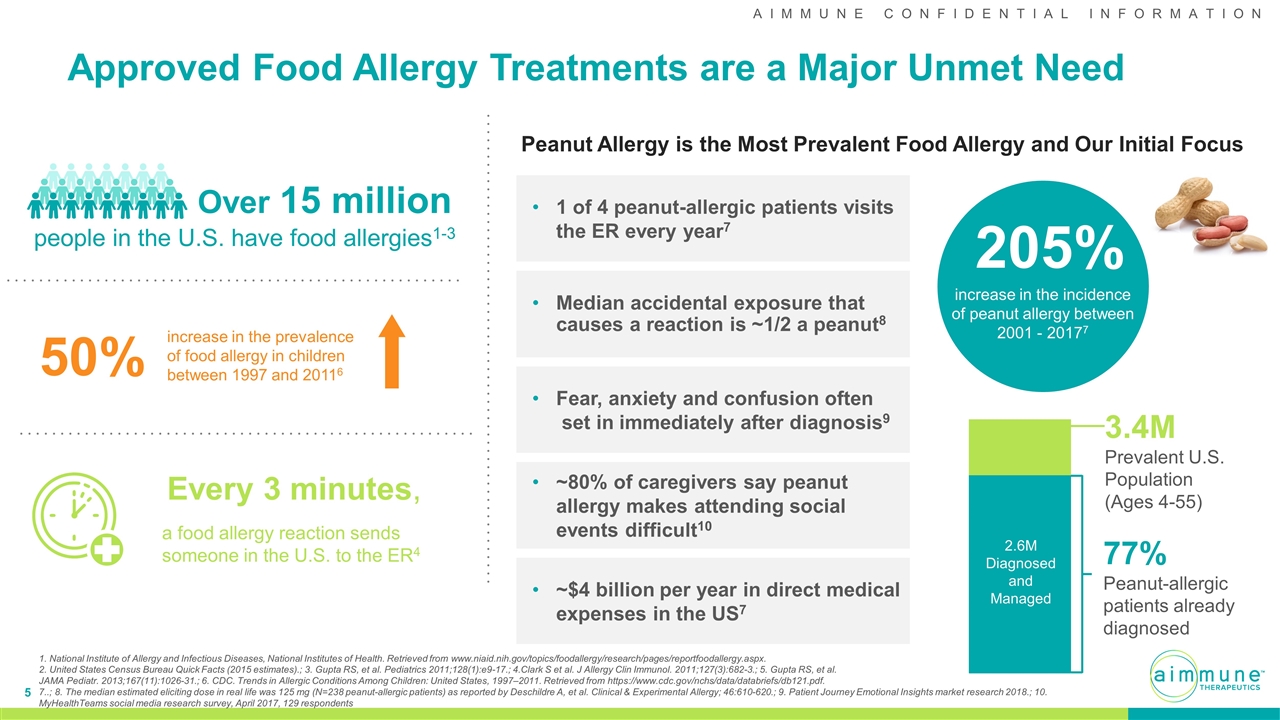
Approved Food Allergy Treatments are a Major Unmet Need Over 15 million people in the U.S. have food allergies1-3 Every 3 minutes, a food allergy reaction sends someone in the U.S. to the ER4 50% increase in the prevalence of food allergy in children between 1997 and 20116 1. National Institute of Allergy and Infectious Diseases, National Institutes of Health. Retrieved from www.niaid.nih.gov/topics/foodallergy/research/pages/reportfoodallergy.aspx. 2. United States Census Bureau Quick Facts (2015 estimates).; 3. Gupta RS, et al. Pediatrics 2011;128(1):e9-17.; 4.Clark S et al. J Allergy Clin Immunol. 2011;127(3):682-3.; 5. Gupta RS, et al. JAMA Pediatr. 2013;167(11):1026-31.; 6. CDC. Trends in Allergic Conditions Among Children: United States, 1997–2011. Retrieved from https://www.cdc.gov/nchs/data/databriefs/db121.pdf. 7..; 8. The median estimated eliciting dose in real life was 125 mg (N=238 peanut-allergic patients) as reported by Deschildre A, et al. Clinical & Experimental Allergy; 46:610-620.; 9. Patient Journey Emotional Insights market research 2018.; 10. MyHealthTeams social media research survey, April 2017, 129 respondents 205% increase in the incidence of peanut allergy between 2001 - 20177 2.6M Diagnosed and Managed 3.4M Prevalent U.S. Population (Ages 4-55) 77% Peanut-allergic patients already diagnosed 1 of 4 peanut-allergic patients visits the ER every year7 Median accidental exposure that causes a reaction is ~1/2 a peanut8 Peanut Allergy is the Most Prevalent Food Allergy and Our Initial Focus Fear, anxiety and confusion often set in immediately after diagnosis9 ~80% of caregivers say peanut allergy makes attending social events difficult10 ~$4 billion per year in direct medical expenses in the US7

