
June 2022 KER-050 Update

Disclaimer Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "anticipates," "believes," "expects," "intends," “plans,” “potential,” "projects,” “would” and "future" or similar expressions are intended to identify forward-looking statements. Examples of these forward-looking statements include statements concerning: Keros’ expectations regarding its growth, strategy, progress and the design, objectives and timing of its preclinical studies and clinical trials for KER-050, KER-047 and KER-012; the potential impact of COVID-19 on Keros’ ongoing and planned preclinical studies, clinical trials, business and operations; and the potential of Keros’ proprietary discovery approach. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among others: Keros’ limited operating history and historical losses; Keros’ ability to raise additional funding to complete the development and any commercialization of its product candidates; Keros’ dependence on the success of its lead product candidates, KER-050 and KER-047; that Keros may be delayed in initiating, enrolling or completing any clinical trials; competition from third parties that are developing products for similar uses; Keros’ ability to obtain, maintain and protect its intellectual property; Keros’ dependence on third parties in connection with manufacturing, clinical trials and pre-clinical studies; and risks relating to the impact on our business of the COVID-19 pandemic or similar public health crises. These and other risks are described more fully in Keros’ filings with the Securities and Exchange Commission (“SEC”), including the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 5, 2022, and its other documents subsequently filed with or furnished to the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except to the extent required by law, Keros undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third -party sources. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. 2

