
Top-Line Results from the Phase 1 CARBON Trial of CTX110™ October 21, 2020 ® Exhibit 99.2

The presentation and other related materials may contain a number of “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding CRISPR Therapeutics’ expectations about any or all of the following: (i) the safety, efficacy and clinical progress of our various clinical programs; (ii) the status of clinical trials (including, without limitation, the expected timing of data releases, announcement of additional programs and activities at clinical trial sites) and expectations regarding the data that is being presented; (iii) the data that will be generated by ongoing and planned clinical trials, and the ability to use that data for the design and initiation of further clinical trials; and (iv) the therapeutic value, development, and commercial potential of CRISPR/Cas9 gene editing technologies and therapies, including as compared to other therapies. Without limiting the foregoing, the words “believes,” “anticipates,” “plans,” “expects” and similar expressions are intended to identify forward-looking statements. You are cautioned that forward-looking statements are inherently uncertain. Although CRISPR Therapeutics believes that such statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those projected or suggested in the forward-looking statements due to various risks and uncertainties. These risks and uncertainties include, among others: the potential for initial and preliminary data from any clinical trial and initial data from a limited number of patients not to be indicative of final trial results; the potential that clinical trial results may not be favorable; potential impacts due to the coronavirus pandemic, such as the timing and progress of clinical trials; that future competitive or other market factors may