Exhibit 99.3
e was
Acknowledgements The 101A/102A Investigators and Research Staff wish to acknowledge and thankthe Patients, their Families, and Caregiversthe Patients, their Families, and CaregiversInformation on the Phase 3 Pegzilarginase Effect on Arginase 1 Deficiency Clinical Endpoints (PEACE) Study for patients with Arginase 1 Deficiency is available at ARG1Dstudy@aegleabio.com 14

Aeglea BioTherapeutics © Aeglea BioTherapeuticsAPRIL 2019

© AegleaBioTherapeutics1© AegleaBioTherapeutics1Forward Looking StatementsThis presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’sbeliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our current and future financial performance, business plans and objectives, current and future clinical and preclinical development activities, timing and success of our ongoing and planned clinical trials and related data, the timing of announcements, updates and results of our clinical trialsand related data, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our lead product candidate and our other product candidates, potential growth opportunities, financing plans, use and adequacy of financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors including, but not limited to, those related to the success, cost and timing of our product candidate development activities and ongoing and planned clinical trials; our plans to develop and commercialize targeted therapeutics, including our lead product candidate pegzilarginase; the progress of patient enrollment and dosing in the Phase 3 PEACE trial, the ability of pegzilarginaseto achieve applicable endpoints in the Phase 3 PEACE trial, the ability for patients who participate in the Phase 3 PEACE trial to participate in a long-term extension study, the safety profile of pegzilarginasein our Phase 3 PEACE trial, the potential for data from the Company’s clinical trials of pegzilarginaseto support a marketing application, as well as the timing of these events, the potential for preclinical studies to be predictive of current or future clinical trials, our ability to obtain funding for our operations, development and commercialization of our product candidates; the timing of and our ability to obtain and maintain regulatory approvals; the rate and degree of market acceptance and clinical utility of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; our commercialization, marketing and manufacturing capabilities and strategy; future agreements with third parties in connection with the commercialization of our product candidates; our expectations regarding our ability to obtain and maintain intellectual property protection; our dependence on third party manufacturers; our ability to develop our own commercial manufacturing facility; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our ability to identify additional product candidates with significant commercialpotential consistent with our commercial objectives; and our estimates regarding expenses, future