Exhibit 6.14

The Promotion of Coal Combustion Reactivity Using Additives
Phase I — Lab-scale Feasibility Studies
A Report Submitted to:
Advance Green Energy, Inc.
523 US Hwy 41 S
Inverness, FL 34450
352-765-3850
mike@advancegreenenergy.us
Submitted by:
Yan Cao, Ph.D., Professor
SIGNATURE
/s/ Yan Cao
Institute for Combustion Science and Environmental Technology
Department of Chemistry, Western Kentucky University
Bowling Green, KY 42101
Phone number: (270)779-0202,E-mail: Yan.Cao@wku.edu
September 29, 2015
Introduction of WKU Combustion Facility
The WKU Thermal Analysis Laboratory was established in 1986. The Combustion Laboratory was established in 1993. The Mercury Emissions and Control Laboratory was established in 2001 after a Mobile Mercury Monitoring Laboratory was constructed. Following strong efforts to maintain excellence in WKU programs and to create a hands-on learning environment for science students through the establishment of the Applied Research and Technology Program of Distinction, these prominent laboratories have grown and evolved very rapidly. In light of this growth and the changing needs of both our educational and economic climates, on March 1, 2004, these three laboratories formed the Institute for Combustion Science and Environmental Technology (ICSET). Later on in 2013, the thermal-physics laboratory has been established and integrated into ICSET
Thedistinctive and unique featuresof the Institute are:
| · | According to senior officers of the North American Thermal Analysis Society, the ICSET has the best-equipped Thermal Analysis Laboratory in the U.S., and possibly the world. |
| · | The ICSET Combustion Laboratory has more than 20 years of research experience with fossil fuel combustion including high sulfur and high chlorine coals, MSW (RDF), and other fuels. ICSET has also operated its multifunctional fluidized-bed combustion (FBC) system nearly 8,000 hours. |
| · | At the Combustion Laboratory the multifunctional circulating fluidized bed combustion (0.6MW CFBC) system is capable of making combustion runs with various fuels under varying conditions to analyze and monitor air pollutant emissions for industrial partners. |
| · | A 10 kW chemical looping facility has been built-up and operated for over 2 years. It's fully functional and capable of the flow dynamics study of solid recirculation and evaluation of oxygen carriers. |
| · | A new thermal-physics laboratory facilities the most advanced instrument for the thermal physical properties' studies, including rheology and viscosity, thermal conductivity and density over a wide temperature ranges. |
| · | The ICSET Mobile Mercury Emissions and Control Laboratory has worked with about 135 existing coal fired power plants in the US to address their technical problems. |
| · | ICSET has had over 300 industrial clients in the 2004-2007 periods. Many of these clients have visited the labs to discuss projects. |
| · | All ICSET labs are multifunctional facilities used for teaching, research, and service work. |
| · | All ICSET labs are self-supported. |
| · | There have been over 80 graduates (Ph. D., M.S. and B.S.) who were employed as students in the labs of ICSET. After graduation, 45% of these students entered graduate and professional degree programs. The other graduates were all professionally employed within two months of graduation. |
| · | Studies are being conducted to sequester Carbon Dioxide gas. |
| · | Studies are being conducted on pyrolysis, combustion and gasification for different fuels (e.g. coal and biomass). |
| · | Different catalysts are being evaluated for chemical, combustion and biofuel processes. |
| · | Developing Clean Coal Technology using Horizontal Swirling Fluidized Bed Boiler. |
| · | Developing the novel gas-solid reactors. |
| · | Efforts are underway to investigate the incineration of different waste materials including coal waste, biomass and waste liquids. |
| · | ICSET is investigating the possibility of multi-utilization of chicken waste for activated carbon applications. |
ICSET had grown to the point where we needed to and could successfully move into a new, larger and more modern facility in the WKU Research and Development Center (2413 Nashville Road). In 2005, the Combustion Laboratory moved into the new facility, a total of over 13,500 square feet that includes fuel preparation and analysis equipment, as well as the newest circulating fluidized bed combustion system (CFBC). In 2006, the Thermal analysis laboratory also moved into the new facility (around 1,700 square feet). In 2010, a new 10 kW CLC facility has been added in. in 2013, a new thermal-physics laboratory has been established.
The Combustion Laboratory, established in 1993, focuses on the behavior of chlorine, sulfur, and mercury during combustion. Since the construction of the 0.1MWth laboratory scale fluidized-bed combustor (FBC system) in 1995, over a half million dollars in research funding have been received from the U.S. Department of Energy, EPRI, the Illinois Clean Coal Institute, and the Tennessee Valley Authority. The FBC system has been involved in over 8000 hours of testing. This amount of testing time is the longest that has been conducted by any university FBC system in the United States.
The Combustion Laboratory was also awarded a two million dollar grant from the U.S. Department of Energy for their project on "Establishment of an Environmental Control Technology Laboratory with a Circulating Fluidized Bed Combustion System" in the year 2004. The primary objective of this project is to establish an Environmental Control Technology Laboratory (ECTL) using a multi-functional circulating fluidized bed combustion (CFBC) system. The system can be easily configured to make combustion runs with various fuels (such as coal, coal fine, biomass, solid wastes and RDF) under varying conditions to analyze and monitor air pollutant emissions, as requested by the lab's industrial partners. The successful development of these technologies will provide scientific data on atmospheric pollutants resulting from combustion systems and the methodologies required to reduce the emission of these pollutants across the United States.
