
Corporate Presentation January 2023 Exhibit 99.1

Forward-looking Statement This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, the potential regulatory path forward for tebipenem HBr and the potential approval of tebipenem HBr by the U.S. Food and Drug Administration (FDA) and the timing thereof; the potential commercialization of tebipenem HBr and its future value, the potential receipt of milestone payments, and royalties on future sales of tebipenem HBr under the GlaxoSmithKline Intellectual Property (No. 3) Limited (GSK) license agreement; Spero's cash runway and estimated cash balance; the future development and commercialization of SPR206 and SPR720; the potential number of patients who could be treated by tebipenem HBr and SPR720 and market demand for tebipenem HBr and SPR720 generally; the effectiveness of tebipenem HBr and its potential impact on healthcare resource utilizations; expected broad access across payer channels for tebipenem HBr; the anticipated shift in treating patients from intravenous to oral administration; the initiation, timing, progress and results of the Company’s preclinical studies and clinical trials and its research and development programs, including management’s assessment of such results; the direct and indirect impact of the pandemic caused by an outbreak of a strain of coronavirus (COVID-19) on the Company’s business and operations; the timing of the availability of data from the Company’s clinical trials; the timing of the Company’s filings with regulatory agencies; product candidate benefits; competitive position; business strategies; objectives of management; potential growth opportunities; potential market size; reimbursement matters; possible or assumed future results of operations; projected costs; and the Company’s cash forecast and the availability of additional non-dilutive funding from governmental agencies beyond any initially funded awards. In some cases, forward-looking statements can be identified by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. All statements other than statements of historical facts contained in this presentation are forward-looking statements. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various factors, including whether the FDA will ultimately approve tebipenem HBr and, if so, the timing of any such approval; whether the FDA will require any additional clinical data or place labeling restrictions on the use of tebipenem HBr that would add costs for the Company, delay approval and/or reduce the commercial prospects of tebipenem HBr; the Company’s need for additional funding; the lengthy, expensive, and uncertain process of clinical drug development; the Company’s reliance on third parties to manufacture, develop, and commercialize its product candidates, if approved; the ability to develop and commercialize the Company’s product candidates, if approved; the potential impact of the COVID-19 pandemic; the Company’s ability to retain key personnel; whether results obtained in preclinical