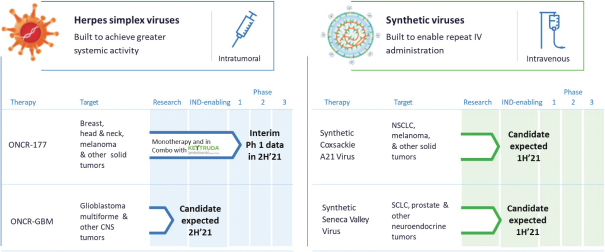
among other methods, pursuing and obtaining patent protection in the United States and in jurisdictions outside of the United States related to our proprietary technology, inventions, improvements, platforms and our product candidates that are important to the development and implementation of our business.
As of December 31, 2020, our patent portfolio consisted of 15 issued U.S. patents, 15 pending U.S. patent applications, and 12 issued foreign patents and approximately 105 pending foreign applications. These patents and patent applications include claims related to our platforms, products, methods, manufacturing processes, and potential future products and developments, with expected expiry dates not earlier than between 2023 and 2041.
oHSV Platform
As of December 31, 2020, our patent portfolio related to our oHSV Platform includes 14 owned or licensed patent families, which relate generally to the composition of our current and potential future products, and their methods of use.
We solely own six patent families, which include two issued U.S. patents, one allowed foreign application, five pending U.S. patent applications, and pending foreign counterparts in Europe, Asia, Canada, Australia, and Central and South America. One issued patent, which expires on January 27, 2037, includes claims directed at particular microRNA-attenuated HSV vectors, expression of certain therapeutic payloads, and their methods of use in the treatment of cancer. The other issued U.S. patent, which expires on June 30, 2037, includes claims directed at HSV vectors comprising particular combinations of certain therapeutic payloads. The pending applications include additional claims for microRNA-attenuated HSV vectors including the HSV vector utilized in our ONCR-177 product candidate, HSV vectors encoding particular therapeutic payloads, and their methods of use in the treatment of cancer. Patent applications are pending in these families in more than 17 jurisdictions worldwide, including Argentina, Australia, Brazil, Canada, China, Europe, Israel, India, Japan, Korea, Mexico, New Zealand, Russia, Singapore, Taiwan and South Africa. Any patents that may issue from these pending applications are expected to expire between 2037 and 2038, absent any patent term adjustments or extensions.
We have exclusively licensed from the University of Pittsburgh rights in three patent families related to oHSV Platform vectors, including certain glycoprotein modifications, a deletion of repeated HSV genes, certain micro-RNA-attenuated HSV vectors, and expression of certain therapeutic proteins from HSV vectors, and their methods of use. These patent families include six issued U.S. patents, seven issued patents in Australia, Europe, Israel, Japan, Russia and Singapore, three pending U.S. patent applications and 22 pending foreign applications pending in various jurisdictions worldwide, including Australia, Brazil, Canada, China, Europe, Israel, India, Japan, Korea, Mexico, New Zealand, Singapore and South Africa. Patents in these families are expected to expire between 2031 and 2037, absent any patent term adjustments or extensions. We have also exclusively licensed from Ospedale San Raffaele S.r.l. and Fondazione Telethon rights in one patent family related to micro-RNA attenuation of therapeutic payloads. This family includes six issued patents and seven pending applications in the U.S. and foreign jurisdictions. Patents in this family are expected to expire in 2026, absent any patent term adjustments or extensions. In addition, we have exclusively licensed from Northwestern University rights in one patent family related to mutations in the UL37 HSV gene. Patents in this family are expected to expire in 2036, absent any patent term adjustments or extensions. We have co-exclusively licensed from University of Chicago rights in two patent families related to HSV glycoprotein mutations. Patents in this family are expected to expire between 2023 and 2024, absent any patent term adjustments or extensions. Finally, we have also exclusively licensed from WuXi Biologics Ireland Limited rights in one patent family related to novel PD-1 antagonist sequences. This family includes six pending applications in the United States, China, Canada, Taiwan, Europe and Japan. Patents in this family are expected to expire in 2039, absent any patent term adjustments or extensions.
Synthetic Platform
As of December 31, 2020, our patent portfolio related to our Synthetic Platform includes five patent families, which relate generally to synthetic virus compositions and methods of use in the treatment of various cancers.
We solely own these five patent families. Two are pending provisional applications that will convert between February 2021 and November of 2021. One family currently has applications pending in the United States and in foreign jurisdictions including Australia, Brazil, Canada, China, Europe, Israel, India, Japan, Korea, Mexico, New Zealand, Singapore and South Africa. Two families have pending PCT applications that will enter national stages between May
124