Keros is Developing Differentiated Clinical Assets in Hematological, Pulmonary, and Musculoskeletal Disorders 3 *Anticipated clinical milestones are subject to the impact of COVID-19 on our business.

KER-050 KER-050 is an investigational modified ActRIIA ligand trap designed to inhibit a subset of TGF-β superfamily ligands, including activin A, activin B, GDF8, and GDF11 In preclinical studies, treatment with a mouse research form of KER-050 (RKER-050) has been shown to increase both erythropoiesis and thrombopoiesis: • Induced red blood cell (RBC) production by promoting multiple stages of erythroid differentiation • Increased platelet production by increasing megakaryocyte progenitors as well as promoting terminal maturation 4

27th Annual Congress of the European Hematology Association KER-050 Presentations: Preclinical • “RKER-050, a novel inhibitor of TGF-β superfamily signaling, induced platelet production in healthy mouse megakaryocytes” Clinical • “A Phase 2, open-label, ascending dose study of KER-050 for the treatment of anemia in patients with very low, low, or intermediate risk myelodysplastic syndromes” 5
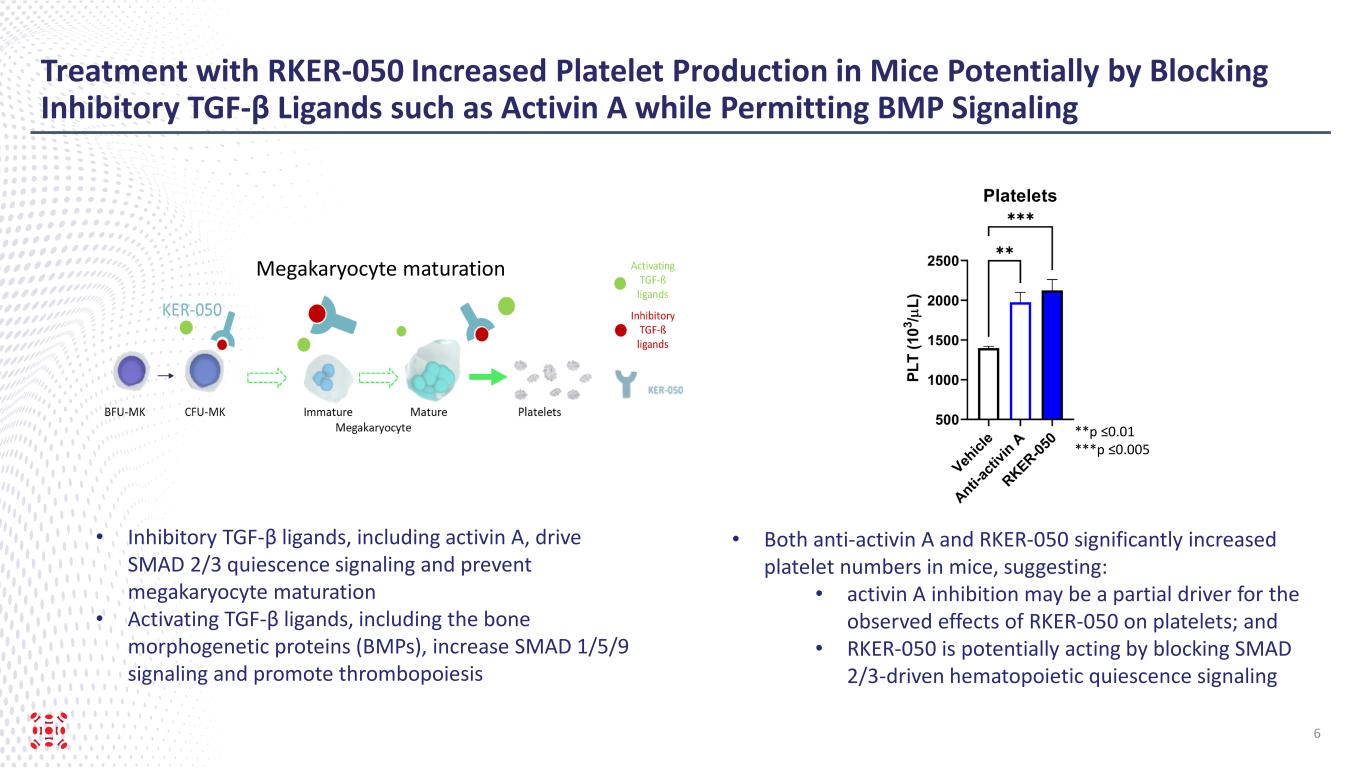
Treatment with RKER-050 Increased Platelet Production in Mice Potentially by Blocking Inhibitory TGF-β Ligands such as Activin A while Permitting BMP Signaling 6 • Both anti-activin A and RKER-050 significantly increased platelet numbers in mice, suggesting: • activin A inhibition may be a partial driver for the observed effects of RKER-050 on platelets; and • RKER-050 is potentially acting by blocking SMAD 2/3-driven hematopoietic quiescence signaling Megakaryocyte maturation • Inhibitory TGF-β ligands, including activin A, drive SMAD 2/3 quiescence signaling and prevent megakaryocyte maturation • Activating TGF-β ligands, including the bone morphogenetic proteins (BMPs), increase SMAD 1/5/9 signaling and promote thrombopoiesis **p ≤0.01 ***p ≤0.005
