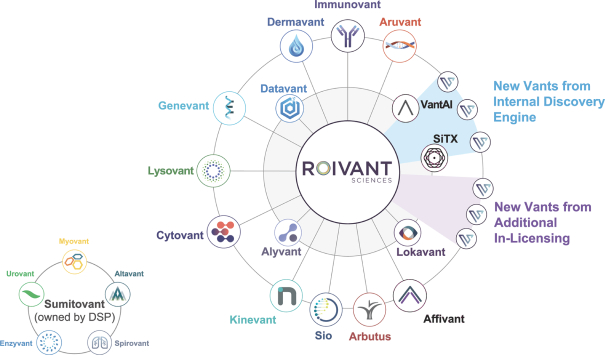
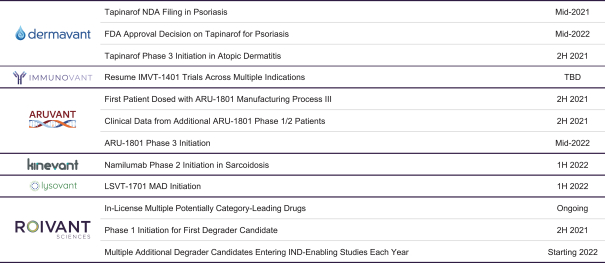
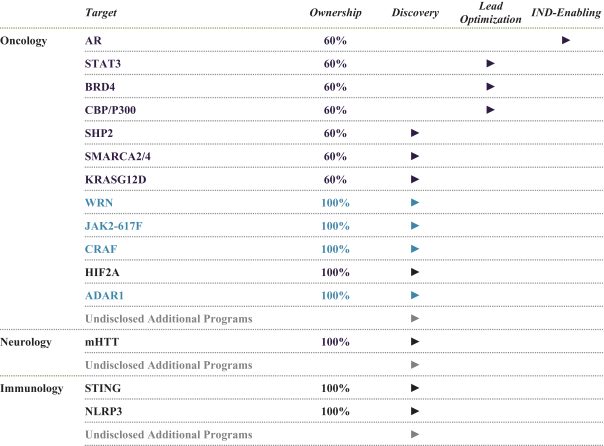
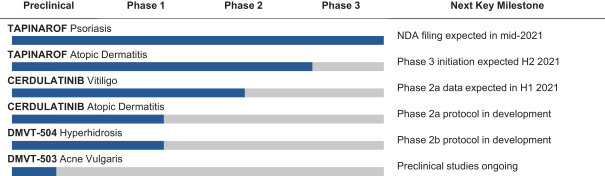
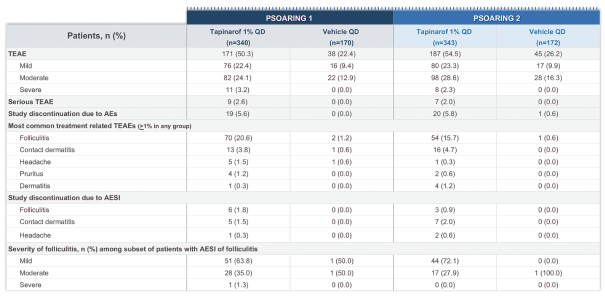
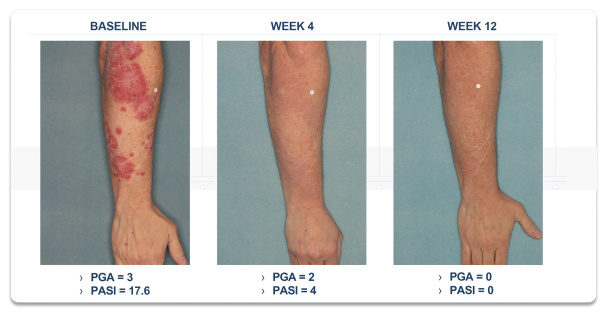
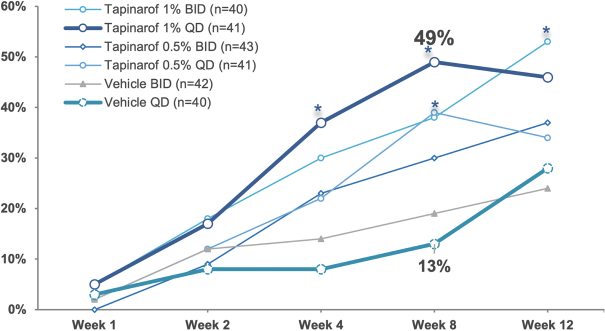
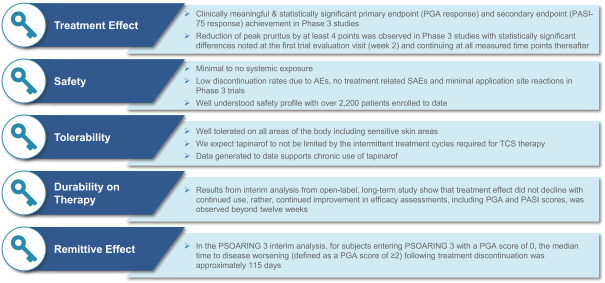
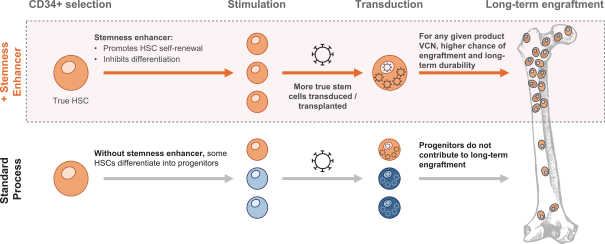
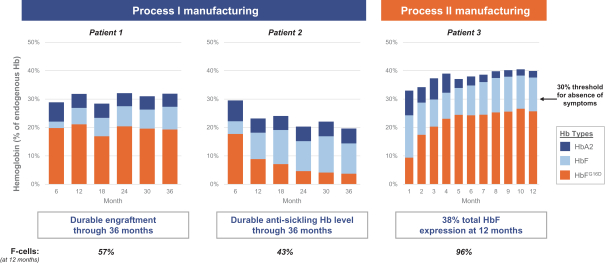
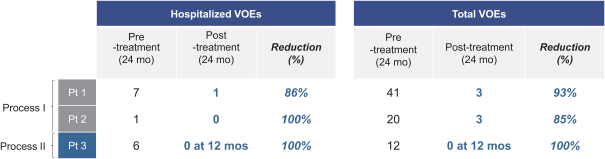
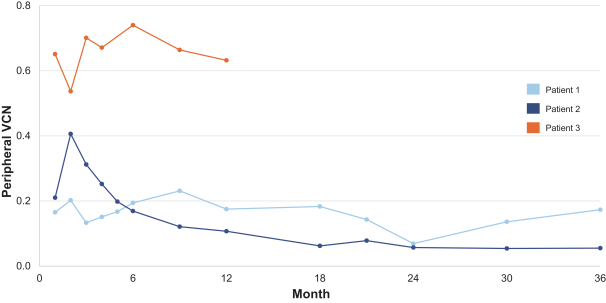
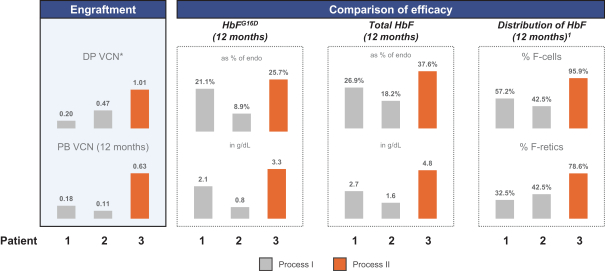
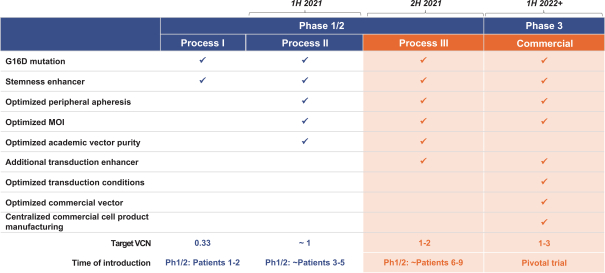
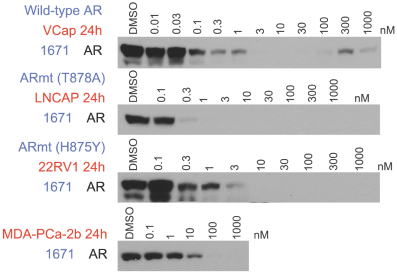
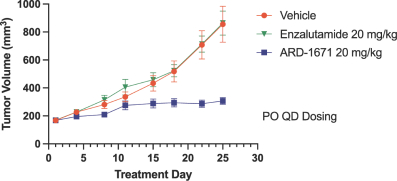
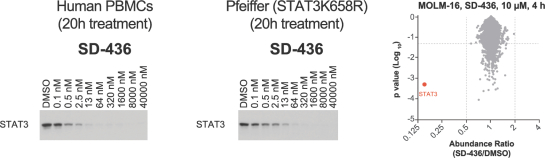
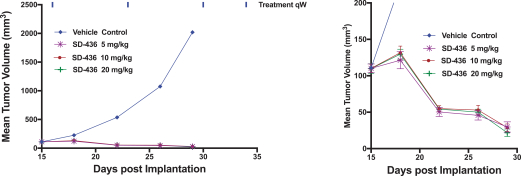
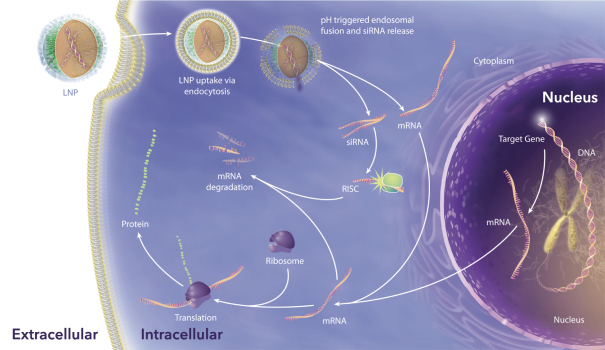
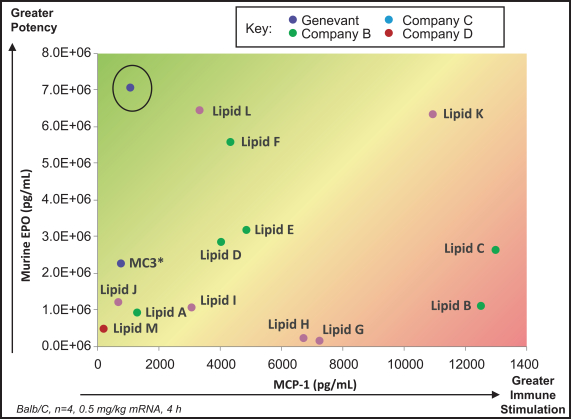
event, development, effect or occurrence, has had or would reasonably be expected to have a material adverse effect on (a) the business, results of operations, assets or financial conditions of the Group Companies, taken as a whole, or (b) the ability of Roivant or Merger Sub to consummate the transactions contemplated by the Business Combination Agreement on the date of Closing (including the Merger and the Pre-Closing Steps); provided, however, that, in the case of clause (a), none of the following shall be taken into account in determining whether a Company Material Adverse Effect has occurred or is reasonably likely to occur: any adverse change, event, development, effect or occurrence arising after the date of the Business Combination Agreement from or related to (i) general business or economic conditions in or affecting the United States, or changes therein, or the global economy generally, (ii) any national or international political or social conditions in the United States or any other country, including the engagement by the United States or any other country in hostilities, whether or not pursuant to the declaration of a national emergency or war, or the occurrence in any place of any military or terrorist attack, sabotage or cyberterrorism, (iii) changes in conditions of the financial, banking, capital or securities markets generally