
Exhibit 99.1 Topline Results for Phase 2 Study of EDP1815 in Mild and Moderate Psoriasis September 27, 2021

Legal Disclaimer This presentation contains forward-looking statements within the meaning of the Private stockholders have the ability to control or significantly influence our business; costs and Securities Litigation Reform Act of 1995. All statements contained in this presentation resources of operating as a public company; unfavorable or no analyst research or that do not relate to matters of historical fact should be considered forward-looking reports; and securities class action litigation against us. statements, including statements concerning the development of EDP1815, the promise These and other important factors discussed under the caption Risk Factors in our and potential impact of EDP1815, the timing of and plans for clinical studies, and the Quarterly Report on Form 10-Q for the three months ended June 30, 2021, and our timing and results of clinical trial readouts. other reports filed with the SEC could cause actual results to differ materially from those These forward-looking statements are based on management's current expectations. indicated by the forward-looking statements made in this presentation. Any such These statements are neither promises nor guarantees, but involve known and unknown forward-looking statements represent management's estimates as of the date of this risks, uncertainties and other important factors that may cause our actual results, presentation. While we may elect to update such forward-looking statements at some performance or achievements to be materially different from any future results, point in the future, except as required by law, we disclaim any obligation to do so, even if performance or achievements expressed or implied by the forward-looking statements, subsequent events cause our views to change. These forward-looking statements including, but not limited to, the following: the impact of the COVID-19 pandemic on our should not be relied upon as representing our views as of any date subsequent to the operations, including our preclinical studies and clinical trials, and the continuity of our date of this presentation. business; we have incurred significant losses, are not currently profitable and may never Certain information contained in this presentation relates to or is based on studies, become profitable; our need for additional funding; our cash runway; our limited publications, surveys and other data obtained from third-party sources and our own operating history; our unproven approach to therapeutic intervention; the lengthy, internal estimates and research. While we believe these third-party sources to be expensive, and uncertain process of clinical drug development, including potential reliable as of the date of this presentation, we have not independently verified, and we delays in regulatory approval; our reliance on third parties and collaborators to expand make no representation as to the adequacy, fairness, accuracy or completeness of any our microbial library, conduct our clinical trials, manufacture our product candidates, and information obtained from third-party sources. In addition, all of the market data included develop and commercialize our product candidates, if approved; our lack of experience in this presentation involves a number of assumptions and limitations, and there can be in manufacturing, selling, marketing, and distributing our product candidates; failure to no guarantee as to the accuracy or reliability of such assumptions. Finally, while we compete successfully against other drug companies; protection of our proprietary believe our own internal research is reliable, such research has not been verified by any technology and the confidentiality of our trade secrets; potential lawsuits for, or claims independent source. of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; risks associated with international operations; our ability to retain key personnel and to manage our growth; the potential volatility of our common stock; our management and principal Legal Disclaimer This presentation contains forward-looking statements within the meaning of the Private stockholders have the ability to control or significantly influence our business; costs and Securities Litigation Reform Act of 1995. All statements contained in this presentation resources of operating as a public company; unfavorable or no analyst research or that do not relate to matters of historical fact should be considered forward-looking reports; and securities class action litigation against us. statements, including statements concerning the development of EDP1815, the promise These and other important factors discussed under the caption Risk Factors in our and potential impact of EDP1815, the timing of and plans for clinical studies, and the Quarterly Report on Form 10-Q for the three months ended June 30, 2021, and our timing and results of clinical trial readouts. other reports filed with the SEC could cause actual results to differ materially from those These forward-looking statements are based on management's current expectations. indicated by the forward-looking statements made in this presentation. Any such These statements are neither promises nor guarantees, but involve known and unknown forward-looking statements represent management's estimates as of the date of this risks, uncertainties and other important factors that may cause our actual results, presentation. While we may elect to update such forward-looking statements at some performance or achievements to be materially different from any future results, point in the future, except as required by law, we disclaim any obligation to do so, even if performance or achievements expressed or implied by the forward-looking statements, subsequent events cause our views to change. These forward-looking statements including, but not limited to, the following: the impact of the COVID-19 pandemic on our should not be relied upon as representing our views as of any date subsequent to the operations, including our preclinical studies and clinical trials, and the continuity of our date of this presentation. business; we have incurred significant losses, are not currently profitable and may never Certain information contained in this presentation relates to or is based on studies, become profitable; our need for additional funding; our cash runway; our limited publications, surveys and other data obtained