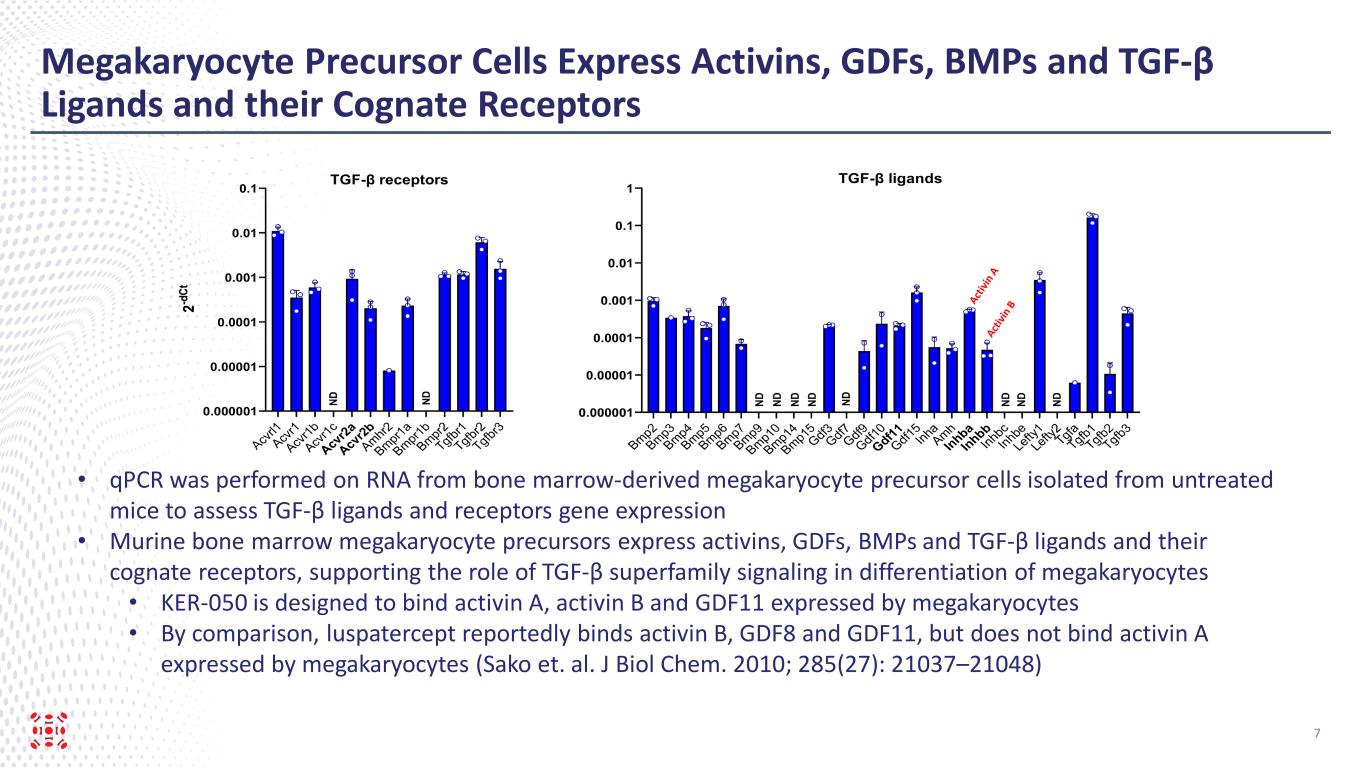
Megakaryocyte Precursor Cells Express Activins, GDFs, BMPs and TGF-β Ligands and their Cognate Receptors 7 • qPCR was performed on RNA from bone marrow-derived megakaryocyte precursor cells isolated from untreated mice to assess TGF-β ligands and receptors gene expression • Murine bone marrow megakaryocyte precursors express activins, GDFs, BMPs and TGF-β ligands and their cognate receptors, supporting the role of TGF-β superfamily signaling in differentiation of megakaryocytes • KER-050 is designed to bind activin A, activin B and GDF11 expressed by megakaryocytes • By comparison, luspatercept reportedly binds activin B, GDF8 and GDF11, but does not bind activin A expressed by megakaryocytes (Sako et. al. J Biol Chem. 2010; 285(27): 21037–21048)

KER-050 Overview • In a Phase 1 clinical trial of KER-050, treatment led to rapid, sustained and dose-dependent increases in RBCs and platelets • Inhibition of activin A in the bone resulted in increases in bone specific alkaline phosphatase (BSAP), a marker of osteoblast activity, which is supportive of change in the bone marrow microenvironment • We believe that data from our preclinical studies and our Phase 1 clinical trial support that treatment with KER-050 has the potential to address ineffective hematopoiesis in diseases like myelodysplastic syndromes and myelofibrosis 8
Myelodysplastic Syndromes (MDS) • MDS are a heterogeneous group of myeloid neoplasms characterized by clonal proliferation of hematopoietic stem cells, recurrent genetic abnormalities, myelodysplasia and ineffective hematopoiesis • Abnormalities in the bone marrow microenvironment, including altered hematopoietic–stromal interactions, results in ineffective hematopoiesis • This results in peripheral blood cytopenias with approximately 90% of lower risk MDS (LR-MDS) patients