Exhibit 99.1

Corporate Presentation Inflammation & Immunology Platforms XPro / CORDStrom / INKmune ® INMB Nasdaq February 2025 v19

FORWARD LOOKING STATEMENTS This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events. When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements. Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook. Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions. Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward - looking statements. They are neither statements of historical fact nor guarantees of assurance of future performance. We caution you therefore against relying on any of these forward - looking statements. Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations; our ability to protect our intellectual property rights; the impact of any infringement actions or other litigation brought against us; competition from other providers and products; our ability to develop and commercialize products and services; changes in government regulation; our ability to complete capital raising transactions; and other factors relating to our industry, our operations and results of operations. There is no guarantee that any specific outcome will be achieved. Investment results are speculative and there is a risk of loss, potentially all loss of investments. Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results. 2
a Three Novel Platforms with Data in 2025 » CORDStrom Completed Blinded Randomized Trial in Recessive Dystrophic Epidermolysis Bullosa (RDEB) o Clinical benefit in RDEB patients treated with CORDStrom o US BLA Submission December 2025 o Granted Orphan Drug and Rare Pediatric Disease Designations from FDA » XPro : Treating Neuroinflammation without Immunosuppression o Phase 2 Alzheimer’s fully enrolled o Top - line cognition results, June 2025 » INKmune : Creates memory - like NK Cells to Kill Cancer o Phase I dose escalation cohorts complete o Open label Phase 2 Metastatic Castrate Resistant Prostate Cancer with ongoing data readouts in 2025 3
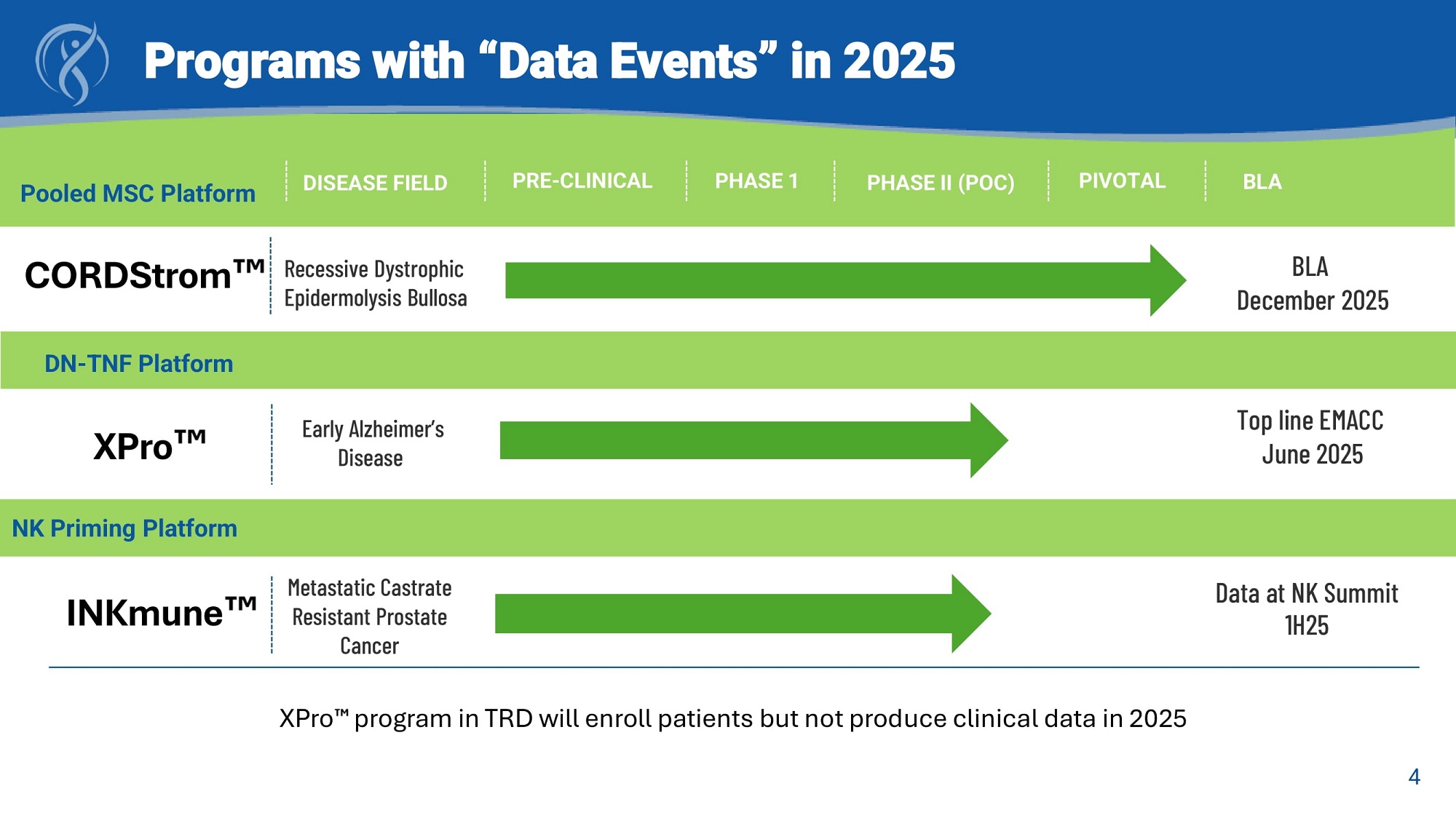
DISEASE FIELD PRE - CLINICAL PHASE 1 PHASE II (POC) PIVOTAL BLA Early Alzheimer’s Disease Top line EMACC June 2025 Recessive Dystrophic Epidermolysis Bullosa BLA December 2025 NK Priming Platform Data at NK Summit 1H25 Programs with “Data Events” in 2025 CORDStrom XPro INKmune Metastatic Castrate Resistant Prostate Cancer XPro program in TRD will enroll patients but not produce clinical data in 2025 Pooled MSC Platform 4 DN - TNF Platform
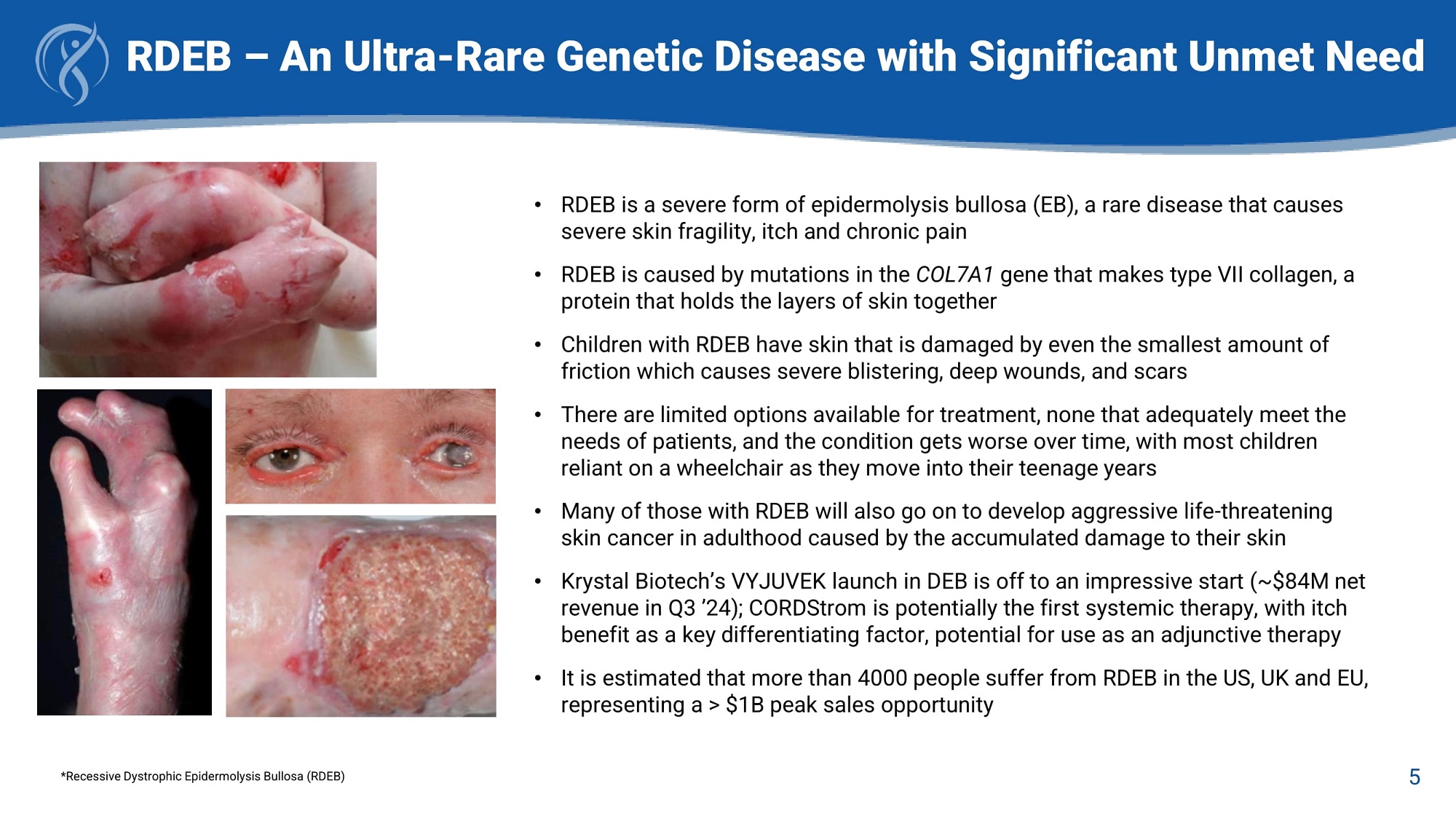
RDEB – An Ultra - Rare Genetic Disease with Significant Unmet Need • RDEB is a severe form of epidermolysis bullosa (EB), a rare disease that causes severe skin fragility, itch and chronic pain • RDEB is caused by mutations in the COL7A1 gene that makes type VII collagen, a protein that holds the layers of skin together • Children with RDEB have skin that is damaged by even the smallest amount of friction which causes severe blistering, deep wounds, and scars • There are limited options available for treatment, none that adequately meet the needs of patients, and the condition gets worse over time, with most children reliant on a wheelchair as