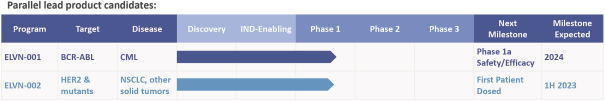
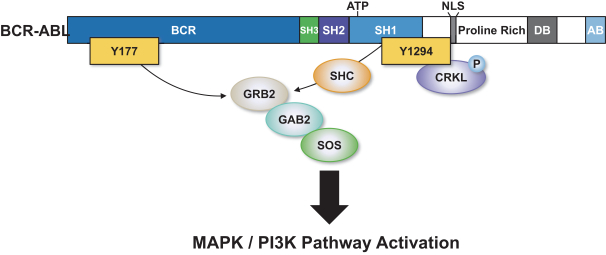
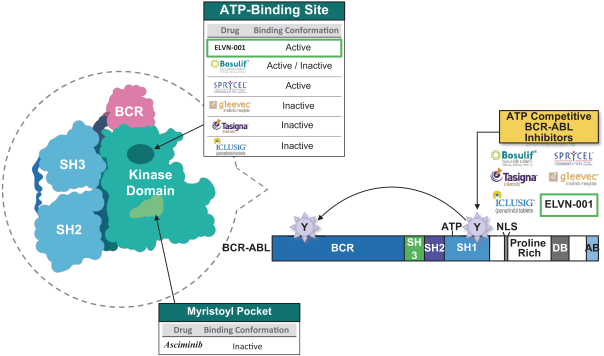
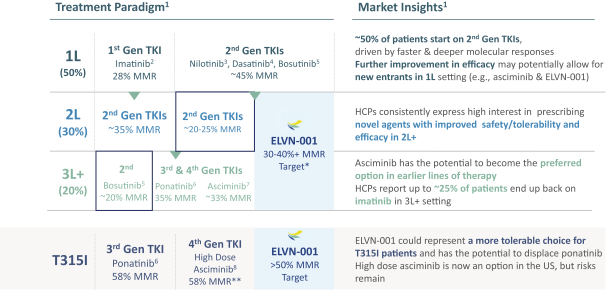
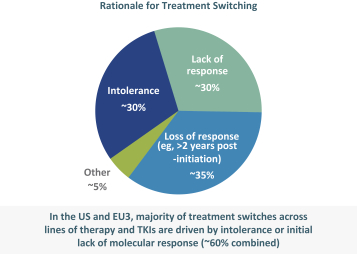
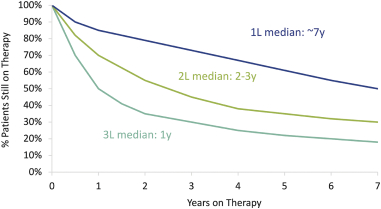
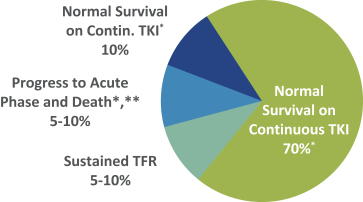
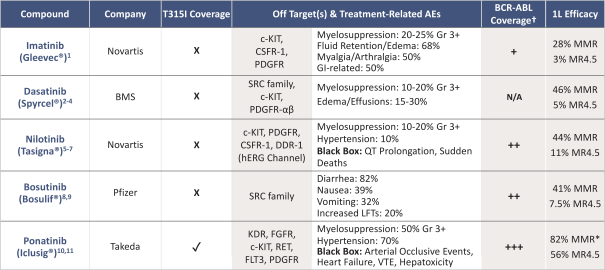
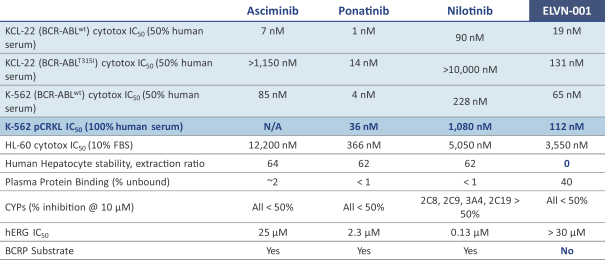
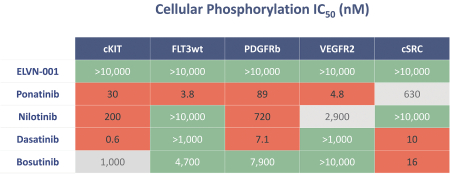
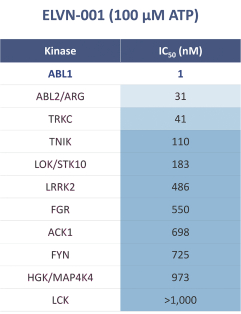
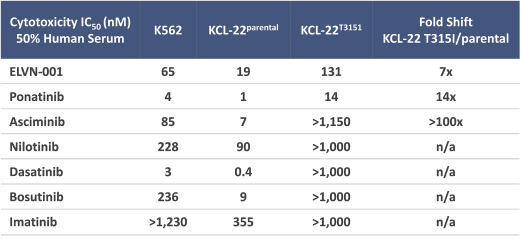
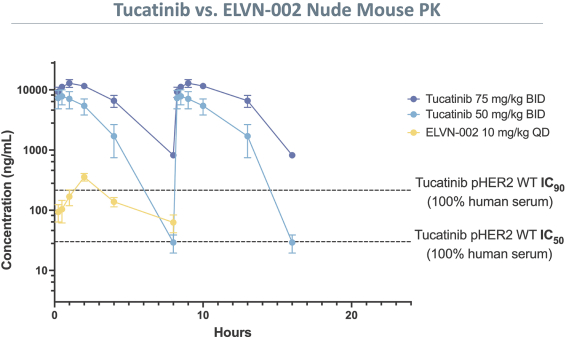
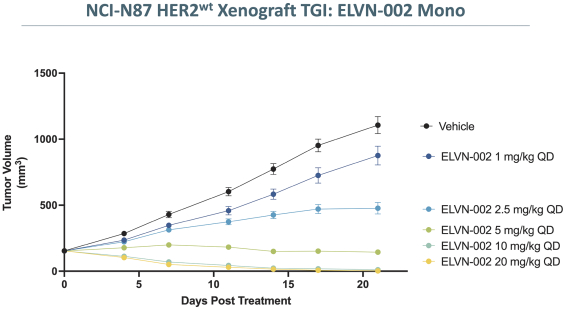
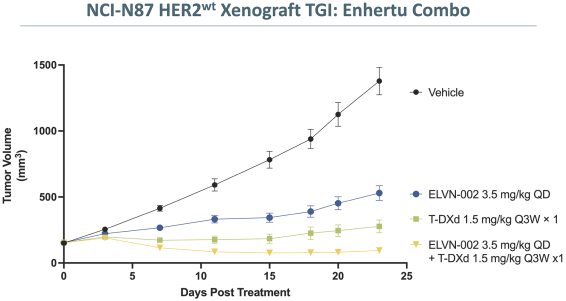
third-party CMOs will generally provide us with necessary quantities of API and drug product on a project-by-project basis based on our development needs.
As we advance our product candidates through development, we plan to explore adding backup suppliers for the API and drug product for each of our product candidates in order to protect against any potential supply disruptions.
Intellectual Property
Our commercial success depends in part on our ability to obtain and maintain proprietary or intellectual property protection for our product candidates, technology and know-how, to operate without infringing the proprietary or intellectual property rights of others and to prevent others from infringing our proprietary or intellectual property rights. We expect that we will seek to protect our proprietary and intellectual property position by, among other methods, pursuing and obtaining patent protection in the United States and in jurisdictions outside of the United States related to our proprietary technology, inventions, improvements and product candidates that are important to the development and implementation of our business. We may also rely on trade secrets, know-how, trademarks, continuing technological innovation and licensing opportunities to develop and maintain our proprietary and intellectual property position.
As of February 1, 2023, our patent portfolio includes pending patent applications that we own related to our HER2 and BCR-ABL programs. In total, as of that date for the HER2 and BCR-ABL programs, we owned one pending U.S. provisional patent application, six pending Patent Cooperation Treaty, or PCT, applications, three pending U.S. non-provisional patent applications, one pending non-provisional Argentinian patent application and one pending non-provisional Taiwanese patent application.
