reasonable cost, in a timely manner or in all jurisdictions where protection may be commercially advantageous, or we may financially not be able to protect our proprietary rights at all. Despite our efforts to protect our proprietary rights, unauthorized parties may be able to obtain and use information we regard as proprietary.
The issuance of a patent does not ensure that it is valid or enforceable. Therefore, even if we are issued a patent, it may not be valid or enforceable against third parties. Issued patents may be challenged, narrowed, invalidated or circumvented. In addition, court decisions may introduce uncertainty in the enforceability or scope of patents owned by pharmaceutical and biotechnology companies. Thus, any of our patents, including patents that we may rely on to protect our market for approved drugs, may be held invalid or unenforceable by a court of final jurisdiction.
In addition, the issuance of a patent does not give us the right to practice the patented invention. Third parties may have blocking patents that prevent marketing of our products or working our own technology. We endeavor to identify early third-party patents and patent applications which may be blocking to a product or technology, to minimize this risk. However, relevant documents may be overlooked or missed, which may in turn impact of the freedom to commercialize the relevant asset.
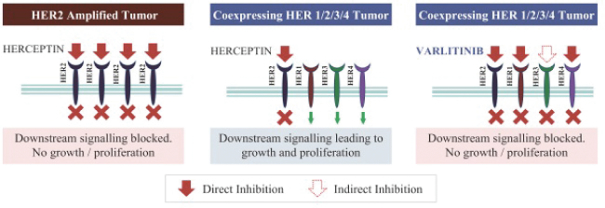
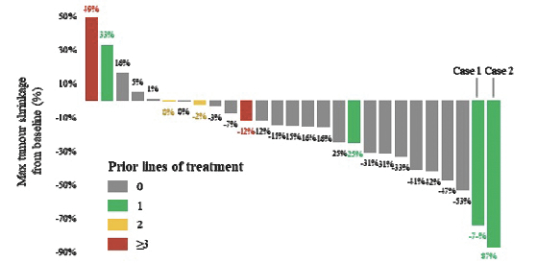
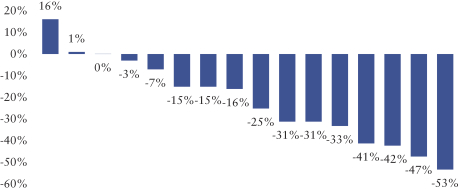
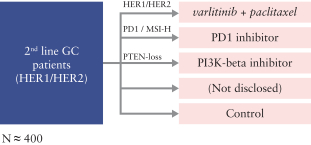
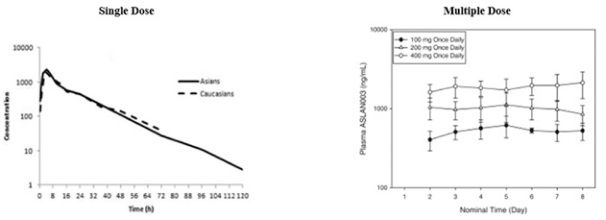
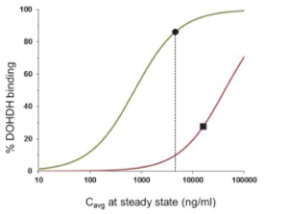
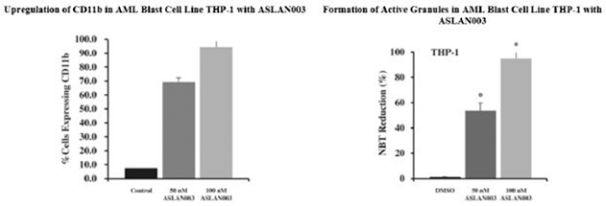
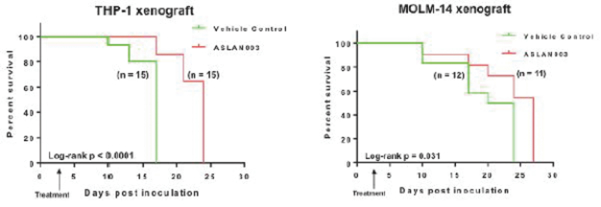
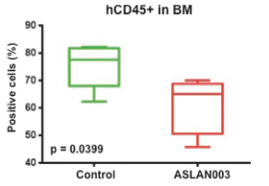
Varlitinib
Licensed from Array
On July 12, 2011, we entered into a collaboration and license agreement with Array, relating to Array’span-HER inhibitor,ARRY-543, which we refer to as ASLAN001 orvarlitinib, pursuant to which we obtained an exclusive, worldwide license to develop products incorporatingvarlitinibas an active ingredient for the treatment or prevention of any diseases or conditions in humans, pursuant to an agreed development plan, and an exclusive, worldwide license to pursue a commercial licensing program in relation to such products. On January 3, 2018, we entered into a new license agreement with Array, which replaces and supersedes our previous collaboration and license agreement, pursuant to which we obtained an exclusive, worldwide license to develop, manufacture and commercializevarlitinibfor all human and animal therapeutic, diagnostic and prophylactic uses.