from third-party sources and our own operating history; our unproven approach to therapeutic intervention; the lengthy, internal estimates and research. While we believe these third-party sources to be expensive, and uncertain process of clinical drug development, including potential reliable as of the date of this presentation, we have not independently verified, and we delays in regulatory approval; our reliance on third parties and collaborators to expand make no representation as to the adequacy, fairness, accuracy or completeness of any our microbial library, conduct our clinical trials, manufacture our product candidates, and information obtained from third-party sources. In addition, all of the market data included develop and commercialize our product candidates, if approved; our lack of experience in this presentation involves a number of assumptions and limitations, and there can be in manufacturing, selling, marketing, and distributing our product candidates; failure to no guarantee as to the accuracy or reliability of such assumptions. Finally, while we compete successfully against other drug companies; protection of our proprietary believe our own internal research is reliable, such research has not been verified by any technology and the confidentiality of our trade secrets; potential lawsuits for, or claims independent source. of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; risks associated with international operations; our ability to retain key personnel and to manage our growth; the potential volatility of our common stock; our management and principal

Positive Phase 2 data of EDP1815 confirms ability to harness the small intestinal axis, SINTAX ™, to treat systemic inflammatory diseases Clinically and statistically significant improvement in PASI-50 score achieved Oral EDP1815 can drive potent effects with placebo-like safety and tolerability EDP1815 advancing towards registration studies in psoriasisPositive Phase 2 data of EDP1815 confirms ability to harness the small intestinal axis, SINTAX ™, to treat systemic inflammatory diseases Clinically and statistically significant improvement in PASI-50 score achieved Oral EDP1815 can drive potent effects with placebo-like safety and tolerability EDP1815 advancing towards registration studies in psoriasis
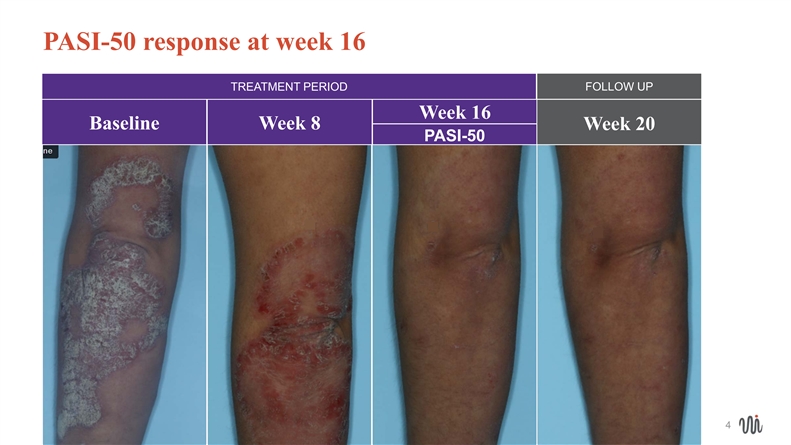
PASI-50 response at week 16 TREATMENT PERIOD FOLLOW UP Week 16 Baseline Week 8 Week 20 PASI-50 4 4PASI-50 response at week 16 TREATMENT PERIOD FOLLOW UP Week 16 Baseline Week 8 Week 20 PASI-50 4 4
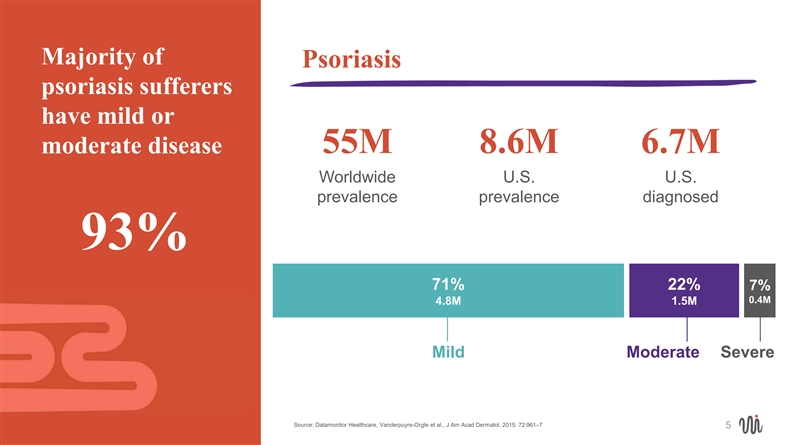
Majority of Psoriasis psoriasis sufferers have mild or moderate disease 55M 8.6M 6.7M Worldwide U.S. U.S. prevalence prevalence diagnosed 93% 71% 22% 7% 0.4M 4.8M 1.5M Mild Moderate Severe Source: Datamonitor Healthcare, Vanderpuyre-Orgle et al., J Am Acad Dermatol. 2015: 72:961–7 5Majority of Psoriasis psoriasis sufferers have mild or moderate disease 55M 8.6M 6.7M Worldwide U.S. U.S. prevalence prevalence diagnosed 93% 71% 22% 7% 0.4M 4.8M 1.5M Mild Moderate Severe Source: Datamonitor Healthcare, Vanderpuyre-Orgle et al., J Am Acad Dermatol. 2015: 72:961–7 5
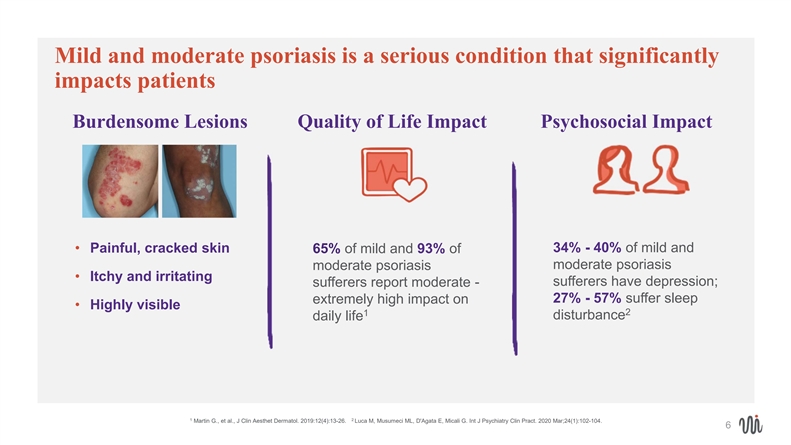
Mild and moderate psoriasis is a serious condition that significantly impacts patients Burdensome Lesions Quality of Life Impact Psychosocial Impact • Painful, cracked skin 34% - 40% of mild and 65% of mild and 93% of moderate psoriasis moderate psoriasis • Itchy and irritating sufferers report moderate - sufferers have depression; 27% - 57% suffer sleep extremely high impact on • Highly visible 2 1 disturbance daily life 1 2 Martin G., et al., J Clin Aesthet Dermatol. 2019:12(4):13-26. Luca M, Musumeci ML, D'Agata E, Micali G. Int J Psychiatry Clin Pract. 2020 Mar;24(1):102-104. 6Mild and moderate psoriasis is a serious condition that significantly impacts patients Burdensome Lesions Quality of Life Impact Psychosocial Impact • Painful, cracked skin 34% - 40% of mild and 65% of mild and 93% of moderate psoriasis moderate psoriasis • Itchy and irritating sufferers report moderate - sufferers have depression; 27% - 57% suffer sleep extremely high impact on • Highly visible 2 1 disturbance daily life 1 2 Martin G., et al., J Clin Aesthet Dermatol. 2019:12(4):13-26. Luca M, Musumeci ML, D'Agata E, Micali G. Int J Psychiatry Clin Pract. 2020 Mar;24(1):102-104. 6
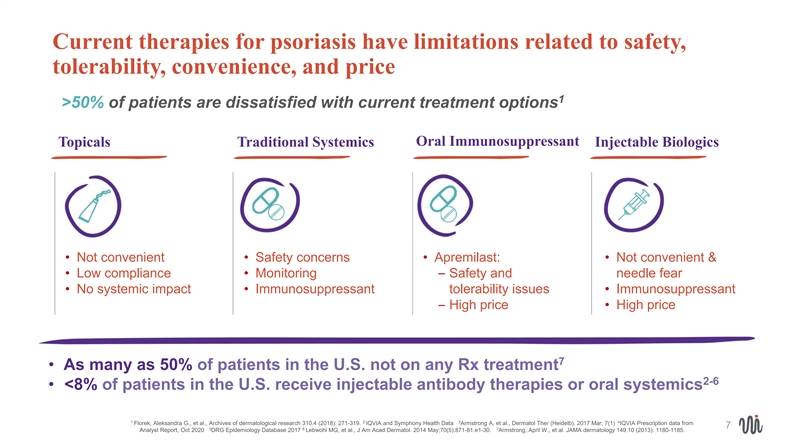
Current therapies for psoriasis have limitations related to safety, tolerability, convenience, and price 1 >50% of patients are dissatisfied with current treatment options Oral Immunosuppressant Topicals Traditional Systemics Injectable Biologics • Not convenient • Safety concerns • Apremilast: • Not convenient & • Low compliance • Monitoring– Safety and needle fear • No systemic impact • Immunosuppressant tolerability issues • Immunosuppressant – High price • High price 7 • As many as 50% of patients in the U.S. not on any Rx treatment 2-6 • <8% of patients in the U.S. receive injectable antibody therapies or oral systemics 1 2 3 4 Florek, Aleksandra G., et al., Archives of dermatological research 310.4 (2018): 271-319. IQVIA and Symphony Health Data Armstrong A, et al., Dermatol Ther (Heidelb). 2017 Mar; 7(1) IQVIA Prescription data from 7 5 6 7 Analyst Report, Oct 2020 DRG Epidemiology Database 2017 Lebwohl MG, et al., J Am Acad Dermatol. 2014 May;70(5):871-81.e1-30. Armstrong, April W., et al. JAMA dermatology 149.10 (2013): 1180-1185. Current therapies for psoriasis have limitations related to safety, tolerability, convenience, and price 1 >50% of patients are dissatisfied with current treatment options Oral Immunosuppressant Topicals Traditional Systemics Injectable Biologics • Not convenient • Safety concerns • Apremilast: • Not convenient & • Low compliance • Monitoring– Safety and needle fear • No systemic impact • Immunosuppressant tolerability issues • Immunosuppressant – High price • High price 7 • As many as 50% of patients in the U.S. not on any Rx treatment 2-6 • <8% of patients in the U.S. receive injectable antibody therapies or oral systemics 1 2 3 4 Florek, Aleksandra G., et al., Archives of dermatological research 310.4 (2018): 271-319. IQVIA and Symphony Health Data Armstrong A, et al., Dermatol Ther (Heidelb). 2017 Mar; 7(1) IQVIA Prescription data from 7 5 6 7 Analyst Report, Oct 2020 DRG Epidemiology Database 2017 Lebwohl MG, et al., J Am Acad Dermatol. 2014 May;70(5):871-81.e1-30. Armstrong, April W., et al. JAMA dermatology 149.10 (2013): 1180-1185.
