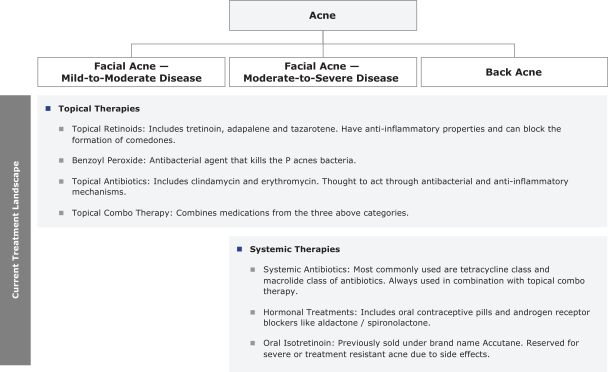
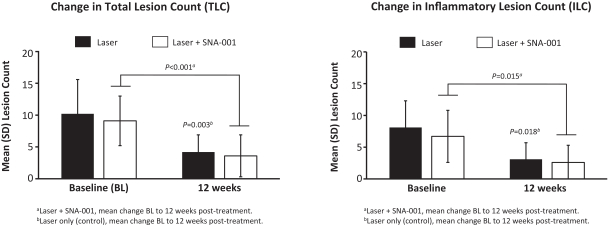
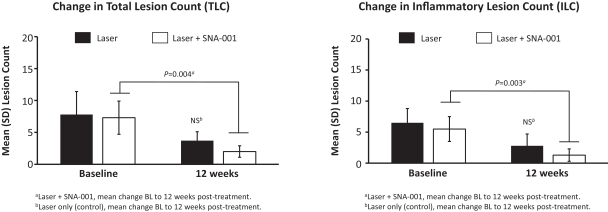
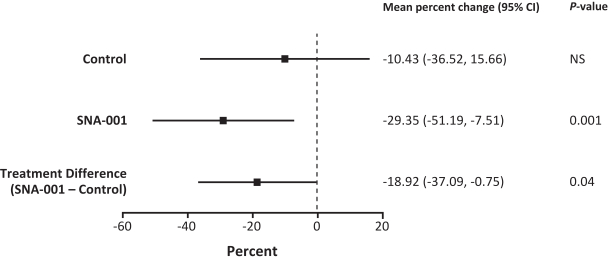
Commercial Operations
We currently have no sales organization. We have retained global rights to all of our product candidates, and, if one of our product candidates is approved by the FDA, expect to access the market in North America through a specialty dermatology-focused sales force. We expect that our sales force will be supported by sales management, internal sales support, an internal marketing group and distribution support. We intend to invest in our commercial capabilities prudently by focusing our marketing efforts on the dermatologists who have the highest prescription volumes and those with a medical focus who have installed laser capabilities with which to use SNA-001. We will also evaluate licensing and partnering with third parties to help us reach other sales channels, such as medispas and laser treatment centers, and geographic markets outside of North America.
Intellectual Property
Our success depends in large part upon our ability to obtain and maintain proprietary protection for our products and technologies and to operate without infringing the proprietary rights of others. Our policy is to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also use other forms of protection, such as confidential information, know-how and trademarks to protect our intellectual property, particularly where we do not believe patent protection is appropriate or obtainable.
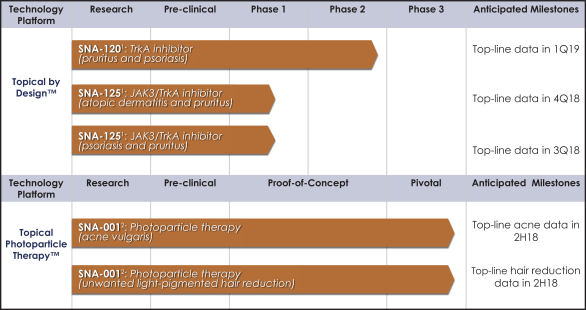
Our Topical by DesignTM and Topical Photoparticle TherapyTM patent portfolios consist of a combination of issued patents and pending patent applications that are owned by us (and our subsidiaries) or licensed from third parties. As of December 31, 2017, we own or have an exclusive license in certain fields of use to over 200 patents and patent applications in the United States and internationally.
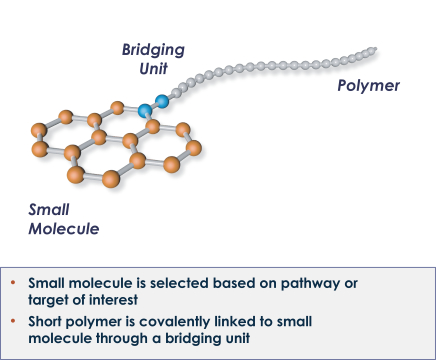
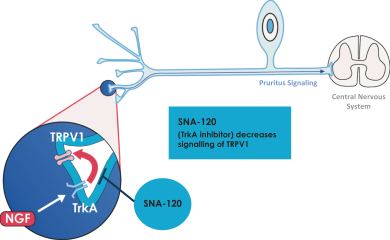
Our Topical by DesignTM patent portfolio includes patents and patent applications that are directed to our Topical by DesignTM technology, including our lead compounds SNA-120 and SNA-125, and as of December 31, 2017, includes approximately six issued U.S. patents and 112 issued international patents in Australia, Canada, China, Albania, Austria, Bosnia & Herzegovina, Belgium, Bulgaria, Switzerland & Liechtenstein, Cyprus, Czech Republic, Germany, Denmark, Estonia, Spain, Finland, France, Great Britain, Greece, Croatia, Hungary, Ireland, Iceland, Italy, Lithuania, Luxembourg, Latvia, Monaco, Macedonia, Malta, Netherlands, Norway, Poland, Portugal, Romania, Serbia, Sweden, Slovenia, Slovakia, San Marino, Turkey, Hong Kong, Israel, India, Indonesia, Japan, South Korea, Mexico, New Zealand, Russia, South Africa, Singapore, and Ukraine; and 17 pending U.S. patent applications and eight pending international patent applications in Brazil, Egypt, Malaysia, Japan and Europe. Our Topical by DesignTM patent portfolio includes patents and patent applications directed to compositions of matter, formulations, use and process. In general, patents have a term of 20 years from the application filing date or earliest claimed non-provisional priority date. Several of our issued U.S. and international patents that relate to our SNA-120 and SNA-125 technologies are scheduled to expire in approximately 2026 through 2032 and may be extended by up to five years in certain countries via patent term extension depending on the regulatory pathway. Certain other patents and patent applications directed to our Topical by DesignTM portfolio, if they were to issue, may have later expirations.
Our Topical Photoparticle TherapyTM patent portfolio includes patents and patent applications that are directed to our Topical Photoparticle TherapyTM technology, including our lead compound SNA-001, and as of December 31, 2017, includes approximately 21 issued U.S. patents and 44 issued international patents in Australia, Japan, China, Germany, France, United Kingdom, Ireland, Italy, Monaco, Netherlands, Spain, Sweden, Switzerland & Liechtenstein, Turkey, Norway, Denmark, Finland, Poland, Austria, Greece, Hungary, Belgium, Czech Republic, and Romania; and five pending U.S. patent applications and 25 pending international patent applications in Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, India, Japan, Mexico, Russia and South Korea. Our Topical Photoparticle TherapyTM patent portfolio includes patents and patent applications directed to compositions of matter, use and process. In general, patents have a term of 20 years from the
34