Since 2002, the Combustion Laboratory has been participating in an ongoing carbon dioxide (CO2) sequestration research project using an aqueous ammonia scrubbing technology with China and other countries. CO2 produced from combustion sources, such as fossil fuel-fired power plants, is captured from the flue gas. The CO2 reacts with aqueous ammonia to form ammonium bicarbonate (ABC), which can act as a "CO2 carrier" to "transport" CO2 from the combustion of fossil fuels to soil structure and crops in the farmlands due to its water solubility. ICSET scientists have investigated the fate of carbon distribution after the ABC fertilizer is applied to soil. It was found that a considerable amount (up to 10%) of the carbon source is absorbed by plants with increased biomass production. The majority of the unused carbon source (up to 76%) percolated into the aquifer to form stable carbonates. Of those 76% carbon, up to 88% was in the form of insoluble salts (i.e., CaCO3) in alkaline soils. Ammonia scrubbing in a slipstream reactor in real flue gas condition is under investigation at the Combustion Laboratory.
The Combustion Laboratory has investigated two approaches aimed at reducing the environmental impact and human health risk associated with animal confined feeding operations. They are (1) maximizing beneficial utilization of animal waste and (2) reducing ammonia emissions from animal feeding operations. Over the past year, ICSET at WKU developed processes for preparing activated carbon from chicken waste and coal for mercury capture. Low-cost activated carbon samples were prepared from a co-process of chicken wastes. Also, a continuous NH3 emission monitoring study of confined feeding operation (CFO) facilities was carried out using 4 commercially available NH3 monitoring systems. During a two-week monitoring period, it was found that the concentration of NH3 in the test poultry house showed an opposite trend to the ambient temperature. High ambient temperature affected the operation of the venting system, which brought air from outside of the test facility and resulted in the observed lowering in NH3 concentration due to dilution.
Kentucky is ranked second in the nation in installed flue gas desulfurization (FGD) scrubber capacity for coal-fired power plants'. As a result, large amounts of FGD by-products are produced annually. An increase in the utilization of FGD by-products (e.g. agricultural land application) creates significant economic opportunities for the state of Kentucky. However, concerns about the release of hazardous elements have inhibited the usage. The Combustion Laboratory is evaluating the environmental impact associated with land application of the FGD by-product. In this project, the emission, leaching, and bioaccumulation of Mercury (Hg) and other environmentally-concerned trace elements (e.g. Arsenic (As), Selenium (Se), and Chromium (Cr)) from soil, which are amended using FGD by-products, will be quantitatively and mechanically determined. The benefit of using FGD by-products in improving plant growth and soil properties will also be systematically demonstrated.
A laboratory scale gasification unit has been constructed at the Combustion Laboratory. The gasification system has developed a number of important gasification programs, including Advanced Gasification Syngas Multi-Contaminant Cleanup Technologies and Novel Gasification Concepts (e.g. chemical looping gasification and co-gasifying coal with CBM to produce a synthesis gas with an adjustable H2/CO ratio). Additional benefits may include economical abatement of sulfur emissions and the production of a potential mercury sorbent. This process is based on some key chemical reaction mechanisms and their cooperative effects.
Other research projects, "Development for Clean Coal Technology-Horizontal Swirling Fluidized Bed Boiler," and "Application of a Circulating Fluidized Bed Process for the Chemical Looping Combustion of Solid Fuels" are also conducted in this laboratory. Currently, a 10 kW CLC facility has been built-up and operated for over 2 years. The evaluated fuels include natural gas, bitumen, coals and biomass. Copper based on oxygen carriers are major focus in ICSET.
Part A. Determination of Combustion Characteristic Parameters
1. Experimental
The selected coal with/without TMT-13"additive were tested using a micro reactor setup for evaluation of changes of combustion reactivity of coal samples, and determine if the additive is functional on promotion of combustion reactivity. Three typical US coals were selected in this study, and they are coals of different ranks with different combustion reactivity. The reason for this coal sample selection is to see how TMT-13' the provided additive promotes combustion efficiency in what extent.
The proximate analytic data of three kinds of coals were summarized in Table 1. The diameter of selected char particles was approximately 200 km. The coal combustion with TMT-13TM the provided additive was carried out in the micro reactor-interfaced to Mass for the determination of evolved gas. In the coal combustion process, 20 mg coal was placed in an inert crucible boat, then heated to 950°C under normal combustion atmosphere using air. The gas flow rates of air were 100 mL/min and the heating rate was 10°C/min. The micro reactor provided data regarding the change of mass of a tested coal sample with/without additive TMT-13TM at different temperatures. The conversion efficiency of the tested coal sample with/without additive TMT-13TM can be calculated based on:
m°--initial weight of the original coal sample, m0 -- the instantaneous weight of the coal sample.
Table 1. The proximate and ultimate analyses of coal samples
| | proximate analysis/% | | ultimate analysis/%, dry basis |
| Moisture | fixed
Carbon | volatiles | ash | C | H | N | 0 | |
| PRB | 11.41 | 44.2 | 39.09 | 5.30 | 63.27 | 4.16 | 0.97 | 25.45 | 0.17 |
| EKPC | 5.06 | 49.46 | 35.02 | 10.46 | 65.52 | 4.52 | 1.43 | 13.94 | 3.57 |
| ICSET2# | 1.06 | 41.9 | 15.13 | 41.91 | 53.95 | 2.73 | 1.64 | | |
Ignition temperature (Ti), burnout temperature (Tf), and peak temperature (Tp) are the combustion characteristic parameters reflecting thermal behavior during the combustion process. They can be derived from the microreactor tests (TG and DTG curves). T1 is defined as the temperature at which a sample starts burning, and determined by the TG-DTG tangent method.Tfis taken as the point immediately before the combustion reaction is completed, when the rate of weight loss becomes less than 1 wt %/min. Tp is the temperature corresponding to the peak of the DTG profile, which is the point presenting the maxmium reaction rate at the applied testing conditions.
