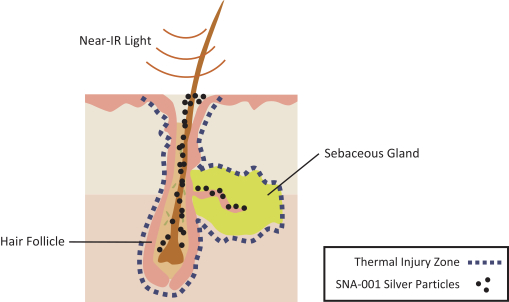
Our patent portfolio also includes patents and patent applications that are directed to our Topical Photoparticle TherapyTM technology, includingSNA-001, and as of December 31, 2018, includes approximately 21 issued U.S. patents and 71 issued international patents in Australia, Japan, China, Germany, France, United Kingdom, Ireland, Italy, Monaco, Netherlands, Spain, Sweden, Switzerland & Liechtenstein, Turkey, Norway, Denmark, Finland, Poland, Austria, Greece, Hungary, Belgium, Czech Republic, and Romania; and five pending U.S. patent applications and 24 pending international patent applications in Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, India, Japan, Mexico, Russia and South Korea. Our Topical Photoparticle TherapyTM patent portfolio includes patents and patent applications directed to compositions of matter, use and process. In general, patents have a term of 20 years from the application filing date or earliest claimednon-provisional priority date. Several of our issued U.S. and international patents that relate to ourSNA-001 technologies expire in 2031 through 2033. Certain other patents and patent applications directed to our Topical Photoparticle TherapyTM portfolio, if they were to issue, may have later expirations.
Most of our issued patents and pending patent applications are owned by us or one of our subsidiaries. Some of our issued and pending Topical Photoparticle TherapyTM patent applications are exclusively licensed to us in certain fields of use from nanoComposix, Inc., or nanoComposix. These patents and pending patent applications are solely owned by nanoComposix or jointly owned by us and nanoComposix, and are generally directed to technologies related to the production of silver nanoplates used in the Topical Photoparticle TherapyTM technology forSNA-001.
Our continuing research and development, technicalknow-how, and contractual arrangements supplement our intellectual property protection in an effort to maintain our competitive position. Our policy is to require inventors who are identified on any Company-owned patent applications to assign rights to us. We also rely, in part, on confidentiality agreements with our employees, consultants, and other advisors to protect our proprietary information. Our policy is to require third parties that receive material confidential information to enter into confidentiality agreements with us.
We also protect our brand through procurement of trademark rights. As of December 31, 2018, we own approximately 53 registered trademarks around the world, as well as 16 U.S. and 120 international pending trademark applications, including registrations in the European Union, Switzerland, Norway, Turkey, Russia, China, Colombia, India, Japan, Mexico, South Korea, Singapore, Vietnam, Australia, Argentina, Israel, New Zealand, Monaco, and the Philippines. Our trademarks SIENNA®, SIENNA BIOPHARMACEUTICALS®, TPT®, TOPICAL BY DESIGN®, LSE®, and SCIENCE YOU CAN SEE. CONFIDENCE YOU CAN FEEL.® are registered trademarks that we own in certain international countries. In order to supplement protection of our brand, we have also registered several internet domain names.
