
THE COBALT-LYM STUDY OF CTX130: A PHASE 1 DOSE ESCALATION STUDY OF CD70-TARGETED ALLOGENEIC CRISPR-CAS9–ENGINEERED CAR T CELLS IN PATIENTS WITH RELAPSED/REFRACTORY (R/R) T-CELL MALIGNANCIES Swaminathan P. Iyer1, R. Alejandro Sica2, P. Joy Ho3, Boyu Hu4, Jasmine Zain5, Anca Prica6, Wen-Kai Weng7, �Youn H. Kim8, Michael S. Khodadoust9, M. Lia Palomba10, Francine M. Foss11, Kimberly Tipton12, �Erika L. Cullingford12, Qiuling He12, Anjali Sharma12, Steven M. Horwitz10 1Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, 2Department of Oncology, Montefiore Medical Center, Albert Einstein Cancer Center, Bronx, United States of America, 3Institute of Haematology, Royal Prince Alfred Hospital, Camperdown, Australia, 4Division of Hematology and Hematologic Malignancies, Huntsman Cancer Institute, Salt Lake City, 5Department of Hematology and Hematopoietic Cell Transplantation, City of Hope, Duarte, United States of America, 6Princess Margaret Cancer Centre, Toronto, Canada, 7Division of Blood and Marrow Transplantation and Cellular Therapy, 8Department of Dermatology, 9Division of Oncology, Department of Medicine, Stanford University School of Medicine, Stanford, 10Memorial Sloan Kettering Cancer Center, New York, 11Department of Dermatology, Yale School of Medicine, New Haven, 12CRISPR Therapeutics, Cambridge, United States of America Presented at the European Hematology Association Annual Meeting. 11 June 2022 Exhibit 99.3
Disclosures The COBALT™ – LYM study of CTX130TM is sponsored by CRISPR Therapeutics Dr. Swaminathan P. Iyer is a Professor, Lead of the T Cell Lymphoma Program, Department of Lymphoma/Myeloma, Division of Cancer Medicine at The University of Texas MD Anderson Cancer Center Dr. Iyer receives research support from CRISPR Therapeutics, Merck, Seagen, Rhizen, Acrotech, Legend, Innate Pharma, Astra Zeneca, Dren Bio, Yingli, and Secura Bio; participates in scientific advisory boards for Seagen, Yingli, and Secura Bio; and participates in Biocure’s and Targeted Oncology’s speaker bureaus as a speaker Presented at the European Hematology Association Annual Meeting. 11 June 2022