they move into their teenage years • Many of those with RDEB will also go on to develop aggressive life - threatening skin cancer in adulthood caused by the accumulated damage to their skin • Krystal Biotech’s VYJUVEK launch in DEB is off to an impressive start (~$84M net revenue in Q3 ’24); CORDStrom is potentially the first systemic therapy, with itch benefit as a key differentiating factor, potential for use as an adjunctive therapy • It is estimated that more than 4000 people suffer from RDEB in the US, UK and EU, representing a > $1B peak sales opportunity 5 *Recessive Dystrophic Epidermolysis Bullosa (RDEB)
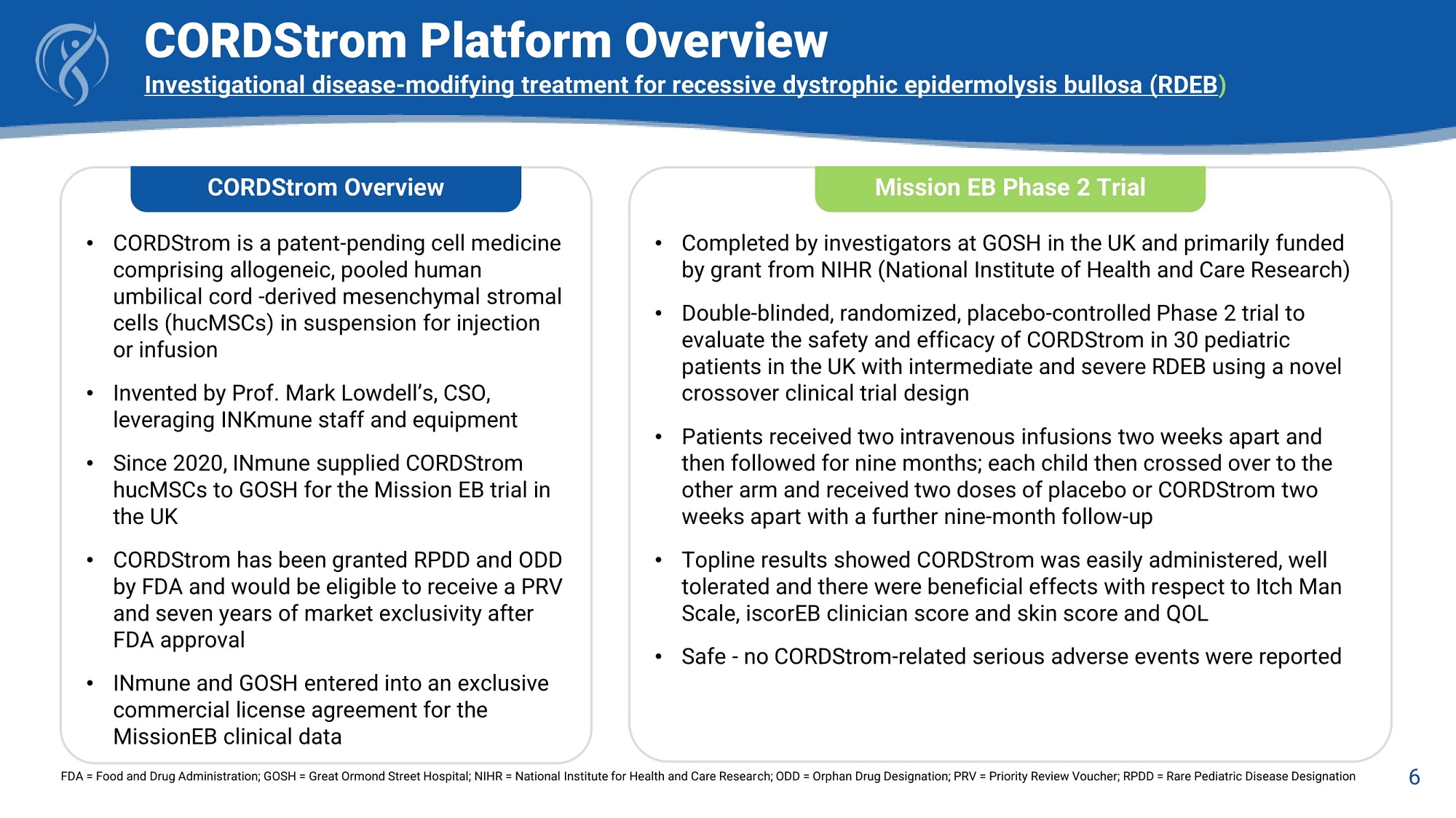
CORDStrom Platform Overview Investigational disease - modifying treatment for recessive dystrophic epidermolysis bullosa (RDEB ) CORDStrom Overview • CORDStrom is a patent - pending cell medicine comprising allogeneic, pooled human umbilical cord - derived mesenchymal stromal cells (hucMSCs) in suspension for injection or infusion • Invented by Prof. Mark Lowdell’s, CSO, leveraging INKmune staff and equipment • Since 2020, INmune supplied CORDStrom hucMSCs to GOSH for the Mission EB trial in the UK • CORDStrom has been granted RPDD and ODD by FDA and would be eligible to receive a PRV and seven years of market exclusivity after FDA approval • INmune and GOSH entered into an exclusive commercial license agreement for the MissionEB clinical data Mission EB Phase 2 Trial 6 FDA = Food and Drug Administration; GOSH = Great Ormond Street Hospital; NIHR = National Institute for Health and Care Research; ODD = Orphan Drug Designation; PRV = Priority Review Voucher; RPDD = Rare Pediatric Disease Designation • Completed by investigators at GOSH in the UK and primarily funded by grant from NIHR (National Institute of Health and Care Research) • Double - blinded, randomized, placebo - controlled Phase 2 trial to evaluate the safety and efficacy of CORDStrom in 30 pediatric patients in the UK with intermediate and severe RDEB using a novel crossover clinical trial design • Patients received two intravenous infusions two weeks apart and then followed for nine months; each child then crossed over to the other arm and received two doses of placebo or CORDStrom two weeks apart with a further nine - month follow - up • Topline results showed CORDStrom was easily administered, well tolerated and there were beneficial effects with respect to Itch Man Scale, iscorEB clinician score and skin score and QOL • Safe - no CORDStrom - related serious adverse events were reported
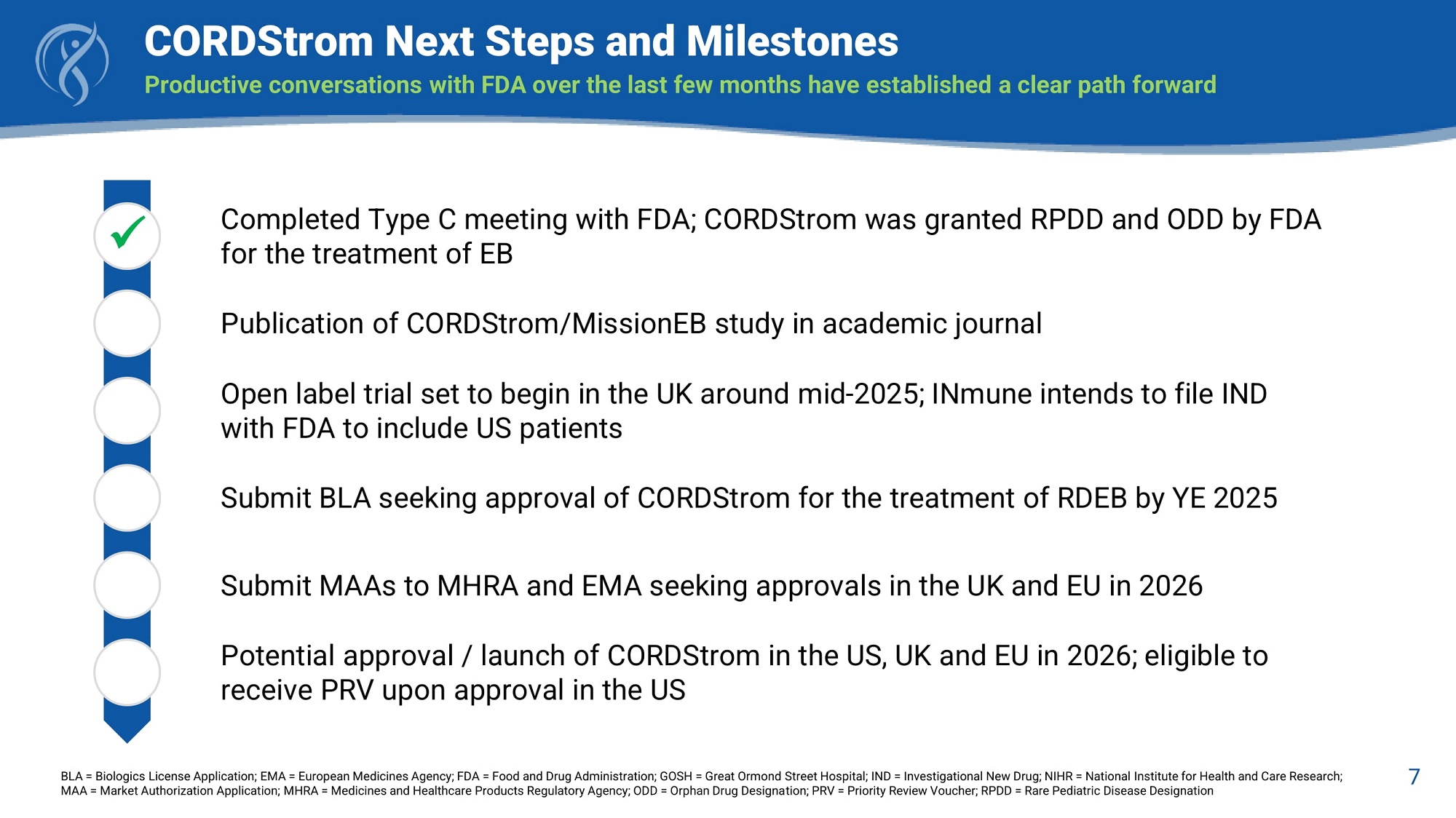
7 CORDStrom Next Steps and Milestones Productive conversations with FDA over the last few months have established a clear path forward BLA = Biologics License Application; EMA = European Medicines Agency; FDA = Food and Drug Administration; GOSH = Great Ormond Street Hospital; IND = Investigational New Drug; NIHR = National Institute for Health and Care Research; MAA = Market Authorization Application; MHRA = Medicines and Healthcare Products Regulatory Agency; ODD = Orphan Drug Designation; PRV = Priority Review Voucher; RPDD = Rare Pediatric Disease Designation Completed Type C meeting with FDA; CORDStrom was