revenue, capital requirements and needs for additional financing. Moreover, we operate in a very competitive and rapidly changing environment, and new risks may emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed herein may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Further information on these and other factors that could affect these forward-looking statements is contained in our most recent Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (SEC), and other reports filed by the SEC. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. We undertake no obligation to publicly update any forward-looking statements, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.We report all financial information required in accordance with U.S. generally accepted accounting principles (GAAP). We are reporting “non-GAAP” financial measures for our cash and marketable securities. We believe that these “non-GAAP” financial measures, when taken together with the corresponding GAAP financial measures, provide meaningful supplemental information regarding our performance and believe that investors benefit from referring to these “non-GAAP” financial measures in assessing our cash and marketable securities. Please see the footnotes on slides 2 and 32 regarding “Non-GAAP” inclusions for cash and marketable securities. The presentation of these non-GAAP financial measures is not intended to be considered in isolation from, as a substitute for, or superior to, the financial information prepared and presented in accordance with GAAP, and may be different from “non-GAAP” financial measures used by other companies.

© AegleaBioTherapeutics2Clinical-Stage Biotechnology Company that Engineers Next Generation Human Enzymes with Enhanced Properties and Novel Activity to Provide Solutions for Diseases with Unmet Medical NeedSpecificityActivityStabilityAegleaBioTherapeutics, Inc. | NASDAQ: AGLE | Austin, TexasEmployees61Pipeline2Homocystinuria CystinuriaLead Investigational ProgramPegzilarginase–Phase3 ARG1-D“Non-GAAP” cash and marketable securities at December 31, 2018$139.01million (no debt);1.Includes $64.5M net proceeds from equity offering in February 2019 along with cash, cash equivalents, and marketable securities of $74.5M as of December 31, 2018. This amount reflects Aeglea’sestimates based solely upon information available to it as of the date of the Annual Report, and the amount reported is not a comprehensive statement of itsfinancial results or position as of December 31, 2018.2.Morris AAM et al 2017, Castro Pereira DJ et al2015
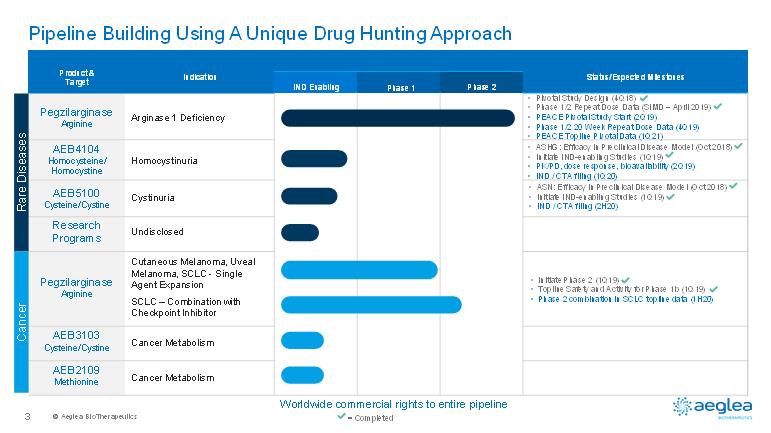
