UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2020
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
001-38875
(Commission file number)
Greenlane Holdings, Inc.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | | 83-0806637 |
State or other jurisdiction of
incorporation or organization | | (I.R.S. Employer
Identification No.) |
| | | | | | | | | | | |
| 1095 Broken Sound Parkway, | Suite 300 | | |
| Boca Raton, | FL | | 33487 |
| (Address of principal executive offices) | | (Zip Code) |
(877) 292-7660
Registrant’s telephone number, including area code
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Class A Common Stock, $0.01 par value per share | | GNLN | | Nasdaq Global Market |
Securities registered pursuant to Section 12 (g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15 (d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No £
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | |
| Large accelerated filer | £ | Accelerated filer | £ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the common equity held by non-affiliates of the registrant as of June 30, 2020, the last business day of the registrant's most recently completed second fiscal quarter, was approximately $48.8 million based upon the closing price reported for such date on the Nasdaq Global Select Market.
As of March 26, 2021, Greenlane Holdings, Inc. had 16,341,897 shares of Class A common stock outstanding, 2,443,437 shares of Class B common stock outstanding and 72,064,218 shares of Class C common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's Proxy Statement for the 2020 Annual Meeting of Stockholders are incorporated herein by reference in Part III of this Form 10-K to the extent stated herein. Such proxy statement will be filed with the Securities and Exchange Commission within 120 days of the registrant's fiscal year ended December 31, 2020.
Greenlane Holdings, Inc.
Form 10-K
For the Fiscal Year Ended December 31, 2020
TABLE OF CONTENTS
| | | | | | | | |
| | Page |
| |
| | |
| | |
| Item 1. | | |
| Item 1A. | | |
| Item 1B. | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| | |
| | |
| Item 5. | | |
| Item 6. | | |
| Item 7. | | |
| Item 7A. | | |
| Item 8. | | |
| Item 9. | | |
| Item 9A. | | |
| Item 9B. | | |
| | |
| PART III | | |
| Item 10. | | |
| Item 11. | | |
| Item 12. | | |
| Item 13. | | |
| Item 14. | | |
| | |
| PART IV | | |
| Item 15. | | |
| Item 16. | | |
| Signatures | |
NOTE ABOUT FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K ("Form 10-K") contains forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, that involve risks and uncertainties. Many of the forward-looking statements are located in Part, Item 7 of this Form 10-K under the heading "Management's Discussion and Analysis of Financial Condition and Results of Operations." Forward-looking statements provide current expectations of future events based on certain assumptions and include any statement that does not directly relate to any historical or current fact. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could” and similar expressions. Examples of forward-looking statements include, without limitation:
•the impacts of the novel coronavirus ("COVID-19") pandemic and measures intended to prevent or mitigate its spread, and our ability to accurately assess and predict such impacts on our results of operations, financial condition, acquisition and disposition activities, and growth opportunities;
•statements regarding our growth and other strategies, results of operations or liquidity;
•statements concerning projections, predictions, expectations, estimates or forecasts as to our business, financial and operational results and future economic performance;
•statements regarding our industry;
•statements of management’s goals and objectives;
•projections of revenue, earnings, capital structure and other financial items;
•assumptions underlying statements regarding us or our business; and
•other similar expressions concerning matters that are not historical facts.
Forward-looking statements should not be read as a guarantee of future performance or results and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. Forward-looking statements are based on information available at the time those statements are made or management’s good faith belief as of that time with respect to future events and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to, those discussed in Part I, Item 1A of this Form 10-K under the heading “Risk Factors" and in other documents that we file from time to time with the Securities and Exchange Commission (the "SEC").
Forward-looking statements involve estimates, assumptions, known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from any future results, performances, or achievements expressed or implied by the forward-looking statements. These risks include, but are not limited to, those listed below and those discussed in greater detail in Part I, Item 1A of this Form 10-K under the heading “Risk Factors."
•our strategy, outlook and growth prospects;
•general economic trends and trends in the industry and markets in which we operate;
•public heath crises, including the COVID-19 pandemic;
•our dependence on, and our ability to establish and maintain business relationships with, third-party suppliers and service suppliers;
•the competitive environment in which we operate;
•our vulnerability to third-party transportation risks;
•the impact of governmental laws and regulations and the outcomes of regulatory or agency proceedings;
•our ability to accurately estimate demand for our products and maintain appropriate levels of inventory;
•our ability to maintain or improve our operating margins and meet sales expectations;
•our ability to adapt to changes in consumer spending and general economic conditions;
•our ability to use or license certain trademarks;
•our ability to maintain consumer brand recognition and loyalty of our products;
•our and our customers’ ability to establish or maintain banking relationships;
•fluctuations in U.S. federal, state, local and foreign tax obligation and changes in tariffs;
•our ability to address product defects;
•our exposure to potential various claims, lawsuits and administrative proceedings;
•contamination of, or damage to, our products;
•any unfavorable scientific studies on the long-term health risks of vaporizers, electronic cigarettes, e-liquids products or hemp-derived products, including cannabidiol (“CBD”);
•failure of our information technology systems to support our current and growing business;
•our ability to prevent and recover from Internet security breaches;
•our ability to generate adequate cash from our existing business to support our growth;
•our ability to protect our intellectual property rights;
•our dependence on continued market acceptance of our products by consumers;
•our sensitivity to global economic conditions and international trade issues;
•our ability to comply with certain environmental, health and safety regulations;
•our ability to successfully identify and complete strategic acquisitions;
•natural disasters, adverse weather conditions, operating hazards, environmental incidents and labor disputes;
•increased costs as a result of being a public company; and
•our failure to maintain adequate internal controls over financial reporting.
Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition or operating results.
The forward-looking statements speak only as of the date on which they are made, and, except as required by law, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Consequently, you should not place undue reliance on forward-looking statements.
Summary Risk Factors
Our business is subject to a number of risks, including risks that may prevent us from achieving our business objectives or may materially and adversely affect our business, financial condition, results of operations, cash flows and prospects. These risks are discussed more fully in Item 1A. Risk Factors herein. These risks include, but are not limited to, the following:
•We have at times experienced rapid growth, both domestically and internationally, and expect continued future growth, including growth from additional acquisitions. If we fail to manage our growth effectively, we may be unable to execute our business plan, maintain high levels of service or address competitive challenges adequately. Furthermore, our corporate culture has contributed to our success, and if we cannot maintain this culture as we grow, we could lose the innovation, creativity, and teamwork fostered by our culture, and our business may be harmed.
•The market for vaporizer products and related items is a niche market, subject to a great deal of uncertainty and is still evolving.
•We depend on third-party suppliers for our products and may experience unexpected supply shortages.
•A significant percentage of our revenue is dependent on sales of products from a relatively small number of key suppliers, and a decline in sales of products from these suppliers could materially harm our business.
•The FDA has expressed growing concern about the popularity among youth of the products of JUUL Labs and other manufactures of ENDS products and has imposed significant regulation on ENDS products. Additional regulatory actions may further impact our ability to sell these products in the United States or online.
•There is uncertainty related to the regulation of vaporization products and certain other consumption accessories at all levels of government. Significant increases in state and local regulation of our vaporizer products have been proposed and enacted, and are likely to continue to be proposed and enacted in numerous jurisdictions. Increased regulatory compliance burdens could have a material adverse impact on our business development efforts and our operations.
•Demand for the products we distribute could decrease if the suppliers of these products were to substantially the amount of goods sold directly to consumers in the sectors we serve.
•We may not be able to maintain existing supplier relationships or exclusive distributor status with our suppliers, which may affect our ability to offer a broad selection of products at competitive prices and negatively impact our results of operations.
•We do not have long-term contracts with most of our customers. The agreements that we do have generally do not commit our customers to any minimum purchase volume. The loss of a significant customer may have a material adverse effect on us.
•Because a majority of our revenues are derived from sales to consumers indirectly through third-party retailers who operate traditional brick-and-mortar locations, the shift of sales to more online retail business could harm our market share and our revenues in certain sectors.
•We may not be successful in maintaining the consumer brand recognition and loyalty of our products.
•Some of the products we sell contain nicotine, which is considered to be a highly-addictive substance, or other chemicals that some jurisdictions have determined to cause cancer and birth defects or other reproductive harm.
•Public health epidemics, pandemics or outbreaks, including the recent COVID-19 pandemic, could adversely affect our business.
•Our business depends partly on continued purchases by businesses and individuals selling or using cannabis pursuant to state laws in the United States or national and provincial laws in Canada.
•The federal and state regulatory landscape regarding products containing hemp-derived products is uncertain and evolving, and new or changing laws or regulations relating to hemp and hemp-derived products could have a material adverse effect on our business, financial condition and results of operations.
•We are subject to legislative uncertainty that could slow or halt the legalization and use of cannabis, which could negatively affect our business.
•Our business, and the business of the suppliers from which we acquire the products we sell, requires compliance with many laws and regulations in many jurisdictions globally across multiple product categories. Failure to comply with these laws and regulations could subject us or such suppliers to regulatory or agency proceedings, prosecutions, or investigations and could also lead to damage awards, fines and penalties.
•While we believe that our business and sales do not violate the Federal Paraphernalia Law, legal proceedings alleging violations of such law or changes in such law or interpretations thereof could materially and adversely affect our business, financial condition or results of operations.
•Officials of the U.S. Customs and Border Protection agency (“CBP”) have broad discretion regarding products imported into the United States, and the CBP has on occasion seized imported products on the basis that such products violate the Federal Paraphernalia Law. While we believe the products that we import do not violate such law, any such seizure of the products we sell could have a material adverse effect on our business operations or our results of operations.
•Because our business is dependent, in part, upon continued market acceptance of cannabis by consumers, any negative trends could materially and adversely affect our business, financial conditions or results of operations.
•Recently adopted laws prohibit the mailing of certain vaporizer products through the United States Postal Service (“USPS”) and place certain regulatory requirements on shipment of those products through other carriers. Additionally, carriers including UPS and FedEx have imposed policies restricting the shipment of vaporizers. If the products we carry cannot be shipped by the USPS or private carriers, or we must comply with burdensome policies and regulations, our shipping costs could increase materially and we could lose our ability to deliver products to customers in a timely and economical matter.
•We and our customers may have difficulty accessing the service of banks, which may make it difficult for us and for them to sell our products.
•The scientific community has not yet extensively studied the long-term health effects of the use of vaporizers, electronic cigarettes or e-liquids products.
•Two of our senior executives, Aaron LoCascio and Adam Schoenfeld, have control over all stockholder decisions because collectively they control a substantial majority of the voting power of our common stock. This will limit or preclude your ability to influence corporate matters, including the election of directors, amendments of our organizational documents and any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.
•The market price of our Class A common stock has been volatile and has declined significantly since our initial public offering and may face more volatility and price declines in the future. As a result, you may not be able to resell your shares at or above the price at which you have acquired or will acquire shares of our Class A common stock.
•We have not paid dividends in the past and have no current plans to pay dividends in the future, and any return on investment may be limited to the value of our common stock.
PART I
ITEM 1. BUSINESS
General
We are one of the largest global sellers of premium cannabis accessories and liquid nicotine products in the world. We operate as a third-party brand accelerator, a powerful house of brands, and a distribution platform for consumption devices and lifestyle brands serving the global cannabis, hemp-derived CBD, and liquid nicotine markets. We have an established track record of partnering with brands through all stages of the product lifecycle, and serve an expansive customer base covering over 8,000 locations, which includes over 1,100 licensed cannabis businesses and 4,100 smoke and vape shops. We supply our products to stores around the globe, offering only the most desired, high-quality products.
We are the partner of choice for many of the industry’s leading players including PAX Labs, Grenco Science, Storz & Bickel, Firefly, DaVinci, Santa Cruz Shredder, Cookies, among others. We have also set out to develop a world-class portfolio of our own proprietary brands ("Greenlane Brands") that we believe, over time will, deliver higher margins and create long-term value. Our Greenlane Brands include VIBES Rolling Papers, Pollen Gear, the Marley Natural accessory line, Aerospaced & Groove grinders, K. Haring Glass Collections, and Higher Standards, which serves as both upscale product line and an innovative retail experience with flagship stores at New York City’s famed Chelsea Market and a location in California's iconic Malibu Village. Subsequent to December 31, 2020, we added Eyce to our Greenlane Brands lineup through the acquisition of substantially all of the assets of Eyce LLC effective March 2, 2021. We also own and operate several industry-leading e-commerce platforms, including Vapor.com, Higherstandards.com, Aerospaced.com, Canada.vapor.com and Vaposhop.com, among others. These e-commerce platforms offer our consumers a convenient and flexible shopping solution.
We have a diverse source of revenue from both business-to-business ("B2B") transactions through wholesale distribution to retailers and business-to-consumer ("B2C") transactions through e-commerce and brick-and-mortar retail sales in three geographically distinct operating segments, which include our United States, Canada, and European operations. For the years ended December 31, 2020 and 2019, sales generated by our United States operating segment accounted for approximately 81.3% and 86.6% of net sales, respectively. Total net sales generated by our Canadian operations for the years ended December 31, 2020 and 2019 accounted for approximately 11.2% and 12.0%, respectively, and our European operations accounted for approximately 7.5% and 1.4% of net sales over the same periods. European operations did not commence until completion of the Conscious Wholesale acquisition in September 2019; therefore, the 2019 results reflect only three months of net sales for this operating segment. Given the recency of the acquisition, we expect our European operating segment to continue increasing as a percentage of our consolidated net sales. Refer to "Note 11— Segment Reporting" within Item 8 for additional information on our reportable segments.
Our diversity in the source of our revenue is further apparent through our increasingly low customer concentration, with our top ten customers accounting for only 9.8% and 17.3% of our net sales for the years ended December 31, 2020 and 2019, respectively, and no single customer accounting for more than 10% of our net sales over the two-year period ended December 31, 2020. While we distribute our products to a number of large national and regional retailers in the United States, Canada and Europe, our typical B2B customer is an independent retailer operating in a single market. Our sales teams regularly interact with our customers, as most of them have frequent restocking needs. We believe our high-touch customer service model strengthens relationships, builds loyalty and drives repeat business. In addition, we believe our premium product lines, broad product portfolio and strategic distribution network position us well to meet the needs of our customers and ensure timely delivery of products.
For the year ended December 31, 2020, revenues derived from B2B, B2C, and Supply & Packaging ("S&P") transactions represented approximately 60.4%, 14.3%, and 11.5% of net sales, respectively, compared to approximately 78.1%, 5.9%, and 10.8%, respectively, of net sales for the same period in 2019. Channel sales and drop-ship revenues derived from the sales and shipment of our products to the customers of third-party website operations and providing other services to our customers represented approximately 13.8% of net sales for the year ended December 31, 2020, compared to approximately 5.2% for the same period in 2019.
Organization
Greenlane Holdings, Inc. (“Greenlane” and, collectively with the Operating Company (as defined below) and its consolidated subsidiaries, the “Company”, "we", "us" and "our") was formed as a Delaware corporation on May 2, 2018. We are a holding company that was formed for the purpose of completing an underwritten initial public offering (“IPO”) of shares of our Class A common stock on April 23, 2019 and other related transactions in order to carry on the business of Greenlane Holdings, LLC (the “Operating Company”). The Operating Company was organized under the laws of the state of Delaware on September 1, 2015, and is based in Boca Raton, Florida. Refer to "Note 1—Business Operations and Organizations" within Item 8 for further information on the Company's organization and the IPO and related transactions. We are the sole manager of the Operating Company and, as of December 31, 2020, owned a 31.6% interest in the Operating Company.
Our Business Relating to the Cannabis Industry
The information included below is based on the most recent information available to the Company and, except as expressly stated below, does not give effect to the continued impact of the COVID-19 pandemic; the long-term impacts of which remain uncertain as of the date of this Form 10-K.
While we do not cultivate, distribute or dispense marijuana as that term is defined by the Controlled Substances Act, several of the products we distribute, such as vaporizers, pipes, rolling papers and storage solutions, can be used with marijuana or marijuana derivatives, as well as several other legal substances.
We believe the global cannabis industry is experiencing a transformation from a state of prohibition toward a state of legalization. We expect the number of states, countries and other jurisdictions implementing legalization legislation to continue to increase, which will create numerous and sizable opportunities for market participants, including us.
Global Landscape
A September 2020 report of Arcview Market Research and BDS Analytics, two of the leading market research firms in the cannabis industry, estimated that spending in the global legal cannabis market was approximately $14.9 billion in 2019 and reached approximately $19.7 billion as of September 2020, representing growth of approximately 32.2%. The report projects that by 2025, spending in the global legal cannabis market will reach $47.2 billion, representing a compound annual growth rate of approximately 22% over the six-year period from 2019. Our experience and awareness of the markets in which we operate lead us to believe that demand for the types of products we distribute will grow in tandem with the industry.
The North American Cannabis Landscape
United States and Territories. Thirty-eight states and the District of Columbia have legalized medical cannabis in some form and have a formal cannabis program. Fifteen of these states, and the District of Columbia, have legalized cannabis for non-medical adult use with additional states, such as New York, actively considering the legalization of cannabis for non-medical adult use. Eleven additional states have legalized high CBD, low tetrahydrocannabinol ("THC") oils for a limited class of patients. Only three states continue to prohibit cannabis entirely. Notwithstanding the continued trend toward further state legalization, cannabis continues to be categorized as a Schedule I controlled substance under the Federal Controlled Substances Act (the “CSA”) and, accordingly, the cultivation, processing, distribution, sale and possession of cannabis violate federal law in the United States as discussed further in Item 1A under the heading "Risk Factors." Our business depends partly on continued purchases by businesses and individuals selling or using cannabis pursuant to state laws in the United States or Canadian and provincial laws.
We believe support for cannabis legalization in the United States is gaining momentum. According to an October 2020 poll by Gallup, public support for the legalization of cannabis in the United States has increased from approximately 12% in 1969 to approximately 68% in 2020. In 2020, five states passed ballot initiatives legalizing either adult use or medical cannabis, further evidencing the public's support for cannabis legalization U.S. legal cannabis sales are projected to represent approximately 73% of total global sales by 2025.