granted RPDD and ODD by FDA for the treatment of EB Publication of CORDStrom/MissionEB study in academic journal Open label trial set to begin in the UK around mid - 2025; INmune intends to file IND with FDA to include US patients Submit BLA seeking approval of CORDStrom for the treatment of RDEB by YE 2025 Submit MAAs to MHRA and EMA seeking approvals in the UK and EU in 2026 Potential approval / launch of CORDStrom in the US, UK and EU in 2026; eligible to receive PRV upon approval in the US x
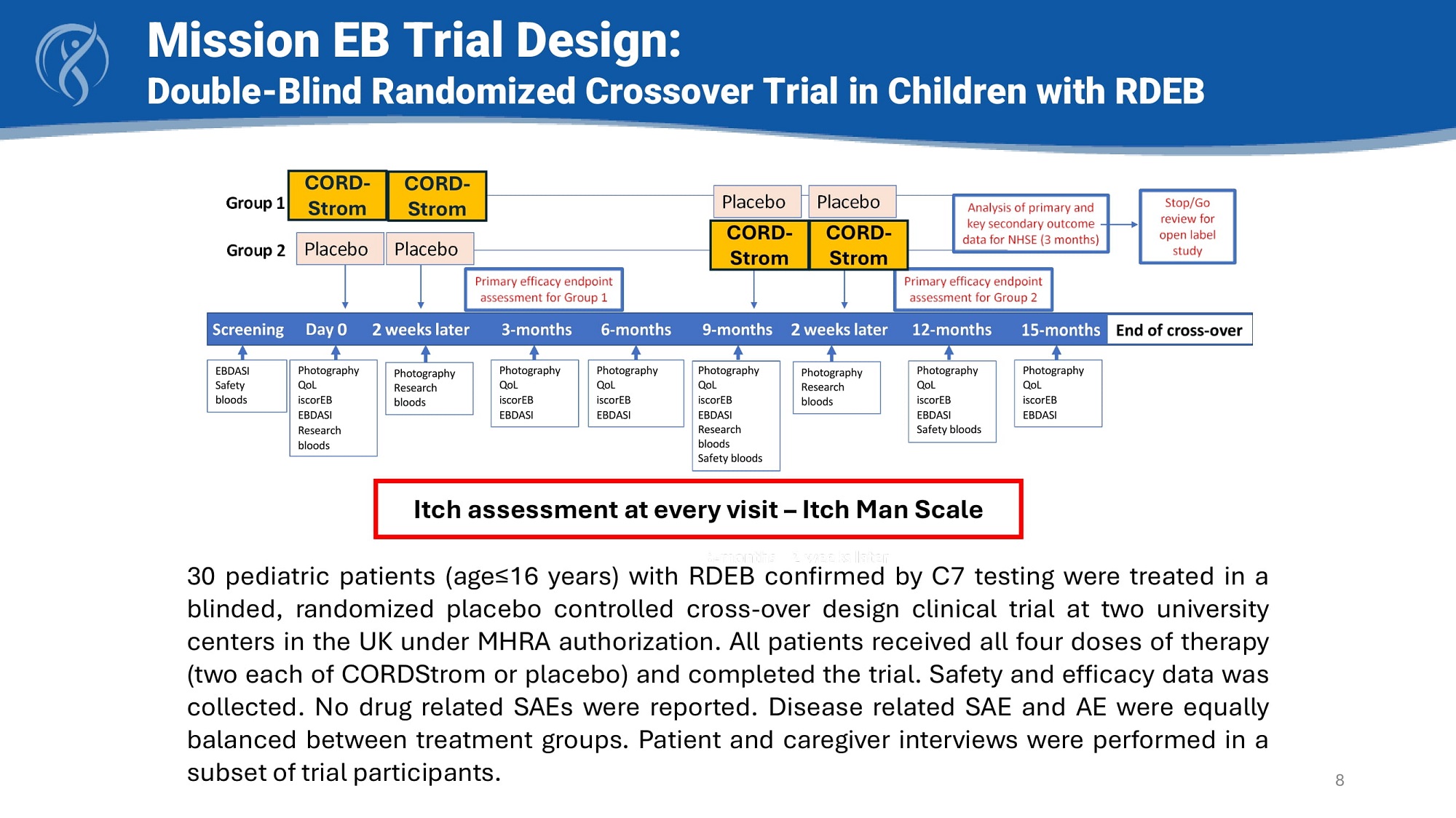
Photography QoL iscorEB EBDASI Research bloods Photography QoL iscorEB EBDASI Photography Research bloods Photography QoL iscorEB EBDASI Safety bloods Photography Research bloods Photography QoL iscorEB EBDASI Photography QoL iscorEB EBDASI EBDASI Safety bloods Photography QoL iscorEB EBDASI Research bloods Safety bloods 30 pediatric patients (age≤ 16 years) with RDEB confirmed by C 7 testing were treated in a blinded, randomized placebo controlled cross - over design clinical trial at two university centers in the UK under MHRA authorization . All patients received all four doses of therapy (two each of CORDStrom or placebo) and completed the trial . Safety and efficacy data was collected . No drug related SAEs were reported . Disease related SAE and AE were equally balanced between treatment groups . Patient and caregiver interviews were performed in a subset of trial participants . Mission EB Trial Design: Double - Blind Randomized Crossover Trial in Children with RDEB 8 CORD - Strom CORD - Strom CORD - Strom CORD - Strom Itch assessment at every visit – Itch Man Scale
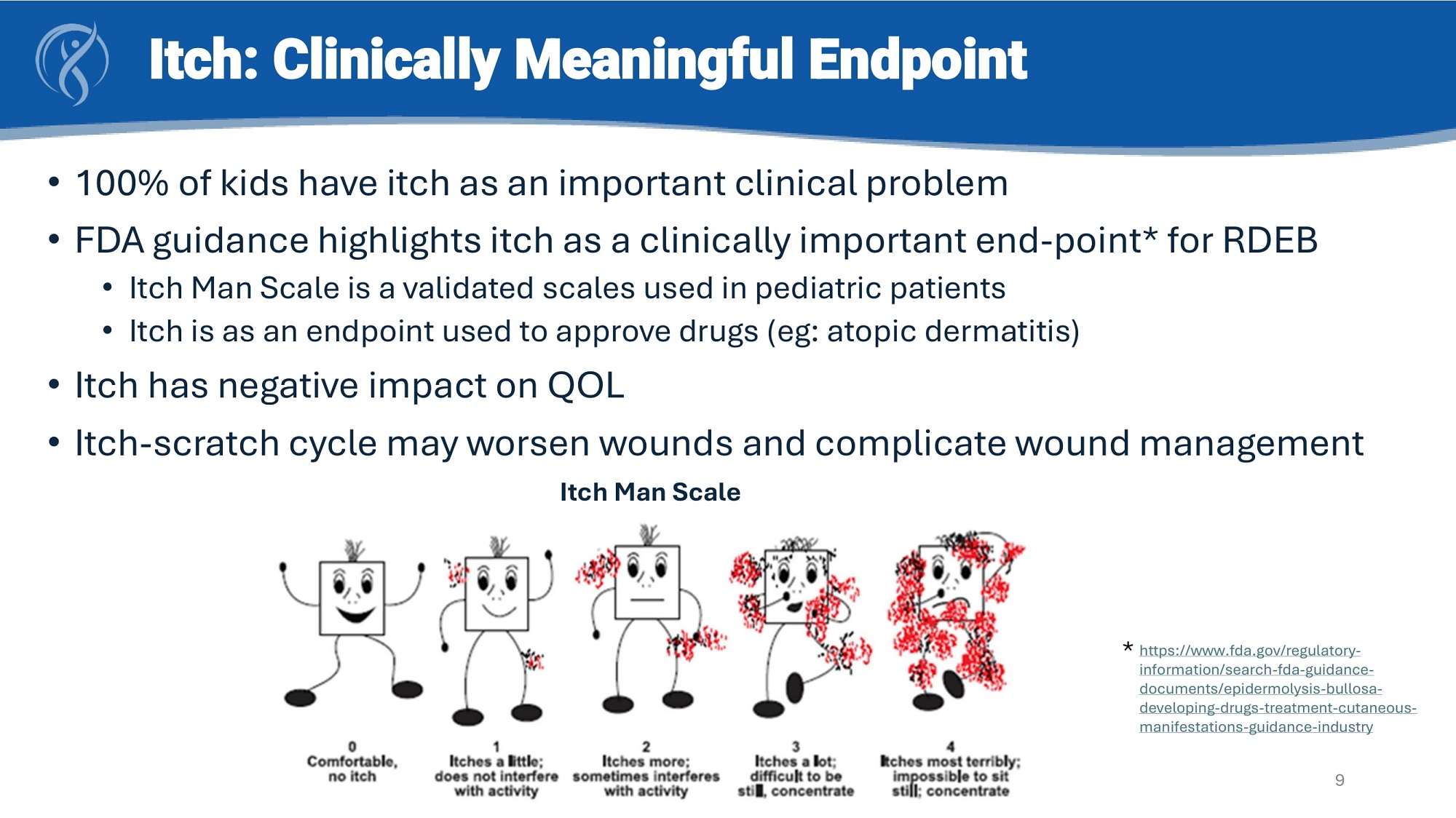
Itch: Clinically Meaningful Endpoint • 100% of kids have itch as an important clinical problem • FDA guidance highlights itch as a clinically important end - point* for RDEB • Itch Man Scale is a validated scales used in pediatric patients • Itch is as an endpoint used to approve drugs (eg: atopic dermatitis) • Itch has negative impact on QOL • Itch - scratch cycle may worsen wounds and complicate wound management Itch Man Scale * https://www.fda.gov/regulatory - information/search - fda - guidance - documents/epidermolysis - bullosa - developing - drugs - treatment - cutaneous - manifestations - guidance - industry 9
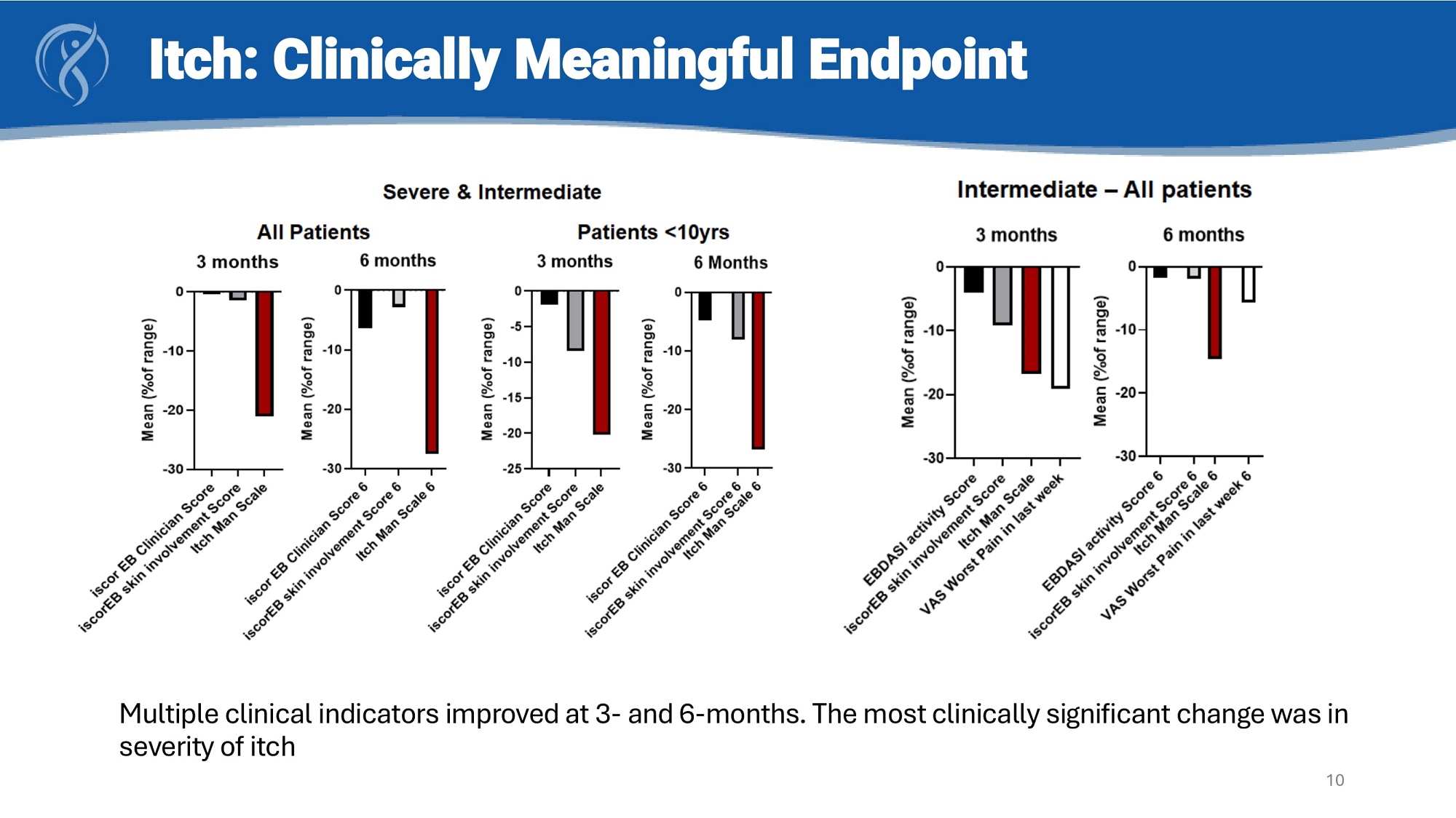
Multiple clinical indicators improved at 3 - and 6 - months. The most clinically significant change was in severity of itch Itch: Clinically Meaningful Endpoint 10
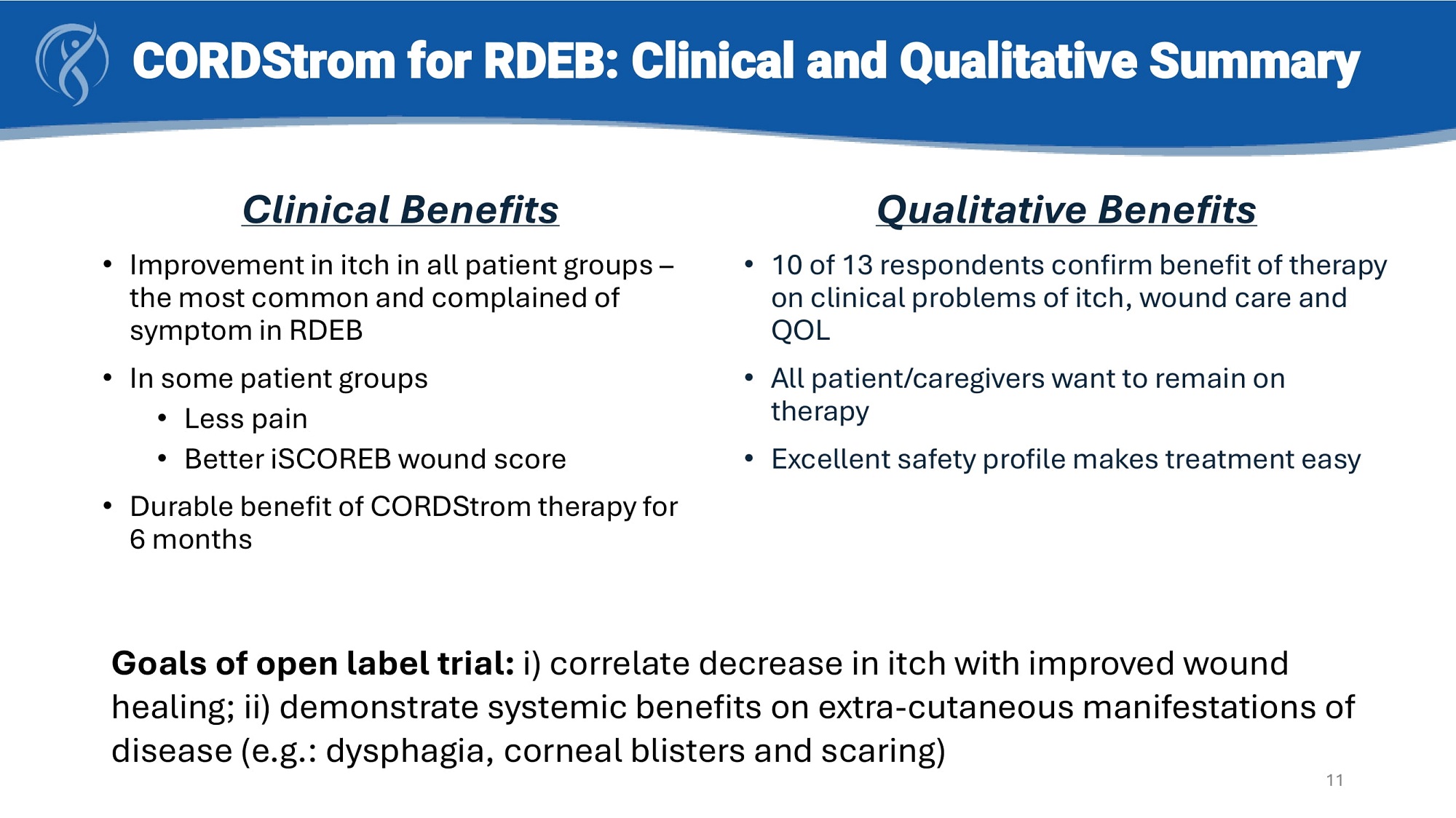
CORDStrom for RDEB: Clinical and Qualitative Summary Clinical Benefits • Improvement in itch in all patient groups – the most common and complained of symptom in RDEB • In some patient groups • Less pain • Better iSCOREB wound score • Durable benefit of CORDStrom therapy for 6 months Qualitative Benefits • 10 of 13 respondents confirm benefit of therapy on clinical problems of itch, wound care and QOL • All patient/caregivers want to remain on therapy • Excellent safety profile makes treatment easy Goals of open label trial: i) correlate decrease in itch with improved wound healing; ii) demonstrate systemic benefits on extra - cutaneous manifestations of disease (e.g.: dysphagia, corneal blisters and scaring) 11
