
Autolus AACR Investor and Analyst Call April 2nd, 2019 Exhibit 99.2

These slides and the accompanying oral presentation made by Autolus Therapeutics plc (“Autolus” or the “Company”) contain forward-looking statements within the meaning of the “safe harbor” provisions of The Private Securities Litigation Reform Act of 1995, including statements about the Company's ongoing and planned clinical trials, including its clinical development of AUTO1, the anticipated benefits of the Company’s product candidates, the timing and availability of data from clinical trials, the timing and ability to obtain and maintain regulatory approvals for the Company’s product candidates and the size and growth potential of the markets for its product candidates. All statements other than statements of historical fact contained in this presentation, including statements regarding the Company’s future results of operations and financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. In some cases, you can identify forward- looking statements by terms such as “may,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. These statements involve known and unknown risks, uncertainties and other important factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Factors that may cause actual results to differ materially from any future results expressed or implied by any forward looking statements include the risks described in the “Risk Factors” section of the Company’s Annual Report on Form 20-F for the year ended September 30, 2018 filed with the SEC on November 23, 2018, as well as those set forth from time to time in the Company’s other SEC filings, available at www.sec.gov. The forward-looking statements contained in this presentation reflect the Company’s views as of the date of this presentation regarding future events, except as required by law, and the Company does not assume any obligation to update any forward-looking statements. You should, therefore, not rely on these forward-looking statements as representing the Company’s views as