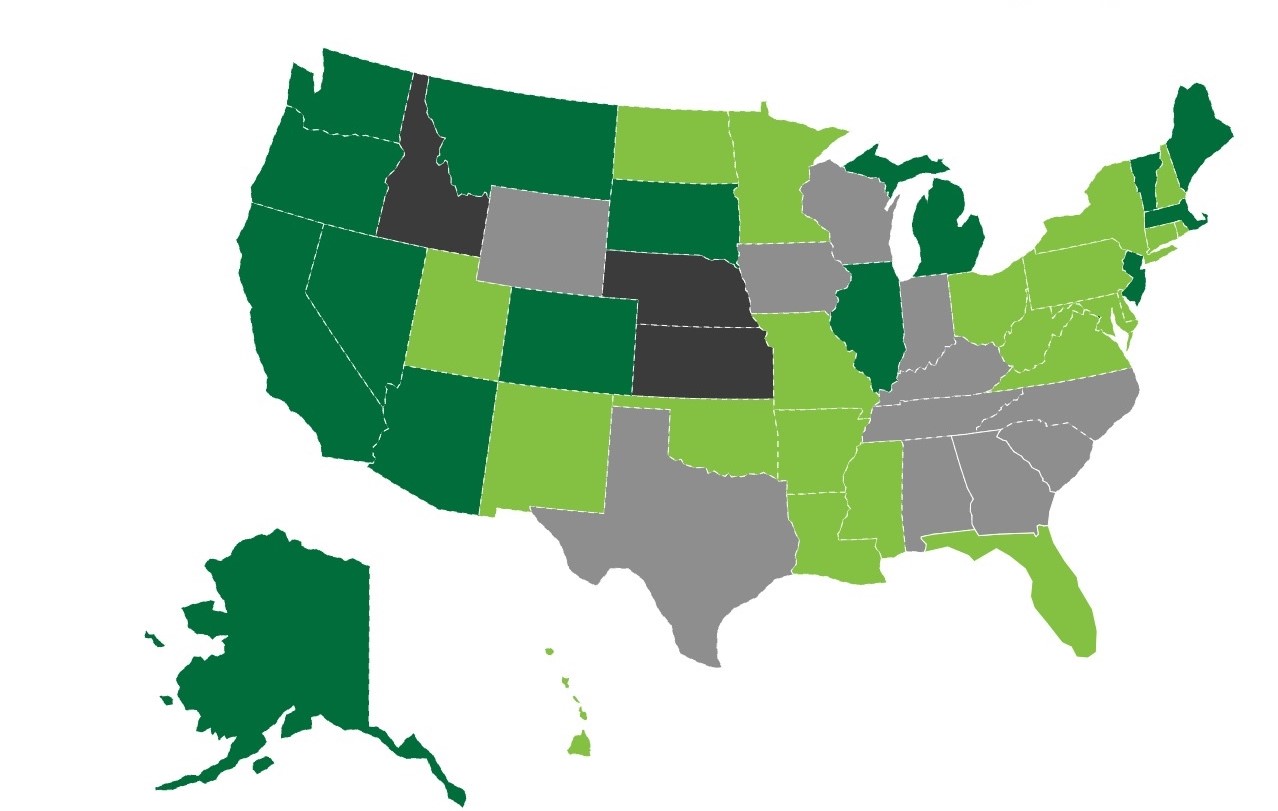
The following map from the National Cannabis Industry Association illustrates the states that have fully legalized adult-use cannabis (for medical and recreational purposes), states that have partially legalized cannabis (for medical purposes only), and states that have legalized cannabis use in a limited capacity (as of February 8, 2021).
U.S. CBD Landscape
In December 2018, the Farm Bill was signed into law in the United States which specifically removed hemp from the definition of “marijuana” under the Controlled Substances Act. In addition, the Farm Bill designated hemp as an agricultural commodity and permits the lawful cultivation of hemp in all states and territories of the United States. Federal and state laws and regulations concerning the cultivation and sale of hemp and hemp-derived products (including CBD) continue to evolve.
Canada.
Legal access to dried cannabis for medical purposes was first allowed in Canada in 1999. The Cannabis Act (the “Cannabis Act”) currently governs the production, sale and distribution of medical cannabis and related oil extracts in Canada.
On April 13, 2017, the Government of Canada introduced Bill C-45, which proposed the enactment of the Cannabis Act to legalize and regulate access to cannabis. The Cannabis Act proposed a strict legal framework for controlling the production, distribution, sale and possession of medical and recreational adult-use cannabis in Canada. On June 21, 2018, the Government of Canada announced that Bill C-45 received Royal Assent. On July 11, 2018, the Government of Canada published the Cannabis Regulations under the Cannabis Act. The Cannabis Regulations provide more detail on the medical and recreational regulatory regimes for cannabis, including regarding licensing, security clearances and physical security requirements, product practices, outdoor growing, security, packaging and labelling, cannabis-containing drugs, document retention requirements, reporting and disclosure requirements, the new access to cannabis for medical purposes regime and industrial hemp. The majority of the Cannabis Act and the Cannabis Regulations came into force on October 17, 2018, with additional Cannabis regulations coming into effect on October 17, 2019.
While the Cannabis Act provides for the regulation by the federal government of, among other things, the commercial cultivation and processing of cannabis for recreational purposes, it provides the provinces and territories of Canada with the authority to regulate in respect of the other aspects of recreational cannabis, such as distribution, sale, minimum age requirements, places where cannabis can be consumed, and a range of other matters.
The governments of every Canadian province and territory have implemented regulatory regimes for the distribution and sale of cannabis for recreational purposes. Most provinces and territories have announced a minimum age of 19 years old, except for Québec and Alberta, where the minimum age will be 18. Certain provinces, such as Ontario, have legislation in place that restricts the packaging of vapor products and the manner in which vapor products are displayed or promoted in stores.
The European Cannabis Landscape
Europe’s population is larger than that of the U.S. and Canadian markets combined, suggesting the potential of a very significant market. The changes in regulations for cannabis products across Europe are expected to result in a market growth of approximately $37.0 billion in annual sales by 2027, a significant growth from approximately $3.5 billion in 2020.
Currently, Germany, Italy, Austria, Czech Republic, Finland, Portugal, Spain, the Netherlands, Denmark, Greece, Croatia, North Macedonia, Poland, Turkey, Malta, Romania, Belgium, Estonia, Lithuania, Moldova, Norway, San Marino, Sweden, Switzerland, Luxembourg, Cyprus, France, the U.K and Ireland allow cannabis use for medicinal purposes, with some of those countries operating pilot programs. It has been widely reported that other countries are considering following suit.
Product Information
Consumers of cannabis, herbs, flavored compounds, aromatherapy oils and nicotine require the types of products we distribute, including vaporizers, pipes, rolling papers and packaging. We believe we distribute the “picks & shovels” for these rapidly-growing industries. As the world of cannabis and its respective aesthetic continues to expand, we strive to keep our product mix relevant, popular, and innovative; offering an array of products from vaporizers, grinders, to rolling papers and apparel lines. As our product offerings continue to develop and expand, we expect our revenue by categories to increase accordingly.
Inhalation Delivery Methods
There are two prevalent types of inhalation methods for cannabis and nicotine: combustion and vaporization. Vaporizers are devices that heat materials to temperatures below the point of combustion, extracting the flavors, aromas and effects of dry herbs and concentrates in the form of vapor. Measured by revenue, vaporizers are our largest product category.
The Science and Popularity of Vaporization
Vaporizers have elements that are designed to quickly heat material, causing vaporization to occur without the carbon dioxide that is typically generated through any combustion. The vapor byproduct is then immediately inhaled through the mouthpiece on the device itself, or through a hose or an inflatable bag. Vaporizers can heat a variety of dry materials, viscous liquids and waxes, and provide a convenient way for users to consume the active ingredients. Common ingredients used in vaporizers include tobacco, nicotine extracts, legal herbs, hemp-derived CBD, aromatherapy oils, cannabis and propylene glycol and glycerin blends.
Vaporization Technology. Consumers have a wide array of vaporization devices at their disposal, which can be broadly categorized into two primary categories: desktop and portable vaporizers. Our vaporizer offering spans over 260 distinct products across 90 brands.
Desktop Vaporizers. Vaporizers were first developed as desktop models that were powered through traditional electric power sources. Desktop vaporizers are capable of heating the material to a more precise temperature choice determined by the consumer or as advised by a health practitioner. Some models dispense the vapor through a pipe or wand, and others into an inflatable bag in order to allow users to more accurately monitor their consumption.
Portable Vaporizers. With the development of lithium batteries, vaporizers have now become portable. Technological advances are resulting in lighter, sleeker and more visually-appealing units that are capable of quickly heating the material to the user’s desired temperature setting. The temperature setting can be fixed by the manufacturer or set manually by the consumer or via Bluetooth connection to the consumer’s smartphone. Portable vaporizers, of which pens are a sub-set, are differentiated by many features, including output, battery life, recharge time, material, capacity and design.
Other Methods of Consumption. In addition to vaporizers, consumers have a wide array of methods of consumption at their disposal, including, among others, hand pipes, water pipes, rolling papers, and oral and topical delivery methods.
Hand and Water Pipes. We offer a diverse portfolio of over 200 hand and water pipes across 27 brands, including our own proprietary Higher Standards, Marley Natural and K. Haring Glass brands. Many display iconic, licensed logos and artwork, as pipes have grown into an artistic expression and are available in countless creative forms and functionality. Hand pipes are small, portable and simple to use, and function by trapping the smoke produced from burning materials, which is then inhaled by the user. Water pipes include large table-top models, bubblers and rigs, and are more complex because they incorporate the cooling effects of water to the burning materials before inhalation.
Rolling Papers. Rolling papers are a traditional consumption method used to smoke dried plant material in a "roll-your-own" application. These include papers, cones and wraps. Our rolling papers category is comprised of over 100 products across 17 brands, inclusive of Greenlane Brand's own Vibes Rolling Papers brand, not including accessories such as rolling trays or tips.
Our Competitive Strengths
We attribute our success to the following competitive strengths:
A Clear Market Leader in an Attractive Industry.
We are a leading North American distributor of premium vaporization products and consumption accessories, reaching over 1,000 licensed cannabis cultivators, processors and dispensaries. We also own and operate one of the industry’s most visited North American direct-to-consumer e-commerce websites, Vapor.com. Vapor.com was launched in April 2019 when we consolidated our previously owned websites, Vapeworld.com and Vapornation.com, into one homogeneous website. The latter website, VaporNation, was acquired as part of our February 2019 purchase of Better Life Holdings, LLC. We also own and operate several industry-leading e-commerce platforms, including Higherstandards.com, Aerospaced.com, Vaposhop.com, and most recently Eycemolds.com.
Market Knowledge and Understanding.
Because of our experience and our extensive, long-term industry relationships, we believe we have a deep understanding of customer needs and desires in our B2B, B2C and S&P channels. This allows us to influence customer demand and the pipeline between product manufacturers, suppliers, advertisers and the marketplace.
Broadest Product Offering.
We believe we offer the industry’s most comprehensive portfolio of vaporization products and consumption accessories with over 5,000 SKUs (stock-keeping units) from more than 300 suppliers. This broad product offering creates a “one-stop shop" for our customers and positively distinguishes us from our competitors. In addition, we have carefully cultivated a portfolio of well-known brands and premium products and have helped many of the brands we distribute to become established names in the industry.
Entrepreneurial Culture.
We believe our entrepreneurial, results-driven culture fosters highly-dedicated employees who provide our customers with superior service. We invest in our talent by providing every sales representative with an extensive and ongoing education, and have successfully developed programs that provide comprehensive product knowledge and the tools needed to have a unique understanding of our customers’ personalities and decision-making processes.
Customers. We believe we offer superior services and solutions due to our comprehensive product offering, proprietary industry data and analytics, product expertise and quality of service. We deliver products to our customers in a precise, safe and timely manner with complementary support from our dedicated sales and service teams.
Suppliers. Our industry knowledge, market reach and resources allow us to establish trusted professional relationships with many of our product suppliers. We generate substantially all of our net sales from products manufactured by others. We have strong relationships with many large, well-established suppliers, and seek to establish distribution relationships with smaller or more recently established manufacturers in our industry. While we purchase our products from over 300 suppliers, a significant percentage of our net sales is dependent on sales of products from a small number of key suppliers. We believe there is a trend of suppliers in our industry to consolidate their relationships to do more business with fewer distributors. We believe our ability to help maximize the value and extend the distribution of our suppliers’ products has allowed us to benefit from this trend. The efforts of our senior management team have been integral to our relationships with our suppliers.
Employees. We provide our employees with an entrepreneurial culture, a safe work environment, financial incentives and career development opportunities.
Experienced and Proven Management Team Driving Organic and Acquisition Growth.
We believe our management team is among the most experienced in the industry. Our senior management team brings experience in accounting, mergers and acquisitions, financial services, consumer-packaged goods, retail operations, third-party logistics, information technology, product development and specialty retail, and an understanding of the cultural nuances of the industry that we serve.
Our Operating Strategies
We intend to leverage our competitive strengths to increase shareholder value through the following core strategies:
Build Upon Strong Customer and Supplier Relationships to Expand Organically.
Our North American footprint and broad supplier relationships, combined with our regular interaction with our large and diverse customer base, provides us key insights and positions us to be a critical link in the supply chain for premium vaporization products and consumption accessories. Our suppliers benefit from access to more than 8,000 brick and mortar locations and more than 1.8 million B2C customers, as we are a single point of contact for improved production, planning and efficiency. Our customers, in turn, benefit from our market leadership, talented sales associates, broad product offerings, high inventory availability, timely delivery and exceptional customer services. We believe our strong customer and supplier relationships will enable us to expand and broaden our market share in the premium vaporization products and consumption accessories marketplace and expand into new categories. For example, in November 2020, we entered into a partnership with Studenglass, which brought the Gravity Hookah to consumers and wholesale purchasers in the United States, Canada, and Europe.
Expand Our Operations Internationally.
We currently focus our marketing and sales efforts on the United States, Canada, and Europe, with the United States and Canada representing the two largest and most developed markets for our products. While we currently support and ship certain products to customers in Australia and parts of South America on a limited basis, we are aware of the growth opportunities in these markets. As we continue to expand our marketing, supplier relationships, sales bandwidth and expertise, we anticipate capturing market share in those regions by opening our own distribution centers, acquiring existing international distributors and/or partnering with local operators. In September 2019, we acquired Conscious Wholesale, a leading European wholesaler and retailer of consumption accessories, vaporizers, and other high-quality products. We assumed control of their existing warehouse facility located in Amsterdam, Netherlands, which is expected to facilitate the expansion of our European operations.
In February 2021, we opened three new Higher Standards shop-in-shop retail locations in Uruguay in a collaboration with the Kaya Herb Group. Further expanding Greenlane’s global footprint, these high-profile locations mark our first physical footprint in the South American market.
Expand our E-Commerce Reach and Capabilities.
We own and operate one of the leading direct-to-consumer e-commerce websites in our industry, Vapor.com. This site is one of the most visited within our industry according to SEMrush, a leading data analytics firm, and as of December 31, 2020, we ranked in the top five in 58 key mobile search terms and in the top ten in 93 key mobile search terms, On desktop computer searches, we ranked in the top five in 54 key search terms, and in the top ten in 96 key search terms. We recently expanded our E-Commerce platform into Canada through the launch of "Canada.Vapor.com" on October 26, 2020. Refer to Item 7 - Results of Operations for further detail on Canada.Vapor.com.
Pursue Value-Enhancing Strategic Acquisitions.
Through our acquisitions of VaporNation (Better Life Holdings, LLC), Pollen Gear LLC, and Conscious Wholesale, we have added new markets within the United States and Europe, new product lines, talented employees and operational best practices. Effective March 2, 2021, we acquired substantially all the assets of Eyce LLC, which further diversified our Greenlane Brand offerings through the integration of Eyce premium silicon smoking products and accessories. We intend to continue pursuing strategic acquisitions to grow our market share and enhance leadership positions by taking advantage of our scale, operational experience and acquisition know-how to pursue and integrate attractive targets. We believe we have significant opportunities to add product categories through our knowledge of our industry and possible acquisition targets.
Enhance Our Operating Margins.
We expect to enhance our operating margins as our business expands through a combination of additional product purchasing discounts, reduced inbound and outbound shipping and handling rates, reduced transaction processing fees, increased operating efficiencies and realization of benefits through leveraging our existing assets and distribution facilities. Additionally, we expect that our operating margins will increase as our product mix continues to evolve to include a greater portion of our proprietary branded products. We are committed to supporting our proprietary brands, such as Higher Standards, VIBES and Pollen Gear, which offer significantly higher gross margins than supplier-branded products.
Developing A World-Class Portfolio of Proprietary Brands.
We intend to continue to develop a portfolio of our own proprietary brands, which over time has helped to improve our blended margins and create long-term value. Our brand development is based upon our proprietary industry intelligence that allows us to identify market opportunities for new brands and products. We leverage our distribution infrastructure and
customer relationships to penetrate the market quickly with our proprietary brands and to gain placement in thousands of stores. Currently, we sell such products directly to consumers through our brand websites and our e-commerce properties. Our existing proprietary brands include VIBES Rolling Papers, Pollen Gear, the Marley Natural accessory line, Aerospaced & Groove grinders, Marley Natural, K. Haring Glass Collections, and Higher Standards. Effective March 2021, we added the Eyce product line to our proprietary brands.
In addition to absorbing the Marley Natural accessory line as a house brand, we are making other strides to ensure we take full advantage of the opportunities given to us as a company. We intend to extend the price points of the Higher Standards line to include a wider customer base, and in doing so, increase the presence of our house brands. To synergize with the direction of Higher Standards, and the K. Haring Glass Collection, both brands will sit under the Higher Standards brand umbrella. Taking this step is expected to ensure the brands are not competing against each other, and that we maximize market penetration for all our brands. With all these changes comes expansion into new markets; we are taking steps to ensure that all our proprietary brands are prepared to enter new markets in Europe during the upcoming year. In creating, acquiring, and expanding our proprietary brands, we intend to stay mindful of our key supplier relationships and to identify opportunities within our product portfolio and in the market where we can introduce or acquire compelling products that do not directly compete with the products of our core suppliers.
Execute on Identified Operational Initiatives.
We continue to evaluate operational initiatives to improve our profitability, enhance our supply chain efficiency, strengthen our pricing and category management capabilities, streamline and refine our marketing process and invest in more sophisticated information technology systems and data analytics. In addition, we continue to further automate our distribution facilities and improve our logistical capabilities. We are also taking steps to transition to a more centralized model with fewer, larger, highly automated facilities. During 2020, we made significant progress towards this goal through consolidating our distribution facilities in the United States into one primary streamlined centrally-located facility and one facility in California, primarily serving our S&P customers, which will reduce costs going forward. We believe we will continue to benefit from these and other operational improvements.
Be the Employer of Choice.
We believe our employees are the key drivers of our success, and we aim to recruit, train, promote and retain the most talented and success-driven personnel in the industry. Our size and scale enable us to offer structured training and career path opportunities for our employees, while in our sales and marketing teams, we have built a vibrant and entrepreneurial culture that rewards performance. We are committed to being the employer of choice in our industry.
Business Seasonality
While our B2B and B2C customers typically operate in highly-seasonal businesses, our Channel & Dropship and S&P divisions are less affected by the holidays. We have historically experienced only moderate seasonality in our business, particularly during the fourth quarter, which coincides with Cyber Monday (the first Monday after Thanksgiving, when online retailers typically offer holiday discounts), and as our customers build up their inventories in anticipation of the holiday season and for which we have related promotional marketing campaigns. Additionally, plans for growth in E-Commerce and more regularity in the B2B division will likely result in more consistent trends around the winter months, and the industry related holidays. For the year ended December 31 2020, seasonality was largely impacted by the COVID-19 pandemic. While we typically experience a substantial increase in sales for the "4/20" industry holiday, we saw a very minimal increase as our retail [brick and mortar] operations were closed and many of our B2B customers were closed for business as well. We also noticed increased revenue in the third quarter of 2020, as compared to the second quarter of 2020, which was largely driven by the lifting of quarantine restrictions and reopening of many businesses that previously were closed temporarily due to the pandemic. If these trends continue, we expect B2B and B2C retail sales to return to and exceed pre-COVID-19 revenue figures as the COVID-19 vaccines become more readily available.
Human Capital Resources
As of December 31, 2020, we had 264 full-time employees. Approximately 189 were employed in the U.S. and 75 were employed in Europe. None of our employees are represented by a labor union. We have never experienced a labor-related work stoppage.
As we mention in our core operating strategies, we aim to be the employer of choice, as our employees are the key drivers of our success. We aim to recruit, train, promote and retain the most talented and success-driven personnel in the industry. Our industry knowledge and scale provide opportunities for our employees to obtain structured training and career path opportunities across all departments and positions. We are a company built and based on trust, sincerity, respect, commitment, and fairness, and we strive to create a work environment that is friendly, open, and co-operative.