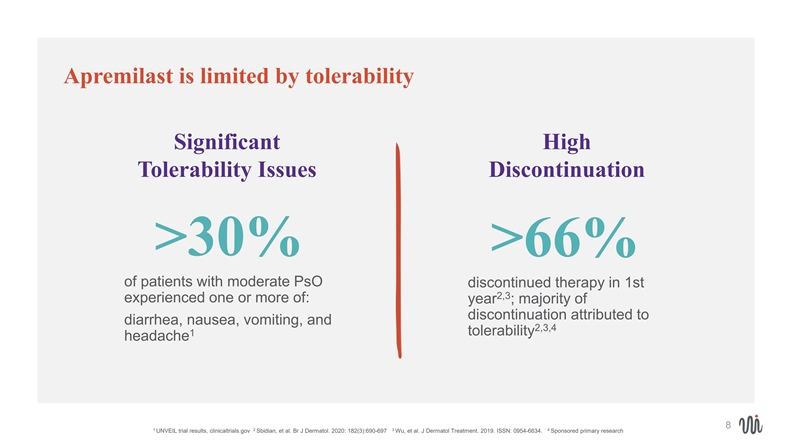
Apremilast is limited by tolerability Significant High Tolerability Issues Discontinuation >30% >66% of patients with moderate PsO discontinued therapy in 1st 2,3 experienced one or more of: year ; majority of discontinuation attributed to diarrhea, nausea, vomiting, and 2,3,4 tolerability 1 headache 8 1 2 3 4 UNVEIL trial results, clinicaltrials.gov Sbidian, et al. Br J Dermatol. 2020: 182(3):690-697 Wu, et al. J Dermatol Treatment. 2019. ISSN: 0954-6634. Sponsored primary researchApremilast is limited by tolerability Significant High Tolerability Issues Discontinuation >30% >66% of patients with moderate PsO discontinued therapy in 1st 2,3 experienced one or more of: year ; majority of discontinuation attributed to diarrhea, nausea, vomiting, and 2,3,4 tolerability 1 headache 8 1 2 3 4 UNVEIL trial results, clinicaltrials.gov Sbidian, et al. Br J Dermatol. 2020: 182(3):690-697 Wu, et al. J Dermatol Treatment. 2019. ISSN: 0954-6634. Sponsored primary research
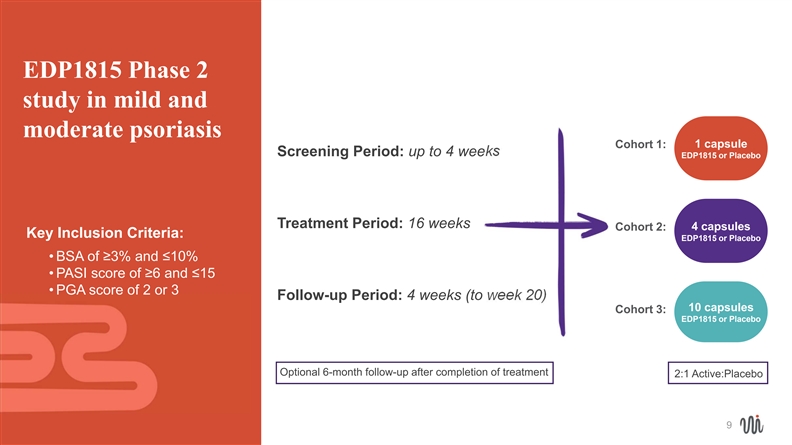
EDP1815 Phase 2 study in mild and moderate psoriasis 1 capsule Cohort 1: Screening Period: up to 4 weeks EDP1815 or Placebo Treatment Period: 16 weeks Cohort 2: 4 capsules Key Inclusion Criteria: EDP1815 or Placebo • BSA of ≥3% and ≤10% • PASI score of ≥6 and ≤15 • PGA score of 2 or 3 Follow-up Period: 4 weeks (to week 20) 10 capsules Cohort 3: EDP1815 or Placebo Optional 6-month follow-up after completion of treatment 2:1 Active:Placebo 9EDP1815 Phase 2 study in mild and moderate psoriasis 1 capsule Cohort 1: Screening Period: up to 4 weeks EDP1815 or Placebo Treatment Period: 16 weeks Cohort 2: 4 capsules Key Inclusion Criteria: EDP1815 or Placebo • BSA of ≥3% and ≤10% • PASI score of ≥6 and ≤15 • PGA score of 2 or 3 Follow-up Period: 4 weeks (to week 20) 10 capsules Cohort 3: EDP1815 or Placebo Optional 6-month follow-up after completion of treatment 2:1 Active:Placebo 9
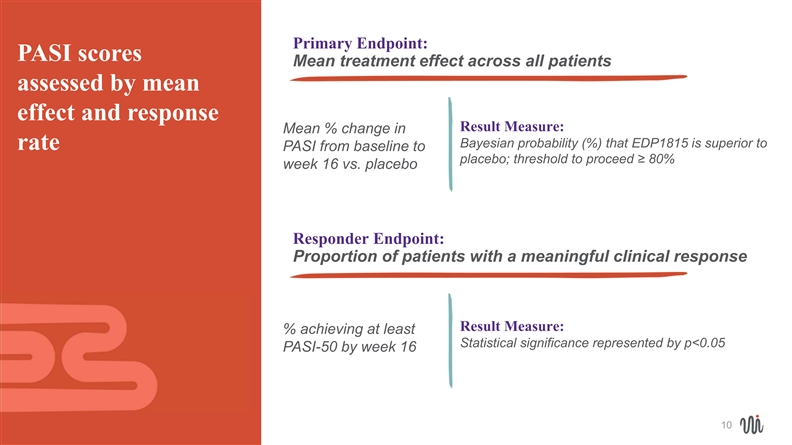
Primary Endpoint: PASI scores Mean treatment effect across all patients assessed by mean effect and response Result Measure: Mean % change in Bayesian probability (%) that EDP1815 is superior to rate PASI from baseline to placebo; threshold to proceed ≥ 80% week 16 vs. placebo Responder Endpoint: Proportion of patients with a meaningful clinical response Result Measure: % achieving at least Statistical significance represented by p<0.05 PASI-50 by week 16 10Primary Endpoint: PASI scores Mean treatment effect across all patients assessed by mean effect and response Result Measure: Mean % change in Bayesian probability (%) that EDP1815 is superior to rate PASI from baseline to placebo; threshold to proceed ≥ 80% week 16 vs. placebo Responder Endpoint: Proportion of patients with a meaningful clinical response Result Measure: % achieving at least Statistical significance represented by p<0.05 PASI-50 by week 16 10

