Exhibit 99.2

Sustained Reductions in Plasma Arginine Following Pegzilarginase Administration in Patients with Arginase-1 Deficiency are Accompanied by Improvements in Mobility and Adaptive Behavior Diaz GA1, Schulze A2, McNutt MC3, Teles EL4, Merritt II JL5, Enns GM6, Batzios SP7, Conway RL8, Bechter M9, Eckert S9, Bubb G9, WooldridgeJE9, Zori RT10 1Icahn School of Medicine at Mt. Sinai, New York City, NY, USA, 2University of Toronto and The Hospital for Sick Children, Toronto, ON, Canada, 3UT Southwestern Medical Center, Dallas, TX, USA, 4Centro Hospitalar de São João, Porto, Portugal, 5University of Washington, Seattle, WA, USA, 6Stanford University, Stanford, CA, 7Great Ormond Street Hospital NHS Trust, London, UK, 8Children’s Hospital of Michigan, Detroit, MI, USA, 9Aeglea BioTherapeutics, Austin, TX, USA, 10University of Florida, Gainesville, FL, USA

Disclosures • Grant/Research Support: Urea Cycle Disorders Consortium, NYCKidSeq Consortium, N-Carbamylglutamate Consortium • Consultant: Aeglea BioTherapeutics, Horizon Pharma, Moderna Therapeutics, Synlogic Therapeutics, Ultragenyx • Major Shareholder: None I will be discussing investigational use of Pegzilarginase 2
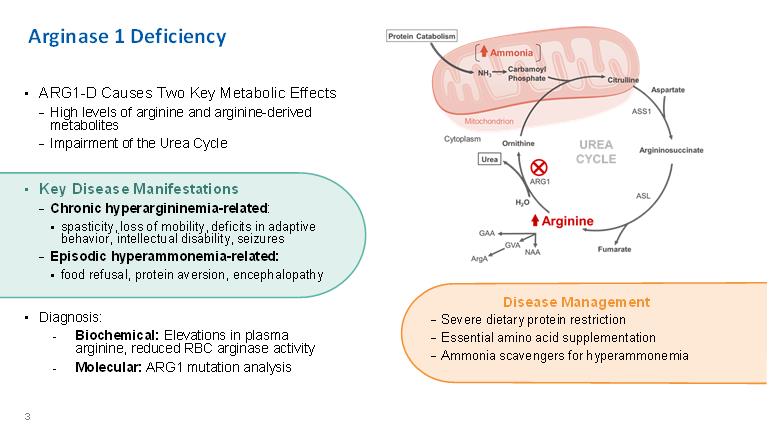
Arginase 1 Deficiency • ARG1-D Causes Two Key Metabolic Effects − High levels of arginine and arginine-derived metabolites − Impairment of the Urea Cycle• Key Disease Manifestations − Chronic hyperargininemia-related: ▪ spasticity, loss of mobility, deficits in adaptive behavior, intellectual disability, seizures − Episodic hyperammonemia-related: ▪ food refusal, protein aversion, encephalopathy • Diagnosis: - Biochemical: Elevations in plasma arginine, reduced RBC arginase activity - Molecular: ARG1 mutation analysis Disease Management − Severe dietary protein restriction − Essential amino acid supplementation − Ammonia scavengers for hyperammonemia 3
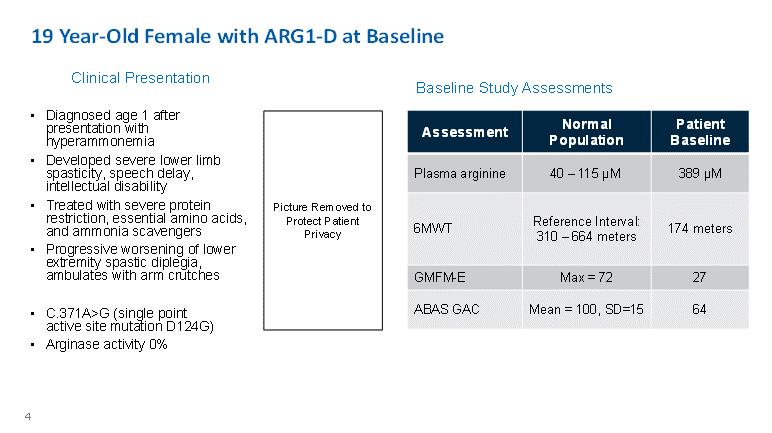
