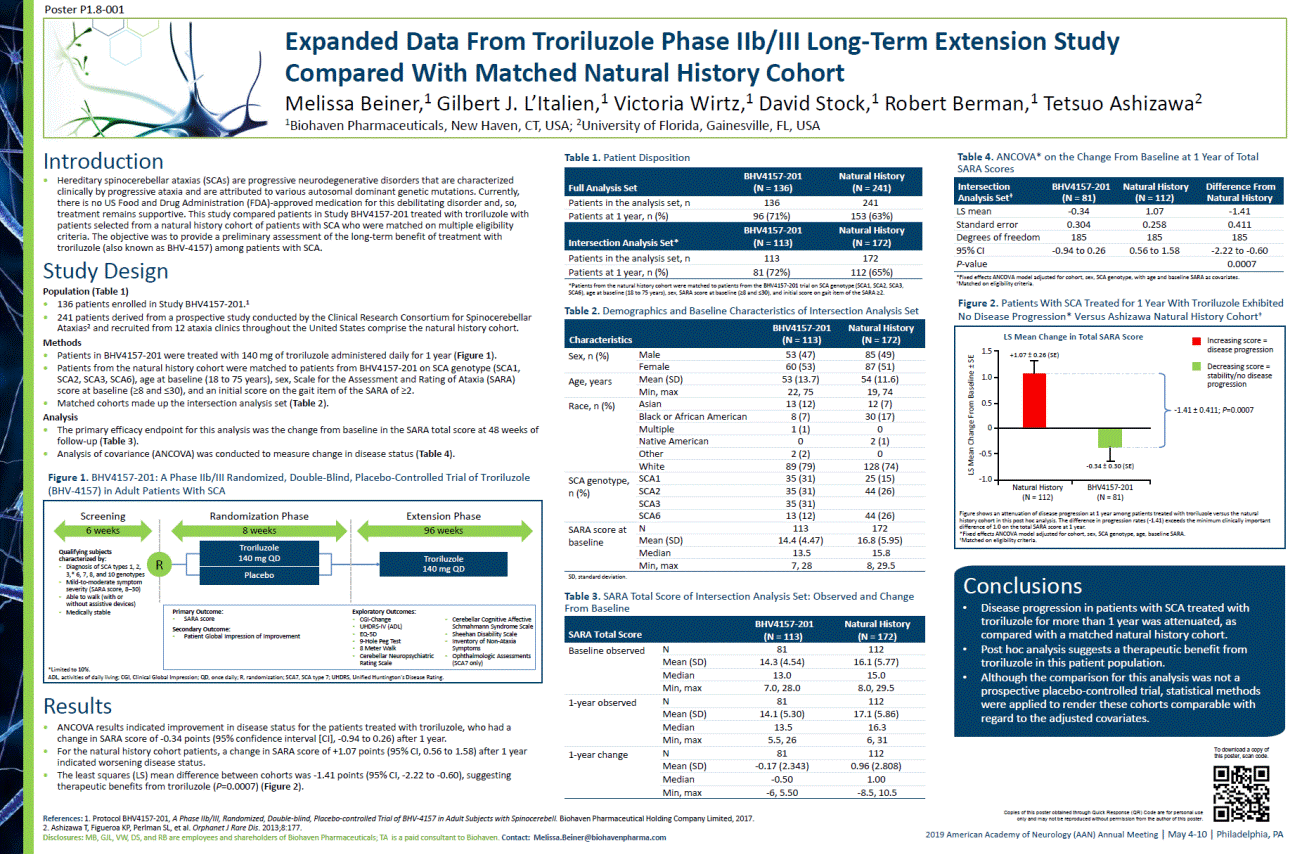
2019 American Academy of Neurology (AAN) Annual Meeting | May 4-10 | Philadelphia, PA References: 1. Protocol BHV4157-201, A Phase IIb/III, Randomized, Double-blind, Placebo-controlled Trial of BHV-4157 in Adult Subjects with Spinocerebell.Biohaven Pharmaceutical Holding Company Limited, 2017. 2. Ashizawa T, Figueroa KP, Perlman SL, et al. Orphanet J Rare Dis. 2013;8:177. Disclosures: MB, GJL, VW, DS, and RB are employees and shareholders of BiohavenPharmaceuticals; TA is a paid consultant to Biohaven.Contact: Melissa.Beiner@biohavenpharma.com •Hereditary spinocerebellar ataxias (SCAs) are progressive neurodegenerative disorders that are characterized clinically by progressive ataxia and are attributed to various autosomal dominant genetic mutations. Currently, there is no US Food and Drug Administration (FDA)-approved medication for this debilitating disorder and, so, treatment remains supportive. This study compared patients in Study BHV4157-201 treated with troriluzolewith patients selected from a natural history cohort of patients with SCA who were matched on multiple eligibility criteria. The objective was to provide a preliminary assessment of the long-term benefit of treatment with troriluzole (also known as BHV-4157) among patients with SCA. Introduction Results •ANCOVA results indicated improvement in disease status for the patients treated with troriluzole, who had a change in SARA score of -0.34 points (95% confidence interval [CI], -0.94 to 0.26) after 1 year. •For the natural history cohort patients, a change in SARA score of +1.07 points (95% CI, 0.56 to 1.58) after 1 year indicated worsening disease status. •The least squares (LS) mean difference between cohorts was -1.41 points (95% CI, -2.22 to -0.60), suggesting therapeutic benefits from troriluzole (P=0.0007) (Figure 2). Conclusions •Disease progression in patients with SCA treated with troriluzolefor more than 1 year was attenuated, as compared with a matched natural history cohort. •Post hoc analysis suggests a therapeutic benefit from troriluzole in this patient population. •Although the comparison for this analysis was not a prospective placebo-controlled trial, statistical methods were applied to render these cohorts comparable with regard to the adjusted covariates. Table 1. Patient Disposition Table 3. SARA Total Score of Intersection Analysis Set: Observed and Change From Baseline Study Design Population (Table 1) •136 patients enrolled in Study BHV4157-201.1 •241 patients derived from a prospective study conducted by the Clinical Research Consortium for Spinocerebellar Ataxias2and recruited