Baseline characteristics are well-balanced across treatment groups Placebo Cohort 1 Cohort 2 Cohort 3 Parameter All (pooled) (1 capsule) (4 capsules) (10 capsules) Number 249 83 56 55 55 Age, mean years 44 44.4 44.5 41.2 45.5 Female (%) 36.9 39.8 39.3 43.6 23.6 2 BMI, mean kg/m 29.7 30.2 29.9 28.5 29.7 PGA 3 (%) 62.2 65.1 62.5 54.5 65.4 PASI mean 8.5 8.7 8.4 8.7 8.3 BSA mean % 7.3 7.3 7.4 7.3 7.1 11Baseline characteristics are well-balanced across treatment groups Placebo Cohort 1 Cohort 2 Cohort 3 Parameter All (pooled) (1 capsule) (4 capsules) (10 capsules) Number 249 83 56 55 55 Age, mean years 44 44.4 44.5 41.2 45.5 Female (%) 36.9 39.8 39.3 43.6 23.6 2 BMI, mean kg/m 29.7 30.2 29.9 28.5 29.7 PGA 3 (%) 62.2 65.1 62.5 54.5 65.4 PASI mean 8.5 8.7 8.4 8.7 8.3 BSA mean % 7.3 7.3 7.4 7.3 7.1 11
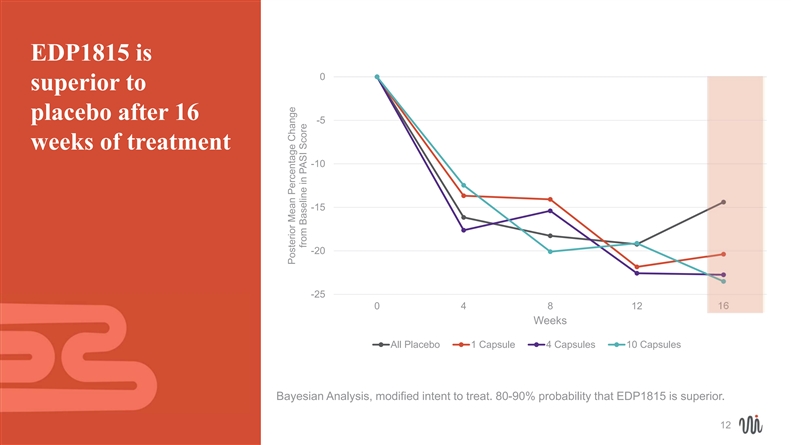
EDP1815 is 0 superior to placebo after 16 -5 weeks of treatment -10 -15 -20 -25 04 8 12 16 Weeks All Placebo 1 Capsule 4 Capsules 10 Capsules Bayesian Analysis, modified intent to treat. 80-90% probability that EDP1815 is superior. 12 Posterior Mean Percentage Change from Baseline in PASI Score
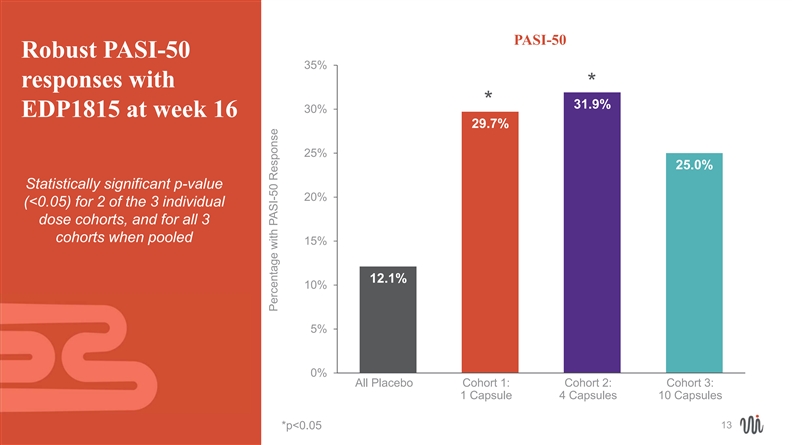
PASI-50 Robust PASI-50 35% responses with * * 31.9% 30% EDP1815 at week 16 29.7% 25% 25.0% Statistically significant p-value 20% (<0.05) for 2 of the 3 individual dose cohorts, and for all 3 cohorts when pooled 15% 12.1% 10% 5% 0% All Placebo Cohort 1: Cohort 2: Cohort 3: 1 Capsule 4 Capsules 10 Capsules 13 *p<0.05 Percentage with PASI-50 ResponsePASI-50 Robust PASI-50 35% responses with * * 31.9% 30% EDP1815 at week 16 29.7% 25% 25.0% Statistically significant p-value 20% (<0.05) for 2 of the 3 individual dose cohorts, and for all 3 cohorts when pooled 15% 12.1% 10% 5% 0% All Placebo Cohort 1: Cohort 2: Cohort 3: 1 Capsule 4 Capsules 10 Capsules 13 *p<0.05 Percentage with PASI-50 Response

