
GEM-3: Phase 3 Safety and Immunogenicity Results of Beremagene Geperpavec (B-VEC), an Investigational, Topical Gene Therapy for Dystrophic Epidermolysis Bullosa (DEB) 1Stanford University, Redwood City, CA, USA; 2University of Miami, Miami, FL, USA; 3Mission Dermatology Center, Children’s Hospital of Orange County, University of California, Irvine, Department of Dermatology, Rancho Santa Margarita, CA, USA; 4Savio Group Analytics & Statistics, Hockessin, DE, USA; 5Krystal Biotech, Inc., Pittsburgh, PA, USA M. Peter Marinkovich,1 Mercedes E. Gonzalez,2 Shireen V. Guide,3 I. Sinem Bagci,1 Surya Chitra,4 Brittani Agostini,5 Hubert Chen,5 Trevor Parry,5 Suma Krishnan5 Exhibit 99.2

Disclosures M. Peter Marinkovich reports the following disclosures: – Krystal Biotech (Investigator), Abeona Therapeutics (Investigator), CastleCreek (Investigator), Phoenix Tissue Repair (Investigator), WINGS Therapeutics (Investigator) This study was funded by Krystal Biotech, Inc. 2
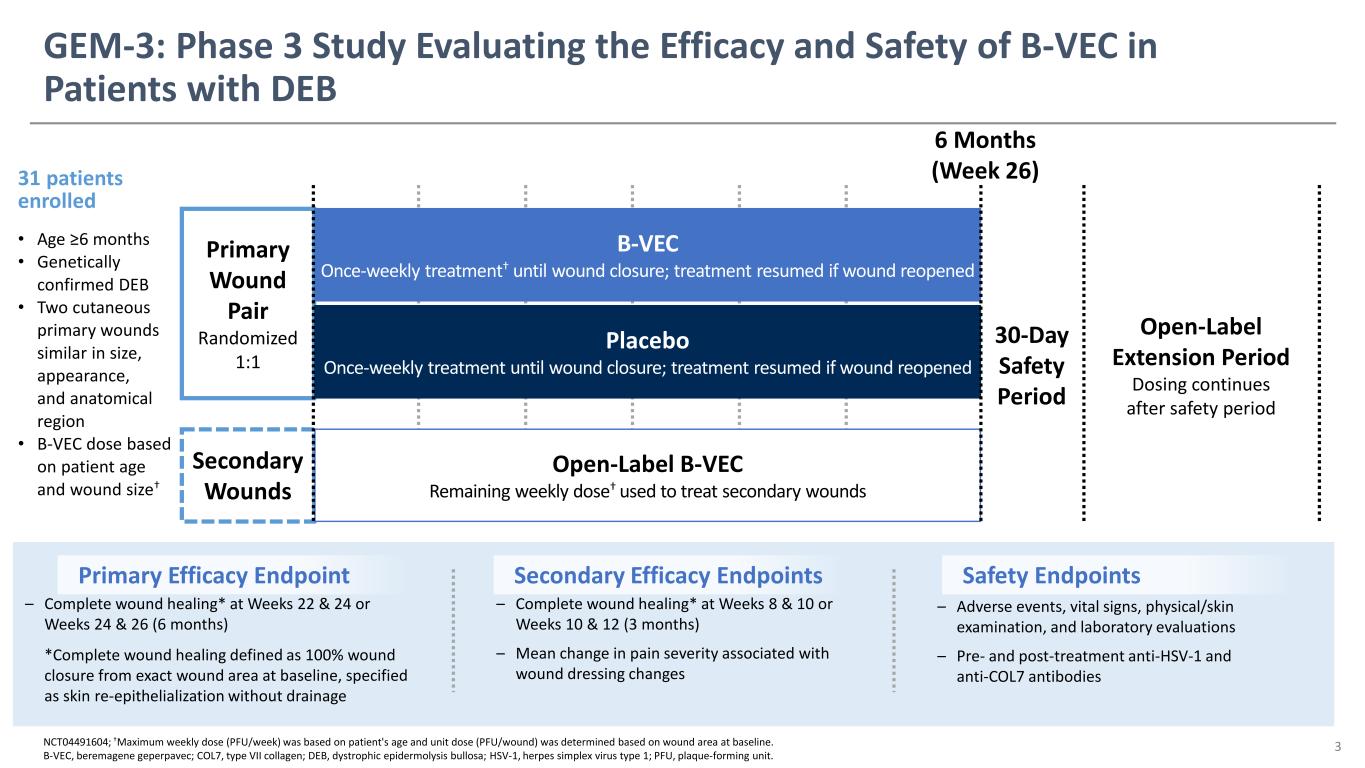
NCT04491604; †Maximum weekly dose (PFU/week) was based on patient's age and unit dose (PFU/wound) was determined based on wound area at baseline. B-VEC, beremagene geperpavec; COL7, type VII collagen; DEB, dystrophic epidermolysis bullosa; HSV-1, herpes simplex virus type 1; PFU, plaque-forming unit. Primary Efficacy Endpoint Secondary Efficacy Endpoints – Complete wound healing* at Weeks 8 & 10 or Weeks 10 & 12 (3 months) – Mean change in pain severity associated with wound dressing changes – Complete wound healing* at Weeks 22 & 24 or Weeks 24 & 26 (6 months) *Complete wound healing defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage Safety Endpoints – Adverse events, vital signs, physical/skin examination, and laboratory evaluations – Pre- and post-treatment anti-HSV-1 and anti-COL7 antibodies Secondary Wounds Primary Wound Pair Randomized 1:1 Open-Label Extension Period Dosing continues after safety period 30-Day Safety Period 31 patients enrolled • Age ≥6 months • Genetically confirmed DEB • Two cutaneous primary wounds similar in size, appearance, and anatomical region • B-VEC dose based on patient age and wound size† B-VEC Once-weekly treatment† until wound closure; treatment resumed if wound reopened Placebo Once-weekly treatment until wound closure; treatment resumed if wound reopened Open-Label B-VEC Remaining weekly dose† used to treat secondary wounds GEM-3: Phase 3 Study Evaluating the Efficacy and Safety of B-VEC in Patients with DEB 3 6 Months (Week 26)