adversely affect the commercial potential for CRISPR Therapeutics’ product candidates; uncertainties regarding the intellectual property protection for CRISPR Therapeutics’ technology and intellectual property belonging to third parties, and the outcome of proceedings (such as an interference, an opposition or a similar proceeding) involving all or any portion of such intellectual property; and those risks and uncertainties described under the heading "Risk Factors" in CRISPR Therapeutics’ most recent annual report on Form 10-K, quarterly report on Form 10-Q and in any other subsequent filings made by CRISPR Therapeutics with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. CRISPR Therapeutics disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this presentation, other than to the extent required by law. CRISPR THERAPEUTICS® standard character mark and design logo, CTX001™, CTX110™, CTX120™, and CTX130™ are trademarks and registered trademarks of CRISPR Therapeutics AG. All other trademarks and registered trademarks are the property of their respective owners. Forward-Looking Statements

Presenters on Today’s Call Samarth Kulkarni, PhD Chief Executive Officer CRISPR Therapeutics Tony Ho, MD Executive Vice President, Head of Research & Development CRISPR Therapeutics Ewelina Morawa, MD Vice President, Clinical Development CRISPR Therapeutics Joseph McGuirk, DO Professor of Medicine and Division Director of Hematologic Malignancies and Cellular Therapeutics University of Kansas Medical Center
Built the leading CRISPR company: 4 programs in the clinic; >300 employees; >$1B cash balance Demonstrated, for the first time, the power of CRISPR gene editing in rare diseases: initial data with CTX001™ supportive of a potential functional cure for sickle cell disease and beta thalassemia Advanced three gene-edited