PASI-75 responses PASI-75 increase with time 15 Placebo 1 capsule 4 capsules 10 capsules 10 Studies of longer treatment with EDP1815 may lead to deeper responses 5 0 Week 16 Week 20 Follow-up period (off study drug) 14 Difference from placebo not statistically significant at week 16 Percent RespondersPASI-75 responses PASI-75 increase with time 15 Placebo 1 capsule 4 capsules 10 capsules 10 Studies of longer treatment with EDP1815 may lead to deeper responses 5 0 Week 16 Week 20 Follow-up period (off study drug) 14 Difference from placebo not statistically significant at week 16 Percent Responders
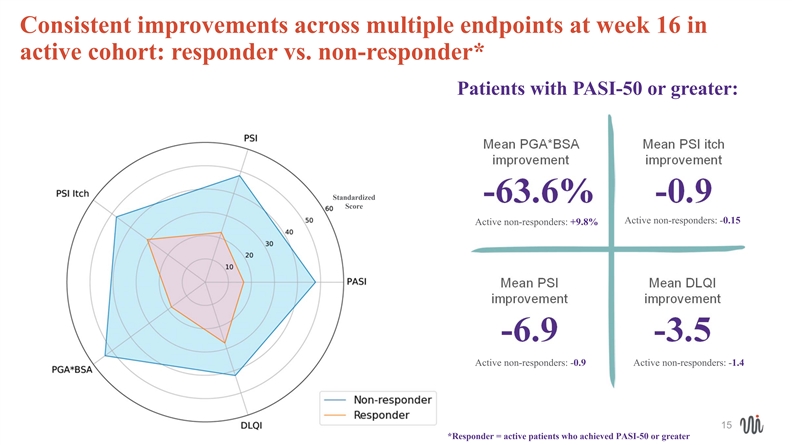
Consistent improvements across multiple endpoints at week 16 in active cohort: responder vs. non-responder* Patients with PASI-50 or greater: Mean PGA*BSA Mean PSI itch improvement improvement Standardized -63.6% -0.9 Score Active non-responders: -0.15 Active non-responders: +9.8% Mean PSI Mean DLQI improvement improvement -6.9 -3.5 Active non-responders: -0.9 Active non-responders: -1.4 15 *Responder = active patients who achieved PASI-50 or greater Consistent improvements across multiple endpoints at week 16 in active cohort: responder vs. non-responder* Patients with PASI-50 or greater: Mean PGA*BSA Mean PSI itch improvement improvement Standardized -63.6% -0.9 Score Active non-responders: -0.15 Active non-responders: +9.8% Mean PSI Mean DLQI improvement improvement -6.9 -3.5 Active non-responders: -0.9 Active non-responders: -1.4 15 *Responder = active patients who achieved PASI-50 or greater
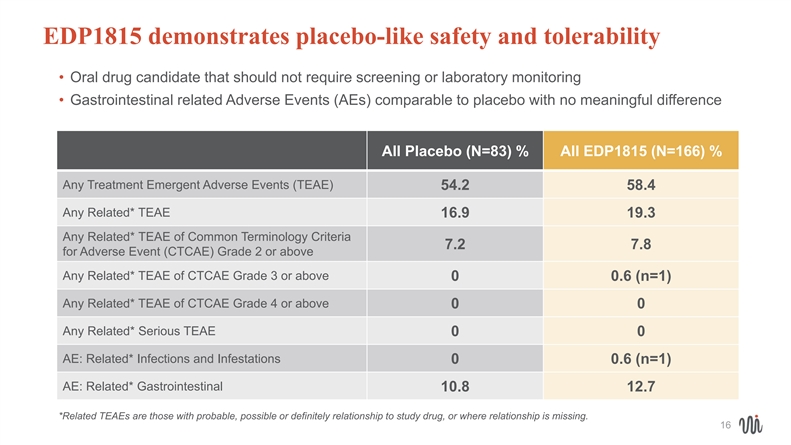
EDP1815 demonstrates placebo-like safety and tolerability • Oral drug candidate that should not require screening or laboratory monitoring • Gastrointestinal related Adverse Events (AEs) comparable to placebo with no meaningful difference All Placebo (N=83) % All EDP1815 (N=166) % Any Treatment Emergent Adverse Events (TEAE) 54.2 58.4 Any Related* TEAE 16.9 19.3 Any Related* TEAE of Common Terminology Criteria 7.2 7.8 for Adverse Event (CTCAE) Grade 2 or above Any Related* TEAE of CTCAE Grade 3 or above 00.6 (n=1) Any Related* TEAE of CTCAE Grade 4 or above 00 Any Related* Serious TEAE 00 AE: Related* Infections and Infestations 00.6 (n=1) AE: Related* Gastrointestinal 10.8 12.7 *Related TEAEs are those with probable, possible or definitely relationship to study drug, or where relationship is missing. 16EDP1815 demonstrates placebo-like safety and tolerability • Oral drug candidate that should not require screening or laboratory monitoring • Gastrointestinal related Adverse Events (AEs) comparable to placebo with no meaningful difference All Placebo (N=83) % All EDP1815 (N=166) % Any Treatment Emergent Adverse Events (TEAE) 54.2 58.4 Any Related* TEAE 16.9 19.3 Any Related* TEAE of Common Terminology Criteria 7.2 7.8 for