from 12 ataxia clinics throughout the United States comprise the natural history cohort. Methods •Patients in BHV4157-201 were treated with 140 mg of troriluzole administered daily for 1 year (Figure 1). •Patients from the natural history cohort were matched to patients from BHV4157-201 on SCA genotype (SCA1, SCA2, SCA3, SCA6), age at baseline (18 to 75 years), sex, Scale for the Assessment and Rating of Ataxia (SARA) score at baseline (>8 and <30), and an initial score on the gait item of the SARA of >2. •Matched cohorts made up the intersection analysis set (Table 2). Analysis •The primary efficacy endpoint for this analysis was the change from baseline in the SARA total score at 48 weeks of follow-up (Table 3). •Analysis of covariance (ANCOVA) was conducted to measure change in disease status (Table 4). PosterP1.8-001 Expanded Data From Troriluzole Phase IIb/III Long-Term Extension Study Compared With Matched Natural History Cohort Melissa Beiner,1Gilbert J. L’Italien,1Victoria Wirtz,1David Stock,1Robert Berman,1Tetsuo Ashizawa2 1Biohaven Pharmaceuticals, New Haven, CT, USA; 2University of Florida, Gainesville, FL, USA Figure 1. BHV4157-201: A Phase IIb/III Randomized, Double-Blind, Placebo-Controlled Trial of Troriluzole (BHV-4157) in Adult Patients With SCA Full Analysis Set BHV4157-201 (N = 136) Natural History (N = 241) Patientsin the analysis set, n 136 241 Patientsat 1 year,n (%) 96 (71%) 153 (63%) Intersection Analysis Set* BHV4157-201 (N = 113) Natural History (N = 172) Patients in the analysis set, n 113 172 Patients at 1 year,n (%) 81 (72%) 112 (65%) *Patients from the natural history cohort were matched to patients from the BHV4157-201 trial on SCA genotype (SCA1, SCA2, SCA3,SCA6), age at baseline (18 to 75 years), sex, SARA score at baseline (>8 and <30), and initial score on gait item of the SARA >2. Characteristics BHV4157-201 (N = 113) Natural History (N = 172) Sex, n (%) Male 53 (47) 85 (49) Female 60 (53) 87 (51) Age, years Mean (SD) 53 (13.7) 54 (11.6) Min, max 22, 75 19, 74 Race, n(%) Asian 13 (12) 12 (7) Black or African American 8 (7) 30 (17) Multiple 1 (1) 0 Native American 0 2 (1) Other 2 (2) 0 White 89 (79) 128 (74) SCA genotype, n (%) SCA1 35 (31) 25 (15) SCA2 35 (31) 44 (26) SCA3 35 (31) SCA6 13 (12) 44 (26) SARA score at baseline N 113 172 Mean (SD) 14.4 (4.47) 16.8 (5.95) Median 13.5 15.8 Min, max 7, 28 8, 29.5 SD, standard deviation. Table 2. Demographics and Baseline Characteristics of Intersection Analysis Set SARA TotalScore BHV4157-201 (N = 113) Natural History (N = 172) Baseline observed N 81 112 Mean (SD) 14.3 (4.54) 16.1 (5.77) Median 13.0 15.0 Min, max 7.0, 28.0 