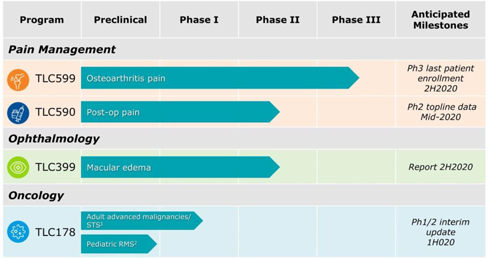
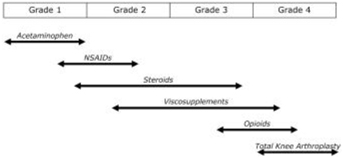
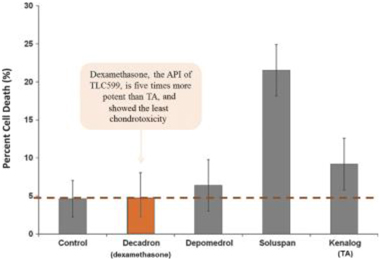
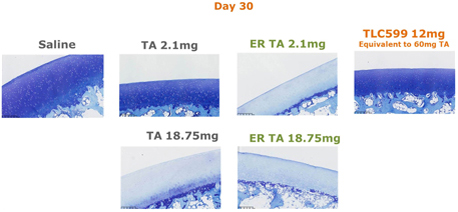
We can provide no assurance that our patent applications or those of our licensors will result in additional patents being issued or that issued patents will afford sufficient protection against competitors with similar technologies, nor can there be any assurance that the patents issued will not be infringed, designed around or invalidated by third parties. Even issued patents may later be found unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. We cannot provide any assurance that any patents will be issued from our pending or any future applications or that any potentially issued patents will our intellectual property.
As of December 31, 2019, we had been granted 126 utility patents worldwide, including ten Taiwan patents, 12 United States patents, seven Japan patents, five New Zealand patents, five China patents, five Australia patents, six Korea patents, three Russian patents, five Hong Kong patents, four European patents, two South Africa patent and two Canada patent. We also have 71 patent applications which are under review in the above major markets (including five U.S. provisional patent applications) plus India, Brazil and Singapore jurisdictions, as well as 12 pending PCT patent application, relating to our product candidates. The patent terms for TLC599 and TLC399 extend into 2033 and the patent term for TLC178 extends into 2034. If issued, the patent term for TLC590 and additional patent families of TLC599 would extend into 2040 and 2039.
We own all of the patents and patent applications relating to our four lead product candidates.
TLC599
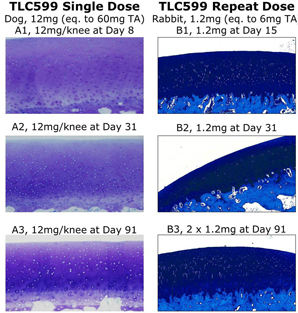
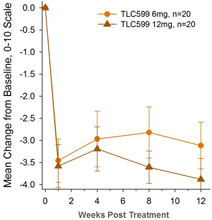
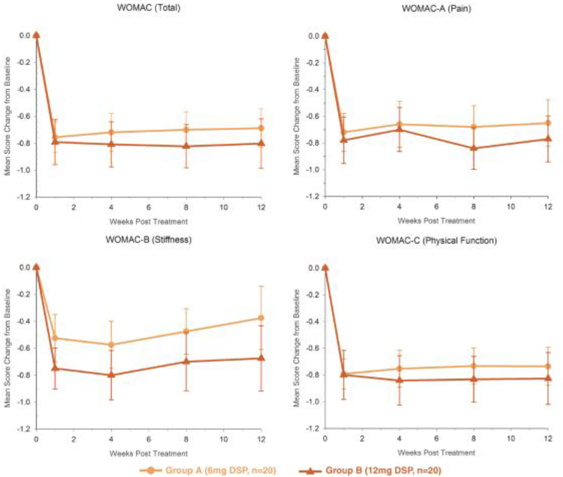
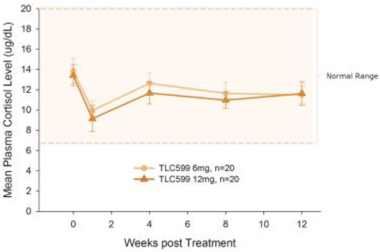
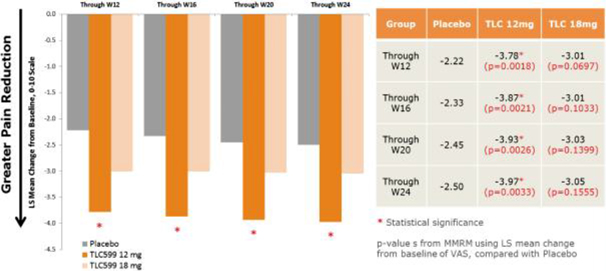
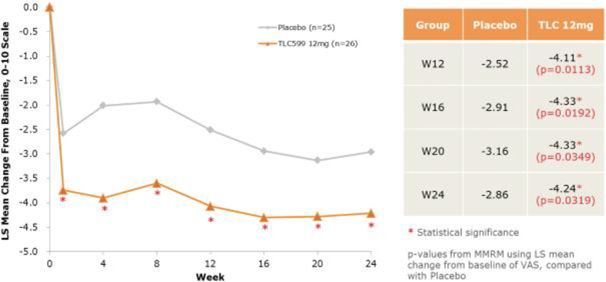
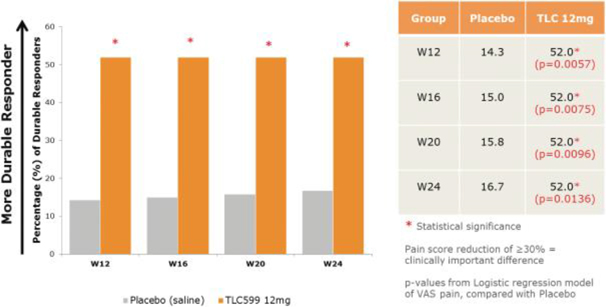
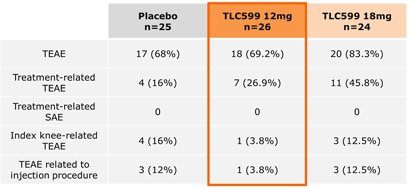
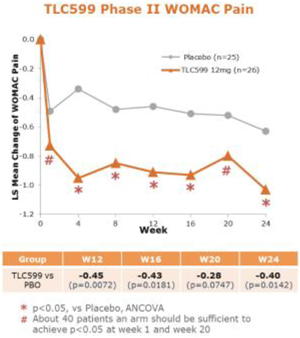
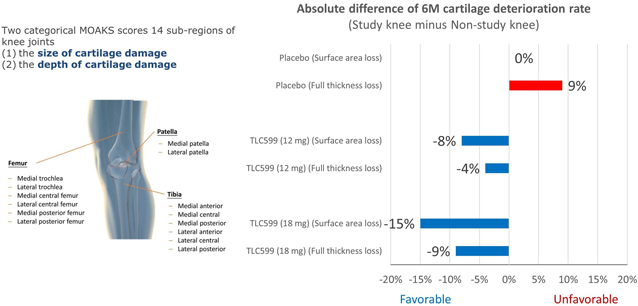
Our TLC599 intellectual property portfolio includes nine issued patents and 13 patent applications. Our issued patents cover the methods of use of TLC599, were granted in the United States, Australia, New Zealand, Russia, Japan, China/Hong Kong and Taiwan, and are expected to expire in 2033. Our patent applications cover composition as well as methods of use of TLC599 and are pending and under review in the United States, South Africa, Taiwan, Singapore, Russia, Korea, Japan, India, Hong Kong, Europe, Canada and Brazil, with expected expiry dates of 2039.