Adverse Event (CTCAE) Grade 2 or above Any Related* TEAE of CTCAE Grade 3 or above 00.6 (n=1) Any Related* TEAE of CTCAE Grade 4 or above 00 Any Related* Serious TEAE 00 AE: Related* Infections and Infestations 00.6 (n=1) AE: Related* Gastrointestinal 10.8 12.7 *Related TEAEs are those with probable, possible or definitely relationship to study drug, or where relationship is missing. 16
Photographs of EDP1815 treatment responders • Mild or moderate psoriasis • Illustrating PASI-50 and greater responses at week 16 (on daily EDP1815) • All maintained or improved response at week 20 (4 weeks after cessation of EDP1815) • No use of topical steroidsPhotographs of EDP1815 treatment responders • Mild or moderate psoriasis • Illustrating PASI-50 and greater responses at week 16 (on daily EDP1815) • All maintained or improved response at week 20 (4 weeks after cessation of EDP1815) • No use of topical steroids
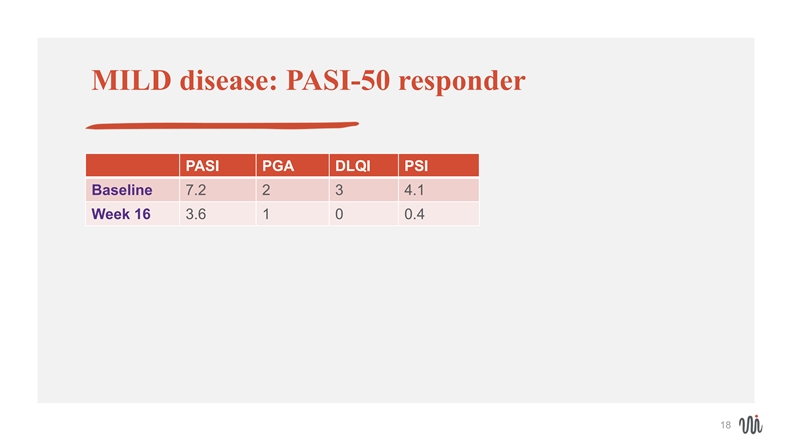
MILD disease: PASI-50 responder PASI PGA DLQI PSI Baseline 7.2 2 3 4.1 Week 16 3.6 1 0 0.4 18MILD disease: PASI-50 responder PASI PGA DLQI PSI Baseline 7.2 2 3 4.1 Week 16 3.6 1 0 0.4 18
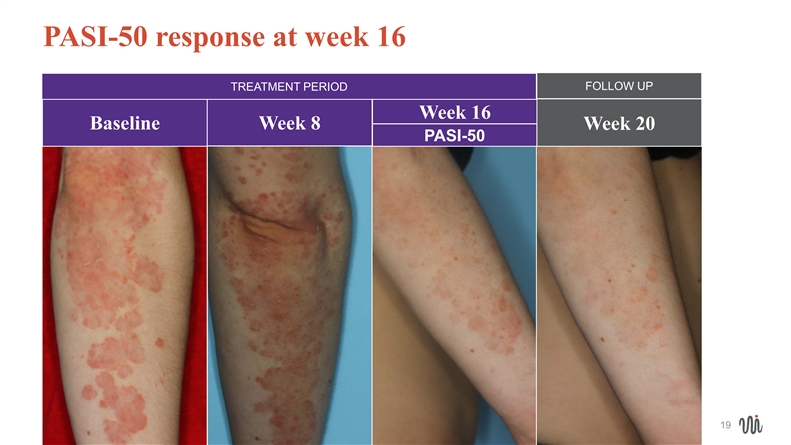
PASI-50 response at week 16 FOLLOW UP TREATMENT PERIOD Week 16 Baseline Week 8 Week 20 PASI-50 19 19PASI-50 response at week 16 FOLLOW UP TREATMENT PERIOD Week 16 Baseline Week 8 Week 20 PASI-50 19 19
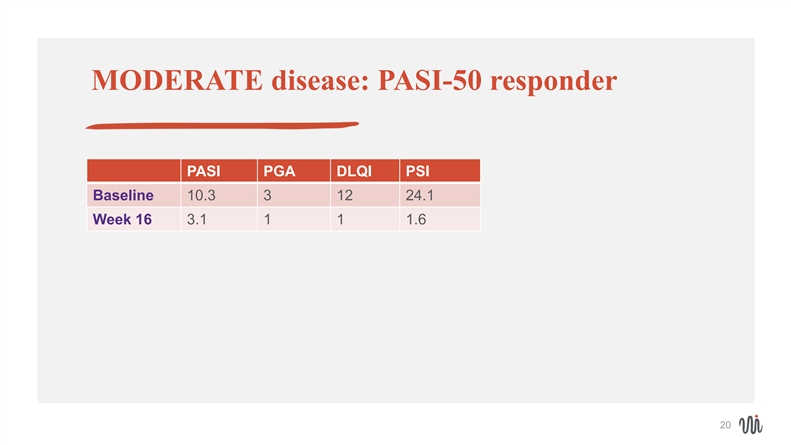
MODERATE disease: PASI-50 responder PASI PGA DLQI PSI Baseline 10.3 3 12 24.1 Week 16 3.1 1 1 1.6 20

PASI-50 response at week 16 TREATMENT PERIOD FOLLOW UP Week 16 Baseline Week 8 Week 20 PASI-50 21 21PASI-50 response at week 16 TREATMENT PERIOD FOLLOW UP Week 16 Baseline Week 8 Week 20 PASI-50 21 21
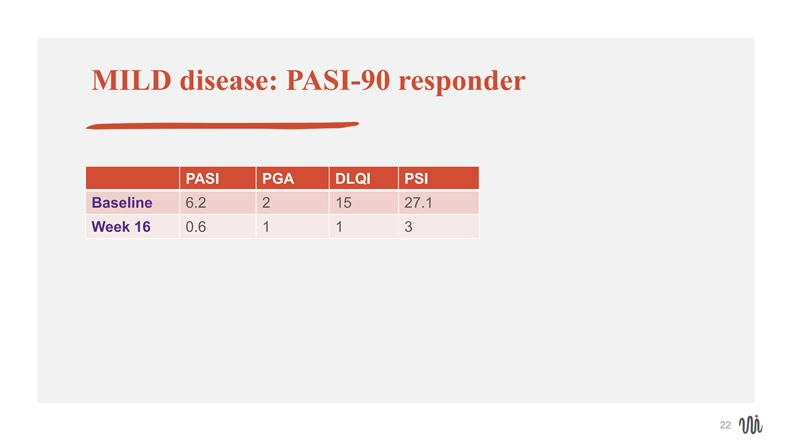
MILD disease: PASI-90 responder PASI PGA DLQI PSI Baseline 6.2 2 15 27.1 Week 16 0.6 1 1 3 22MILD disease: PASI-90 responder PASI PGA DLQI PSI Baseline 6.2 2 15 27.1 Week 16 0.6 1 1 3 22
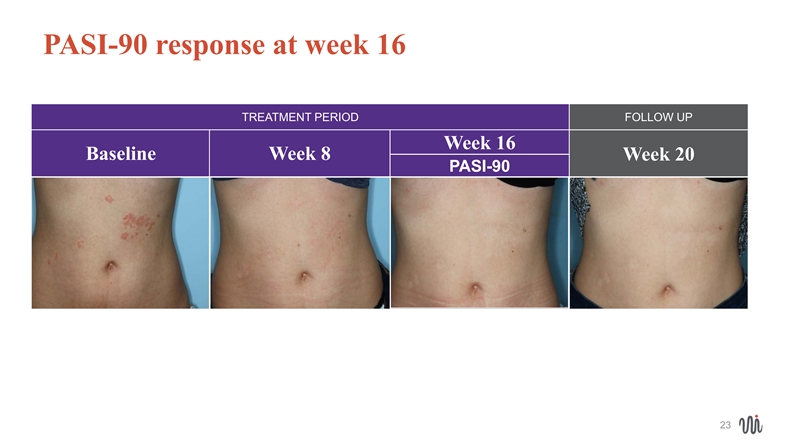
PASI-90 response at week 16 TREATMENT PERIOD FOLLOW UP Week 16 Baseline Week 8 Week 20 PASI-90 23 23PASI-90 response at week 16 TREATMENT PERIOD FOLLOW UP Week 16 Baseline Week 8 Week 20 PASI-90 23 23
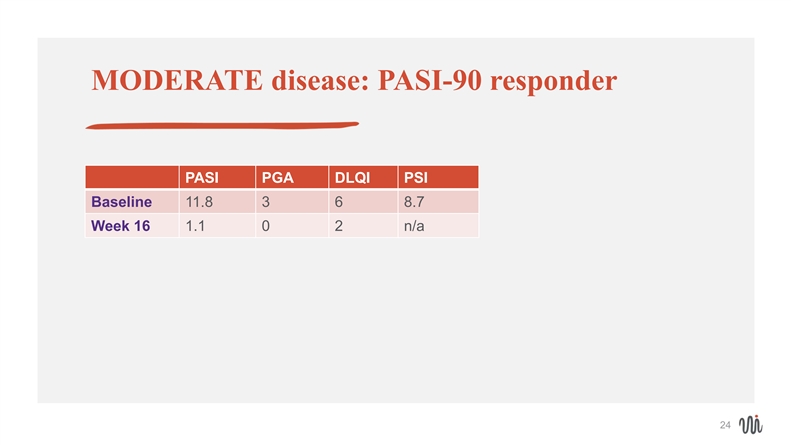
MODERATE disease: PASI-90 responder PASI PGA DLQI PSI Baseline 11.8 3 6 8.7 Week 16 1.1 0 2 n/a 24MODERATE disease: PASI-90 responder PASI PGA DLQI PSI Baseline 11.8 3 6 8.7 Week 16 1.1 0 2 n/a 24
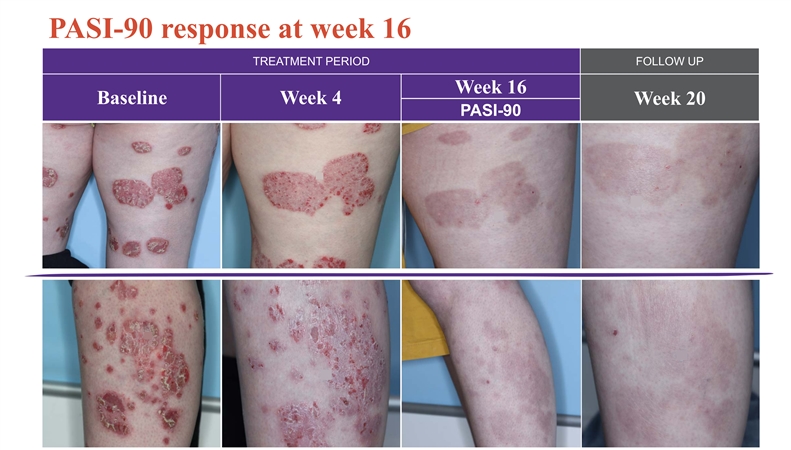