Overview PTCL and CTCL are complex diseases with significant unmet need and limited approved systemic therapies. Few therapies effectively treat all disease compartments (lymph nodes, skin, blood) or achieve meaningful CR rates. For patients with R/R PTCL and transformed CTCL, median OS is 1-2.5 and <5 years, respectively1-5 CTX130TM is a first-in-class, CD70-targeting allogeneic CAR T therapy that represents the first potential cell therapy for TCL patients. Allogeneic cellular therapy approaches for TCL have greater potential to meet the unmet need in this patient population given the patients’ own T cells are not suitable for autologous manufacturing6 CD70 is a ligand for CD27 with transient expression on activated lymphocytes and is highly expressed in many TCLs7-10 Preliminary data from dose escalation of CTX130 shows promising efficacy, including a 70% ORR and a 30% CR rate at DL≥3 (≥3x108 cells), with an acceptable safety profile CAR, chimeric antigen receptor; CR, complete response; CTCL, cutaneous T cell lymphoma; ORR, overall response rate; OS, overall survival; PTCL, peripheral T cell lymphoma; R/R, relapsed/refractory; TCL, T cell lymphoma. References: 1. Fleischer LC, et al. J Hematol Oncol. 2019;12:141. 2. Toki H, et al. Jpn J Clin Oncol. 1986;16:41-48.3. Lansigan F, et al. Acta Hematol. 2020;143:40-50. 4. Scarisbrick JJ, et al. J Clin Oncol. 2015;33:3766-3733. 5. Lansigan F, et al. Clin Lymphoma Myeloma Leuk. 2020;20:744-748. 6. Alcantara M, et al. Leukemia. 2018; 32, 2307–2315. 7. Wajant H. Expert Opin Ther Targets. 2016;20:959-973. 8. Hintzen RQ, et al. Int Immunol. 1994;6:477-480. 9. Lens SM, et al. Immunology. 1997;90:38-45. 10. Marques-Piubelli M, et al. Histopathology. 2022 Apr 26. doi: 10.1111/his.14670. Online ahead of print. Presented at the European Hematology Association Annual Meeting. 11 June 2022
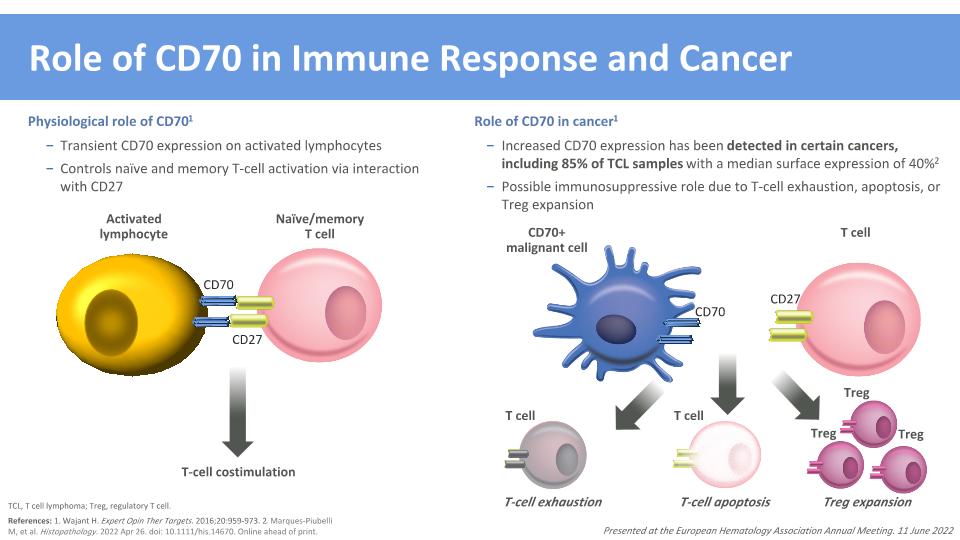