2.Results and Discussion
The TG curves of EKPC coal as a function of temperature with 10°C/min heating rate are presented in Figure 1. With temperature increasing, it can be observed that TG profiles of weight loss of the burnout process of tested samples. This weight loss curve reveals a simulating combustion process in its whole, corresponding to the involvement, the ignition and the combustion of volatile matters in coal, and then followed-up the ignition and burnout of the fixed carbon content in coal, as well as some other events involved, such as decompositions of mineral matters to generate later major constituents in fly ash. It can be observed that the provided additive TMT-13TM produces a significant effect on the coal combustion process: 1) the coal combustion started earlier with the use of the provided additive in a minimal amount, such as 200 ppm mixing ratio; 2) reaction rate has increased significantly when the provided TMT-13" additive is used. The corresponding maximum reaction rates were around 4.5 %/min without TMT-13TM additive contrasting to 8.0 %/min with TMT-13TM.
The combustion characteristics parameters of first tested coal, EKPC coal, are listed in Table 2. As shown in the Table 2, the provided additive impact the combustion performances of the target coal. The ignition temperatures (Ti) of EKPC coal without the additive TMT-13TM is 260°C which is higher than that of EKPC coal mixed with 200 ppm additive TMT-13", such as 200°C. The peak temperature (Tp) of EKPC coal without mixing with additive TMT-13" the provided TMT-13™ additive is 510°C. With the provided TMT-13™ additive is mixed, in a ratio of the additive TMT-13™ to the target coal of 200 ppm, the peak temperature (Tp) significantly dropped additive to about 270°C. The burnout temperatures of EKPC coal without and with the provided TMT-13" additive are 620°C and 440°C, respectively. Test results reveals a significant impact of the provided TMT-13™ additive on combustion performances of the first selected coal, EKPC coal. The outcome of a minimal addition of the provdied TMT-13™ additive apparently change, somehow, the combustion reaction toward a much earlier inginition, intensive combustion, and an earlier burnout. Referring to the reaction engineering mechanism, the highly reactive and less residence time of combustion organized by mixing of the provided TMT-13™ additive with the EKPC coal could be expected. The direct outcome of the use of TMT-13™ additive likely be correlative to the much less carbon content (lower LOI) in fly ash in the full utility application of EKPC coal.
We assumed aforementioned combustion phenomena is closely relative to behaviors of devolatilization process of the target coal. The oxygen functional groups and the pore structures of the treated coal with TMT-13™ additive likely occurs. This is followed up by early and ??inensive ignitions of volatile materials coming out of coal at a lower temperatures, which later provides necessary energy to move the fixed carbon in the target coal to be combusted. These two processes coincided at the a narrow temperature windows, produce a syngestic effect on both individual process, especially the combustion process of the fixed carbon. This is very beneficial for decreasing carbon residue in fly ash in the coal utility, especially when aged coal is used, such as Kentucky bituminous coal. The pursuit of promotion of combustion readditive??, just like the provided TMT-13' additive did in this test, is generally preferable in coal utilities, in order to bolster the combustion efficiency and generate more electricity and less CO2 and other air pollutants, in less cost. This TMT-13 additive helped on that, in a significant efficiency.
Table 2. Combustion Characteristics Parameters of EKPC coal Sample
| samples | Tp(°C) | Ti(°C) | Tf(°C) |
| blank | 510 | 260 | 620 |
| 200 ppm | 270 | 200 | 440 |

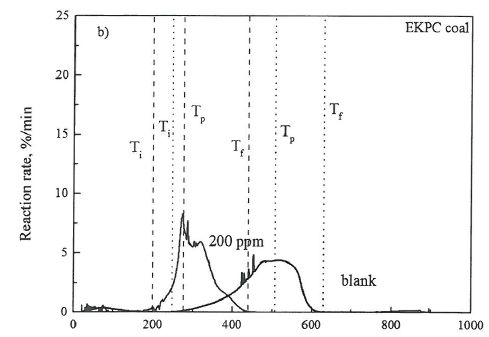
Figure 1 Thermogravimetry and differential thermogravimetry curves of the reduction reaction of EKPC coal combustion.
The mass result of the EKPC coal combustion process was shown in Fig. 2. In this case, we focused on the CO, CO2, NO, NO2, SO2 and SO3emission during the coal combustion. It can be found that there was no CO produced during the combustion, carbon was completely transferred to CO2 in this reaction.
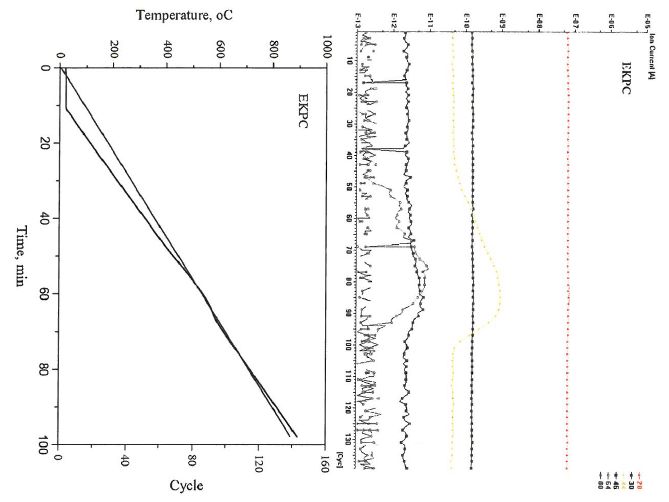
Figure 2 Mass results of the EKPC combustion (It noted that the figures indicated the relationship between cycle and temperature, and this can help to understand the relationship between the mass results and temperature; The 28, 30, 44, 46, 64 and 80 represent to CO, NO, CO2, NO2, SO2 and SO3, respectively.)