studies and clinical trials will be indicative of results obtained in future clinical trials and whether preliminary data from the Company’s clinical trials will be predictive of final results from such trials; whether the Company’s product candidates will advance through the preclinical development and clinical trial process on a timely basis, or at all, taking into account such factors as the effects of possible regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, clinical trial design, clinical data requirements and clinical outcomes; whether the results of such clinical trials will warrant submission for approval from the FDA or equivalent foreign regulatory agencies; decisions made by the FDA and equivalent foreign regulatory agencies with respect to the development and commercialization of the Company’s product candidates; the commercial potential of the Company’s product candidates; the Company’s ability to obtain adequate third-party reimbursement for its product candidates; whether the Company will satisfy all of the pre-conditions to receipt of the milestone payments under its various license and collaboration agreements; whether BARDA elects to exercise its second option under the Company’s agreement with BARDA; the Company’s ability to implement its strategic plans; the Company’s ability to obtain, maintain and enforce intellectual property and other proprietary rights for its product candidates; the risks and uncertainties related to market conditions; whether the Company’s cash resources will be sufficient to fund its continuing operations for the periods and/or trials anticipated; and other factors discussed in the “Risk Factors” section of the Company’s periodic reports filed with the U.S. Securities and Exchange Commission (SEC), and risks described in other filings the Company may make with the SEC in the future. The forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation.
Spero Therapeutics, Inc. A Leader in Infectious and Rare Diseases SPR720: Potential Novel, First Line, Oral Therapy for NTM Infections Strong Balance Sheet Supported by World Class Partnerships for Tebipenem HBr, SPR206 NTM = non-tuberculous mycobacterial; MDR = multidrug resistant infections; Tebipenem HBr = tebipenem pivoxil hydrobromide (formerly SPR994), 1. CHEST Foundation, About Nontuberculous Mycobacteria (NTM) lung infections; Kevin L. Winthrop et al., Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large United States Managed Care Health Plan, 2008-2015 (Feb. 2020) 2. As of Dec 31, 2022, This figure is unaudited, and considered a preliminary estimate that is subject to change Three late-stage medicines, targeting indications with high unmet need and strong commercial prospects Seasoned executive team, with significant clinical, regulatory and operational experience (10+ credited drug approvals at prior companies) NTM: Large orphan disease patient population (approximately 240k patients worldwide1). No approved first line therapies Oral therapy targeting bacterial DNA replication Initiated Phase 2 proof-of-concept study in 4Q 2022 Phase 1 SAD/MAD study and in vivo efficacy data support potential for NTM patients $600M global partnership with GSK fully supports tebipenem HBr clinical program through potential approval Partnership milestones, non-dilutive funding, and over $100 million2 in available cash provides operating runway beyond 2024