Role of CD70 in Immune Response and Cancer CD70+�malignant cell CD70 Physiological role of CD701 Transient CD70 expression on activated lymphocytes Controls naïve and memory T-cell activation via interaction with CD27 Role of CD70 in cancer1 Increased CD70 expression has been detected in certain cancers, including 85% of TCL samples with a median surface expression of 40%2 Possible immunosuppressive role due to T-cell exhaustion, apoptosis, or Treg expansion References: 1. Wajant H. Expert Opin Ther Targets. 2016;20:959-973. 2. Marques-Piubelli M, et al. Histopathology. 2022 Apr 26. doi: 10.1111/his.14670. Online ahead of print. TCL, T cell lymphoma; Treg, regulatory T cell. T-cell exhaustion Treg expansion T-cell apoptosis Activated lymphocyte CD70 CD27 Naïve/memory �T cell T-cell costimulation T cell T cell Treg Treg Treg T cell CD27 Presented at the European Hematology Association Annual Meeting. 11 June 2022
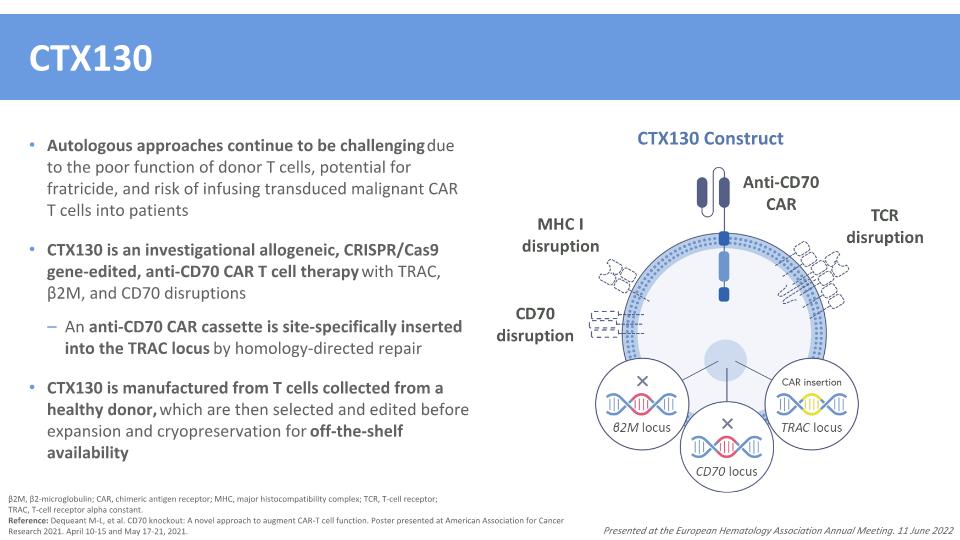
CTX130 Autologous approaches continue to be challenging due to the poor function of donor T cells, potential for fratricide, and risk of infusing transduced malignant CAR T cells into patients CTX130 is an investigational allogeneic, CRISPR/Cas9 gene-edited, anti-CD70 CAR T cell therapy with TRAC, β2M, and CD70 disruptions An anti-CD70 CAR cassette is site-specifically inserted into the TRAC locus by homology-directed repair CTX130 is manufactured from T cells collected from a healthy donor, which are then selected and edited before expansion and cryopreservation for off-the-shelf availability β2M, β2-microglobulin; CAR, chimeric antigen receptor; MHC, major histocompatibility complex; TCR, T-cell receptor; �TRAC, T-cell receptor alpha constant. Reference: Dequeant M-L, et al. CD70 knockout: A novel approach to augment CAR-T cell function. Poster presented at American Association for Cancer Research 2021. April 10-15 and May 17-21, 2021. Presented at the European Hematology Association Annual Meeting. 11 June 2022 CTX130 Construct
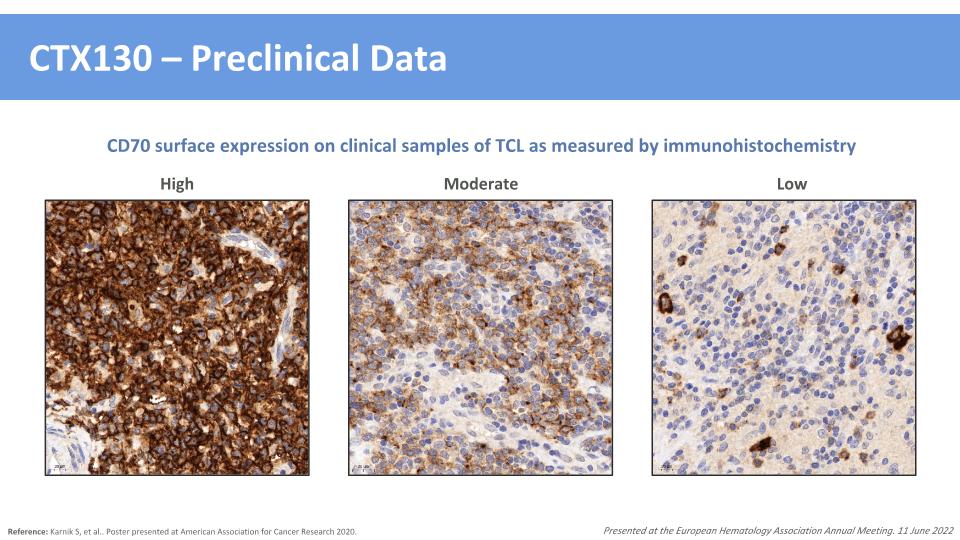
CTX130 – Preclinical Data Reference: Karnik S, et al.. Poster presented at American Association for Cancer Research 2020. Presented at the European Hematology Association Annual Meeting. 11 June 2022 High Moderate Low CD70 surface expression on clinical samples of TCL as measured by immunohistochemistry