© AegleaBioTherapeutics3Rare DiseasesCancerPipeline Building Using A Unique Drug Hunting ApproachPegzilarginaseArginineProduct &TargetIndicationIND EnablingPhase 1Phase 2Status/Expected MilestonesArginase 1 DeficiencyCutaneous Melanoma, Uveal Melanoma, SCLC -Single Agent ExpansionSCLC –Combination with Checkpoint InhibitorCancer MetabolismAEB2109MethionineAEB3103Cysteine/CystinePegzilarginaseArginineResearchProgramsAEB4104Homocysteine/ HomocystineHomocystinuriaCystinuriaUndisclosedCancer Metabolism•Pivotal Study Design (4Q18)•Phase 1/2 Repeat Dose Data (SIMD –April 2019) •PEACE Pivotal Study Start (2Q19)•Phase 1/2 20 Week Repeat Dose Data (4Q19)•PEACE Topline Pivotal Data (1Q21)•ASHG: Efficacy in Preclinical Disease Model (Oct 2018)•Initiate IND-enabling Studies (1Q19)•PK/PD, dose response, bioavailability (2Q19)•IND / CTA filing (1Q20)•Initiate Phase 2 (1Q19)•Topline Safety and Activity for Phase 1b (1Q19)•Phase 2 combination in SCLC topline data (1H20)Worldwide commercial rights to entire pipeline= Completed•ASN: Efficacy in Preclinical Disease Model (Oct 2018)•Initiate IND-enabling Studies (1Q19)•IND / CTA filing (2H20)AEB5100Cysteine/Cystine

© AegleaBioTherapeutics4© AegleaBioTherapeutics4Strong Momentum With Multiple Anticipated Near-term CatalystsRecent Milestones•Consistent, substantial and sustained control of plasma arginine, the key driver of disease manifestations in ARG1-D with pegzilarginaseoSafety profile supports progression to a pivotal study•Capturing Phase 1/2 clinical responses with mobility & adaptive behavior assessments•FDA grants rare pediatric disease designation to pegzilarginasefor ARG1-D: PRV eligible•AEB4104 for homocystinuria demonstrated decreases in plasma homocysteine levels, corrected important disease-related abnormalities and improved survival in a preclinical model•AEB5100 for cystinuria demonstrated reductions in plasma and urine cystine levels, accompanied by reduced kidney stone formation in a preclinical model•Pegzilarginasemonotherapy demonstrated anti-tumor activity in heavily pre-treated patients with melanomaUpcoming Expected MilestonesPegzilarginase: ARG1-D •PEACE Pivotal trial start 2Q2019•Phase 1/2 20 week repeat dose data 4Q2019•Topline Pivotal data 1Q2021AEB4104 for Homocystinuria•PK/PD, dose response and bioavailability 2Q2019•IND / CTA filing 1Q2020AEB5100 for Cystinuria•IND / CTA filing 2H2020Pegzilarginase: Cancer•Phase 2 combination in SCLC topline data 1H2020

© AegleaBioTherapeutics5Highly Experienced Leadership Team In Drug Discovery & DevelopmentAnthony G. Quinn, MD PhDChief Executive OfficerJames Wooldridge, MDChief Medical OfficerLeslie Sloan, PhDChief Operating OfficerCharles N. York II, MBAChief Financial OfficerAaron Schuchart, MBAChief Business OfficerScott Rowlinson, PhDVice President of ResearchRocheLillyNOVARTISPWCAMGENAstrazenecaIPSENPfizerCoherusSynageva

© AegleaBioTherapeutics66Arginase 1 DeficiencyAutosomal recessive disorder of the urea cycle that causes toxic levels of arginine to accumulate in the bloodPegzilarginase: Lead Program