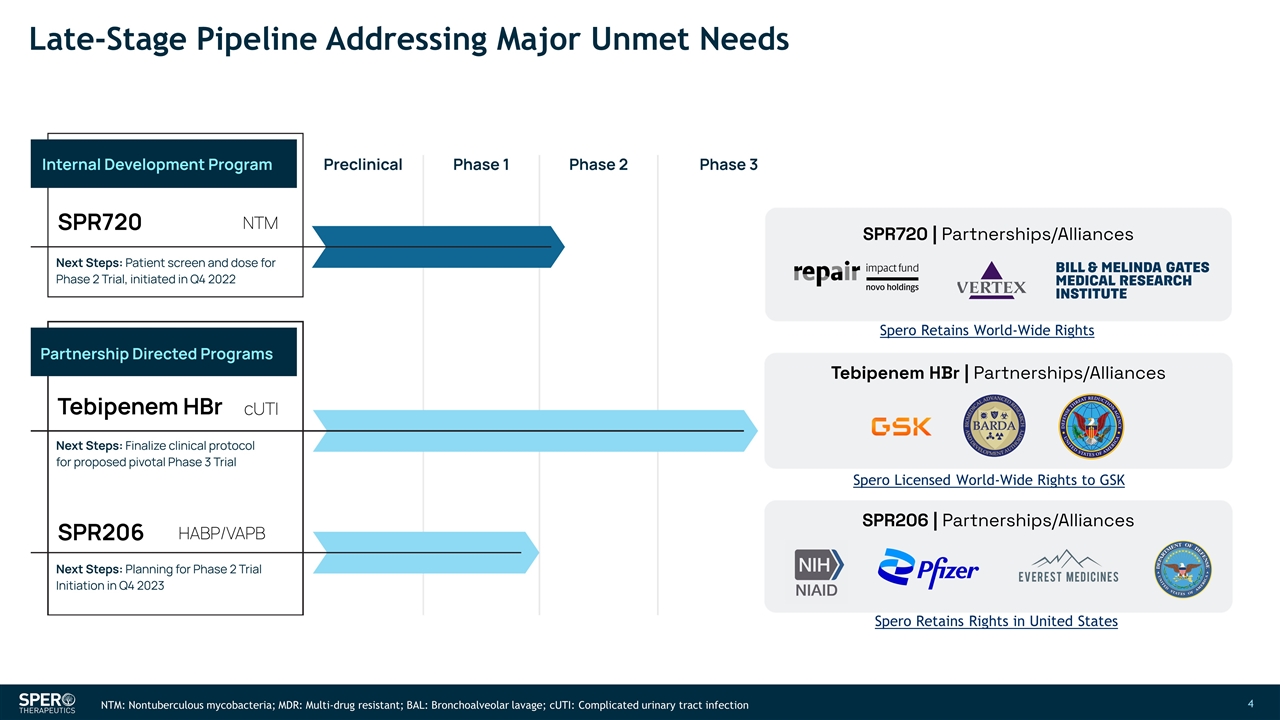
Late-Stage Pipeline Addressing Major Unmet Needs NTM: Nontuberculous mycobacteria; MDR: Multi-drug resistant; BAL: Bronchoalveolar lavage; cUTI: Complicated urinary tract infection Spero Retains World-Wide Rights Spero Licensed World-Wide Rights to GSK Spero Retains Rights in United States
Spero Strategy: Invest in diseases and settings of care supportive of blockbuster growth Non-DRG reimbursement High unmet need with strong economic benefit $1 B+ Sales* *Estimated Peak Year Worldwide Sales *Trademarks are properties of their respective owners
SPR720
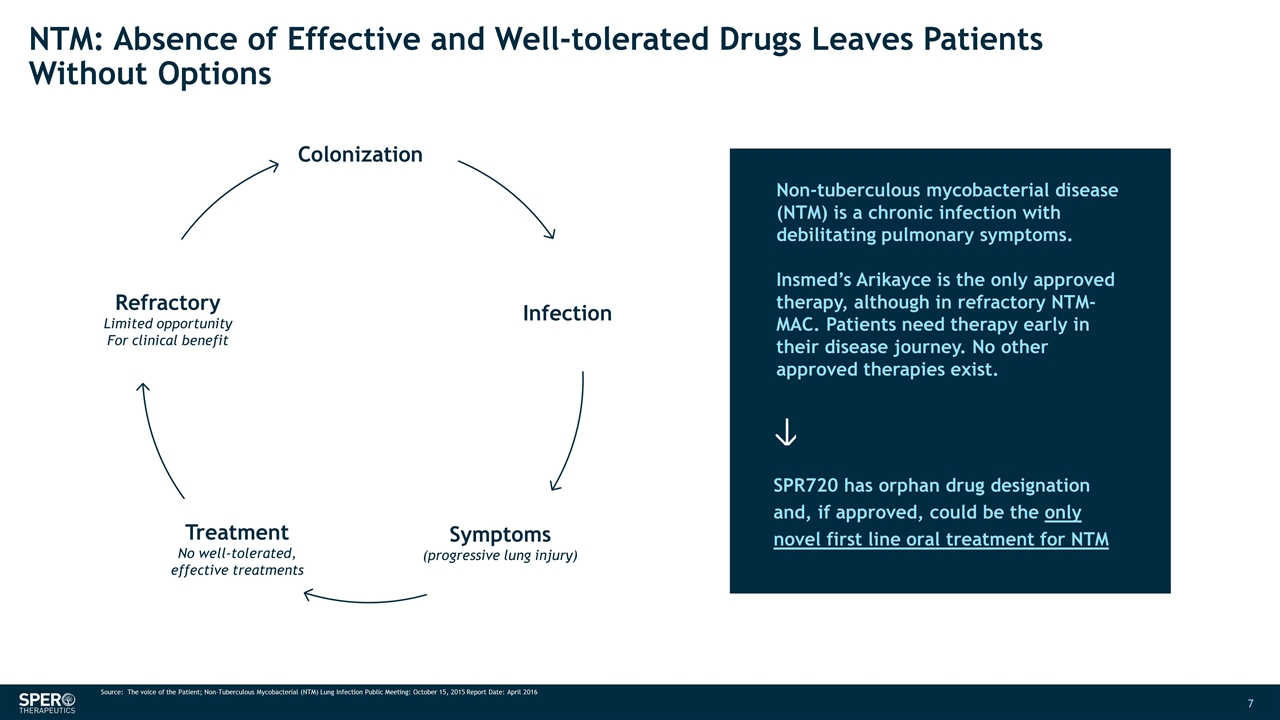
NTM: Absence of Effective and Well-tolerated Drugs Leaves Patients Without Options SPR720 has orphan drug designation and, if approved, could be the only novel first line oral treatment for NTM Infection Colonization Symptoms (progressive lung injury) Treatment No well-tolerated, effective treatments Refractory Limited opportunity For clinical benefit Non-tuberculous mycobacterial disease (NTM) is a chronic infection with debilitating pulmonary symptoms. Insmed’s Arikayce is the only approved therapy, although in refractory NTM-MAC. Patients need therapy early in their disease journey. No other approved therapies exist. Source: The voice of the Patient; Non-Tuberculous Mycobacterial (NTM) Lung Infection Public Meeting: October 15, 2015 Report Date: April 2016
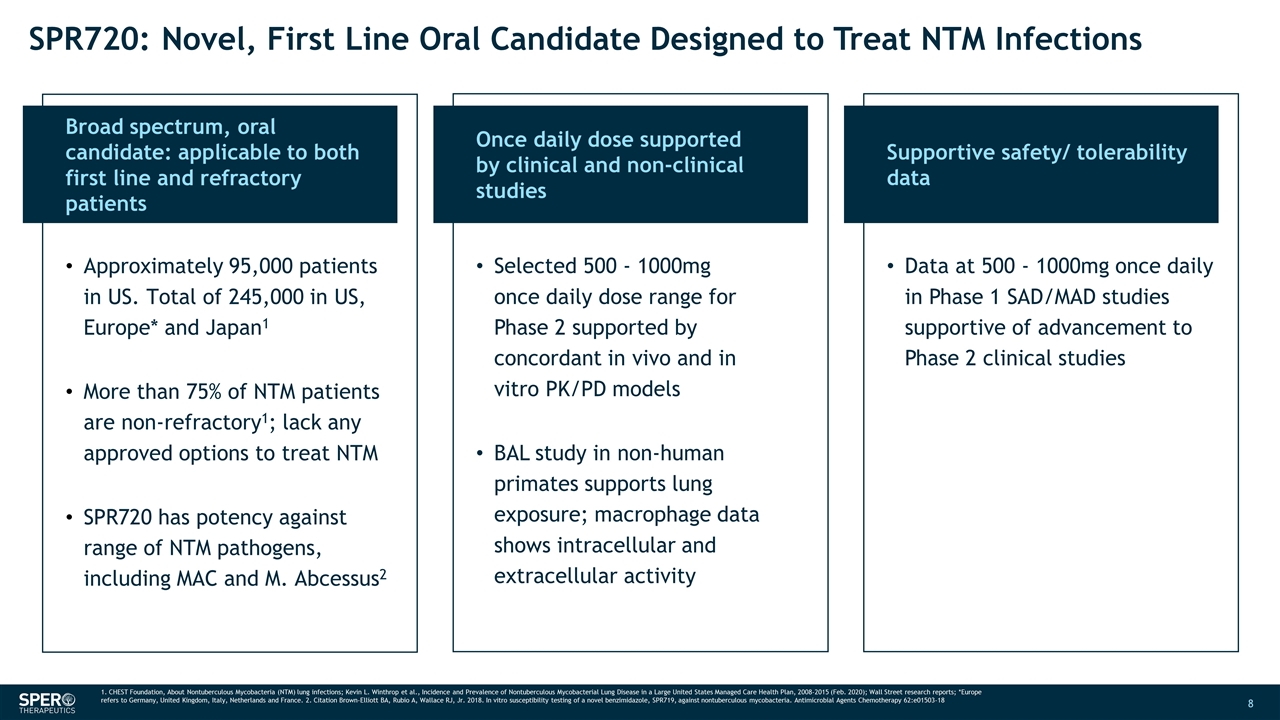
SPR720: Novel, First Line Oral Candidate Designed to Treat NTM Infections Broad spectrum, oral candidate: applicable to both first line and refractory patients Approximately 95,000 patients in US. Total of 245,000 in US, Europe* and Japan1 More than 75% of NTM patients are non-refractory1; lack any approved options to treat NTM SPR720 has potency against range of NTM pathogens, including MAC and M. Abcessus2 Once daily dose supported by clinical and non-clinical studies Supportive safety/ tolerability data Selected 500 - 1000mg once daily dose range for Phase 2 supported by concordant in vivo and in vitro PK/PD models BAL study in non-human primates supports lung exposure; macrophage data shows intracellular and extracellular activity Data at 500 - 1000mg once daily in Phase 1 SAD/MAD studies supportive of advancement to Phase 2 clinical studies 1. CHEST Foundation, About Nontuberculous Mycobacteria (NTM) lung infections; Kevin L. Winthrop et al., Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large United States Managed Care Health Plan, 2008-2015 (Feb. 2020); Wall Street research reports; *Europe refers to Germany, United Kingdom, Italy, Netherlands and France. 2. Citation Brown-Elliott BA, Rubio A, Wallace RJ, Jr. 2018. In vitro susceptibility testing of a novel benzimidazole, SPR719, against nontuberculous mycobacteria. Antimicrobial Agents Chemotherapy 62:e01503-18