in the United States or any other country or region in the world, or changes therein, including changes in interest rates in the United States or any other country and changes in exchange rates for the currencies of any countries, (iv) changes in any applicable Laws, (v) any change, event, development, effect or occurrence that is generally applicable to the industries or markets in which any of Roivant or any of its Subsidiaries (each a “Group Company”) operates, (vi) the execution or public announcement of the Business Combination Agreement or the pendency or consummation of the transactions contemplated by the Business Combination Agreement, including the impact thereof on the relationships, contractual or otherwise, of any Group Company with employees, customers, investors, contractors, lenders, suppliers, vendors, partners, licensors, licensees, payors or other third parties related thereto (provided that the exception in this clause (vi) shall not apply to the representations and warranties set forth in Section 3.5(b) of the Business Combination Agreement to the extent that its purpose is to address the consequences resulting from the public announcement or pendency or consummation of the transactions contemplated by the Business Combination Agreement or the condition set forth in Section 6.2(a) of the Business Combination Agreement to the extent it relates to such representations and warranties), (vii) any failure by any Group Company to meet, or changes to, any internal or published budgets, projections, forecasts, estimates or predictions (although the underlying facts and circumstances resulting in such failure may be taken into account to the extent not otherwise excluded from this definition, (viii) any hurricane, tornado, flood, earthquake, tsunami, natural disaster, mudslides, wild fires, epidemics, pandemics (including COVID-19) or quarantines, acts of God or other natural disasters or comparable events in the United States or any other country or region in the world, or any escalation of the foregoing, or (ix) any regulatory, preclinical, clinical, pricing or reimbursement changes, effects, developments or occurrences arising after