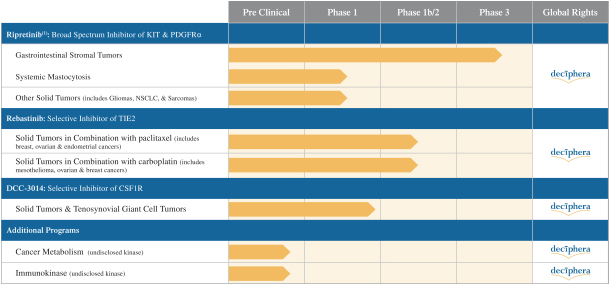
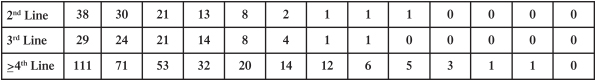
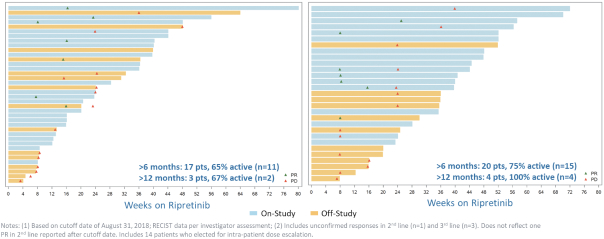
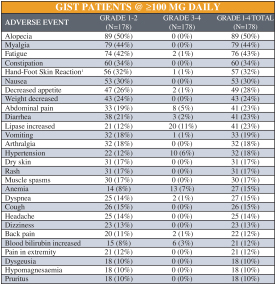
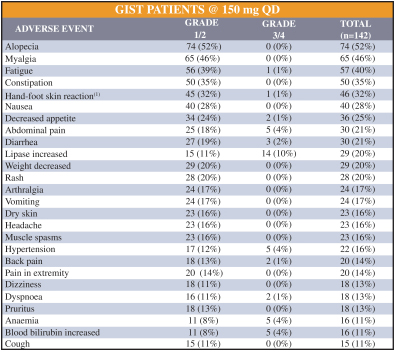
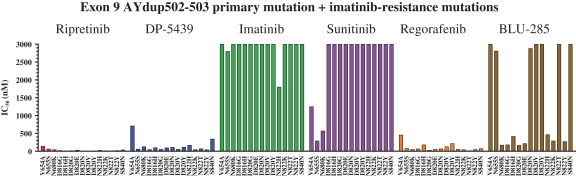
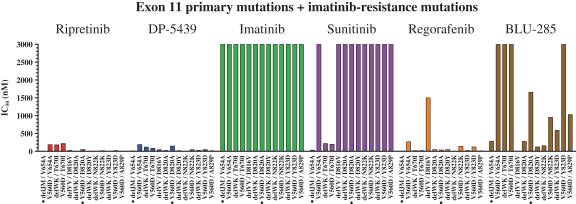
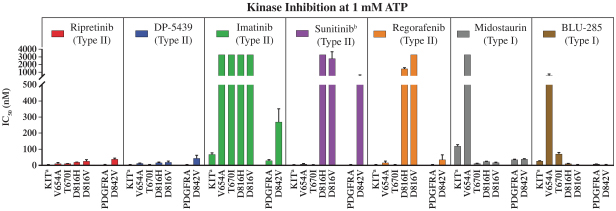
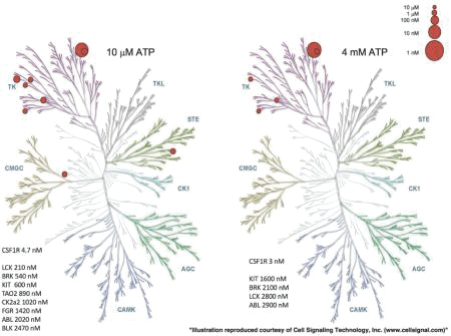
Competition for Ripretinib
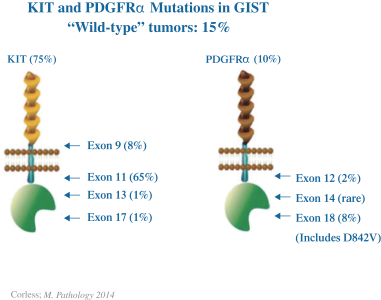
We are initially developing ripretinib for patients with GIST, ASM and other solid tumors with mutations in KIT and PDGFRα.