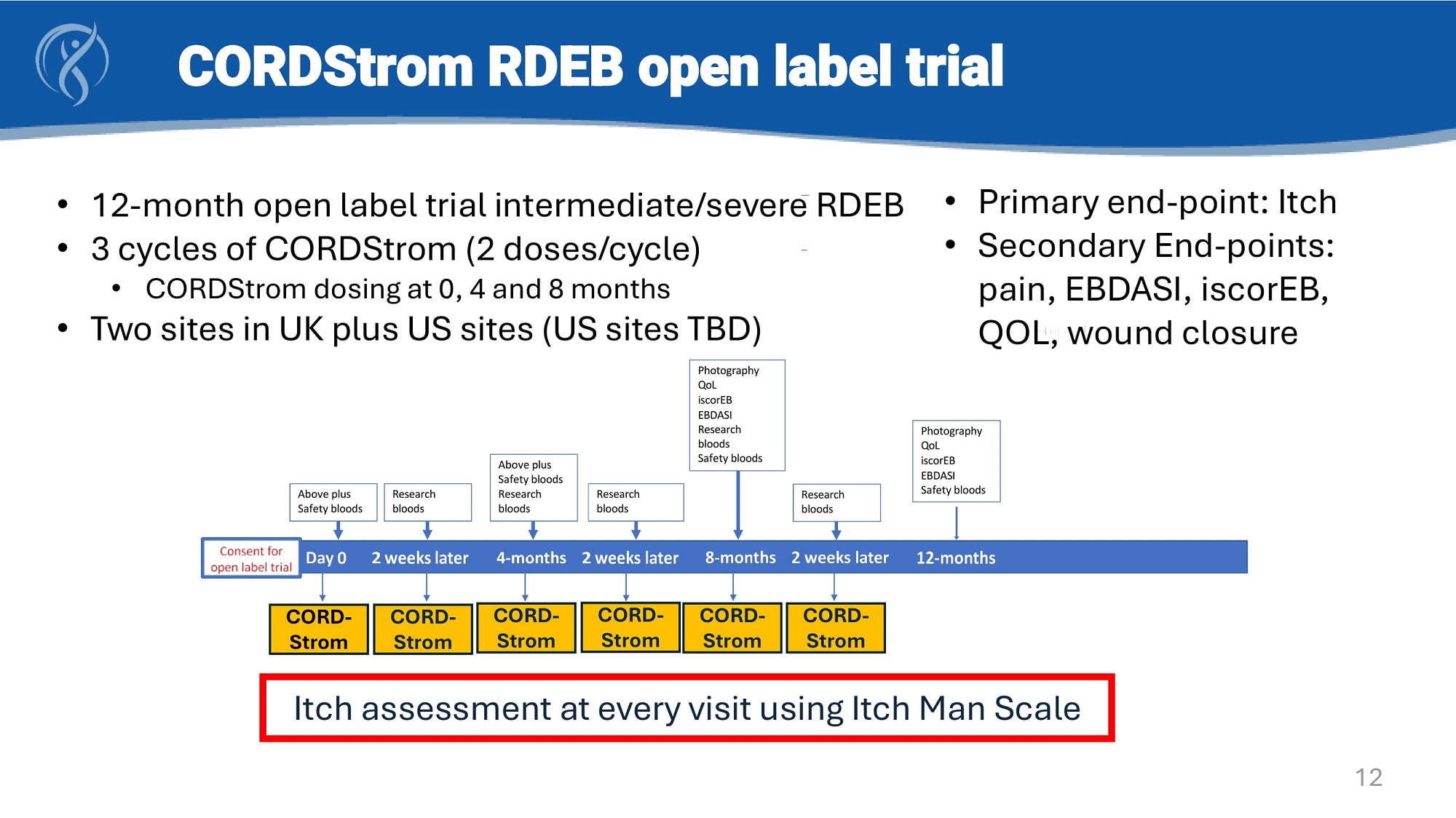
Photography QoL iscorEB EBDASI Safety bloods Research bloods Safety bloods Above plus Safety bloods Above plus Safety bloods Research bloods Research bloods Research bloods • 12 - month open label trial intermediate/severe RDEB • 3 cycles of CORDStrom (2 doses/cycle) • CORDStrom dosing at 0, 4 and 8 months • Two sites in UK plus US sites (US sites TBD) Photography QoL iscorEB EBDASI Research bloods CORDStrom RDEB open label trial CORD - CORD - CORD - Strom CORD - CORD - CORD - Strom Strom Strom Strom Strom • Primary end - point : Itch • Secondary End - points : pain, EBDASI, iscorEB, QOL, wound closure Itch assessment at every visit using Itch Man Scale 12
13 for AD Treating Alzheimer’s as an Immunologic Disease Driven by Neuroinflammation
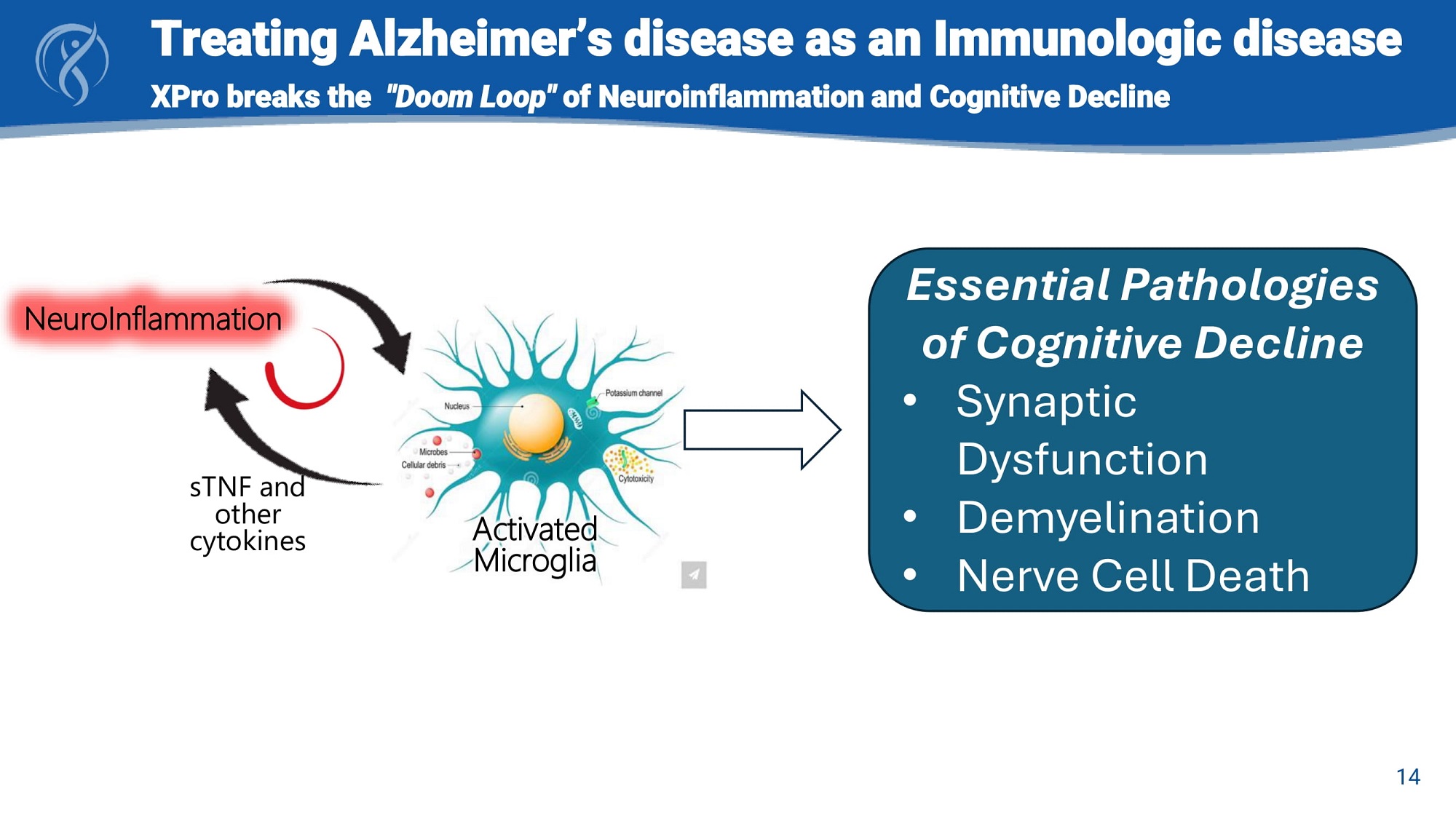
Activated Microglia sTNF and other cytokines NeuroInflammation Essential Pathologies of Cognitive Decline • Synaptic Dysfunction • Demyelination • Nerve Cell Death Treating Alzheimer’s disease as an Immunologic disease 14 XPro breaks the "Doom Loop" of Neuroinflammation and Cognitive Decline
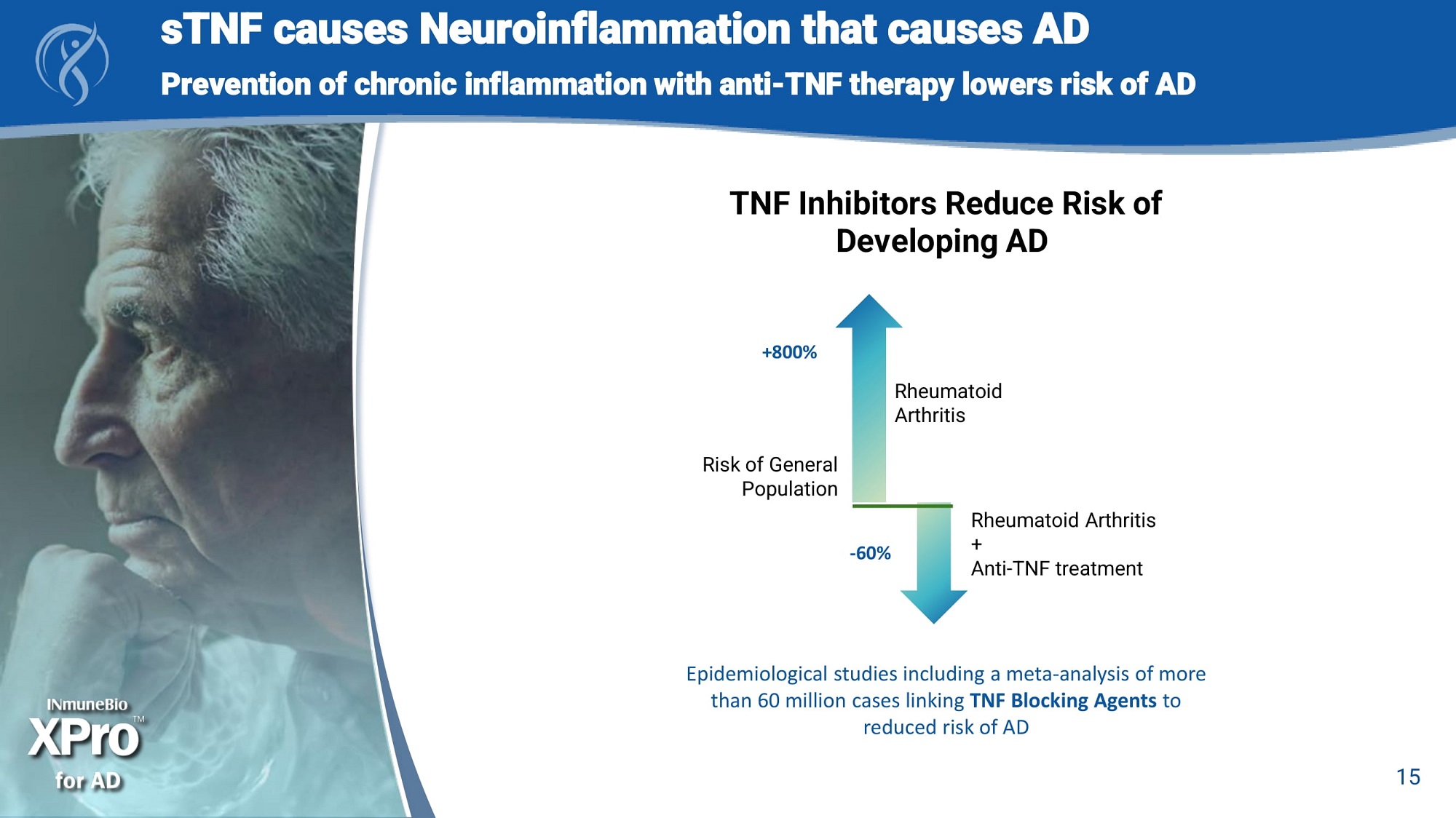
a Epidemiological studies including a meta - analysis of more than 60 million cases linking TNF Blocking Agents to reduced risk of AD +800% Rheumatoid Arthritis Risk of General Population Rheumatoid Arthritis + Anti - TNF treatment - 60% 15 TNF Inhibitors Reduce Risk of Developing AD sTNF causes Neuroinflammation that causes AD Prevention of chronic inflammation with anti - TNF therapy lowers risk of AD
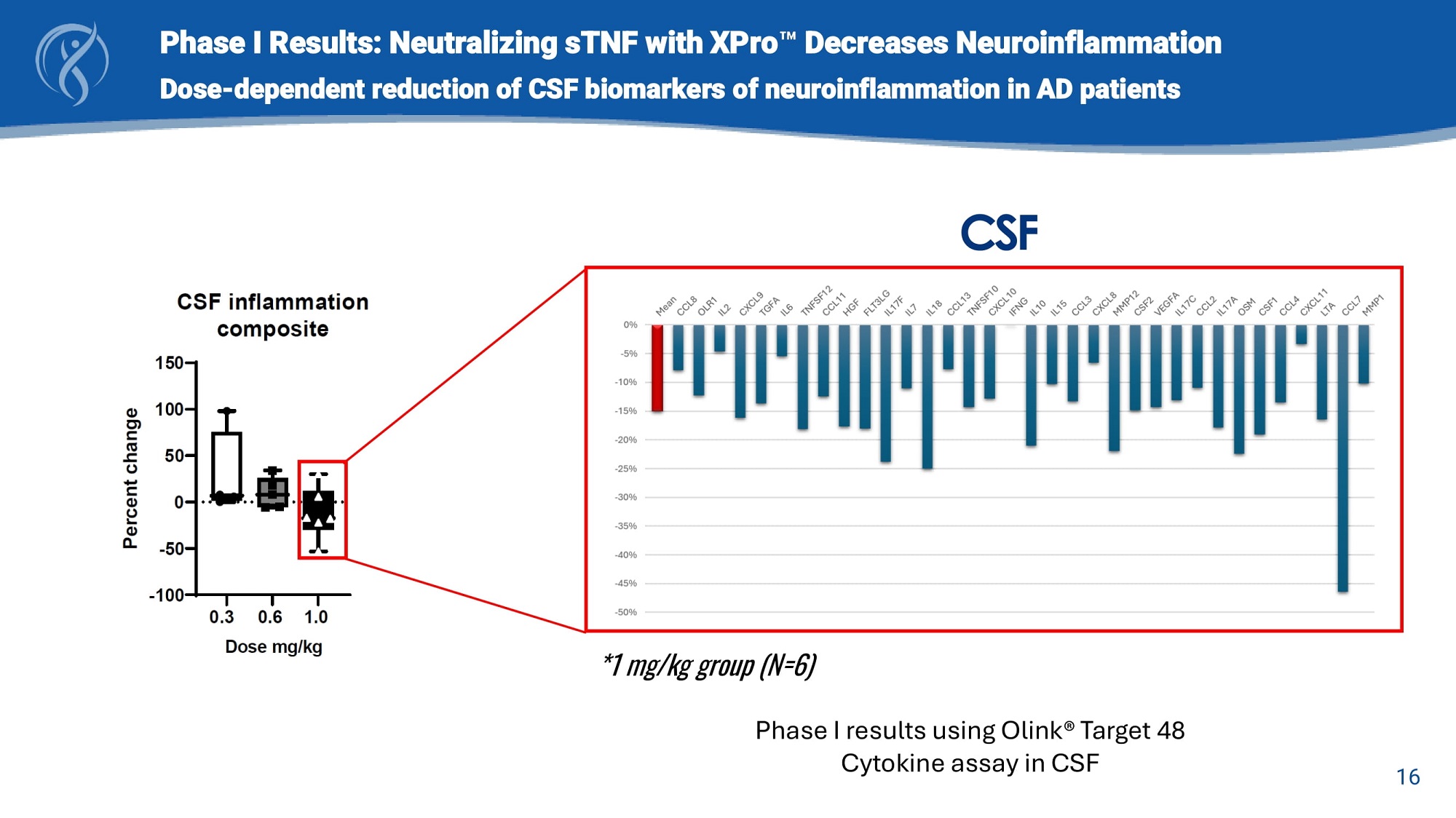
16 Phase I Results: Neutralizing sTNF with XPro Decreases Neuroinflammation Dose - dependent reduction of CSF biomarkers of neuroinflammation in AD patients CSF *1 mg/kg group (N=6) Phase I results using Olink® Target 48 Cytokine assay in CSF
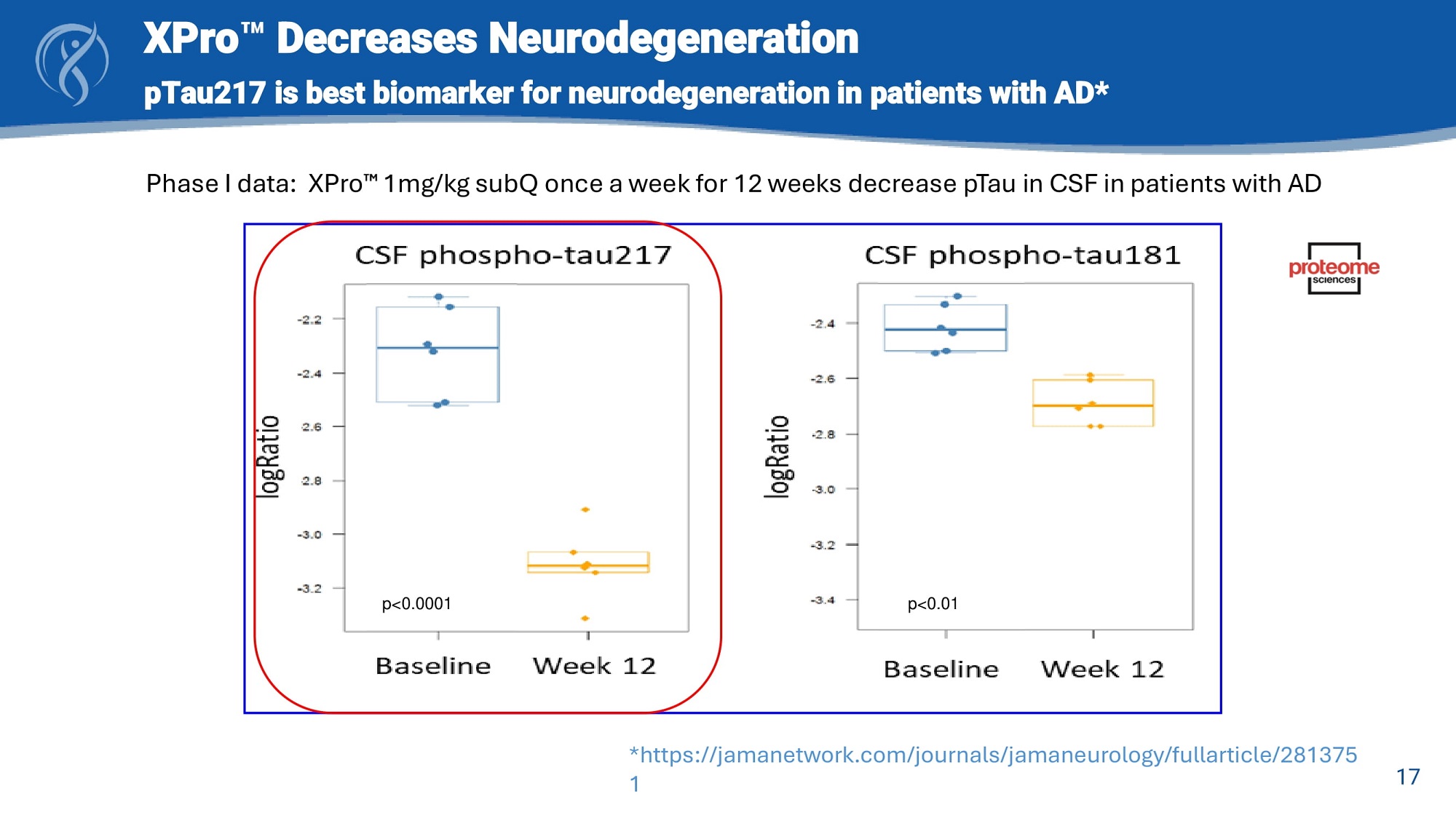
17 XPro Decreases Neurodegeneration pTau217 is best biomarker for neurodegeneration in patients with AD* p<0.0001 p<0.01 Phase I data: XPro 1mg/kg subQ once a week for 12 weeks decrease pTau in CSF in patients with AD *https://jamanetwork.com/journals/jamaneurology/fullarticle/281375 1
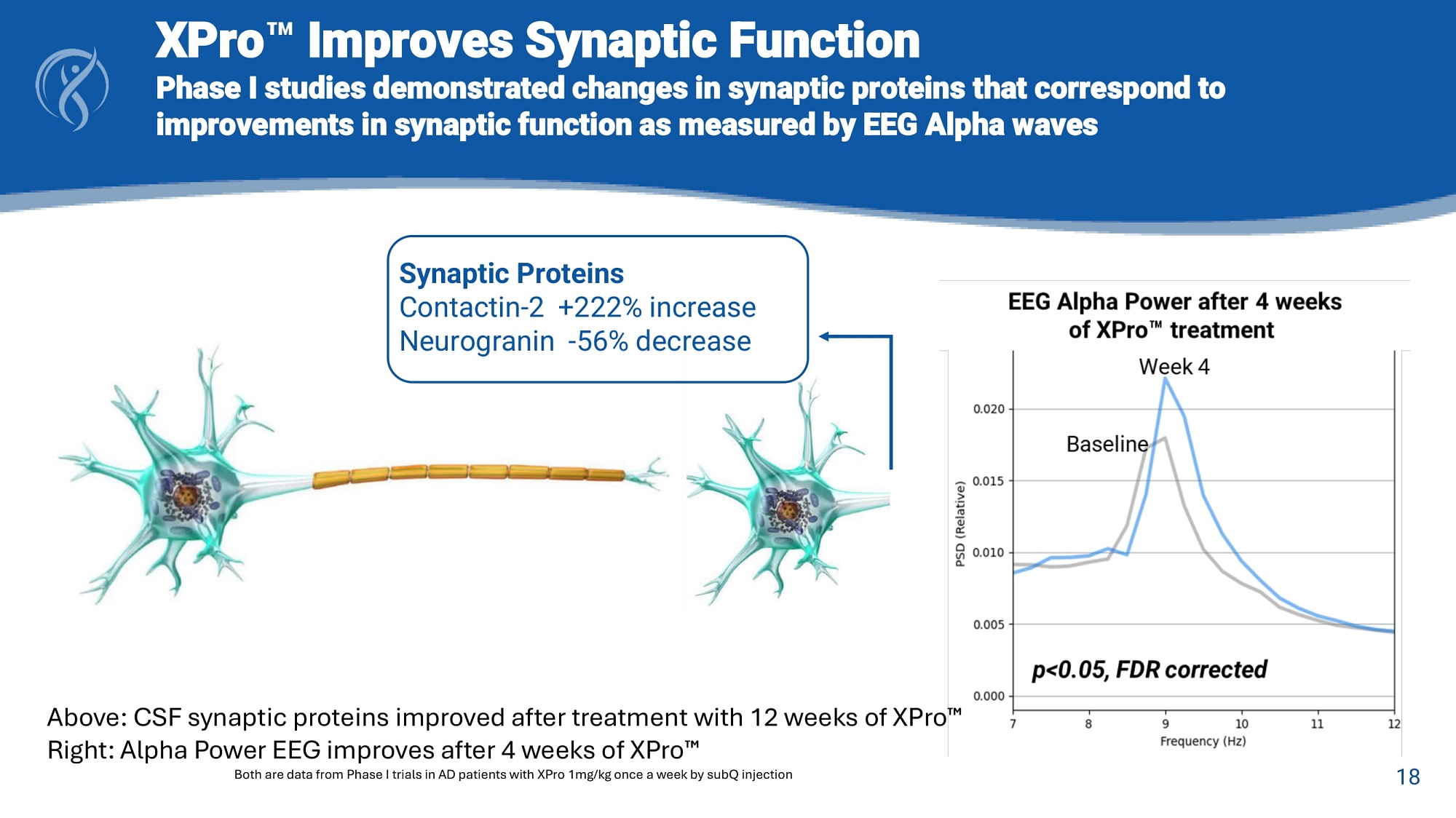
18 XPro Improves Synaptic Function Phase I studies demonstrated changes in synaptic proteins that correspond to improvements in synaptic function as measured by EEG Alpha waves Synaptic Proteins Contactin - 2 +222% increase Neurogranin - 56% decrease Above: CSF synaptic proteins improved after treatment with 12 weeks of XPro Right: Alpha Power EEG improves after 4 weeks of XPro Both are data from Phase I trials in AD patients with XPro 1mg/kg once a week by subQ injection
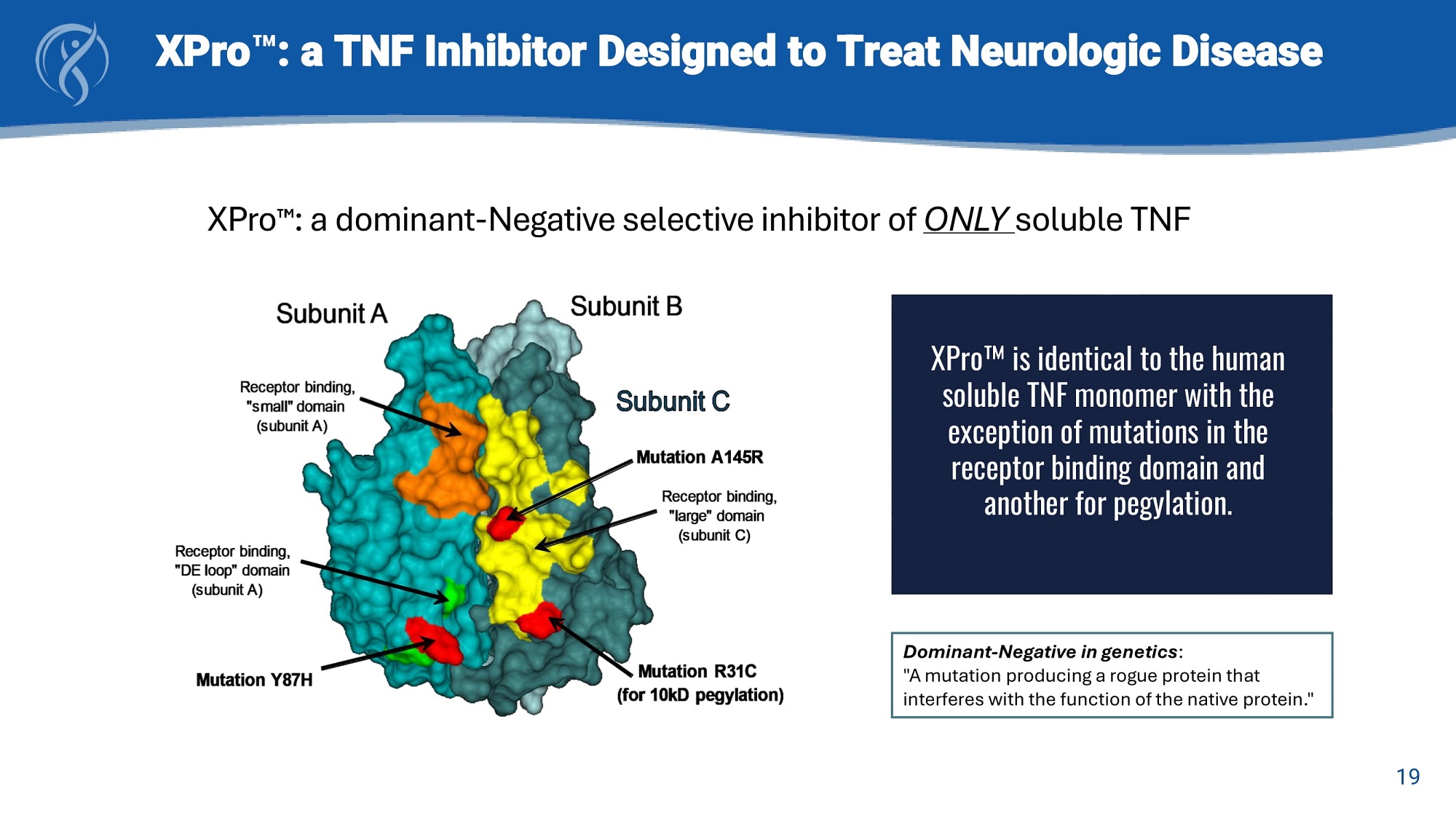
19 XPro : a TNF Inhibitor Designed to Treat Neurologic Disease XPro is identical to the human soluble TNF monomer with the exception of mutations in the receptor binding domain and another for pegylation. XPro : a dominant - Negative selective inhibitor of ONLY soluble TNF Dominant - Negative in genetics : "A mutation producing a rogue protein that interferes with the function of the native protein."