Employee Health and Safety during COVID-19
The health and safety of our employees is a top priority for us. During COVID-19, we were deemed an essential industry and as a result, we were very active in monitoring and tracking all relevant data, including guidance from local, national, and international health agencies. Our actions included:
•Allowing employees to work remotely where feasible;
•Implemented enhanced safety measures including mandatory face coverings, physical distance requirements, temperature checks, deep cleaning and disinfectant protocols, and hand sanitizing stations for employees continuing critical on-site work at all locations;
•Provide employee-wide training on COVID-19 safety measures;
•Restrict company travel to essential business travel that requires prior multi-level approvals.
Our Human Resources department is continuing to communicate to our employees as more information is available and continues to evaluate our operations considering federal, state, and local guidance.
Diversity and Inclusion
We are committed to diversity and inclusion across all aspects of our company. We have developed a diversity and inclusion committee (led by our employees) that is centered on educating our employees on the benefits of a diverse workforce, reducing the risk of bias and ensuring that everyone owns responsibility for inclusive behaviors and actions across the organization. We have established hiring principles that focus on our mission to hire people from diverse backgrounds who will add to our culture.
Culture and Engagement
Everything we do is powered by our vision and core values and our culture reflects that. As a result, we enjoy a highly motivated and skilled work force committed to our company. In 2020, we held our first employee engagement survey, and in consultation with our employees we have addressed several opportunities to further improve our culture. By being open, honest, and transparent, our employees feel more actively engaged in our success.
Total Rewards and Pay Equity
We strive to attract and retain diverse, high caliber employees who raise the talent bar by offering competitive compensation and benefit packages, regardless of their gender, race, or other personal characteristics. We regularly review and survey our compensation and benefit programs against the market to ensure we remain competitive in our hiring practices. We provide employee salaries that are competitive and consider factors such as an employee’s role and experience, the location of their job and their performance. In addition to our competitive salaries, to enhance our employees’ sense of participation in the company and to further align their interests with those of our stockholders, we offer equity packages to a broad set of key employees.
Development and Retention
We strive to hire, develop, and retain talent that continuously raises the performance bar. We encourage, support, and compensate our employees based on our philosophy of recognizing and rewarding exceptional performance. We believe that performance and development is an ongoing process in which all employees should be active participants. In 2020, we rolled out key performance goals for all employees linked to their compensation and have begun work on a Greenlane Learning and Development curriculum that will include a blended approach to both in person and virtual learning.
Competition
Business-to-Business. We operate in an evolving industry in which the market and its participants remain highly fragmented. Although it is difficult to find reliable independent research, we believe there is a vast number of potential B2B customers in North America comprised of independent retail shops, specialty retailers, licensed cannabis dispensaries and regional retailer chains.. We currently serve over 8,000 of these locations. Our B2B customers compete primarily on the basis of the breadth, style, quality, pricing and availability of merchandise, the level of customer service, brand recognition and loyalty. We successfully reach our B2B customers through our direct sales force and other marketing initiatives, and provide them with our strategically-curated mix of brands and products, merchandise planning strategies and exceptional customer service. Among vaporizer product distributors, we compete against both suppliers and other distributors. A number of suppliers choose to distribute directly in some sales channels and may also operate their own e-commerce platforms. We face competition from many small privately-owned regional distributors that carry a narrow range of products. We believe there are only a select few wholesale distributors carrying a complete line of premium vaporization products and consumption accessories.
Business-to-Consumer. A number of suppliers of vaporizers and specialized consumption products and accessories operate their own e-commerce websites through which they sell their items directly to end consumers. Additionally, there are hundreds of websites that sell products similar to those we offer in North America, Europe, Australia and other parts of the world. We believe we compete effectively with other e-commerce websites. Further, we provide fulfillment services to the owners of some of these websites as they do not carry their own inventory, are not able to ship as efficiently as we do and are unable to meet certain regulatory requirements, such as sales tax collection. Our competitors’ websites rank in many search categories below our primary e-commerce website, Vapor.com, which has its own dedicated design, social media and search engine optimization ("SEO") teams. We believe our market knowledge, large product selection, relationships with vaporizer brands, in-house search engine optimization teams, social media focus and distribution facilities will enable us to remain a market leader in e-commerce.
Trademarks
We own a number of registered trademarks and service marks, including without limitation, Greenlane, Higher Standards, VIBES, Aerospaced, Groove, Pollen GearTM, and most recently Eyce. Solely for convenience, trademarks and trade names referred to in this Form 10-K may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. In addition, this Form 10-K contains trade names, trademarks and service marks of other companies that we do not own. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, these other companies. We believe our largest trademarks are widely recognized throughout the world and have considerable value. The duration of trademark registrations varies from country to country. However, trademarks are generally valid and may be renewed indefinitely as long as they are in use and/or their registrations are properly maintained.
Insurance
We carry a broad range of insurance coverages, including general liability, real and personal property, workers’ compensation, directors’ and officers’ liability and other coverages we believe are customary. Our exposure to loss for insurance claims is generally limited to the per-incident deductible under the related insurance policy. We do not expect the impact of any known casualty, property, environmental or other contingency to have a material impact on our financial conditions, results of operations or cash flows.
Our directors’ and officers’ liability insurance policy we chose to maintain covers only non-indemnifiable individual executive liability, often referred to as “Side A,” and does not provide individual or corporate reimbursement coverage, often referred to as “Side B” and “Side C,” respectively. The Side A policy covers directors and officers directly for loss, including defense costs, when corporate indemnification is unavailable. Side A-only coverage cannot be exhausted by payments to the Company, as the Company is not insured for any money it advances for defense costs or pays as indemnity to the insured directors and officers. As a result, we currently do not have insurance coverage for, and directly self-funded with cash on hand, our litigation defense costs for actions like those described under "Item 3—Legal Proceedings".
Regulatory Developments
Our operating results and prospects will be impacted, directly and indirectly, by regulatory developments at the local, state, and federal levels. Certain changes in local, state, national, and international laws and regulations, such as increased legalization of cannabis, create significant opportunities for our business. However, other changes to laws and regulations result in restrictions on which products we are permitted to sell and the manner in which we market our products, increased taxation of our products, and negative changes to the public perceptions of our products, among other effects.
We believe the continuing trend of states’ legalization of medicinal and adult-use cannabis is likely to contribute to an increase in the demand for many of our products. In the 2020 election, voters approved ballot initiatives legalizing adult-use cannabis in New Jersey, Arizona, Montana and South Dakota. Voters also approved initiatives legalizing medical marijuana in Mississippi and South Dakota. Other states appear likely to legalize either medical or adult-use cannabis in 2021 and beyond. However, we can provide no assurances that additional states will legalize cannabis.
Recently, the identification of many cases of e-cigarette or vaping product use associated lung injury (“EVALI”) has led to significant scrutiny of e-cigarette and other vaporization products. Additionally, certain academic studies and news reports have suggested that smoking or vaping may increase the risk of complications for individuals who contract COVID-19. EVALI, COVID-19 and other public health concerns could contribute to negative perceptions of vaping and smoking, which in turn could lead consumers to avoid certain of our products, which would materially and adversely affect our results of operations.
In response to health concerns and concerns about people under the age of eighteen using vaping products, several localities, states, and the federal government have enacted measures restricting the sale of certain types of vaping products. For
example, on December 20, 2019, legislation was signed into law that raised the federal minimum age of sale for tobacco products from 18 to 21. As another example, on January 2, 2020, the United States Food and Drug Administration ("FDA") announced a new policy prioritizing enforcement against certain unauthorized flavored e-cigarette products that appeal to minors, including fruit and mint flavors, as well as of any other products that are targeted to minors. More recently, as discussed below, the FDA announced its intention to take enforcement measures related to ENDS products offered for sale after September 9, 2020 for which the manufacturer has not submitted a premarket tobacco product application ("PMTA"). Additionally, some state, provincial, and local governments have enacted or plan to enact laws and regulations that restrict the sale of certain types of vaping products. For example, several states and localities have implemented bans on certain flavored vaping products in an effort to reduce the appeal of such products to minors and some localities have banned the sale of nicotine vaping products entirely. Other states, including Arkansas, Maine, Utah, and Vermont have banned the sale of vaporizers direct to consumers through mail. These new vaping laws are rapidly shifting and, in some instances, have been repealed or narrowed as the result of successful legal challenges. Laws banning certain vaping products or restricting the manner in which they may be sold have been adopted in Arkansas, Massachusetts, New York, New Jersey, Maryland, Rhode Island, Vermont, Utah and Maine among other jurisdictions. Taken together, these federal, state, and provincial restrictions on vaping products materially and adversely affect our revenues. The ultimate impact of these policy developments will depend upon, among other things, the types and quantities of products we sell that are encompassed by each ban, the success of legal challenges to the bans, our suppliers' actions to adapt to actual and potential regulatory changes, and our ability to provide alternative products.
In addition, 27 states and the District of Columbia have recently adopted laws imposing taxes on liquid nicotine. Additionally, at least eleven states have adopted laws imposing taxes on vaporizers. These taxes will result in increased prices to end consumers, which may adversely impact the demand for our products. We expect these taxes would impact our competitors similarly, assuming their compliance with applicable laws.
The Consolidated Appropriations Act, 2021, which was signed into law on December 27, 2020, contains provisions that prohibit the mailing of electronic nicotine delivery systems ("ENDS") through the United States Postal Service (“USPS”) and place certain regulatory requirements on shipment of ENDS through other carriers. Certain private carriers, including UPS and FedEx, also have policies restricting or prohibiting the shipment of many vaporization products we sell. If a substantial volume of the products we carry cannot be shipped by the USPS or private carriers, or we must comply with burdensome policies and regulations, our shipping costs could increase materially and we could lose our ability to deliver products to customers in a timely and economical matter.
Corporate Information
Our executive offices are located at 1095 Broken Sound Parkway, Suite 300, Boca Raton, Florida 33487. Our telephone number at our executive offices is (877) 292-7660.
Available Information
The Company’s Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to reports filed pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), are filed with the SEC. We are subject to the informational requirements of the Exchange Act and file or furnish reports, proxy statements and other information with the SEC. Such reports and other information filed by us with the SEC are available free of charge at investor.gnln.com/financial-information/sec-filings when such reports are available on the SEC’s website. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at www.sec.gov. We periodically provide other information for investors on our corporate website, www.gnln.com, and our investor relations website, investor.gnln.com. This includes press releases and other information about financial performance, information on corporate governance and details related to our annual meeting of shareholders. The information contained on the websites referenced in this Form 10-K is not incorporated by reference into this filing. Further, our references to website URLs are intended to be inactive textual references only.
ITEM 1A. RISK FACTORS
Our operations and financial results are subject to various risks and uncertainties, including but not limited to those described below, which could harm our business, reputation, financial condition, and operating results. The following is a description of what we consider the key challenges and material risks to our business and an investment in our Class A common stock.
Risks Related to Our Business and Industry
We have experienced rapid growth, both domestically and internationally, and expect continued future growth, including growth from additional acquisitions. If we fail to manage our growth effectively, we may be unable to execute our business plan, maintain high levels of service or address competitive challenges adequately. Furthermore, our corporate culture has contributed to our success, and if we cannot maintain this culture as we grow, we could lose the innovation, creativity, and teamwork fostered by our culture, and our business may be materially and adversely affected.
We intend to continue to grow our business. Our success will depend, in part, on our ability to manage this growth, both domestically and internationally. Any growth in, or expansion of, our business is likely to continue to place a strain on our management and administrative resources, infrastructure and systems. As with other growing businesses, we expect that we will need to further refine and expand our business development capabilities, our systems and processes and our access to financing sources. We will also need to hire, train, supervise, and manage new employees. These processes are time consuming and expensive and will increase management responsibilities and divert management attention. We cannot assure that we will be able to:
•expand our product offerings effectively or efficiently or in a timely manner, if at all;
•allocate our human resources optimally;
•meet our capital needs;
•identify and hire qualified employees or retain valued employees;
•effectively incorporate the components of any business or product line that we may acquire in our effort to achieve growth; or
•continue to grow our business.
Our inability or failure to manage our growth and expansion effectively could harm our business and materially adversely affect our operating results and financial condition. In addition, we believe that an important contributor to our success has been and will continue to be our corporate culture, which we believe fosters innovation, teamwork and a passion for our products and customers. As a result of our rapid growth, we may find it difficult to build and maintain our strong corporate culture, which could limit our ability to innovate and operate effectively. Any failure to preserve our culture could also negatively affect our ability to retain current and recruit new personnel, continue to perform at current levels or execute on our business strategy.
The market for vaporizer products and related items is a niche market, subject to a great deal of uncertainty and is still evolving.
Vaporizer products comprise a significant portion of our product portfolio. Many of these products have only recently been introduced to the market and are at an early stage of development. These products represent core components of a niche market that is evolving rapidly, is characterized by a number of market participants and is subject to regulatory oversight and a potentially fluctuating regulatory framework. Rapid growth in the use of, and interest in, vaporizer products is recent, and may not continue on a lasting basis. The demand and market acceptance for these products is subject to a high level of uncertainty, including, but not limited to, changes in governmental regulation, developments in product technology, perceived safety and efficacy of our products, perceived advantages of competing products and sale and use of materials that can be vaporized, including in the expanding legal state cannabis markets. For example, recent concerns about EVALI and youth use of vaporizers have, by some metrics, negatively impacted demand for vaporizers and led to laws and regulations restricting the sale of certain products in different markets. Therefore, we are subject to many of the business risks associated with a new enterprise in a niche market. Continued technical evolution, market uncertainty, evolving regulation and the resulting risk of failure of our new and existing product offerings in this market could have a material adverse effect on our ability to build and maintain market share and on our business, results of operations and financial condition. Further, there can be no assurance that we will be able to continue to compete effectively in this marketplace.
We depend on third-party suppliers for our products and may experience unexpected supply shortages which could have a material adverse effect on our business.
We depend on third-party suppliers for our vaporization products and consumption accessories product offerings. Our customers associate certain characteristics of our products, including the weight, feel, draw, flavor, packaging and other unique attributes, to the brands we market, distribute and sell. In the future, we may have difficulty obtaining the products we need from our suppliers as a result of unexpected demand or production difficulties that might extend lead times. Also, products may not be available to us in quantities sufficient to meet our customer demand. Any interruption in supply and/or consistency of these products may adversely impact our ability to deliver products to our customers, may harm our relationships and reputation with our customers, and may have a material adverse effect on our business, results of operations and financial condition. Interruptions in supply or consistency of products could arise for a number of reasons, including but not limited to economic and civil unrest, epidemics/pandemics, such as coronavirus (COVID-19), embargoes, and sanctions.
A significant percentage of our revenue is dependent on sales of products from a relatively small number of key suppliers, and a decline in sales of products from these suppliers could materially harm our business.
A significant percentage of our revenue is dependent on sales of products, primarily vaporizers and related components, that we purchase from a small number of key suppliers, including JUUL Labs, PAX Labs, Grenco Science, and Storz & Bickel. For example, products manufactured by JUUL Labs represented approximately 8.8% and 38.6% of our net sales in the years ended December 31, 2020 and 2019, respectively, and products manufactured by PAX Labs represented
approximately 14.5% and 11.1% of our net sales in the years ended December 31, 2020 and 2019, respectively. Products manufactured by Grenco Science represented approximately 13.5% and 6.3% of our net sales in the years ended December 31, 2020 and 2019, respectively, and products manufactured by Storz & Bickel represented approximately 12.7% and 7.4% of our net sales in the years ended December 31, 2020 and 2019, respectively. A decline in sales of any of our key suppliers’ products, whether due to decreases in supply of, or demand for, their products, termination of our agreements with them, regulatory actions or otherwise, could have a material adverse impact on our sales and earnings and adversely affect our business.
The FDA has and may continue to implement regulations that significantly limit our ability to sell certain types of ENDS products. Additional regulatory actions may further impact our ability to sell these products in the United States or online.
Any regulatory action by the FDA that adversely affects the sale or distribution of Electronic Nicotine Delivery Systems ("ENDS") products may have a material adverse effect on our business, results of operations and financial condition.
For many years, ENDS in general, and ENDS produced by JUUL Labs in particular, have been subject to intense regulatory scrutiny at all levels of government. Dating back to at least 2018, the FDA has undertaken a number of initiatives designed to limit the appeal and availability of ENDS to minors. These actions include crackdowns on retailers identified as having sold ENDS to minors, among other steps. As a result of public pressure, JUUL Labs took numerous steps to address regulators' concerns, including deleting social media accounts and ceasing the sale of certain flavored JUUL products.
On December 20, 2019, the President signed legislation to amend the Federal Food, Drug, and Cosmetic Act (“FFDCA”), and raise the federal minimum age of sale of tobacco products (including ENDS products) from 18 to 21 years. Although many states had already established a minimum age of 21 years, our sales could be adversely impacted by this change in federal law.
In January 2020, FDA issued a guidance document titled Enforcement Priorities for Electronic Nicotine Delivery Systems ("ENDS") and Other Deemed Products on the Market Without Premarket Authorization (“ENDS Enforcement Guidance”). According to the ENDS Enforcement Guidance, FDA intends to prioritize enforcement against (1) flavored, cartridge-based ENDS products (except tobacco or menthol flavored products), (2) all other ENDS products for which the manufacturer has or is failing to take adequate measures to prevent minors’ access, and (3) any ENDS product targeted to minors or whose marketing is likely to promote use by minors. FDA also intends to prioritize any ENDS product offered for sale after May 12, 2020 for which the manufacturer has not submitted a premarket application. As discussed below, this date was later shifted to September 9, 2020. The FDA has taken actions against manufacturers that have continued to market ENDS without submitting a premarket authorization application.
The ENDS Enforcement Guidance had the effect of prohibiting the sale of certain products we sell, including mint flavored products from JUUL Labs and other flavored ENDS. We expect that our sales will be adversely impacted by this prohibition. Products impacted by the ENDS Enforcement Guidance represented less than 0.1% and 17.8% of our net sales for the years ended December 31, 2020 and 2019, respectively.
In February 2020, FDA published a notice seeking data and information related to the use of vaping products associated with recent lung injuries. FDA seeks information relating to product design and ways to prevent consumers from modifying or adding substances to these products that are not intended by the manufacturers. The notice states that FDA may use the information in future rule making, review of premarket authorization applications, or other regulatory activity. The notice further states that FDA has not found one product or substance implicated in all of the cases of injury, and that FDA is following all potential leads and will take appropriate actions as additional facts emerge. The FDA's actions resulting from this request for information could adversely affect our sales of ENDS products and may have a material adverse effect on our business, results of operations and financial condition.
There is uncertainty related to the regulation of vaporization products and certain other consumption accessories. Increased regulatory compliance burdens could have a material adverse impact on our business development efforts and our operations.
United States
There is uncertainty regarding whether, in what circumstances, how and when the FDA will seek to enforce the tobacco-related provisions of the FFDCA relative to vaporizer hardware and accessories that can be used to vaporize cannabis and other material, including electronic cigarettes, rolling papers and glassware, in light of the potential for dual use with tobacco.
Through amendments to the FFDCA, the Tobacco Control Act established, by statute, that the FDA has oversight over specific types of tobacco products (cigarettes, cigarette tobacco, roll-your-own (“RYO”) tobacco, and smokeless tobacco) and granted the FDA the authority to “deem” other types of tobacco products as subject to the statutory requirements. In addition to establishing authority, defining key terminology, and setting adulteration and misbranding standards, the Tobacco Control Act
established FDA’s authority over tobacco products in a number of areas such as: submission of health information to the FDA; registration with the FDA; premarket authorization requirements; good manufacturing practice requirements; tobacco product standards; notification, recall, corrections, and removals; records and reports; marketing considerations and restrictions; post-market surveillance and studies; labeling and warnings; and recordkeeping and tracking.
