Filed Pursuant to Rule 424(b)(3)
Registration Statement No. 333-218626
Prospectus Supplement No. 3
(To Prospectus dated June 16, 2017)
110,494 Shares of Common Stock
220,988 Shares of Common Stock issuable upon the
exercise of outstanding warrants
This prospectus supplement supplements the prospectus dated June 16, 2017, relating to an aggregate of 331,482 shares of our common stock, par value $0.0001 per share, consisting of (i) 110,494 shares that are currently issued and outstanding, and (ii) 220,988 shares that are issuable upon the exercise of warrants to purchase shares of common stock originally issued on March 31, 2017.
This prospectus supplement incorporates into our prospectus the information contained in our attached Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on December 01, 2017.
You should read this prospectus supplement in conjunction with the accompanying prospectus, including any supplements and amendments thereto. This prospectus supplement is qualified by reference to the accompanying prospectus except to the extent that the information in the prospectus supplement supersedes the information contained in the accompanying prospectus.
Investing in our common stock involves risks. See the information under the captions "Risk Factors" beginning on page 8 of the accompanying prospectus. You should also read carefully and consider any additional risk factors included in documents that we file with the Securities and Exchange Commission that are incorporated by reference in this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state or other securities commission has approved or disapproved of these securities nor passed upon the accuracy of this prospectus supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus supplement is December 01, 2017.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 01, 2017
AIT Therapeutics, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | | 000-55759 | | 47-3812456 |
| (State or Other Jurisdiction of Incorporation) | | (Commission File Number) | | (IRS Employer Identification No.) |
2 Ilan Ramon, Science Park Ness Ziona, 7403635 Israel |
| (Address of Principal Executive Office) |
+972.8.684.3313
(Registrant's telephone number, including area code)
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01. Other Events.
AIT Therapeutics, Inc. (the "Company") has announced that preliminary results from its NO-NTM Phase 2 study in Nontuberculous Mycobacteria (NTM) targeting patients suffering from Mycobacterium Abscessus Complex (MABSC) indicate that the primary endpoint of safety was met, with no Nitrous Oxide (NO)-related serious adverse events (SAEs) observed.
All patients in the trial were refractory to the standard-of-care for MABSC and had underlying cystic fibrosis. Patients were treated with inhaled NO in addition to background antibiotic therapy at the physicians' discretion. NO was delivered at a concentration of 160 ppm for 30 minutes intermittently for 21 days (5 times per day for 14 days and then 3 times per day for a subsequent 7 days), after which no further NO treatments were provided. As per the protocol, all patients will be followed for 81 days. Nine patients were enrolled in the study and all have completed the 21-day dosing schedule. Data presented below are through Day 51 (30 days post completion of treatment) for the endpoints of 6-minute walk (6 MW) and forced expiratory volume (FEV1). The primary endpoint of safety, as well as the endpoints of mycobacterium abscessus (M. abscessus) load in sputum and quality-of-life (QoL), assessed at Day 21, are discussed below. The Company anticipates having all data collected by the end of the first quarter of 2018. Key findings from the data collected to date include:
| · | The primary endpoint of safety was achieved: there were no NO-related SAEs reported over the 21-day treatment period, which included more than 800 inhalations. |
| · | Based on unaudited preliminary results, one of nine patients achieved culture conversion at Day 21. |
| · | QoL questionnaire SF-36 was used in the trial and all relevant data collected to date have trended positive. |
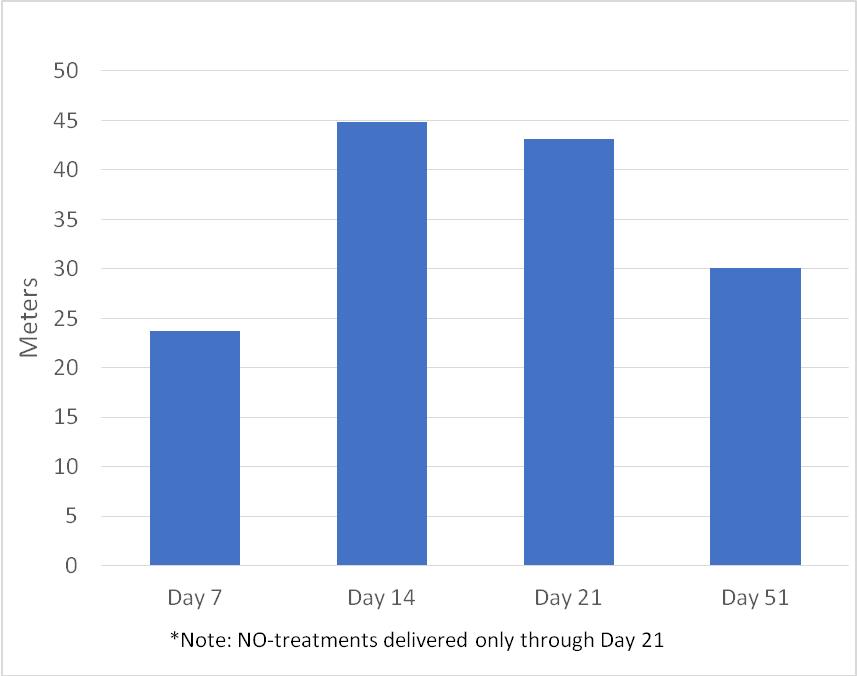
| · | The mean change in distance versus baseline for the 6 MW test at various timepoints is presented in Figure 1. Patients received therapy through Day 21, and the measurement at Day 51 occurred during the follow-up period post treatment. |
Figure 1: 6-Minute Walk Test Mean Increase in Distance (meters) versus Baseline*
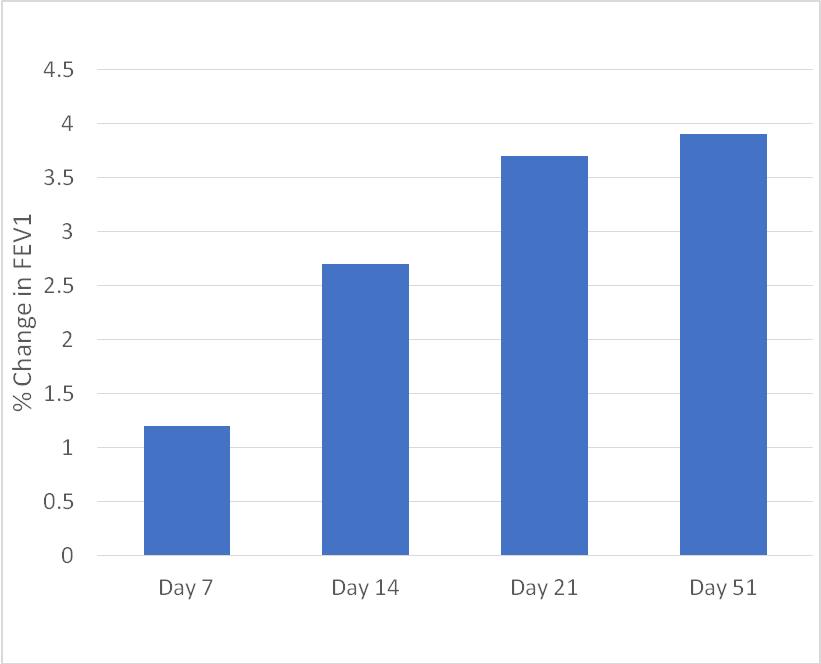
| · | The mean % change in FEV1 versus baseline at various timepoints is presented in Figure 2. Patients received therapy through Day 21, and the measurement at Day 51 occurred during the follow-up period post treatment. |
Figure 2: Mean Percentage Change in FEV1 versus Baseline*
*Note: NO-treatments delivered only through Day 21
The Company plans to continue developing its inhaled NO therapy for this indication and expects to begin enrollment in a potential pivotal study in the U.S. in the second half of 2018.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this report that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding the timing of potential pivotal trials; the anticipated timing of release of clinical trial data; and the Company's abilities and prospects.
These forward-looking statements are based on management's current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: our approach to discover and develop novel drugs, which is unproven and may never lead to marketable products; our ability to fund and the results of further pre-clinical and clinical trials; obtaining, maintaining and protecting intellectual property utilized by our products; our ability to enforce our patents against infringers and to defend our patent portfolio against challenges from third parties; our ability to obtain additional funding to support our business activities; our dependence on third parties for development, manufacture, marketing, sales, and distribution of products; the successful development of our product candidates, all of which are in early stages of development; obtaining regulatory approval for products; competition from others using technology similar to ours and others developing products for similar uses; our dependence on collaborators; our short operating history; and the other risks and uncertainties described under the captions ''Cautionary Note Regarding Forward-Looking Statements'' and "Risk Factors" in our filings with the Securities and Exchange Commission. Any such forward-looking statements represent management's estimates as of the date of this report. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this report.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | AIT THERAPEUTICS, INC. |
| | |
Date: December 01, 2017 | By: /s/ Steven Lisi Name: Steven Lisi Title: Chief Executive Officer |