allogeneic CAR-T programs into the clinic across four trials In parallel, expanded into regenerative medicine and progressed our in vivo efforts Created a sustainable innovation engine with pre-eminent capabilities And today, we show the promise and potential of CRISPR-edited cell therapies in the fight against cancer We Have Made Tremendous Progress Over the Past 5 Years
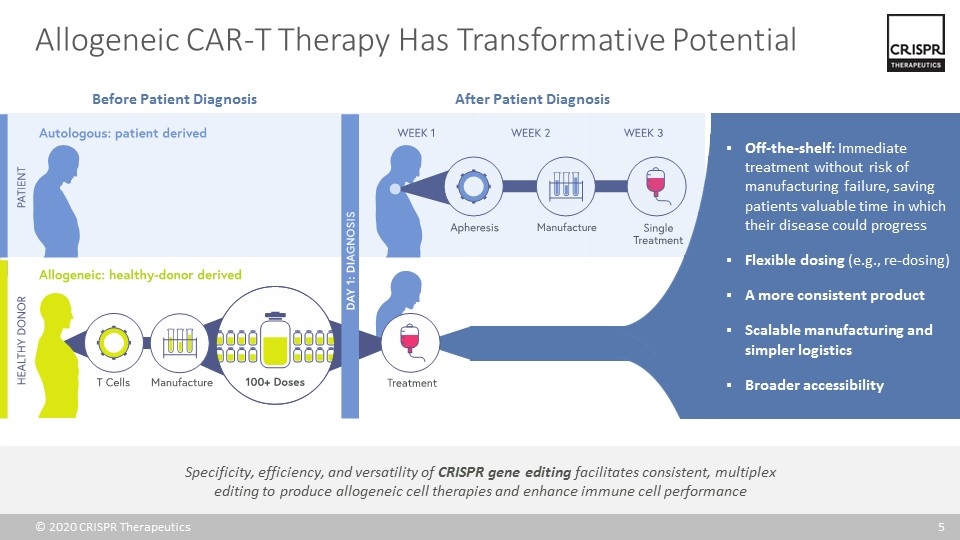
Allogeneic CAR-T Therapy Has Transformative Potential Specificity, efficiency, and versatility of CRISPR gene editing facilitates consistent, multiplex editing to produce allogeneic cell therapies and enhance immune cell performance Before Patient Diagnosis After Patient Diagnosis Off-the-shelf: Immediate treatment without risk of manufacturing failure, saving patients valuable time in which their disease could progress Flexible dosing (e.g., re-dosing) A more consistent product Scalable manufacturing and simpler logistics Broader accessibility
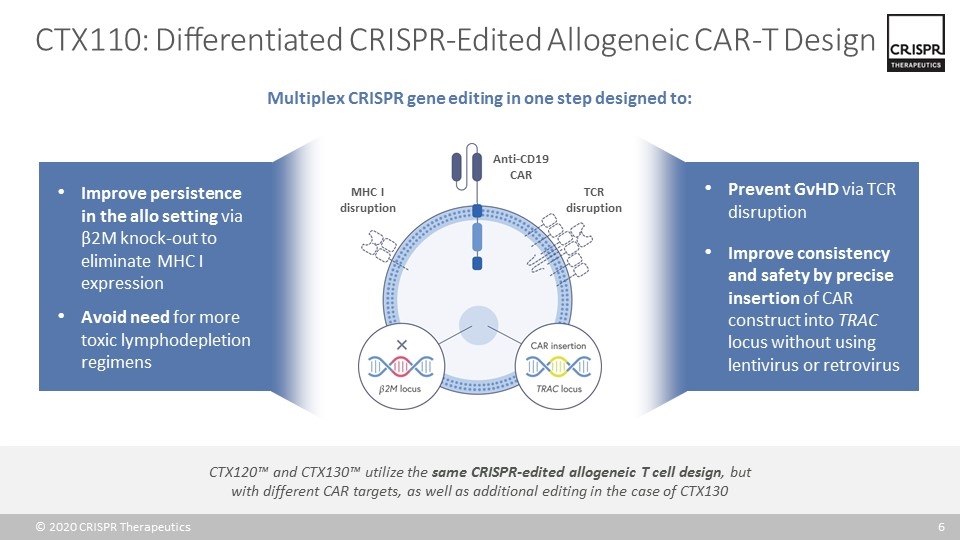
CTX110: Differentiated CRISPR-Edited Allogeneic CAR-T Design Multiplex CRISPR gene editing in one step designed to: Anti-CD19 CAR TCR disruption MHC I disruption Prevent GvHD via TCR disruption Improve consistency and safety by precise insertion of CAR construct into TRAC locus without using lentivirus or retrovirus Improve persistence in the allo setting via β2M knock-out to eliminate MHC I expression Avoid need for more toxic lymphodepletion regimens CTX120™ and CTX130™ utilize the same CRISPR-edited allogeneic T cell design, but with different CAR targets, as well as additional editing in the case of CTX130
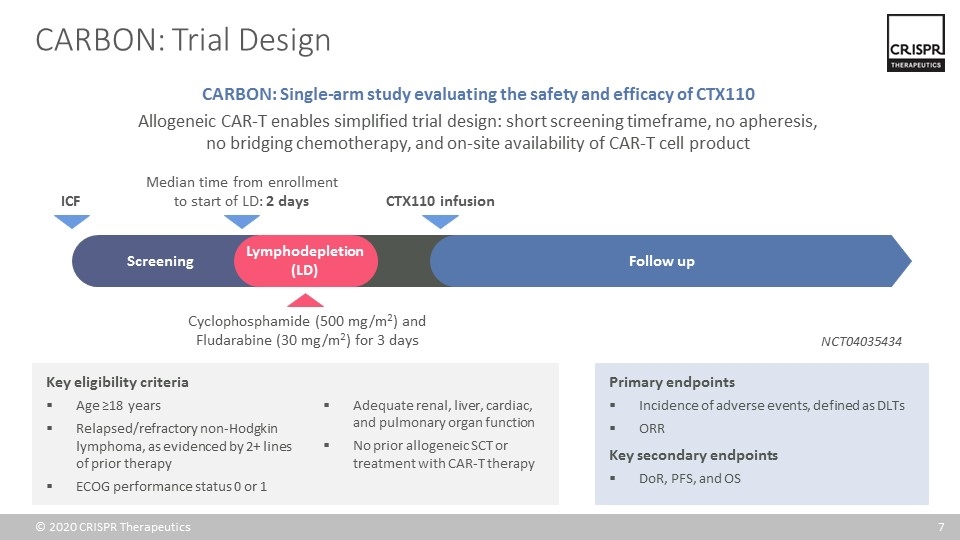