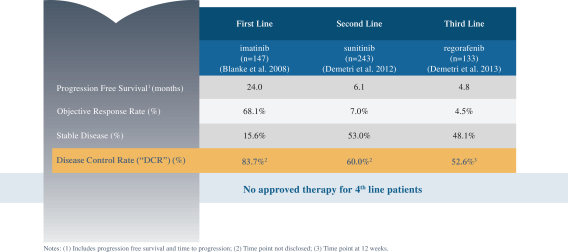
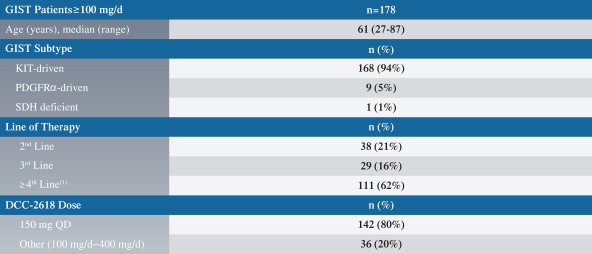
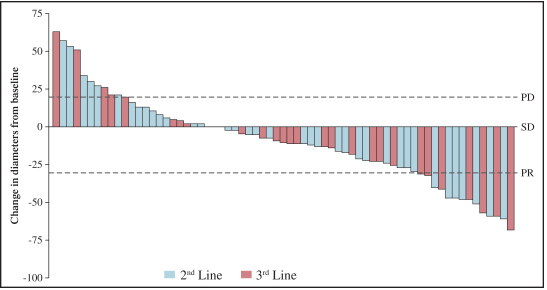
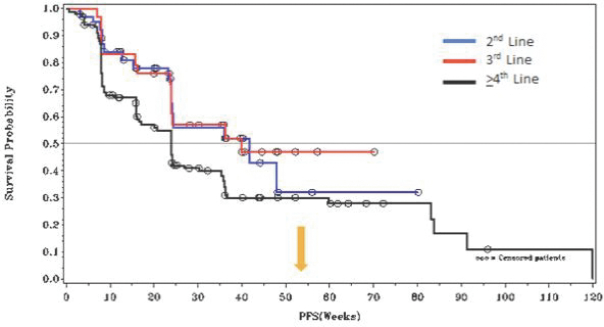
In GIST, the current approved standards of care for unresectable or metastatic patients are first-line imatinib, followed by second-line sunitinib upon imatinib progression, followed by third-line regorafenib upon sunitinib progression. If ripretinib receives marketing approval for GIST, we may also face competition from drug candidates in clinical trials including Arog Pharmaceuticals, Inc., Blueprint Medicines Corporation, Plexxikon Inc., a wholly owned subsidiary of Daiichi Sankyo Company, Limited, ARIAD Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceuticals U.S.A., Bristol-Myers Squibb Company, Celldex Therapeutics, Inc., Novartis AG and Xencor, Inc.
For ASM, the only approved drugs that inhibit KIT are imatinib for patients without the KIT D816V mutation or mutational status unknown and midostaurin (Novartis AG). If ripretinib receives marketing approval, in addition to midostaurin it may face competition from other drug candidates in clinical trials for ASM, including drug candidates from AB Science S.A., Allakos Inc., Blueprint Medicines Corporation, Celldex Therapeutics, Inc., Plexxikon Inc., a wholly owned subsidiary of Daiichi Sankyo Company, Limited.