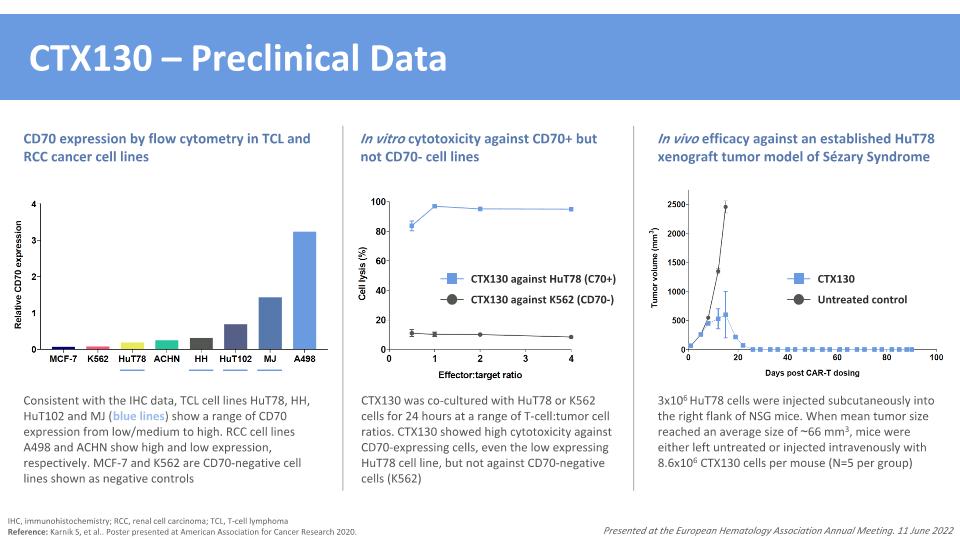
CTX130 – Preclinical Data IHC, immunohistochemistry; RCC, renal cell carcinoma; TCL, T-cell lymphoma Reference: Karnik S, et al.. Poster presented at American Association for Cancer Research 2020. Presented at the European Hematology Association Annual Meeting. 11 June 2022 Consistent with the IHC data, TCL cell lines HuT78, HH, HuT102 and MJ (blue lines) show a range of CD70 expression from low/medium to high. RCC cell lines A498 and ACHN show high and low expression, respectively. MCF-7 and K562 are CD70-negative cell lines shown as negative controls 3x106 HuT78 cells were injected subcutaneously into the right flank of NSG mice. When mean tumor size reached an average size of ~66 mm3, mice were either left untreated or injected intravenously with 8.6x106 CTX130 cells per mouse (N=5 per group) CTX130 Untreated control In vivo efficacy against an established HuT78 xenograft tumor model of Sézary Syndrome CTX130 was co-cultured with HuT78 or K562 cells for 24 hours at a range of T-cell:tumor cell ratios. CTX130 showed high cytotoxicity against CD70-expressing cells, even the low expressing HuT78 cell line, but not against CD70-negative cells (K562) CTX130 against HuT78 (C70+) CTX130 against K562 (CD70-) In vitro cytotoxicity against CD70+ but not CD70- cell lines CD70 expression by flow cytometry in TCL and RCC cancer cell lines
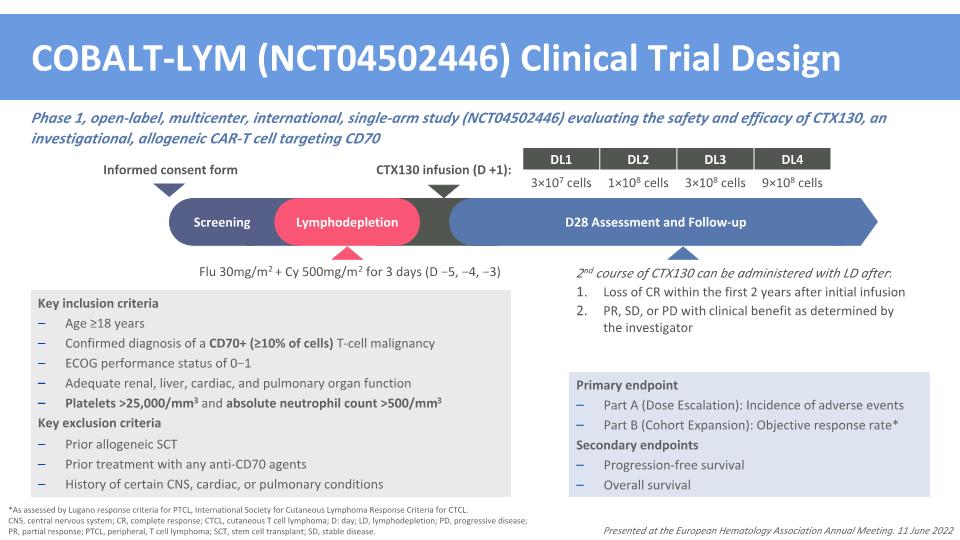
COBALT-LYM (NCT04502446) Clinical Trial Design Phase 1, open-label, multicenter, international, single-arm study (NCT04502446) evaluating the safety and efficacy of CTX130, an investigational, allogeneic CAR-T cell targeting CD70 *As assessed by Lugano response criteria for PTCL, International Society for Cutaneous Lymphoma Response Criteria for CTCL. CNS, central nervous system; CR, complete response; CTCL, cutaneous T cell lymphoma; D: day; LD, lymphodepletion; PD, progressive disease; PR, partial