CARBON: Trial Design Key eligibility criteria Age ≥18 years Relapsed/refractory non-Hodgkin lymphoma, as evidenced by 2+ lines of prior therapy ECOG performance status 0 or 1 Adequate renal, liver, cardiac, and pulmonary organ function No prior allogeneic SCT or treatment with CAR-T therapy Primary endpoints Incidence of adverse events, defined as DLTs ORR Key secondary endpoints DoR, PFS, and OS CARBON: Single-arm study evaluating the safety and efficacy of CTX110 Allogeneic CAR-T enables simplified trial design: short screening timeframe, no apheresis, no bridging chemotherapy, and on-site availability of CAR-T cell product Median time from enrollment to start of LD: 2 days Cyclophosphamide (500 mg/m2) and Fludarabine (30 mg/m2) for 3 days CTX110 infusion Screening Lymphodepletion (LD) Follow up ICF NCT04035434
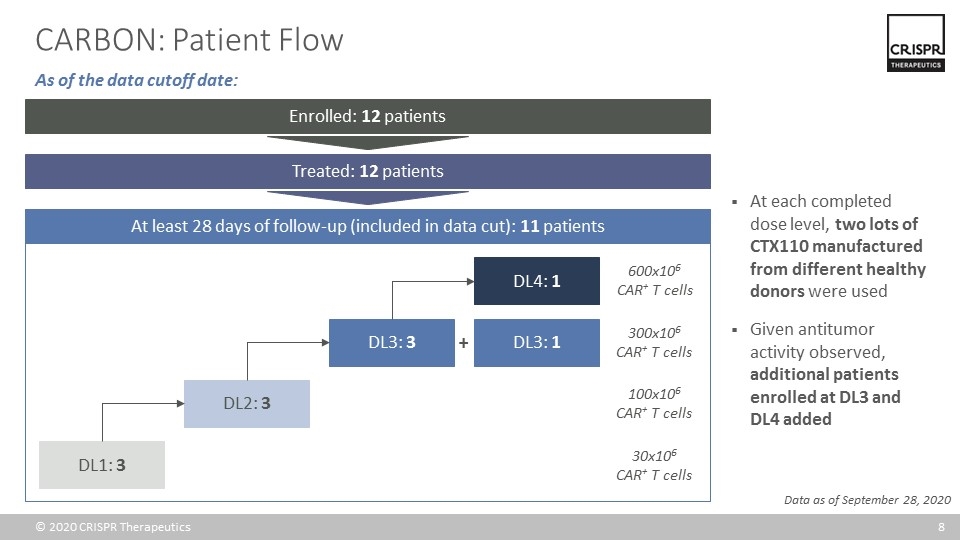
CARBON: Patient Flow At each completed dose level, two lots of CTX110 manufactured from different healthy donors were used Given antitumor activity observed, additional patients enrolled at DL3 and DL4 added Data as of September 28, 2020 At least 28 days of follow-up (included in data cut): 11 patients Enrolled: 12 patients DL1: 3 DL2: 3 DL3: 3 DL4: 1 DL3: 1 + 300x106 CAR+ T cells 100x106 CAR+ T cells 30x106 CAR+ T cells 600x106 CAR+ T cells Treated: 12 patients As of the data cutoff date:
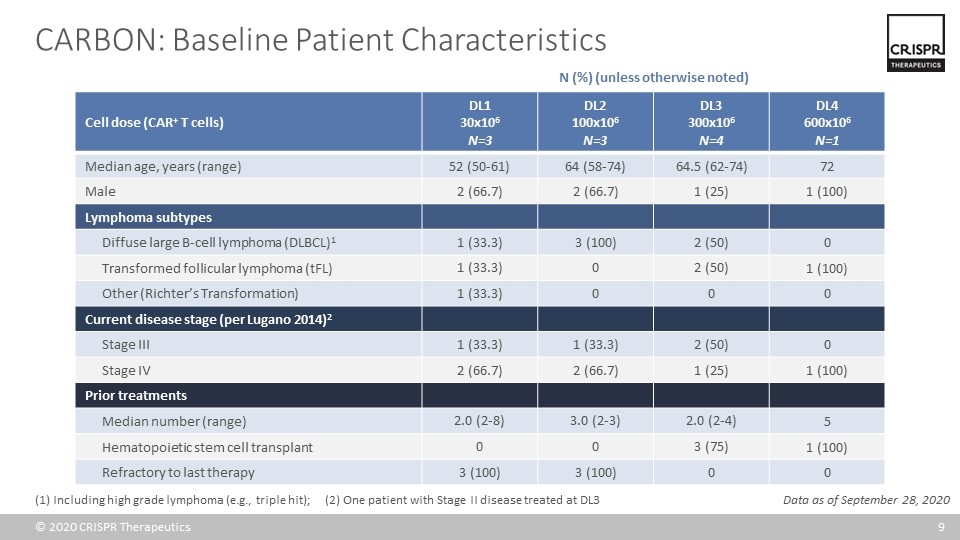
CARBON: Baseline Patient Characteristics Cell dose (CAR+ T cells) DL1 30x106 N=3 DL2 100x106 N=3 DL3 300x106 N=4 DL4 600x106 N=1 Median age, years (range) 52 (50-61) 64 (58-74) 64.5 (62-74) 72 Male 2 (66.7) 2 (66.7) 1 (25) 1 (100) Lymphoma subtypes Diffuse large B-cell lymphoma (DLBCL)1 1 (33.3) 3 (100) 2 (50) 0 Transformed follicular lymphoma (tFL) 1 (33.3) 0 2 (50) 1 (100) Other (Richter’s Transformation) 1 (33.3) 0 0 0 Current disease stage (per Lugano 2014)2 Stage III 1 (33.3) 1 (33.3) 2 (50) 0 Stage IV 2 (66.7) 2 (66.7) 1 (25) 1 (100) Prior treatments Median number (range) 2.0 (2-8) 3.0 (2-3) 