PASI-90 response at week 16 TREATMENT PERIOD FOLLOW UP Week 16 Baseline Week 4 Week 20 PASI-90 25 25PASI-90 response at week 16 TREATMENT PERIOD FOLLOW UP Week 16 Baseline Week 4 Week 20 PASI-90 25 25

MODERATE disease: Week 20 PASI-50 response PASI PGA DLQI PSI Baseline 10.5 3 3 4.9 Week 16 5.7* 2 0 2.0 26MODERATE disease: Week 20 PASI-50 response PASI PGA DLQI PSI Baseline 10.5 3 3 4.9 Week 16 5.7* 2 0 2.0 26
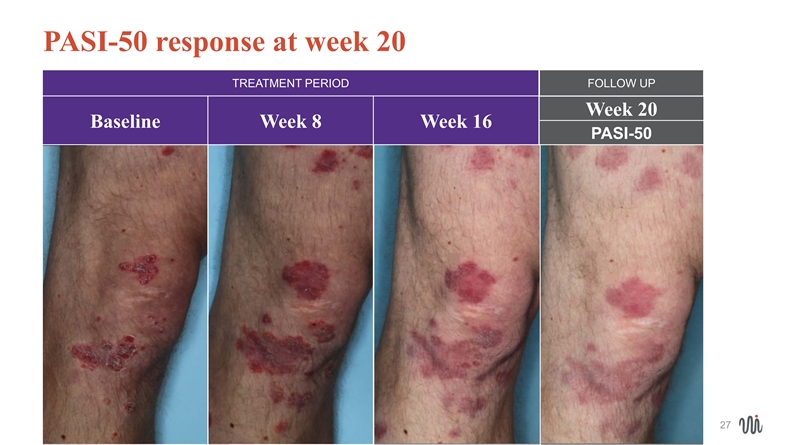
PASI-50 response at week 20 TREATMENT PERIOD FOLLOW UP Week 20 Baseline Week 8 Week 16 PASI-50 27 27PASI-50 response at week 20 TREATMENT PERIOD FOLLOW UP Week 20 Baseline Week 8 Week 16 PASI-50 27 27
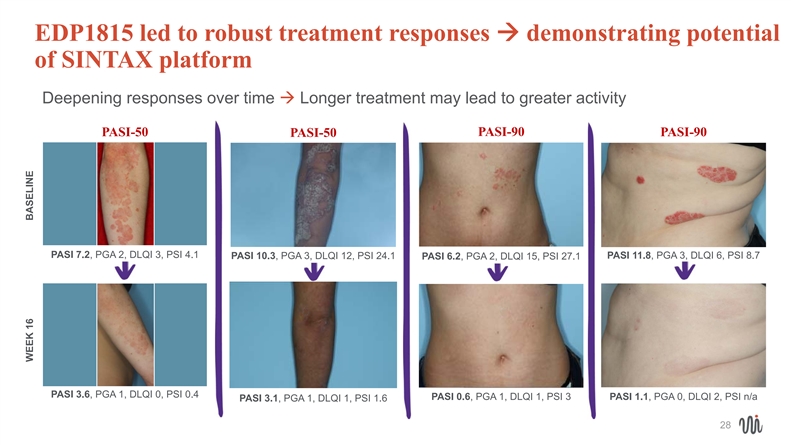
EDP1815 led to robust treatment responses à demonstrating potential of SINTAX platform Deepening responses over timeà Longer treatment may lead to greater activity PASI-50 PASI-50 PASI-90 PASI-90 PASI 7.2, PGA 2, DLQI 3, PSI 4.1 PASI 11.8, PGA 3, DLQI 6, PSI 8.7 PASI 10.3, PGA 3, DLQI 12, PSI 24.1 PASI 6.2, PGA 2, DLQI 15, PSI 27.1 PASI 3.6, PGA 1, DLQI 0, PSI 0.4 PASI 0.6, PGA 1, DLQI 1, PSI 3 PASI 1.1, PGA 0, DLQI 2, PSI n/a PASI 3.1, PGA 1, DLQI 1, PSI 1.6 28 WEEK 16 BASELINE

Summary of additional Phase 1b studies • Mild and moderate psoriasis using EDP1815 capsule dosage form • Results from this 8-week study were consistent with the 8-week time point in Phase 2 • Successful bridge to future studies using the commercial manufacturing process • Human scintigraphy of capsule and tablet dosage forms • Tablets take longer to release EDP1815 than capsules • Results underscore the importance of an optimized release profile • Mild and moderate psoriasis using EDP1815 tablet dosage form • There was no clear evidence of a drug effect after 8 weeks of treatment • Likely explanation is the different release profile of tablets vs. capsules 29Summary of additional Phase 1b studies • Mild and moderate psoriasis using EDP1815 capsule dosage form • Results from this 8-week study were consistent with the 8-week time point in Phase 2 • Successful bridge to future studies using the commercial manufacturing process • Human scintigraphy of capsule and tablet dosage forms • Tablets take longer to release EDP1815 than capsules • Results underscore the importance of an optimized release profile • Mild and moderate psoriasis using EDP1815 tablet dosage form • There was no clear evidence of a drug effect after 8 weeks of treatment • Likely explanation is the different release profile of tablets vs. capsules 29
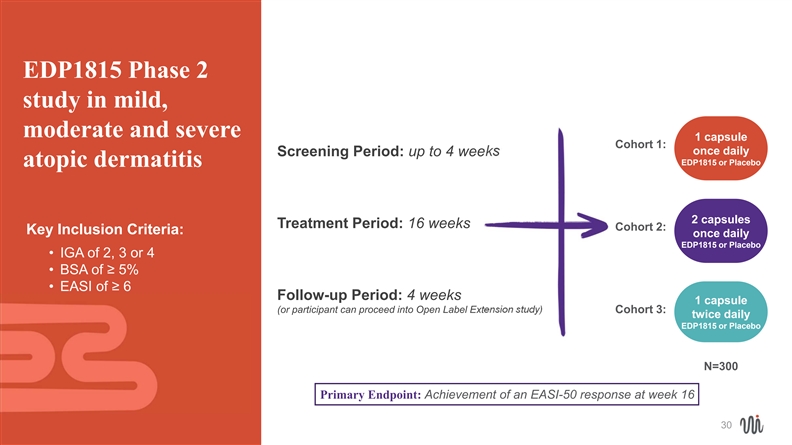