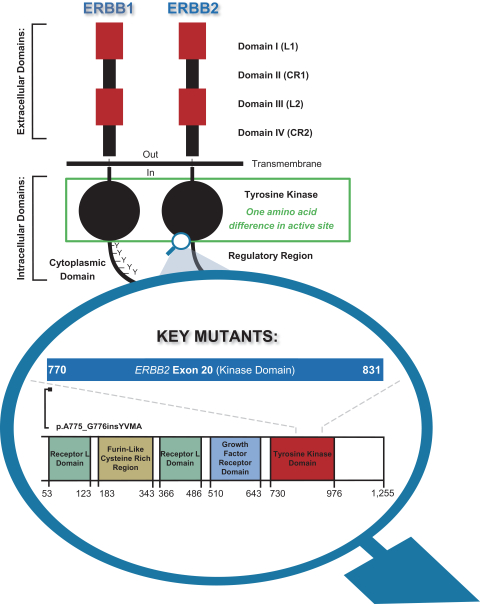
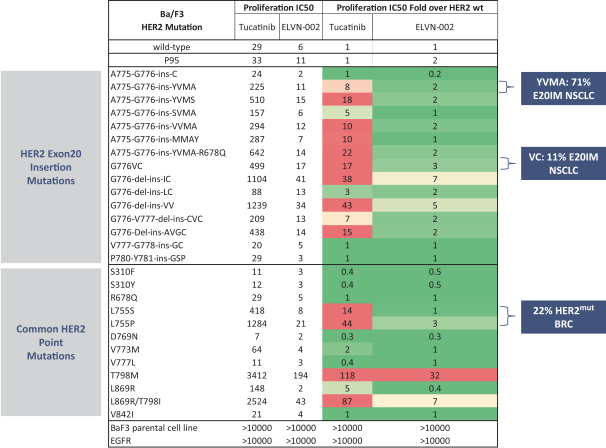
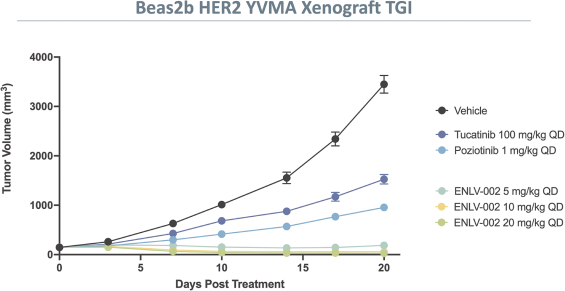
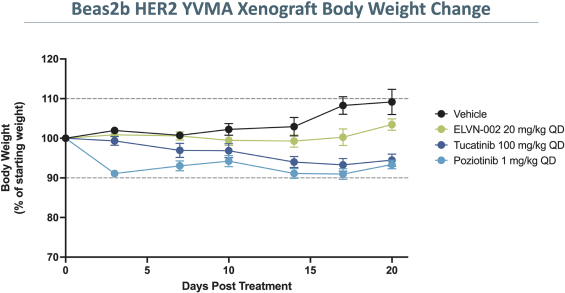
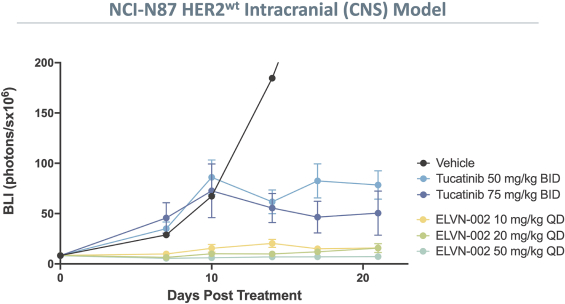
More specifically, with respect to our HER2 program, we own three pending PCT applications, two pending U.S. non-provisional patent applications, one pending U.S. provisional patent application, one pending non-provisional Argentinian patent application and one pending non-provisional Taiwanese patent application with claims directed to our HER2 selective inhibitory compounds as composition of matter, as well as claims directed to pharmaceutical compositions and combinations comprising such compounds and uses of such compounds, e.g., for treatment of cancers, such as NSCLC, including cancers associated with E20IMs. Any patents that may issue from our pending patent applications are expected to expire between 2041-2043, absent any patent term adjustments or patent term extensions for regulatory delay.
With respect to the BCR-ABL program, we own three pending PCT applications and one pending U.S. non-provisional patent application with claims directed to BCR-ABL tyrosine-kinase inhibitory compounds as composition of matter, as well as claims directed to pharmaceutical compositions and combinations comprising such compounds and uses of such compounds, e.g., treatment of CML, acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), or mixed phenotype acute leukemia, including refractory leukemias associated with a T315I mutation in BCR-ABL. Any patents that may issue from our pending patent applications are expected to expire in 2041 or 2042, absent any patent term adjustments or patent term extensions for regulatory delay.
The term of individual patents depends upon the legal term for patents in the countries in which they are granted. In most countries in which we file, the patent term is generally 20 years from the earliest date of filing a non-provisional patent application. In the United States, the patent term may, in certain cases, be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in examining and granting a patent or may be shortened if a patent is terminally disclaimed over a commonly owned patent or a patent naming a common inventor and having an earlier expiration date. Additionally, the Hatch-Waxman Act permits patent term extension of up to five years beyond the expiration date of a U.S. patent as partial compensation for the length of time a drug is under regulatory review while a patent that covers the drug is in force. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent applicable to each regulatory review period may be extended and only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended.
52