© AegleaBioTherapeutics7Pegzilarginase: An Innovative Approach To Treat Arginase 1 DeficiencyIndication•Arginase 1 Deficiency (ARG1-D)Patient Population(Addressable Markets)•Greater than600patients•Greater than170 patients identified to dateDiagnosis (Blood Arginine Levels)•Symptomatic cases: spasticity, developmental delay, seizures, hyperammonemia•Newborn screening: currently available in 34 of 50 US statesUnmet Medical Need •Very high -no effective therapy•Early mortality and serious complications due to continued disease progressionMechanism of Action•Extracellular depletion of plasma arginine•Reduces high plasma arginine levels that cause neurotoxicity Development status•Pivotal trial initiation planned for 2Q2019 •Phase 1/2 20 week repeat dose data 4Q2019•PEACE topline data expected 1Q2021
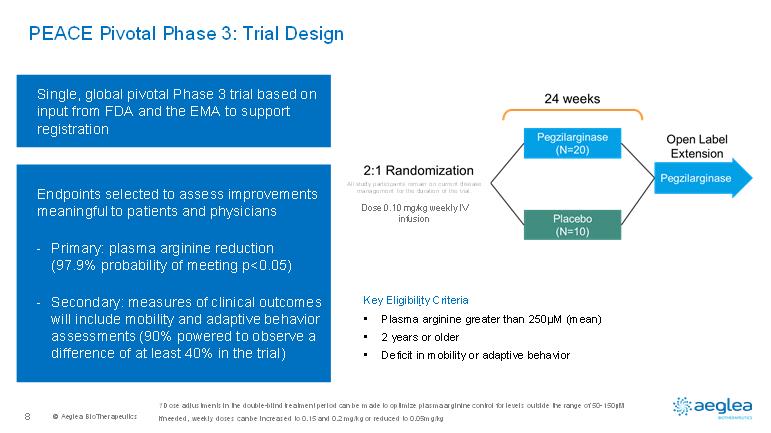
© AegleaBioTherapeutics8PEACE Pivotal Phase 3: Trial DesignSingle, global pivotal Phase 3 trialbased on input from FDA and the EMA to support registrationEndpoints selected to assess improvements meaningful to patients and physicians-Primary: plasma arginine reduction (97.9% probability of meeting p<0.05)-Secondary: measures of clinical outcomes will includemobility and adaptive behavior assessments (90% powered to observe a difference of at least 40% in the trial)Key Eligibility Criteria•Plasma arginine greater than 250µM (mean)•2 years or older•Deficit in mobility or adaptive behaviorAll study participants remain on current disease management for the duration of the trial. Dose 0.10 mg/kg weekly IV infusion1 Dose adjustments in the double-blind treatment period can be made to optimize plasma arginine control for levels outside the range of 50-150µM If needed, weekly doses can be increased to 0.15 and 0.2 mg/kg or reduced to 0.05mg/kg
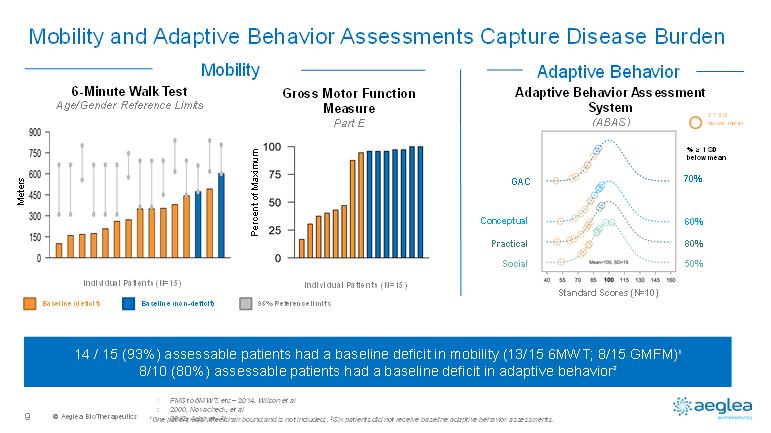
© AegleaBioTherapeutics9Mobility and Adaptive Behavior Assessments Capture Disease Burden1One patient was wheelchair bound and is not included;. 2Six patients did not receive baseline adaptive behavior assessments. Gross Motor Function Measure Part E6-Minute Walk TestAge/Gender Reference LimitsIndividual Patients (N=15)Individual Patients (N=15)Baseline (non-deficit)Baseline (deficit)95% Reference limitsMobilityAdaptive BehaviorAdaptive Behavior Assessment System(ABAS)= 1 SD below mean14 / 15 (93%) assessable patients had a baseline deficit in mobility (13/15 6MWT; 8/15 GMFM)18/10 (80%) assessable patients had a baseline deficit in adaptive behavior2Standard Scores (N=10)GACConceptualPracticalSocial70%60%80%50%% = 1 SD below meanMetersPercent of Maximum1.FMS to 6MWT, etc –2014, Wilson et al2.2000, Novacheck, et al3.2012, Adair et. Al.
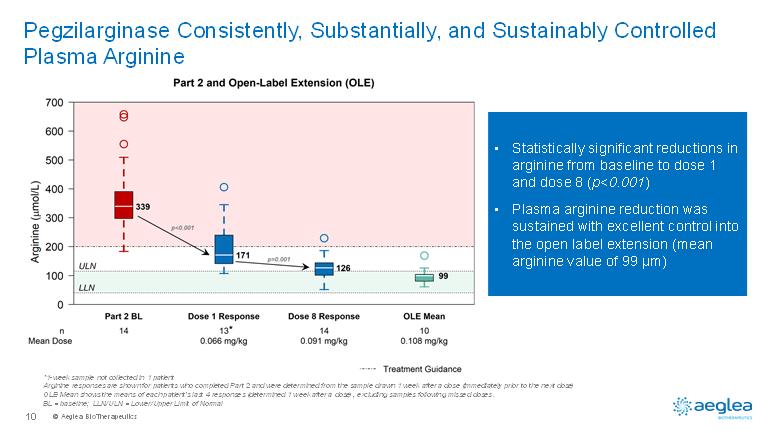
© AegleaBioTherapeutics10PegzilarginaseConsistently, Substantially, and Sustainably Controlled Plasma Arginine•Statistically significant reductions in arginine from baseline to dose 1 and dose 8 (p<0.001)•Plasma arginine reduction was sustained with excellent control into the open label extension (mean arginine value of 99 µm)*1-week sample not collected in 1 patientArginine responses are shown for patients who completed Part 2 and were determined from the sample drawn 1 week after a dose (immediately prior to the next dose) OLE Mean shows the means of each patient’s last 4 responses (determined 1 week after a dose), excluding samples following misseddoses.BL = baseline; LLN/ULN = Lower/Upper Limit of Normal
