1.60. “GSP” means all applicable Good Supply Practice standards, including, as applicable, as set forth in the then current good supply practice standards promulgated or endorsed by the FDA as defined in Good Supply Practice for Pharmaceutical Licensed Products or the equivalent Applicable Laws in the Region in the Licensed Territory, each as may be amended and applicable from time to time.
1.61. “HGR Approval” means any and all necessary record filings with, and approvals, licenses, and/or permits issued by, the Human Genetics Resources Administration of the PRC or any other Governmental Authorities in the PRC required for Development activities (including Clinical Trials) and data transfer and sharing under this Agreement with respect to the Exploitation of Licensed Product in the Field in the PRC.
1.62. “ICC Rules” has the meaning set forth in Section 14.4(a).
1.63. “IFRS” means international financial reporting standards, consistently applied.
1.64. “IND” means an investigational new drug application, or equivalent application such as a clinical trial applications filed with the applicable Regulatory Authority in a Region in the Licensed Territory (a “CTA”), which application is required to commence Clinical Trials in the Licensed Territory.
1.65. “Indemnifying Party” has the meaning set forth in Section 11.3.
1.66. “Indemnitee” has the meaning set forth in Section 11.3.
1.67. “Indication” means a separate and distinct disease or condition, or sign or symptom of a disease or medical condition in the Field. For clarity, different lines of treatment or the treatment of separate stages or forms or different population (e.g., adult vs pediatric) of the same disease or medical condition shall not constitute separate Indications.
1.68. “Initial MAH” has the meaning set forth in Section 5.1(e).
1.69. “Invention” means any and all inventions, discoveries and developments, whether or not patentable, which are created, conceived, developed or made in the course of performance of this Agreement, whether created, conceived, developed or made solely by, or on behalf of, Karuna, Zai, the Parties jointly or jointly with a Third Party, or any Affiliate of the same.
1.70. “Joint Global Study” has the meaning set forth in Section 4.4(b).
1.71. “JSC” has the meaning set forth in Section 3.1(a).
1.72. “Karuna” has the meaning set forth in the preamble of this Agreement.
1.73. “Karuna Indemnitee(s)” has the meaning set forth in Section 11.1.
1.74. “Karuna Product Marks” has the meaning set forth in Section 7.5.
1.75. “Karuna Sponsored Regulatory and Commercial Activities” has the meaning set forth in Section 5.1(e)(ii).
1.76. “Karuna Sponsored Study” has the meaning set forth in Section 5.1(d).
1.77. “Know-How” means any proprietary scientific or technical information, results and data of any type whatsoever, in any tangible or intangible form whatsoever, including databases, safety information, practices, methods, techniques, specifications, formulations, formulae, knowledge, know-how, skill, experience, test data including pharmacological, medicinal chemistry, biological, chemical, biochemical, toxicological and clinical test data, analytical and quality control data, stability data, studies and procedures, and manufacturing process and development information, results and data.
6
[***] = CERTAIN CONFIDENTIAL INFORMATION OMITTED
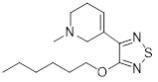 .. Trospium chloride has the IUPAC name of [(1R,5S)-spiro[8-azoniabicyclo[3.2.1]octane-8,1’-azolidin-1-ium]-3-yl] 2-hydroxy-2,2-diphenylacetate chloride and the structure of
.. Trospium chloride has the IUPAC name of [(1R,5S)-spiro[8-azoniabicyclo[3.2.1]octane-8,1’-azolidin-1-ium]-3-yl] 2-hydroxy-2,2-diphenylacetate chloride and the structure of ..
..