response; PTCL, peripheral, T cell lymphoma; SCT, stem cell transplant; SD, stable disease. Primary endpoint Part A (Dose Escalation): Incidence of adverse events Part B (Cohort Expansion): Objective response rate* Secondary endpoints Progression-free survival Overall survival Key inclusion criteria Age ≥18 years Confirmed diagnosis of a CD70+ (≥10% of cells) T-cell malignancy ECOG performance status of 0−1 Adequate renal, liver, cardiac, and pulmonary organ function Platelets >25,000/mm3 and absolute neutrophil count >500/mm3 Key exclusion criteria Prior allogeneic SCT Prior treatment with any anti-CD70 agents History of certain CNS, cardiac, or pulmonary conditions Flu 30mg/m2 + Cy 500mg/m2 for 3 days (D −5, −4, −3) DL1 DL2 DL3 DL4 3×107 cells 1×108 cells 3×108 cells 9×108 cells CTX130 infusion (D +1): Screening Lymphodepletion D28 Assessment and Follow-up Informed consent form 2nd course of CTX130 can be administered with LD after: Loss of CR within the first 2 years after initial infusion PR, SD, or PD with clinical benefit as determined by the investigator Presented at the European Hematology Association Annual Meeting. 11 June 2022
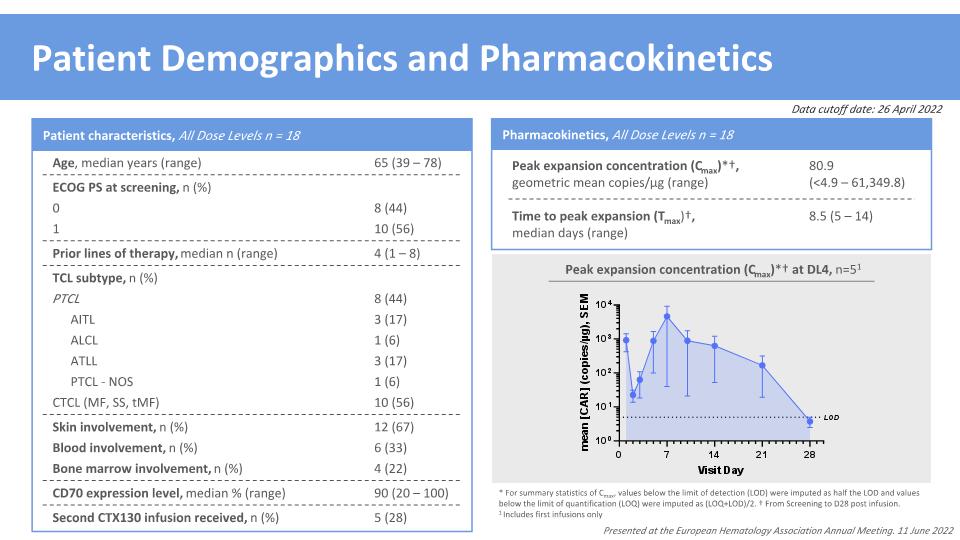
Time to peak expansion (Tmax)†, median days (range) 8.5 (5 – 14) * For summary statistics of Cmax, values below the limit of detection (LOD) were imputed as half the LOD and values below the limit of quantification (LOQ) were imputed as (LOQ+LOD)/2. † From Screening to D28 post infusion. 1 Includes first infusions only Patient Demographics and Pharmacokinetics Pharmacokinetics, All Dose Levels n = 18 Peak expansion concentration (Cmax)*†, geometric mean copies/μg (range) 80.9 �(<4.9 – 61,349.8) Peak expansion concentration (Cmax)*† at DL4, n=51 Presented at the European Hematology Association Annual Meeting. 11 June 2022 CD70 expression level, median % (range) 90 (20 – 100) Bone marrow involvement, n (%) 4 (22) Patient characteristics, All Dose Levels n = 18 Age, median years (range) 65 (39 – 78) ECOG PS at screening, n (%) Prior lines of therapy, median n (range) 4 (1 – 8) TCL subtype, n (%) Blood involvement, n (%) 6 (33) 0 8 (44) 1 10 (56) PTCL 8 (44) AITL 3 (17) ALCL 1 (6) ATLL 3 (17) PTCL - NOS 1 (6) CTCL (MF, SS, tMF) 10 (56) Skin involvement, n (%) 12 (67) Second CTX130 infusion received, n (%) 5 (28) Data cutoff date: 26 April 2022