Competition for Rebastinib
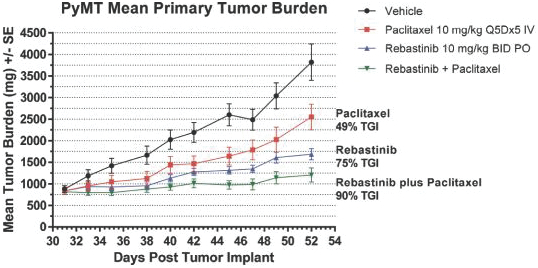
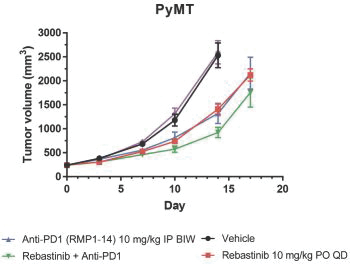
We are initially developing rebastinib, a TIE2 inhibitor, to control immunosuppressive TAMs expressing TIE2 to assist the immune system in targeting tumor cells. While rebastinib is a novel molecule, we may face competition from drug candidates in clinical trials that are not TIE2 inhibitors, but aim to achieve similar effects on the immune system. These include small molecule drug candidates in clinical trials from Array BioPharma Inc., Bristol-Myers Squibb Company, Novartis AG, Plexxikon Inc., a wholly owned subsidiary of Daiichi Sankyo Company, Limited, and from antibody therapeutics from Amgen Inc., Eli Lilly and Company, Roche Holding Ltd, Five Prime Therapeutics, Inc., Novartis AG, and Syndax Pharmaceuticals, Inc.
Competition forDCC-3014
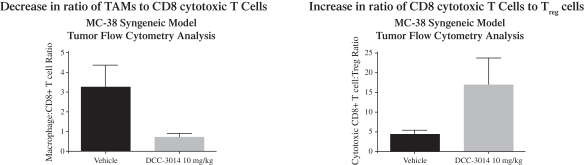
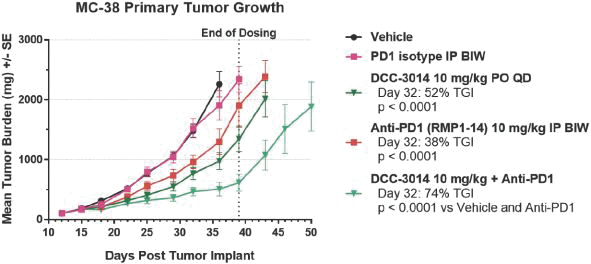
We are initially developingDCC-3014, an inhibitor of CSF1R, to control immunosuppressive tumor-associated macrophages to assist the immune system in targeting tumor cells. IfDCC-3014 receives marketing approval, it may face competition from other drug candidates in clinical trials that target CSF1R, including small molecule drug candidates in clinical trials from Array BioPharma, Inc., Novartis AG, Plexxikon Inc., a wholly owned subsidiary of Daiichi Sankyo Company, Limited, and from antibody therapeutics including those from Amgen Inc., Eli Lilly and Company, Roche Holding Ltd, Five Prime Therapeutics, Inc., Novartis AG, and Syndax Pharmaceuticals, Inc.
Intellectual Property
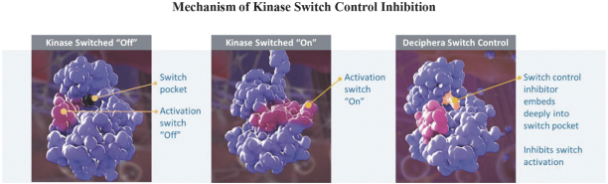
We strive to protect the proprietary technologies that we believe are important to our business, including pursuing and maintaining patent protection intended to cover the composition of matter of our drug candidates, for example, ripretinib,DCC-3014 and rebastinib, their methods of use, related technologies and other inventions that are important to our business. In addition to patent protection, we also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, including our proprietary kinase switch control inhibitor platform.
Our commercial success depends in part upon our ability to obtain and maintain patent and other proprietary protection for our drug candidates and other commercially important technologies, inventions andknow-how related to our business, defend and enforce our intellectual property rights, in particular, our patent rights, preserve the confidentiality of our trade secrets and operate without infringing valid and enforceable intellectual property rights of others.
35