The TG curves of PRB coal as a function of temperature with 10°C/min heating rate are presented in Figure 1. With temperature increasing, it can be observed that TG profiles of weight loss of the burnout process of tested samples. This weight loss curve reveals a simulating combustion process in its whole, corresponding to the involvement, the ignition and the combustion of volatile matters in coal, and then followed-up the ignition and burnout of the fixed carbon content in coal, as well as some other events involved, such as decompositions of mineral matters to generate later major constituents in fly ash. It can be observed that the provided TMT-13" additive produces a significant effect on the coal combustion process: 1) the coal combustion started at about earlier with the use of the provided TMT-13' additive in a minimal amount, such as 200 ppm mixing ratio; 2) reaction rate has increased significantly when the provided TMT-13' additive is used. The corresponding maximum reaction rates were around 10 %/min without TMT-13" additive contrasting to 87 %/min with TMT-13" additive.
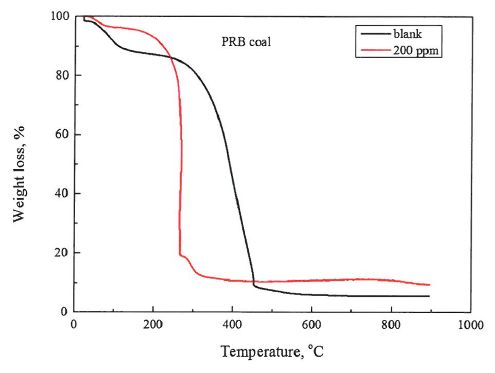

Figure 3. Thermogravimetry and differential thermogravimetry curves of the reduction reaction of PRB coal combustion.
The combustion characteristics parameters of second tested coal, PRB coal, are listed in Table 3. As shown in the Table 3, the provided TMT-13" additive impact the combustion performances of the target coal. The ignition temperatures (TO of PRB coal without the TMT-13' additive is 250°C, which is higher than that of PRB coal mixed with 200 ppm TMT-13' additive, such as 180°C. The peak temperature (Tp) of PRB coal without mixing with the provided TMT-13" additive is 350°C. When the provided TMT-13" additive is mixed, in a ratio of the TMT-13" additive to the target coal of 200 ppm, the peak temperature (Tp) significantly dropped to about 260°C. The burnout temperatures of PRB coal without and with the provided TMT-13" additive are 470°C and 320°C, respectively. Test results reveals a significant impact of the provided TMT-13' additive on combustion performances of the second selected coal, PRB coal. The outcome of a minimal addition of the provdied TMT-13" additive apparently change, somehow, the combustion reaction toward a much earlier inginition, intensive combustion, and an earlier burnout. Referring to the reaction engineering mechanism, the highly reactive and less residence time of combustion organized by mixing of the provided TMT-13TM additive with the PRB coal could be expected. The direct outcome of the use of TMT-13" additive would likely be correlative to the much less carbon content (lower L01) in fly ash in the full utility application of PRB coal.
We assumed aforementioned combustion phenomena is closely relative to behaviors of devolatilization process of the target coal. The oxygen functional groups and the pore structures of the treated coal with the TMT-13TM additive likely occurs. This is followed up by early and intensive ignitions of volatile materials coming out of coal at lower temperatures, which later provides necessary energy to move the fixed carbon in the target coal to be combusted. These two processes coincided at the a narrow temperature windows, produce a syngestic effect on both individual process, especially the combustion process of the fixed carbon. The pursuit of promotion of combustion readditive, just like the provided TMT-13TM additive did in this test, is generally perferable in coal utilities, in order to bolster the combustion efficiency and generate more electricity and less CO2 and other air pollutants, in less cost. This TMT-13TM additive helped on that, in a significant efficiency.
Table 3.Combustion Characteristics Parameters of PRB coal Samples
| samples | Tp(°C) | T,(°C) | Tf(°C) |
blank
200 ppm | 350 260 | 250 180 | 470 320 |
The mass result of the PRB coal combustion process was shown in Figure 2. In this case, we focused on the emissions of CO, NO, NO2, SO2 and SO3, as well as the expected combustion product, CO2, during the coal combustion. It can be found that there was no CO produced during the combustion, carbon was completely oxidized to CO2 in this process. Furthermore, it helped to identify there is no emission of nitrogen oxide emissions, such as NO and NO2, when compared in three mass graphs below. In addition, the tendency of each graphs changed gentler compared with blank one: 1) peaks shifted left, which was coordinate with TGA data; 2) intensity of peaks and corresponding areas decreased when concentrations increased.