Significant Transaction—Acquisition of Creabilis plc
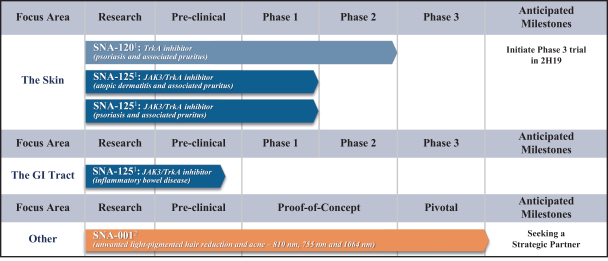
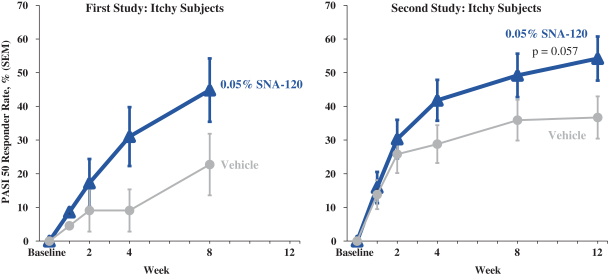
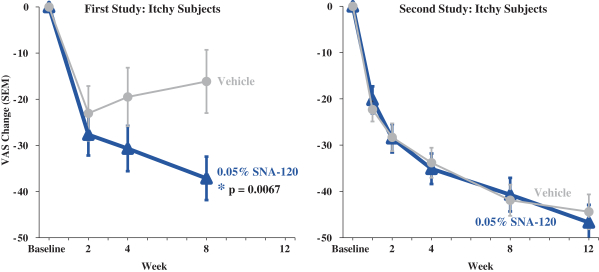
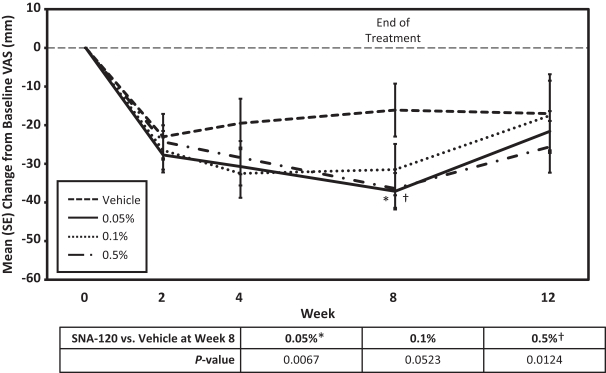
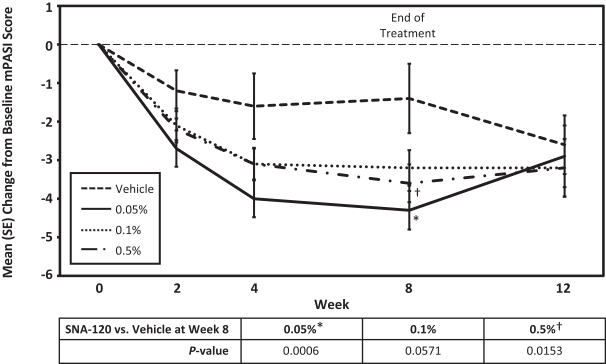
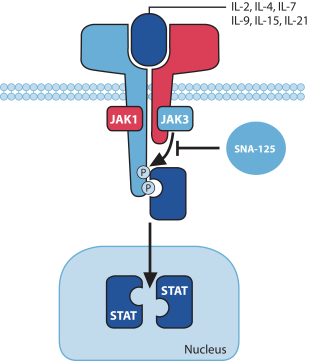
In December 2016, we entered into a Share Purchase Agreement to acquire the entire issued share capital of Creabilis plc, which, upon closing, became our direct wholly-owned subsidiary. Through the acquisition of Creabilis we obtained our proprietary technology platform and related product candidates, includingSNA-120 andSNA-125. In connection with closing, we made an upfront payment of $0.2 million in cash, issued 1,407,679 shares of our SeriesA-3 Preferred Stock to the former Creabilis shareholders and settled approximately $6.7 million of Creabilis liabilities. In October 2017, we commenced our additional Phase 2b clinical trial forSNA-120, triggering our first contingent milestone payment of $5.0 million, less certain offsets totaling approximately $0.3 million, which we satisfied by issuing an aggregate of 201,268 shares of common stock to the former Creabilis shareholders in December 2017 pursuant to the terms of the Share Purchase Agreement.
Upon the achievement of certain additional development, approval and sales milestones forSNA-120 andSNA-125, we are obligated to pay the former Creabilis shareholders up to an aggregate of $53.0 million, which consists of an aggregate of $25.0 million in cash and $28.0 million in shares of our common stock, including our
36