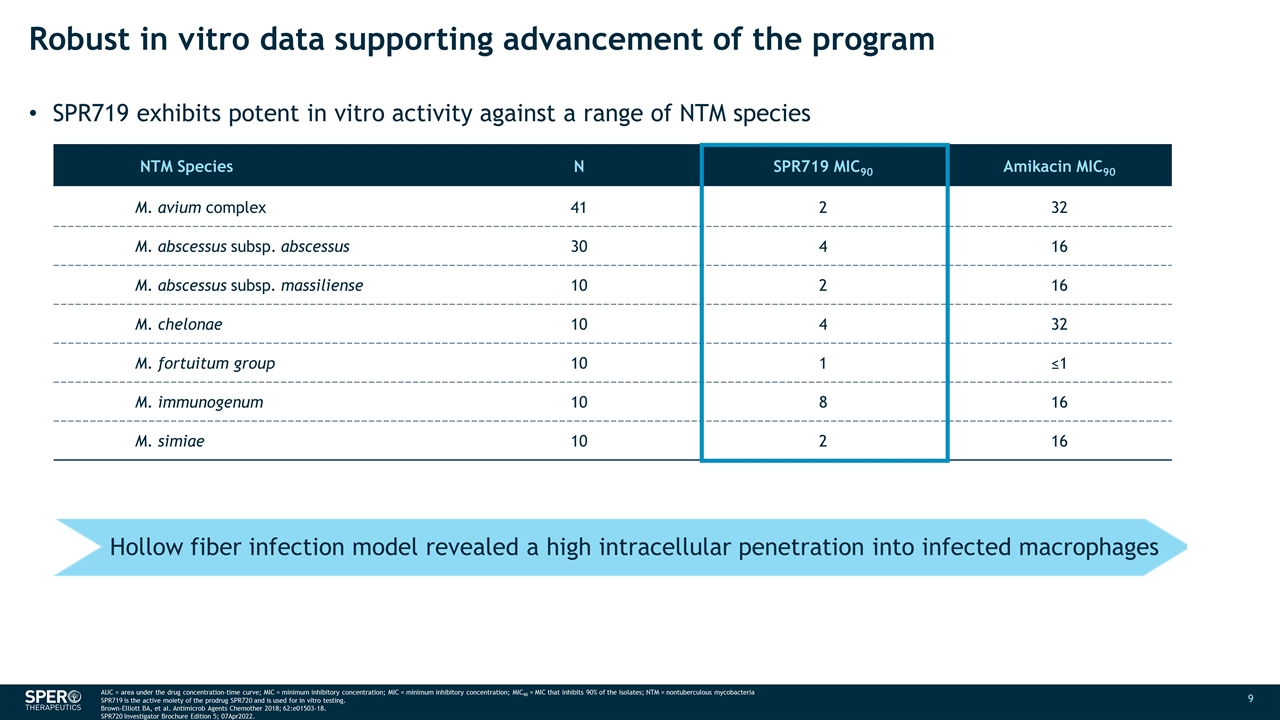
Robust in vitro data supporting advancement of the program SPR719 exhibits potent in vitro activity against a range of NTM species NTM Species N SPR719 MIC90 Amikacin MIC90 M. avium complex 41 2 32 M. abscessus subsp. abscessus 30 4 16 M. abscessus subsp. massiliense 10 2 16 M. chelonae 10 4 32 M. fortuitum group 10 1 ≤1 M. immunogenum 10 8 16 M. simiae 10 2 16 AUC = area under the drug concentration-time curve; MIC = minimum inhibitory concentration; MIC = minimum inhibitory concentration; MIC90 = MIC that inhibits 90% of the isolates; NTM = nontuberculous mycobacteria SPR719 is the active moiety of the prodrug SPR720 and is used for in vitro testing. Brown-Elliott BA, et al. Antimicrob Agents Chemother 2018; 62:e01503-18. SPR720 Investigator Brochure Edition 5; 07Apr2022. Hollow fiber infection model revealed a high intracellular penetration into infected macrophages
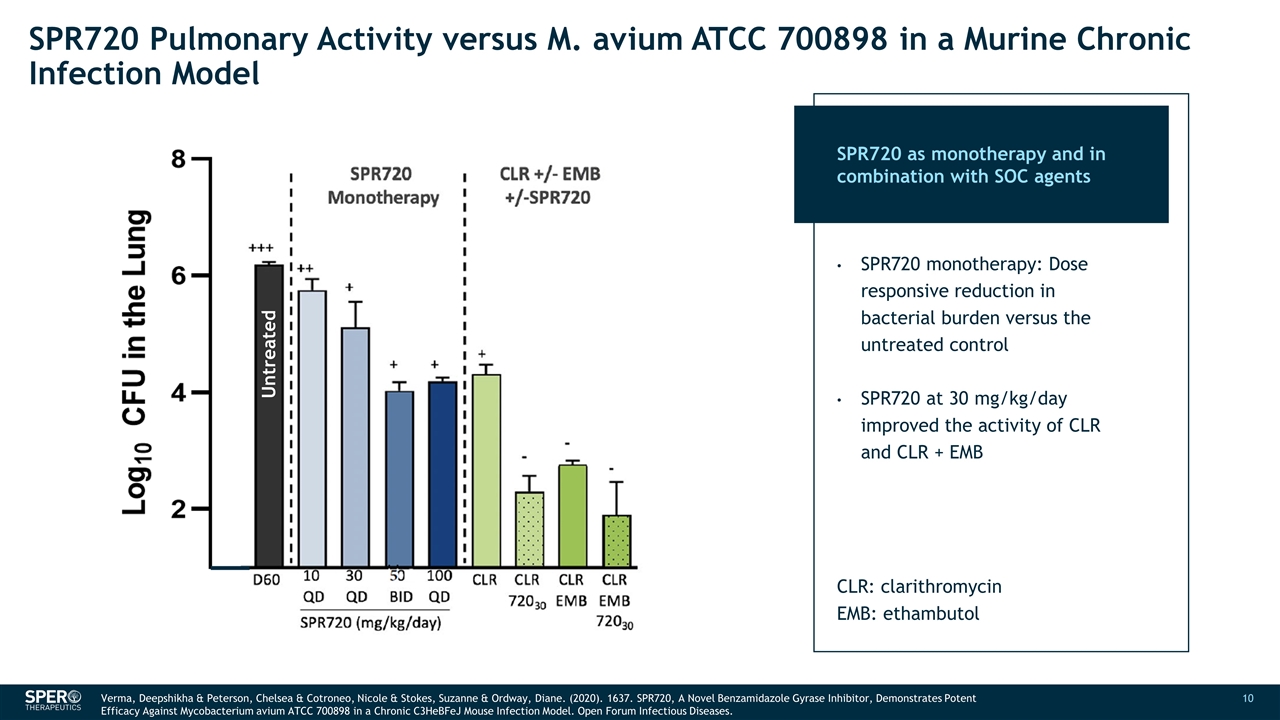
SPR720 Pulmonary Activity versus M. avium ATCC 700898 in a Murine Chronic Infection Model ++ - +++ ++ + + + + - - - + + - + SPR720 Monotherapy CLR +/- EMB +/-SPR720 CLR + RFB +/- EMB +/-SPR720 D1 D27 D60 SPR720 (mg/kg/day) 10 QD 30 QD 100 BID 100 QD CLR CLR 72030 CLR EMB CLR EMB 72030 CLR RFB CLR RFB 72030 CLR RFB EMB CLR RFB EMB 72030 Untreated SPR720 as monotherapy and in combination with SOC agents SPR720 monotherapy: Dose responsive reduction in bacterial burden versus the untreated control SPR720 at 30 mg/kg/day improved the activity of CLR and CLR + EMB CLR: clarithromycin EMB: ethambutol Verma, Deepshikha & Peterson, Chelsea & Cotroneo, Nicole & Stokes, Suzanne & Ordway, Diane. (2020). 1637. SPR720, A Novel Benzamidazole Gyrase Inhibitor, Demonstrates Potent Efficacy Against Mycobacterium avium ATCC 700898 in a Chronic C3HeBFeJ Mouse Infection Model. Open Forum Infectious Diseases. Untreated
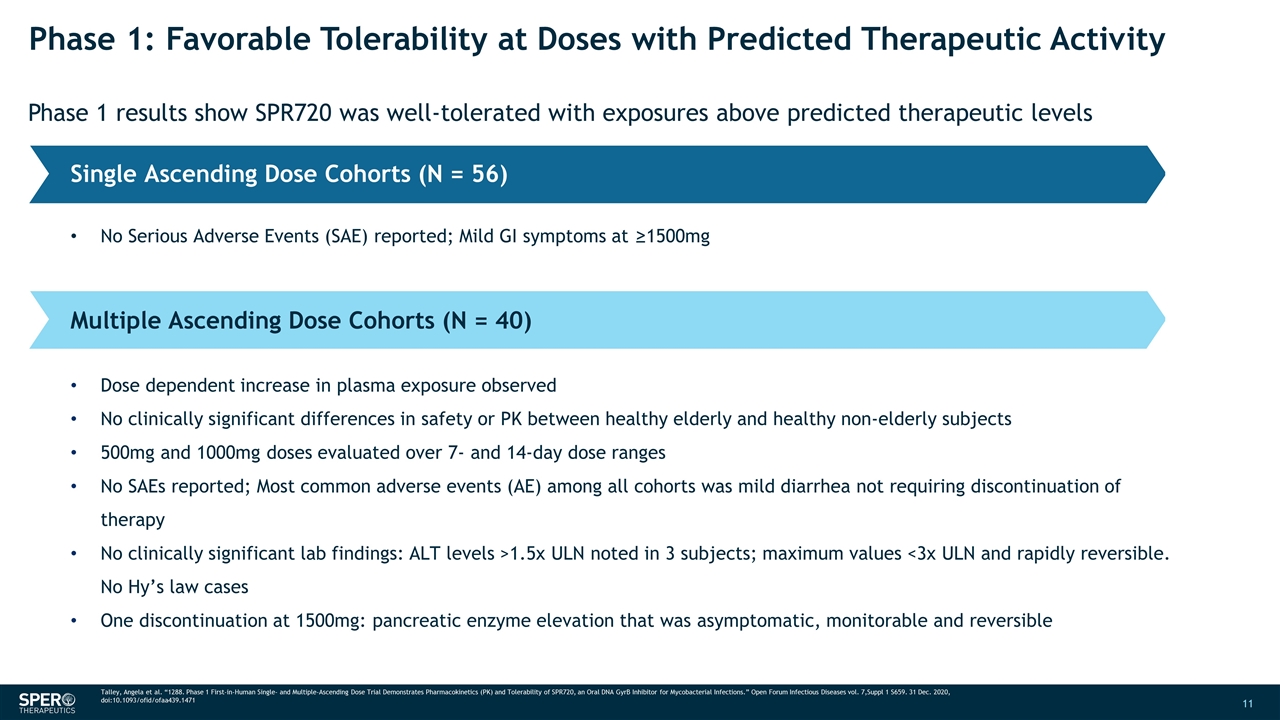
Phase 1: Favorable Tolerability at Doses with Predicted Therapeutic Activity Phase 1 results show SPR720 was well-tolerated with exposures above predicted therapeutic levels Single Ascending Dose Cohorts (N = 56) Multiple Ascending Dose Cohorts (N = 40) No Serious Adverse Events (SAE) reported; Mild GI symptoms at ≥1500mg Dose dependent increase in plasma exposure observed No clinically significant differences in safety or PK between healthy elderly and healthy non-elderly subjects 500mg and 1000mg doses evaluated over 7- and 14-day dose ranges No SAEs reported; Most common adverse events (AE) among all cohorts was mild diarrhea not requiring discontinuation of therapy No clinically significant lab findings: ALT levels >1.5x ULN noted in 3 subjects; maximum values <3x ULN and rapidly reversible. No Hy’s law cases One discontinuation at 1500mg: pancreatic enzyme elevation that was asymptomatic, monitorable and reversible Talley, Angela et al. “1288. Phase 1 First-in-Human Single- and Multiple-Ascending Dose Trial Demonstrates Pharmacokinetics (PK) and Tolerability of SPR720, an Oral DNA GyrB Inhibitor for Mycobacterial Infections.” Open Forum Infectious Diseases vol. 7,Suppl 1 S659. 31 Dec. 2020, doi:10.1093/ofid/ofaa439.1471