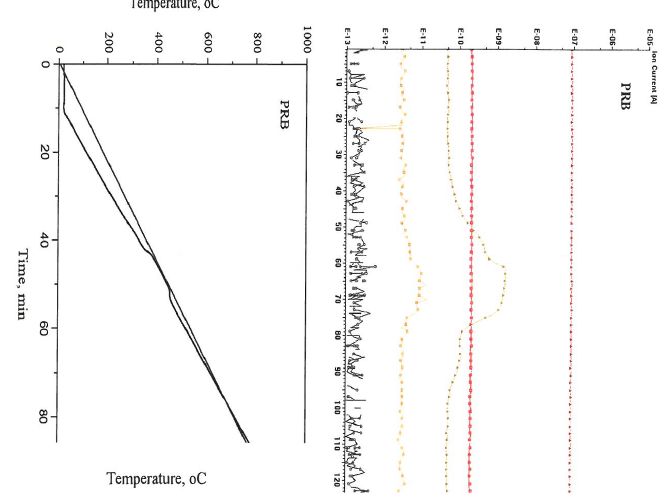
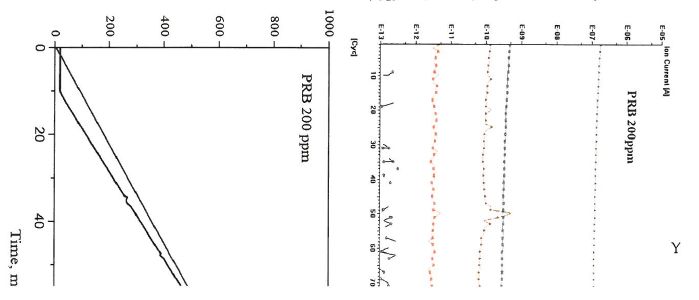
Figure 4. Mass results of the PRB combustion (It noted that the figures indicated the relationship between cycle and temperature, and this can help to understand the relationship between the mass results and temperature; The 28, 30, 44, 46, 64 and 80 represent to CO, NO, CO2, NO2, SO2 and SO3, respectively.)
The combustion process of ICSET 2# coal with & without TMT-13" additive in response to variation in temperature with 10°C/min heating rate was presented in Figure 5. With temperature increasing, it can be observed that TG profiles of weight loss of the burnout process of tested samples. This weight loss curve reveals a simulating combustion process in its whole, corresponding to the involvement, the ignition and the combustion of volatile matters in coal, and then followed-up the ignition and burnout of the fixed carbon content in coal, as well as some other events involved,such as decompositions of mineral matters to generate later major consituents in fly ash. It can be observed that the provided TMT-13TM additive produces a significant effect on the coal combustion process: 1) the coal combustion started at earlier with the use of the provided TMT-13TM additive in a minimal amount, such as 200 ppm mixing ratio; 2) reaction rate has increased significantly when the the provided TMT-13TM additive was used. The corresponding maximum reaction rates were around 5.0 %/min without TMT-13Tm additive contrasting to 8.0 %/min with additive.


Figure 5. Thermogravimetry and differential thermogravimetry curves of the reduction reaction of ICSET 2# coal combustion.
The combustion characteristics parameters of first tested coal, ICSET 2# coal, are listed in Table 4. As shown in the Table 4, the provided TMT-13TM additive impact the combustion performances of the target coal. The ignition temperatures (T,) of 2# coal without the TMT-13TM additive is 330°C, which is higher than that of 2# coal mixed with 00 ppm TMT-13TM additive, such as 260°C. The peak temperature (Tp) of 2# coal without mixing with the provided TMT-13' additive is 520°C. When the provided TMT-13" additive is mixed, in a ratio of the TMT-13TM additive to the target coal of 200 ppm, the peak temperature (Tp) significantly dropped to about 380°C. The burnout temperatures of 2# coal without and with the provided TMT-13TM additive are 610°C and 470°C, respectively. Test results reveals a significant impact of the provided TMT-13TM additive on combustion performances of the first selected coal, 2# coal. The outcome of a minimal addition of the provdied TMT-13TM additive apparently change, somehow, the combustion reaction toward a much earlier ingnition, intensive combustion, and an earlier burnout. Referring to the reaction engineering mechanism, the highly reactive and less residence time of combustion organized by mixing of the provided TMT-13TM additive with the 2# coal could be expected. The direct outcome of the use of TMT-13TM additive likely be correlative to the much less carbon content (lower [01) in fly ash in the full utility application of 2# coal.
We assumed aforementioned combustion phenomena is closely relative to behaviors of devolatilization process of the target coal. The oxygen functional groups and the pore structures of the treated coal with TMT-13TM additive likely occurs. This is followed up by early and intensive ignitions of volatile materials coming out of coal at a lower temperatures, which later provides necessary energy to move the fixed carbon in the target coal to be combusted. These two processes coincided at the a narrow temperature windows, produce a syngestic effect on both individual process, especially the combustion process of the fixed carbon. The pursuit of promotion of combustion readditive, just like the provided TMT-13TM additive did in this test, is generally perferable in coal utilities, in order to bolster the combustion efficiency and generate more electricity and less CO2 and other air pollutants, in less cost. This TMT-13TM additive helped on that, in a significant efficiency.
| Table 4.Combustion Characteristics Parameters of ICSET 2# coal Samples |
| samples | Tp(°C) | Ti(°C) | Tf(°C) |
blank
200 ppm | 520 380 | 330 260 | 610 470 |
The mass result of the 2# coal combustion process was shown in Figure 6. In this case, we focused on the CO, CO2, NO, NO2, SO2 and SO3 emission during the coal combustion. It can be found that there was no CO produced during the combustion, carbon was completely oxidized to CO2 in this process.
3. Conclusion
This research discussed the effect of the TMT-13TM additive on the coal combustion with integrated micro reactor with on-line gas analyzers. The results showed that the TMT-13TM additive helps increasing the reaction rate of the combustion. During the combustion process, the TMT-13TM additive burns first to generate heat and initialize the follow-up coal ignition. It facilitates lowering the ignition and combustion temperatures of coal and increasing the combustion reaction rate. Results looks promising enough to be considered proof-of-concept for the TMT-13TM additive in all three coal types tested and we strongly recommend further tests in the scaled-up combustion facility.