In a final rule effective August 8, 2016 (“Deeming Rule”), the FDA deemed all products that meet the Tobacco Control Act’s definition of “tobacco product,” including components and parts but excluding accessories, to be subject to the tobacco control requirements of the FFDCA and the FDA’s implementing regulations. Accordingly, as of the Deeming Rule’s effective date, deemed tobacco products that are “new” (i.e., those that were not commercially marketed in the United States as of February 15, 2007) are subject to the premarket authorization requirements. Deemed new tobacco products that remain on the market without authorization are marketed unlawfully.
Deemed new tobacco products include, among other things: products such as electronic cigarettes, electronic cigars, electronic hookahs, vape pens, vaporizers and e-liquids and their components or parts (such as tanks, coils and batteries) (“ENDS”). The FDA’s interpretation of components and parts of a tobacco product includes any assembly of materials intended or reasonably expected to be used with or for the human consumption of a tobacco product. In a 2017 decision of the D.C. Circuit court, the court upheld the FDA’s authority to regulate ENDS even though they do not actually contain tobacco, and even if the products could be used with nicotine-free e-liquids.
The Tobacco Control Act and FDA’s implementation of regulations require regulatory approvals before certain products may be sold and restrict the way tobacco product manufacturers, retailers, and distributors can advertise and promote tobacco products, including a prohibition against free samples or the use of vending machines, requirements for presentation of warning information, and age verification of purchasers.
Newly-deemed tobacco products are also subject to the other requirements of the Tobacco Control Act, such as that they not be adulterated or misbranded. The FDA has been directed under the Tobacco Control Act to establish specific good manufacturing practice (“GMP”) regulations for tobacco products, and could do so in the future, which could have a material adverse impact on the ability of some of our suppliers to manufacture, and the cost to manufacture, certain of our products. Even in the absence of specific GMP regulations, a facility’s failure to maintain sanitary conditions or to prevent contamination of products could result in the FDA deeming the products produced there adulterated.
The FDA has announced its intention to take enforcement measures related to ENDS products offered for sale after September 9, 2020 for which the manufacturers had not submitted a PMTA. Following that date, the FDA did in fact take actions against certain manufacturers of ENDS products for which a PMTA had not been submitted. Accordingly, and in light of the laws noted above, premarket authorizations will be necessary for us to continue our distribution of certain vaporizer hardware and accessories that meet the FDA's definition of ENDS. While we do not believe vaporizers intended for use with non-tobacco substances meet the FDA's definition of ENDS, it is possible that the FDA could require premarket authorization for such products.
Our suppliers who make vaporizers subject to FDA regulation must timely file applications for the appropriate authorizations so that we may continue selling their products in the United States. We have no control over the content of those applications, and we have no assurances that the outcome of the FDA’s review will result in authorization of the marketing of these products. If the FDA establishes or applies review standards or processes that our suppliers are unable or unwilling to comply with, our business, results of operations, financial condition and prospects would be adversely affected.
The anticipated costs to our suppliers of complying with future FDA regulations will be dependent on the rules issued by the FDA (which have yet to be issued), the timing and clarity of any new rules or guidance documents accompanying these rules, the reliability and simplicity (or complexity) of the electronic systems utilized by the FDA for information and reports to be submitted, and the details required by the FDA for such information and reports with respect to each regulated product. Any failure to comply with existing or new FDA regulatory requirements could result in significant financial penalties to us or our suppliers, which could ultimately have a material adverse effect on our business, results of operations, financial condition and ability to market and sell our products. Compliance and related costs could be substantial and could significantly increase the costs of operating in the vaporization products and certain other consumption accessories markets.
In addition, failure to comply with the Tobacco Control Act and with FDA regulatory requirements could result in litigation, criminal convictions or significant financial penalties and could impair our ability to market and sell some of our vaporizer products. At present, we are not able to predict whether the Tobacco Control Act will impact our business to a greater degree than competitors in the industry, thus affecting our competitive position.
Additionally, as discussed elsewhere in these Risk Factors and under the heading Regulatory Developments, the Consolidated Appropriations Act, 2021 expanded the range of products encompassed by the Prevent All Cigarette Trafficking
Act to include ENDS. This development could severely restrict our ability to ship many of the products we sell, as well as place costly regulatory burdens on such shipments.
At the state level, over 25 states have implemented statewide regulations that prohibit vaping in public places. As discussed elsewhere in these Risk Factors and under the heading Regulatory Developments, a number of states and cities have also implemented bans or restrictions on the sale of flavored tobacco products, including vaping liquids and menthol cigarettes. There may, in the future, also be increased regulation of additives in smokeless products and internet sales of vaporization products and certain other consumption accessories. The application of either or both of these federal laws, and of any new laws or regulations which may be adopted in the future at a state, provincial or local level, to vaporization products, consumption accessories or such additives could result in additional expenses and require us to change our advertising and labeling, and methods of marketing and distribution of our products, any of which could have a material adverse effect on our business, results of operations and financial condition.
Canada
On May 23, 2018, the Tobacco and Vaping Products Act (“TVPA”) became effective, and now governs the manufacture, sale, labeling and promotion of vaping products sold in Canada. The TVPA replaced the former Tobacco Act (Canada) and established a legislative framework that applies to vaping products, whether or not they contain nicotine. While the TVPA prescribes high-level requirements in relation to vaping products, the Government of Canada has yet to implement regulations the full set of regulations that will ultimately address the standards, testing methods, reporting requirements, packaging and labeling requirements, and other obligations with which vaping products will be required to comply. Accordingly, absent any such regulations, there is a lack of visibility as to the specific compliance regime that will apply to vaping products in the future. As such, there can be no assurance that we will initially be in total compliance, remain competitive, or financially able to meet future requirements administered pursuant to the TVPA.
Prior to the TVPA becoming effective, Health Canada had taken the position that electronic smoking products (i.e., electronic products for the vaporization and administration of inhaled doses of nicotine, including electronic cigarettes, cigars, cigarillos and pipes, as well as cartridges of nicotine solutions and related products) fell within the scope of the Food and Drugs Act (Canada) (“Food and Drugs Act”). Vaping products with therapeutic or health-related claims are subject to the Food and Drugs Act and related regulations.
It is not presently clear what implications the enactment of the TVPA will have for Health Canada’s role in authorizing vaping products, or on the degree to which it will remain subject to the provisions of Food and Drugs Act. Until regulations are published and enacted pursuant to the TVPA, a significant degree of uncertainty will remain with respect to compliance landscape for vaping products.
On December 21, 2019, Health Canada issued a Regulatory Impact Analysis Statement titled “Vaping Products Promotion Regulations.” The Impact Analysis addressed two proposed new regulations that would place stricter limits on the advertising and promotion of nicotine vaping products and make health warnings on nicotine vaping products mandatory (the “Proposed Regulations”). The Proposed Regulations would: (1) prohibit the promotion of nicotine vaping products and nicotine vaping product-related brand elements by means of advertising that is done in a manner that can be seen or heard by youth, including the display of nicotine vaping products a points of sale where can be seen by youth; and (2) require that all nicotine vaping advertising convey a health warning about the health hazards of nicotine vaping product use.
In the wake of these proposed regulations and additional pressure in both the United States and Canada, JUUL Labs confirmed on January 14, 2020, that it had sent a letter to Canadian retailers outlining a plan to stop selling its mango, vanilla, fruit, and cucumber pods on a temporary basis while allowing retailers to sell remaining inventory. This hold continues. We expect that our sales will be adversely impacted by JUUL Lab’s decision regarding the sales of flavored JUUL products.
On July 1, 2020, Health Canada’s “Vaping Products Labeling and Packaging Regulations” (the “VPLPR”) came into effect; requiring (1) all vaping products containing nicotine to display a standardized nicotine concentration statement and health warning about the addictiveness of nicotine; (2) products containing nicotine to be packaged in child-resistant containers and display a toxicity warning and first aid treatment statement; and (3) the display of a list of ingredients contained in the vaping substances, regardless of nicotine content. On July 14, 2020, Health Canada issued a guidance document on vaping products titled, “Industry Guide to vaping products subject to the Canada Consumer Product Safety Act” (the “CCPA Guidance”). The CCPA Guidance provided clarity on requirements under the Canada Consumer Product Safety Act (“CCPSA”) for vaping products that are manufactured, imported, advertised, or sold in Canada. The CCPA Guidance provided clarity on the requirements of the VPLPR and the authority of the CCPSA to address safety issues posed by a vaping product not marketed for therapeutic use or by a cannabis accessory (such as a vaporizer represented to be used in the consumption of cannabis) not marketed for a therapeutic use.
In addition to federal regulations, several provinces, including Alberta, British Columbia, Nova Scotia, Ontario, Prince Edward Island (“PEI”), Quebec, and Saskatchewan, have passed regulations fully restricting or limiting the advertising and sales of certain types of nicotine vaping products. Notably, in Prince Edward Island, as of March 1, 2020, the minimum age for purchasing nicotine products increased to age 21, and on August 11, 2020, PEI adopted a regulation to ban the sale of all flavored vaping products, effective March 1, 2021. Other provinces continue to review the prospect of adopting new regulations addressing nicotine vaping products.
In addition to the federal level and provincial regulations noted above, on December 18, 2020, Health Canada proposed regulations that would lower the allowable nicotine concentration in nicotine products from 66mg/ml to 20 mg/ml, mirroring the cap in the European Union. Health Canada provided a 75-day public consultation on the proposed new regulations, which ends on March 4, 2021. In addition, Health Canada continues to consider new rules governing flavored vaping products, which could include restricting the use of flavor in nicotine products. The Council of Chief Medical Officers of Health has also issued a statement supporting federal action to create national consistency and provided recommendations for individual provinces and territories.
These developments, together with the passed and proposed federal and provincial regulations may have a material adverse effect on our business, results of operations, and financial condition.
Europe
Throughout Europe, several countries’ laws implementing the European Union Tobacco Products Directive (“TPD”) impose strict regulations on the approval, sale, and advertising of e-cigarettes. While we do not sell or market products that we believe fall within the definition of e-cigarettes in Europe, if vaporization products we sell are found to fall within the scope of laws implementing the TPD, we would be unable to continue selling those products in certain countries, which may have a material adverse effect on our business, results of operations, and financial condition.
We may be unable to identify or contract with new suppliers in the event of a disruption to our supply.
In the event of a disruption to our supply of products, we would have to identify new suppliers that can meet our needs. Such a disruption may occur for many reasons, including but not limited to the current COVID-19 pandemic. Only a limited number of suppliers may have the ability to produce certain products we sell at the volumes we need, and it could be costly or time-consuming to locate and approve such alternative sources. Moreover, it may be difficult or costly to find suppliers to produce small volumes of products in the event we are looking only to supplement our current supply as suppliers may impose minimum order requirements. In addition, we may be unable to negotiate pricing or other terms with our existing or new suppliers as favorable as those we currently enjoy. We cannot guarantee that a failure to adequately replace or supplement our existing suppliers would not have a material adverse effect on our business, results of operations and financial condition.
Demand for the products we distribute could decrease if the trend of our suppliers selling products directly to consumers continues or accelerates.
Retailers and consumers of vaporization products and consumption accessories have historically purchased certain amounts of these products directly from suppliers. Recently, direct to consumer sales of vaporization products and consumption accessories have accelerated, consistent with broader sales trends. If our customers were to increase their purchases of products directly from suppliers, or if suppliers further increase their efforts to sell such products directly to consumers, we could experience a significant decrease in our business, results of operations and financial condition. These, or other developments that remove us from, or limit our role in, the distribution chain, may harm our competitive position in the marketplace and reduce our sales and earnings and adversely affect our business.
We are vulnerable to third-party transportation risks, including governmental laws and common carriers' policies that prevent the shipment of the types of products we sell.
We depend on fast and efficient shipping services to distribute our products. Any prolonged disruption of these services may have a material adverse effect on our business, financial condition and results of operations. Rising costs associated with transportation services used by us to receive or deliver our products, including tariffs, as well as delays as a results of factors outside of our control, including the COVID-19 pandemic, may also have a material adverse effect on our business, financial condition and results of operations.
The Consolidated Appropriations Act, 2021, which was signed into law on December 27, 2020, contains provisions that prohibit the mailing of ENDS through the United States Postal Service (“USPS”) and place certain regulatory requirements on shipment of ENDS through other carriers. Certain private carriers, including UPS and FedEx, also have policies restricting or prohibiting the shipment of many vaporization products we sell. If a substantial volume the products we carry cannot be shipped by the USPS or private carriers, or we must comply with burdensome policies and regulations, our shipping costs could
increase materially and we could lose our ability to deliver products to customers in a timely and economical matter. Additionally, rising costs associated with transportation services used by us to receive or deliver our products (including tariffs) and prohibitions on the use of certain shipping services for specified products, may also have a material adverse effect on our business, financial condition and results of operations.
We do not have long-term agreements or guaranteed price or delivery arrangements with most of our suppliers. The loss of a significant supplier would require us to rely more heavily on our other existing suppliers or to develop relationships with new suppliers. Such a loss may have an adverse effect on our product offerings and our business.
While we have exclusive, and non-exclusive, long-term distribution agreements with certain of our suppliers, consistent with industry practice, we do not have guaranteed price or delivery arrangements with most of our suppliers. We generally make our purchases through purchase orders. As a result, we have experienced and may in the future experience inventory shortages or price increases on certain products. Furthermore, our industry occasionally experiences significant product supply shortages, and we sometimes experience customer order backlogs due to the inability of certain suppliers to make available to us certain products as needed. We cannot provide assurances that suppliers will maintain an adequate inventory of products to fulfill our orders on a timely basis, or at all, or that we will be able to obtain particular products on favorable terms, or at all. Additionally, we cannot provide assurances that product lines currently offered by suppliers will continue to be available to us. A decline in the supply or continued availability of the products of our suppliers, or a significant increase in the price of those products, could reduce our sales and negatively affect our operating results.
In addition, some of our suppliers have the ability to terminate their relationships with us at any time, or to decide to sell, or increase their sales of, their products through other resellers or channels. Although we believe there are numerous suppliers with the capacity to supply the products we distribute, the loss of one or more of our major suppliers could have an adverse effect on our product offerings and our business. Such a loss would require us to rely more heavily on our other existing suppliers, develop relationships with new suppliers or undertake our own manufacturing, which may cause us to pay higher prices for products due to, among other things, a loss of volume discount benefits currently obtained from our major suppliers. Any termination, interruption or adverse modification of our relationship with a key supplier or a significant number of other suppliers would likely adversely affect our operating income, cash flow and future prospects.
If we fail to maintain proper inventory levels, our business could be harmed.
We purchase key products from suppliers prior to the time we receive purchase orders from customers. We do this to minimize purchasing costs, the time necessary to fill customer orders, and the risk of non-delivery. However, we may be unable to sell the products we have purchased in advance. Inventory levels in excess of customer demand have previously and may in the future, result in inventory write-downs, and the sale of excess inventory at discounted prices could significantly impair our brand image and have a material adverse effect on our business, results of operations and financial condition. Conversely, if we underestimate demand for our products or if we fail to acquire the products that we require at the time we need them, we may experience inventory shortages. Inventory shortages might delay shipments to customers, reduce revenue, negatively impact customer relationships and diminish brand loyalty, which in turn could have a material adverse effect on our business, results of operations and financial condition.
Certain of our suppliers provide us with incentives and other assistance that reduce our operating costs, and any decline in these incentives and other assistance could materially harm our operating results.
Certain of our suppliers, including PAX Labs and Storz & Bickel, provide us with trade credit or substantial incentives in the form of discounts, credits and cooperative advertising, among other benefits. We have agreements with many of our suppliers under which they provide us, or they have otherwise consistently provided us, with market price discounts to subsidize portions of our advertising, marketing and distribution costs based upon the amount of coverage we give to their respective products in our catalogs or other advertising and marketing mediums. Any termination or interruption of our relationships with one or more of these suppliers, or modification of the terms or discontinuance of our agreements or arrangements with these suppliers, could adversely affect our operating income and cash flow. For example, the incentives we receive from a particular supplier may be impacted by a number of events outside of our control, including acquisitions, divestitures, management changes or economic pressures affecting such supplier, any of which could materially affect or eliminate the incentives we receive from such supplier.
Our success is dependent in part upon our ability to distribute popular products from new suppliers, as well as the ability of our existing suppliers to develop and market products that meet changes in market demand or regulatory requirements.
Many of the products we sell are generally subject to rapid changes in marketplace demand and regulatory requirements. For example, recent laws and regulations have prohibited the sale of certain types of ENDS products that we previously sold. Our success is dependent, in part, upon the ability of our suppliers to develop and market products that meet these changes. Our success is also dependent on our ability to develop relationships with and sell products from new suppliers
that address these changes in market demand or regulatory requirements. To the extent products that address recent changes are not available to us, or are not available to us in sufficient quantities or on acceptable terms, we could encounter increased competition, which would likely adversely affect our business, results of operations and financial condition.
We may not be able to maintain existing supplier relationships or exclusive distributor status with our suppliers, which may affect our ability to offer a broad selection of products at competitive prices and negatively impact our results of operations.
We purchase products for resale both directly from manufacturers and, on occasion, from other sources, all of whom we consider our suppliers. We also maintain certain exclusive relationships with several of our suppliers, which provide us with exclusive rights to distribute their products in certain geographic areas or sales channels, preferred pricing, training, support, preferred access and other significant benefits. In some cases, suppliers require us to meet certain minimum standards in order to retain these qualifications and our exclusive distributor status, and in some instances, we have failed to achieve those minimum standards. If we do not maintain our existing relationships or exclusive distributor status, or if we fail to build new relationships with suppliers on acceptable terms, including our exclusive distribution rights, favorable pricing, manufacturer incentives or reseller qualifications, we may not be able to offer a broad selection of products or continue to offer products from these suppliers at competitive prices, or at all. From time to time, suppliers may be acquired by other companies, terminate our right to sell some or all of their products, modify or terminate our exclusive distributor or qualification status, change the applicable terms and conditions of sale or reduce or discontinue the incentives or supplier consideration that they offer us. Any termination or reduction of our exclusive distributor status with any of our major suppliers, or our failure to build new supplier relationships, could have a negative impact on our operating results. Further, some products may be subject to allocation by the supplier, which could limit the number of units of those products that are available to us and may adversely affect our operating results.
We do not have long-term contracts with most of our customers. The agreements that we do have generally do not commit our customers to any minimum purchase volume. The loss of a significant customer may have a material adverse effect on us.
Our customers generally place orders on an as-needed basis. Consistent with industry practice, we do not have long-term contracts with most of our customers, other than certain retail chains in Canada and certain state-licensed cannabis businesses in the United States. In addition, our agreements generally do not commit our customers to any minimum purchase volume. Accordingly, we are exposed to risks from potential adverse financial conditions in the vaporization products and consumption accessories industry, a potentially shifting legal landscape, the general economy, a competitive landscape, a changing technological landscape or changing customer needs or any other change that may affect the demand for our products. We cannot assure you that our customers will continue to place orders with us in similar volumes, on the same terms, or at all. Our customers may terminate their relationships with us or reduce their purchasing volume at any time. Our ten largest customers, in the aggregate, represented approximately 9.8% and 17.3% of our net sales for the years ended December 31, 2020 and 2019, respectively. The loss of a significant number of customers, or a substantial decrease in a significant customer’s orders, may have an adverse effect on our revenue.
Changes in our customer, product or competition mix could cause our product margin and results of operations to fluctuate.