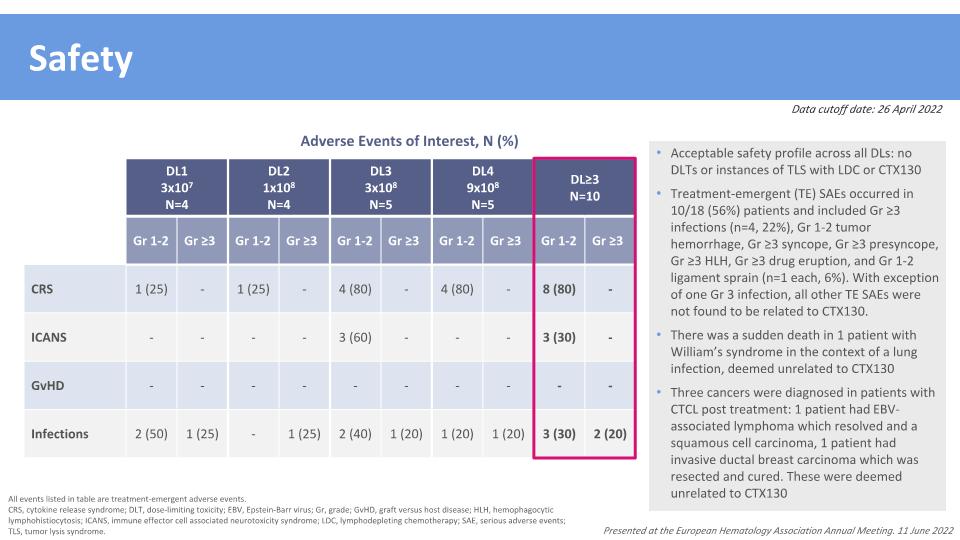
Safety All events listed in table are treatment-emergent adverse events. CRS, cytokine release syndrome; DLT, dose-limiting toxicity; EBV, Epstein-Barr virus; Gr, grade; GvHD, graft versus host disease; HLH, hemophagocytic lymphohistiocytosis; ICANS, immune effector cell associated neurotoxicity syndrome; LDC, lymphodepleting chemotherapy; SAE, serious adverse events; TLS, tumor lysis syndrome. Data cutoff date: 26 April 2022 DL1 3x107 N=4 DL2 1x108 N=4 DL3 3x108 N=5 DL4 9x108 N=5 DL≥3 N=10 Gr 1-2 Gr ≥3 Gr 1-2 Gr ≥3 Gr 1-2 Gr ≥3 Gr 1-2 Gr ≥3 Gr 1-2 Gr ≥3 CRS 1 (25) - 1 (25) - 4 (80) - 4 (80) - 8 (80) - ICANS - - - - 3 (60) - - - 3 (30) - GvHD - - - - - - - - - - Infections 2 (50) 1 (25) - 1 (25) 2 (40) 1 (20) 1 (20) 1 (20) 3 (30) 2 (20) Adverse Events of Interest, N (%) Acceptable safety profile across all DLs: no DLTs or instances of TLS with LDC or CTX130 Treatment-emergent (TE) SAEs occurred in 10/18 (56%) patients and included Gr ≥3 infections (n=4, 22%), Gr 1-2 tumor hemorrhage, Gr ≥3 syncope, Gr ≥3 presyncope, Gr ≥3 HLH, Gr ≥3 drug eruption, and Gr 1-2 ligament sprain (n=1 each, 6%). With exception of one Gr 3 infection, all other TE SAEs were not found to be related to CTX130. There was a sudden death in 1 patient with William’s syndrome in the context of a lung infection, deemed unrelated to CTX130 Three cancers were diagnosed in patients with CTCL post treatment: 1 patient had EBV-associated lymphoma which resolved and a squamous cell carcinoma, 1 patient had invasive ductal breast carcinoma which was resected and cured. These were deemed unrelated to CTX130 Presented at the European Hematology Association Annual Meeting. 11 June 2022
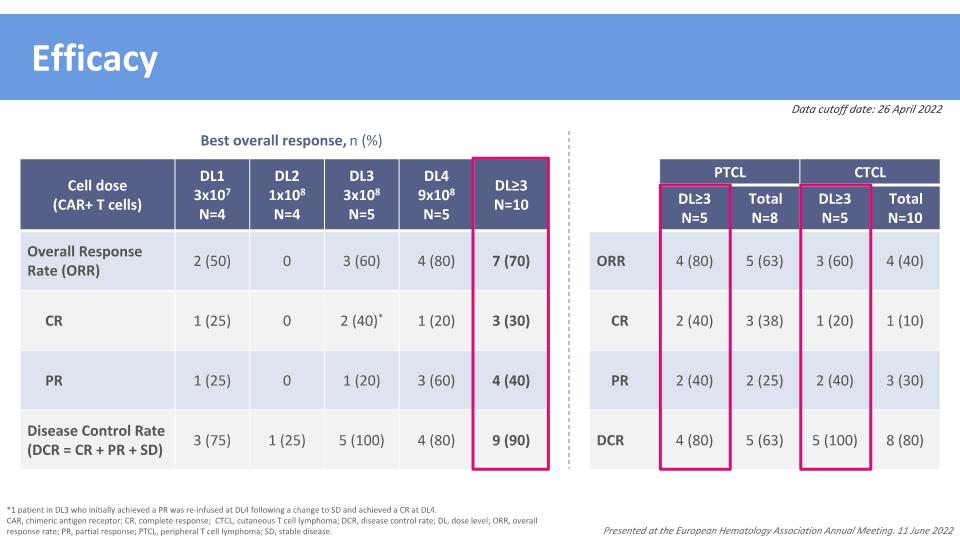