of any date subsequent to the date of this presentation. Certain data in this presentation was obtained from various external sources. Such data speak only as of the date referenced in this presentation and neither the Company nor its affiliates, advisors or representatives make any representation as to the accuracy or completeness of that data or undertake to update such data after the date of this presentation. Such data involve risks and uncertainties and are subject to change based on various factors. Disclaimer
AUTO1 CAR T cell therapy designed to reduce the risk of severe CRS and improve the safety profile of the CD19 binder CD19-targeting programmed T cell therapy with a new binder (CAT19) with fast-off kinetics Similar structure to Kymriah®, 41BB endo-domain, lentivial vector Key difference is the binder In pre-clinical studies, AUTO1 has shown better proliferation, cytotoxicity, less exhaustion and cytokine production AUTO1 behaves more like a natural T cell Currently in ongoing Phase 1 clinical trial at University College London
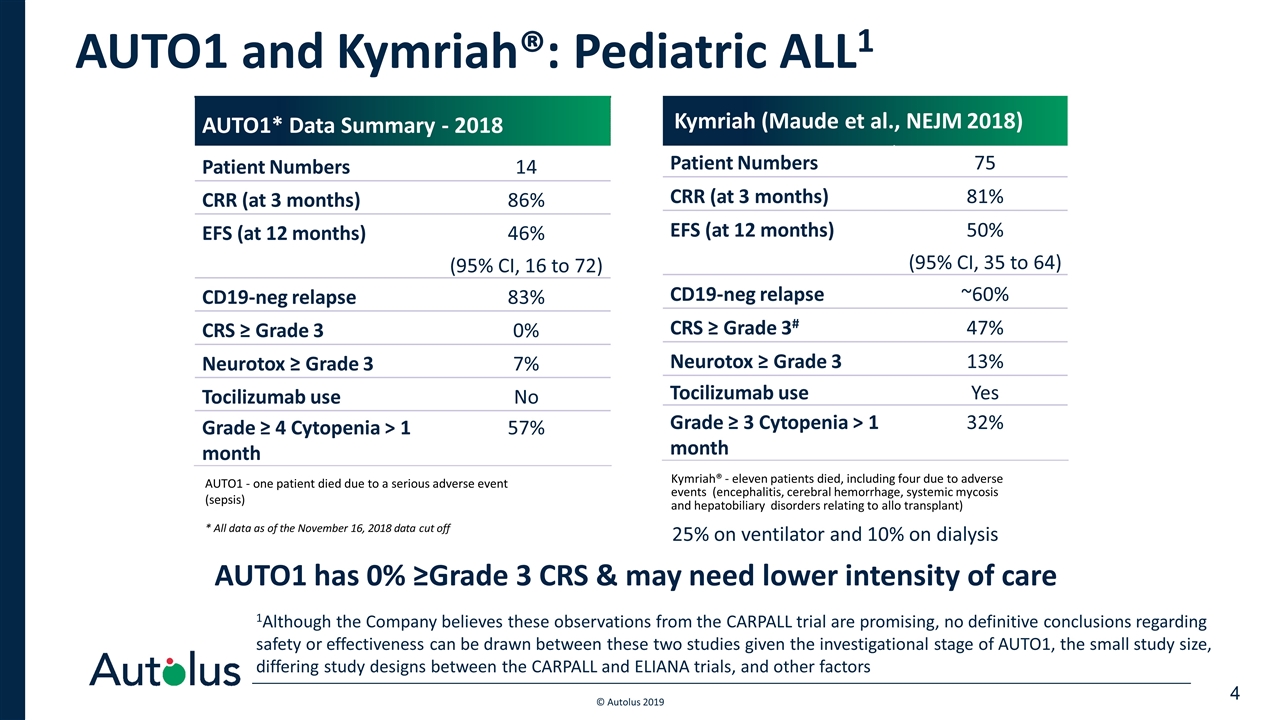
AUTO1 and Kymriah®: Pediatric ALL1 AUTO1 has 0% ≥Grade 3 CRS & may need lower intensity of care AUTO1 - one patient died due to a serious adverse event (sepsis) * All data as of the November 16, 2018 data cut off Kymriah® - eleven patients died, including four due to adverse events (encephalitis, cerebral hemorrhage, systemic mycosis and hepatobiliary disorders relating to allo transplant) AUTO1* Data Summary - 2018 Patient Numbers 14 CRR (at 3 months) 86% EFS (at 12 months) 46% (95% CI, 16 to 72) CD19-neg relapse 83% CRS ≥ Grade 3 0% Neurotox ≥ Grade 3 7% Tocilizumab use No Grade ≥ 4 Cytopenia > 1 month 57% Kymriah (Maude et al., NEJM 2018) Patient Numbers 75 CRR (at 3 months) 81% EFS (at 12 months) 50% (95% CI, 35 to 64) CD19-neg relapse ~60% CRS ≥ Grade 3# 47% Neurotox ≥ Grade 3 13% Tocilizumab use Yes Grade ≥ 3 Cytopenia > 1 month 32% 25% on ventilator and 10% on dialysis 1Although the Company believes these observations from the CARPALL trial are promising, no definitive conclusions regarding safety or effectiveness can be drawn between these two studies given the investigational stage of AUTO1, the small study size, differing study designs between the CARPALL and ELIANA trials, and other factors