From time to time, we may experience changes in our customer mix, our product mix or our competition mix. Changes in our customer mix may result from geographic expansion or contractions, legislative, regulatory or enforcement priority changes affecting the products we distribute, selling activities within current geographic markets and targeted selling activities to new customer sectors. Changes in our product mix may result from marketing activities to existing customers, the needs of existing and prospective customers and from regulatory and legislative changes. Changes in our competition mix may result from well-financed competitors entering into our business segment or existing competitors growing their operations. If customer demand for lower-margin products increases and demand for higher-margin products decreases, our business, results of operations and financial condition may suffer.
Because a majority of our revenues are derived from sales to consumers indirectly through third-party retailers who operate traditional brick-and-mortar locations, the shift of sales to more online retail business could harm our market share and our revenues in certain sectors.
Our current B2B model includes selling our products through third-party retailers. These third-party retailers operate physical brick-and-mortar locations to sell our product to consumers. The current shift in purchasing demographics due to to many factors, including the COVID-19 pandemic and the changing preferences of consumers who are moving from in-store purchases to online purchases creates the additional risks of our current revenue streams being impacted negatively and an overall decrease of market share.
Further, laws in some jurisdictions in which we operate could make collection of receivables difficult, time consuming or expensive. We generally do not require collateral in support of our trade receivables. While we maintain reserves for
expected credit losses, we cannot assure these reserves will be sufficient to meet write-offs of uncollectible receivables or that our losses from such receivables will be consistent with our historical performance. Significant write-offs may affect our business, results of operations and financial condition. As we begin selling our products indirectly through large retailers, customer credit risks will expand.
Our ability to distribute certain licensed brands and to use or license certain trademarks may be terminated or not renewed.
We are reliant upon brand recognition in the markets in which we compete, as the industry is characterized by a high degree of brand loyalty and a reluctance of consumers to switch to substitute or unrecognizable brands. Some of the brands we distribute and the trademarks under which products are sold are licensed for a fixed period of time with regard to specified markets.
In the event that the licenses to use the brand names and trademarks for the products we distribute are terminated or are not renewed after the end of the term, there is no guarantee we or our suppliers will be able to find suitable replacement brands or trademarks, or that if a replacement is found, that it will be on favorable terms. Any loss in brand-name appeal to our existing customers as a result of the lapse or termination of our licenses or the licenses of our suppliers could have a material adverse effect on our business, results of operations and financial condition.
We may not be successful in maintaining the consumer brand recognition and loyalty of our products.
We compete in a market that relies on innovation and the ability to react to evolving consumer preferences. The vaporization products and consumption accessories industry, as well as the nicotine industries, are subject to changing consumer trends, demands and preferences. Therefore, products once favored may, over time, become disfavored by consumers or no longer perceived as the best option. Consumers in the vaporizer market have demonstrated a degree of brand loyalty, but suppliers must continue to adapt their products in order to maintain their status among customers as the market evolves. Our continued success depends in part on our ability and our supplier’s ability to continue to differentiate the brand names we represent, own or license and maintain similarly high levels of recognition with target consumers. Trends within the vaporization products and consumption accessories industry change often and our failure to anticipate, identify or react to changes in these trends could, among other things, lead to reduced demand for our products. Factors that have previously and may continue to affect consumer perception of our products include health trends and attention to health concerns associated with tobacco, nicotine, herbs, cannabis or other materials used with vaporizers, price-sensitivity in the presence of competitors’ products or substitute products and trends in favor of new vaporization products or technology consumption accessories products that are currently being researched and produced by participants in our industry. For example, in recent years, we have witnessed a shift in consumer purchases from vaporizers designed for dry herbs to those designed for liquids or wax type concentrates. A failure to react to similar trends in the future could enable our competitors to grow or establish their brands’ market share in these categories before we have a chance to respond.
Regulations have recently been and are likely to continue to be enacted in the future that would make it more difficult to appeal to consumers or to leverage the brands that we distribute, own or license. Furthermore, even if we are able to continue to distinguish our products, there can be no assurance that the sales, marketing and distribution efforts of our competitors will not be successful in persuading consumers of our products to switch to their products. Some of our competitors have greater access to resources than we do, which better positions them to conduct market research in relation to branding strategies or costly marketing campaigns. Any loss of consumer brand loyalty to our products or in our ability to effectively brand our products in a recognizable way will have a material effect on our ability to continue to sell our products and maintain our market share, which could have a material adverse effect on our business, results of operations and financial condition.
We may not be able to establish sustainable relationships with large retailers or regional or national chains.
In connection with efforts to enter new sales channels, including large retailers and chains, we may have to pay slotting fees based on the number of stores in which our products will be carried. We may not be able to develop these relationships or continue to maintain relationships with large retailers or national chains. Our inability to develop and sustain relationships with large retailers and chains may impede our ability to develop brand and product recognition and increase sales volume and, ultimately, require us to continue to rely on local and more fragmented sales channels, which may have a material adverse effect on our business, results of operations and financial condition. In addition, if we are unable to develop or maintain relationships with large retailers and national chains and such large retailers or chains take market share from the smaller local and more fragmented sales channels, our business, results of operations and financial condition will be adversely impacted.
New products face intense media attention and public pressure.
Many of our vaporizers and other products are new to the marketplace. Since their introduction, certain members of the media, politicians, government regulators and advocacy groups, including independent doctors, have called for and driven the adoption of stringent regulation of the sale of certain products and in some cases, an outright ban of such products pending increased regulatory review and a further demonstration of safety. For example, local and state governments have banned
certain types of vaporization products, such as those containing flavored liquid nicotine and flavored hemp-derived CBD. Additional bans of this type would likely have the effect of terminating our sales and marketing efforts of certain products in jurisdictions in which we may currently market or have plans to market such products. Such bans would also likely cause public confusion as to which products are the subject of bans, which confusion could also have a material adverse effect on our business, results of operations and financial condition.
Our success depends, in part, on the quality and safety of our products, as well as the perception of quality and safety in the vaporization products and consumption accessories industry generally.
Our success depends, in part, on the quality and safety of the products we sell, including manufacturing issues, health concerns about the substances consumed using the products we sell, and unforeseen product misuse. Even a single incident of product defect or misuse, whether relating to products sold by us or just to our industry generally, could result in significant harm to our reputation. For example, incidents of EVALI have, by some metrics, negatively impacted demand for vaporizers. If any of our products are found to be, or are perceived to be, defective or unsafe, or if they otherwise fail to meet our customers’ standards, our relationship with our customers could suffer, our reputation or the appeal of our brands could be diminished, and we could lose market share and/or become subject to liability claims, any of which could result in a material adverse effect on our business, results of operations and financial condition.
Damage to our reputation, or that of any of our key suppliers or their brands, could affect our business performance.
The success of our business depends in part upon the positive image that consumers have of the third-party brands we distribute. Incidents, publicity or events arising accidentally or through deliberate third-party action that harm the integrity or consumer support of our products could affect the demand for our products. Unfavorable media, whether accurate or not, related to our industry, to us, to our customers, or to the products we sell could negatively affect our corporate reputation, stock price, ability to attract high-quality talent, or the performance of our business. For example, JUUL Labs has been the subject of significant negative publicity. Additional negative publicity or commentary on social media outlets also could cause consumers to react rapidly by avoiding our products and brands or by choosing brands offered by our competitors, which could have a material adverse effect on our business, results of operations and financial condition.
We are subject to substantial and increasing regulation regarding the tobacco industry.
The tobacco industry, of which some of our vaporizer products are deemed to be a part, has been under public scrutiny for many years. Industry critics include special interest groups, and many legislators and regulators at the state, federal and provincial levels. A wide variety of federal, state or provincial and local laws limit the advertising, sale and use of tobacco and these laws have proliferated in recent years. Together with changing public attitudes towards tobacco and nicotine consumption, the constant expansion of regulations has been a major cause of the overall decline in the consumption of tobacco products since the early 1970s. These regulations relate to, among other things, the importation of tobacco products and shipping throughout the North American market, increases in the minimum age to purchase tobacco products, imposition of taxes, sampling and advertising bans or restrictions, flavor bans or restrictions, ingredient and constituent disclosure requirements and media campaigns and restrictions on where tobacco can be consumed. Additional restrictions may be legislatively imposed or agreed to in the future. These limitations may make it difficult for us to maintain the sales levels of our regulated vaporizer products.
Moreover, the current trend is toward increasing regulation of the tobacco industry, which is likely to differ between the various U.S. states and Canadian provinces in which we currently conduct business. The continued promulgation of extensive and inconsistent regulation by multiple states or provinces and at different governmental levels could prove to be particularly disruptive to our business as well, as we may be unable to accommodate such regulations in a cost-effective manner that will allow us to continue to compete in an economically-viable way. Tobacco regulations are often introduced without the tobacco industry’s input and have been a significant reason behind reduced sales volumes and increased illicit trade in the tobacco industry. Such regulations also may impact our sales volumes to the extent they apply to the vaporizer products we sell.
On June 22, 2009, the Family Smoking Prevention and Tobacco Control Act (the “Tobacco Control Act”) amended the FFDCA to authorize the FDA to regulate the tobacco industry and amended the Federal Cigarette Labeling and Advertising Act, which governs how cigarettes can be advertised and marketed. In addition to the FDA, we are subject to regulation by numerous other federal agencies, including the Federal Trade Commission, the Alcohol and Tobacco Tax and Trade Bureau, the Federal Communications Commission, the U.S. Environmental Protection Agency, the U.S. Department of Agriculture, U.S. Customs and Border Protection and the U.S. Center for Disease Control and Prevention’s Office on Smoking and Health. There have also been adverse legislative and political decisions and other unfavorable developments concerning cigarette smoking and the tobacco industry, which have received widespread public attention. There can be no assurance as to the ultimate content, timing or effect of any regulation of tobacco or nicotine products by governmental bodies, nor can there be any assurance that potential corresponding declines in demand resulting from negative media attention would not have a material adverse effect on our business, results of operations and financial condition.
Some of the products we sell contain nicotine, which is considered to be a highly-addictive substance, or other chemicals that some jurisdictions have determined to cause cancer and birth defects or other reproductive harm.
Some of our products, like the JUUL nicotine vaporizers, contain nicotine, a chemical that is considered to be highly addictive. The Tobacco Control Act empowers the FDA to regulate the amount of nicotine found in tobacco products (including vaporizers), but not to require the reduction of nicotine yields of a tobacco product to zero ; similar legislation in Canada empowers the Canadian government and provincial governments to limit the amount of nicotine in tobacco and vaporizer products. . In addition, the State of California has determined that some chemicals found in certain vaporizers, as well as materials frequently consumed by using vaporizers (such as cannabis), cause cancer and birth defects or other reproductive harm. New federal, state or provincial regulations, whether of nicotine levels or other product attributes, may require us to recall and/or discontinue certain of the products we sell, which may have a material adverse effect on our ability to market our products and have a material adverse effect on our business, results of operations and financial condition.
Significant increases in state and local regulation of our vaporizer products have been proposed and enacted, and are likely to continue to be proposed and enacted in numerous jurisdictions.
As discussed under the heading "Regulatory Developments" above, there has been increasing activity on the state, provincial and local levels with respect to scrutiny of vaporizer products. State and local governmental bodies across the United States have indicated that vaporization products and certain other consumption accessories may become subject to new laws and regulations at the state and local levels. For example, in January 2015, the California Department of Health declared electronic cigarettes and certain other vaporizer products a health threat that should be strictly regulated like tobacco products. Further, many states and cities have enacted regulations that require retailers to obtain a tobacco retail license in order to sell electronic cigarettes and vaporizer products. Many states, provinces and some cities have passed laws restricting the sale of electronic cigarettes and certain other vaporizer products. If one or more states or provinces from which we generate or anticipate generating significant sales of vaporizer products bring actions that prevent us from selling certain or all of our vaporizer products, we would be required to cease sales and distribution of certain products to those states, which could have a material adverse effect on our business, results of operations and financial condition. Additionally, if one or more states or provinces from which we generate or anticipate generating significant sales of vaporizer products bring actions that require us to obtain certain licenses, approvals or permits, and if we are not able to obtain the necessary licenses, approvals or permits for financial reasons or otherwise and/or any such license, approval or permit is determined to be overly burdensome to us, then we may be required to cease sales and distribution of our products to those states, which could have a material adverse effect on our business, results of operations and financial condition.
Certain states, provinces and cities have already restricted the use of electronic cigarettes and vaporizer products in smoke free venues. Additional city, state, provincial or federal regulators, municipalities, local governments and private industry may enact rules and regulations restricting the use of electronic cigarettes and vaporizer products in those same places where cigarettes cannot be smoked. Because of these restrictions, our customers may reduce or otherwise cease using our vaporization products or certain other consumption accessories, which could have a material adverse effect on our business, results of operations and financial condition.
Certain provinces of Canada have passed or propose to pass legislation which will restrict the extent to which e-cigarettes, e-liquid and other vaping products may be displayed or sold. Additionally, Canadian laws require health warnings to be placed on certain vaporizer products, which could reduce the appeal of these products. These regulations and future regulations could have a material adverse effect on our business, results of operations and financial condition.
Based on regulations surrounding health-related concerns related to the use of some of our vaporizer products, especially e-cigarettes and those used for tobacco and nicotine intake, possible new or increased taxes by government entities intended to reduce use of our products or to raise revenue, additional governmental regulations concerning the marketing, labeling, packaging or sale of some of our products, negative publicity resulting from actual or threatened legal actions against us or other companies in our industry, all may reduce demand for, or increase the cost of, certain of our products, which could adversely affect our profitability and ultimate success.
Public health epidemics, pandemics or outbreaks, including the recent COVID-19 pandemic, could materially and adversely affect our business.
Public health epidemics, pandemics or outbreaks, and the resulting business or economic disruptions resulting therefrom, could adversely impact our business as well as our ability to raise capital. In December 2019, COVID-19 was identified in Wuhan, China. The virus has been declared a pandemic by the World Health Organization. The impact of this pandemic has been and will likely continue to be extensive in many aspects of society, which has resulted in and will likely continue to result in significant disruptions to the global economy, as well as businesses and capital markets around the world. The extent to which COVID-19 impacts our business will depend on future developments, which are highly uncertain and
cannot be predicted with confidence, including the duration, spread and intensity of the pandemic, the timing and effectiveness of vaccines and other treatments, possible resurgences in COVID-19 cases, and the duration of government measures to mitigate the pandemic, all of which are uncertain and difficult to predict. COVID-19 has and will likely continue to result in social, economic and labor instability in the countries in which we or the third parties with whom we engage operate. While we cannot presently predict the scope and severity of any potential business shutdowns or disruptions, if we or any of the third parties with whom we engage, including the suppliers, manufacturers and other third parties in our global supply chain, were to experience shutdowns or other significant business disruptions, our ability to conduct our business in the manner presently planned could be materially and negatively impacted. For example, our Higher Standards stores in California and New York were closed for several months in 2020 as a result of COVID-19.
The COVID-19 pandemic has also caused, and is likely to continue to cause, severe economic, market and other disruptions worldwide. We cannot assure you that conditions in the bank lending, capital and other financial markets will not deteriorate as a result of the pandemic, or that our access to capital and other sources of funding will not become constrained, which could adversely affect the availability and terms of future borrowings, renewals or refinancings.
Adverse U.S., Canadian and global economic conditions could materially and adversely our business, prospects, results of operations, financial condition or cash flows.
Our business and operations are sensitive to global economic conditions. These conditions include interest rates, energy costs, inflation, international trade relationships, recession, fluctuations in debt and equity capital markets and the general condition of the U.S., Canadian and global economy. A material decline in the economic conditions affecting consumers, such as the recent downturn in the global economy due to COVID-19, which cause a reduction in disposable income for the average consumer, may change consumption patterns, and may result in a reduction in spending on vaporization products and consumption accessories or a switch to cheaper products or products obtained through illicit channels. Vaporizer, e-cigarette and e-liquid products are relatively new to the market and may be regarded by consumers as a novelty item and expendable. As such, demand for our vaporizer products may be particularly sensitive to economic conditions such as inflation, recession, high energy costs, unemployment, changes in interest rates and money supply, changes in the political environment and other factors beyond our control, any combination of which could result in a material adverse effect on our business, results of operations and financial condition.
Our business depends partly on continued purchases by businesses and individuals selling or using cannabis pursuant to state laws in the United States or Canadian and provincial laws.
Because some of our B2C customers use some of the items that we sell to consume cannabis and some of our B2B customers operate in the legal national and state cannabis industry, our business depends partly on federal, state, provincial and local laws, regulations, guidelines and enforcement pertaining to cannabis. In both the United States and Canada, those factors are in flux.
United States
Currently, in the United States, 38 states and the District of Columbia permit some form of cannabis cultivation, sales, and use for certain medical purposes (“medical states”). Fifteen of those states and the District of Columbia have also legalized cannabis for adults for non-medical purposes (sometime referred to as recreational use). Several medical states may extend legalization to adult use.
States’ cannabis programs have proliferated and grown even though the cultivation, sale and possession of cannabis is considered illegal under U.S. federal law. Under the CSA, cannabis is a Schedule I drug, meaning that the Drug Enforcement Administration recognizes no accepted medical use for cannabis, and the substance is considered illegal under federal law.
In an effort to provide guidance to U.S. Attorneys’ offices regarding the enforcement priorities associated with cannabis in the United States, the U.S. Department of Justice (the “DOJ”) has issued a series of memoranda detailing its suggested enforcement approach. During the administration of former President Obama, each memorandum acknowledged the DOJ’s authority to enforce the CSA in the face of state laws, but noted that the DOJ was more committed to using its limited investigative and prosecutorial resources to address the most significant threats associated with cannabis in the most effective, consistent, and rational way.
On August 29, 2013, the DOJ issued what came to be called the “Cole Memorandum,” which gave U.S. Attorneys the discretion not to prosecute federal cannabis cases that were otherwise compliant with applicable state law that had legalized medical or adult-use cannabis and that have implemented strong regulatory systems to control the cultivation, production, and distribution of cannabis. The eight federal priorities were preventing:
•The distribution of cannabis to minors;
•Revenue from the sale of cannabis from going to criminal enterprises, gangs, and cartels;
•The diversion of cannabis from states where it is legal under state law in some form to other states;
•State-authorized cannabis activities from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity;
•Violence and the use of firearms in the cultivation and distribution of cannabis;
•Drugged driving and exacerbation of other adverse public health consequences associated with cannabis use;
•Growing cannabis on public lands and the attendant public safety and environmental dangers posed by cannabis production on public lands; and
•Cannabis possession or use on federal property.
Accordingly, the Cole Memorandum provided lawful cannabis-related enterprises a tacit federal go-ahead in states with legal cannabis programs, provided that the state had adopted and was enforcing strict regulations and oversight of the medical or adult-use cannabis program in accordance with the specific directives of the Cole Memorandum.
On January 4, 2018, Attorney General Jeff Sessions issued a memorandum that rescinded previous DOJ guidance on the state legal cannabis industry, including the Cole Memorandum. Attorney General Sessions wrote that the previous guidance on cannabis law enforcement was unnecessary, given the well-established principles governing federal prosecution that are already in place. As a result, federal prosecutors could and still can use their prosecutorial discretion to decide whether to prosecute even state-legal cannabis activities.
Since the Cole Memorandum was rescinded, however, U.S. Attorneys have refrained from prosecuting state law compliant marijuana businesses. Current Attorney General Merrick Garland during his confirmation hearings expressed that "It does not seem to me useful the use of limited resources that we have to be pursuing prosecutions in states that have legalized and are regulating the use of marijuana, either medically or otherwise."
Since December 2014, companies that are strictly complying with state medical cannabis laws have been protected against enforcement for that activity by an amendment (originally called the Rohrabacher-Blumenauer Amendment, now called the Joyce Amendment) to the Omnibus Spending Bill, which prevents federal prosecutors from using federal funds to impede the implementation of medical cannabis laws enacted at the state level. Federal courts have interpreted the provision to bar the DOJ from prosecuting any person or entity in strict compliance with state medical cannabis laws.