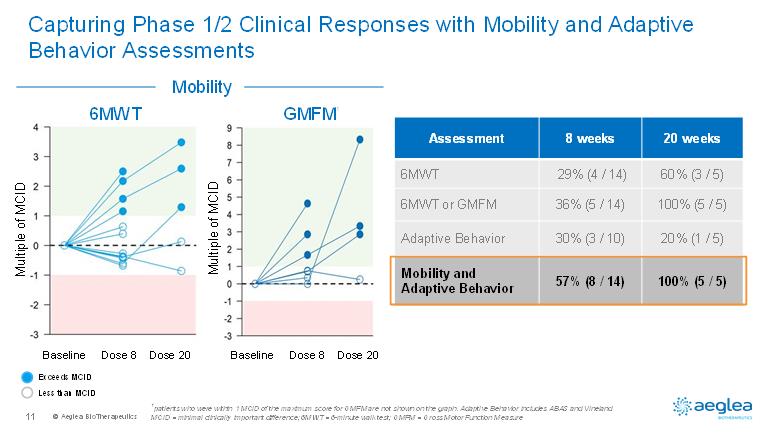
© AegleaBioTherapeutics11Capturing Phase 1/2 Clinical Responses with Mobility and Adaptive Behavior Assessments1patients who were within 1 MCID of the maximum score for GMFM are not shown on the graph. Adaptive Behavior includes ABAS andVinelandMCID = minimal clinically important difference; 6MWT = 6-minute walk test; GMFM = Gross Motor Function MeasureExceeds MCIDLess than MCIDAssessment8 weeks20 weeks6MWT29% (4 / 14)60% (3 / 5)6MWT or GMFM 36% (5 / 14)100% (5 / 5)Adaptive Behavior30% (3 / 10)20% (1 / 5)Mobility and Adaptive Behavior57% (8 / 14)100% (5 / 5)6MWTGMFM1Baseline Dose 8 Dose 20Baseline Dose 8 Dose 20Multiple of MCIDMultiple of MCIDMobility

© AegleaBioTherapeutics12Safety•Pegzilarginasewas generally well tolerated-> 300 infusions administered-83% of infusions were = 30 minutes duration•Hypersensitivity reactions were infrequent, managed with standard treatment and did not lead to any patient discontinuations. •Low-titer, emergent ADAs were detected in 8/16 patients; all titers became undetectable by end of the Phase 1/2 trial

© AegleaBioTherapeutics13Key Highlights from New Phase 1/2 Data•Pegzilarginasewas highly effective in consistently, substantially and sustainablylowering elevated plasma arginine, a key driver of ARG1-D disease manifestations and progression•Plasma arginine reductions were accompanied by improvements in mobility and adaptive behavior after only 8 weeks of repeat dosing with additional improvements after longer dosing•Pegzilarginasewas well tolerated; hypersensitivity reactions were manageable with standard measures and did not lead to treatment discontinuation•Mobility and adaptive behavior assessments effectively captured treatment related clinical improvements
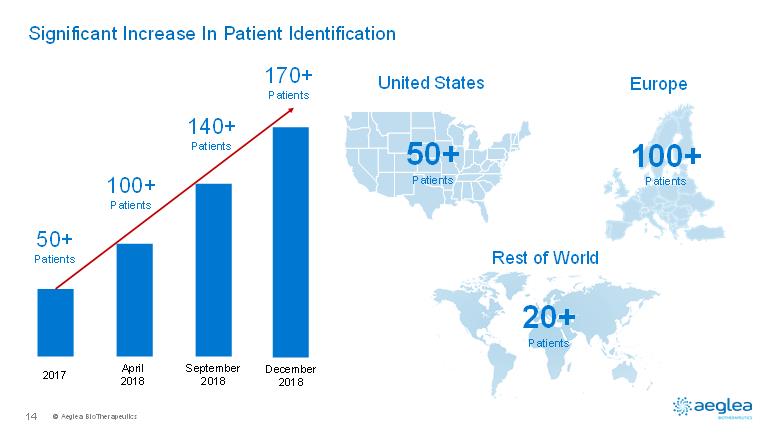
© AegleaBioTherapeutics14Significant Increase In Patient Identification United StatesEuropeRest of World50+Patients100+Patients20+Patients2017April 2018September 201850+Patients100+Patients140+PatientsDecember2018170+Patients
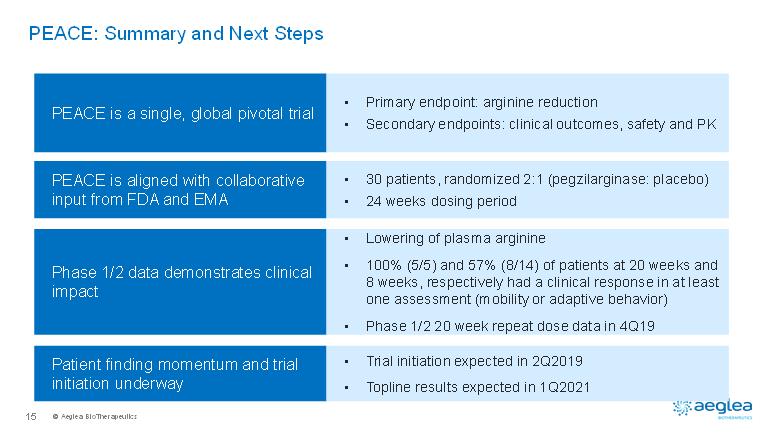
© AegleaBioTherapeutics15PEACE: Summary and Next StepsPEACE is aligned with collaborative input from FDA and EMAPEACE is a single, global pivotal trialPhase 1/2 data demonstrates clinical impact •30 patients, randomized 2:1 (pegzilarginase: placebo)•24 weeks dosing period•Primary endpoint: arginine reduction•Secondary endpoints: clinical outcomes, safety and PK•Lowering of plasma arginine•100% (5/5) and 57% (8/14) of patients at 20 weeks and 8 weeks, respectively had a clinical response in at least one assessment (mobility or adaptive behavior)•Phase 1/2 20 week repeat dose data in 4Q19Patient finding momentum and trial initiation underway•Trial initiation expected in 2Q2019•Topline results expected in 1Q2021

© AegleaBioTherapeutics16© AegleaBioTherapeutics16PipelineDrug Hunting In Human Enzyme Space
