Exhibit 99.4
ENLIVEN MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
On February 23, 2023, Enliven Therapeutics, Inc. (formerly Imara Inc.) (the “Company”) completed its business combination with Enliven Inc. (formerly Enliven Therapeutics, Inc.) (“Enliven”) in accordance with the terms of Agreement and Plan of Merger, dated as of October 13, 2022 (the “Merger Agreement”), pursuant to which, subject to the terms and conditions thereof, a wholly owned subsidiary of Imara, Iguana Merger Sub, Inc. merged with and into Enliven, with Enliven surviving as a wholly owned subsidiary of the Company, and the surviving corporation of the merger (the “Merger”). Effective at 5:00 p.m. Eastern Time on February 23, 2023, the Company effected a 1-for-4 reverse stock split of its common stock (the “Reverse Stock Split”) and implemented a reduction in the number of authorized shares of common stock to 100,000,000 shares; effective at 5:01 p.m. Eastern Time, the Company completed the Merger; and effective at 5:02 p.m. Eastern Time, the Company changed its name to “Enliven Therapeutics, Inc.” Following the completion of the Merger, the business conducted by the Company became primarily the business conducted by Enliven, which is a clinical-stage biopharmaceutical company focused on the discovery and development of small molecule inhibitors to help patients with cancer. The references to share and per share amounts in this Exhibit 99.4 to the Company’s Current Report on Form 8-K do not reflect the Reverse Stock Split. The discussion in this Exhibit 99.4 to the Company’s Current Report on Form 8-K beginning with “Components of our Results of Operations” are as of September 30, 2022 for Enliven.
In this section, references to “we,” “our,” “us” and “our company” refer to Enliven.
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes appearing in Exhibits 99.5, 99.6 and 99.7 to the Company’s Current Report on Form 8-K of which this Exhibit 99.4 is a part. Some of the information contained in this discussion and analysis or set forth in the Company’s definitive proxy statement/prospectus filed with the Securities and Exchange Commission (the “SEC”) on January 23, 2023 (the “definitive proxy statement/prospectus”), including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks, uncertainties and assumptions. As a result of many factors, including those factors set forth in the “Risk Factors” in Exhibit 99.2 to the Company’s Current Report on Form 8-K of which this Exhibit 99.4 is a part, our actual results could differ materially from the results described in or implied by these forward-looking statements. You should carefully read the “Risk Factors” in Exhibit 99.2 to the Company’s Current Report on Form 8-K of which this Exhibit 99.4 is a part to gain an understanding of the factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section titled “Cautionary Statement Concerning Forward-Looking Statements and Market and Industry Data” in the definitive proxy statement/prospectus.
Capitalized terms not defined herein shall have the meaning granted to them in the definitive proxy statement/prospectus.
Overview
We are a clinical-stage biopharmaceutical company focused on the discovery and development of small molecule inhibitors to help patients with cancer live not only longer, but better. We aim to address existing and emerging unmet needs with a precision oncology approach that improves survival and enhances overall patient well-being. Our discovery process combines deep insights from clinically validated biological targets and differentiated chemistry with the goal of designing therapies for unmet needs. By combining clinically validated targets and specific TPPs with disciplined clinical trial design and regulatory strategy, we aim to develop drugs with an increased probability of clinical and commercial success. Clinically validated targets refers to biological targets that have demonstrated statistical significance on efficacy endpoints in published third-party clinical trials which we believe supports the development of our product candidates by increasing our probability of success. We have assembled a team of seasoned drug hunters with significant expertise in discovery and development of small molecule kinase inhibitors. Our team includes leading chemists who have been the primary or co-inventor of over 20 product candidates that have been advanced to clinical trials, including four FDA-approved products: Koselugo (selumetinib), Mektovi (binimetinib), Tukysa (tucatinib), and Retevmo (selpercatinib). We are currently advancing two parallel lead product candidates, ELVN-001 and ELVN-002, as well as pursuing several additional research stage opportunities that align with our development approach.
The following table summarizes our product candidate pipeline:
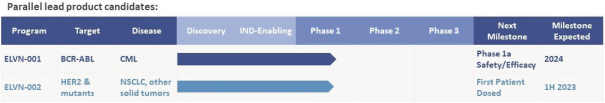
We were incorporated in the State of Delaware in June 2019 and are headquartered in Boulder, Colorado. Since our inception, we have devoted substantially all of our resources to research and development activities, including with respect to our BCR-ABL and HER2 programs and our other programs, business planning, establishing and maintaining our intellectual property portfolio, hiring personnel, raising capital, and providing general and administrative support for these activities.
We also do not own or operate, and currently have no plans to establish, any manufacturing facilities. We rely, and expect to continue to rely, on third parties for the manufacture of our product candidates for clinical and
1
