| | • | | published PCT application WO2017/037300 filed September 5, 2016 relates to use ofvarlitinib in treatment of resistant cancers; and |
| | • | | published PCT application WO2017/184086 filed April 21, 2017 relates to use of thevarlitinib in the treatment of HCC. |
Normal expiration of these patents, if granted, is 2036 or 2037 subject to the payment of renewal fees. It is not clear what claims may be granted, if any, when these patents are pursued at the national and regional phase.
There is one unpublished PCT application and at least four unpublished Singapore priority patent applications relating to use ofvarlitinib. These patent applications are at an early stage of filing and it is not possible to predict what claims may be ultimately granted, if any from these patent applications.
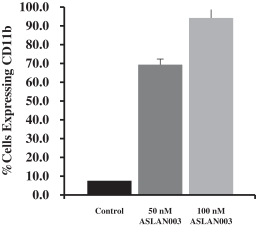
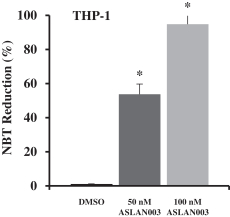
ASLAN003
Licensed from Almirall
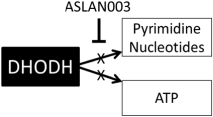
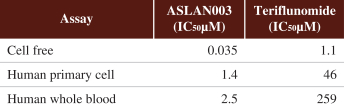
On May 16, 2012, we entered into a development and license agreement with Almirall, pursuant to which we obtained an exclusive, worldwide license to certain patents,know-how and other intellectual property controlled by Almirall to a DHODH inhibitor, LAS186323, which we refer to as ASLAN003. On December 21, 2015, we entered into an amended development and license agreement with Almirall which replaced the previous agreement. This was further amended by an amendment agreement entered into on March 16, 2018. Under the amended agreement as so amended, we obtained from Almirall an expanded exclusive, worldwide license to develop, manufacture and commercialize ASLAN003 products for all human diseases with primary focus on oncology diseases, excluding topically-administered products embodying the compound for keratinocyte hyperproliferative disorders, and the non-melanoma skin cancers basal cell carcinoma, squamous cell carcinomas and Gorlin Syndrome.
The basic compound protection for ASLAN003 is provided by the composition of matter family of patents derived from WO2008/077639. As of December 6, 2017, this family of patents included patents issued in Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Mexico, New Zealand, Nigeria, Russia, South Africa, South Korea, Taiwan, and the United States (two patents). In addition, as of December 6, 2017, this family of patents included patent applications filed in Argentina, Bolivia, Chile, Colombia, Ecuador, Egypt, Norway, Pakistan, Peru, Philippines, Singapore, Thailand, Ukraine, Uruguay, Venezuela and Vietnam. The scope of the claims may differ in different countries. The normal expiration of this family of patents is December 2027, subject to the payment of renewal fees.
Owned by Us
We have four unpublished Singapore priority patent applications related to specific uses of ASLAN003.
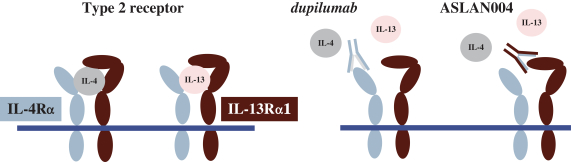
ASLAN004
On May 12, 2014, we entered into a license agreement with CSL, pursuant to which we obtained an exclusive, worldwide license to certain patents,know-how and other intellectual property owned or controlled by CSL related to CSL’s anti-IL13 receptor monoclonal antibody, CSL334, which we refer to as ASLAN004, and antigen binding fragments thereof.
The basic compound protection for ASLAN004 is provided by a species (specific sequence) composition of matter family of patents is derived from WO2008/060813, filed October 19, 2007. As of December 6, 2017, this family of patents included patents issued in Australia (two patents), Canada, China, Europe (two patents), Japan (two patents), and the United States (four patents). In addition, as of December 6, 2017, this family of patents included patent applications filed in Hong Kong (two applications). The normal expiration of this family of patents is October 2027, subject to the payment of renewal fees.
105

 .” In China, we have a trademark registration for “
.” In China, we have a trademark registration for “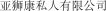 .” We also have a pending application in China to protect the following Chinese character version of the wordvarlitinib: “
.” We also have a pending application in China to protect the following Chinese character version of the wordvarlitinib: “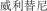 ” (wei li ti ni). This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
” (wei li ti ni). This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
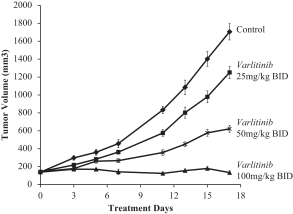
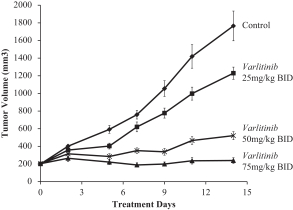

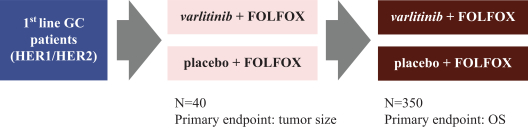
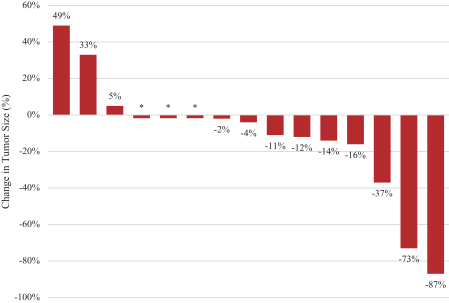






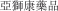 .” In China, we have a trademark registration for “
.” In China, we have a trademark registration for “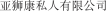 .” We also have a pending application in China to protect the following Chinese character version of the wordvarlitinib: “
.” We also have a pending application in China to protect the following Chinese character version of the wordvarlitinib: “