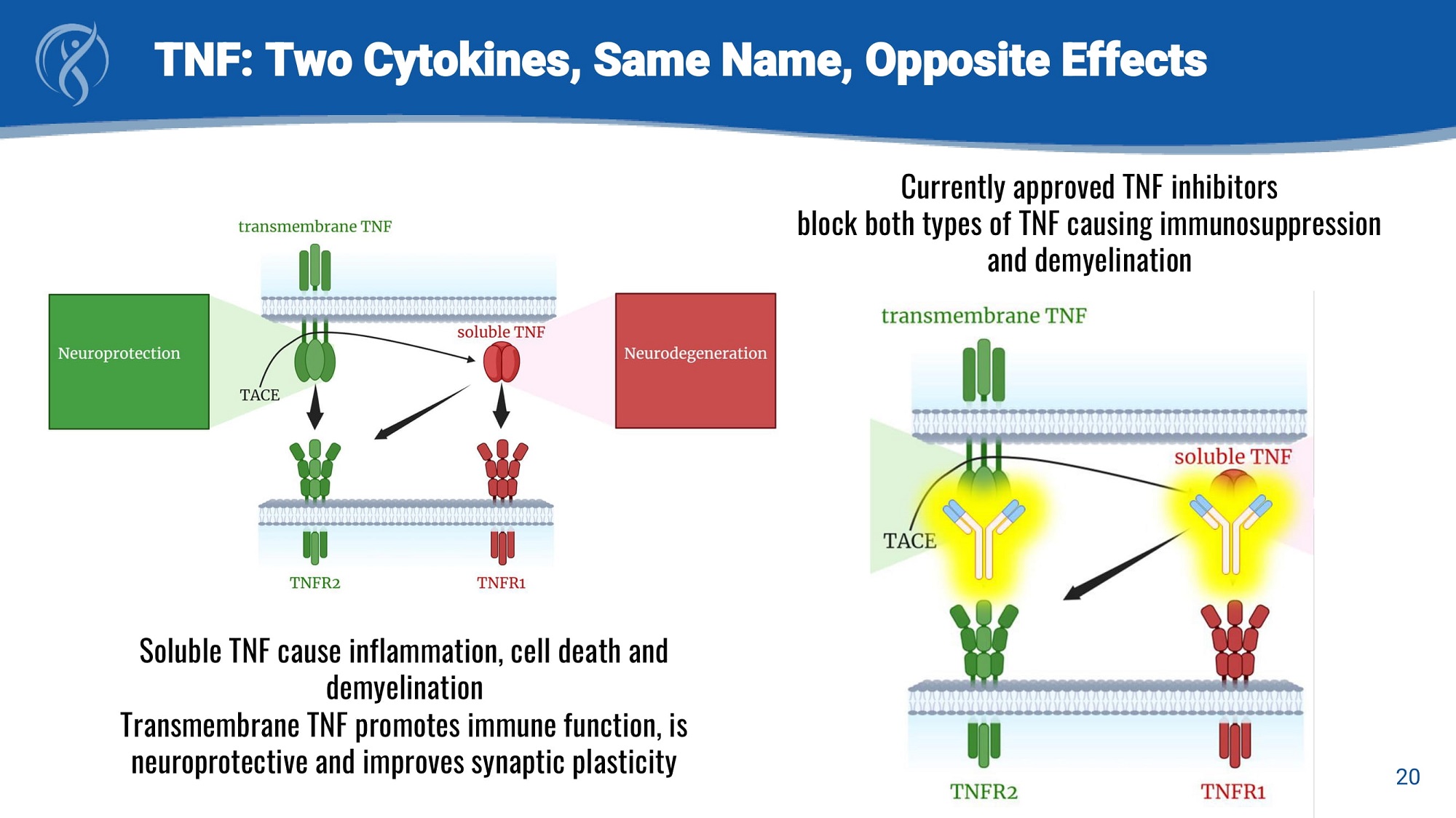
20 TNF: Two Cytokines, Same Name, Opposite Effects Currently approved TNF inhibitors block both types of TNF causing immunosuppression and demyelination Soluble TNF cause inflammation, cell death and demyelination Transmembrane TNF promotes immune function, is neuroprotective and improves synaptic plasticity

XPro unique Mechanism of Action XPro neutralizes sTNF without affecting tmTNF using dominant - negative technology XPro Targeting sTNF exchanges with sTNF monomers to form inactive heterotrimers Inflammatory TNF eliminated No paracrine signaling through receptors Preserving tmTNF Function tmTNF homotrimers are anchored to the cell membrane; XPro cannot exchange Purpose Built for Treating CNS Disease: XPro neutralizes sTNF without affecting tmTNF Beneficial TNF signaling preserved Improved immune and CNS function 21
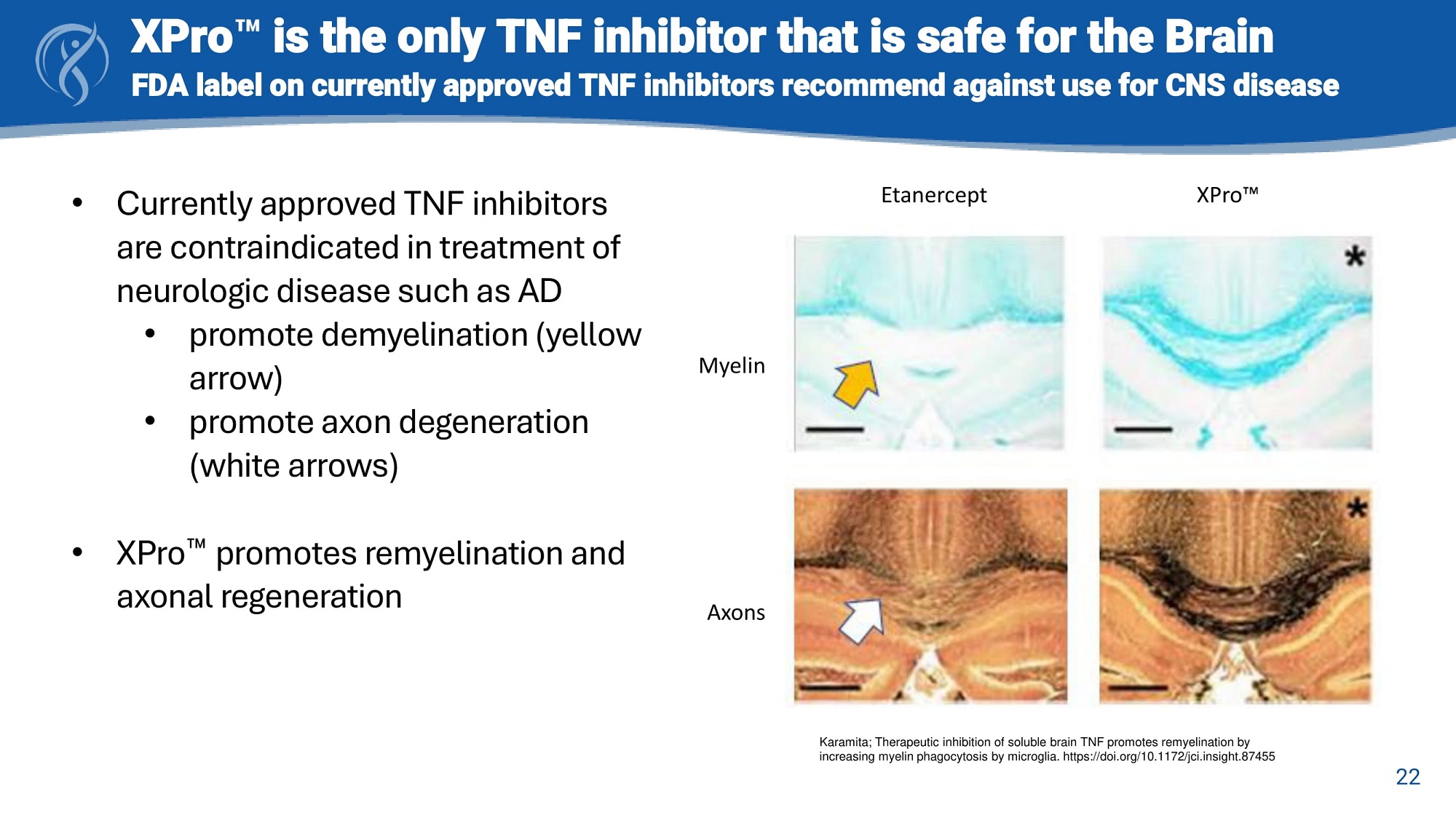
• Currently approved TNF inhibitors are contraindicated in treatment of neurologic disease such as AD • promote demyelination (yellow arrow) • promote axon degeneration (white arrows) • XPro promotes remyelination and axonal regeneration Karamita; Therapeutic inhibition of soluble brain TNF promotes remyelination by increasing myelin phagocytosis by microglia. https://doi.org/10.1172/jci.insight.87455 Etanercept XPro Myelin 22 Axons XPro is the only TNF inhibitor that is safe for the Brain FDA label on currently approved TNF inhibitors recommend against use for CNS disease
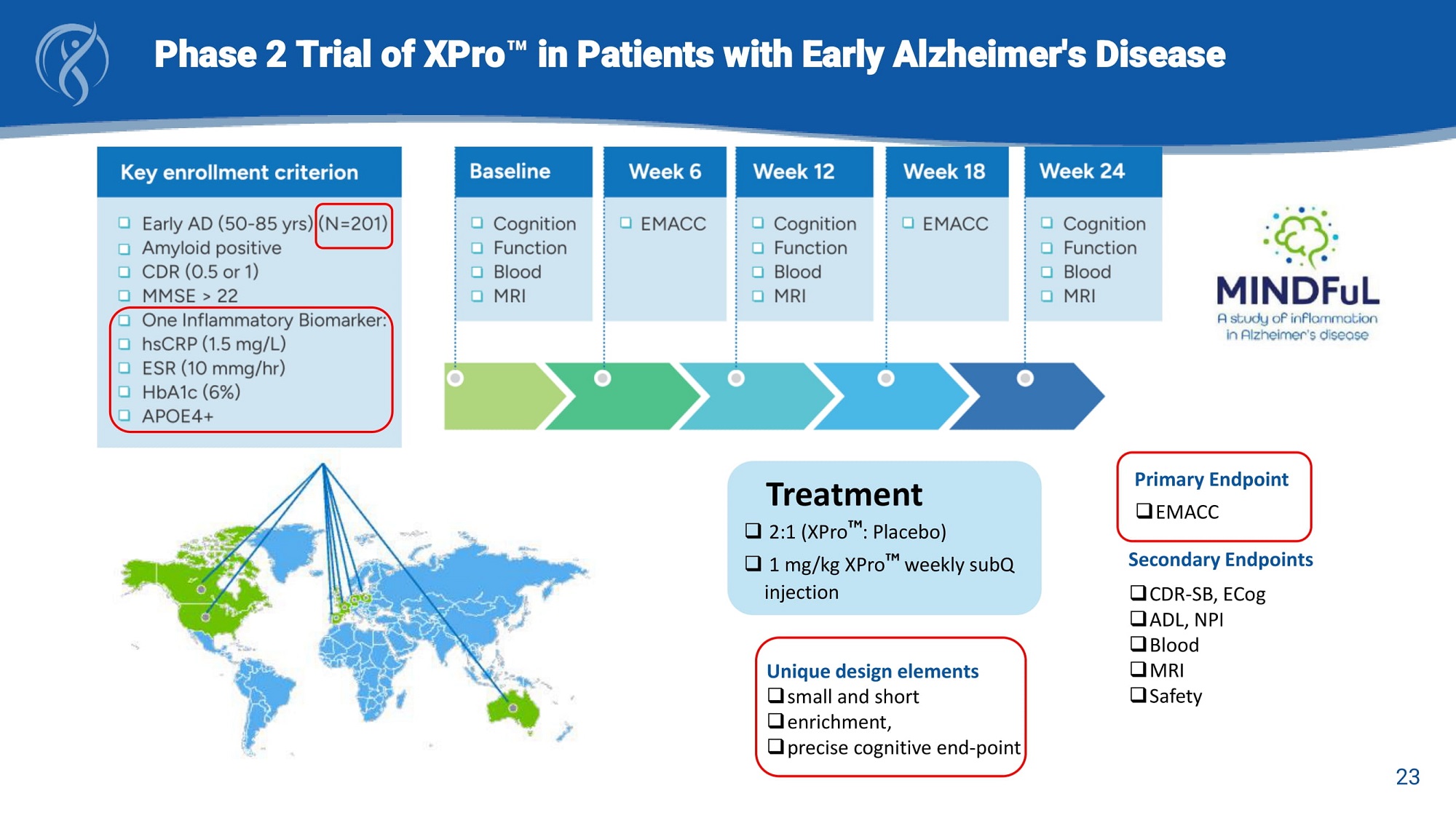
Phase 2 Trial of XPro in Patients with Early Alzheimer's Disease Primary Endpoint □ EMACC Secondary Endpoints □ CDR - SB, ECog □ ADL, NPI □ Blood □ MRI □ Safety Treatment □ 2:1 (XPro : Placebo) □ 1 mg/kg XPro weekly subQ injection Unique design elements □ small and short □ enrichment, □ precise cognitive end - point 23
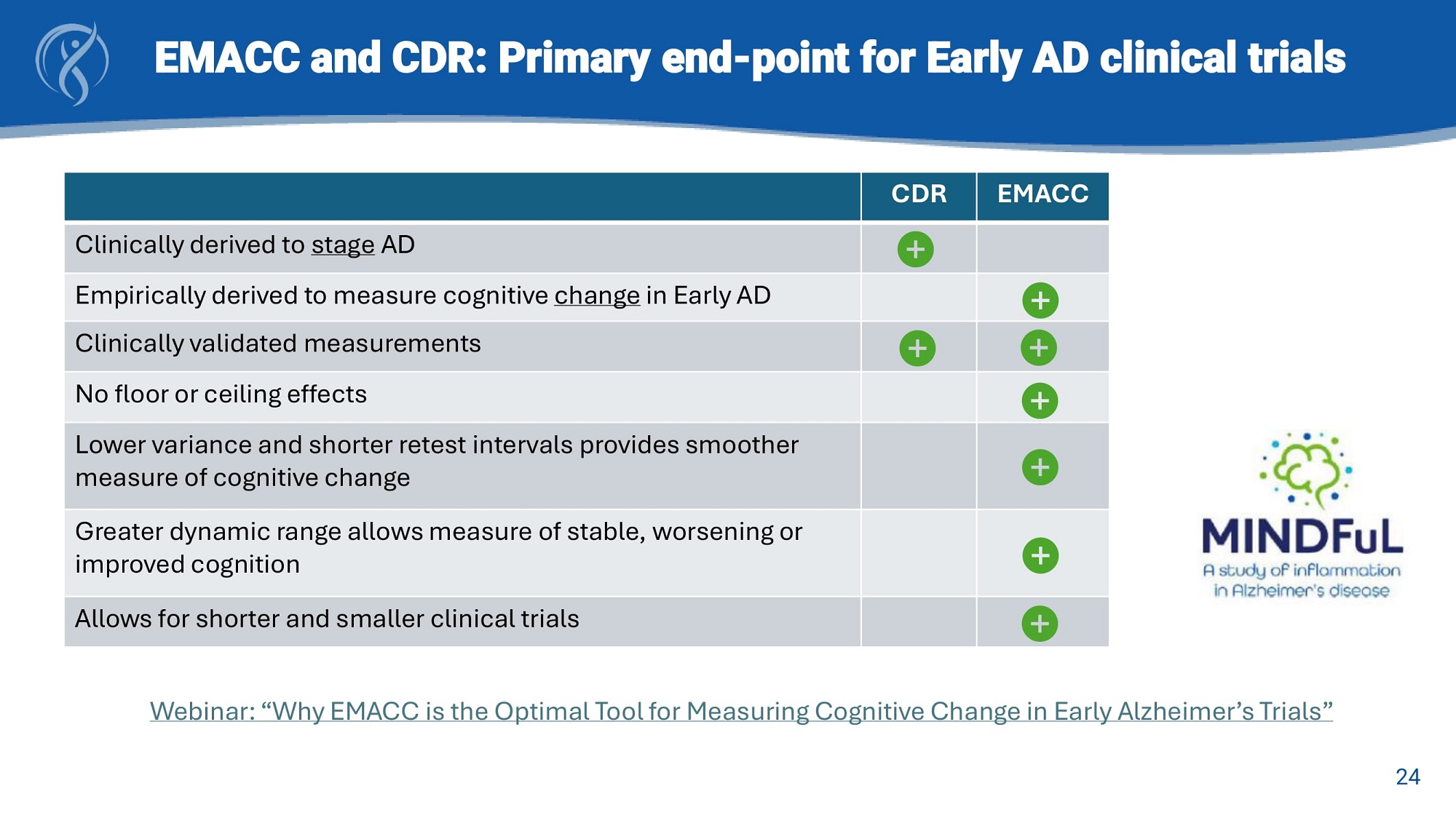
EMACC and CDR: Primary end - point for Early AD clinical trials EMACC CDR Clinically derived to stage AD Empirically derived to measure cognitive change in Early AD Clinically validated measurements No floor or ceiling effects Lower variance and shorter retest intervals provides smoother measure of cognitive change Greater dynamic range allows measure of stable, worsening or improved cognition Allows for shorter and smaller clinical trials Webinar: “Why EMACC is the Optimal Tool for Measuring Cognitive Change in Early Alzheimer’s Trials” 24
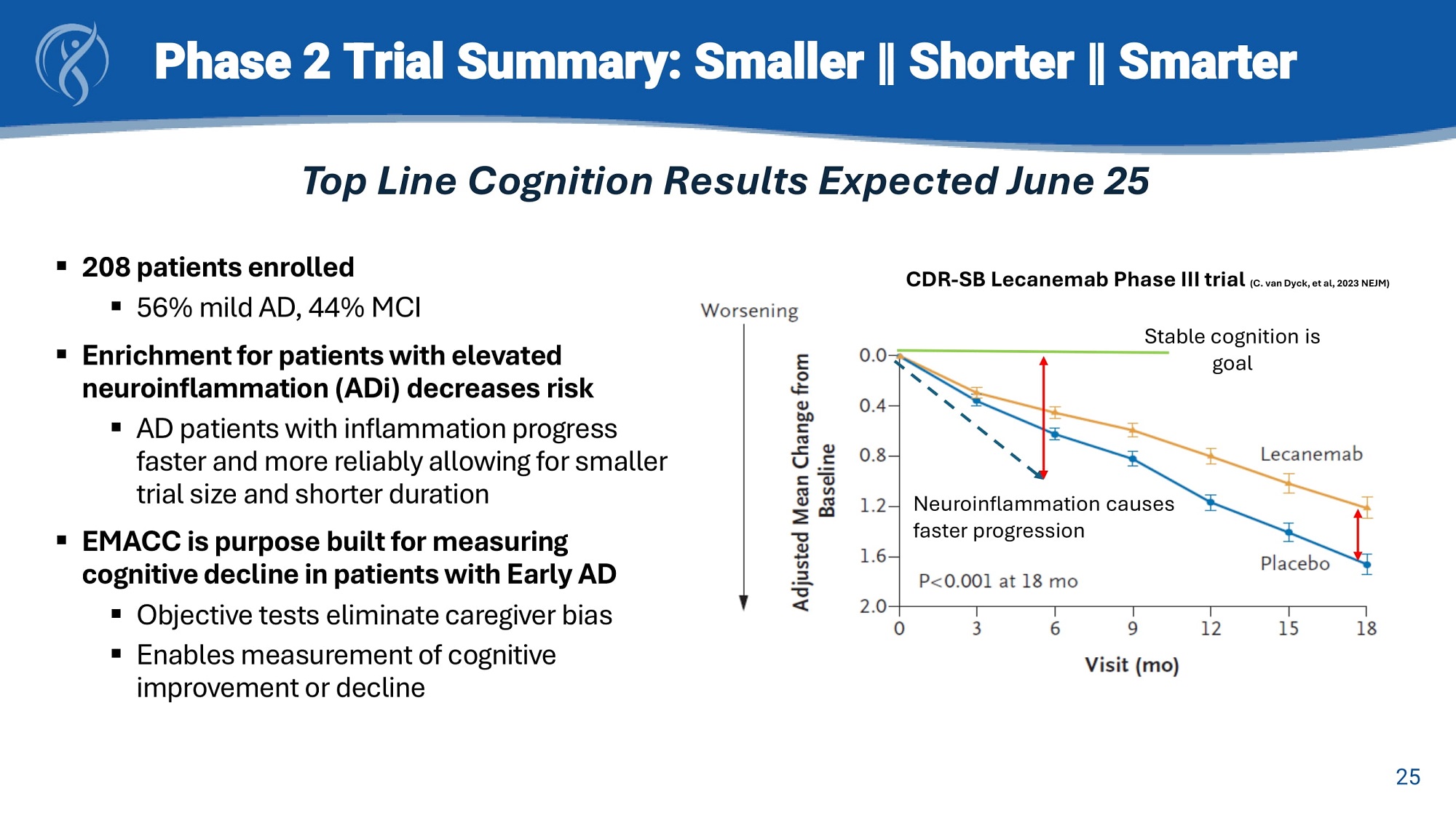
Phase 2 Trial Summary: Smaller ‖ Shorter ‖ Smarter ▪ 208 patients enrolled ▪ 56% mild AD, 44% MCI ▪ Enrichment for patients with elevated neuroinflammation (ADi) decreases risk ▪ AD patients with inflammation progress faster and more reliably allowing for smaller trial size and shorter duration ▪ EMACC is purpose built for measuring cognitive decline in patients with Early AD ▪ Objective tests eliminate caregiver bias ▪ Enables measurement of cognitive improvement or decline Top Line Cognition Results Expected June 25 Sta ble cognition is goal Neuroinflammation causes faster progression 25 CDR - SB Lecanemab Phase III trial (C. van Dyck, et al, 2023 NEJM)

26 Off - the - Shelf NK Therapy Converts Patient’s Resting NK cells into Cancer Killing memory like NK cells