Over 1,200 patients participated in clinical trials resulting in the largest dataset ever assembled for peanut allergy; only program to meet its pre-specified primary efficacy endpoint OIT recognized as promising approach to deliver reliable protection against accidental exposure for food allergy patients Called for rigorous pharmaceutical development and approval of an OIT product Aimmune Therapeutics: A Call to Action for an FDA-Approved Oral Immunotherapy (OIT) Product First approved treatment for any food allergy 2020 2011 meeting with patient advocates, FDA, NIH, academic leaders, and industry representatives 2011

Our Vision: Power Over Food Allergies At Aimmune, we aspire to become the global leader in developing curative therapies and solutions for patients with food allergies.

To realize our vision, we intend to: Our 2028 Strategy DRIVE THE MOST COMPELLING FOOD ALLERGY RESEARCH Pursue research collaborations Build capabilities DIVERSIFY OUR FOOD ALLERGY PORTFOLIO Explore alternate antigen delivery approaches Evaluate options to repurpose assets in adjacencies into food allergy * EoE= Eosinophilic Esophagitis; EG = Eosinophilic Gastritis MAXIMIZE CODIT PLATFORM ACROSS FOOD ALLERGIES Near-term focus on PALFORZIA launch excellence Advance LCM programs, evaluate CODIT combination opportunities EXPAND TO FOOD ALLERGY ADJACENCIES Monitor opportunities in EoE* and EG* Continue monitoring opportunities
Aimmune Values Help Drive Our Success
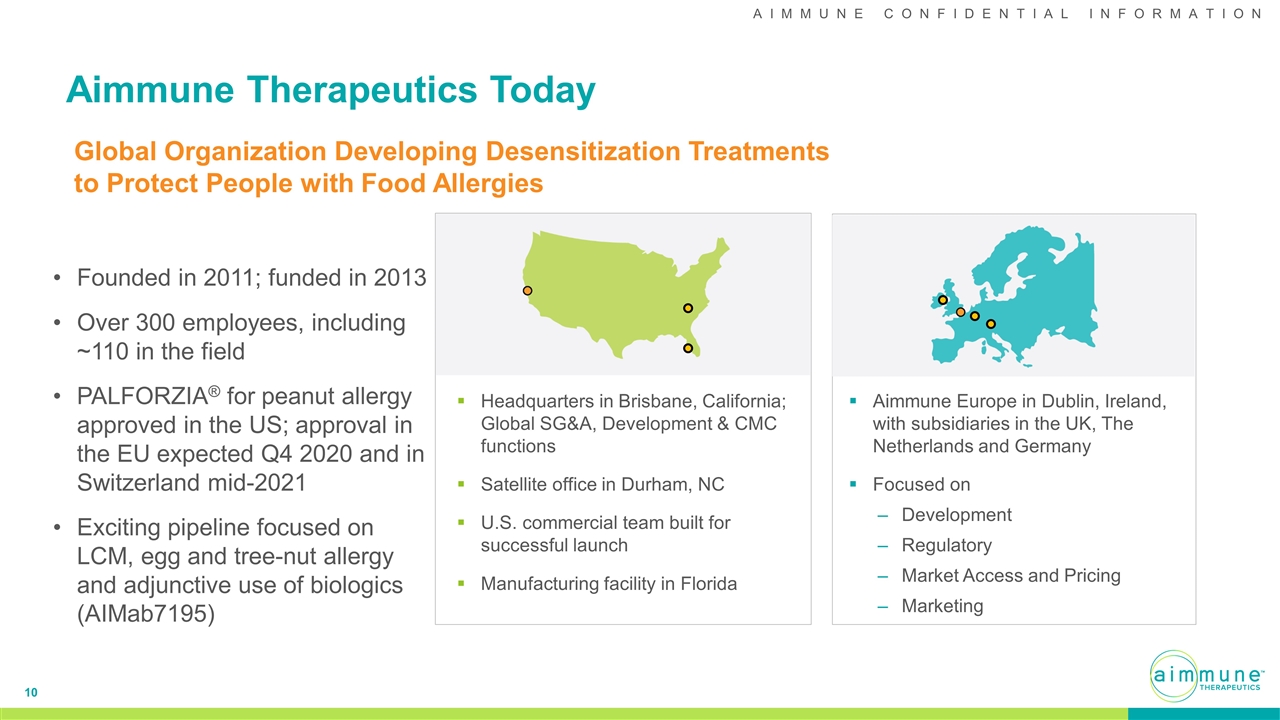
Aimmune Therapeutics Today Headquarters in Brisbane, California; Global SG&A, Development & CMC functions Satellite office in Durham, NC U.S. commercial team built for successful launch Manufacturing facility in Florida Aimmune Europe in Dublin, Ireland, with subsidiaries in the UK, The Netherlands and Germany Focused on Development Regulatory Market Access and Pricing Marketing Global Organization Developing Desensitization Treatments to Protect People with Food Allergies Founded in 2011; funded in 2013 Over 300 employees, including ~110 in the field PALFORZIA® for peanut allergy approved in the US; approval in the EU expected Q4 2020 and in Switzerland mid-2021 Exciting pipeline focused on LCM, egg and tree-nut allergy and adjunctive use of biologics (AIMab7195)