With respect tovarlitinib, we exclusively licensed from Array a family of patents which includes composition of matter patents. These patents disclose a genus and also explicitly disclosesvarlitinib. More specifically, as of May 15, 2019, this patent family included four issued patents in the United States, 58 issued patents in a number of foreign countries and jurisdictions, including Argentina, Australia, Canada, China (at least three patents), Chile, Colombia, Europe, Hong Kong, Indonesia, India, Iceland, Israel, Japan, South Korea, Macau, Mexico, Norway, New Zealand, Philippines, Russia, Singapore, Ukraine, South Africa, and Taiwan, one pending patent application in the United States and three pending patent applications in a number of foreign countries, including Brazil, Egypt and Venezuela. The scope of the claims may differ in the various countries. The issued patents in this family and the pending patent applications, if issued, are expected to expire in August 2023 in the United States and August 2024 outside the United States, subject to the payment of renewal fees, excluding any additional term for patent term adjustments or patent term extensions.
The first patent application filed in China was not granted based on a technicality of Chinese practice. Subsequently filed divisional patent applications were granted. If the validity of one or more of the granted divisional patents is challenged, then one or more of these patents may ultimately be considered invalid.
In addition, we exclusively licensed from Array a family of patents derived from WO2007/059257, filed November 15, 2006, which relate to the synthetic process of makingvarlitiniband a key intermediate in that process. As of May 15, 2019, this patent family includes two issued patents in the United States, 17
122

 ..” In China, we have a trademark registration for “
..” In China, we have a trademark registration for “ ..” We also have a trademark registration in China to protect the following Chinese character version of the wordvarlitinib: “
..” We also have a trademark registration in China to protect the following Chinese character version of the wordvarlitinib: “ ” (wei li ti ni). This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
” (wei li ti ni). This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.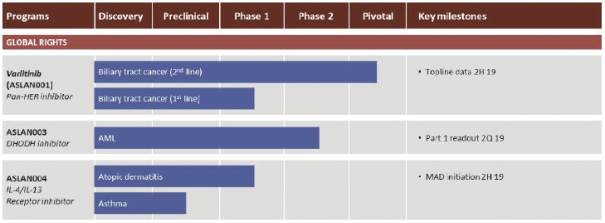





 ”. In China, we have a trademark registration for “
”. In China, we have a trademark registration for “ ”. We also have a trade mark registration in China to protect the following Chinese character version of the wordvarlitinib: “
”. We also have a trade mark registration in China to protect the following Chinese character version of the wordvarlitinib: “ ” (wei li ti ni). We have a portfolio of 20 domain names, which includes: aslanpharma.com, aslanpharma.com.sg, aslanpharma.com.tw, aslanpharma.asia, aslanpharma.org, and aslanpharma.biz.
” (wei li ti ni). We have a portfolio of 20 domain names, which includes: aslanpharma.com, aslanpharma.com.sg, aslanpharma.com.tw, aslanpharma.asia, aslanpharma.org, and aslanpharma.biz.