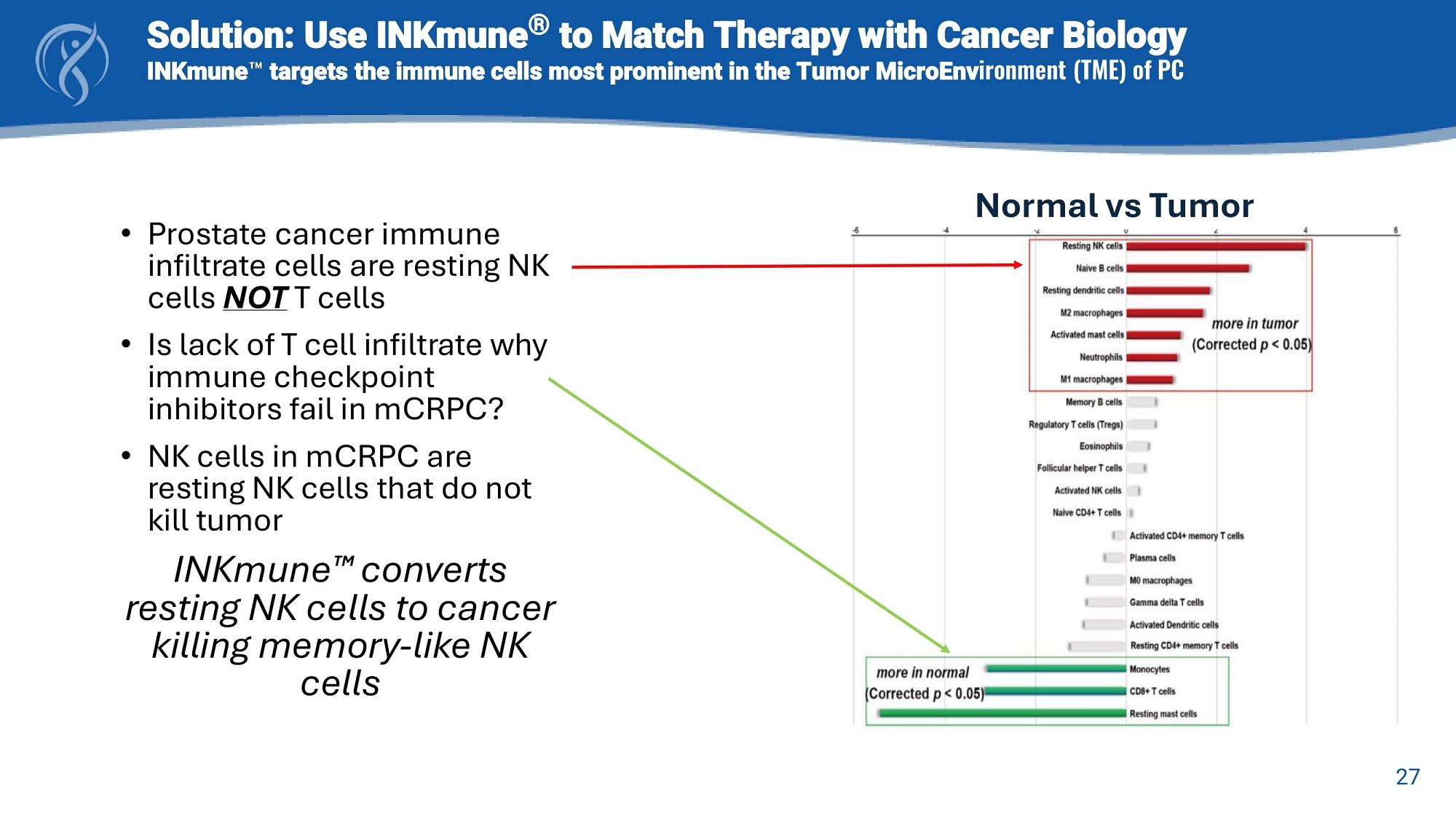
Solution: Use INKmune ® to Match Therapy with Cancer Biology INKmune targets the immune cells most prominent in the Tumor MicroEnv ironment (TME) of PC • Prostate cancer immune infiltrate cells are resting NK cells NOT T cells • Is lack of T cell infiltrate why immune checkpoint inhibitors fail in mCRPC? • NK cells in mCRPC are resting NK cells that do not kill tumor INKmune converts resting NK cells to cancer killing memory - like NK cells Normal vs Tumor 27
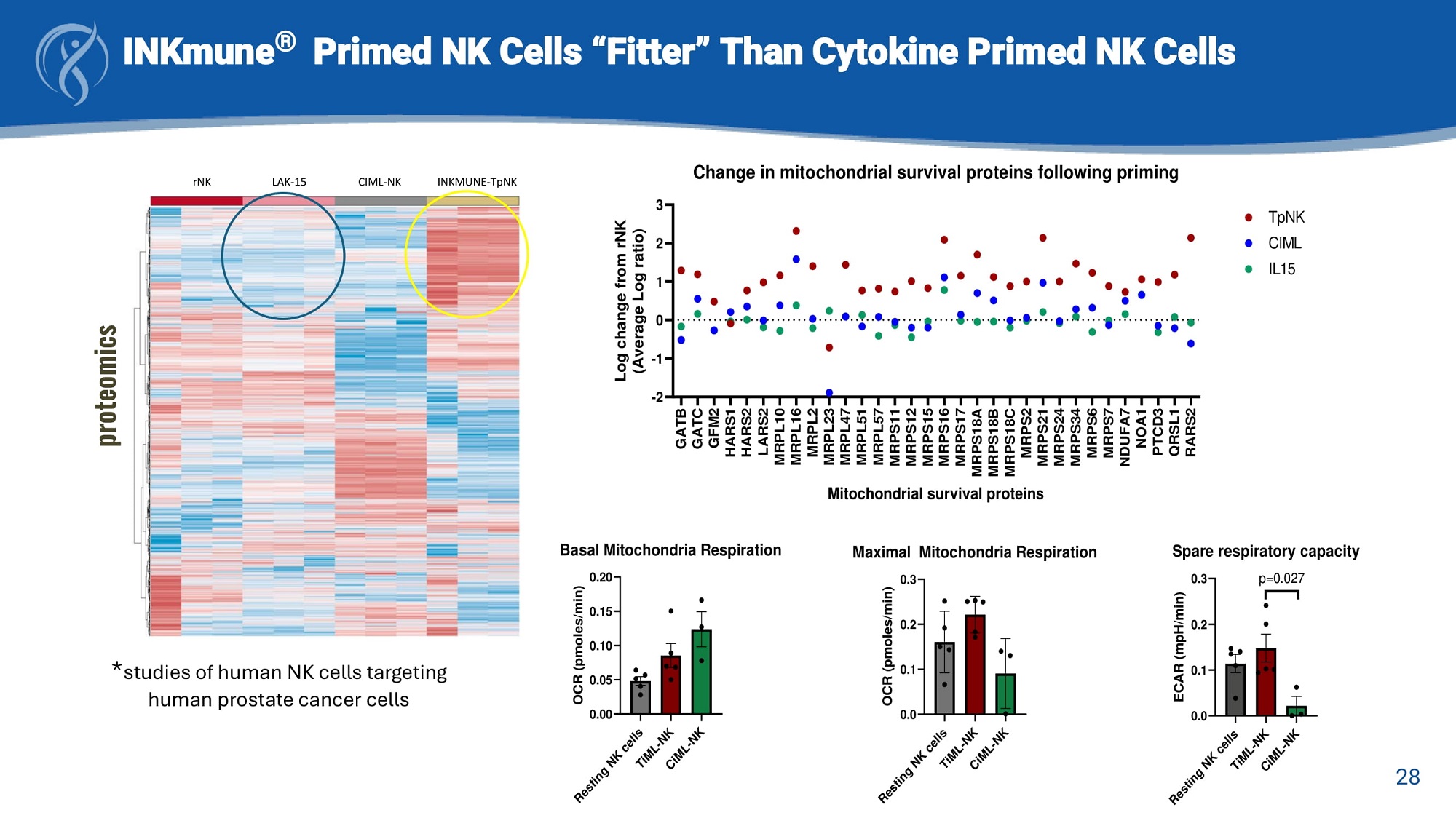
INKmune ® Primed NK Cells “Fitter” Than Cytokine Primed NK Cells rNK LAK - 15 CIML - NK INKMUNE - TpNK proteomic s GATB GATC GFM2 HARS1 HARS2 LARS2 MRPL10 MRPL16 MRPL2 MRPL23 MRPL47 MRPL51 MRPL57 MRPS11 MRPS12 MRPS15 MRPS16 MRPS17 MRPS18A MRPS18B MRPS18C MRPS2 MRPS21 MRPS24 MRPS34 MRPS6 MRPS7 NDUFA7 NOA1 PTCD3 QRSL1 RARS2 1 0 - 1 - 2 2 3 Change in mitochondrial survival proteins following priming Mitochondrial survival proteins Log change from rNK (Average Log ratio) TpNK CIML IL 15 0.00 0.05 0.10 0.15 0.20 OCR (pmoles/min) Basal Mitochondria Respiration 0.0 0.1 0.2 0.3 OCR (pmoles/min) Maximal Mitochondria Respiration 0.0 0.1 0.2 ECAR (mpH/min) 28 Spare respiratory capacity 0.3 p=0.027 * studies of human NK cells targeting human prostate cancer cells
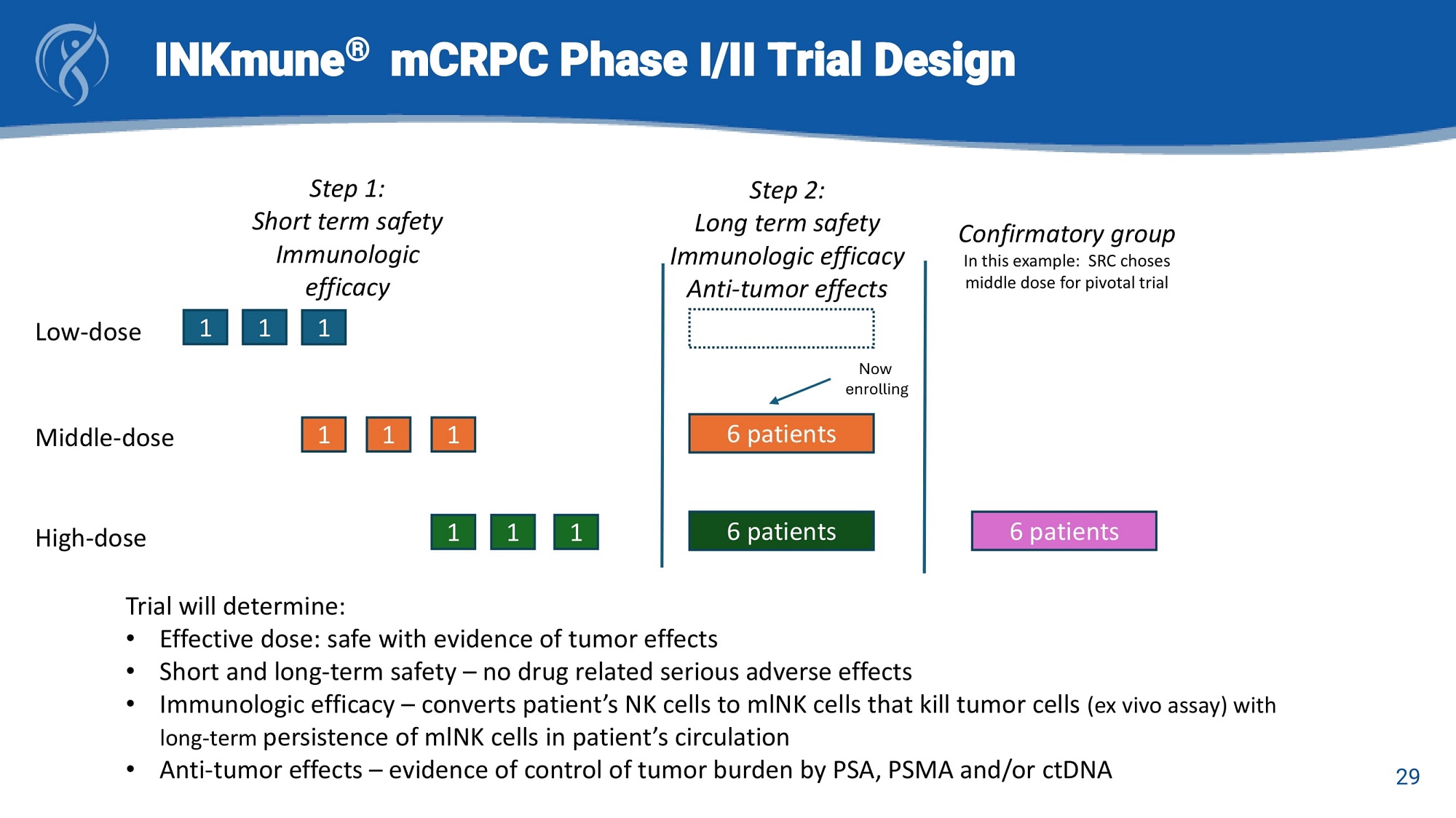
INKmune ® mCRPC Phase I/II Trial Design Low - dose Middle - dose High - dose 1 1 1 1 1 1 1 1 1 Step 1: Short term safety Immunologic efficacy 6 patients 6 patients Step 2: Long term safety Immunologic efficacy Anti - tumor effects 6 patients Confirmatory group In this example: SRC choses middle dose for pivotal trial Trial will determine: • Effective dose: safe with evidence of tumor effects • Short and long - term safety – no drug related serious adverse effects • Immunologic efficacy – converts patient’s NK cells to mlNK cells that kill tumor cells (ex vivo assay) with long - term persistence of mlNK cells in patient’s circulation Now enrolling • Anti - tumor effects – evidence of control of tumor burden by PSA, PSMA and/or ctDNA 29

Anticipated Milestones in 2024 and 2025 EXPECTED TIMING EVENT June 2025 Topline Cognition data Phase 2 AD 3/4Q 2025 End of Phase 2 FDA Meeting AD 1H 2025 First patient TRD study Dec 2025 BLA submission RDStrom Jan 2025 3Q 2025 Completed Phase 1 mCRPC Enrollment Complete Phase 2 mCRPC Enrollment Ongoing Open Label Phase 2 mCRPC Data Key Upcoming Clinical & Regulatory Milestones CO 30

Contact Us: 31 INmune Bio Inc. 225 NE Mizner Blvd.Suite 640 Boca Raton, FL 33432 (858) 964 - 3720 info@inmunebio.com v19 Symbol: INMB (Nasdaq) Inflammation and Immune Repair
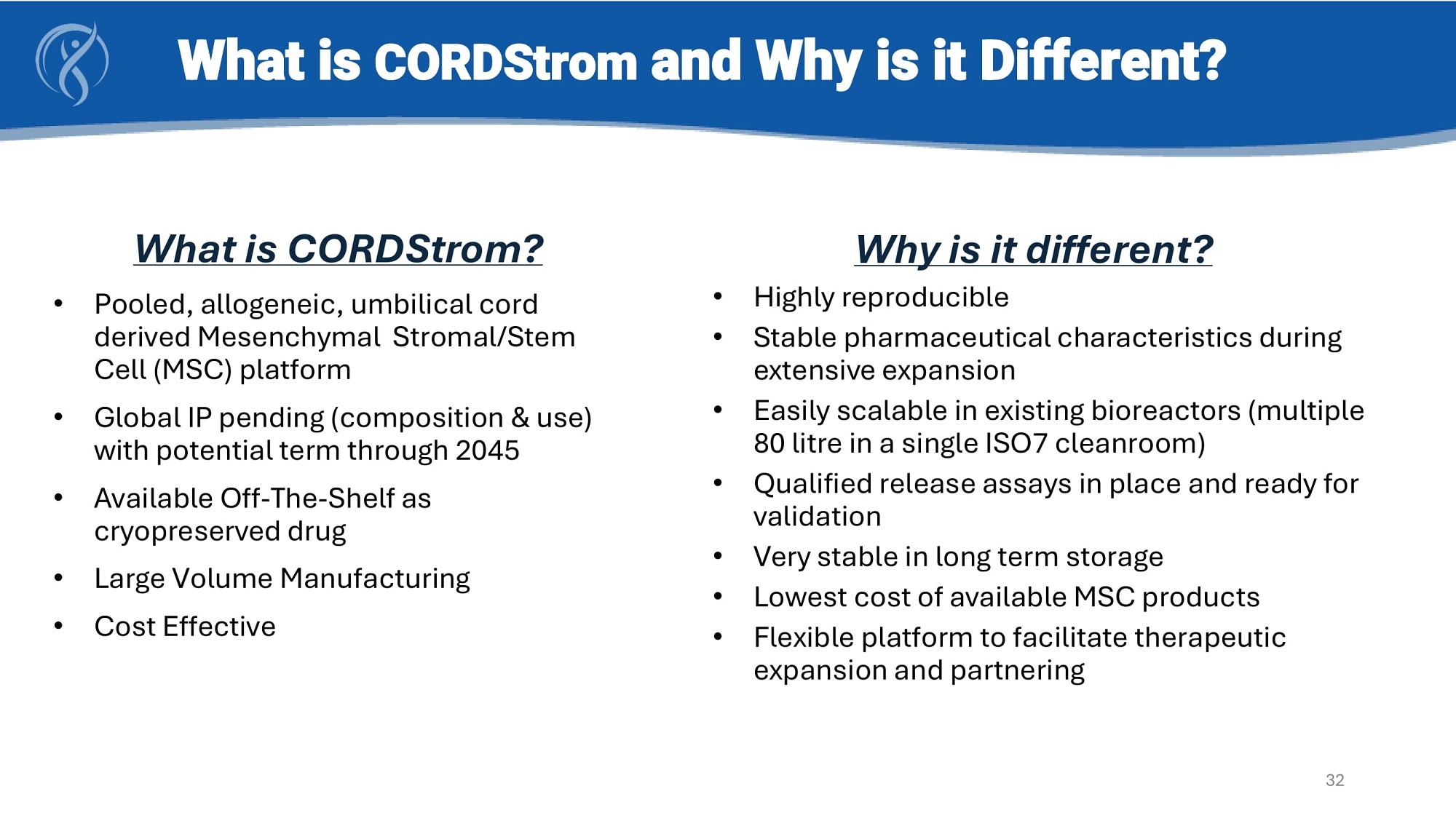
What is CORDStrom and Why is it Different? What is CORDStrom? • Pooled, allogeneic, umbilical cord derived Mesenchymal Stromal/Stem Cell (MSC) platform • Global IP pending (composition & use) with potential term through 2045 • Available Off - The - Shelf as cryopreserved drug • Large Volume Manufacturing • Cost Effective Why is it different? • Highly reproducible • Stable pharmaceutical characteristics during extensive expansion • Easily scalable in existing bioreactors (multiple 80 litre in a single ISO7 cleanroom) • Qualified release assays in place and ready for validation • Very stable in long term storage • Lowest cost of available MSC products • Flexible platform to facilitate therapeutic expansion and partnering 32
