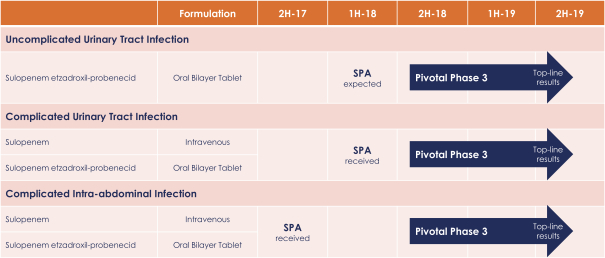
We believe the key competitive factors that will affect the development and commercial success of oral sulopenem and sulopenem, if approved, will be efficacy, coverage of drug-resistant strains of bacteria, safety and tolerability profile, reliability, convenience of oral dosing, price, availability of reimbursement from governmental and other third-party payers and susceptibility to drug resistance.
If approved, oral sulopenem would compete with several oral antibiotics currently in clinical development, including ceftibuten clavulanate from Achaogen, Inc., tebipenem pivoxil from Spero Therapeutics, Inc., eravacycline from Tetraphase Pharmaceuticals, Inc., delafloxacin from Melinta Therapeutics and omadacycline from Paratek Pharmaceuticals, Inc.
We also expect that oral sulopenem, if approved, would compete with future and current generic versions of marketed oral antibiotics. If approved, we believe that oral sulopenem would compete effectively against these compounds on the basis of sulopenem’s potential:
| | • | | broad range of activity against a wide variety of resistant and MDR gram-negative bacteria; |
| | • | | low probability of drug resistance; |
| | • | | a favorable safety and tolerability profile; |
| | • | | a convenient oral dosing regimen and opportunity to step down from IV-administered therapy; and |
| | • | | as a monotherapy treatment for resistant and MDR gram-negative infections. |
If approved, sulopenem would compete with several IV-administered product candidates marketed for the treatment of gram-negative infections, including Avycaz from Allergan plc and Pfizer and Zerbaxa from Merck & Co. There are also a number of IV-administered product candidates in late-stage clinical development that are intended to treat gram-negative infections, including plazomicin from Achaogen Inc., meropenem-vaborbactam from Melinta Therapeutics, cefiderocol from Shionogi & Co. Ltd., eravacycline IV from Tetraphase Pharmaceuticals, Inc. and relabactam from Merck & Co.
If approved, we believe that sulopenem would compete effectively and potentially occupy an earlier place in treatment against these compounds on the basis of sulopenem’s potential, including:
| | • | | allows physicians to stay in the same molecule with step down therapy to oral sulopenem; |
| | • | | convenient once a day dosing over a three-hour infusion period; |
| | • | | broad spectrum activity against a wide variety of resistant and MDR gram-negative bacteria; |
| | • | | low probability of drug resistance; and |
| | • | | a favorable safety and tolerability profile. |
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and in other countries, extensively regulate, among other things, the research, development, clinical trials, testing, manufacture, including any manufacturing changes, authorization, pharmacovigilance, adverse event reporting, recalls, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, import and export of pharmaceutical products and product candidates such as those we are developing. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
United States Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance
104