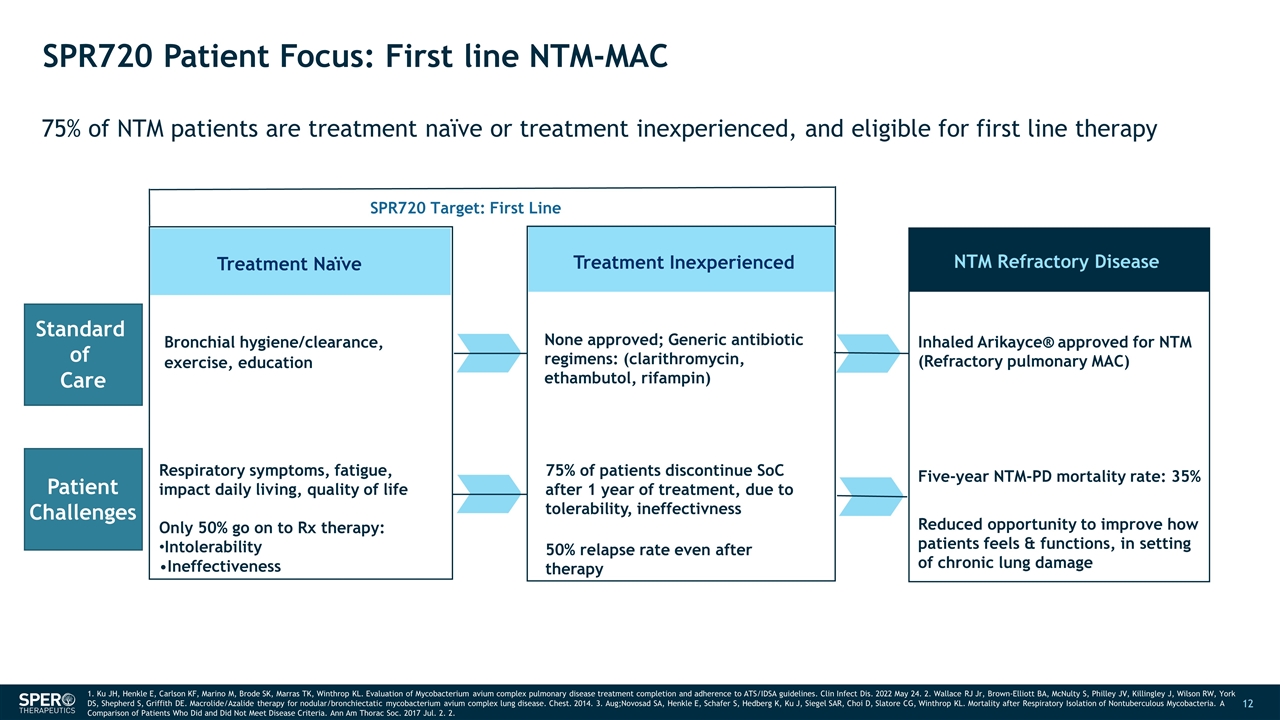
SPR720 Patient Focus: First line NTM-MAC 75% of NTM patients are treatment naïve or treatment inexperienced, and eligible for first line therapy Treatment Naïve Bronchial hygiene/clearance, exercise, education Treatment Inexperienced NTM Refractory Disease None approved; Generic antibiotic regimens: (clarithromycin, ethambutol, rifampin) Inhaled Arikayce® approved for NTM (Refractory pulmonary MAC) Five-year NTM-PD mortality rate: 35% SPR720 Target: First Line 1. Ku JH, Henkle E, Carlson KF, Marino M, Brode SK, Marras TK, Winthrop KL. Evaluation of Mycobacterium avium complex pulmonary disease treatment completion and adherence to ATS/IDSA guidelines. Clin Infect Dis. 2022 May 24. 2. Wallace RJ Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, York DS, Shepherd S, Griffith DE. Macrolide/Azalide therapy for nodular/bronchiectatic mycobacterium avium complex lung disease. Chest. 2014. 3. Aug;Novosad SA, Henkle E, Schafer S, Hedberg K, Ku J, Siegel SAR, Choi D, Slatore CG, Winthrop KL. Mortality after Respiratory Isolation of Nontuberculous Mycobacteria. A Comparison of Patients Who Did and Did Not Meet Disease Criteria. Ann Am Thorac Soc. 2017 Jul. 2. 2. Standard of Care Patient Challenges Respiratory symptoms, fatigue, impact daily living, quality of life Only 50% go on to Rx therapy: Intolerability Ineffectiveness 75% of patients discontinue SoC after 1 year of treatment, due to tolerability, ineffectivness 50% relapse rate even after therapy Reduced opportunity to improve how patients feels & functions, in setting of chronic lung damage
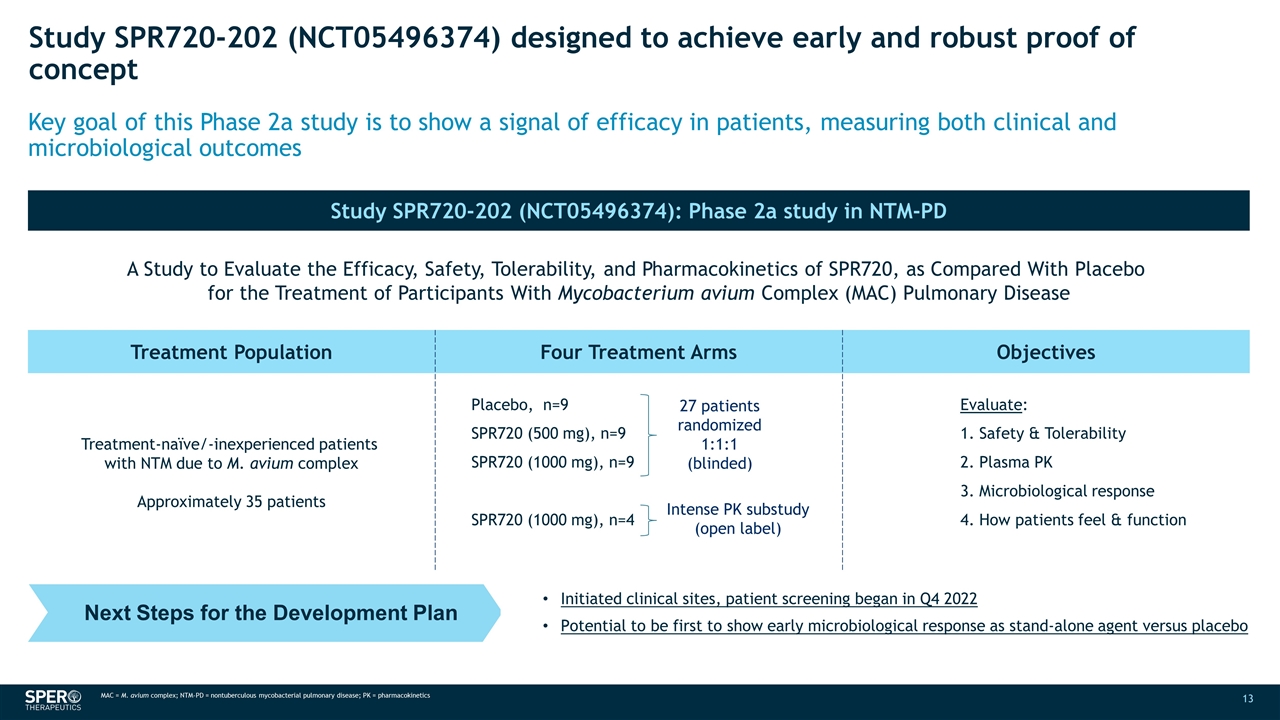
Study SPR720-202 (NCT05496374) designed to achieve early and robust proof of concept Study SPR720-202 (NCT05496374): Phase 2a study in NTM-PD N SPR719 MIC50 A Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacokinetics of SPR720, as Compared With Placebo for the Treatment of Participants With Mycobacterium avium Complex (MAC) Pulmonary Disease 30 2 Treatment Population Four Treatment Arms Objectives Treatment-naïve/-inexperienced patients with NTM due to M. avium complex Approximately 35 patients Placebo, n=9 SPR720 (500 mg), n=9 SPR720 (1000 mg), n=9 SPR720 (1000 mg), n=4 Evaluate: 1. Safety & Tolerability 2. Plasma PK 3. Microbiological response 4. How patients feel & function Key goal of this Phase 2a study is to show a signal of efficacy in patients, measuring both clinical and microbiological outcomes 27 patients randomized 1:1:1 (blinded) Intense PK substudy (open label) MAC = M. avium complex; NTM-PD = nontuberculous mycobacterial pulmonary disease; PK = pharmacokinetics Next Steps for the Development Plan Initiated clinical sites, patient screening began in Q4 2022 Potential to be first to show early microbiological response as stand-alone agent versus placebo