While the protection of the Joyce Amendment prevents prosecutions of state law compliant medical cannabis activities, it does not make cannabis legal. The protection of the Joyce Amendment depends on its continued inclusion in the federal omnibus spending bill, or in some other legislation, and entities’ strict compliance with the state medical cannabis laws. That protection has been extended through September 30, 2021 through recent appropriations bill. While industry observers expect Congress to extend the protection in future Omnibus Spending Bills, there can be no assurance that it will do so.
Although several cannabis law reform bills are pending in the U.S. Congress, passage of any of them and ultimately the President’s support and approval remain uncertain. Unless and until the U.S. Government changes the law with respect to cannabis, and particularly if Congress does not extend the protection of state medical cannabis programs, there is a risk that federal authorities could enforce current federal cannabis law. An increase in federal enforcement against companies licensed under state cannabis laws would negatively impact the state cannabis industries and, in turn, our revenues, profits, financial condition, and business model.
Canada
On April 13, 2017, the Government of Canada introduced Bill C-45, which proposed the enactment of the Cannabis Act to legalize and regulate access to cannabis. The Cannabis Act proposed a strict legal framework for controlling the production, distribution, sale and possession of medical and recreational adult-use cannabis in Canada. On June 21, 2018, the Government of Canada announced that Bill C-45, received Royal Assent. On July 11, 2018, the Government of Canada published the Cannabis Regulations under the Cannabis Act. The Cannabis Regulations provide more detail on the medical and recreational regulatory regimes for cannabis, including regarding licensing, physical security requirements, product practices, outdoor growing, security, packaging and labelling (including for cannabis accessories), cannabis-containing drugs, document retention requirements, reporting and disclosure requirements, the new access to cannabis for medical purposes regime and industrial hemp. The majority of the Cannabis Act and the Cannabis Regulations came into force on October 17, 2018; additional Cannabis Regulations took effect on October 17, 2017..
While the Cannabis Act provides for the regulation by the federal government of, among other things, the commercial cultivation and processing of cannabis for recreational purposes, it provides the provinces and territories of Canada with the authority to regulate with respect to the other aspects of recreational cannabis, such as distribution, sale, minimum age requirements, places where cannabis can be consumed, and a range of other matters.
The governments of every Canadian province and territory have implemented regulatory regimes for the distribution and sale of cannabis for recreational purposes. In most provinces and territories, the minimum age is 19 years old, except for Québec and Alberta, where the minimum age is 18. Certain provinces, such as Ontario, have legislation in place that restricts the packaging of vapor products and the manner in which vapor products are displayed or promoted in stores.
The Cannabis Act is a relatively new regime that has no close precedent in Canadian law. The effect of relevant governmental authorities’ administration, application and enforcement of their respective regulatory regimes and delays in obtaining, or failure to obtain, applicable regulatory approvals which may be required may significantly delay or impact the development of markets, products and sales initiatives and could have a material adverse effect on our business, financial condition and results of operations.
The federal and state regulatory landscape regarding products containing hemp-derived CBD and other cannabinoids is uncertain and evolving, and new or changing laws or regulations relating to hemp and hemp-derived products could have a material adverse effect on our business, financial condition and results of operations.
In December 2018, the U.S. government changed the legal status of hemp and its derivatives, including hemp-derived CBD and other cannabinoids. The 2018 Farm Bill, which was signed into law by President Trump on December 20, 2018 (Pub.L. 115-334), established a new framework for the regulation of hemp production (defined in the Farm Bill as Cannabis sativa L. with a THC concentration of not more than 0.3 percent on a dry weight basis) and extracts of hemp, including CBD. The law also removed hemp and extracts of hemp from the federal controlled substances schedules. The section of the Farm Bill establishing a framework for hemp production, however, makes clear explicitly that it does not affect or modify the United States Federal Food, Drug, and Cosmetic Act (the “FDCA”), section 351 of the Public Health Service Act (addressing the regulation of biological products), the authority of the Commissioner of the FDA under those laws, or the Commissioner’s authority to regulate hemp production and sale under those laws.
Since passage of the Farm Bill, the FDA has expressed multiple times its position that any cannabis product, whether derived from hemp or otherwise, marketed with a disease claim (e.g., a claim of therapeutic benefit or disease prevention) must be approved by the FDA for its intended use through one of the drug approval pathways prior to it being introduced into interstate commerce. The FDA has also repeatedly stated its position that introducing food or dietary supplements with added CBD (or THC), regardless of source, into interstate commerce is illegal under the FDCA. Although enforcement under the FDCA may be civil or criminal in nature, the FDA has thus far limited its recent enforcement against companies selling CBD products to warning letters alleging various violations of the FDCA, including that the products bear claims that render the products unapproved and misbranded new drugs, that CBD is excluded from the FDCA’s definition of “dietary supplement,” and that the FDCA prohibits the addition of CBD to food. The FDA also tested some of the products, and found that many did not contain the levels of CBD they claimed to contain, which could be the basis for a separate violation of the FDCA. In addition, some states have taken actions to restrict or prohibit the sale of CBD products under state law. The FDA has signaled that it will likely issue further guidance and/or issue regulations concerning CBD products, although the contents and timing of such guidance and regulations remain unknown.
We currently distribute products containing hemp-derived CBD and other cannabinoids. Although the Farm Bill removed hemp and its derivatives from the definition of “marijuana” under the CSA, uncertainties remain regarding the cultivation, sourcing, production and distribution of hemp and products containing hemp derivatives. Certain states prohibit the sale of all or certain types of products containing hemp. The laws and regulations of states that permit the sale of products containing hemp derivatives, such as CBD, impose various requirements, including requirements to obtain certain permits or licenses, related to the marketing, packaging, safety, and sale of products containing hemp derivatives. These laws and regulations are rapidly developing. We may have to quickly adapt our operations to comply with forthcoming and rapidly-shifting federal and state regulations. These regulations could require significant changes to our business, plans or operations concerning hemp-derived products, and could adversely affect our business, financial condition or results of operations. Additionally, while we believe our current operations with respect to hemp derived products such as CBD comply with existing federal and state laws relating to hemp and hemp-derived products in all material respects, legal proceedings alleging violations of such laws could have a material adverse effect on our business, financial condition and results of operations.
We are subject to legislative uncertainty that could slow or halt the legalization and use of cannabis, which could negatively affect our business.
Continued development of the cannabis industry is dependent upon continued legislative authorization of cannabis at the state level, as well as the U.S. government’s continued non-enforcement of federal cannabis laws against state-law-compliant cannabis businesses. Any number of factors could slow or halt progress in this area. Further, progress, while generally expected, is not assured. Well-funded interests, including businesses in the tobacco, alcohol beverage and the pharmaceutical industries, may have a strong economic opposition to the continued legalization of cannabis. The pharmaceutical industry, for example, is well funded with a strong and experienced lobby that eclipses the funding of the
cannabis movement. Any inroads legalization opponents could make in halting the impending cannabis industry could have a detrimental impact on our business. While there may be ample public support for legislative action, numerous factors impact the legislative process. Any one of those factors could slow or halt the continued legalization and use of cannabis, which would negatively impact our business.
While we believe that our business and sales do not violate the Federal Paraphernalia Law, legal proceedings alleging violations of such law or changes in such law or interpretations thereof could materially and adversely affect our business, financial condition or results of operations.
Under U.S. Code Title 21 Section 863 (the “Federal Paraphernalia Law”), the term “drug paraphernalia” means “any equipment, product or material of any kind which is primarily intended or designed for use in manufacturing, compounding, converting, concealing, producing, processing, preparing, injecting, ingesting, inhaling, or otherwise introducing into the human body a controlled substance.” That law exempts “(1) any person authorized by local, State, or Federal law to manufacture, possess, or distribute such items” and “(2) any item that, in the normal lawful course of business, is imported, exported, transported, or sold through the mail or by any other means, and traditionally intended for use with tobacco products, including any pipe, paper, or accessory.” Any nonexempt drug paraphernalia offered or sold by any person in violation of the Federal Paraphernalia Law can be subject to seizure and forfeiture upon the conviction of such person for such violation, and a convicted person can be subject to fines under the Federal Paraphernalia Law and even imprisonment.
We believe our sales do not violate the Federal Paraphernalia Law in any material respect. First, we understand that a substantial majority of the products we offer and sell were and are not primarily intended or designed for any purpose not permitted by the Federal Paraphernalia Law. Indeed, most of the manufacturers whose products we sell disclaim that the products are for use with cannabis. Second, we restrict the sale of certain products — those that may have been primarily intended or designed for use with cannabis — to comply with the Federal Paraphernalia Law’s exemption for sales authorized by state law. In particular, we (a) do not sell those products at all into the six states that have maintained complete or near complete cannabis prohibition and (b) limit the sale of those products to licensed cannabis businesses, such as dispensaries, cultivators, and manufacturers, in the nine states that authorize sales of cannabis paraphernalia only through state-licensed cannabis businesses. Third, we have been in business for many years without facing even threatened legal action under the Federal Paraphernalia Law.
While we believe that our business and sales are legally compliant with the Federal Paraphernalia Law in all material respects, any legal action commenced against us under such law could result in substantial costs and could have an adverse impact on our business, financial condition or results of operations. In addition, changes in cannabis laws or interpretations of such laws are difficult to predict, and could materially and adversely affect our business.
Officials of the U.S. Customs and Border Protection agency (“CBP”) have broad discretion regarding products imported into the United States, and the CBP has on occasion seized imported products on the basis that such products violate the Federal Paraphernalia Law. While we believe the products that we import do not violate such law, any such seizure of the products we sell could have a material adverse effect on our business operations or our results of operations.
Officials of the CBP have broad discretion regarding products imported into the United States. Individual shipments of imported products we distribute, as well as similar products, have been detained or seized by the CBP for a variety of reasons, including because the CBP officials inspecting the goods believed such goods were marketed as drug paraphernalia and therefore violated the Federal Paraphernalia Law. Although suppliers or distributors of such products have at times successfully contested such actions of the CBP, such challenges are costly and time consuming. While we would disagree with any conclusion of the CBP that our product sales violate the Federal Paraphernalia Law, we cannot give any assurance that the CBP will not take seize our imports, or that if the CBP seizes any of our goods that the CBP would not seek to impose penalties related to such imports. Should we elect to contest any such seizure, the costs of doing so could be substantial and there are no assurances we would prevail in a contested proceeding. Additionally, the cost and/or results of any such contest could adversely impact our business, financial condition or results of operations. Additionally, if the CBP fails to release seized products, we may no longer be able to ensure a sellable supply of some of our products, which could have a material adverse impact on our business, financial condition and results of operations.
Because our business is dependent, in part, upon continued market acceptance of cannabis by consumers, any negative trends could materially and adversely affect our business, financial conditions or results of operations.
We are dependent on public support, continued market acceptance and the proliferation of consumers in the legal cannabis markets. While we believe that the market and opportunity in the space continue to grow, we cannot predict the future growth rate or size of the market. Any downturns in, or negative outlooks on, the cannabis industry may materially and adversely affect our business and financial condition.
We and our customers may have difficulty accessing the service of banks, which may make it difficult for us and for them to sell our products.
Financial transactions involving proceeds generated by cannabis-related activities can form the basis for prosecution under the U.S. federal money laundering statutes, unlicensed money transmitter statutes and the U.S. Bank Secrecy Act. Guidance issued by FinCEN clarifies how financial institutions can provide services to cannabis-related businesses consistent with their obligations under the Bank Secrecy Act. Furthermore, since the rescission by U.S. Attorney General Jeff Sessions on January 4, 2018 of the Cole Memorandum, U.S. federal prosecutors have had greater discretion when determining whether to charge institutions or individuals with any of the financial crimes described above based upon cannabis-related activity. As a result, given these risks and their own related disclosure requirements, some banks remain hesitant to offer banking services to cannabis-related businesses. Consequently, those businesses involved in the cannabis industry continue to encounter difficulty establishing banking relationships. Indeed, we have been asked to close bank accounts due to our activity in the cannabis industry. We may become unable maintain stable banking relationships, which would create significant challenges in operating our business, increase our operating costs, pose additional operational, logistical and security challenges, and result in our inability to implement our business plan. Additionally, if our more significant customers to are unable maintain their current banking relationships, we might not be able to continue transacting with such customers.
Our payments system and the payment systems of our customers depend on third-party providers and are subject to evolving laws and regulations.
We and our retail customers have engaged third-party service providers to perform underlying credit and debit card processing, currency exchange, identity verification and fraud analysis services. If these service providers do not perform adequately or if our relationships, or the relationships of our retail customers with these service providers, were to terminate, our ability or the ability of such retail customers to process payments could be adversely affected and our business would be harmed.
The laws and regulations related to payments are complex and are potentially impacted by tensions between federal and state treatment of the vaporization, tobacco, nicotine and cannabis industries. These laws and regulations also vary across different jurisdictions in the United States, Canada and globally. As a result, we are required to spend significant time and effort to comply with those laws and regulations. Any failure or claim of our failure to comply, or any failure by our third-party service providers to comply, could cost us substantial resources, could result in liabilities, or could force us to stop offering our customers the ability to pay with credit cards, debit cards and bank transfers. As we expand the availability of these payment methods or offer new payment methods to our customers in the future, we may become subject to additional regulations and compliance requirements.
Further, through our agreement with our third-party credit card processors, we are indirectly subject to payment card association operating rules and certification requirements, including restrictions on product mix and the Payment Card Industry Data Security Standard, 02 PCIDSS. We also are subject to rules governing electronic funds transfers. Any change in these rules and requirements could make it difficult or impossible for us to comply.
Due to our acceptance of credit cards in our e-commerce business, we are subject to the Payment Card Industry Data Security Standard, designed to protect the information of credit card users. We have had a security incident in the past, which we do not believe reached the level of a breach, that would be reportable under state laws or our other obligations; however there can be no assurance that our determination was correct. In the event our determination is challenged and found to have been incorrect, we may be subject to claims by one or more state attorney generals, federal regulators, or private plaintiffs and we may additionally be subject to claims or fines from credit associations.
We are subject to certain U.S. federal regulations relating to cash reporting.
The U.S. Bank Secrecy Act, enforced by the Financial Crimes Enforcement Network (“FinCEN”), a division of the U.S. Department of the Treasury, requires a party in trade or business to file with the U.S. Internal Revenue Service (the “IRS”) a Form 8300 report within 15 days of receiving a cash payment of over $10,000. While we receive very few cash payments for the products we sell, if we fail to comply with these laws and regulations, the imposition of a substantial penalty could have a material adverse effect on our business, results of operations and financial condition.
Increases in tobacco-related taxes have been proposed or enacted and are likely to continue to be proposed or enacted in numerous jurisdictions.
Tobacco products, premium cigarette papers and tubes have long been subject to substantial federal, state, provincial and local excise taxes. Such taxes have frequently been increased or proposed to be increased, in some cases significantly, to fund various legislative initiatives or further disincentivize smoking.
In addition to federal excise taxes, every state and certain city and county governments have imposed substantial excise taxes on sales of tobacco products, and many have raised or proposed to raise excise taxes in recent years. Tax increases,
depending on their parameters, may result in consumers switching between tobacco products or depress overall tobacco consumption, which is likely to result in declines in overall sales volumes in certain of our products.
Any future enactment of increases in federal, provincial or state excise taxes on our tobacco products or rulings that certain of our products should be categorized differently for excise tax purposes could adversely affect demand for our products and may result in consumers switching between tobacco products or a depression in overall tobacco consumption, which could have a material adverse effect on our business, results of operations and financial condition.
If countries, states, and provinces continue the trend of imposing, expanding, and increasing taxes on vaporizer products, it could materially and adversely affect our business.
Supply to our customers is sensitive to increased sales taxes and economic conditions affecting their disposable income. Discretionary consumer purchases, such as of vaporization products and consumption accessories, may decline during recessionary periods or at other times when disposable income is lower and taxes may be higher.
As discussed under "Regulatory Developments" above, the sale of vaporization products and certain other consumption accessories is, in certain jurisdictions, subject to federal, state, provincial and local excise taxes like the sale of conventional cigarettes or other tobacco products, all of which generally have high tax rates and have faced significant increases in the amount of taxes collected on their sales. Other jurisdictions are contemplating similar legislation and other restrictions on electronic cigarettes and certain other vaporizer products. Should federal, state, provincial and local governments and/or other taxing authorities continue to impose excise taxes similar to those levied against conventional cigarettes and tobacco products on vaporization products or consumption accessories, it may have a material adverse effect on the demand for those products, as consumers may be unwilling to pay the increased costs, which in turn could have a material adverse effect on our business, results of operations and financial condition.
We could be required to collect additional sales taxes or be subject to other tax liabilities that may increase the costs our B2C customers would have to pay for our product offering, which could materially and adversely affect our operating results.
An increasing number of states have considered or adopted laws that attempt to impose tax collection obligations on out-of-state companies. Additionally, the Supreme Court of the United States recently ruled in South Dakota v. Wayfair, Inc. et al, or Wayfair, that online sellers can be required to collect sales and use tax despite not having a physical presence in the buyer’s state. In response to Wayfair, or otherwise, states or local governments may adopt, or begin to enforce, laws requiring us to calculate, collect, and remit taxes on sales in their jurisdictions. A successful assertion by one or more states requiring us to collect taxes where we presently do not do so, or to collect more taxes in a jurisdiction in which we currently do collect some taxes, could result in substantial tax liabilities, including taxes on past sales, as well as penalties and interest. The imposition by state governments or local governments of sales tax collection obligations on out-of-state sellers could also create additional administrative burdens for us, put us at a competitive disadvantage if they do not impose similar obligations on our competitors and decrease our future sales, which could have a material adverse impact on our business, financial condition and results of operations.
We may become involved in regulatory or agency proceedings, investigations, prosecutions, and audits.
Our business, and the business of the suppliers from which we acquire the products we sell, requires compliance with many laws and regulations in many jurisdictions globally across multiple product categories and regulatory regimes. Failure to comply with these laws and regulations could subject us or such suppliers to regulatory or agency proceedings, investigations, or prosecutions, and could also lead to damage awards, fines and penalties. We or such suppliers may become involved in a number of government proceedings, investigations and audits. The outcome of any government proceedings, investigations, prosecutions, audits, and other contingencies could harm our reputation or the reputations of the brands that we sell, require us to take, or refrain from taking, actions that could harm our operations or require us to pay substantial amounts of money, harming our financial condition. There can be no assurance that any pending or future regulatory or agency proceedings, investigations and audits will not result in substantial costs or a diversion of management’s attention and resources or have a material adverse impact on our business, financial condition and results of operations.
We are subject to increasing international control and regulation.
The World Health Organization’s Framework Convention on Tobacco Control (“FCTC”) is the first international public health treaty that establishes a global agenda to reduce initiation of tobacco use and regulate tobacco in an effort to encourage tobacco cessation. Over 170 governments worldwide have ratified the FCTC, including Canada. The FCTC has led to increased efforts to reduce the supply of and demand for tobacco products and to encourage governments to further regulate the tobacco industry. The tobacco industry and others expect significant regulatory developments to take place over the next few years, driven principally by the FCTC.
If the United States ratifies the FCTC and/or national laws are enacted in the United States that reflect the major elements of the FCTC, our business, results of operations and financial condition could be materially and adversely affected. In addition, if any of our vaporization products or consumption accessories become subject to one or more of the significant regulatory initiatives proposed under the FCTC or any other international treaty, our business, results of operations and financial condition may also be materially adversely affected.