Part C. Mercury additive test
1. Experimental
Test facility,applied in this study, is presented in Figure 1-1 and 1-2. Four major parts were included into this test facility, 1) the fixed bed reactor, 2) the preparation of simulating flue gas source, 3) system control, and 4) Hg measurement. The tested sample was loaded into this fixed bed test facility. The prepared flue gas simulates the specific flue gas constituents of eastern bituminous flue gas (derived from eastern bituminous coal) after treatment with FGD (nominally 100-200 ppm 502, saturated H2O, 5-6% 02, 13-14% CO2, 10 tig/NM3 Hg, balance N2.
Test conditions:
1) Feed rate: 1L/min;
2) Operation temperature: 150°C;
3) Feed Simulating flue gas compositions: 150 ppm SO2, saturated H2O, 5.5 % 02, 13.5 % CO2, balance N2;
4) Mercury concentration in the simulating flue gas: around 100 [Ag/NM3 Hg
5) Sample: coating on a filter in 2 inch of diameter, the total amount of sample in 02984 gram.
Test procedure:
| 1) | Sample was loaded into the fixed bed facility (0.2984g on 2 inch filter); |
| 2) | Sample was tested under the simulating flue gas under required test conditions. The experiment was run until effluent Hg concentration reaches the feed Hg concentration. When Hg analysis was on a continuous basis (according to the instrument's capability). |
| 3) | After adsorbent was statured with mercury, the feed was changed to mercury-free flue gas, with the balance composition the same as the original Hg-containing feed gas. |
| 4) | The feed gas temperature was ramped to 150°C under Hg-free flue gas flow while continuously monitoring the Hg composition of the effluent The feed temperature was then held at 150°C until the mercury level dropped to below the detection limit. |
| 5) | The adsorbent sample was again placed into Hg-containing flue gas for a second breakthrough experiment similar to the first run. |
| 6) | During the period of the adsorption tests, the system blank (air stream without mercury) and mercury concentrations at reactor inlet, which were for the quality control purpose, were checked once by daily. |
| 7) | During the period of desorption test, re-calibration was conducted to insure the mercury peak was measured accurately. |
2. Test Results
Test result on mercury adsorption is presented in Figure 2. Under the current test condition, it took around 300 mins to make tested sample totally breakthrough. During the test period, QA/QC procedures were performed, including system blank check and mercury concentration check at the inlet of the reactor. QA/QC tests indicated system runs in good condition. The mercury concentration was around 100 ug/NM3 during blank-check tests. The total mercury at the reactor inlet was also kept constant, which was very close to that during initial test setup.
Test result on mercury desorption is presented in Figure 3, and raw data was shown in Table 1. Under the current test conditions (max temperature to about 150°C), the mercury desorption capacity was found to be 0.103 mg/g sorbent, which is equivalent to 103 ppm of mercury captured on sorbent. Contrasting to regular fly ash, the mercury capture capability of the provided sorbent is confirmed. As a reference, fly ash is generally regarded as a baseline of mercury capture sorbent in the coal utility. Its mercury capture capability is generally about a few ppm. That confirmed the provided sorbent should perform well in coal fired utility with over 100 times more capacity of mercury capture than that of self-generated fly ash, which also can be considered proof-of-concept for the additive.
Figure 1-1. Schematic of test setup
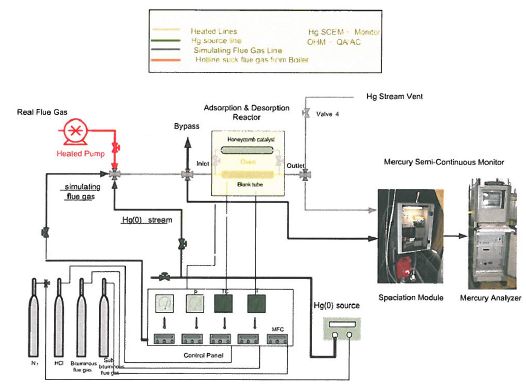

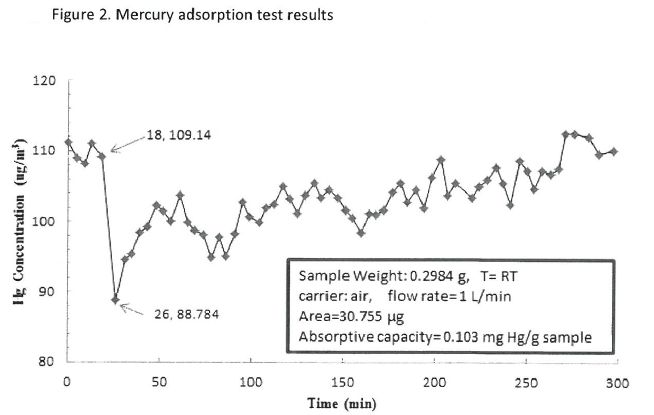
Table 1. The raw data record of mercury concentration at the outlet of adsorption bed.