19 Year-Old Female with ARG1-D at Baseline Clinical Presentation Baseline Study Assessments • Diagnosed age 1 after presentation with hyperammonemia • Developed severe lower limb spasticity, speech delay, intellectual disability • Treated with severe protein restriction, essential amino acids, and ammonia scavengers • Progressive worsening of lower extremity spastic diplegia, ambulates with arm crutches • C.371A>G (single point active site mutation D124G) • Arginase activity 0% Picture Removed to Protect Patient Privacy Assessment Normal Population Patient Baseline Plasma arginine 40 – 115 μM 389 μM 6MWT Reference Interval: 310 – 664 meters 174 meters GMFM-E Max = 72 27 ABAS GAC Mean = 100, SD=15 64 4
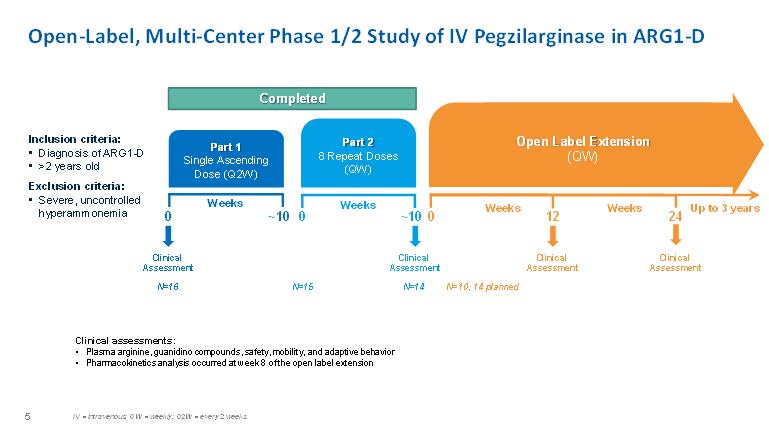
Open-Label, Multi-Center Phase 1/2 Study of IV Pegzilarginase in ARG1-DInclusion criteria: • Diagnosis of ARG1-D • >2 years old Exclusion criteria: • Severe, uncontrolled hyperammonemia Completed Part 1 Single Ascending Dose (Q2W) Part 2 8 Repeat Doses (QW) Open Label Extension (QW) Clinical assessments: • Plasma arginine, guanidino compounds, safety, mobility, and adaptive behavior • Pharmacokinetics analysis occurred at week 8 of the open label extension Weeks Weeks Weeks Weeks Up to 3 years 24 12 0 0 0 ~10 ~10 Clinical Assessment Clinical Assessment Clinical Assessment Clinical Assessment N=16 N=15 N=14 N=10 14 planned IV = intravenous; QW = weekly; Q2W = every 2 weeks 5
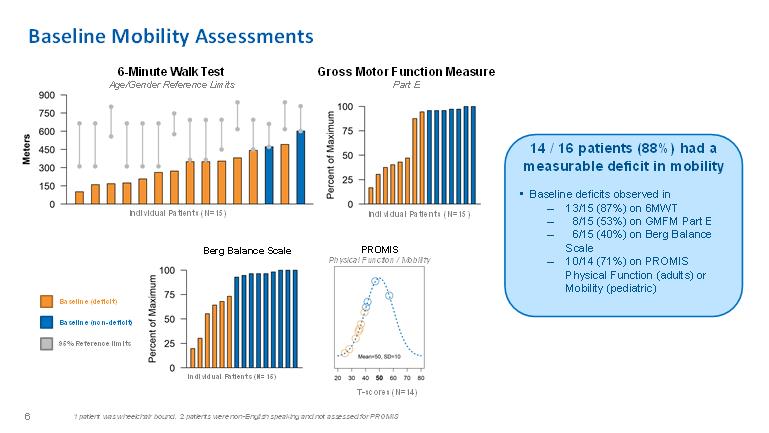
Baseline Mobility Assessments6-Minute Walk Test Age/Gender Reference Limits6-Minute Walk Test Age/Gender Reference Limits Berg Balance ScalePROMIS Physical Function / MobilityT-scores (N=14) patient was wheelchair bound. 2 patients were non-English speaking and not assessed for PROMIS1 patient was wheelchair bound. 2 patients were non-English speaking and not assessed for PROMIS 6
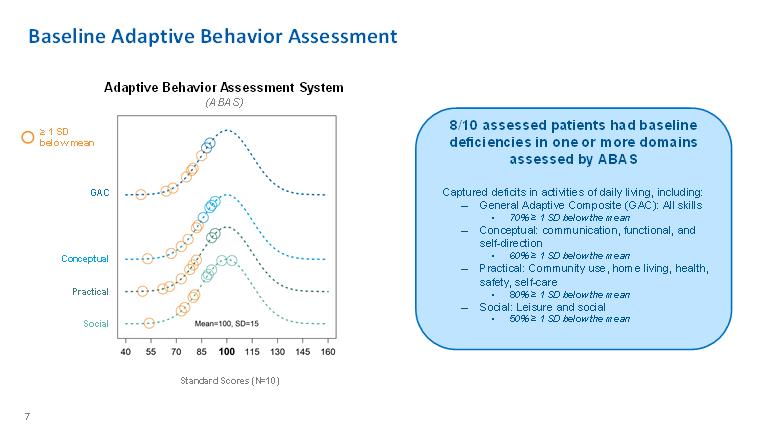
Baseline Adaptive Behavior Assessment Adaptive Behavior Assessment System(ABAS) ≥ 1 SD below mean GAC Conceptual Practical Social GAC Conceptual Practical Social8/10 assessed patients had baseline deficiencies in one or more domains assessed by ABAS Captured deficits in activities of daily living, including: – General Adaptive Composite (GAC): All skills • 70% ≥ 1 SD below the mean – Conceptual: communication, functional, and self-direction • 60% ≥ 1 SD below the mean – Practical: Community use, home living, health, safety, self-care • 80% ≥ 1 SD below the mean – Social: Leisure and social • 50% ≥ 1 SD below the mean 7
