Exhibit 99.1

PRESS RELEASE
(restated August 18, 2023)
EBLASAKIMAB MONTHLY DOSING SHOWS POTENTIAL FOR BEST-IN-CLASS THERAPY IN POSITIVE PHASE 2B STUDY IN ATOPIC DERMATITIS
| | • | | Eblasakimab is the first biologic in moderate-to-severe atopic dermatitis to demonstrate a competitive efficacy profile with once-monthly dosing from initiation comparable to once every two weeks, supporting advancement to Phase 3. |
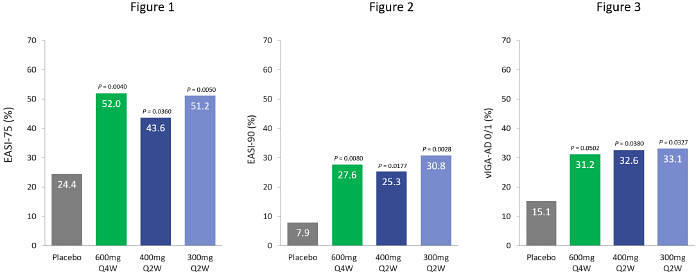
| | • | | 600mg eblasakimab dosed once every four weeks in the TREK-AD study met the primary endpoint with statistical significance, with 52.0% of patients achieving EASI-75, 27.6% reaching EASI-90 and 31.2% achieving vIGA-AD of 0 or 1. |
| | • | | Regimens dosing once every two weeks also met the primary endpoint with statistical significance, as well as meeting key secondary endpoints. |
| | • | | Unique loading dose regimen observed to deliver rapid onset of action with statistically significant improvement in EASI score by week 4. |
| | • | | Eblasakimab was generally well-tolerated at all dose levels, with low rates of conjunctivitis and injection site reactions supporting the potential for a differentiated safety profile. |
| | • | | Management to host webcast today, July 6, at 8:30 a.m. ET / 8:30 p.m. SGT. |
San Mateo, California, and Singapore, July 6, 2023 – ASLAN Pharmaceuticals (NASDAQ: ASLN), a clinical-stage, immunology-focused biopharmaceutical company developing innovative treatments to transform the lives of patients, today announced positive topline data from its Phase 2b dose-ranging study of eblasakimab in adult patients with moderate-to-severe atopic dermatitis (AD), the TREK-AD (TRials with EblasaKimab in Atopic Dermatitis) study. Eblasakimab met the primary endpoint of percent change from baseline in the Eczema Area and Severity Index (EASI) score at week 16 versus placebo with statistical significance in three dosing arms: 600mg dosed once every 4 weeks (600mg Q4W), which was numerically the best performing arm, 400mg dosed once every 2 weeks (400mg Q2W) and 300mg dosed once every 2 weeks (300mg Q2W).
Eblasakimab is a novel monoclonal antibody targeting the IL-13 receptor subunit of the Type 2 receptor, a key pathway driving several allergic inflammatory diseases. Eblasakimab’s unique mechanism of action has been observed to enable specific blockade of the Type 2 receptor, preventing signaling through both interleukin 4 (IL-4) and interleukin 13 (IL-13) – the key drivers of inflammation in AD, while sparing the Type 1 receptor.
“This is the first time we’ve seen a once-a-month treatment option deliver competitive efficacy data, which would be a game-changer for patients with AD,” said Eric L. Simpson, M.D., Frances J. Storrs Professor of Medical Dermatology at the Oregon Health and Science University and Lead Investigator in the TREK-AD study. “We haven’t seen much in the way of advancement since the launch of dupilumab, and there remains a huge unmet burden of disease experienced by patients. These results support eblasakimab’s potential to be a leading therapy for the treatment of AD, if approved.”
“We are delighted to announce these positive topline data from the TREK-AD study, which support recent translational studies we have published showing eblasakimab’s unique mechanism of action may provide a more efficient and targeted approach to blocking Type 2 signaling, which is responsible for allergic inflammation. Based on these results, we believe that eblasakimab could be a leading treatment in AD, if approved, getting more patients to EASI-75 or EASI-90, with fewer unwanted side effects, a rapid onset of action, and – uniquely – once-monthly dosing convenience,” said Dr Carl Firth, Chief Executive Officer of ASLAN Pharmaceuticals. “We look forward to advancing quickly into a Phase 3 program in AD, and exploring the broad range of indications where we would expect this drug candidate to be successful. We are grateful to the patients, investigators, and our team who have made such an important contribution to the development of eblasakimab.”