experiencing anemia • Patients have a high risk of evolution to acute myeloid leukemia (AML) • Patients are also at high risk of morbidity and mortality, including increased risk of infection, hemorrhage, iron overload due to transfusions and cardiovascular disease • Treatment options for LR-MDS patients include erythroid stimulating agents (ESA), erythroid maturation agent (EMA), RBC transfusions and iron chelation therapy • However, the benefit of RBC agents is limited in certain patients including patients with high transfusion burden 9

KER050-MD-201 A Phase 2 Clinical Trial Of KER-050 For The Treatment Of Anemia In Patients With Very Low, Low Or Intermediate Risk Myelodysplastic Syndromes (MDS) 10
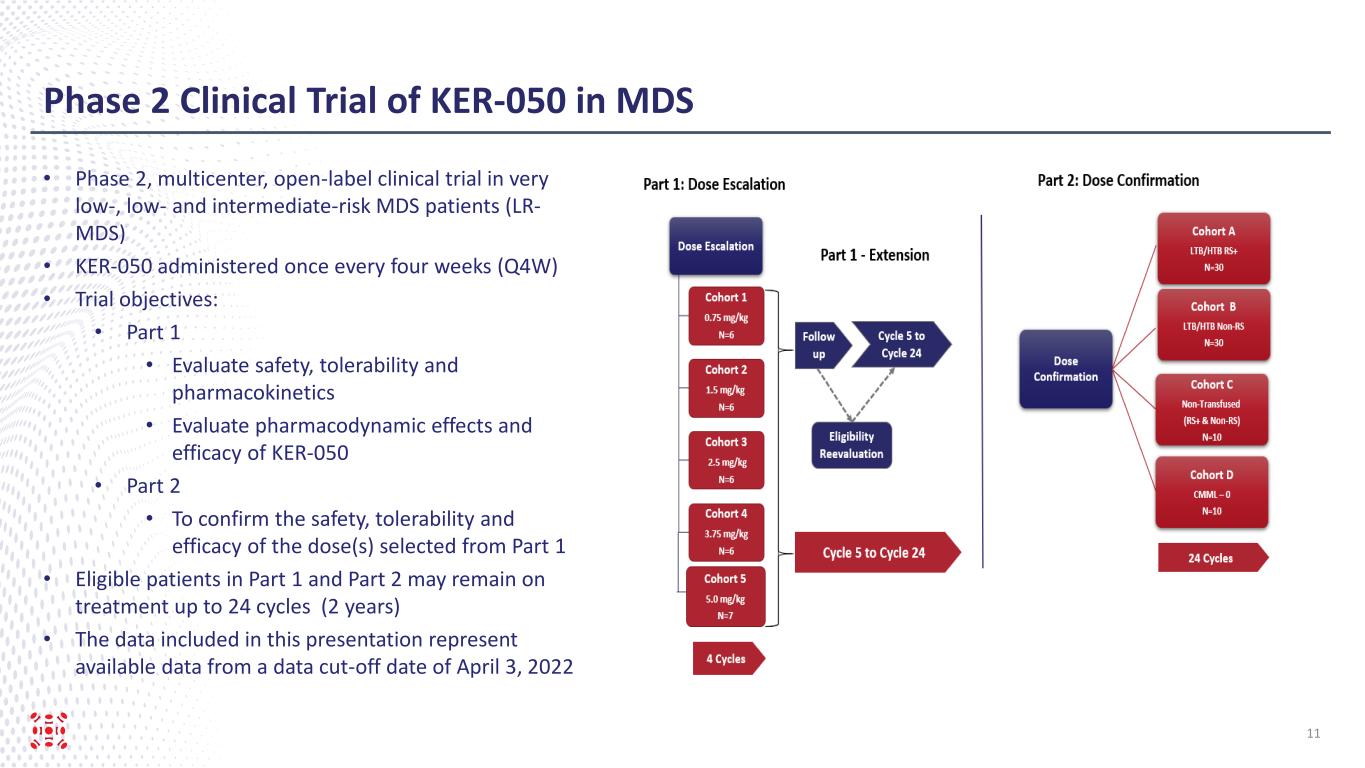
Phase 2 Clinical Trial of KER-050 in MDS • Phase 2, multicenter, open-label clinical trial in very low-, low- and intermediate-risk MDS patients (LR- MDS) • KER-050 administered once every four weeks (Q4W) • Trial objectives: • Part 1 • Evaluate safety, tolerability and pharmacokinetics • Evaluate pharmacodynamic effects and efficacy of KER-050 • Part 2 • To confirm the safety, tolerability and efficacy of the dose(s) selected from Part 1 • Eligible patients in Part 1 and Part 2 may remain on treatment up to 24 cycles (2 years) • The data included in this presentation represent available data from a data cut-off date of April 3, 2022 11

Phase 2 Clinical Trial of KER-050 in MDS Key Eligibility Criteria: • MDS with very low-, low-, or intermediate-risk disease, as classified by the International Prognostic Scoring System- Revised, including both patients that did not have ring sideroblasts (non-RS) and patients that have ring sideroblasts (RS+) • ESA naïve and experienced patients are eligible • No prior treatment with azacitidine, decitabine, lenalidomide, luspatercept or sotatercept • Anemia, categorized in one of the following three groups: • Non-transfused (NT): hemoglobin (Hgb) ≤10 g/dL • Low transfusion burden (LTB): 1-3 units of RBC/8 weeks for Hgb ≤9 g/dL • High transfusion burden (HTB): ≥4 units of RBC/8 weeks for Hgb ≤9 g/dL Select Efficacy Endpoints: • IWG 2006 Hematological improvement-erythroid (HI-E) • Hemoglobin increase of ≥1.5 g/dL for 8 weeks (in NT and LTB patients) • Reduction of ≥4 RBC units transfused over 8 weeks compared to baseline (in HTB patients) • Transfusion independence (TI) for at least 8 weeks in patients who require ≥ 2 RBC units transfused at baseline 12

Demographics and Baseline Characteristics from Part 1 Dose Escalation KER-050 Dose Level (mg/kg) 0.75 (N=6) 1.5 (N=6) 2.5 (N=6) 3.75 (N=6) 5 (N=7) All (N=31) Age, Mean (range) 75.5 68.3 72 73.3 76.7 73.3 (55-88) Female, n (%) 5 1 4 2 0 12 (38.7%) RS positive 3 3 3 4 4 17 (54.8%) Transfusion Burden Non-Transfused (NT), 0 units Low Transfusion Burden (LTB), <4 units High Transfusion Burden (HTB), ≥4 units 3 2 1 0 0 6 1 1 4 1 2 3 0 3 4 5 (16.1%) 8 (25.8%) 18 (58.1%) WHO MDS Classification, n (%) MDS-MLD MDS-RS-MLD MDS-RS-SLD MDS with isolated del(5q) N/A 3 2 0 1 0 3 2 1 0 0 3 3 0 0 0 1 4 0 0 1 3 3 0 0 1 13 (41.9%) 14 (45.2%) 1 (3.2%) 1 (3.2%) 2 (6.5%) Prior ESA Therapy, n (%) 0 0 2 1 0 3 (9.7%) Iron chelator, n (%) 0 2 2 2 1 7 (22.6%) Efficacy Evaluable Patients*, n(%) 6 4 6 6 5 27 (87.1%) Efficacy Evaluable HTB Patients**, n (%) 1 4 4 3 4 16/18 (88.9%) April 3, 2022 data cutoff *Patients with at least 8 weeks of post-treatment hemoglobin and transfusion assessments were defined as efficacy evaluable. ** Percentage was based on all HTB patients. 13

n (%) KER-050 Dose Level (mg/kg) 0.75 (N=6) 1.5 (N=6) 2.5 (N=6) 3.75 (N=6) 5 (N=7) All (N=31) TEAE 6 (100) 6 (100) 6 (100) 5 (83.3) 6 (85.7) 29 (93.5) Related TEAE 1 (16.7) 0 1 (16.7) 1 (16.7) 2 (28.6) 5 (16.1) Grade ≥3 1 (16.7) 1 (16.7) 3 (50.0) 2 (33.3) 5 (71.4) 12 (38.7) SAE 1 (16.7) 2 (33.3) 1 (16.7) 2 (33.3) 4 (57.1) 