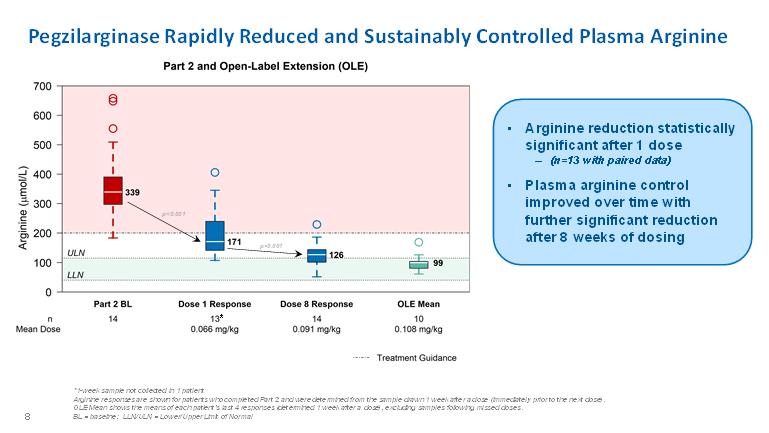
Pegzilarginase Rapidly Reduced and Sustainably Controlled Plasma Arginine• Arginine reduction statistically significant after 1 dose – (n=13 with paired data) • Plasma arginine control improved over time with further significant reduction after 8 weeks of dosing 8 *1-week sample not collected in 1 patient Arginine responses are shown for patients who completed Part 2 and were determined from the sample drawn 1 week after a dose (immediately prior to the next dose). OLE Mean shows the means of each patient’s last 4 responses (determined 1 week after a dose), excluding samples following missed doses. BL = baseline; LLN/ULN = Lower/Upper Limit of Normal
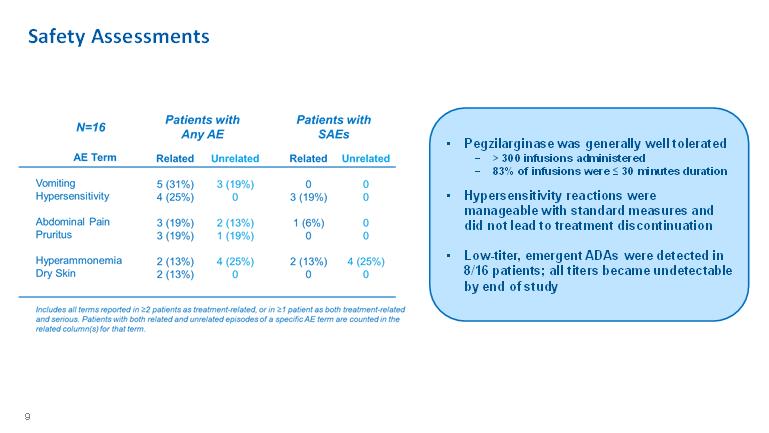
• Pegzilarginase was generally well tolerated − > 300 infusions administered − 83% of infusions were ≤ 30 minutes duration • Hypersensitivity reactions were manageable with standard measures and did not lead to treatment discontinuation • Low-titer, emergent ADAs were detected in 8/16 patients; all titers became undetectable by end of study Safety Assessments 9
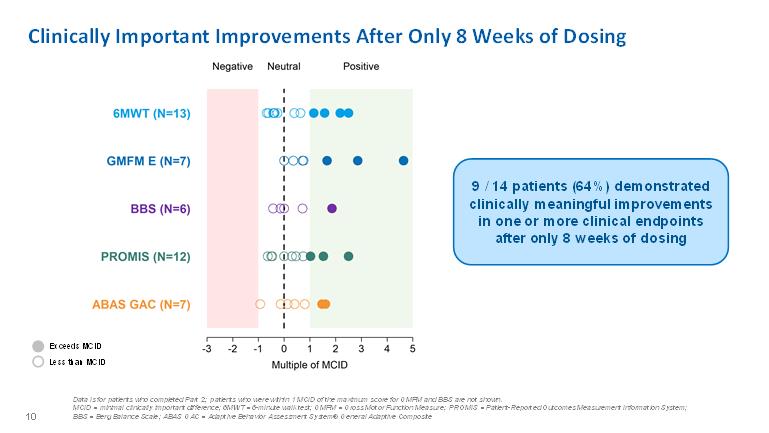
Clinically Important Improvements After Only 8 Weeks of Dosing9 / 14 patients (64%) demonstrated clinically meaningful improvements in one or more clinical endpoints after only 8 weeks of dosingExceeds MCID Less than MCID 10 Data is for patients who completed Part 2; patients who were within 1 MCID of the maximum score for GMFM and BBS are not shown. MCID = minimal clinically important difference; 6MWT = 6-minute walk test; GMFM = Gross Motor Function Measure; PROMIS = Patient-Reported Outcomes Measurement Information System; BBS = Berg Balance Scale; ABAS GAC = Adaptive Behavior Assessment System® General Adaptive Composite

Continued Improvements in Mobility were Observed at Dose 205 / 5 (100%) patients demonstrated clinically meaningful improvements in one or more clinical endpoints at 20 weeks of dosing GMFM enhances the ability to capture objective improvements in mobility14 patients have completed Part 2, and 5 patients have completed 20 weeks of dosing. MCID = minimal clinically important difference 11