the date of the Business Combination Agreement and relating to or affecting any product candidate, product or platform that is being or has been researched, tested, developed, manufactured, distributed, sold, promoted, advertised or marketed by or on behalf of the Group Companies (“Company Product”) (including (A) any suspension, rejection, refusal of, request to refile or any delay in obtaining or making any regulatory application or filing relating to any Company Product, (B) any negative regulatory actions, requests, recommendations or decisions of any Governmental Entity (as defined in the Business Combination Agreement) relating to any Company Product or the manufacture thereof, or any other regulatory or preclinical or clinical development relating to any Company Product, (C) any preclinical or clinical studies, trials, tests, results or adverse events, or announcements of any of the foregoing, with respect to any Company Product, (D) any delay, hold or termination of any preclinical or clinical study, trial or test or any delay, hold or termination of any planned application for investigational new drug application or application for marketing approval with respect to any Company Product, (E) any preclinical or clinical studies, trials, tests, results or adverse events, or announcements of any of the foregoing, with respect to any product or product candidate competitive with or related to any Company Product, (F) FDA approval (or other preclinical or clinical or regulatory developments), market entry or threatened market entry of any product or product candidate competitive with or related to any Company Product or (G) any recommendations, statements, decisions or other pronouncements made, published or proposed by professional medical organizations, payors, Governmental Entities or representatives of the foregoing, or any panel or advisory body empowered or appointed thereby, relating to any Company Product or any products or product candidates of any competitors of the Company), in each case, as applicable and solely to the extent not resulting from or arising out of any fraud or intentional and material violation of any applicable
161.6