We currently distribute products across Canada and Europe, in addition to distributing products in select international markets. As part of our strategy, we anticipate further international expansions. Future expansions may subject us to additional or increasing international regulation, either by that country’s legal requirements or through international regulatory regimes, such as the FCTC, to which those countries may be signatories.
Countries’ laws implementing the European Union Tobacco Products Directive (“TPD”) impose strict regulations on the approval, sale, and advertising of e-cigarettes. Although we do not sell or market any material quantities of products classified as e-cigarettes in Europe, countries could enact new laws implementing the TPD or other laws or regulations that re-classify and/or restrict the products we may sell or market in Europe. Any future measures that limit our ability to market or sell vaporization products or other consumption accessories in Europe may have a material adverse effect on our business, results of operations, and financial condition.
To the extent our existing or future products become subject to international regulatory regimes that we are unable to comply with or fail to comply with, they may have a material adverse effect on our business, results of operations and financial condition.
We face intense competition and may fail to compete effectively.
The vaporization products and consumption accessories industry is characterized by brand recognition and loyalty, with product quality features, price, marketing and packaging constituting the primary methods of competition. Substantial marketing support, merchandising display, competitive pricing and other financial incentives generally are required to introduce a new brand or to improve or maintain a brand’s market position. Our principal competitors may be significantly larger than us and aggressively seek to limit the distribution or sale of our products.
Competition in the vaporization products and consumption accessories industry is particularly intense, and the market is highly fragmented. In addition, some competitors still have the ability to access sales channels through the mail, which is no longer available to us and may place us at a competitive disadvantage.
“Big tobacco” and other well-resources competitors are continuing to establish its presence in the vaporization products and consumption accessories market. There can be no assurance that our products will be able to compete successfully against these companies or any of our other competitors, some of which have far greater resources, capital, experience, market penetration, sales and distribution channels than us. In addition, if large online retailers such as Amazon establish their presence in the vaporization products and consumption accessories market, our sales through both our direct to consumer e-commerce channel and our business-to-business wholesale channel may be harmed. Competitors, including “big tobacco” and large online retailers, may also have more resources than us for advertising, which could have a material adverse effect on our ability to build and maintain market share, and thus have a material adverse effect on our business, results of operations and financial condition.
Our narrow margins may magnify the impact of variations in operating costs and of adverse or unforeseen events on operating results.
We are subject to intense price competition. As a result, our gross and operating margins have historically been narrow, and we expect them to continue to be narrow. Narrow margins magnify the impact of variations in operating costs and of gross margin and of unforeseen adverse events on operating results. Future increases in costs, such as the cost of merchandise, wage levels, shipping rates, import duties and fuel costs, may negatively impact our margins and profitability. We are not always able to raise the sales price to offset cost increases or to effect increased operating efficiencies in response to increasing costs. If we are unable to maintain our margins in the future, it could have a material adverse effect on our business, results of operations and financial condition. If we become subject to increased price competition in the future, we cannot assure you that we will not lose market share, that we will not be forced to reduce our prices and further reduce our margins, or that we will be able to compete effectively.
Additionally, promotional activities can significantly increase net sales in the periods in which they are initiated and net sales can be adversely impacted in the periods after a promotion. Accordingly, based upon the timing of our marketing and promotional initiatives, we have and may continue to experience significant variability in our month-to-month results, which could affect our ability to formulate strategies that allow us to maintain our market presence across volatile months. If our monthly sales fluctuations obscure our ability to track important trends in our key markets, it may have a material adverse effect on our business, results of operations and financial condition.
We experience variability in our net sales and net income on a quarterly basis as a result of many factors.
We experience variability in our net sales and net income on a quarterly basis as a result of many factors. These factors include:
•the relative mix of vaporization products and consumption accessories sold during the period;
•the general economic environment and competitive conditions, such as pricing;
•the timing of procurement cycles by our customers;
•seasonality in customer spending and demand for products we provide;
•variability in supplier programs;
•the introduction of new and upgraded products;
•changes in prices from our suppliers;
•trade show attendance;
•promotions;
•the loss or consolidation of significant suppliers or customers;
•our ability to control costs;
•the timing of our capital expenditures;
•the condition of our industry in general;
•regulatory developments that limit or expand the products we may sell, or the manner in which those products may be transported;
•any inability on our part to obtain adequate quantities of products;
•delays in the release by suppliers of new products and inventory adjustments;
•delays in the release of imported products by customs authorities;
•our expenditures on new business ventures and acquisitions;
•performance of acquired businesses;
•adverse weather conditions, natural disasters, pandemics, or other events that affect supply or customer response;
•distribution or shipping to our customers; and
•geopolitical events.
Our planned operating expenditures each quarter are based on sales forecasts for the quarter. If our sales do not meet expectations in any given quarter, our operating results for that quarter may be materially adversely affected. Our narrow margins may magnify the impact of these factors on our operating results. We believe that period-to-period comparisons of our operating results are not necessarily a good indication of our future performance. In addition, our results in any quarterly period are not necessarily indicative of results to be expected for a full fiscal year. In future quarters, our operating results may be below the expectations of public market analysts or investors and, as a result, the market price of our Class A common stock could be materially adversely affected.
Product defects could increase our expenses, damage our reputation or expose us to liability.
We may not be able to adequately address product defects. Product defects in vaporizers and other accessories may harm the health or safety of our end-consumers. In addition, remedial efforts could be particularly time-consuming and expensive if product defects are only found after we have sold the defective product in volume. Any actual or perceived defects in our products could result in unsold inventory, product recalls, repairs or replacements, damage to our reputation, increased customer service costs and other expenses, as well as divert management attention and expose us to liabilities. Furthermore, a product liability claim brought against us by our customers or end-consumers could be time-consuming and costly to defend and, if successful, could require us to make significant payments.
Contamination of, or damage to, our products could adversely impact sales volume, market share and profitability.
Our market position may be affected through the contamination of our products, as well as the material used during the manufacturing processes of the products we sell, or at different points in the entire supply chain. We keep significant amounts of inventory of our products in warehouses and it is possible that this inventory could become contaminated prior to arrival at our premises or during the storage period. If contamination of our inventory or packaged products occurs, whether as a result of a failure in quality control by us or by one of our suppliers, we may incur significant costs in replacing the inventory and recalling products. We may be unable to meet customer demand and may lose customers who purchase alternative brands or products. In addition, consumers may lose confidence in the affected product.
Under the terms of our contracts, we generally impose requirements on our suppliers to maintain quality and comply with product specifications and requirements, and with all federal, state and local laws. Our suppliers, however, may not continue to produce products that are consistent with our standards or that are in compliance with applicable laws, and we cannot guarantee that we will be able to identify instances in which our suppliers fail to comply with our standards or applicable
laws. A loss of sales volume from a contamination event may occur, and such a loss may affect our ability to supply our current customers and to recapture their business in the event they are forced to switch products or brands, even if on a temporary basis. We may also be subject to legal action as a result of a contamination, which could result in negative publicity and affect our sales. During this time, our competitors may benefit from an increased market share that could be difficult and costly to regain. Such a contamination event could have a material adverse effect on our business, results of operations and financial condition.
We may not have adequate insurance for potential liabilities, including liabilities arising from litigation.
In the ordinary course of business, we have and in the future may become the subject of various claims, lawsuits and governmental proceedings seeking damages or other remedies concerning our commercial operations, the products we distribute, our employees and other matters, including potential claims by individuals alleging exposure to hazardous materials as a result of the products we distribute. Some of these claims may relate to the activities of businesses that we have acquired, even though these activities may have occurred prior to our acquisition of the businesses. The products we distribute may contain lithium ion or similar type batteries that can explode or release hazardous substances. In addition, defects in the products we distribute could result in death, personal injury, property damage, pollution, release of hazardous substances or damage to equipment and facilities. Actual or claimed defects in the products we distribute may give rise to claims against us for losses and expose us to claims for damages.
We maintain insurance to cover certain of our potential losses, and we are subject to various self-retentions, deductibles and caps under our insurance. We face the following risks with respect to our insurance coverage:
•we may not be able to continue to obtain insurance on commercially reasonable terms;
•we may incur losses from interruption of our business that exceed our insurance coverage;
•we may be faced with types of liabilities that will not be covered adequately or at all by our insurance;
•our insurance carriers may not be able to meet their obligations under the policies;
•our existing Directors & Officers insurance does not provide coverage for claims against the company or identifiable claims against its officers and directors, including the types of claims asserted against us in the securities litigation discussed in Item 3 of this Form 10-K; accordingly, we are required to fund the costs of defending such litigation and the costs of any recovery by the plaintiffs in the event that such litigation is resolved in a manner adverse to us; or
•the dollar amount of any liabilities may exceed our policy limits.
Even a partially uninsured claim, if successful and of significant size, could have a material adverse effect on us. Finally, even in cases where we maintain insurance coverage, our insurers may raise various objections and exceptions to coverage that could make uncertain the timing and amount of any possible insurance recovery.
Due to our position in the supply chain of vaporization products and consumption accessories, we are subject to personal injury, product liability and environmental claims involving allegedly defective products.
Our customers use certain products we distribute in potentially hazardous applications that can result in personal injury, product liability and environmental claims. A catastrophic occurrence at a location at which consumers use the products we distribute may result in our company being named as a defendant in lawsuits asserting potentially large claims, even though we did not manufacture such products or even if such products were not used in the manner recommended by the manufacturer. Applicable law may render us liable for damages without regard to negligence or fault. Certain of these risks are reduced by the fact that we are a distributor of products that third-party manufacturers produce, and, thus, in certain circumstances, we may have third-party warranty or other claims against the manufacturer of products alleged to have been defective. However, there is no assurance that these claims could fully protect us or that the manufacturer would be financially able to provide protection. There is no assurance that our insurance coverage will be adequate to cover the underlying claims. Our insurance does not provide coverage for all liabilities (including liability for certain events involving pollution or other environmental claims).
We may become subject to significant product liability litigation.
The tobacco and e-cigarette industries have experienced and continue to experience significant product liability litigation and other claims, such as those related to marketing of tobacco and e-cigarettes to minors. As a result of their relative novelty, electronic cigarette, vaporizer product and other consumption product manufacturers, suppliers, distributors and sellers have only recently become subject to litigation. While we have not been a party to any product liability litigation, several lawsuits have been brought against other manufacturers and sellers of smokeless products for injuries to health allegedly caused by use of smokeless products. We may be subject to similar claims in the future relating to our vaporizer products. We may also be named as a defendant in product liability litigation against one of our suppliers by association, including in class action lawsuits. In addition, we may see increasing litigation over our vaporizer products or the regulation of our products as the regulatory regimes surrounding these products develop. For example, California’s Proposition 65 (“Prop 65”) requires the State of California to identify chemicals that could cause cancer, birth defects, or reproductive harm, and businesses selling products in California are then required to warn consumers of any possible exposure to the chemicals on the list. The State of California and private plaintiffs have been active in enforcing Prop 65 against companies in the tobacco, nicotine, cannabis, and
vaporization industries. We may face substantial costs due to increased product liability litigation relating to new regulations or other potential defects associated with our vaporizer and other consumption products, including litigation arising out of faulty devices or improper usage, which could have a material adverse effect on our business, results of operations and financial condition.
There can be no assurances that we will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of products.
The scientific community has not yet extensively studied the long-term health effects of the use of vaporizers, electronic cigarettes or e-liquids products.
Vaporizers, electronic cigarettes and related products were recently developed and therefore the scientific community has not had a sufficient period of time to study the long-term health effects of their use. Currently, there is no way of knowing whether these products are safe for their intended use. If the scientific community were to determine conclusively that use of any or all of these products poses long-term health risks, market demand for these products and their use could materially decline. Such a determination could also lead to litigation and significant regulation. Loss of demand for our product, product liability claims and increased regulation stemming from unfavorable scientific studies on these products could have a material adverse effect on our business, results of operations and financial condition.
Reliance on information technology means a significant disruption could affect our communications and operations.
We increasingly rely on information technology systems for our internal communications, controls, reporting and relations with customers, vendors and suppliers, and information technology is becoming a significantly important tool for our sales staff. Our marketing and distribution strategy is dependent upon our ability to closely monitor consumer and market trends on a highly specified level, for which we are reliant on our sophisticated data tracking systems, which are susceptible to disruption or failure. In addition, our reliance on information technology exposes us to cyber-security risks, which could have a material adverse effect on our ability to compete. Security and privacy breaches may expose us to liability and cause us to lose customers, or may disrupt our relationships and ongoing transactions with other entities with whom we contract throughout our supply chain. The failure of our information systems to function as intended, or the penetration by outside parties intent on disrupting business processes, could result in significant costs, loss of revenue, assets or personal or other sensitive data and reputational harm.
Internet security poses a risk to our e-commerce sales.
At present, we generate a portion of our sales through e-commerce sales on our own websites and fulfillment activities through third-party websites. We manage our websites and e-commerce platform internally and, as a result, any compromise of our security or misappropriation of proprietary information could have a material adverse effect on our business, results of operations and financial condition. We rely on encryption and authentication technology licensed from third parties to provide the security and authentication necessary to effect secure Internet transmission of confidential information, such as credit and other proprietary information. Advances in computer capabilities, new discoveries in the field of cryptography or other events or developments may result in a compromise or breach of the technology used by us to protect client transaction data. Anyone who is able to circumvent our security measures could misappropriate proprietary information or cause material interruptions in our operations. We may be required to expend significant capital and other resources to protect against security breaches or to minimize problems caused by security breaches. To the extent that our activities or the activities of others involve the storage and transmission of proprietary information, security breaches could damage our reputation and expose us to a risk of loss and/or litigation. Our security measures may not prevent security breaches. Our failure to prevent these security breaches may result in consumer distrust and may adversely affect our business, results of operations and financial condition.
Security and privacy breaches may expose us to liability and cause us to lose customers.
Federal, provincial and state laws require us to safeguard our customers’ financial information, including credit information, as well as our employees' information. Although we have established security procedures to protect against identity theft and the theft of information of our customers, distributors, consumers, and employees, our security and testing measures may not prevent security breaches and breaches of privacy may occur, which would harm our business. Typically, we rely on encryption and authentication technology licensed from third parties to enhance transmission security of confidential information in relation to financial and other sensitive information that we have on file. Advances in computer capabilities, new discoveries in the field of cryptography, inadequate facility security or other developments may result in a compromise or breach of the technology used by us to protect customer data. Any compromise of our security could harm our reputation or financial condition and therefore, our business. In addition, a party who is able to circumvent our security measures or exploit inadequacies in our security measures, could, among other effects, misappropriate proprietary information, cause interruptions in our operations or expose customers and other entities with which we interact to computer viruses or other disruptions. Actual
or perceived vulnerabilities may lead to claims against us. To the extent the measures we have taken prove to be insufficient or inadequate, we may become subject to litigation or administrative sanctions, which could result in significant fines, penalties or damages and harm to our reputation.
If the methodologies of Internet search engines are modified, traffic to our websites and corresponding consumer origination volumes could decline.
We depend in part on various Internet search engines, including Google® and others to direct a significant amount of traffic to our websites. Our ability to maintain the number of visitors directed to our websites by search engines through which we distribute our content is not entirely within our control. Our competitors’ search engine optimization (“SEO”) efforts may result in their websites receiving a higher search result page ranking than ours, or Internet search engines could revise their methodologies, which could adversely affect the placement of our search result page ranking. If search engine companies modify their search algorithms in ways that are detrimental to our consumer growth or in ways that make it harder for our customers to access or use our websites, or if our competitors’ SEO efforts are more successful than ours, our consumer engagement and number of consumers could decline. Any reduction in the number of consumers directed to our websites could negatively affect our ability to earn revenue. If traffic on our websites declines, we may need to employ more costly resources to replace lost traffic, and such increased expense could adversely affect our business, results of operations and financial condition.
We are a holding company and depend upon our subsidiaries for our cash flow.
We are a holding company. Our subsidiaries conduct all of our operations and own substantially all of our tangible assets. Consequently, our cash flow and our ability to meet our obligations or to make other distributions in the future will depend upon the cash flow of our subsidiaries and our subsidiaries’ payment of funds to us in the form of distributions, dividends, tax sharing payments or otherwise.
The ability of our subsidiaries to make any payments to us will depend on their earnings and cash flow, the terms of their current and future indebtedness, tax considerations and legal and contractual restrictions on their ability to make distributions.
Our subsidiaries are separate and distinct legal entities. Any right that we have to receive any assets of or distributions from any of our subsidiaries upon the bankruptcy, dissolution, liquidation or reorganization, or to realize proceeds from the sale of their assets, will be junior to the claims of that subsidiary’s creditors, including trade creditors and holders of debt that the subsidiary issued.
Changes in our credit profile may affect our relationship with our suppliers, which could have a material adverse effect on our liquidity.
Changes in our credit profile may affect the way our suppliers view our ability to make payments and may induce them to shorten the payment terms of their invoices. Given the large dollar amounts and volume of our purchases from suppliers, a change in payment terms may have a material adverse effect on our liquidity and our ability to make payments to our suppliers and, consequently, may have a material adverse effect on us.
Our intellectual property may be infringed.
We currently rely on trademark and other intellectual property rights to establish and protect the brand names and logos we own or license on the products we distribute. Third parties have in the past infringed, and may in the future infringe, on these trademarks and our other intellectual property rights. Our ability to maintain and further build brand recognition is dependent on the continued use of these trademarks, service marks and other proprietary intellectual property, including the names and logos we own or license. Despite our attempts to ensure these intellectual property rights are protected, third parties may take actions that could materially and adversely affect our rights or the value of this intellectual property. Any litigation concerning our intellectual property rights or the intellectual property rights of our suppliers, whether successful or unsuccessful, could result in substantial costs to us and diversions of our resources. Expenses related to protecting our intellectual property rights or the intellectual property rights of our suppliers, the loss or compromise of any of these rights or the loss of revenues as a result of infringement could have a material adverse effect on our business, results of operations and financial condition, and may prevent the brands we own or license, or are owned or licensed by our suppliers, from growing or maintaining market share. There can be no assurance that any trademarks or common marks that we own or license, or are owned or licensed by our suppliers, will not be challenged in the future, invalidated or circumvented or that the rights granted thereunder or under licensing agreements will provide us or our suppliers competitive advantages. We are dependent on the validity, integrity and intellectual property of our suppliers and their efforts to appropriately register, maintain and enforce intellectual property in all jurisdictions in which their products are sold.
We devote significant resources to the registration and protection of our trademarks and to anti-counterfeiting efforts. Despite these efforts, we regularly discover products that infringe on our proprietary rights or that otherwise seek to mimic or leverage our intellectual property or the intellectual property of our suppliers. Counterfeiting and other infringing activities typically increase as brand recognition increases, especially in markets outside the United States and Canada. Counterfeiting and other infringement of our intellectual property could divert away sales, and association of our brands with inferior counterfeit reproductions or third party labels could adversely affect the integrity and reputation of our brands.
Although we currently hold a number of patents on our products, we generally rely on patents on the products of our suppliers as well as their efforts in successfully defending third-party challenges to such products. Third parties have in the past infringed, and may in the future infringe, on our patents and our suppliers' patents. Our ability to maintain and enforce our patent rights, and the ability of our suppliers, licensors, collaborators and manufacturers to maintain and enforce their patent rights, against third-party challenges to their validity, scope or enforceability plays an important role in determining our future. There can be no assurances that we will ever successfully file or receive any patents in the future, and changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of the intellectual property rights of the products we distribute, license or own. Accordingly, we cannot predict with any certainty the range of claims that may be allowed or enforced concerning the products that we sell.