10 (32.3) TEAE requiring dose modification 0 0 0 1 (16.7) 1 (14.3) 2 (6.5) Death 0 1 (16.7) 0 0 0 1 (3.2) KER-050 was Generally Well-Tolerated at all Doses Tested in Part 1 April 3, 2022 data cutoff • No drug related serious adverse events or dose-limiting toxicities were reported • 10 patients experienced treatment-emergent SAEs • 4 patients discontinued study drug: 1 withdrew consent, 1 death (unrelated to study drug, per autopsy due to obesity-associated heart disease), 2 withdrew due to unrelated TEAE • No patients developed high-risk MDS or progressed to AML 14

Response Summary Response Rate, n/m (%) All evaluable patients HTB evaluable patients Overall Erythroid Response (HI-E or TI) 14/27 (51.8%) 11/16 (68.8%) IWG 2006 HI-E 12/26 (46.2%) 11/16 (68.8%) Transfusion independence (TI*) RS+ Non-RS 9/20 (45%) 6/12 (50%) 3/8 (37.5%) 7/16 (43.8%) 4/9 (44.4%) 3/7 (42.9%) Efficacy Summary of 8-Week Endpoints Achieved in MDS Patients *Baseline Transfusion Requirement ≥2 RBC units n = responders in each category; m = 8-week evaluable population as of data cutoff date 15April 3, 2022 data cutoff
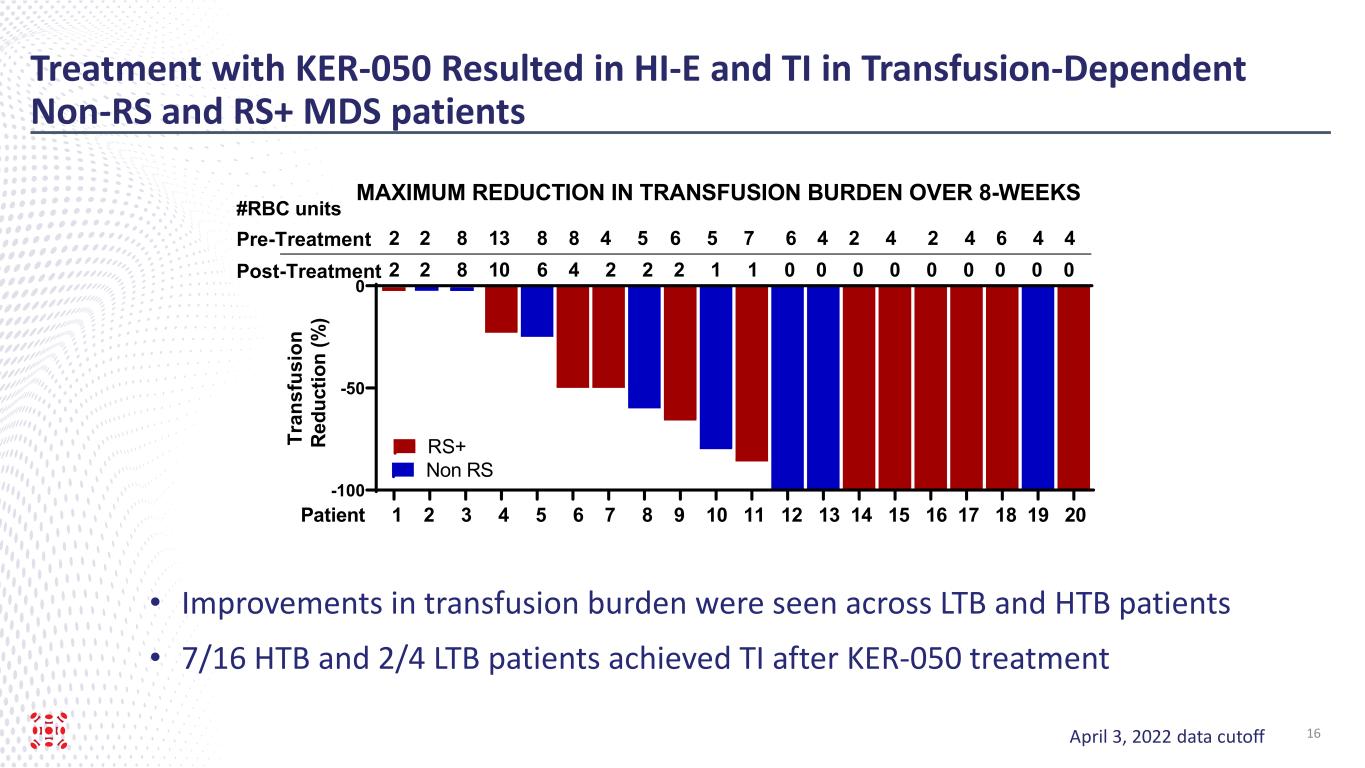
Treatment with KER-050 Resulted in HI-E and TI in Transfusion-Dependent Non-RS and RS+ MDS patients Post-Treatment -100 -50 0 Tr an sf us io n R ed uc tio n (% ) Patient 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 2 2 8 13 8 8 4 5 6 5 7 6 4 2 4 2 4 6 4 4 2 2 8 10 6 4 2 2 2 1 1 0 0 0 0 0 0 0 0 0 #RBC units RS+ Non RS Pre-Treatment MAXIMUM REDUCTION IN TRANSFUSION BURDEN OVER 8-WEEKS April 3, 2022 data cutoff • Improvements in transfusion burden were seen across LTB and HTB patients • 7/16 HTB and 2/4 LTB patients achieved TI after KER-050 treatment 16
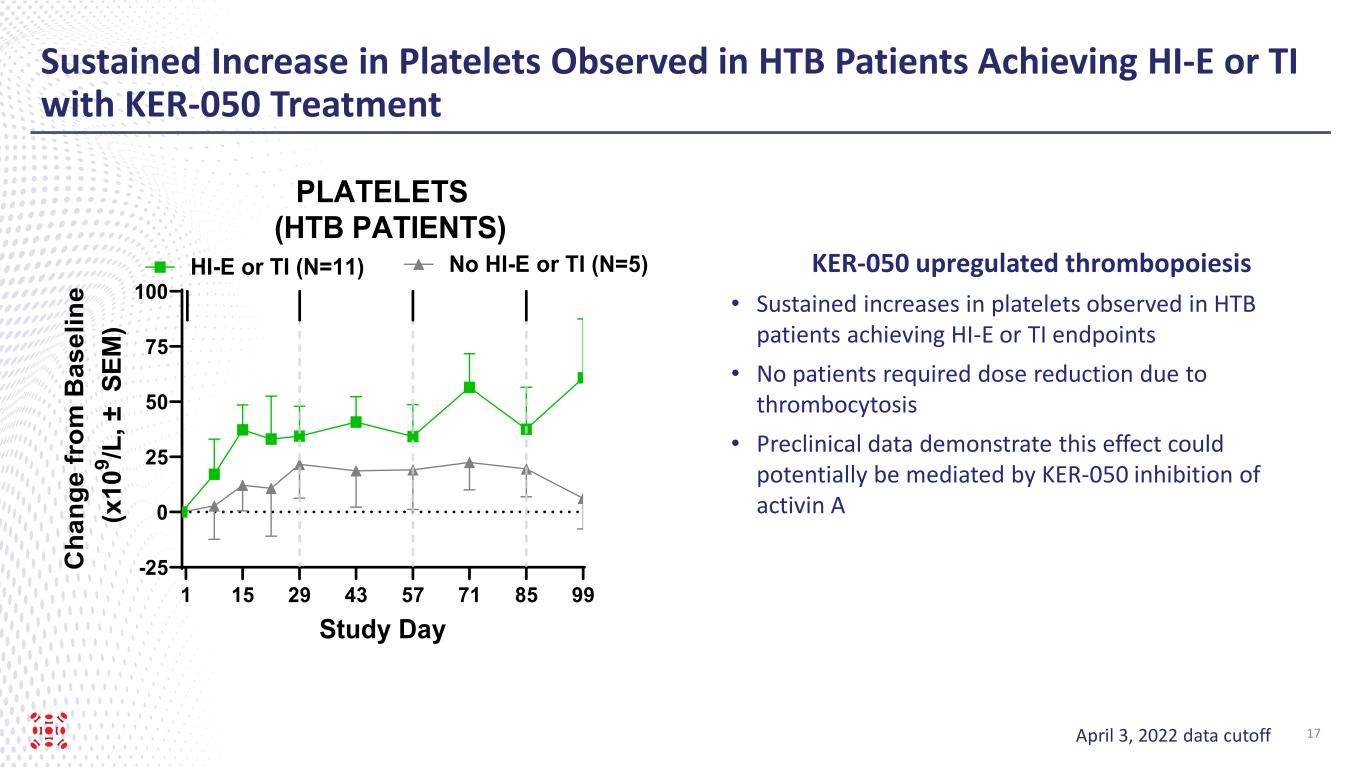