Oral Carbapenem Tebipenem HBr
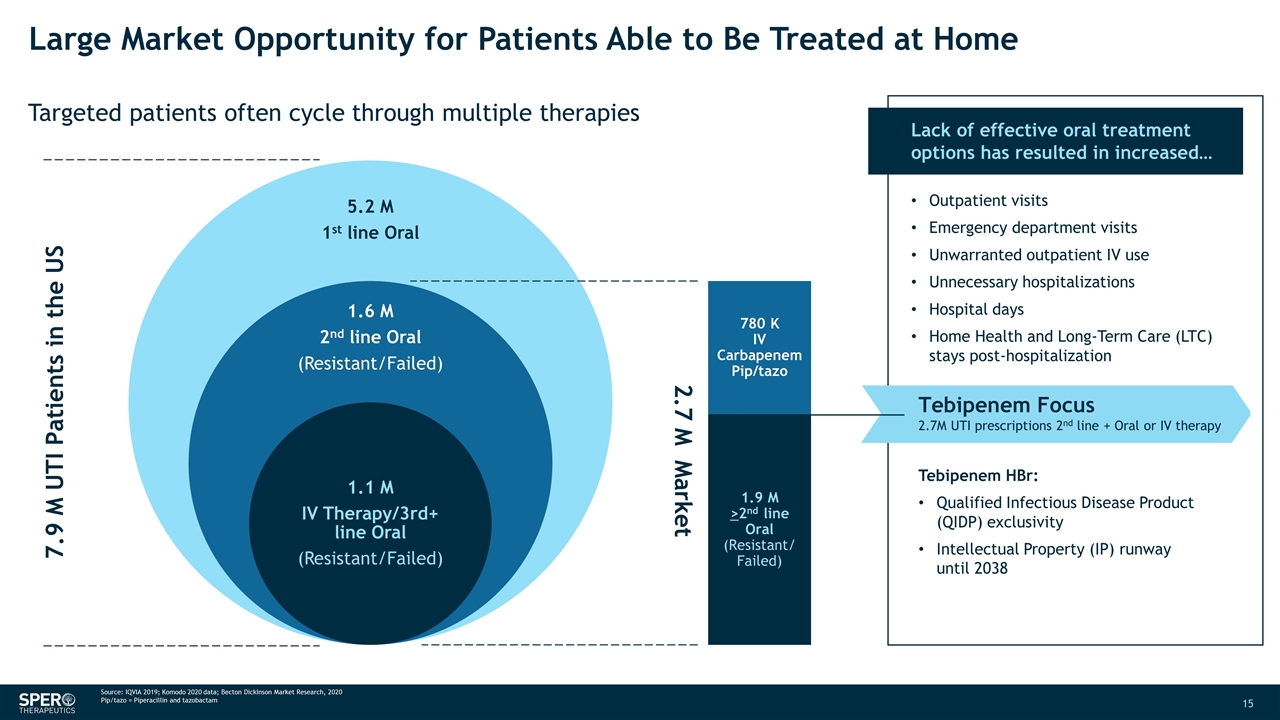
Large Market Opportunity for Patients Able to Be Treated at Home Targeted patients often cycle through multiple therapies 7.9 M UTI Patients in the US 2.7 M Market 780 K IV Carbapenem Pip/tazo 1.9 M >2nd line Oral (Resistant/ Failed) Lack of effective oral treatment options has resulted in increased… Outpatient visits Emergency department visits Unwarranted outpatient IV use Unnecessary hospitalizations Hospital days Home Health and Long-Term Care (LTC) stays post-hospitalization Tebipenem Focus 2.7M UTI prescriptions 2nd line + Oral or IV therapy Tebipenem HBr: Qualified Infectious Disease Product (QIDP) exclusivity Intellectual Property (IP) runway until 2038 Source: IQVIA 2019; Komodo 2020 data; Becton Dickinson Market Research, 2020 Pip/tazo = Piperacillin and tazobactam 5.2 M 1 st line Oral 1.6 M 2 nd line Oral (Resistant/Failed) 1.1 M IV Therapy/3rd+ line Oral (Resistant/Failed)
Clinical Experience Supports Safety, Efficacy of Tebipenem1 Tebipenem pivoxil evaluated in 24 trials enrolling over 2500 subjects Approved in Japan since 2009 Spero experience: Global cUTI Phase 3, enrolling 1372 subjects Meiji Seika experience 741 Adult subjects evaluated, across 17 efficacy and pharmacology trials 440 pediatric subjects evaluated, across 6 efficacy and pharmacology trials Tebipenem pivoxil was generally well tolerated, comparable to common, approved oral beta lactam antibiotics and IV carbapenems Tebipenem pivoxil met its primary endpoint in 3 double blind placebo-controlled efficacy trials in pediatric pneumonia, otitis media, and sinusitis Approved for pediatric pneumonia, otitis media, sinusitis, over 4 million patients dosed to date Extensive post-marketing safety and efficacy surveillance completed, covering 3,331 patients No issues of safety were observed, and adequate efficacy was demonstrated 1. Akash Jain, Luke Utley, Thomas R. Parr, Thomas Zabawa & Michael J. Pucci (2018): Tebipenem, the first oral carbapenem antibiotic, Expert Review of Anti-infective Therapy, DOI: 10.1080/14787210.2018.1496821

Results from Spero’s ADAPT-PO Phase 3 Clinical Trial Published in The New England Journal of Medicine Results from Spero’s Phase 3 ADAPT-PO study, as recently published in The New England Journal of Medicine (NEJM), demonstrated that oral tebipenem HBr was well-tolerated and non-inferior to IV ertapenem, based on its trial protocol, in the treatment of 1372 enrolled adult patients with cUTI or acute pyelonephritis.