AUTO1 and Kymriah®: Pediatric ALL AUTO1 Dose 1mil/kg vs 2-5mil/kg Kymriah Peak expansion : 10x higher than Kymriah AUC: 10x of Kymriah We believe AUTO 1 may have lower toxicity We believe the safety profile may enable AUTO1 to be well-suited for the treatment of adult ALL: older patients, more co-morbidities and less likely to tolerate toxicity

Next Steps for AUTO1 April 2nd, 2019
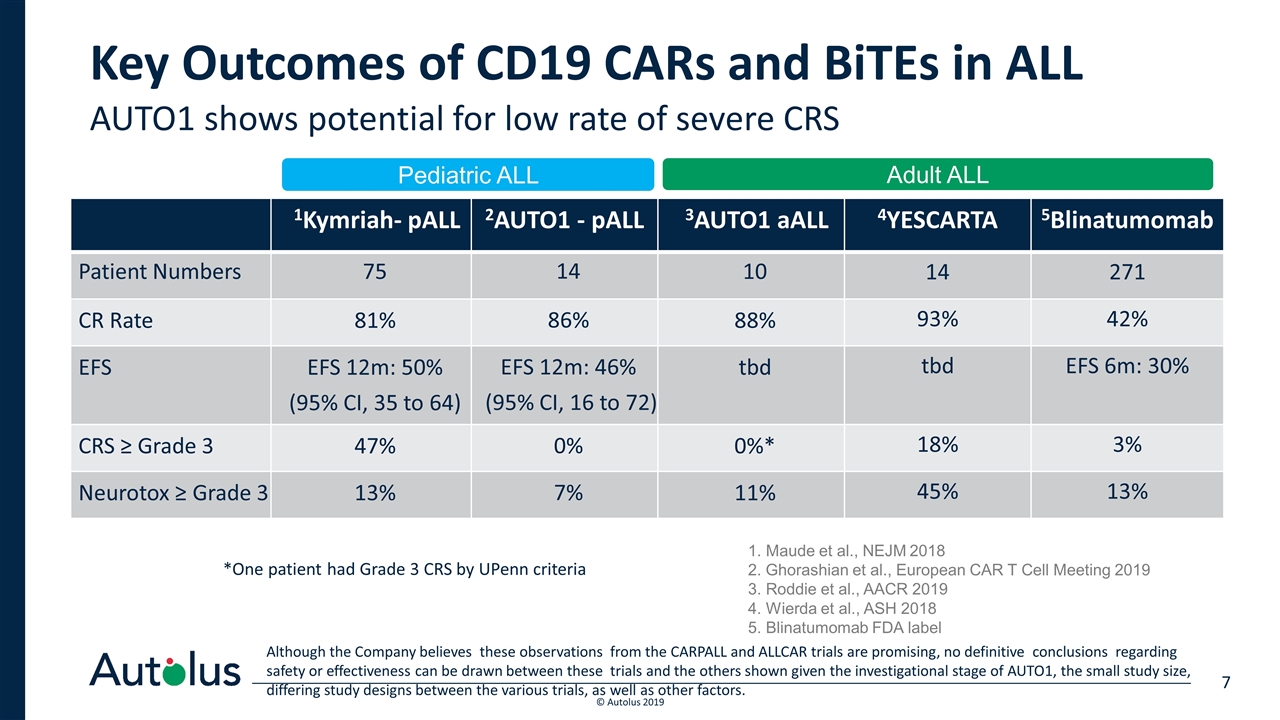
Key Outcomes of CD19 CARs and BiTEs in ALL 1Kymriah- pALL 2AUTO1 - pALL 3AUTO1 aALL 4YESCARTA 5Blinatumomab Patient Numbers 75 14 10 14 271 CR Rate 81% 86% 88% 93% 42% EFS EFS 12m: 50% (95% CI, 35 to 64) EFS 12m: 46% (95% CI, 16 to 72) tbd tbd EFS 6m: 30% CRS ≥ Grade 3 47% 0% 0%* 18% 3% Neurotox ≥ Grade 3 13% 7% 11% 45% 13% AUTO1 shows potential for low rate of severe CRS Pediatric ALL 1. Maude et al., NEJM 2018 2. Ghorashian et al., European CAR T Cell Meeting 2019 3. Roddie et al., AACR 2019 4. Wierda et al., ASH 2018 5. Blinatumomab FDA label Adult ALL Although the Company believes these observations from the CARPALL and ALLCAR trials are promising, no definitive conclusions regarding safety or effectiveness can be drawn between these trials and the others shown given the investigational stage of AUTO1, the small study size, differing study designs between the various trials, as well as other factors. *One patient had Grade 3 CRS by UPenn criteria

AUTO1 Outlook and Next Steps This initial data set for AUTO1 from the Phase 1/2 ALLCAR19 trial in adult r/r B-ALL patients indicates a potential attractive safety profile Further follow up will provide information on durability of treatment effect Level of CD19-negative relapse rate is too early to call Full update of ALLCAR19 trial planned for ASH 2019 Planned transition to Phase 2 in Q4 2019