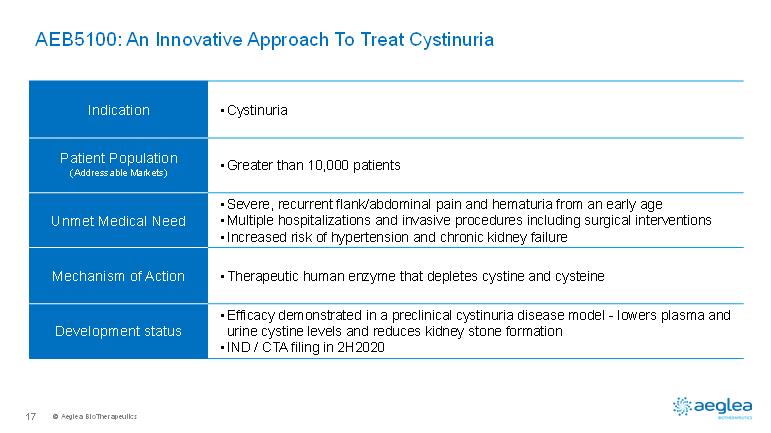
© AegleaBioTherapeutics17AEB5100: An Innovative Approach To Treat CystinuriaIndication•CystinuriaPatient Population(Addressable Markets)•Greater than 10,000 patientsUnmet Medical Need •Severe, recurrent flank/abdominal pain and hematuria from an early age•Multiple hospitalizations and invasive procedures including surgical interventions•Increased risk of hypertension and chronic kidney failureMechanism of Action•Therapeutic human enzyme that depletes cystine and cysteineDevelopment status•Efficacy demonstrated in a preclinical cystinuria disease model -lowers plasma and urine cystine levels and reduces kidney stone formation•IND / CTA filing in 2H2020
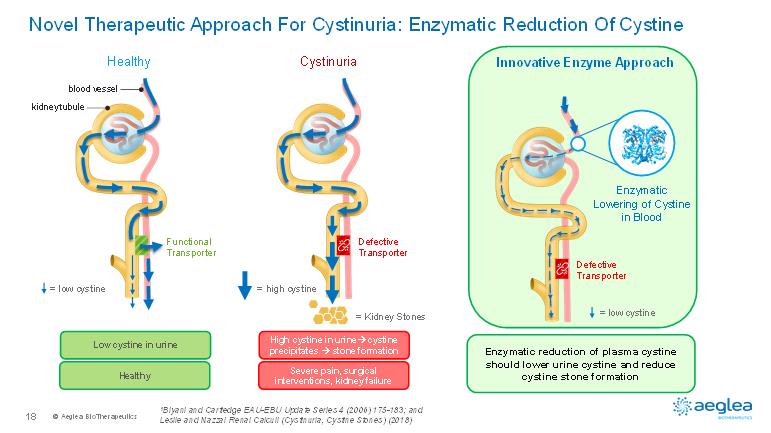
© AegleaBioTherapeutics18Novel Therapeutic Approach For Cystinuria: Enzymatic Reduction Of Cystine1Biyani and Cartledge EAU-EBU Update Series 4 (2006) 175-183; andLeslie and Nazzal Renal Calculi (Cystinuria, Cystine Stones) (2018)CystinuriaHigh cystine in urine.cystine precipitates .stone formationSevere pain, surgical interventions, kidney failureDefectiveTransporter= high cystineFunctionalTransporterkidney tubuleblood vesselHealthyLow cystine in urineHealthy= low cystineDefectiveTransporterInnovative EnzymeApproachEnzymatic Lowering of Cystine in BloodEnzymatic reduction of plasma cystine should lower urine cystine and reduce cystine stone formation= low cystine= Kidney Stones
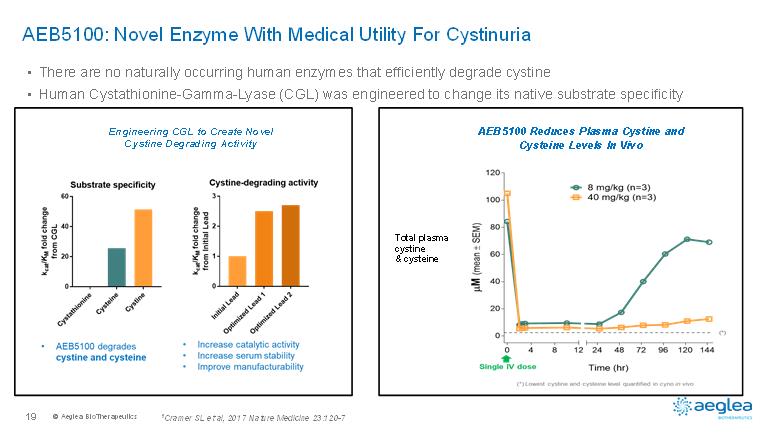
© AegleaBioTherapeutics19•There are no naturally occurring human enzymes that efficiently degrade cystine•Human Cystathionine-Gamma-Lyase (CGL) was engineered to change its native substrate specificityAEB5100: Novel Enzyme With Medical Utility For Cystinuria1Cramer SL et al, 2017 Nature Medicine 23:120-7Engineering CGL to Create Novel Cystine Degrading Activity AEB5100 Reduces Plasma Cystine and Cysteine Levels In Vivo Total plasma cystine & cysteine
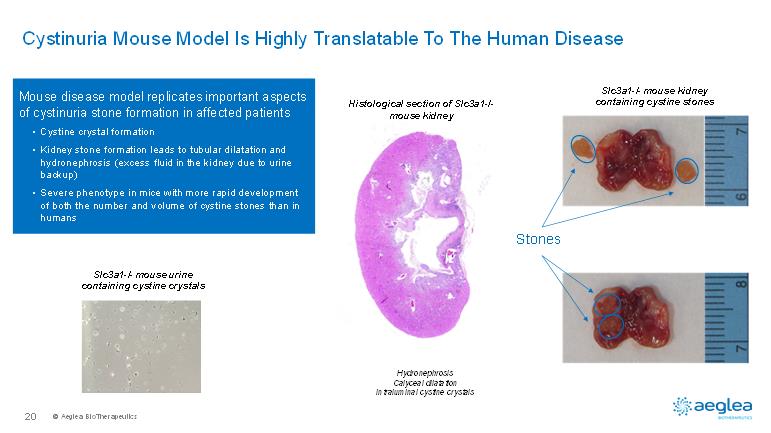
© AegleaBioTherapeutics20Cystinuria Mouse Model Is Highly Translatable To The Human DiseaseMouse disease model replicates important aspects of cystinuria stone formation in affected patients •Cystine crystal formation •Kidney stone formation leads to tubular dilatation and hydronephrosis (excess fluid in the kidney due to urine backup)•Severe phenotype in mice with more rapid development of both the number and volume of cystine stones than in humansHistological section of Slc3a1-/-mouse kidneySlc3a1-/-mouse urine containing cystine crystalsHydronephrosisCalyceal dilatationIntraluminal cystine crystalsStonesSlc3a1-/-mouse kidney containing cystine stones
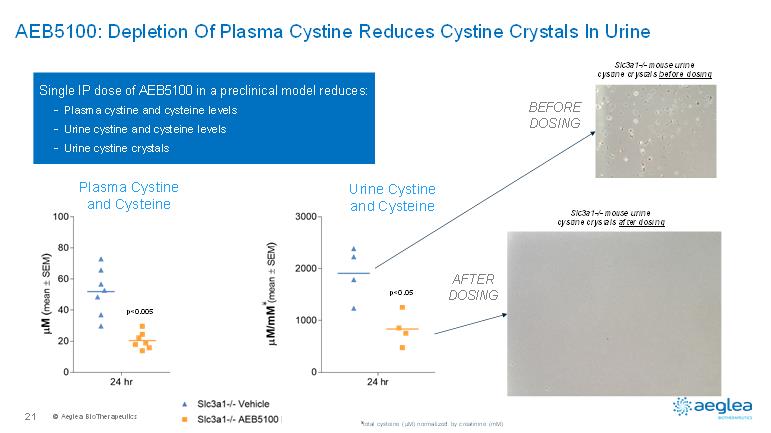
© AegleaBioTherapeutics21AEB5100: Depletion Of Plasma Cystine Reduces Cystine Crystals In UrineSlc3a1-/-mouse urinecystine crystals before dosingSlc3a1-/-mouse urinecystine crystals after dosingSingle IP dose of AEB5100 in a preclinical model reduces:-Plasma cystine and cysteine levels-Urine cystine and cysteine levels-Urine cystine crystalsPlasma Cystine and CysteineUrine Cystine and Cysteine*p<0.005**p<0.05¥total cysteine (µM) normalized by creatinine (mM)BEFORE DOSINGAFTER DOSING