Management Team Led by Industry Experts Experienced Team of Drug Developers with 30+ NDAs, BLAs, MAAs and Approvals Daniel Adelman, MD Chief Medical Officer Andrew Oxtoby Chief Commercial Officer Samina Bari SVP, Corporate Affairs Narinder Singh EVP, Technical Operations Becki Filice SVP, Product & Portfolio Management Alicia Goodman SVP, Human Resources Eric Bjerkholdt Chief Financial Officer Jayson Dallas, MD President & Chief Executive Officer Douglas Sheehy General Counsel & Secretary

Thank you
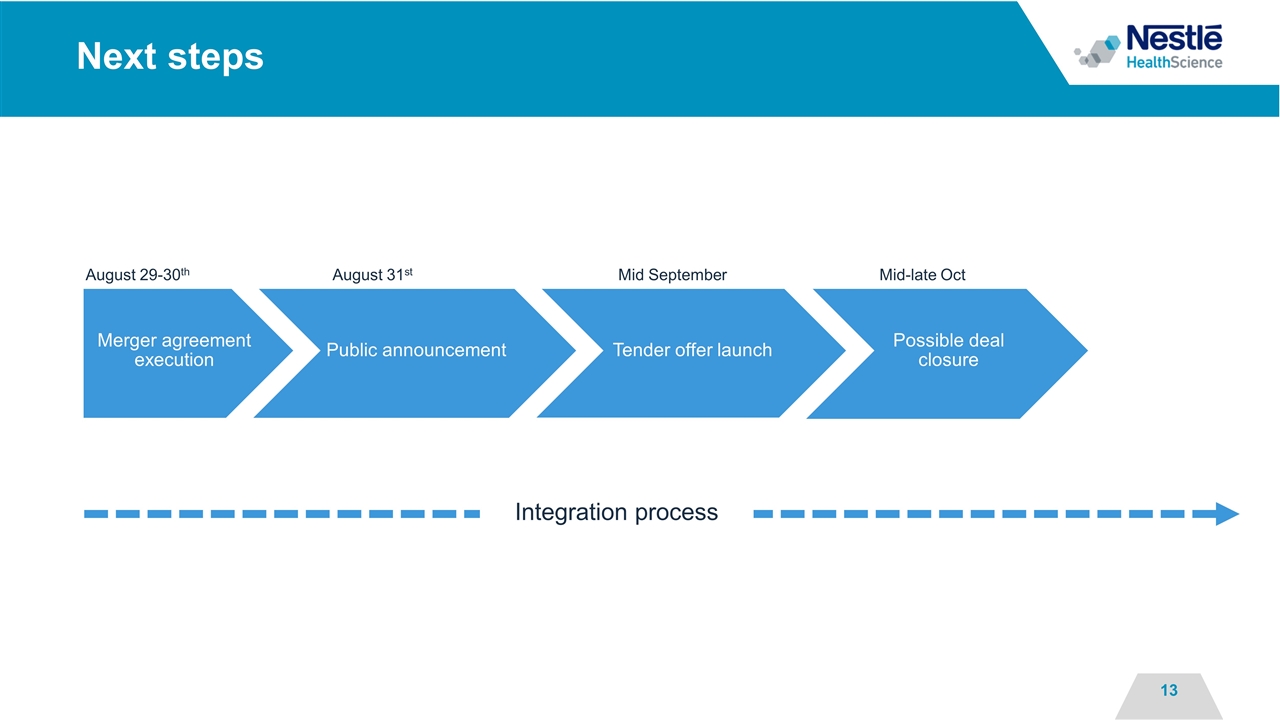
Next steps Integration process August 29-30th August 31st Mid September Mid-late Oct Merger agreement execution Public announcement Tender offer launch Possible deal closure