B-VEC was Generally Well Tolerated Total patients (n=31) Total number of AEs 45 Patients with ≥1 AE, n (%) 18 (58.1) Mild AE 15 (48.4) Moderate AE 3 (9.7) Severe AE 2 (6.5) Serious AE 3 (9.7) Drug-related AE 1 (3.2) AE leading to treatment discontinuation 0 (0) Death 0 (0) *AEs were coded using MedDRA version 24.1. At each level of summarization, a patient was counted once if the patient reported ≥1 event. AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities. The majority of AEs were mild or moderate; no AEs led to treatment discontinuation or death One AE, mild erythema, was considered possibly related to study drug as assessed by the investigator The most frequently reported AEs were pruritus, chills, and squamous cell carcinoma (3 patients each) – All 3 reports of squamous cell carcinoma occurred at sites that were not directly exposed to B-VEC or placebo and were deemed not related to study drug 4 AEs reported in ≥5% of patients by System Organ Class and Preferred Term*, n (%) Total patients (n=31) Skin and subcutaneous disorders Pruritus 3 (9.7) Erythema 2 (6.5) Rash 2 (6.5) General disorders and site conditions Chills 3 (9.7) Neoplasms benign, malignant, and unspecified Squamous cell carcinoma of the skin 3 (9.7) Respiratory, thoracic, and mediastinal disorders Cough 2 (6.5) Rhinorrhea 2 (6.5)

Anti-HSV-1 and Anti-COL7 Antibody Results 22 of 31 patients (71.0%) provided a serum sample at baseline due to the difficulty of blood draws owing to skin fragility – 19 of the 22 patients (86.4%) also had matched serum samples at 6 months At baseline, 14 of the 22 patients (63.6%) were anti-HSV-1 seropositive and 8 were seronegative, in agreement with seropositivity rates of the general US population1 – 6 of 8 (75.0%) baseline seronegative patients seroconverted at 6 months – For baseline seropositive patients, where quantitative differences at study completion could be calculated, antibody responses were not determined to be meaningful At baseline, 1 of 22 patients (4.5%) was positive for anti-COL7 antibodies – 13 of 18 patients (72.2%) with matched serum samples seroconverted by 6 months; no clinically significant immunologic reactions or differences in treatment response were seen 1. Xu F, et al. J Infect Dis. 2002;185(8):1019-1024. COL7; type VII collagen; HSV-1, herpes simplex virus type 1. 5

Treatment Response to B-VEC was Not Associated with Anti-HSV-1 Serostatus at Baseline or with Anti-COL7 Seroconversion Data in figures based on post hoc analyses using imputation; a responder was defined as meeting the primary endpoint of complete wound healing at 6 months. *Seroconversion defined as seronegative at baseline but seropositive when tested at 6 months. B-VEC, beremagene geperpavec; COL7, type VII collagen; HSV-1, herpes simplex virus type 1. 6 Response rates in primary wound pairs at 6 months suggested equivalent efficacy regardless of baseline anti–HSV-1 antibody status At 6 months, treatment response to B-VEC was consistent regardless of anti-COL7 seroconversion 67.9% 57.5% 29.3% 16.3% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Baseline Anti-HSV-1 Seropostive (n=14) Baseline Anti-HSV-1 Seronegative (n=8) Re sp on se R at e (% P at ie nt s) B-VEC Placebo 38.6% absolute difference 41.3% absolute difference 66.2% 60.0% 25.4% 20.0% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Anti-COL7 Seroconversion* (n=13) No Anti-COL7 Seroconversion (n=5) Re sp on se R at e (% P at ie nt s) B-VEC Placebo 40.8% absolute difference 40.0% absolute difference Anti-HSV-1 Anti-COL7
Conclusions B-VEC treatment demonstrated a durable and statistically significant improvement in complete wound healing at 3 and 6 months compared with placebo B-VEC was generally well tolerated, with no treatment-related discontinuations No clinically significant immunologic reactions were reported during the study Treatment response to B-VEC was not associated with anti-HSV-1 serostatus at baseline or with anti-COL7 seroconversion An ongoing open-label extension study is investigating the long-term efficacy and safety of B-VEC in patients with DEB, regardless of prior enrollment in GEM-3 7 Open-label extension study: NCT04917874 B-VEC, beremagene geperpavec; COL7, type VII collagen; DEB, dystrophic epidermolysis bullosa; HSV-1, herpes simplex virus type 1.