8.0, 29.5 1-year observed N 81 112 Mean (SD) 14.1 (5.30) 17.1 (5.86) Median 13.5 16.3 Min, max 5.5, 26 6, 31 1-year change N 81 112 Mean (SD) -0.17 (2.343) 0.96 (2.808) Median -0.50 1.00 Min, max -6, 5.50 -8.5, 10.5 Intersection Analysis Set† BHV4157-201(N = 81) Natural History (N = 112) Difference From Natural History LS mean -0.34 1.07 -1.41 Standard error 0.304 0.258 0.411 Degreesof freedom 185 185 185 95% CI -0.94to 0.26 0.56 to 1.58 -2.22 to -0.60 P-value 0.0007 *Fixed effects ANCOVA model adjusted for cohort, sex, SCA genotype, with age and baseline SARA as covariates.†Matched on eligibility criteria. Figure 2. Patients With SCA Treated for 1 Year With Troriluzole Exhibited No Disease Progression* Versus Ashizawa Natural History Cohort† Figure shows an attenuation of disease progression at 1 year among patients treated with troriluzole versus the natural history cohort in this post hoc analysis. The difference in progression rates (-1.41) exceeds the minimum clinically important difference of 1.0 on the total SARA score at 1 year. *Fixed effects ANCOVA model adjusted for cohort, sex, SCA genotype, age, baseline SARA. †Matched on eligibility criteria. +1.07 ±0.26 (SE) -0.34 ±0.30 (SE) Increasing score = disease progression Decreasing score = stability/no disease progression Table 4. ANCOVA* on the Change From Baseline at 1 Year of Total SARA Scores 6 weeks 8 weeks 96 weeks Screening Randomization Phase Extension Phase Qualifying subjects characterized by: •Diagnosis of SCA types 1, 2, 3,* 6, 7, 8, and 10 genotypes •Mild-to-moderate symptom severity (SARA score, 8–30) •Able to walk (with or without assistive devices) •Medically stable Primary Outcome: •SARA score Secondary Outcome: •Patient Global Impression of Improvement •CGI-Change •UHDRS-IV (ADL) •EQ-5D •9-Hole Peg Test •8 Meter Walk •Cerebellar Neuropsychiatric Rating Scale •Cerebellar Cognitive Affective Schmahmann Syndrome Scale •Sheehan Disability Scale •Inventory of Non-Ataxia Symptoms •Ophthalmologic Assessments (SCA7 only) Troriluzole 140 mg QD *Limited to 10%. ADL, activities of daily living; CGI, Clinical Global Impression; QD, once daily; R, randomization; SCA7, SCA type 7; UHDRS, Unified Huntington's Disease Rating. R Troriluzole 140 mg QD Placebo Exploratory Outcomes: LS Mean Change in Total SARA Score LS Mean Change From Baseline ±SE -1.41 ±0.411; P=0.0007 Natural History(N = 112) BHV4157-201(N = 81) 1.5 1.0 0.5 0 -0.5 -1.0 Copies of this poster obtained through Quick Response (QR) Code are for personal use only and may not be reproduced without permission from the author of this poster. To download a copy of this poster, scan code.