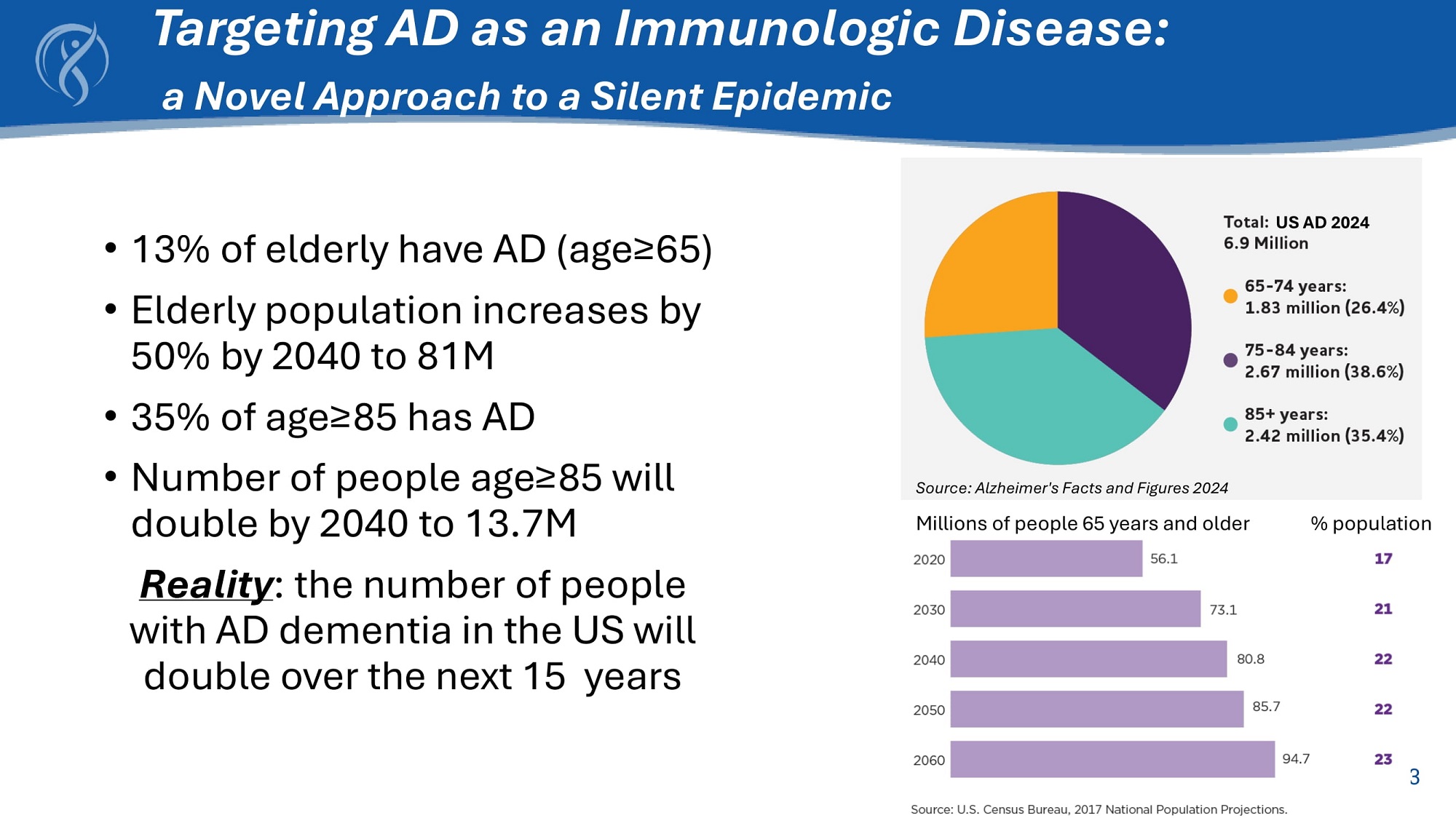
3 Targeting AD as an Immunologic Disease: a Novel Approach to a Silent Epidemic • 13% of elderly have AD (age≥65) • Elderly population increases by 50% by 2040 to 81M • 35% of age≥85 has AD • Number of people age≥85 will double by 2040 to 13.7M Reality : the number of people with AD dementia in the US will double over the next 15 years US AD 2024 Source: Alzheimer's Facts and Figures 2024 Millions of people 65 years and older 3 % population
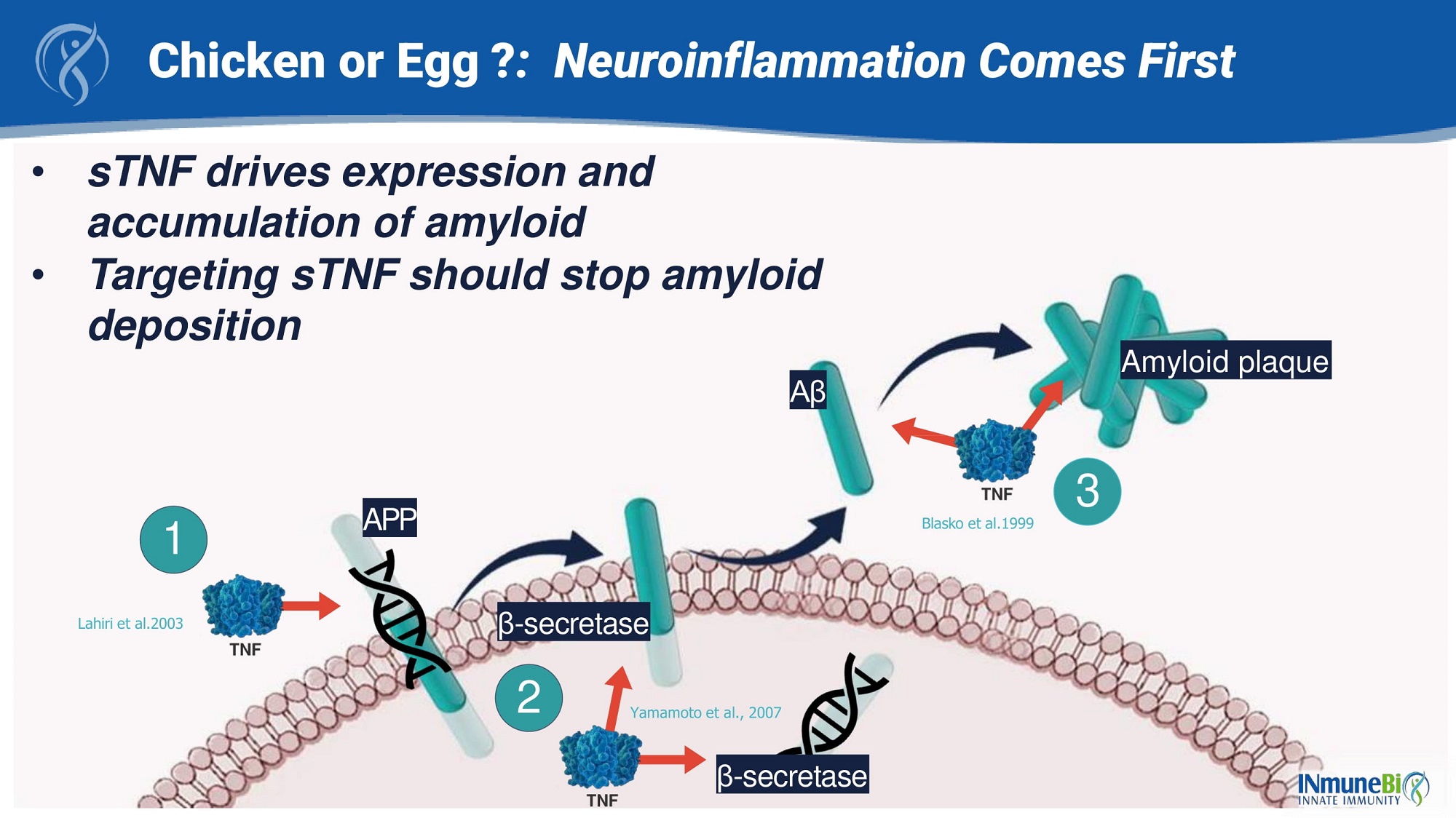
34 • sTNF drives expression and accumulation of amyloid • Targeting sTNF should stop amyloid deposition Aβ Amyloid plaque 3 Blasko et al.1999 β - secretase β - secretase 2 APP 1 Lahiri et al.2003 Chicken or Egg ? : Neuroinflammation Comes First Yamamoto et al., 2007
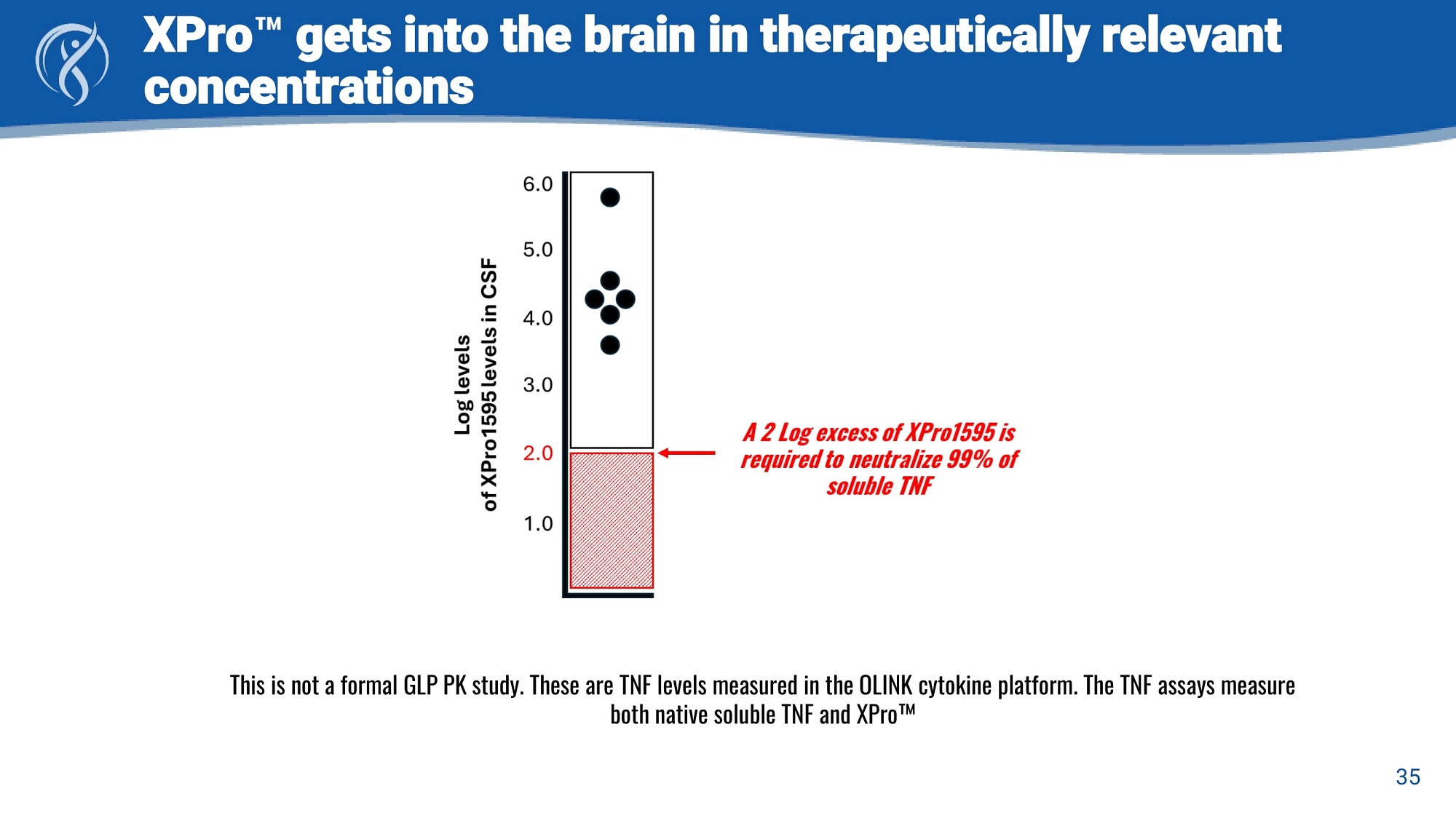
XPro gets into the brain in therapeutically relevant concentrations This is not a formal GLP PK study. These are TNF levels measured in the OLINK cytokine platform. The TNF assays measure both native soluble TNF and XPro 35
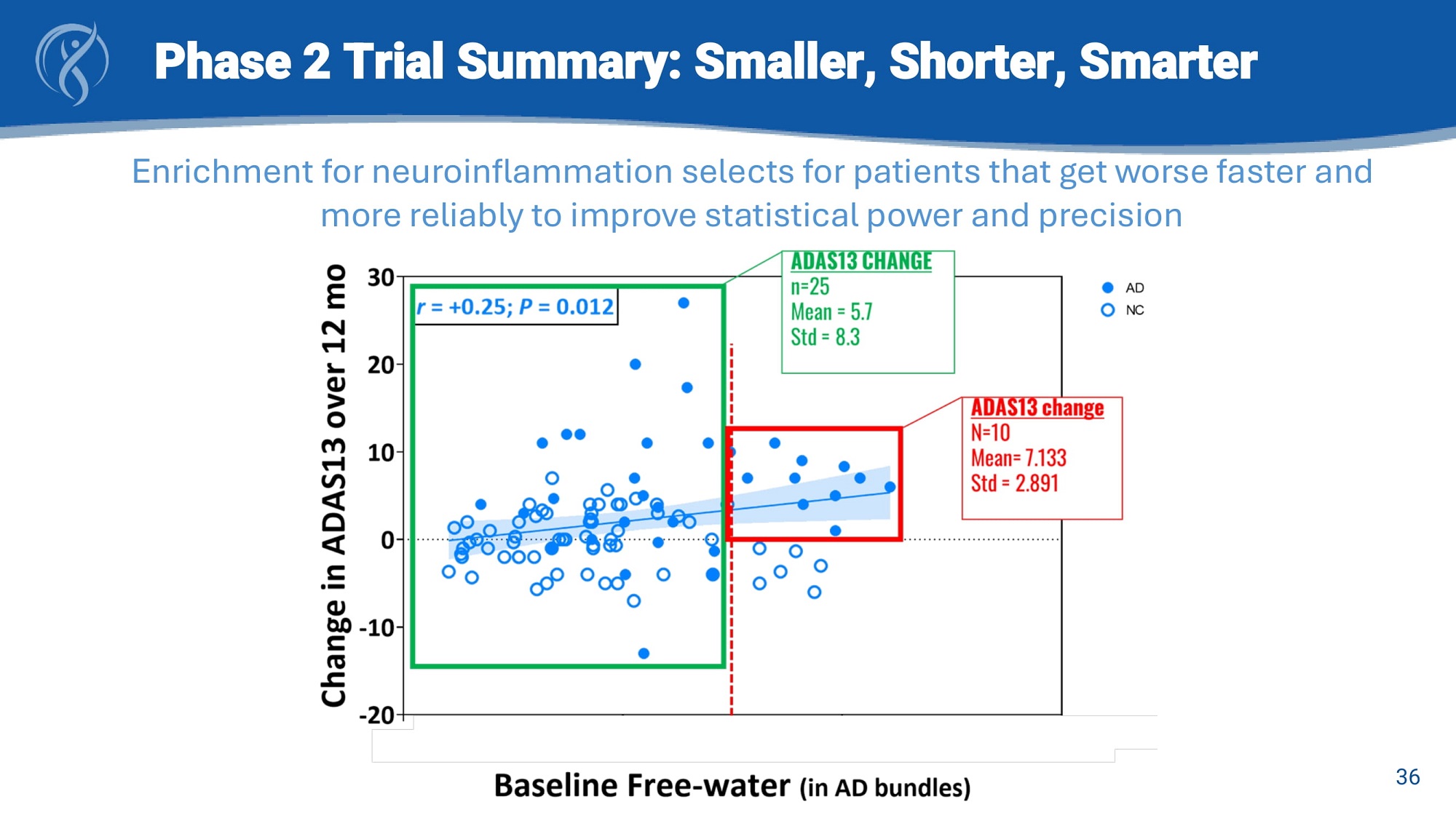
Phase 2 Trial Summary: Smaller, Shorter, Smarter Enrichment for neuroinflammation selects for patients that get worse faster and more reliably to improve statistical power and precision 36
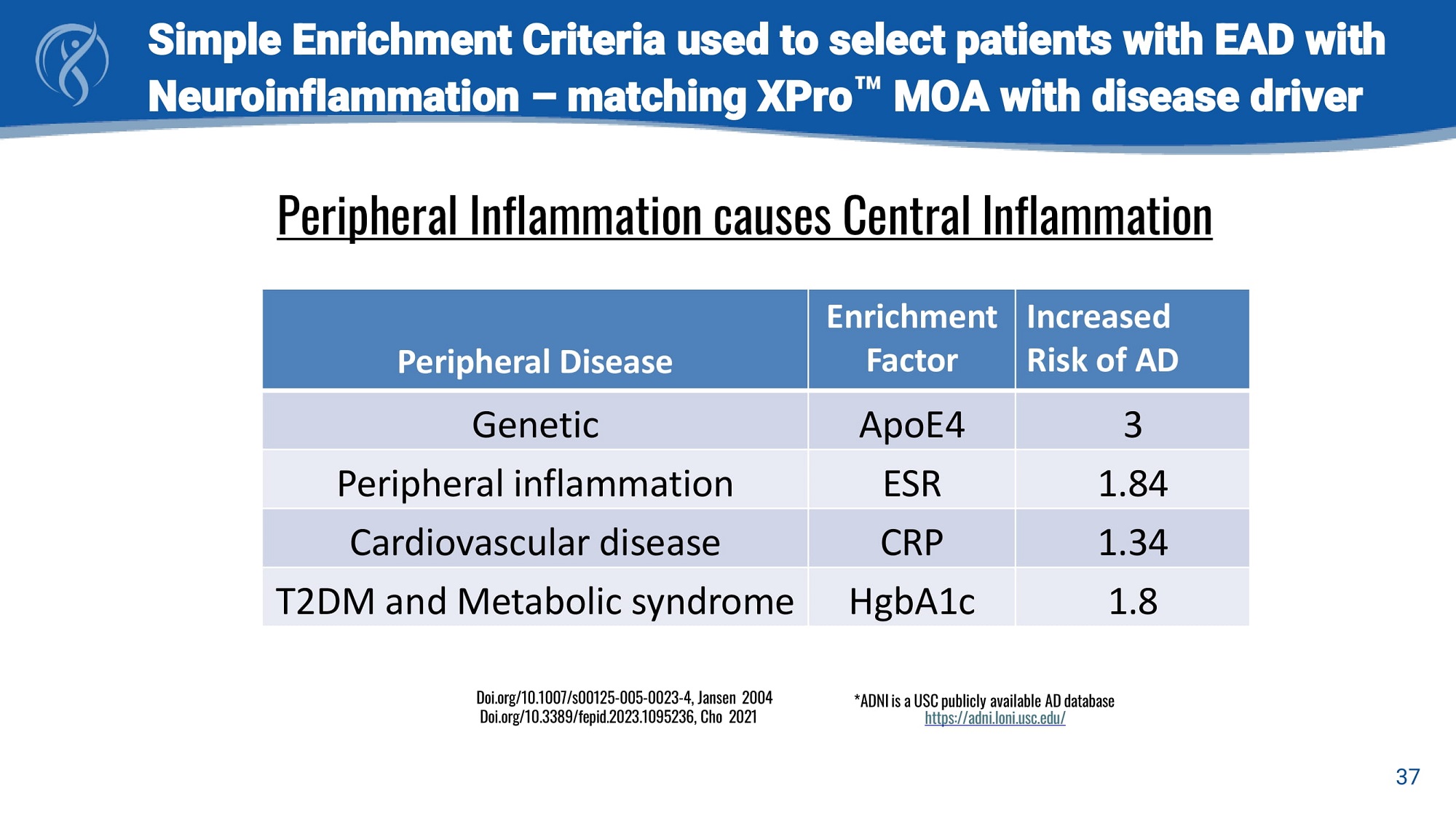
Simple Enrichment Criteria used to select patients with EAD with Neuroinflammation – matching XPro MOA with disease driver 37 Peripheral Inflammation causes Central Inflammation Increased Risk of AD Enrichment Factor Peripheral Disease 3 ApoE4 Genetic 1.84 ESR Peripheral inflammation 1.34 CRP Cardiovascular disease 1.8 HgbA1c T2DM and Metabolic syndrome *ADNI is a USC publicly available AD database https://adni.loni.usc.edu/ Doi.org/10.1007/s00125 - 005 - 0023 - 4, Jansen 2004 Doi.org/10.3389/fepid.2023.1095236, Cho 2021

EMACC – a stress test for the brain! Cognitive test that is “purpose built” for Early AD CDR Relationship 0.39 Treatment Lecanemab vs Placebo 0.45 CDR Frequency of contact 0.57 0.67 38 Sims et al 2023, vanDyck et al 2003, Vargas - Gonzalez et al 2024 Treatment Donanemab vs Placebo Magnitude of effect CDR - SB scale points CDR - SB inserts significant variance into cognition. The informant can affect the data as much as treatment!
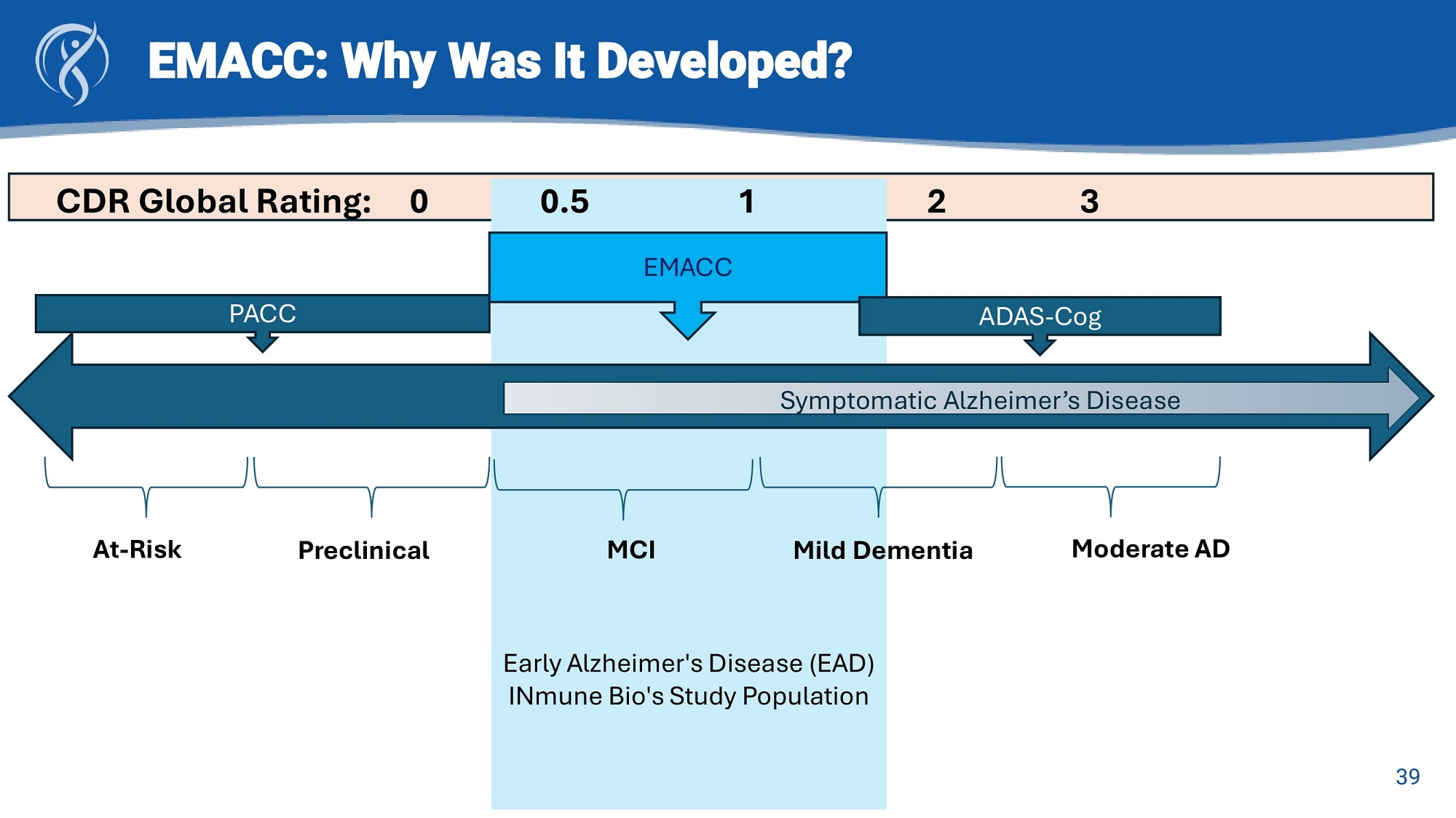
EMACC: Why Was It Developed? Preclinical MCI Symptomatic Alzheimer’s Disease Mild Dementia Moderate AD At - Risk PACC Early Alzheimer's Disease (EAD) INmune Bio's Study Population EMACC ADAS - Cog 39 CDR Global Rating: 0 0.5 1 2 3
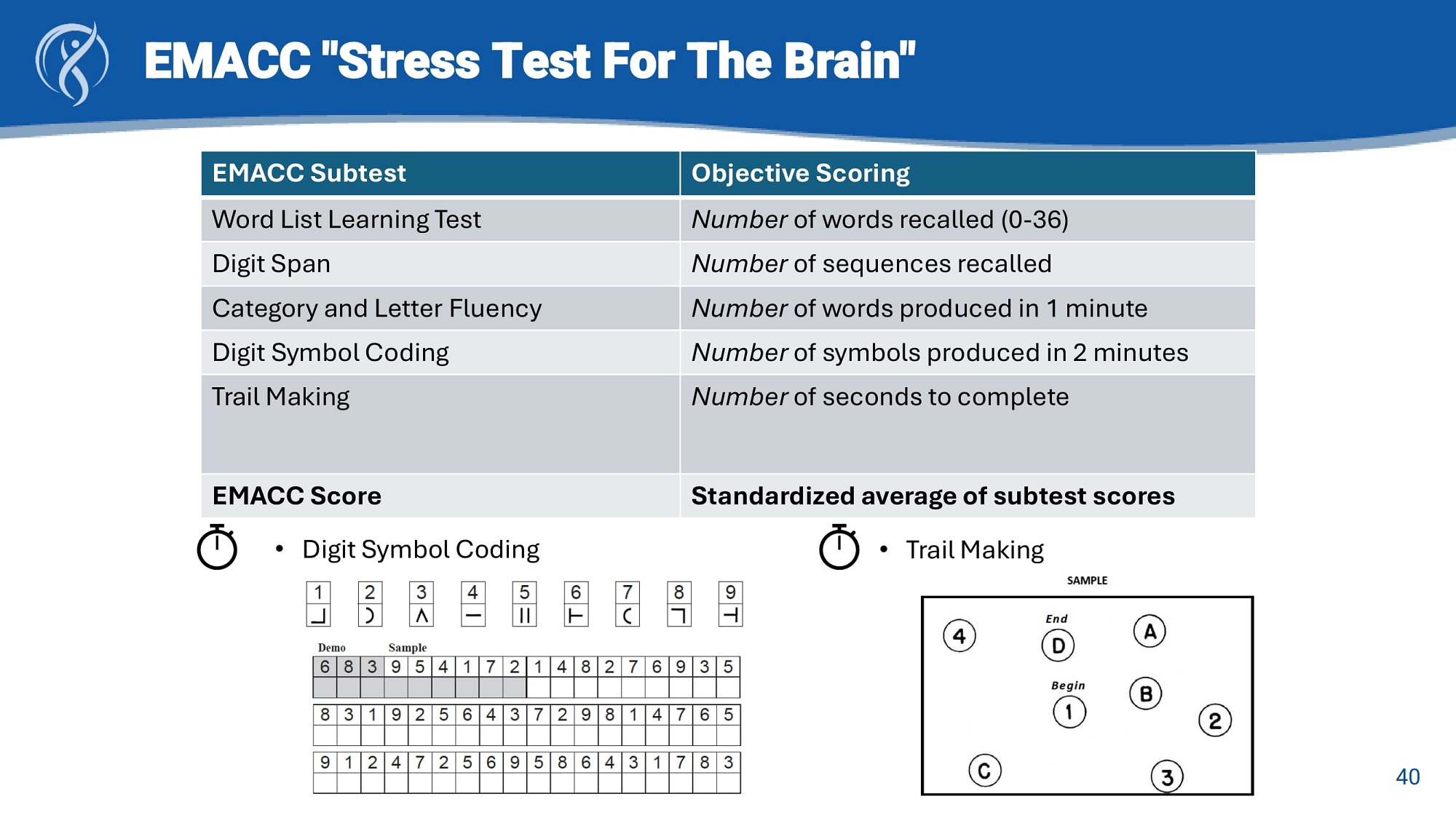
EMACC "Stress Test For The Brain" Objective Scoring EMACC Subtest Number of words recalled (0 - 36) Word List Learning Test Number of sequences recalled Digit Span Number of words produced in 1 minute Category and Letter Fluency Number of symbols produced in 2 minutes Digit Symbol Coding Number of seconds to complete Trail Making Standardized average of subtest scores EMACC Score • Digit Symbol Coding • Trail Making 40