TLC399
Our TLC399 intellectual property portfolio includes 89 issued patents and eight patent applications. Our issued patents cover the composition of matter of TLC399, were granted in Taiwan, the United States, Canada, South Africa, Russia, New Zealand, Korea, Japan, Hong Kong, China, Australia and a series of European countries, and are expected to expire between 2029 and 2033. Our patent applications cover the composition of matter of TLC399 and are pending and under review in the United States, Hong Kong, Europe, China, Canada and Brazil, with expected expiry dates between 2029 and 2033.
TLC590
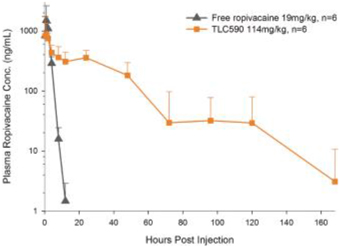
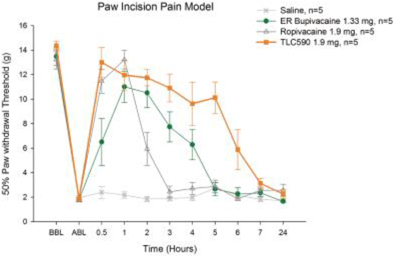
Our TLC590 intellectual property portfolio includes two PCT applications, two Taiwan patents and two U.S. provisional applications, which covers the composition of matter of TLC590, method of manufacturing and method of use of TLC590, and are pending, in the international phase of PCT applications and in Taiwan with expected expiry dates extending into 2040.
TLC178
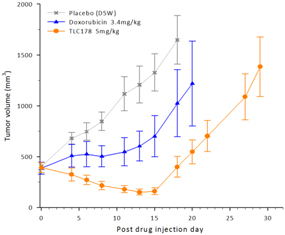
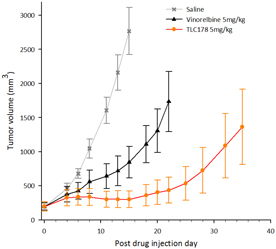
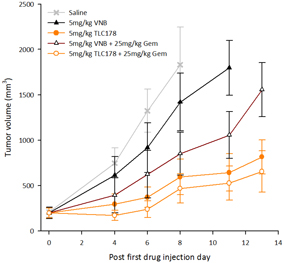
Our material TLC178 intellectual property portfolio includes nine issued patents and 12 patent applications. Our issued patents cover the composition of matter of TLC178, were granted in Taiwan, China/Hong Kong, Japan, Korea, Australia and the United States, and are going to take effect in a serial of European countries and expected to expire in 2034. Our patent applications cover the composition of matter of TLC178 and are pending and under review in Taiwan, the United States, South Africa, Singapore, Russia, New Zealand, Japan, India, Hong Kong, China, Canada and Brazil, with expected expiry dates of 2034.
Individual patents extend for varying periods depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, patents issued for regularly filed applications in the United States are granted a term of 20 years from the earliest effectivenon-provisional filing date. In addition, in certain instances, a patent term can be extended to recapture a portion of the USPTO delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. However, the actual protection afforded by a patent varies on a product by product basis, from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
84