EDP1815 Phase 2 study in mild, moderate and severe 1 capsule Cohort 1: once daily Screening Period: up to 4 weeks EDP1815 or Placebo atopic dermatitis 2 capsules Treatment Period: 16 weeks Cohort 2: Key Inclusion Criteria: once daily EDP1815 or Placebo • IGA of 2, 3 or 4 • BSA of ≥ 5% • EASI of ≥ 6 Follow-up Period: 4 weeks 1 capsule (or participant can proceed into Open Label Extension study) Cohort 3: twice daily EDP1815 or Placebo N=300 Primary Endpoint: Achievement of an EASI-50 response at week 16 30EDP1815 Phase 2 study in mild, moderate and severe 1 capsule Cohort 1: once daily Screening Period: up to 4 weeks EDP1815 or Placebo atopic dermatitis 2 capsules Treatment Period: 16 weeks Cohort 2: Key Inclusion Criteria: once daily EDP1815 or Placebo • IGA of 2, 3 or 4 • BSA of ≥ 5% • EASI of ≥ 6 Follow-up Period: 4 weeks 1 capsule (or participant can proceed into Open Label Extension study) Cohort 3: twice daily EDP1815 or Placebo N=300 Primary Endpoint: Achievement of an EASI-50 response at week 16 30

Positive Phase 2 data of EDP1815 confirms ability to harness the small intestinal axis, SINTAX, to treat systemic inflammatory diseases Clinically and statistically significant improvement in PASI-50 score achieved Oral EDP1815 can drive potent effects with placebo-like safety and tolerability EDP1815 advancing towards registration studies in psoriasisPositive Phase 2 data of EDP1815 confirms ability to harness the small intestinal axis, SINTAX, to treat systemic inflammatory diseases Clinically and statistically significant improvement in PASI-50 score achieved Oral EDP1815 can drive potent effects with placebo-like safety and tolerability EDP1815 advancing towards registration studies in psoriasis
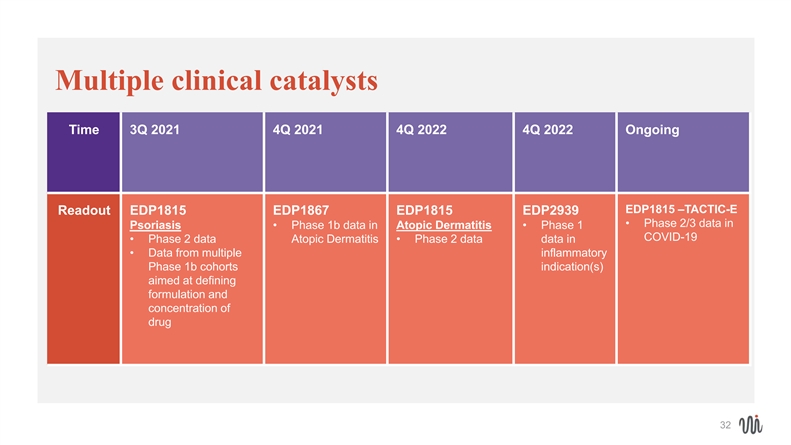
Multiple clinical catalysts Time 3Q 2021 4Q 2021 4Q 2022 4Q 2022 Ongoing EDP1815 –TACTIC-E Readout EDP1815 EDP1867 EDP1815 EDP2939 • Phase 2/3 data in Psoriasis • Phase 1b data in Atopic Dermatitis •Phase 1 COVID-19 • Phase 2 data Atopic Dermatitis •Phase 2 data data in • Data from multiple inflammatory Phase 1b cohorts indication(s) aimed at defining formulation and concentration of drug 32Multiple clinical catalysts Time 3Q 2021 4Q 2021 4Q 2022 4Q 2022 Ongoing EDP1815 –TACTIC-E Readout EDP1815 EDP1867 EDP1815 EDP2939 • Phase 2/3 data in Psoriasis • Phase 1b data in Atopic Dermatitis •Phase 1 COVID-19 • Phase 2 data Atopic Dermatitis •Phase 2 data data in • Data from multiple inflammatory Phase 1b cohorts indication(s) aimed at defining formulation and concentration of drug 32

Q&AQ&A
