| Date/Time | Channel | Signal | Peak Height | Peak Area | Concentration(ug/m^3) | Volume (mls ) Name | | Time(min) |
| 8/17/150:13 | 2 | 84.4 | 77.715 | 732.435 | 48.624 | 21.09 Hg total | | |
| 8/17/15 0:17 | 2 | 78.7 | 72.235 | 575.442 | 49.819 | 19.23 Hgtotal | 4 | |
| 8/17/150:22 | 2 | 138.8 | 132.528 | 910.595 | 92.712 | 18.34 Hgtotal | 8 | |
| 8/17/15 0:26 | 2 | 162.1 | 155.884 | 1015.72 | 111.304 | 17.86 Hg total | 12 | 0 |
| 8/17/15 0:30 | 2 | 158.2 | 152.005 | 1005.522 | 109.058 | 17.79 Hg total | 17 | 5 |
| 8/17/15 0:35 | 2 | 155.9 | 149.749 | 1007.63 | 108.226 | 17.67 Hg total | 21 | 9 |
| 8/17/15 0:39 | 2 | 157.8 | 151.685 | 1013.881 | 111.148 | 17.42 Hg total | 25 | 13 |
| 8/17/15 0:43 | 2 | 152.2 | 146.096 | 988.983 | 109.14 | 17.11 Hg total | 30 | 18 |
| 8/17/15 0:52 | 2 | 105.6 | 99.635 | 691.765 | 88.784 | 14.59 Hg total | 38 | 26 |
| 8/17/15 0:56 | 2 | 110.9 | 105.02 | 734.654 | 94.494 | 14.41 Hg total | 43 | 31 |
| 8/17/15 1:00 | 2 | 110.6 | 104.639 | 744.828 | 95.293 | 14.24 Hg total | 47 | 35 |
| 8/17/15 1:05 | 2 | 112.3 | 106.357 | 753.097 | 98.437 | 14 Hg total | 51 | 39 |
| 8/17/15 1:09 | 2 | 113 | 107.018 | 766.314 | 99.232 | 13.97 Hg total | 55 | 43 |
| 8/17/15 1:13 | 2 | 114.9 | 108.913 | 766.237 | 102.292 | 13.78 Hg total | 60 | 48 |
| 8/17/15 1:18 | 2 | 112.4 | 106.396 | 770.969 | 101.442 | 13.59 Hg total | 64 | 52 |
| 8/17/15 1:22 | 2 | 109.5 | 103.511. | 758.283 | 100.006 | 13.43 Hg total | 68 | 56 |
| 8/17/15 1:26 | 2 | 112.7 | 106.738 | 752.038 | 103.737 | 13.33 Hg total | 73 | 61 |
| 8/17/15 1:31 | 2 | 107.5 | 101.567 | 735.923 | 99.86 | 13.21 Hg total | 77 | 65 |
| 8/17/15 1:35 | 2 | 105.8 | 99.902 | 741.385 | 98.757 | 13.15 Hg total | 81 | 69 |
| 8/17/15 1:39 | 2 | 103 | 97.111 | 728.032 | 98.082 | 12.89 Hg total | 86 | 74 |
| I:SW/lb .I..95 | 1 | 1UU.4 | V4.518 | 719.488 | 94.87 | 12.99 Hg total | 90 | 78 |
| 8/17/15 1:48 | 2 | 99 | 93.157 | 707.144 | 97.717 | 12.44 Hg total | 94 | 82 |
| 8/17/15 1:52 | 2 | 96.9 | 91.107 | 705.717 | 95.001 | 12.53 Hg total | 98 | 86 |
| 8/17/15 1:56 | 2 | 98 | 92.227 | 706.829 | 98.298 | 12.25 Hg total | 103 | 91 |
| 8/17/15 2:01 | 2 | 102.5 | 96.713 | 715533 | 102.723 | 12.26 Hg total | 107 | 95 |
| 8/17/15 2:05 | 2 | 99.1 | 93.342 | 711.616 | 100.569 | 12.11 Hg total | 111 | 99 |
| 8/17/15 2:09 | 2 | 97.1 | 91.37 | 702.896 | 99.883 | 11.95 Hg total | 116 | 104 |
| 8/17/15 2:14 | 2 | 97.8 | 92.079 | 703.408 | 101.893 | 11.8 Hg total | 120 | 108 |
| 8/17/15 2:18 | 2 | 97.2 | 91.458 | 694.429 | 102.372 | 11.67 Hg total | 124 | 112 |
| 8/17/15 2:22 | 2 | 99.8 | 94.055 | 702.824 | 105.023 | 11.68 Hg total | 129 | 117 |
| 8/17/15 2:26 | 2 | 97.9 | 92.18 | 701.223 | 103.223 | 11.66 Hg total | 133 | 121 |
| 8/17/15 2:31 | 2 | 95.8 | 90.095 | 693.744 | 101.194 | 11.64 Hg total | 137 | 125 |
| 8/17/15 2:35 | 2 | 98.7 | 92.974 | 694.606 | 103.705 | 11.7 Hg total | 141 | 129 |
| 8/17/15 2:39 | 2 | 99.1 | 93.378 | 690.418 | 105.483 | 11.55 Hg total | 146 | 134 |
| 8/17/15 2:44 | 2 | 95 | 89.278 | 678.468 | 103.349 | 11.3 Hg total | 150 | 138 |
| 8/17/15 2:48 | 2 | 96.9 | 91.162 | 685.116 | 104.569 | 11.39 Hg total | 154 | 142 |
| 8/17/15 2:52 | 2 | 94.9 | 89.16 | 674.634 | 103.403 | 11.28 Hg total | 159 | 147 |
| 8/17/15 2:57 | 2 | 94.4 | 88.698 | 661.014 | 101.638 | 11.42 Hg total | 163 | 151 |
| 8/17/15 3:01 | 2 | 91.9 | 86.197 | 648.949 | 100.526 | 11.24 Hg total | 167 | 155 |