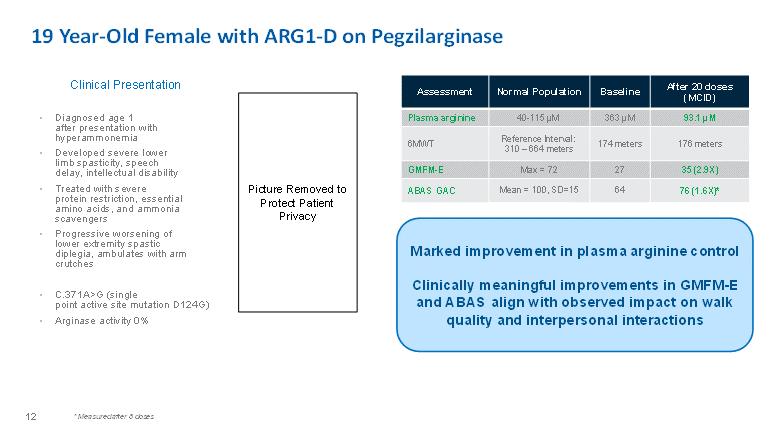
19 Year-Old Female with ARG1-D on PegzilarginaseClinical Presentation• Diagnosed age 1 after presentation with hyperammonemia • Developed severe lower limb spasticity, speech delay, intellectual disability • Treated with severe protein restriction, essential amino acids, and ammonia scavengers • Progressive worsening of lower extremity spastic diplegia, ambulates with arm crutches • C.371A>G (single point active site mutation D124G) • Arginase activity 0%Picture Removed to Protect Patient PrivacyMarked improvement in plasma arginine controlClinically meaningful improvements in GMFM-E and ABAS align with observed impact on walk quality and interpersonal interactionsAssessment Normal Population Baseline After 20 doses (MCID) Plasma arginine 40-115 μM 363 μM 93.1 μM 6MWT Reference Interval: 310 – 664 meters 174 meters 176 meters GMFM-E Max = 72 27 35 (2.9X) ABAS GAC Mean = 100, SD=15 64 76 (1.6X)* * Measured after 8 doses 12

Conclusions • Pegzilarginase was highly effective in sustainably lowering plasma arginine, a key driver of ARG1-D disease manifestations and progression • Plasma arginine reductions were accompanied by improvements in mobility and adaptive behavior after only 8 weeks of repeat dosing with additional improvements after longer dosing • Pegzilarginase was well tolerated; hypersensitivity reactions were manageable with standard measures and did not lead to treatment discontinuation • Mobility and adaptive behavior assessments effectively captured treatment related clinical improvements13

Acknowledgements The 101A/102A Investigators and Research Staff wish to acknowledge and thankthe Patients, their Families, and Caregiversthe Patients, their Families, and CaregiversInformation on the Phase 3 Pegzilarginase Effect on Arginase 1 Deficiency Clinical Endpoints (PEACE) Study for patients with Arginase 1 Deficiency is available at ARG1Dstudy@aegleabio.com 14