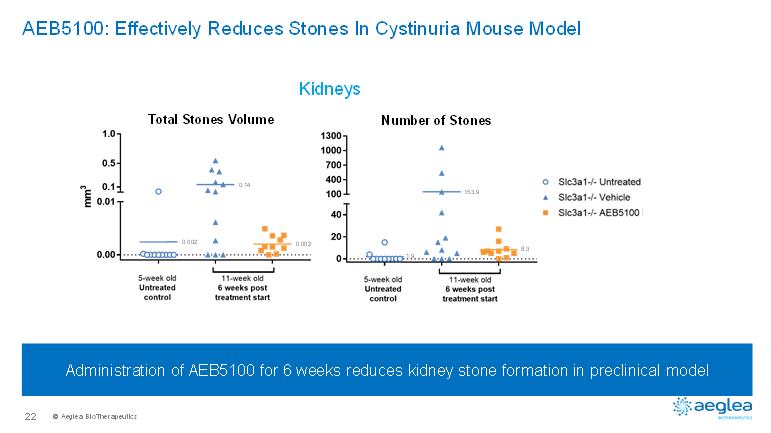
© AegleaBioTherapeutics22AEB5100: Effectively Reduces Stones In Cystinuria Mouse ModelKidneys*Administration of AEB5100 for 6 weeks reduces kidney stone formation in preclinical model Total Stones VolumeNumber of Stones0.0020.140.0021.9153.98.3
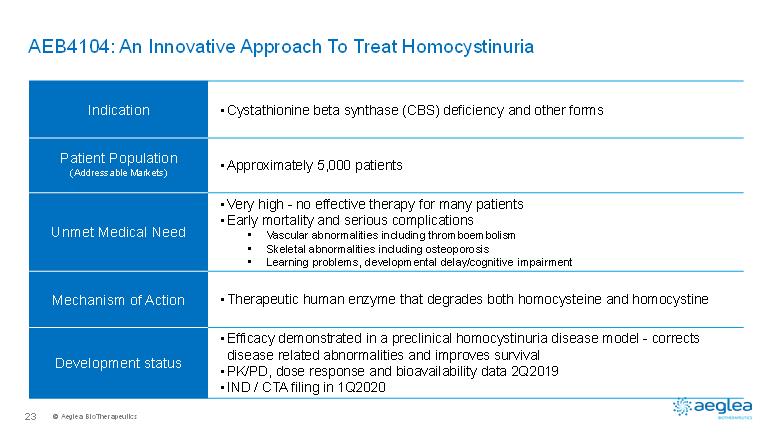
© AegleaBioTherapeutics23AEB4104: An Innovative Approach To Treat HomocystinuriaIndication•Cystathionine beta synthase (CBS) deficiency and other formsPatient Population(Addressable Markets)•Approximately 5,000 patientsUnmet Medical Need •Very high -no effective therapy for many patients•Early mortality and serious complications•Vascular abnormalities including thromboembolism•Skeletal abnormalities including osteoporosis•Learning problems, developmental delay/cognitive impairmentMechanism of Action•Therapeutic human enzyme that degrades both homocysteine and homocystineDevelopment status•Efficacy demonstrated in a preclinical homocystinuria disease model -corrects disease related abnormalities and improves survival•PK/PD, dose response and bioavailability data 2Q2019•IND / CTA filing in 1Q2020

© AegleaBioTherapeutics24AEB4104: Therapeutic Approach To Depletion Of Homocysteine And HomocystineAEB4104 is an engineered CGL enzyme designed to change its native substrate specificity from cystathionine to both homocysteine and homocystineAEB4104 Reduces Plasma Homocysteine & HomocystineLevels in Preclinical Models of Homocystinuria Total plasma homocysteine and homocystineas % of controlTotal plasma homocysteine & homocystineHigh methionine diet-induced homocystinuria modelCBS -/-mouse model of homocystinuria012345670100200300400Days.MTotal homocysteine and homocystine
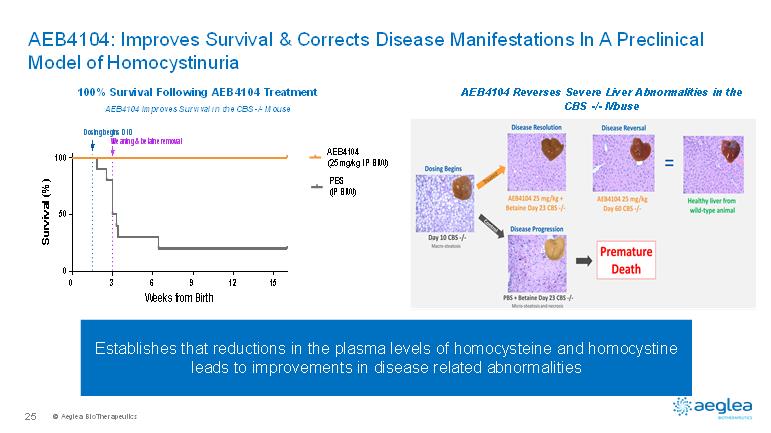
© AegleaBioTherapeutics25AEB4104: Improves Survival & Corrects Disease Manifestations In A Preclinical Model of HomocystinuriaEstablishes that reductions in the plasma levels of homocysteine and homocystineleads to improvements in disease related abnormalitiesAEB4104 Reverses Severe Liver Abnormalities in the CBS -/-Mouse 03691215050100Weeks from BirthSurvival (%)AEB4104(25 mg/kg IP BIW)PBS(IP BIW)Dosing begins D10Weaning & betaine removal100% Survival Following AEB4104 TreatmentAEB4104 Improves Survival in the CBS -/-Mouse

© AegleaBioTherapeutics2626Arginine Dependent CancersFocus on Small Cell Lung Cancer (SCLC) and MelanomaPegzilarginase: Lead Program
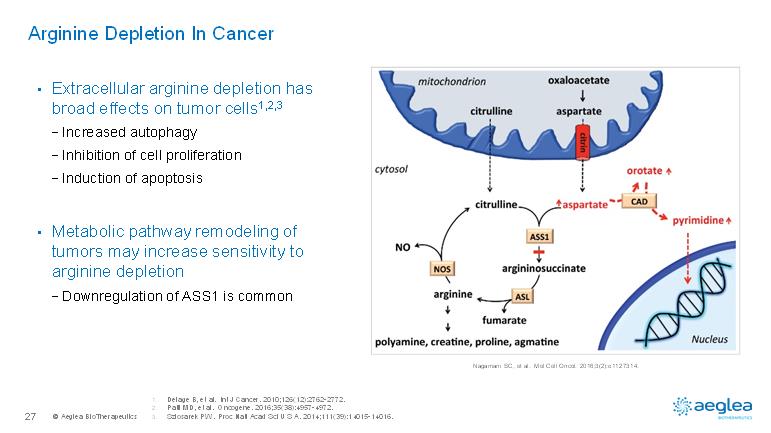
© AegleaBioTherapeutics27Arginine Depletion In Cancer •Extracellular arginine depletion has broad effects on tumor cells1,2,3-Increased autophagy-Inhibition of cell proliferation-Induction of apoptosis•Metabolic pathway remodeling of tumors may increase sensitivity to arginine depletion-Downregulation of ASS1 is commonNagamani SC, et al. Mol Cell Oncol. 2016;3(2):e1127314.1.Delage B, et al. Int J Cancer. 2010;126(12):2762-2772.2.Patil MD, et al. Oncogene. 2016;35(38):4957-4972.3.Szlosarek PW. Proc Natl Acad Sci U S A. 2014;111(39):14015-14016.