Exclusive License Agreement with GSK for Tebipenem HBr and Equity Investment Global Collaboration Financial Terms GSK received exclusive license to develop and commercialize tebipenem pivoxil and tebipenem HBr in all territories, except Japan and certain other Asian countries (Meiji Seika Territories) Spero is responsible for execution and costs of the tebipenem HBr follow up Phase 3 in the United States GSK is responsible for the execution and costs of commercial activities for tebipenem HBr in the United States, as well as all development and commercial activities in territories outside of United States (not including Meiji Seika Territories) Transaction closed November 7, 2022 Spero received an upfront payment of $66 Million and GSK made additional $9 million common stock investment in Spero Spero eligible to receive up to $525 million in development, commercial and sales milestone payments $150 million in development milestone payments (allocated through duration of Phase 3 study and NDA submission) $150 million in commercial milestone payments $225 million in sales related milestone payments Spero to receive tiered low-single digit to low-double digit (if sales exceed $1bn) tiered royalties

Direct Acting IV Potentiator SPR206
SPR206 Has Potential to Address Significant Unmet Need 2-4X mortality rate for carbapenem-resistant versus susceptible Enterobacteriaceae infections Mortality rate of 40-50% for patients infected with carbapenem-resistant Acinetobacter baumannii Severely ill patients are typically treated with multiple agents in parallel: carbapenem, polymyxin, aminoglycoside These agents have liabilities: aminoglycosides and polymyxins cause nephrotoxicity 1 . Avycax, Zerbaxa, IMI-REL 2. Not recommended per IDSA Guidelines https://doi.org/10.1093/cid/ciw353 SPR206 has the potential to fulfill the need for a well-tolerated therapy with the potential for single agent efficacy against carbapenem-resistant pathogens Current standard of care involves drug combinations that are often associated with nephrotoxicity
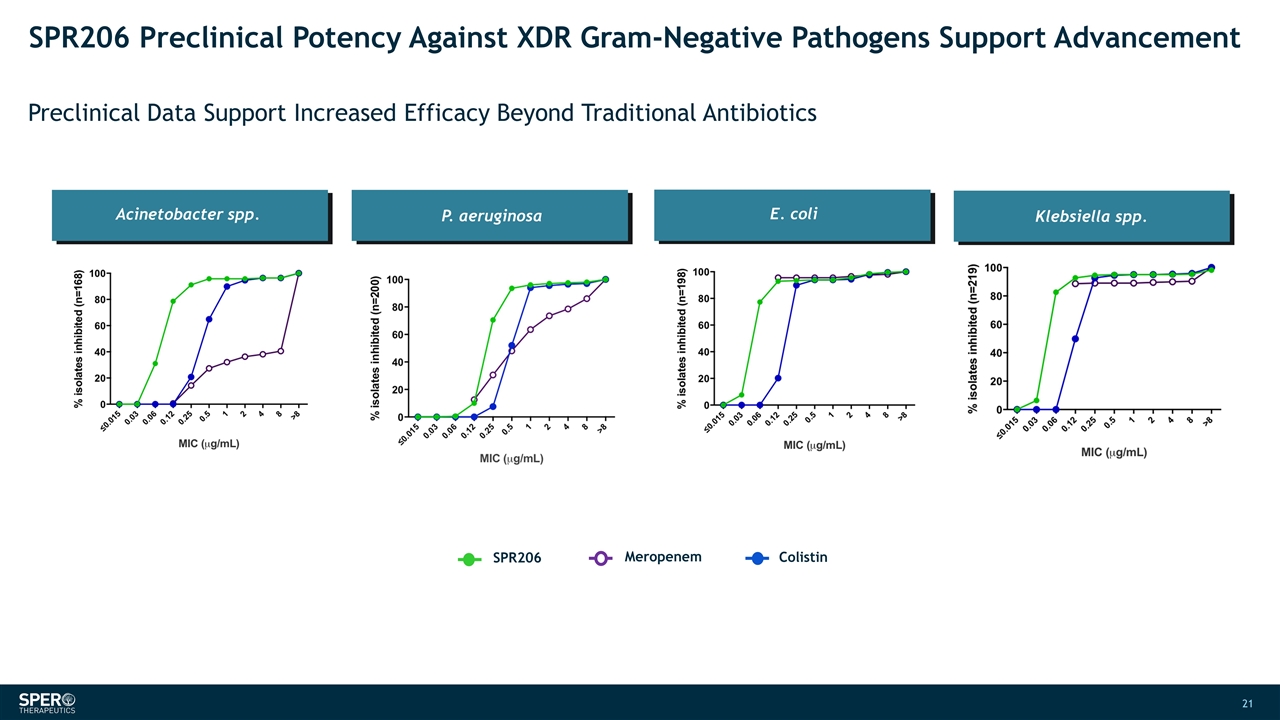
SPR206 Preclinical Potency Against XDR Gram-Negative Pathogens Support Advancement Preclinical Data Support Increased Efficacy Beyond Traditional Antibiotics P. aeruginosa E. coli Acinetobacter spp. Klebsiella spp. SPR206 Colistin Meropenem
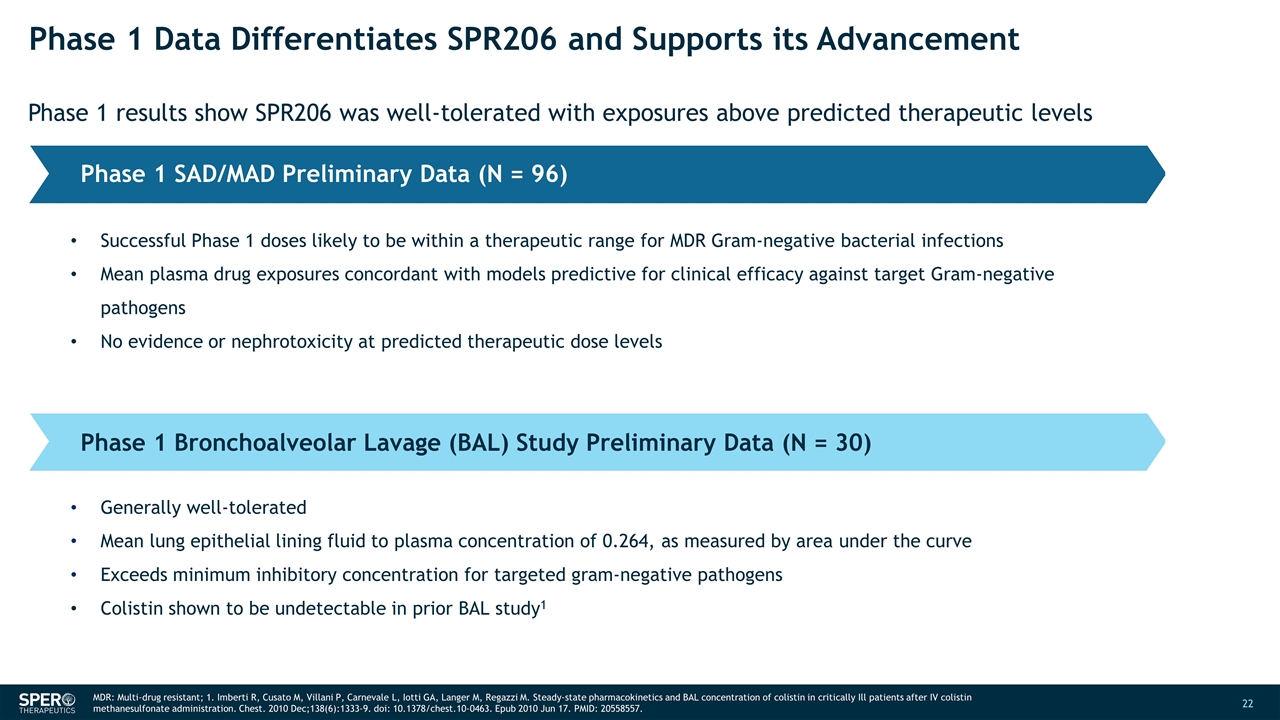
Phase 1 Data Differentiates SPR206 and Supports its Advancement Phase 1 results show SPR206 was well-tolerated with exposures above predicted therapeutic levels Phase 1 SAD/MAD Preliminary Data (N = 96) Phase 1 Bronchoalveolar Lavage (BAL) Study Preliminary Data (N = 30) Successful Phase 1 doses likely to be within a therapeutic range for MDR Gram-negative bacterial infections Mean plasma drug exposures concordant with models predictive for clinical efficacy against target Gram-negative pathogens No evidence or nephrotoxicity at predicted therapeutic dose levels Generally well-tolerated Mean lung epithelial lining fluid to plasma concentration of 0.264, as measured by area under the curve Exceeds minimum inhibitory concentration for targeted gram-negative pathogens Colistin shown to be undetectable in prior BAL study1 MDR: Multi-drug resistant; 1. Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest. 2010 Dec;138(6):1333-9. doi: 10.1378/chest.10-0463. Epub 2010 Jun 17. PMID: 20558557.