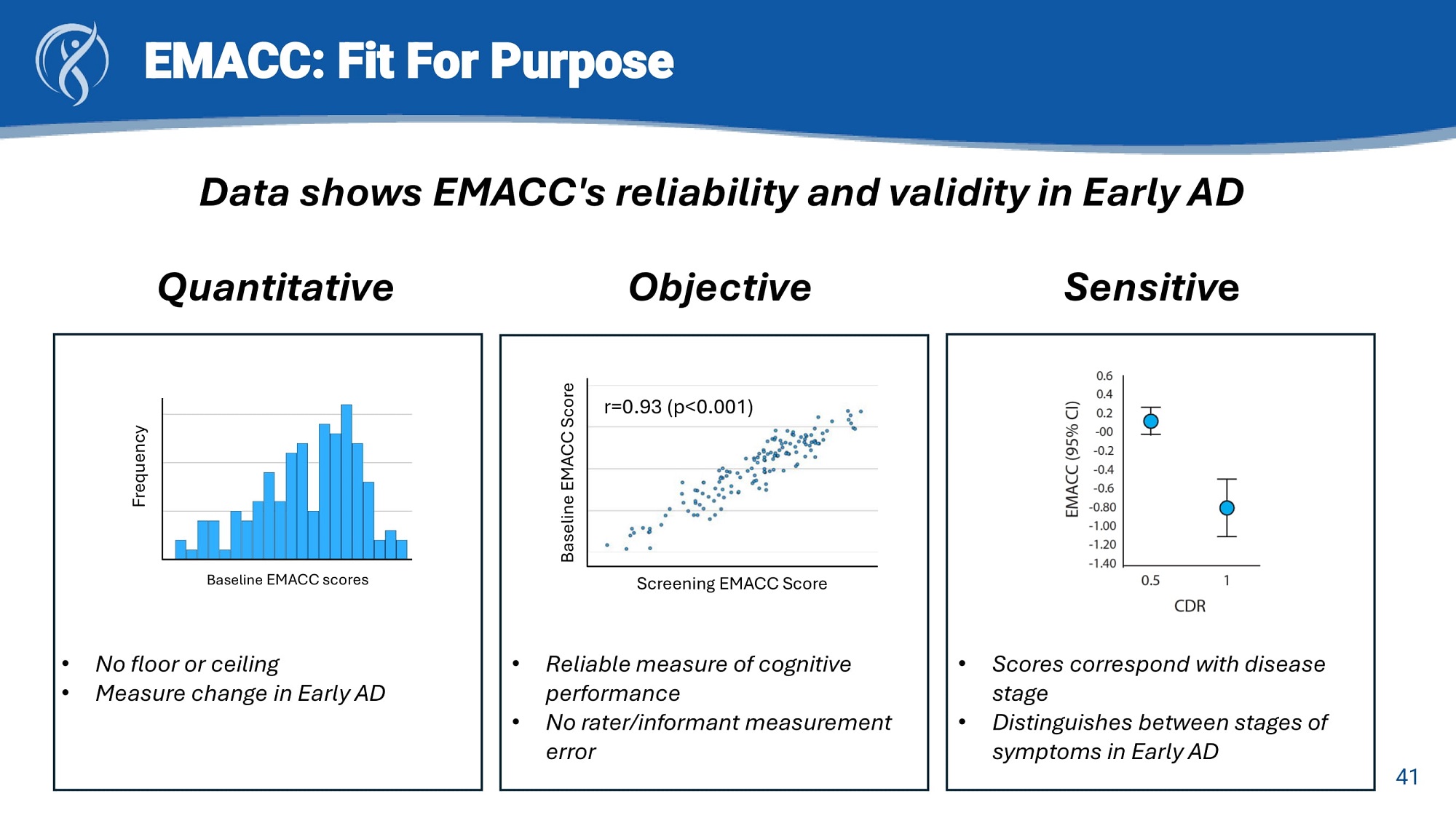
EMACC: Fit For Purpose Data shows EMACC's reliability and validity in Early AD Quantitative Objective Sensitiv e r=0.93 (p<0.001) Baseline EMACC Score Screening EMACC Score • Reliable measure of cognitive performance • No rater/informant measurement error Frequency 41 Baseline EMACC scores • No floor or ceiling • Measure change in Early AD • Scores correspond with disease stage • Distinguishes between stages of symptoms in Early AD
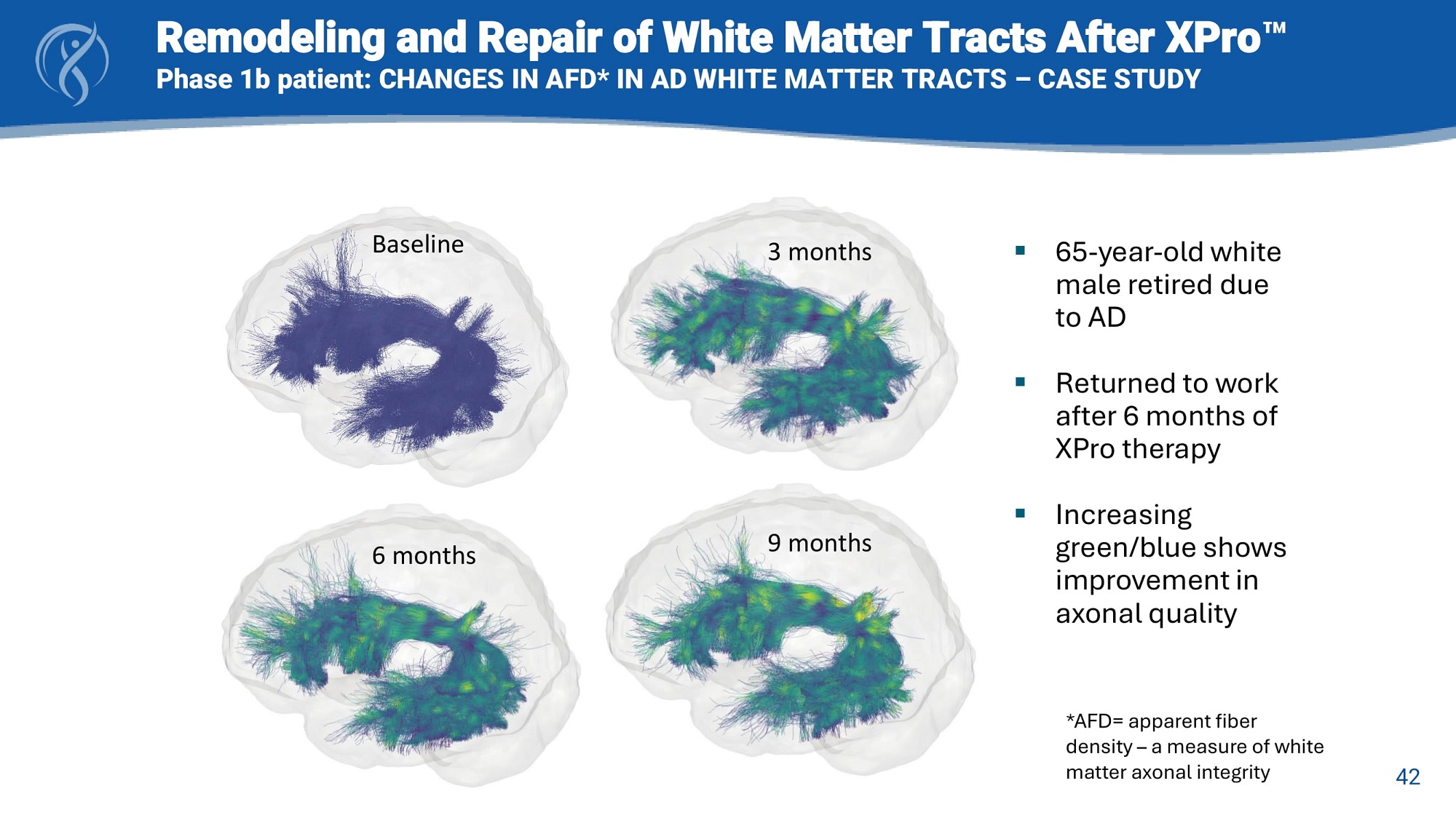
42 Baseline 3 months 6 months 9 months ▪ 65 - year - old white male retired due to AD ▪ Returned to work after 6 months of XPro therapy ▪ Increasing green/blue shows improvement in axonal quality Remodeling and Repair of White Matter Tracts After XPro Phase 1b patient: CHANGES IN AFD* IN AD WHITE MATTER TRACTS – CASE STUDY *AFD= apparent fiber density – a measure of white matter axonal integrity
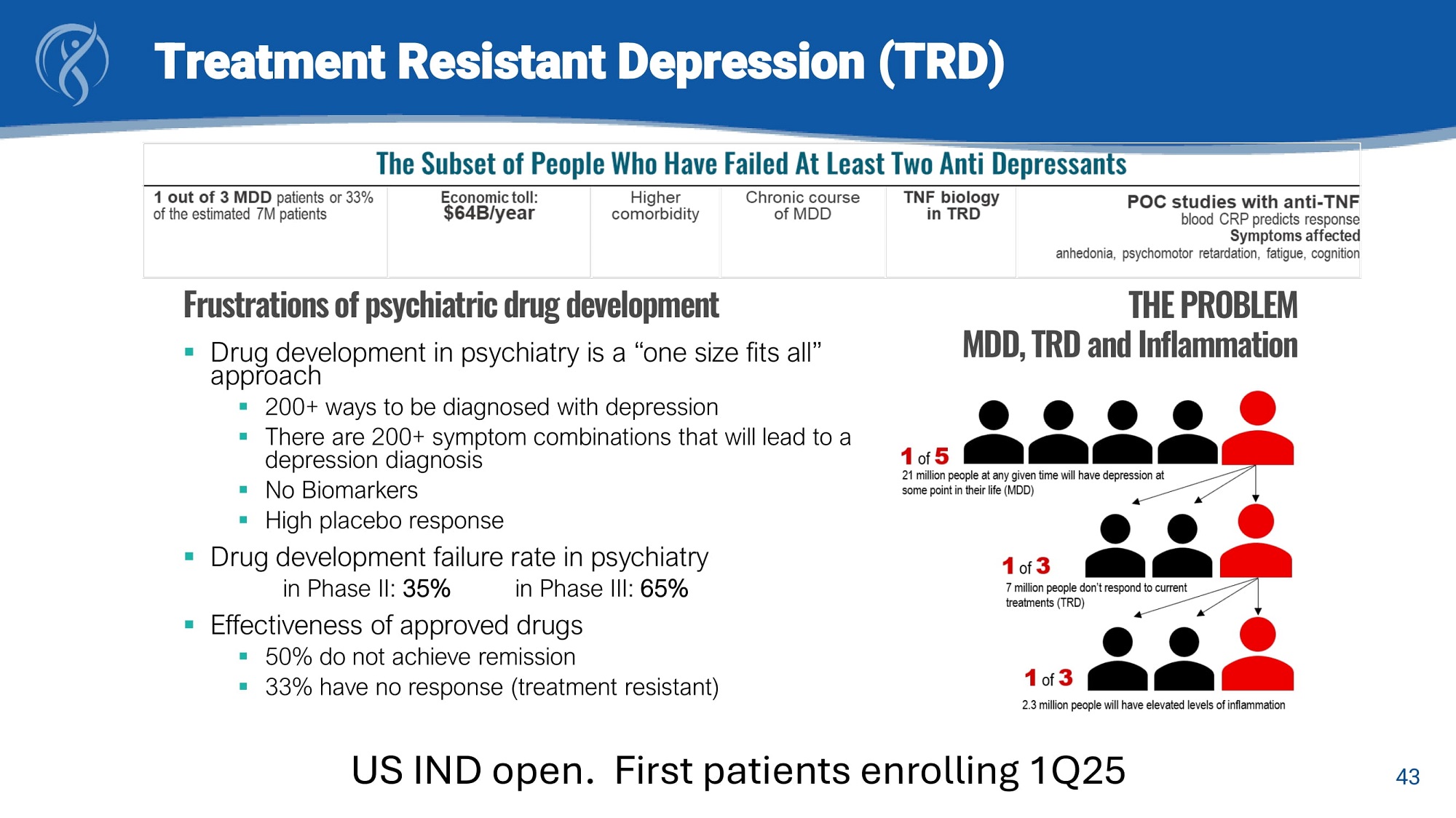
43 Treatment Resistant Depression (TRD) ▪ Drug development in psychiatry is a “one size fits all” approach ▪ 200+ ways to be diagnosed with depression ▪ There are 200+ symptom combinations that will lead to a depression diagnosis ▪ No Biomarkers ▪ High placebo response ▪ Drug development failure rate in psychiatry in Phase II: 35% in Phase III: 65% ▪ Effectiveness of approved drugs ▪ 50% do not achieve remission ▪ 33% have no response (treatment resistant) Frustrations of psychiatric drug development THE PROBLEM MDD, TRD and Inflammation US IND open. First patients enrolling 1Q25
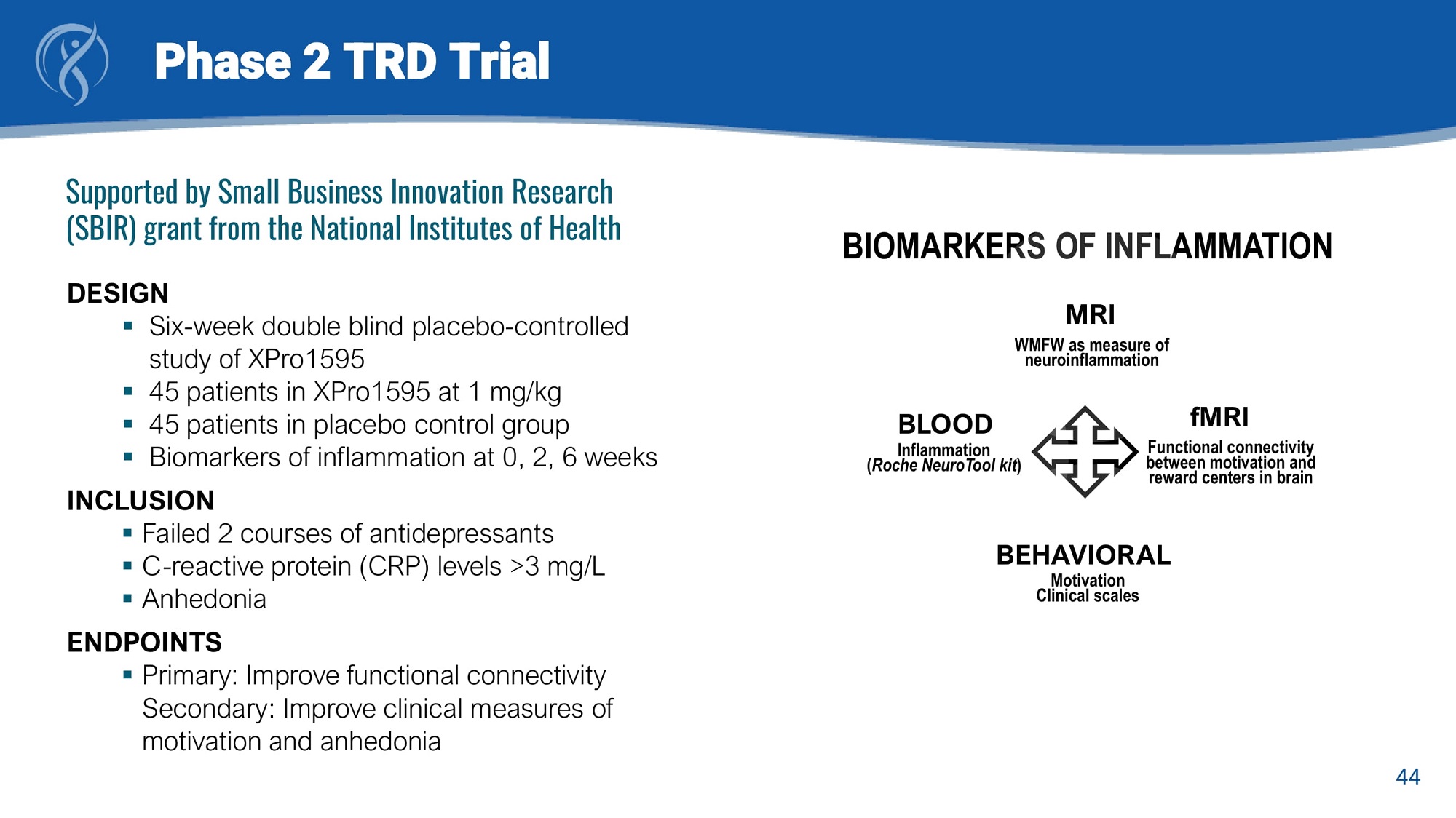
Phase 2 TRD Trial Supported by Small Business Innovation Research (SBIR) grant from the National Institutes of Health DESIGN ▪ Six - week double blind placebo - controlled study of XPro1595 ▪ 45 patients in XPro1595 at 1 mg/kg ▪ 45 patients in placebo control group ▪ Biomarkers of inflammation at 0, 2, 6 weeks INCLUSION ▪ Failed 2 courses of antidepressants ▪ C - reactive protein (CRP) levels >3 mg/L ▪ Anhedonia ENDPOINTS ▪ Primary : Improve functional connectivity Secondary : Improve clinical measures of motivation and anhedonia BIOMARKERS OF INFLAMMATION MRI WMFW as measure of neuroinflammation 44 BLOOD Inflammation ( Roche NeuroTool kit ) fMRI Functional connectivity between motivation and reward centers in brain BEHAVIORAL Motivation Clinical scales
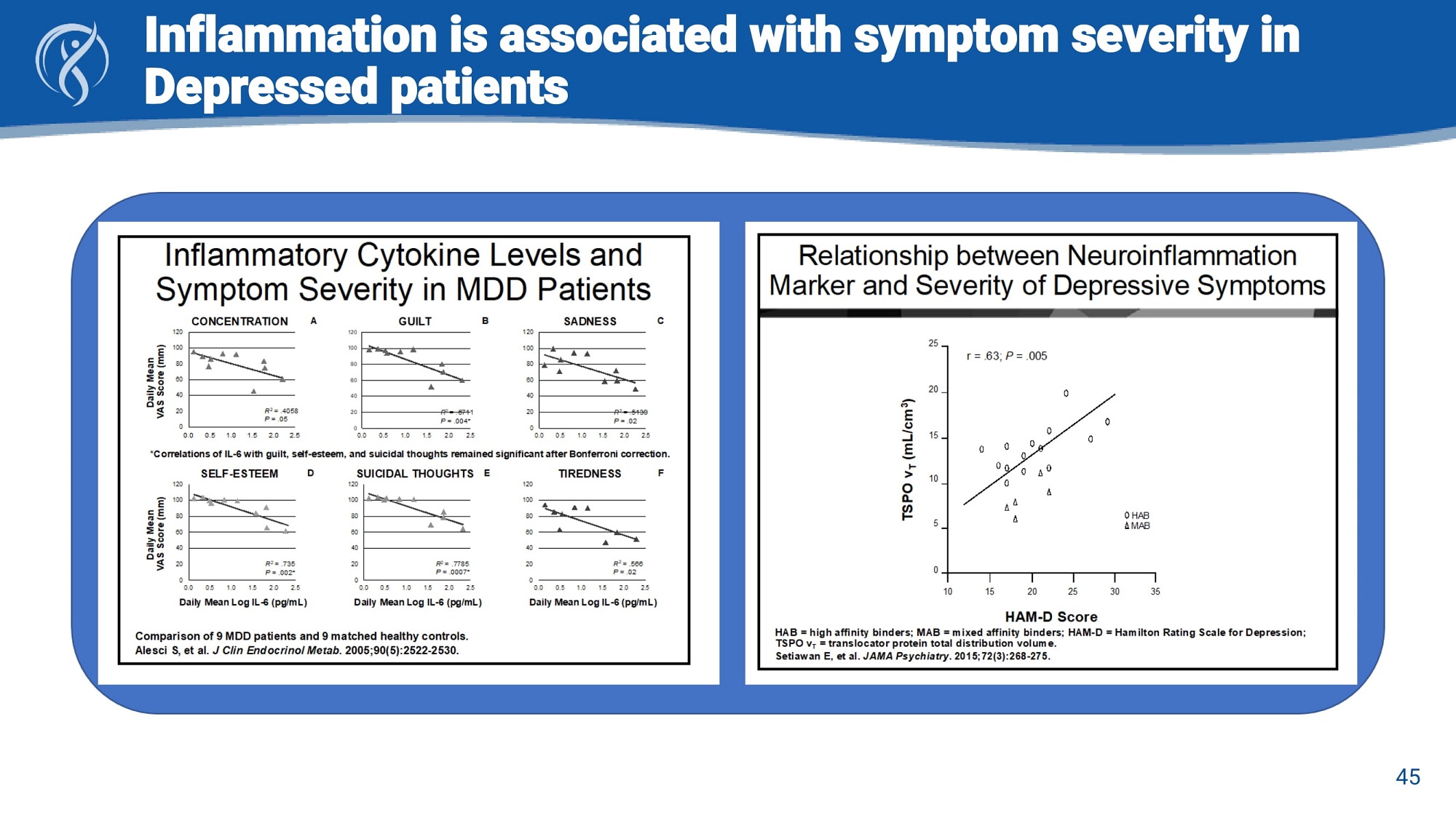
Inflammation is associated with symptom severity in Depressed patients 45
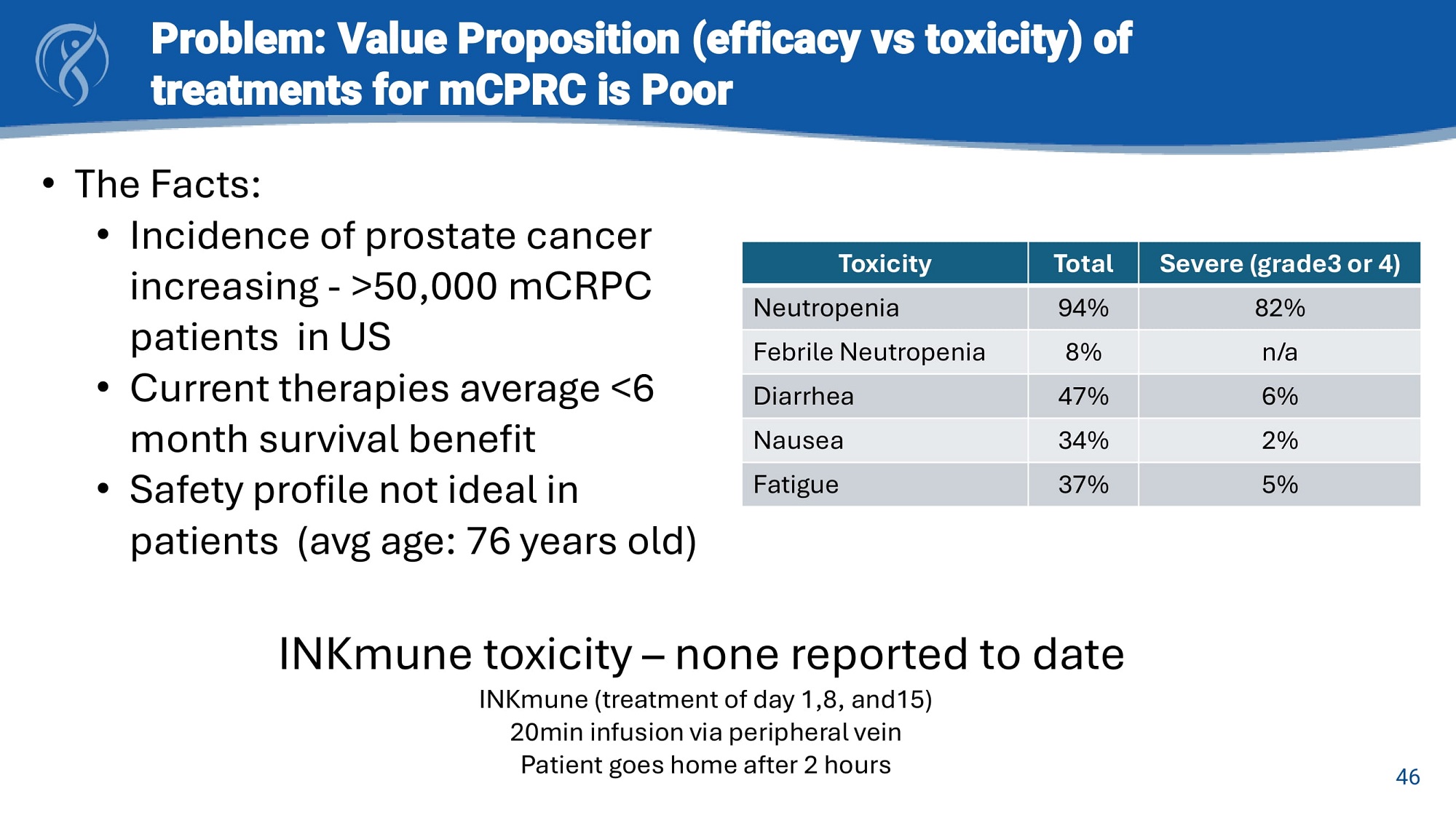
46 Problem: Value Proposition (efficacy vs toxicity) of treatments for mCPRC is Poor • The Facts : • Incidence of prostate cancer increasing - > 50 , 000 mCRPC patients in US • Current therapies average < 6 month survival benefit • Safety profile not ideal in patients (avg age : 76 years old) Severe (grade3 or 4) Total Toxicity 82% 94% Neutropenia n/a 8% Febrile Neutropenia 6% 47% Diarrhea 2% 34% Nausea 5% 37% Fatigue INKmune toxicity – none reported to date INKmune (treatment of day 1,8, and15) 20min infusion via peripheral vein Patient goes home after 2 hours













