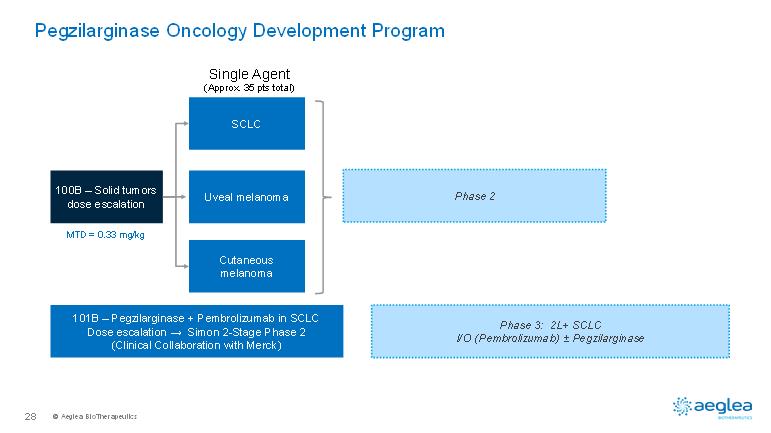
© AegleaBioTherapeutics28PegzilarginaseOncology Development Program100B –Solid tumorsdose escalationSCLCUveal melanomaCutaneous melanoma101B –Pegzilarginase+ Pembrolizumab in SCLCDose escalation . Simon 2-Stage Phase 2(Clinical Collaboration with Merck)Phase 2Phase 3: 2L+ SCLCI/O (Pembrolizumab) ±PegzilarginaseSingle Agent(Approx. 35 pts total)MTD = 0.33 mg/kg
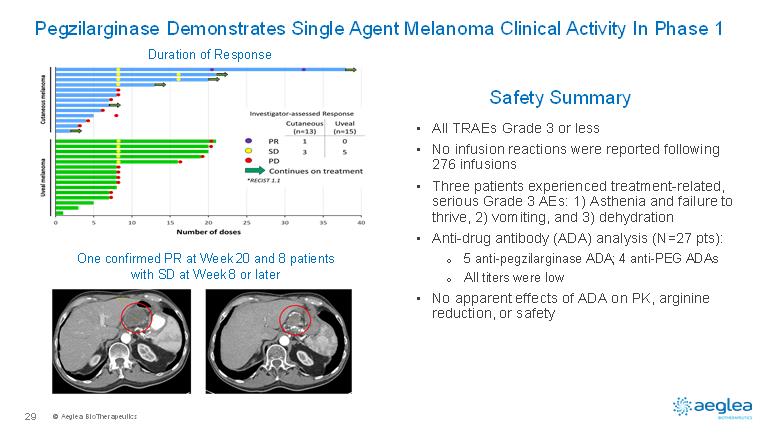
© AegleaBioTherapeutics29•All TRAEs Grade 3 or less•No infusion reactions were reported following 276 infusions•Three patients experienced treatment-related, serious Grade 3 AEs: 1) Asthenia and failure to thrive, 2) vomiting, and 3) dehydration•Anti-drug antibody (ADA) analysis (N=27 pts):o5 anti-pegzilarginaseADA; 4 anti-PEG ADAsoAll titers were low •No apparent effects of ADA on PK, arginine reduction, or safetyPegzilarginase Demonstrates Single Agent Melanoma Clinical Activity In Phase 1Safety SummaryOne confirmed PR at Week 20 and 8 patients with SD at Week 8 or laterDuration of Response
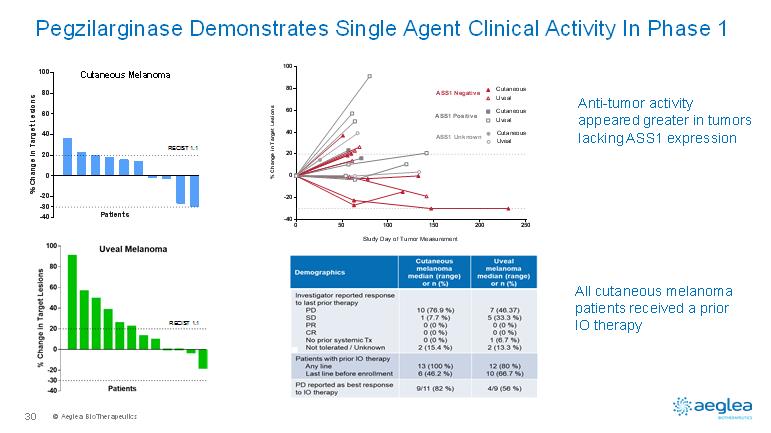
© AegleaBioTherapeutics30-40-20020406080100Cutaneous Melanoma% Change in Target LesionsPatients-30050100150200250-40-20020406080100Study Day of Tumor Measurement% Change in Target LesionsUvealCutaneousCutaneousUvealCutaneousUvealASS1 NegativeASS1 PositiveASS1 UnknownPegzilarginase Demonstrates Single Agent Clinical Activity In Phase 1Anti-tumor activity appeared greater in tumors lacking ASS1 expressionAll cutaneous melanoma patients received a prior IO therapyRECIST 1.1RECIST 1.1

© AegleaBioTherapeutics31© AegleaBioTherapeutics31Arginine Depletionin Cancers Summary12Single agent activity in heavily pre-treated Phase 1 melanoma patient populationPegzilarginasesafely and sustainably depletes plasma arginine3Anti-tumor activity appeared greater in tumors lacking ASS1 expression4Anticipated Upcoming catalystsPhase 2 combination in SCLC topline data 1H2020
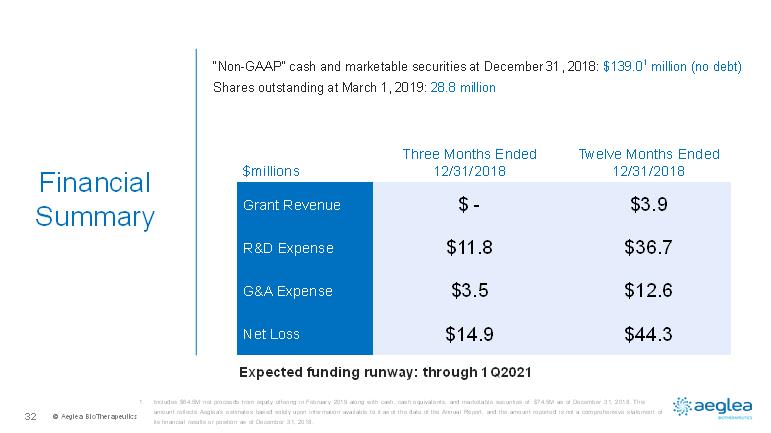
© AegleaBioTherapeutics32FinancialSummary$millionsThree Months Ended 12/31/2018Twelve Months Ended 12/31/2018Grant Revenue$ -$3.9R&D Expense$11.8$36.7G&A Expense$3.5$12.6Net Loss$14.9$44.3Expected funding runway: through 1Q20211.Includes $64.5M net proceeds from equity offering in February 2019 along with cash, cash equivalents, and marketable securities of $74.5M as of December 31, 2018. This amount reflects Aeglea’sestimates based solely upon information available to it as of the date of the Annual Report, and the amount reported is not acomprehensive statement of its financial results or position as of December 31, 2018.“Non-GAAP” cash and marketable securities at December 31, 2018: $139.01million (no debt)Shares outstanding at March 1, 2019: 28.8 million

Aeglea BioTherapeutics

