Sustained Increase in Platelets Observed in HTB Patients Achieving HI-E or TI with KER-050 Treatment 1 15 29 43 57 71 85 99 -25 0 25 50 75 100 PLATELETS (HTB PATIENTS) Study Day C ha ng e fr om B as el in e (x 10 9 /L , ± S EM ) HI-E or TI (N=11) No HI-E or TI (N=5) April 3, 2022 data cutoff KER-050 upregulated thrombopoiesis • Sustained increases in platelets observed in HTB patients achieving HI-E or TI endpoints • No patients required dose reduction due to thrombocytosis • Preclinical data demonstrate this effect could potentially be mediated by KER-050 inhibition of activin A 17
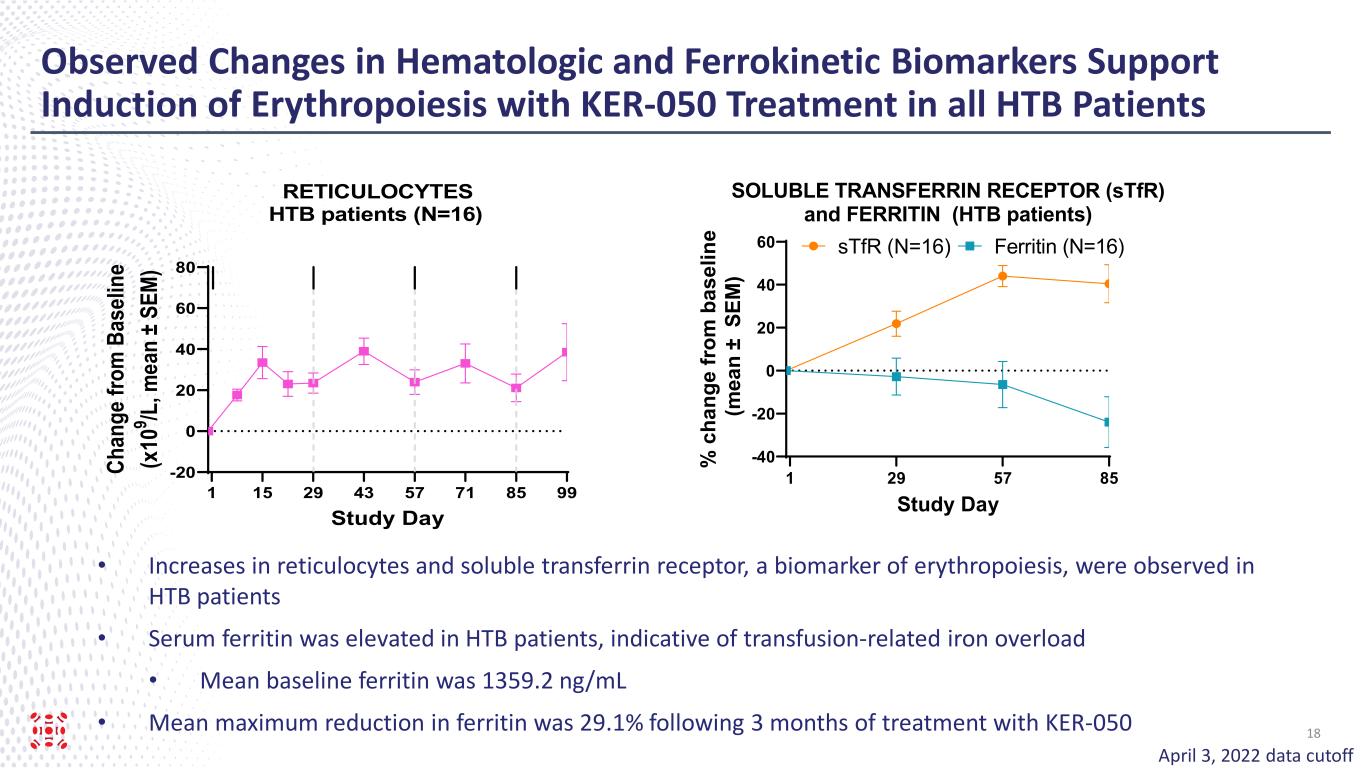
Observed Changes in Hematologic and Ferrokinetic Biomarkers Support Induction of Erythropoiesis with KER-050 Treatment in all HTB Patients 1 29 57 85 -40 -20 0 20 40 60 SOLUBLE TRANSFERRIN RECEPTOR (sTfR) and FERRITIN (HTB patients) Study Day % c ha ng e fr om b as el in e (m ea n ± S EM ) Ferritin (N=16)sTfR (N=16) April 3, 2022 data cutoff • Increases in reticulocytes and soluble transferrin receptor, a biomarker of erythropoiesis, were observed in HTB patients • Serum ferritin was elevated in HTB patients, indicative of transfusion-related iron overload • Mean baseline ferritin was 1359.2 ng/mL • Mean maximum reduction in ferritin was 29.1% following 3 months of treatment with KER-050 1 15 29 43 57 71 85 99 -20 0 20 40 60 80 Study Day Ch an ge fr om B as el in e (x 10 9 /L , m ea n ± S EM ) RETICULOCYTES HTB patients (N=16) 18

Summary • LR-MDS patients enrolled in Part 1 of this Phase 2 clinical trial were primarily transfusion-dependent with multilineage dysplasia • 58% of patients were HTB patients with elevated serum ferritin • KER-050 was generally well-tolerated as of data cut-off date at doses ranging from 0.75 to 5.0 mg/kg Q4W • No drug related SAEs or dose-limiting toxicities were observed • Observed PD effects in reticulocytes, soluble transferrin receptor and platelets support the proposed KER- 050 mechanism of increasing hematopoiesis • HI-E and transfusion independence have been observed in both RS+ and non-RS MDS patients treated