2.0 (2-4) 5 Hematopoietic stem cell transplant 0 0 3 (75) 1 (100) Refractory to last therapy 3 (100) 3 (100) 0 0 (1) Including high grade lymphoma (e.g., triple hit); (2) One patient with Stage II disease treated at DL3 N (%) (unless otherwise noted) Data as of September 28, 2020
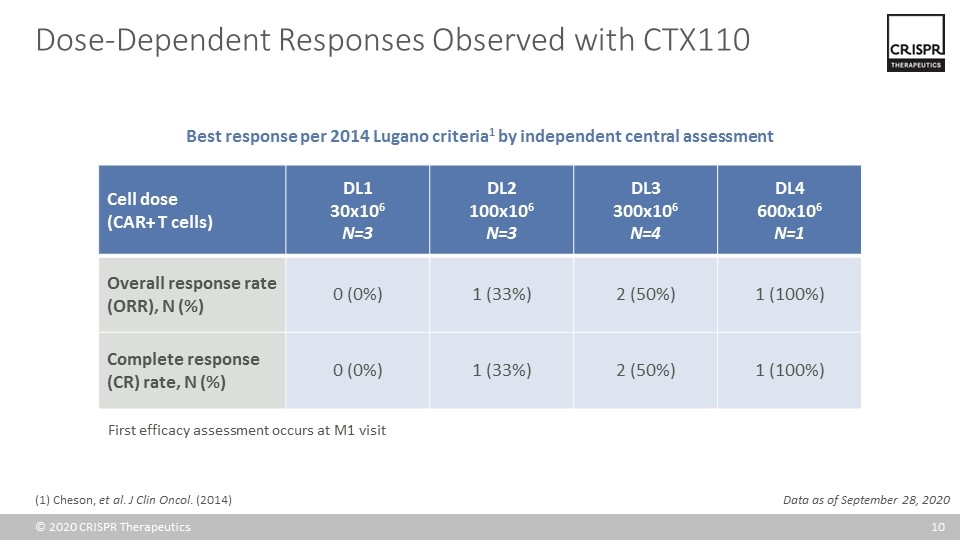
Cell dose (CAR+ T cells) DL1 30x106 N=3 DL2 100x106 N=3 DL3 300x106 N=4 DL4 600x106 N=1 Overall response rate (ORR), N (%) 0 (0%) 1 (33%) 2 (50%) 1 (100%) Complete response (CR) rate, N (%) 0 (0%) 1 (33%) 2 (50%) 1 (100%) Data as of September 28, 2020 Dose-Dependent Responses Observed with CTX110 Best response per 2014 Lugano criteria1 by independent central assessment First efficacy assessment occurs at M1 visit (1) Cheson, et al. J Clin Oncol. (2014)
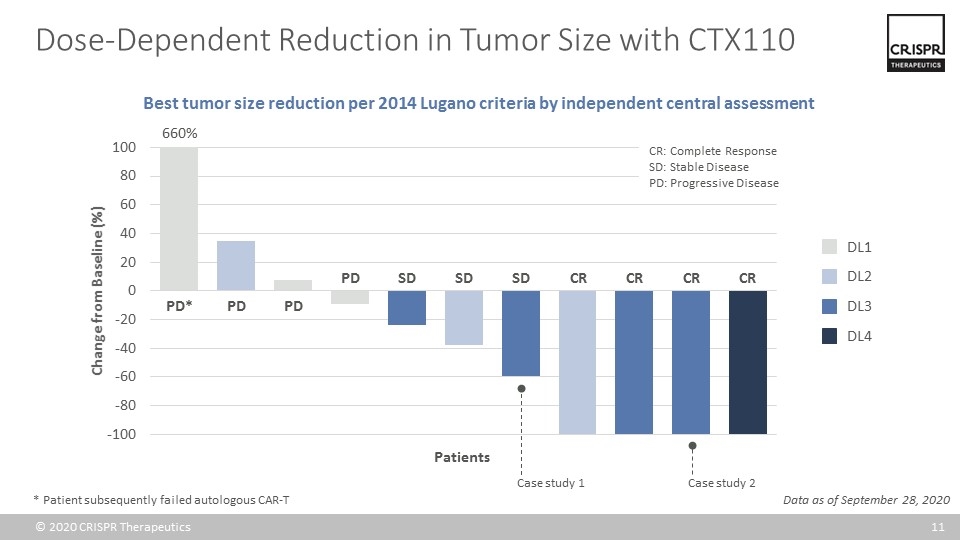
Dose-Dependent Reduction in Tumor Size with CTX110 Data as of September 28, 2020 DL1 DL2 DL3 DL4 660% PD* PD PD SD SD PD SD CR CR CR CR Patients * Patient subsequently failed autologous CAR-T Best tumor size reduction per 2014 Lugano criteria by independent central assessment Case study 1 Case study 2 CR: Complete Response SD: Stable Disease PD: Progressive Disease
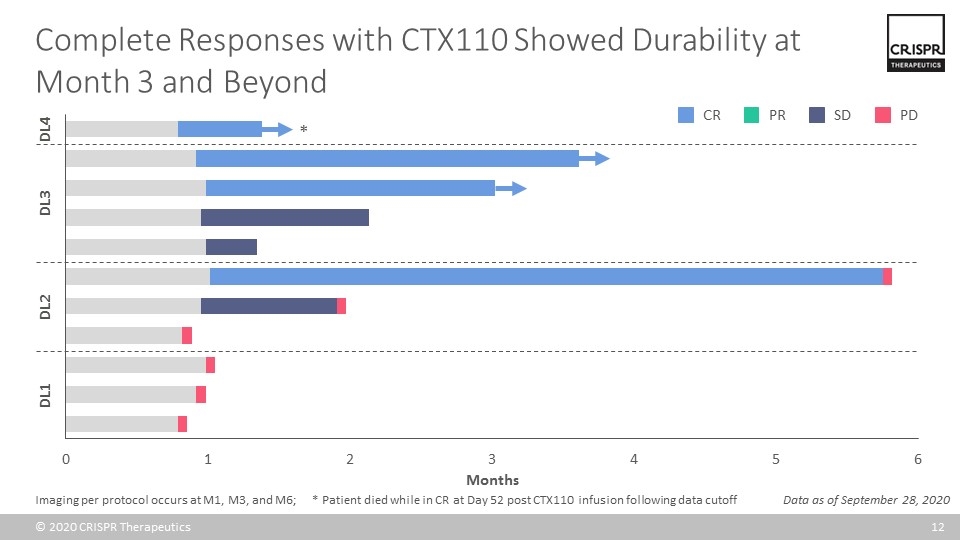
Complete Responses with CTX110 Showed Durability at Month 3 and Beyond Data as of September 28, 2020 CR SD PD PR DL1 DL2 DL3 DL4 * Imaging per protocol occurs at M1, M3, and M6; * Patient died while in CR at Day 52 post CTX110 infusion following data cutoff

Acceptable Safety Profile with CTX110 at DL3 and Below N=10 Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 Graft-versus-Host Disease (GvHD) 0 0 0 0 0 Cytokine Release Syndrome (CRS)1,2 1 (10%) 2 (20%) 0 0 0 ICANS1,3 0 1 (10%) 0 0 0 Infections 0 0 1 (10%) 0 0 (1) Per ASTCT criteria; other AEs graded per CTCAE; (2) Includes two separate episodes