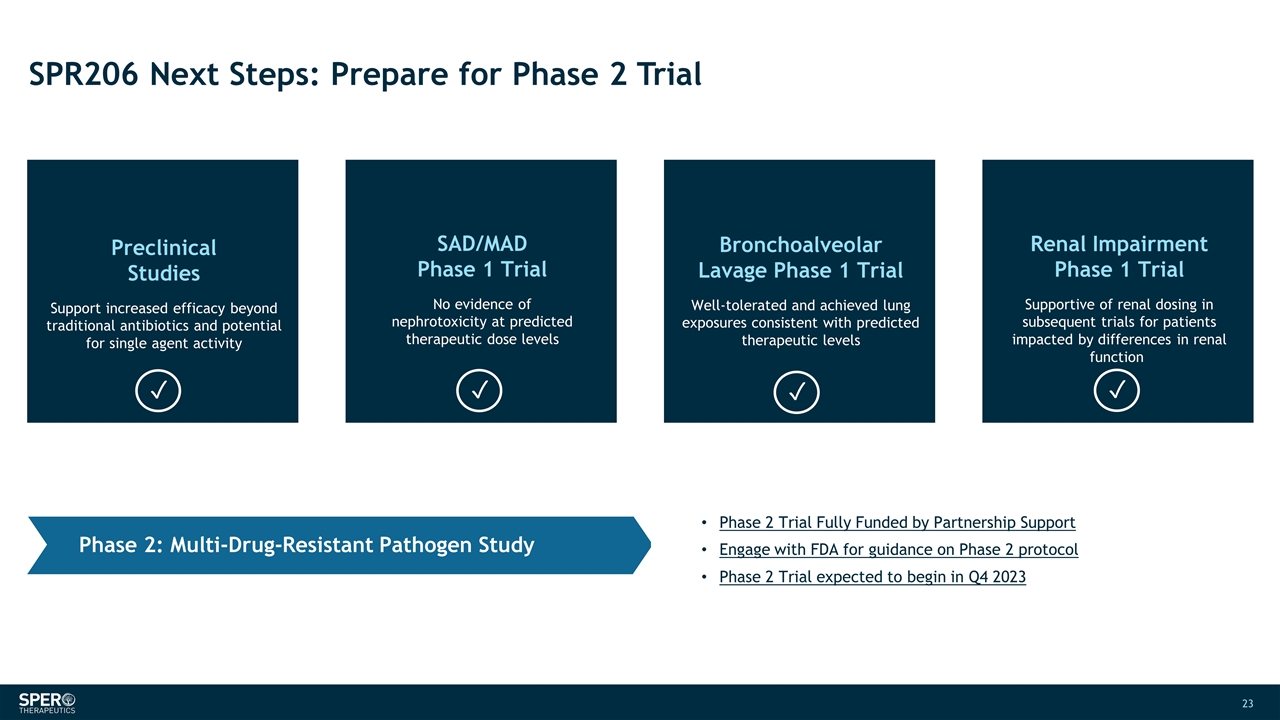
SPR206 Next Steps: Prepare for Phase 2 Trial SAD/MAD Phase 1 Trial No evidence of nephrotoxicity at predicted therapeutic dose levels Bronchoalveolar Lavage Phase 1 Trial Well-tolerated and achieved lung exposures consistent with predicted therapeutic levels Preclinical Studies Support increased efficacy beyond traditional antibiotics and potential for single agent activity Renal Impairment Phase 1 Trial Supportive of renal dosing in subsequent trials for patients impacted by differences in renal function Phase 2 Trial Fully Funded by Partnership Support Engage with FDA for guidance on Phase 2 protocol Phase 2 Trial expected to begin in Q4 2023 Phase 2: Multi-Drug-Resistant Pathogen Study ✓ ✓ ✓ ✓
Spero Corporate Overview Update
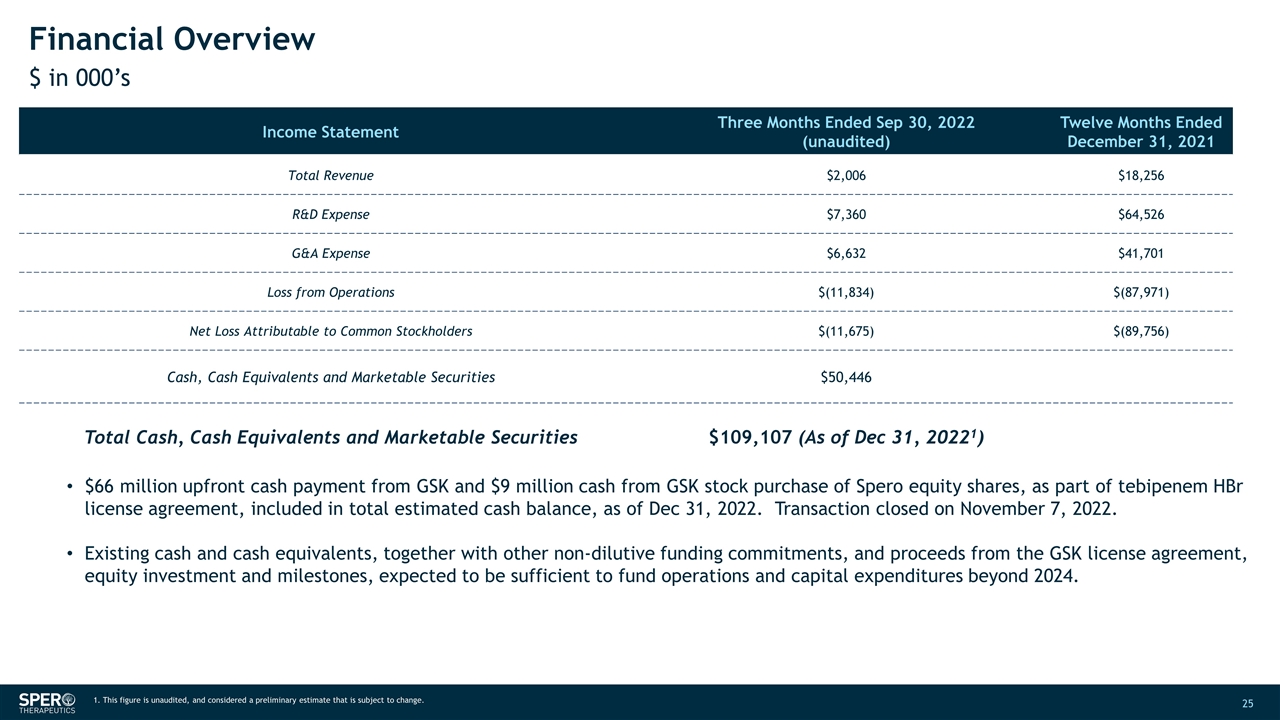
Financial Overview $ in 000’s Income Statement Three Months Ended Sep 30, 2022 (unaudited) Twelve Months Ended December 31, 2021 Total Revenue $2,006 $18,256 R&D Expense $7,360 $64,526 G&A Expense $6,632 $41,701 Loss from Operations $(11,834) $(87,971) Net Loss Attributable to Common Stockholders $(11,675) $(89,756) Cash, Cash Equivalents and Marketable Securities $50,446 Total Cash, Cash Equivalents and Marketable Securities $109,107 (As of Dec 31, 20221) $66 million upfront cash payment from GSK and $9 million cash from GSK stock purchase of Spero equity shares, as part of tebipenem HBr license agreement, included in total estimated cash balance, as of Dec 31, 2022. Transaction closed on November 7, 2022. Existing cash and cash equivalents, together with other non-dilutive funding commitments, and proceeds from the GSK license agreement, equity investment and milestones, expected to be sufficient to fund operations and capital expenditures beyond 2024. 1. This figure is unaudited, and considered a preliminary estimate that is subject to change.

Leadership Team *Trademarks are properties of their respective owners Ankit Mahadevia, MD Chief Executive Officer Prior Venture Partner at Atlas Venture; Genentech, McKinsey Formed eight companies in the life sciences sector; three as Acting CEO. Background in healthcare policy Timothy Keutzer Chief Development Officer Prior VP Program and Portfolio Management, Cubist Extensive antibiotic development experience from pre-clinical to approval Over 30 years in the pharmaceutical industry James Brady Chief Human Resource Officer Prior CHRO at uniQure Therapeutics; Vice President, Human Resources at Intarcia Therapeutics Over 30 years of senior human resources leadership within the life science space Sath Shukla Chief Financial Officer Prior CFO at Ziopharm Oncology; VP and Global Head of Corporate Finance at Vertex Over 20 years of financial leadership, executing within commercial and clinical companies Tamara Joseph, JD, LLM Chief Legal Officer Over 20 years of leadership and legal experience in the biotech sector Prior General Counsel at several biotechnology companies including Millendo Therapeutics, Enzyvant Therapeutics, InVivo Therapeutics, and Cubist Kamal Hamed, MD, MPH, MBA Chief Medical Officer Prior CMO (Lysovant Sciences), Head of Development & Medical Affairs (Basilea) Therapeutic Area Head (Novartis) Led various anti-infective clinical development programs in antibacterials, antivirals, antimalarials, and antifungals Over 20 years in the pharmaceutical industry
Key Investment Highlights Experienced management team Significant near-term catalysts Multiple pipeline products targeting large market opportunities World class partnerships Strong balance sheet and cash runway


























