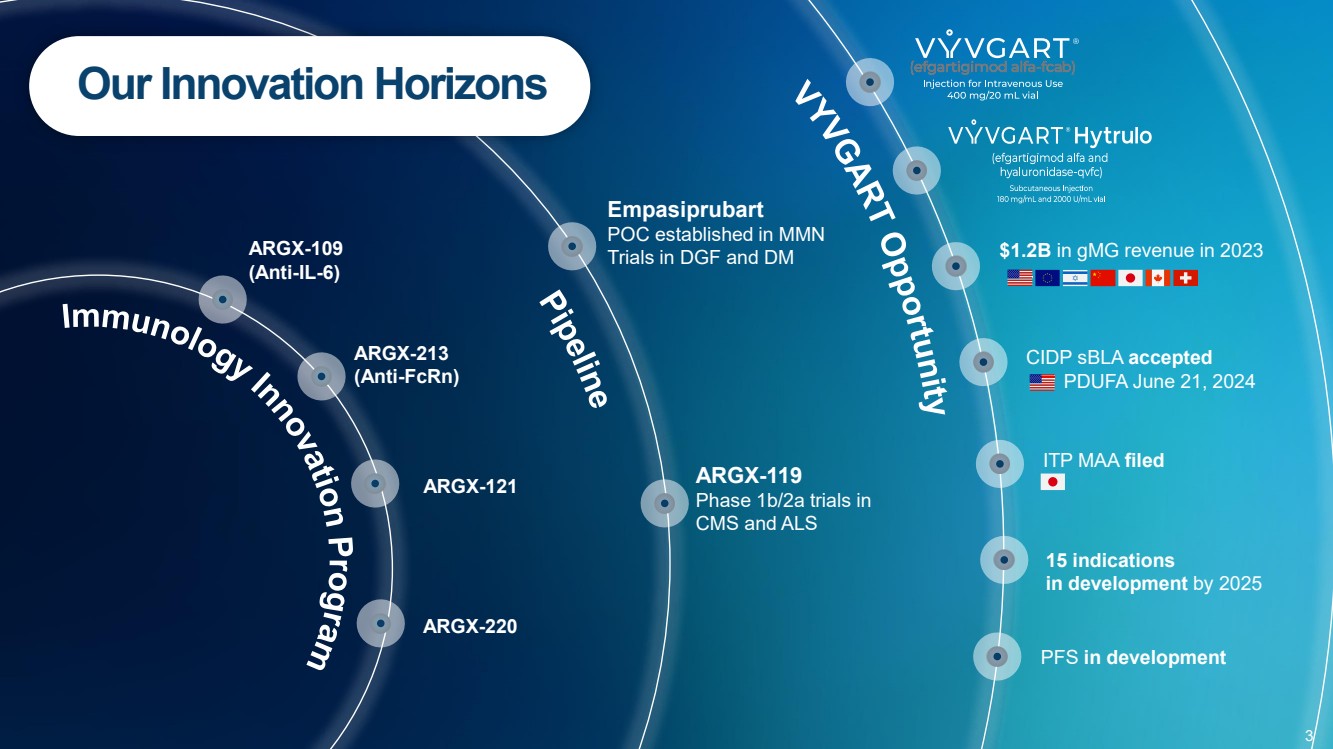
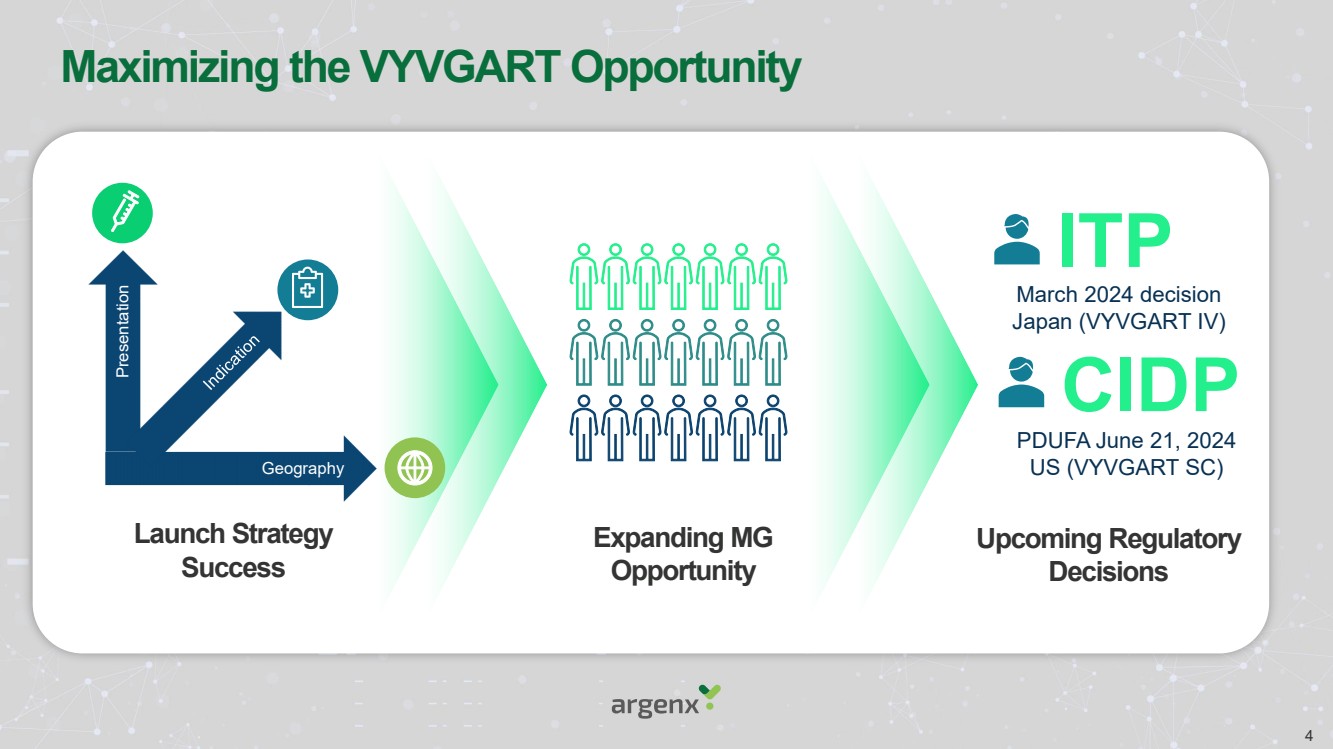
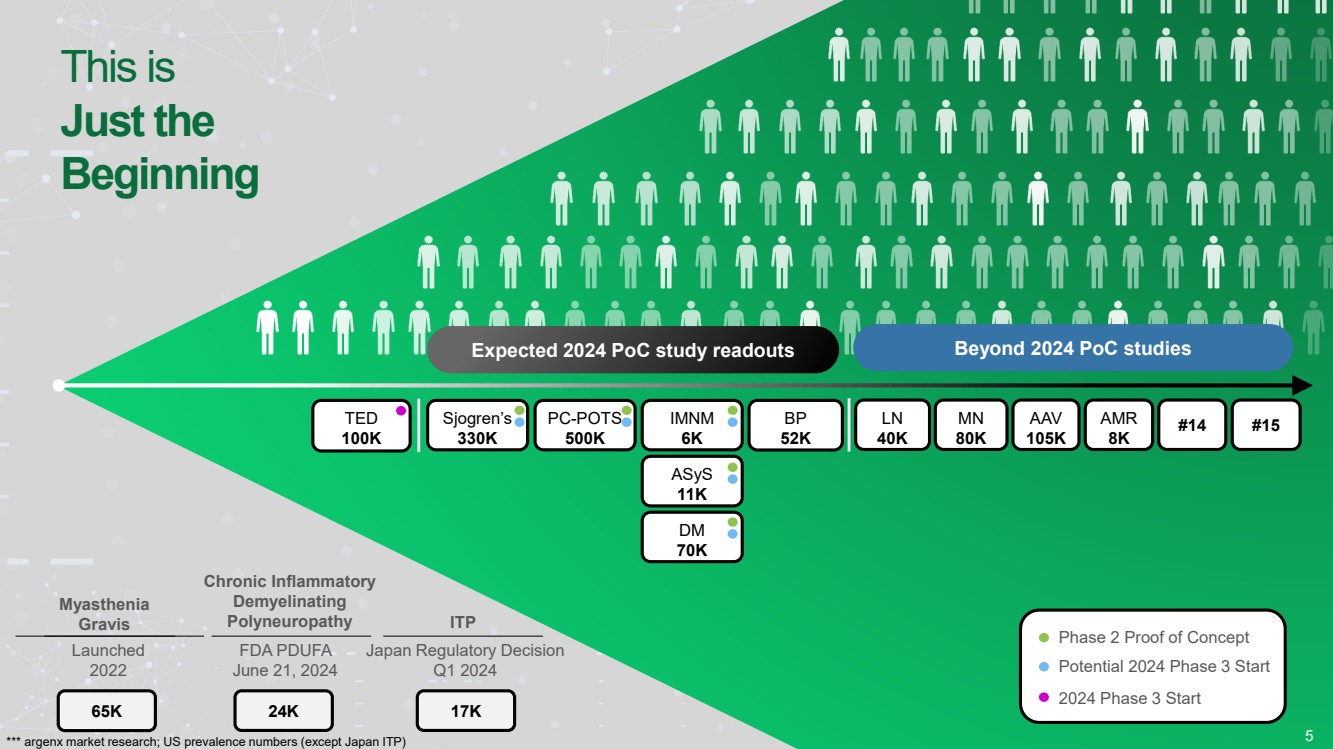
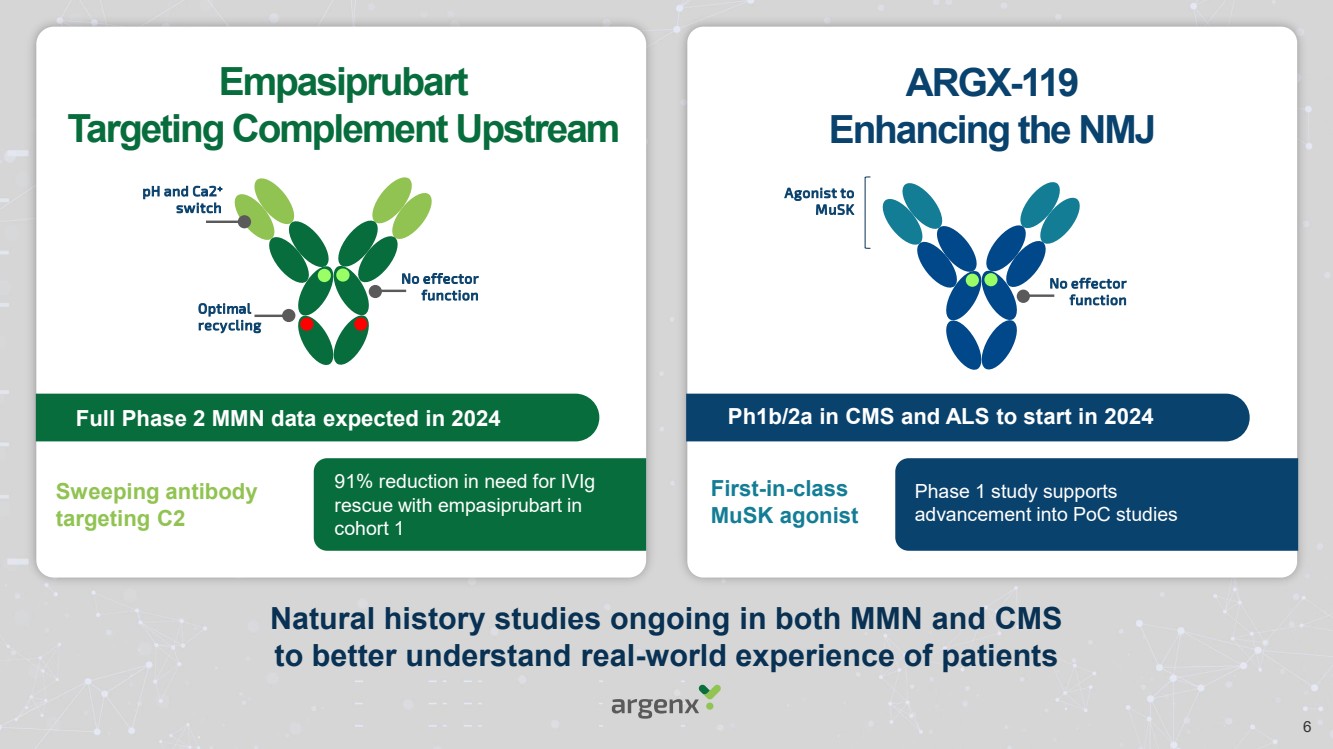
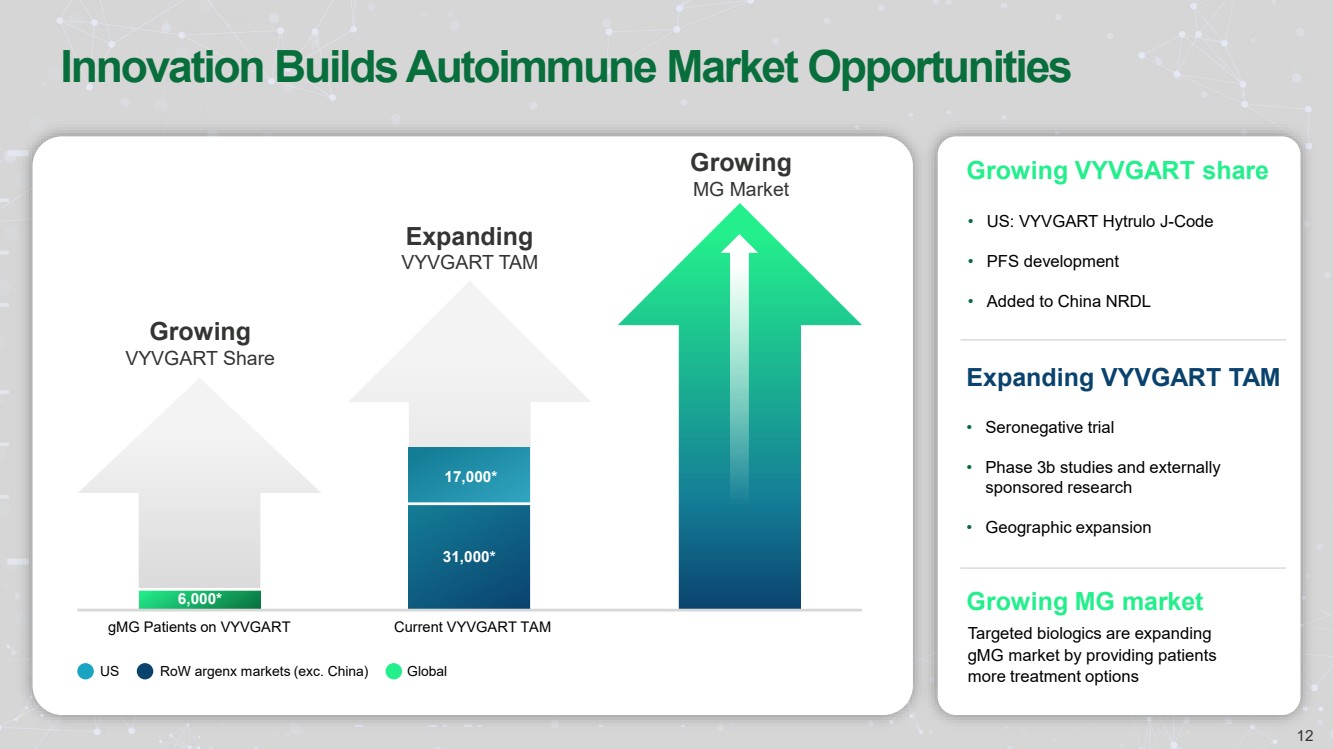
| Forward Looking Statements • This presentation has been prepared by argenx se (“argenx” or the “company”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or the company or any director, employee, agent, or adviser of the company. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the company’s own internal estimates and research. While argenx believes these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of argenx’s internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. • • Certain statements contained in this presentation, other than present and historical facts and conditions independently verifiable at the date hereof, may constitute forward-looking statements. These forward-looking statements can be identified by the use of forward-looking terminology, including the terms “plans,” “aims,” “continues,” “anticipates,” “expects,” “will,” or “commitment” and include statements argenx makes concerning its Immunology Innovation Program and its pipeline, including argenx’s goal to expand technical capabilities through collaboration with different partners to drive internal and external value creation; the expected approval or development in 15 autoimmune indications by 2025; our plans to maximize the VYVGART opportunity by its launch strategy success and expanding opportunities for MG; the advancement of, and anticipated clinical development, data readouts and regulatory milestones and plans, including: (1) the update on pre-filled syringe (“PFS”) development, (2) expected decisions on approval of VYVGART for ITP in Japan in the first quarter of 2024, (3) expected PoC study readouts in 2024 and beyond, (5) expected regulatory submissions of VYVGART SC of CIDP in 2024, (6) the Full Phase 2 MMN data expected in 2024, (7) the planned Phase 1b/2a clinical trials of ARGX-119 in 2024; its plans to expand its patient reach, including through its multidimensional expansion efforts aimed at expanding opportunities for MG and pursuing global regulatory approvals for MG; its goal to continue to drive transformational outcomes for patients and maximize value creation and patient impact by reaching new gMG patients with VYVGART and leveraging MG know-how into future indications; its future financial and operating performance, including its anticipated operating expenses and cash burn for 2024; its autoimmune market opportunities; its goal to address the unseen suffering in CIDP; and its commitment to value creation. By their nature, forward-looking statements involve risks and uncertainties and readers are cautioned that any such forward-looking statements are not guarantees of future performance. argenx’s actual results may differ materially from those predicted by the forward-looking statements as a result of various important factors, including but not limited to, the results of argenx’s clinical trials, expectations regarding the inherent uncertainties associated with development of novel drug therapies, preclinical and clinical trial and product development activities and regulatory approval requirements, the acceptance of our products and product candidates by our patients as safe, effective and cost-effective, and the impact of governmental laws and regulations on our business. A further list and description of these risks, uncertainties and other risks can be found in argenx’s U.S. Securities and Exchange Commission (SEC) filings and reports, including in argenx’s most recent annual report on Form 20-F filed with the SEC as well as subsequent filings and reports filed by argenx with the SEC. Given these uncertainties, the reader is advised not to place any undue reliance on such forward-looking statements. These forward-looking statements speak only as of the date of publication of this document. argenx undertakes no obligation to publicly update or revise the information in this presentation, including any forward-looking statements, except as may be required by law. • • This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. .. 2 |