of CRS (1 G1, 1 G2) in single patient; worst grade reported; (3) Immune effector Cell-Associated Neurotoxicity Syndrome For patients in DL1 through DL3 (N=10): No GvHD despite all patients with ≤3/12 HLA match to CTX110 donors No CRS or ICANS above Grade 2 No infusion reactions 4 serious adverse events (SAEs) following CTX110 infusion not related to disease progression among 3 treated patients: ICANS (n=1), CRS (n=1), periorbital cellulitis (n=1), febrile neutropenia (n=1) Data as of September 28, 2020 Treatment-emergent adverse events (AEs) of special interest in DL1-3, N (%)
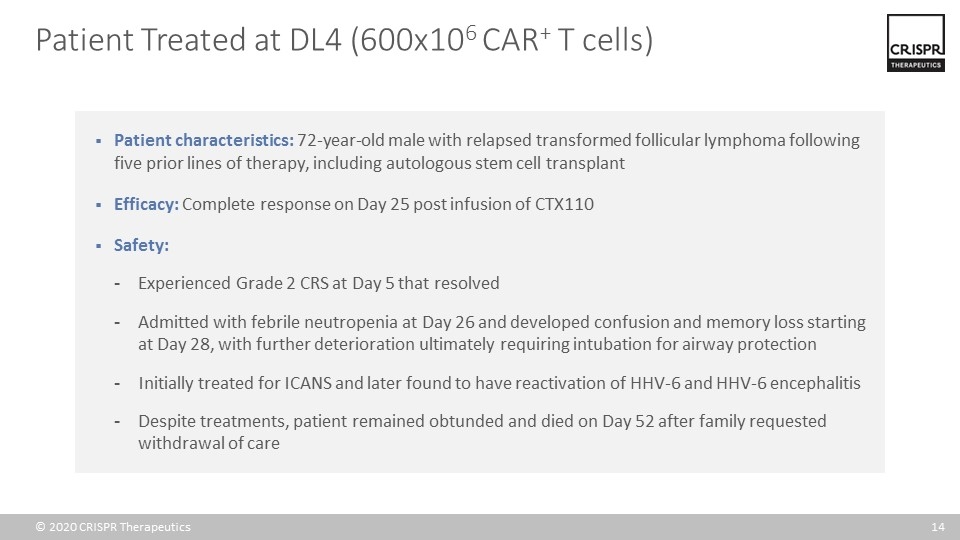
Patient characteristics: 72-year-old male with relapsed transformed follicular lymphoma following five prior lines of therapy, including autologous stem cell transplant Efficacy: Complete response on Day 25 post infusion of CTX110 Safety: Experienced Grade 2 CRS at Day 5 that resolved Admitted with febrile neutropenia at Day 26 and developed confusion and memory loss starting at Day 28, with further deterioration ultimately requiring intubation for airway protection Initially treated for ICANS and later found to have reactivation of HHV-6 and HHV-6 encephalitis Despite treatments, patient remained obtunded and died on Day 52 after family requested withdrawal of care Patient Treated at DL4 (600x106 CAR+ T cells)
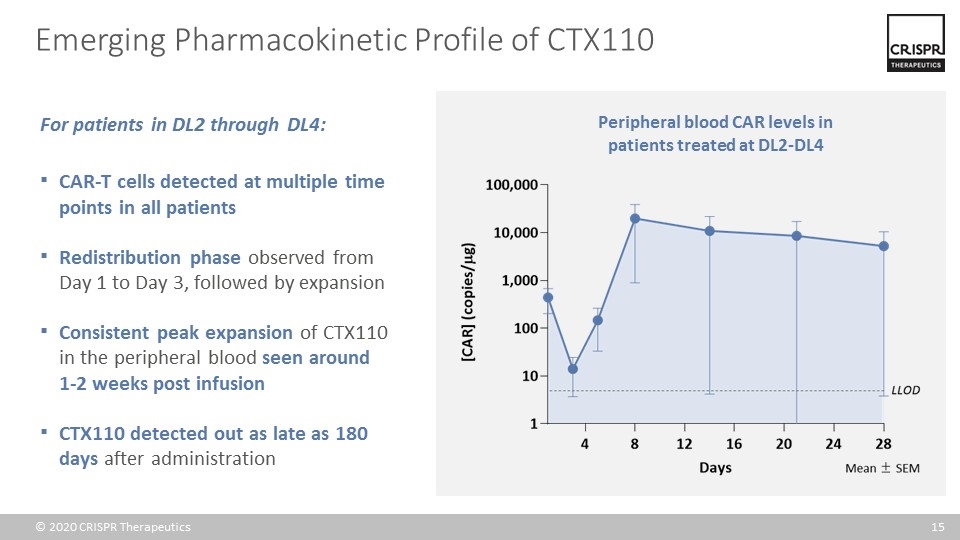
Emerging Pharmacokinetic Profile of CTX110 For patients in DL2 through DL4: CAR-T cells detected at multiple time points in all patients Redistribution phase observed from Day 1 to Day 3, followed by expansion Consistent peak expansion of CTX110 in the peripheral blood seen around 1-2 weeks post infusion CTX110 detected out as late as 180 days after administration Peripheral blood CAR levels in patients treated at DL2-DL4
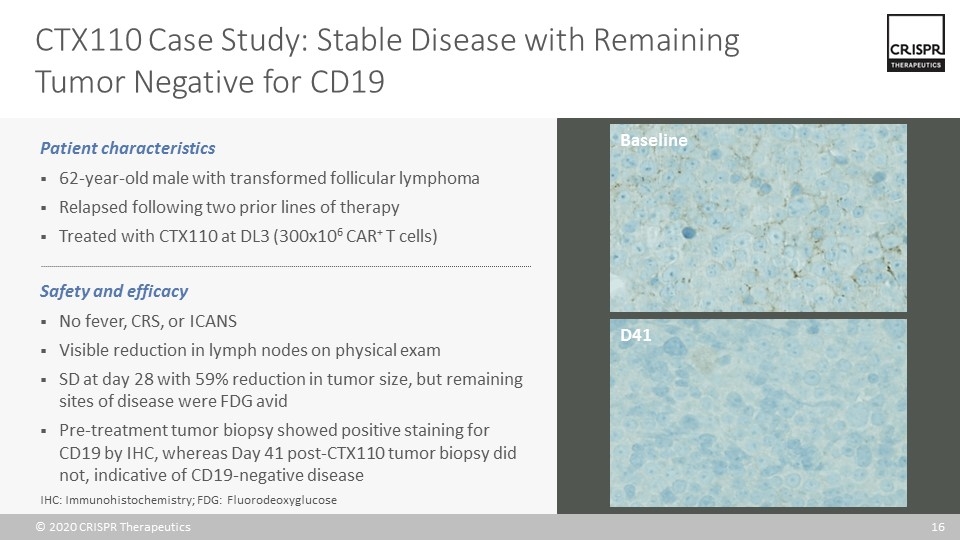