Efficacy Cell dose (CAR+ T cells) DL1 3x107 N=4 DL2 1x108 N=4 DL3 3x108 N=5 DL4 9x108 N=5 DL≥3 N=10 Overall Response Rate (ORR) 2 (50) 0 3 (60) 4 (80) 7 (70) CR 1 (25) 0 2 (40)* 1 (20) 3 (30) PR 1 (25) 0 1 (20) 3 (60) 4 (40) Disease Control Rate �(DCR = CR + PR + SD) 3 (75) 1 (25) 5 (100) 4 (80) 9 (90) *1 patient in DL3 who initially achieved a PR was re-infused at DL4 following a change to SD and achieved a CR at DL4. CAR, chimeric antigen receptor; CR, complete response; CTCL, cutaneous T cell lymphoma; DCR, disease control rate; DL, dose level; ORR, overall response rate; PR, partial response; PTCL, peripheral T cell lymphoma; SD, stable disease. Best overall response, n (%) PTCL DL2 N=X CTCL DL≥3 N=5 Total N=8 DL≥3 N=5 Total N=10 ORR 4 (80) 5 (63) 3 (60) 4 (40) CR 2 (40) 3 (38) 1 (20) 1 (10) PR 2 (40) 2 (25) 2 (40) 3 (30) DCR 4 (80) 5 (63) 5 (100) 8 (80) Data cutoff date: 26 April 2022 Presented at the European Hematology Association Annual Meeting. 11 June 2022
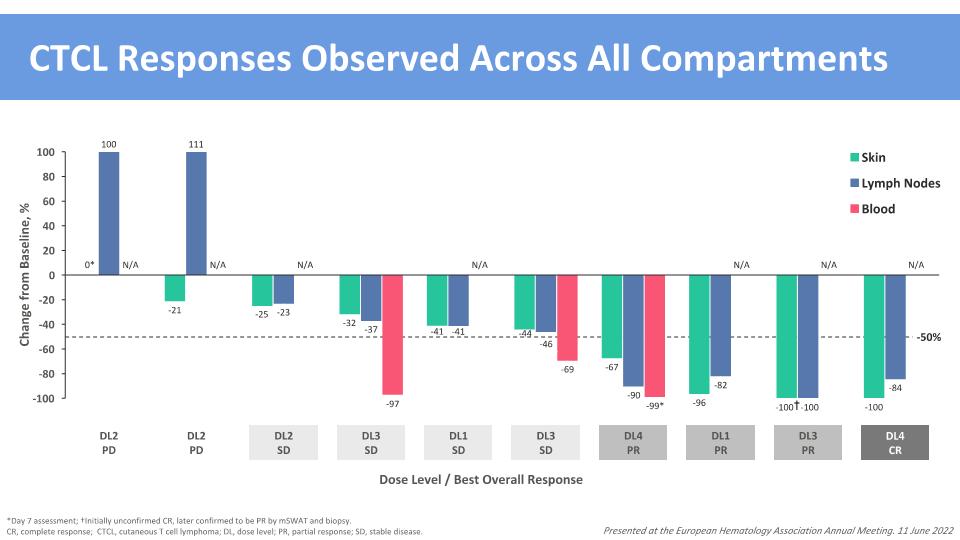
† CTCL Responses Observed Across All Compartments *Day 7 assessment; †Initially unconfirmed CR, later confirmed to be PR by mSWAT and biopsy. CR, complete response; CTCL, cutaneous T cell lymphoma; DL, dose level; PR, partial response; SD, stable disease. Dose Level / Best Overall Response 111 100 -50% Presented at the European Hematology Association Annual Meeting. 11 June 2022 0* -25 -23 -32 -99* -90 -67 -69 -46 -44 -41 -41 -97 -37 -96 -82 -100 -100 -100 -84 -21 N/A N/A N/A N/A N/A N/A N/A DL4 CR
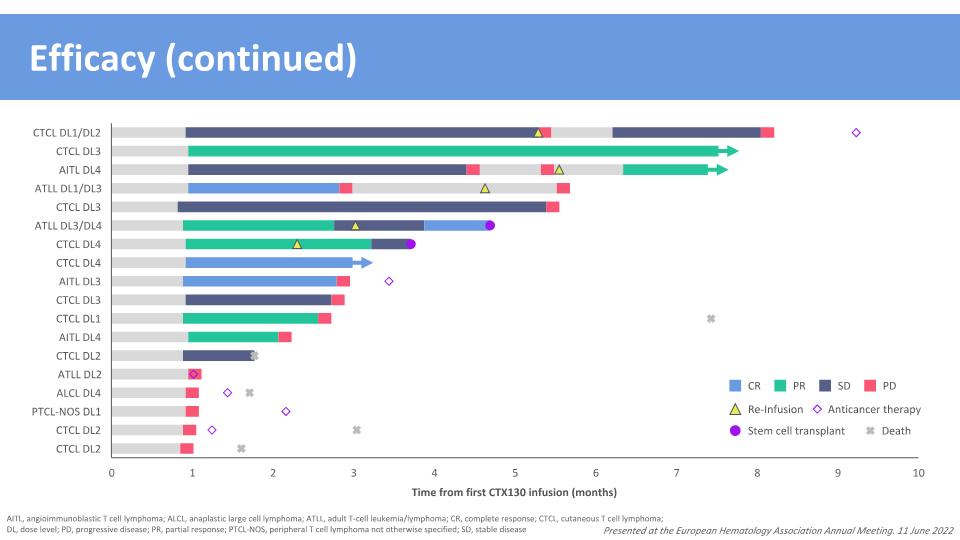
Efficacy (continued) Presented at the European Hematology Association Annual Meeting. 11 June 2022 Time from first CTX130 infusion (months) CR SD PD PR Re-Infusion Death Stem cell transplant Anticancer therapy AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; CR, complete response; CTCL, cutaneous T cell lymphoma; DL, dose level; PD, progressive disease; PR, partial response; PTCL-NOS, peripheral T cell lymphoma not otherwise specified; SD, stable disease
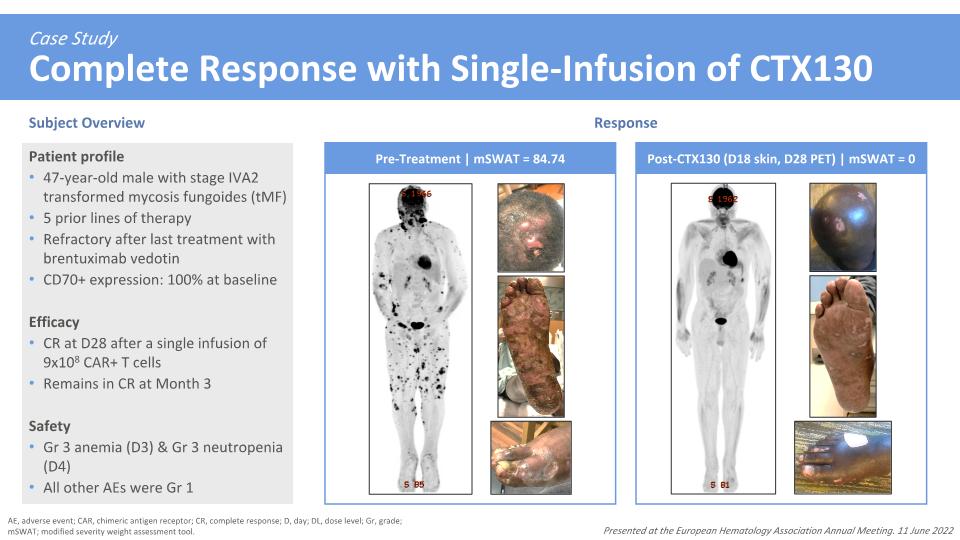