with KER-050 across varying transfusion burdens, with 44% of HTB patients achieving TI during this 3- month treatment trial • Reductions in serum ferritin were also observed in HTB patients • These preliminary data support the potential of KER-050 as a treatment for multilineage cytopenias in LR- MDS, including difficult-to-treat HTB patients 19April 3, 2022 data cutoff
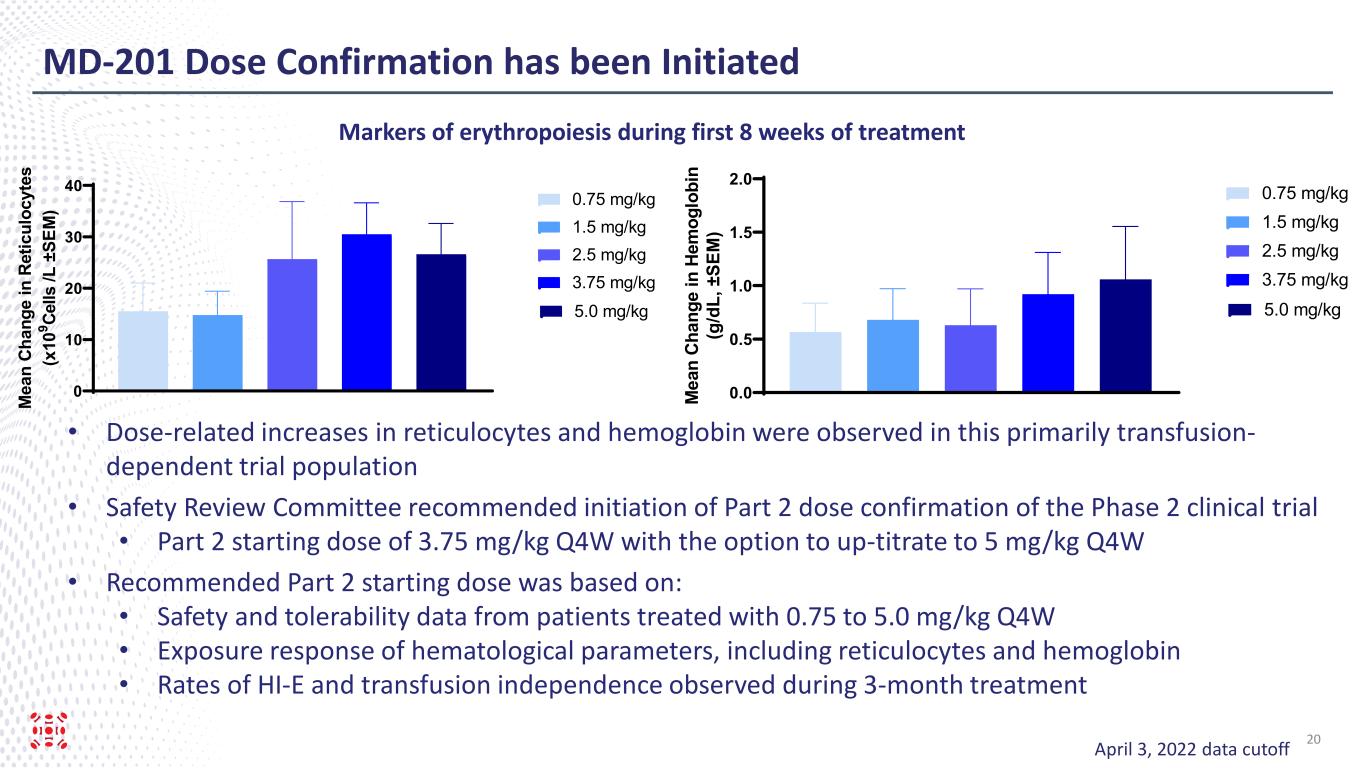
MD-201 Dose Confirmation has been Initiated April 3, 2022 data cutoff • Dose-related increases in reticulocytes and hemoglobin were observed in this primarily transfusion- dependent trial population • Safety Review Committee recommended initiation of Part 2 dose confirmation of the Phase 2 clinical trial • Part 2 starting dose of 3.75 mg/kg Q4W with the option to up-titrate to 5 mg/kg Q4W • Recommended Part 2 starting dose was based on: • Safety and tolerability data from patients treated with 0.75 to 5.0 mg/kg Q4W • Exposure response of hematological parameters, including reticulocytes and hemoglobin • Rates of HI-E and transfusion independence observed during 3-month treatment 0.0 0.5 1.0 1.5 2.0 M ea n Ch an ge in H em og lo bi n (g /d L, ± SE M ) 0.75 mg/kg 1.5 mg/kg 2.5 mg/kg 3.75 mg/kg 5.0 mg/kg 0 10 20 30 40 M ea n Ch an ge in R et ic ul oc yt es (x 10 9 Ce lls /L ± SE M ) 0.75 mg/kg 1.5 mg/kg 2.5 mg/kg 3.75 mg/kg 5.0 mg/kg Markers of erythropoiesis during first 8 weeks of treatment 20

Anticipated Key Milestones* KER-050 • Announce additional data from Phase 2 trial in MDS End 2022 • Announce initial data from Phase 2 trial in myelofibrosis End 2022 KER-047 • Initiate Phase 2 trial in IRIDA H1 2022 (initial data end of 2022) • Initiate Phase 2 trial in IDA Q3 2022 (initial data end of 2022) KER-012 • Announce additional data from Part 2 of Phase 1 trial H2 2022 21*Anticipated clinical milestones are subject to the impact of COVID-19 on our business.

Thank You 22





