CTX110 Case Study: Stable Disease with Remaining Tumor Negative for CD19 Patient characteristics 62-year-old male with transformed follicular lymphoma Relapsed following two prior lines of therapy Treated with CTX110 at DL3 (300x106 CAR+ T cells) Safety and efficacy No fever, CRS, or ICANS Visible reduction in lymph nodes on physical exam SD at day 28 with 59% reduction in tumor size, but remaining sites of disease were FDG avid Pre-treatment tumor biopsy showed positive staining for CD19 by IHC, whereas Day 41 post-CTX110 tumor biopsy did not, indicative of CD19-negative disease Baseline D41 IHC: Immunohistochemistry; FDG: Fluorodeoxyglucose
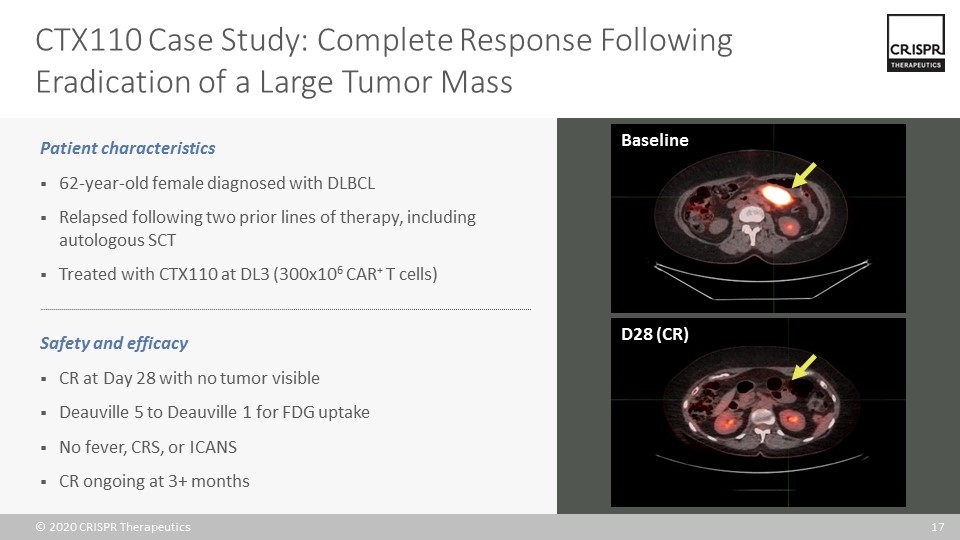
CTX110 Case Study: Complete Response Following Eradication of a Large Tumor Mass Baseline D28 (CR) Patient characteristics 62-year-old female diagnosed with DLBCL Relapsed following two prior lines of therapy, including autologous SCT Treated with CTX110 at DL3 (300x106 CAR+ T cells) Safety and efficacy CR at Day 28 with no tumor visible Deauville 5 to Deauville 1 for FDG uptake No fever, CRS, or ICANS CR ongoing at 3+ months
Initial CTX110 Data Supports Our Approach Early evidence of dose response Complete responses achieved in 4 patients (both DLBCL and tFL) Data in line with early autologous CAR-T trials Dose-dependent antitumor activity Acceptable safety profile at DL3 and below Initial experience demonstrates versatility of allogeneic CAR-T No CRS or ICANS above Grade 2 at DL3 and below; no GvHD at any dose level Responses achieved without the use of more toxic lymphodepletion agents, consistent with CTX110 being engineered for immune evasion All enrolled patients treated rapidly – no need for bridging chemotherapy or risk of manufacturing failure Responses seen across multiple product lots manufactured from different donors Validates our CRISPR-edited allogeneic CAR-T approach