| 8/17/15 3:05 | 2 | 89.3 | 83.596 | 646.934 | 98.377 | 11.16 Hg total | 172 | 160 |
| 8/17/15 3:09 | 2 | 91 | 85.295 | 654.82 | 101.066 | 11.07 Hg total | 176 | 164 |
| 8/17/15 3:14 | 2 | 90.6 | 84.922 | 653.298 | 100.925 | 11.04 Hg total | 180 | 168 |
| 8/17/15 3:18 | 2 | 90.8 | 85.111 | 648.395 | 101.688 | 10.98 Hg total | 184 | 172 |
| 8/17/15 3:22 | 2 | 93.5 | 87.804 | 652.48 | 104.14 | 11.04 Hg total | 189 | 177 |
| 8/17/15 3:27 | 2 | 95 | 89.309 | 655.916 | 105.531 | 11.07 Hg total | 193 | 181 |
| 8/17/15 3:31 | 2 | 91.7 | 86.02 | 647.892 | 102.801 | 10.97 Hg total | 197 | 185 |
| 8/17/15 3:35 | 2 | 90.7 | 85.037 | 649.71 | 104.46 | 10.68 Hg total | 202 | 190 |
| 8/17/15 3:40 | 2 | 88.9 | 83.22 | 643.291 | 101.983 | 10.72 Hg total | 206 | 194 |
| 8/17/15 3:44 | 2 | 93 | 87.316 | 649.022 | 106.192 | 10.77 Hg total | 210 | 198 |
| 8/17/15 3:48 | 2 | 94.8 | 89.128 | 655.938 | 108.769 | 10.72 Hg total | 215 | 203 |
| 8/17/15 3:52 | 2 | 89.7 | 84.05 | 648.892 | 103.71 | 10.64 Hg total | 219 | 207 |
| 8/17/15 3:57 | 2 | 92.7 | 87.068 | 656.926 | 105.42 | 10.82 Hg total | 223 | 211 |
| 8/17/15 4:01 | 2 | 83.7 | 78.073 | 614.636 | 114.178 | 9.02 Hg total | 227 | 215 |
| 8/17/15 4:05 | 2 | 88.6 | 82.978 | 641.337 | 103.442 | 10.54 Hg total | 232 | 220 |
| 8/17/15 4:10 | 2 | 90.3 | 84.652 | 649.623 | 104.9 | 10.59 Hg total | 236 | 224 |
| 8/17/15 4:14 | 2 | 90 | 84.358 | 645.849 | 105.958 | 10.45 Hg total | 240 | 228 |
| 8/17/15 4:18 | 2 | 88.8 | 83.158 | 645.125 | 107.635 | 10.15 Hg total | 245 | 233 |
| 8/17/15 4:23 | 2 | 89 | 83.361 | 649.869 | 105.389 | 10.39 Hg total | 249 | 237 |
| 8/17/15 4:27 | 2 | 88 | 82.351 | 641.857 | 102.418 | 10.57 Hg total | 253 | 241 |
| 8/17/15 4:31 | 2 | 89.7 | 84.085 | 645.929 | 108.652 | 10.16 Hg total | 258 | 246 |
| 8/17/15 4:35 | 2 | 89 | 83.363 | 641.6 | 107.145 | 10.22 Hg total | 262 | 250 |
| 8/17/15 4:40 | 2 | 88.5 | 82.866 | 648.27 | 104.702 | 10.4 Hg total | 266 | 254 |
| 8/17/15 4:44 | 2 | 89.4 | 83.778 | 652.938 | 107.225 | 10.26 Hg total | 270 | 258 |
| 8/17/15 4:48 | 2 | 88.4 | 82.765 | 650.855 | 106.738 | 10.19 Hg total | 275 | 263 |
| 8/17/15 4:53 | 2 | 87.7 | 82.088 | 646.75 | 107.502 | 10.04 Hg total | 279 | 267 |
| 8/17/15 4:57 | 2 | 94 | 88.348 | 654.012 | 112.49 | 10.28 Hg total | 283 | 271 |
| 8/17/15 5:01 | 2 | 92.1 | 86.472 | 650.121 | 112.542 | 10.07 Hg total | 288 | 276 |
| 8/17/15 5:06 | 2 | 86 | 80.401 | 645.184 | 104.704 | 10.11 Hg total | 292 | 280 |
| 8/17/15 5:10 | 2 | 92.6 | 87.003 | 649.964 | 112.079 | 10.17 Hg total | 296 | 284 |
| 8/17/15 5:14 | 2 | 91 | 85.38 | 648.17 | 109.682 | 10.21 Hg total | 301 | 289 |
| 8/17/15 5:18 | 2 | 83 | 77.413 | 614.654 | 123.253 | 8.29 Hg total | 305 | 293 |
| 8/17/15 5:23 | 2 | 89.9 | 84.308 | 656.076 | 110.114 | 10.05 Hg total | 309 | 297 |
| 8/17/15 5:27 | 2 | 93 | 87.399 | 658.678 | 115.744 | 9.89 Hg total | 313 | 301 |
| 8/17/15 5:31 | 2 | 93.6 | 87.989 | 658.665 | 117.309 | 9.82 Hg total | 318 | 306 |
| 8/17/15 5:36 | 2 | 93.9 | 88.316 | 660.9 | 119.299 | 9.69 Hg total | 322 | 310 |
| 8/17/15 5:40 | 2 | 93.6 | 88.008 | 656.603 | 116.033 | 9.93 Hg total | 326 | 314 |
| 8/17/15 5:44 | 2 | 95.5 | 89.942 | 661.343 | 119.272 | 9.86 Hg total | 331 | 319 |
| 8/17/15 5:49 | 2 | 90.3 | 84.717 | 658.815 | 114.137 | 9.74 Hg total | 335 | 323 |
| 8/17/15 5:53 | 2 | 88.6 | 83.022 | 653.324 | 113.038 | 9.65 Hg total | 339 | 327 |