Case Study�Complete Response with Single-Infusion of CTX130 Subject Overview Response AE, adverse event; CAR, chimeric antigen receptor; CR, complete response; D, day; DL, dose level; Gr, grade; mSWAT; modified severity weight assessment tool. Patient profile 47-year-old male with stage IVA2 transformed mycosis fungoides (tMF) 5 prior lines of therapy Refractory after last treatment with brentuximab vedotin CD70+ expression: 100% at baseline Efficacy CR at D28 after a single infusion of 9x108 CAR+ T cells Remains in CR at Month 3 Safety Gr 3 anemia (D3) & Gr 3 neutropenia (D4) All other AEs were Gr 1 Presented at the European Hematology Association Annual Meeting. 11 June 2022 Pre-Treatment | mSWAT = 84.74 Post-CTX130 (D18 skin, D28 PET) | mSWAT = 0
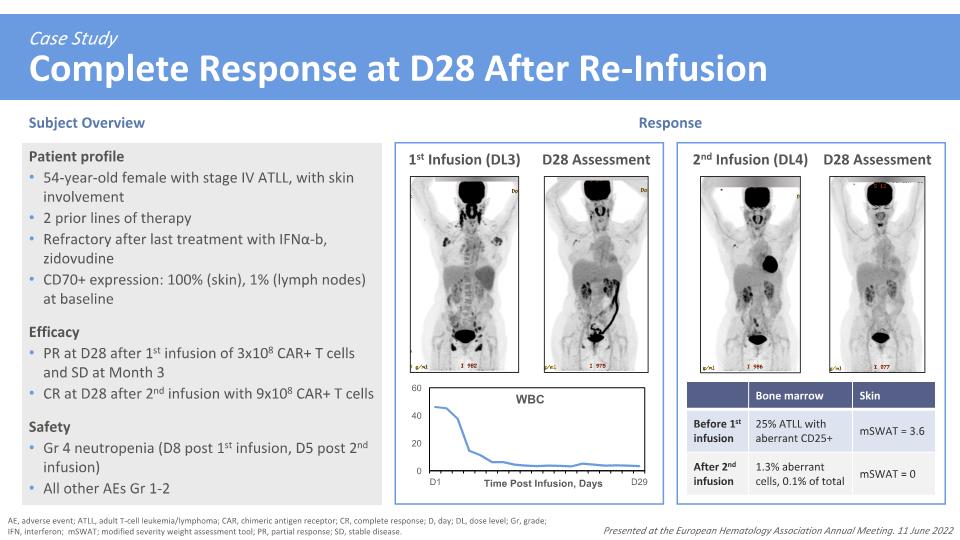
Case Study�Complete Response at D28 After Re-Infusion AE, adverse event; ATLL, adult T-cell leukemia/lymphoma; CAR, chimeric antigen receptor; CR, complete response; D, day; DL, dose level; Gr, grade; IFN, interferon; mSWAT; modified severity weight assessment tool; PR, partial response; SD, stable disease. Presented at the European Hematology Association Annual Meeting. 11 June 2022 Patient profile 54-year-old female with stage IV ATLL, with skin involvement 2 prior lines of therapy Refractory after last treatment with IFNα-b, zidovudine CD70+ expression: 100% (skin), 1% (lymph nodes) at baseline Efficacy PR at D28 after 1st infusion of 3x108 CAR+ T cells and SD at Month 3 CR at D28 after 2nd infusion with 9x108 CAR+ T cells Safety Gr 4 neutropenia (D8 post 1st infusion, D5 post 2nd infusion) All other AEs Gr 1-2 1st Infusion (DL3) D28 Assessment 2nd Infusion (DL4) D28 Assessment Subject Overview Response Bone marrow Skin Before 1st infusion 25% ATLL with aberrant CD25+ mSWAT = 3.6 After 2nd infusion 1.3% aberrant cells, 0.1% of total mSWAT = 0
Acknowledgments Thank you to all the patients, families and investigators involved with the COBALT-LYM Study This study was sponsored by CRISPR Therapeutics COBALT-LYM (NCT04502446) Study Sites Presented at the European Hematology Association Annual Meeting. 11 June 2022