“Full steam ahead” on CTX110 Proceed into expansion cohort following selection of optimal dose Re-dosing now included as an option in all cohorts; one patient re-dosed with CTX110 at DL3 so far Continue rapid progress on CTX120 and CTX130 Dosing ongoing in trial of CTX120 in multiple myeloma Dosing ongoing in trials of CTX130 in renal cell carcinoma and in T and B cell lymphomas Initial data for both programs expected in 2021 Building on the pipeline: announcement of additional programs planned in 2021 Planned Next Steps for CTX110 and Our CRISPR-Edited CAR-T Pipeline
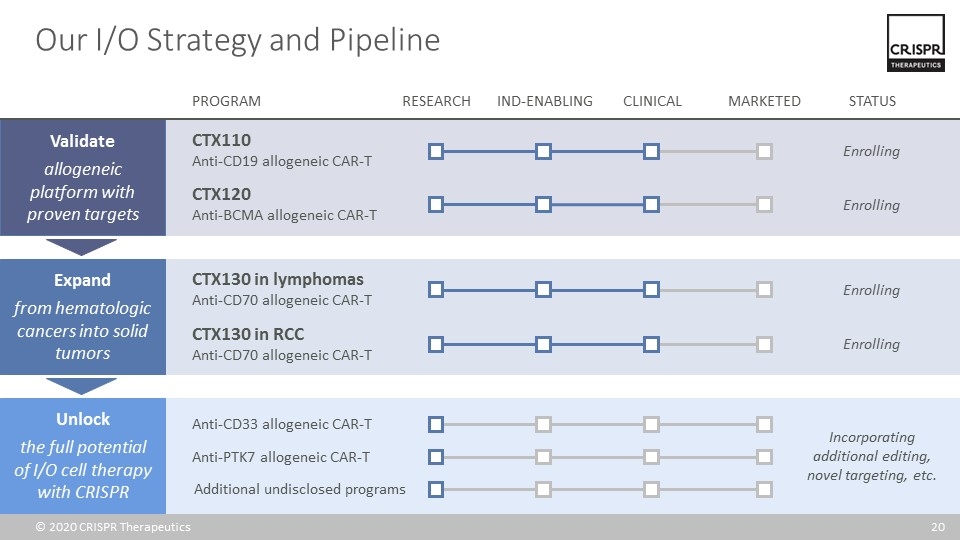
Our I/O Strategy and Pipeline Expand from hematologic cancers into solid tumors Validate allogeneic platform with proven targets Unlock the full potential of I/O cell therapy with CRISPR CTX120 Anti-BCMA allogeneic CAR-T CTX110 Anti-CD19 allogeneic CAR-T Enrolling Enrolling CTX130 in lymphomas Anti-CD70 allogeneic CAR-T Enrolling PROGRAM RESEARCH IND-ENABLING CLINICAL MARKETED STATUS Additional undisclosed programs Anti-CD33 allogeneic CAR-T Anti-PTK7 allogeneic CAR-T Incorporating additional editing, novel targeting, etc. CTX130 in RCC Anti-CD70 allogeneic CAR-T Enrolling
Thank You to Patients and Their Families Thank you to patients and their families, investigators, and site staff United States CTX110 sites Europe Australia University of Kansas Medical Center Westwood, KS Oregon Health and Science University Portland, OR Sarah Cannon Research Institute Nashville, TN University of Chicago Chicago, IL Mayo Clinic Jacksonville, FL Texas Transplant Institute San Antonio, TX University Medical Center Hamburg-Eppendorf Hamburg, Germany Peter MacCallum Cancer Centre Melbourne Royal Prince Alfred Hospital Sydney






