In addition, there can be no assurance that standard intellectual property confidentiality and assignment agreements with employees, consultants and other advisors will not be breached, that we will have adequate remedies for any breach, or that our trade secrets will not otherwise become known to or independently developed by competitors. Furthermore, there can be no assurance that our efforts to protect our intellectual property will prevent others from unlawfully using our trademarks, trade secrets, copyrights and other intellectual property. Our success depends in part, on our continued ability to maintain our intellectual property and those of our suppliers, and to protect our trade secrets. An inability to continue to preserve and protect our intellectual property would likely have a material adverse effect on our business, results of operations and financial condition.
We are subject to the risks of exchange rate fluctuations.
Currency movements and suppliers’ price increases relating to currency exchange rates are significant factors affecting our cost of sales. Many of our products are purchased from suppliers located in foreign countries and we make payments for our products in numerous currencies. Thus, we bear certain foreign exchange rate risk for certain of our inventory purchases. In addition, we recently expanded our footprint in Canada and Europe, and as part of our strategy, we may undertake further international expansion. As a result, in the future, we may be more sensitive to the risks of exchange rate fluctuations, which may have a material adverse effect on our business, results of operations and financial condition.
We may be required to seek additional financing sources, which may not be available to us on attractive terms if at all and could restrict our ability to engage in certain business activities.
Unless and until the market price of our Class A common stock recovers significantly, we may not be in a position to fund our liquidity needs by accessing the public markets. If we are not able to fund our operations with cash on hand, we may be required to seek other financing sources, including debt financing, the amount of which may be substantial. In the past, because of the nature of our industry, we have had difficulties establishing relationships with certain financial institutions and may continue to face such difficulties. As a result, indebtedness or other forms of financing may not be available to us on attractive terms or at all. Furthermore, we may have to seek financing from non-traditional sources such as private equity and hedge funds, which may require us to give up significant governance or other rights or agree to economic and other terms that are not favorable.
In addition, future financing agreements we may enter into in the future may contain customary negative covenants and other financial and operating covenants that, among other things:
•restrict our ability to incur additional indebtedness;
•restrict our ability to incur additional liens;
•restrict our ability to make certain investments (including capital expenditures);
•restrict our ability to merge with another company;
•restrict our ability to sell or dispose of assets;
•restrict our ability to make distributions to stockholders; and
•require us to satisfy minimum financial coverage ratios, minimum net worth requirements and maximum leverage ratios.
We are required to comply with laws and regulations in other countries and are exposed to business risks associated with our international operations.
For the years ended December 31, 2020 and 2019, we derived 20.8% and 16.2%, respectively, of our net sales from outside the United States, primarily in Canada and certain European countries. We intend to increase our international sales, both as to the dollar amount and as a percentage of our net sales and operations in the future. As a result, we are subject to numerous evolving and complex laws and regulations which apply, among other things, to financial reporting standards, corporate governance, data privacy, tax, trade regulations, export controls, competitive practices, labor, health and safety laws, laws regarding controlled substances, laws regarding drug paraphernalia, and regulations in each jurisdiction in which we operate. We are also required to obtain permits and other authorizations or licenses from governmental authorities for certain of our operations and we or our suppliers’ must protect our intellectual property worldwide. In the jurisdictions in which we operate, we need to comply with various standards and practices of different regulatory, tax, judicial and administrative bodies.
There are a number of risks associated with international business operations, including political instability (e.g., the threat of war, terrorist attacks or civil unrest), inconsistent regulations across jurisdictions, unanticipated changes in the regulatory environment, and import and export restrictions. Any of these events may affect our employees, reputation, business or financial results as well as our ability to meet our objectives, including the following international business risks:
•negative economic developments in economies around the world and the instability of governments, or the downgrades in the debt ratings of certain major economies;
•social and political instability;
•complex regulations governing certain of our products;
•potential terrorist attacks;
•adverse changes in governmental policies, especially those affecting trade, tariffs and investment;
•foreign currency exchange, particularly with respect to the Canadian Dollar, Euro, British Pound Sterling and Australian Dollar; and
•threats that our operations or property could be subject to nationalization and expropriation.
We may not be in full compliance at all times with the laws and regulations to which we are subject. Likewise, we may not have obtained or may not be able to obtain the permits and other authorizations or licenses that we need. If we violate or fail to comply with laws, regulations, permits, labor, health and safety regulations or other authorizations or licenses, we could be fined or otherwise sanctioned by regulators. In such a case, or if any of these international business risks were to materialize, our business, results of operations and financial condition could be adversely affected.
New tariffs and the evolving trade policy dispute between the United States and China may adversely affect our business.
On August 14, 2017, then President Trump instructed the U.S. Trade Representative (“USTR”) to determine under Section 301 of the U.S. Trade Act of 1974 (the “Trade Act”) whether to investigate China’s laws, policies, practices or actions that may be unreasonable or discriminatory and that may be harming American intellectual property rights, innovation or technology development. On March 22, 2018, based upon the results of its investigation, the USTR published a report finding that the acts, policies and practices of the Chinese government are unreasonable or discriminatory and burden or restrict U.S. commerce.
On March 8, 2018, President Trump imposed significant tariffs on steel and aluminum imports from a number of countries, including China. Subsequently, the USTR announced an initial proposed list of 1,300 goods imported from China that could be subject to additional tariffs and initiated a dispute with the World Trade Organization against China for alleged unfair trade practices.
On June 15, 2018, the USTR announced a list of products subject to additional tariffs. The list focused on products from industrial sectors that contribute to or benefit from the “Made in China 2025” industrial policy. The list of products consists of two sets of tariff lines. The first set contains 818 tariff lines for which Customs and Border Protection began collecting the additional duties on July 6, 2018. This list includes some of the products we distribute. The second set contains 284 proposed tariff lines that remain subject to further review. On July 10, 2018, the USTR announced that, as a result of China’s retaliation and failure to change its practices, President Trump has ordered the USTR to begin the process of imposing tariffs of 10 percent on an additional $200 billion of Chinese imports, and on September 17, 2018, President Trump announced that such tariffs would go into effect on September 24, 2018 and would increase to 25 percent on January 1, 2019. However, in early December 2018, President Trump agreed to leave the tariffs at the 10 percent rate while the United States and China entered into negotiations regarding various trade-related matters.
These new tariffs and the evolving trade policy dispute between the United States and China may have a significant impact on the industries in which we participate. Many of the products we sell are subject to the 25 percent tariff and such tariff, along with resultant price increases, may negatively impact our pricing and customer demand for these products. A “trade war”
between the United States and China or other governmental action related to tariffs or international trade agreements or policies has the potential to adversely impact demand for our products, our costs, customers, suppliers and/or the United States economy or certain sectors thereof and, thus, to adversely impact our businesses and results of operations.
Our failure to comply with certain environmental, health and safety regulations could materially and adversely affect our business.
The storage, distribution and transportation of some of the products that we sell are subject to a variety of federal, state, provincial and local environmental regulations. We are also subject to operational, health and safety laws and regulations. Our failure to comply with these laws and regulations could cause a disruption in our business, an inability to maintain our warehousing resources, additional and potentially significant remedial costs and damages, fines, sanctions or other legal consequences that could have a material adverse effect on our business, results of operations and financial condition. In addition, changes in environmental, employee health and safety or other laws, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations or give rise to material liabilities, which could have a material adverse effect on our business, financial condition and results of operations.
Our business depends substantially on the continued efforts of our executive officers and key employees, and our business may be severely disrupted if we lose their services.
Our future success depends substantially on the continued efforts of our executive officers, as well as our key employees.
If one or more of our executive officers or key employees were unable or unwilling to continue in their present positions, we may not be able to replace them in a timely manner, or at all. Our business may be severely disrupted, our financial conditions and results of operations may be materially adversely affected and we may incur additional expenses to recruit, train and retain personnel. In addition, if any of our executive officers or key employees joins a competitor or forms a competing company, we may lose customers, suppliers, know-how, key professionals and staff members.
In the future, we may pursue selective acquisitions to complement our organic growth, which may not be successful and may divert financial and management resources.
If we identify appropriate opportunities, we may acquire or invest in technologies, businesses or assets that are strategically important to our business or form alliances with key participants in the vaporization products and consumption accessories industry to further expand our business. However, we may not be successful in identifying suitable acquisition opportunities or completing such transactions. Our competitors may be more effective in executing and closing acquisitions in competitive auctions than us. Our ability to enter into and complete acquisitions may be restricted by, or subject to, various approvals under U.S., Canadian or other applicable law or may not otherwise be possible, may result in a possible dilutive issuance of our securities, or may require us to seek additional financing. We also may experience difficulties integrating acquired operations, technology, and personnel into our existing business and operations. Completed acquisitions may also expose us to potential risks, including risks associated with unforeseen or hidden liabilities, impact to our corporate culture, the diversion of resources from our existing business, and the potential loss of, or harm to, relationships with our suppliers, business relationships or employees as a result of our integration of new businesses. In addition, following completion of an acquisition, our management and resources may be diverted from their core business activities due to the integration process, which diversion may harm the effective management of our business. Furthermore, it may not be possible to achieve the expected synergies or the actual cost of delivering such benefits may exceed the anticipated cost. Any of these factors may have an adverse effect on our business, results of operations and financial condition.
Our operations are subject to natural disasters, adverse weather conditions, operating hazards, environmental incidents and labor disputes.
We may experience earthquakes, floods, typhoons, power outages, labor and trade disputes or similar events beyond our control that would affect our warehousing and distribution operations. The occurrences of such events could result in shutdowns or periods of reduced operations, which could significantly disrupt our business operations, cause us to incur additional costs and affect our ability to deliver our products to our customers as scheduled, which may adversely affect our business, results of operations and financial condition. Moreover, such events could result in severe damage to property, personal injuries, fatalities, regulatory enforcement proceedings or in us being named as a defendant in lawsuits asserting claims for large amounts of damages, which in turn could lead to significant liabilities.
Changes to the base rate on our floating rate indebtedness could increase our borrowing costs.
As of December 31, 2020, approximately $7.8 million of our outstanding indebtedness bears interest at floating rates based on the London interbank offered rate (“LIBOR”) and has maturity dates beyond December 31, 2021. In July 2017, the
United Kingdom's Financial Conduct Authority, which regulates LIBOR, announced that it will stop compelling banks to submit rates for the calculation of LIBOR after 2021. It is not possible to predict the effect of these changes, other reforms or the establishment of alternative reference rates. The discontinuation or modification of LIBOR could result in interest rate increases on our debt, which could adversely affect our cash flow, operating results and ability to make distributions to our stockholders at expected levels or at all.
Risks Related to Our Organizational Structure
Our principal asset is our interest in the Operating Company, and, accordingly, we depend on distributions from The Operating Company to pay our taxes and expenses, including payments under the Tax Receivable Agreement (the “TRA”). The Operating Company’s ability to make such distributions may be subject to various limitations and restrictions.
We are a holding company and have no material assets other than our ownership of Common Units of the Operating Company. As such, we will have no independent means of generating revenue or cash flow. Our ability to pay our operating expenses, including taxes and payments under the TRA, or declare and pay dividends in the future, if any, will be dependent upon the financial results and cash flows of the Operating Company and its subsidiaries and distributions we receive from the Operating Company. There can be no assurance that the Operating Company and its subsidiaries will generate sufficient cash flow to distribute funds to us or that applicable state law and contractual restrictions, including negative covenants, in any future debt instruments, will permit such distributions. In addition, because we are a holding company, our stockholders’ claims as a stockholder will be structurally subordinated to all existing and future liabilities and obligations of the Operating Company. Therefore, in the event of our bankruptcy, liquidation or reorganization, our assets and those of the Operating Company and its subsidiaries will be available to satisfy the claims of our stockholders only after all of our and Greenland Holdings, LLC’s and its subsidiaries’ liabilities and obligations have been paid in full.
The Operating Company is treated as a partnership for U.S. federal income tax purposes and, as such, is not subject to any entity-level U.S. federal income tax. Instead, taxable income is allocated to holders of Common Units, including us. Accordingly, we will incur income taxes on our allocable share of any net taxable income of the Operating Company. Under the terms of the Third Amended and Restated Agreement of the Operating Company (the “Operating Agreement”), the Operating Company will be obligated to make tax distributions to holders of Common Units, including us. In addition to tax expenses, we will also incur expenses related to our operations, including payments under the TRA, which we expect could be significant. We intend, as its manager, to cause the Operating Company to make cash distributions to the owners of Common Units in an amount sufficient to (i) fund their tax obligations in respect of taxable income allocated to them and (ii) cover our operating expenses, including payments under the TRA. However, the Operating Company’s ability to make such distributions may be subject to various limitations and restrictions, such as restrictions on distributions that would either violate any contract or agreement to which the Operating Company is then a party, including debt agreements, or any applicable law, or that would have the effect of rendering the Operating Company insolvent. If we do not have sufficient funds to pay tax or other liabilities or to fund our operations, we may have to borrow funds, which could materially adversely affect our liquidity and financial condition and subject us to various restrictions imposed by any such lenders. To the extent that we are unable to make payments under the TRA for any reason, such payments generally will be deferred and will accrue interest until paid; provided, however, that nonpayment for a specified period may constitute a material breach of a material obligation under the TRA and therefore accelerate payments due under the TRA.
The TRA requires us to make cash payments to them in respect of certain tax benefits to which we may become entitled, and we expect that the payments we will be required to make will be substantial.
Under the TRA we entered into with the Operating Company and the members, including Mr. LoCascio, our Chief Executive Officer, and Mr. Schoenfeld, our Chief Strategy Officer, we are required to make cash payments to the members of the Operating Partnership equal to 85% of the tax benefits, if any, that we actually realize, or in certain circumstances are deemed to realize, as a result of (i) the increases in the tax basis of assets of the Operating Company resulting from any redemptions or exchanges of Common Units from the members and (ii) certain other tax benefits related to our making payments under the TRA. Although the actual timing and amount of any payments that we make to the members under the TRA will vary, we expect those payments will be significant. Any payments made by us to the members under the TRA may generally reduce the amount of overall cash flow that might have otherwise been available to us. Furthermore, our future obligation to make payments under the TRA could make us a less attractive target for an acquisition, particularly in the case of an acquirer that cannot use some or all of the tax benefits that are the subject of the TRA. Payments under the TRA are not conditioned on any member’s continued ownership of Common Units or our Class A common stock.
The actual amount and timing of any payments under the TRA will vary depending upon a number of factors, including the timing of redemptions or exchanges by the holders of Common Units, the amount of gain recognized by such holders of Common Units, the amount and timing of the taxable income we generate in the future, and the federal tax rates then applicable.
Two of our senior executives, Aaron LoCascio and Adam Schoenfeld, have control over all stockholder decisions because collectively they control a substantial majority of the voting power of our common stock. This will limit or preclude your ability to influence corporate matters, including the election of directors, amendments of our organizational documents and any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.
Our Chief Executive Officer, Aaron LoCascio, and our Chief Strategy Officer, Adam Schoenfeld, are senior executives and board members, and they and their affiliates beneficially own 100% of our Class C common stock and thereby collectively control approximately 79.3% of the voting power of our common stock.
As a result, Messrs. LoCascio and Schoenfeld will have the ability to substantially control us, including the ability to control any action requiring the approval of our stockholders, including, but not limited to, the election of directors, the adoption of amendments to our amended and restated certificate of incorporation and bylaws and the approval of any merger or sale of substantially all of our assets. This concentration of ownership and voting power may also delay, defer or even prevent an acquisition by a third party or other change of control of us and may make some transactions more difficult or impossible without their support, even if such events are in the best interests of minority stockholders. This concentration of voting power with Messrs. LoCascio and Schoenfeld may have a negative impact on the market price of our Class A common stock.
As our Chief Executive Officer, Mr. LoCascio has control over our day-to-day management and the implementation of major strategic investments of our company, subject to authorization and oversight by our board of directors. As members of our board of directors, Messrs. LoCascio and Schoenfeld owe fiduciary duties to our company, including those of care and loyalty, and must act in good faith and with a view to the interests of the corporation. However, Delaware law provides that a director or officer shall not be personally liable to a corporation for a breach of fiduciary duty except for an act or omission constituting a breach and which involves intentional misconduct, fraud or a knowing violation of law. In addition, a director or officer is entitled to a presumption that he or she acted in good faith, on an informed basis and with a view to the interests of the corporation, and is not individually liable unless that presumption is found by a trier of fact to have been rebutted. As a stockholder, even a controlling stockholder, each of Messrs. LoCascio and Schoenfeld is entitled to vote his shares, and shares over which he has voting control, in his own interests, which may not always be in the interests of our stockholders generally. Because Messrs. LoCascio and Schoenfeld hold their economic interest in our business through the Operating Company, rather than through the public company, they may have conflicting interests with holders of shares of our Class A common stock. For example, Messrs. LoCascio and Schoenfeld may have different tax positions from us, which could influence their decisions regarding whether and when we should dispose of assets or incur new or refinance existing indebtedness, especially in light of the existence of the TRA, and whether and when we should undergo certain changes of control within the meaning of the TRA or terminate the TRA. In addition, the structuring of future transactions may take into consideration these tax or other considerations even where no similar benefit would accrue to us. In addition, the significant ownership of Messrs. LoCascio and Schoenfeld in us and their resulting ability to effectively control us may discourage someone from making a significant equity investment in us, or could discourage transactions involving a change in control, including transactions in which you as a holder of shares of our Class A common stock might otherwise receive a premium for your shares over the then-current market price.
Under certain circumstances, redemptions of Common Units by members will result in dilution to the holders of our Class A common stock.
Redemptions of Common Units by members in accordance with the terms of the Greenlane Operating Agreement will result in a corresponding increase in our membership interest in the Operating Company, an increase in the number of shares of Class A common stock outstanding and a decrease in the number of shares of Class B common stock or Class C common stock outstanding. In the event that Common Units are exchanged at a time when the Operating Company has made cash distributions to members, including our company, and we have accumulated such distributions and neither reinvested them in the Operating Company in exchange for additional Common Units nor distributed them as dividends to the holders of our Class A common stock, the holders of our Class A common stock would experience dilution with respect to such accumulated distributions.
As of March 26, 2021, the founder members own 72,064,218 shares of Class C common stock, which are exchangeable for 24,021,406 shares of Class A common stock in connection with a redemption of the corresponding Common Units, which would represent approximately 56.1% of our total outstanding Class A common stock if all members exchanged their Common Units for Class A common stock, and the members' corresponding Class B common stock and Class C common stock were cancelled. In addition, as of March 26, 2021, the non-founder members own 2,443,437 shares of Class B common stock (including 93,701 shares subject to certain vesting conditions), which are exchangeable for 2,443,437 shares of Class A common stock in connection with a redemption of the corresponding Common Units, which would represent approximately 5.7% of our total outstanding Class A common stock, under the same assumptions as described above. We are party to a
registration rights agreement between us and the members, which will require us to effect the registration of their shares in certain circumstances.
Furthermore, we cannot predict the timing of any redemption of Common Units or the effect that such redemptions will have on the market price of our Class A common stock.
Our organizational structure, including the TRA, confers certain benefits upon the members that will not benefit Class A common stockholders to the same extent as it will benefit the members.
Our organizational structure, including the TRA, confers certain benefits upon the members that will not benefit the holders of our Class A common stock to the same extent as it will benefit the members. The TRA provides for the payment by us to the members of 85% of the amount of tax benefits, if any, that we actually realize, or in some circumstances are deemed to realize, as a result of (1) the increases in the tax basis of assets of the Operating Company resulting from any redemptions or exchanges of Common Units from the members and (2) certain other tax benefits related to our making payments under the TRA. Although we will retain 15% of the amount of such tax benefits, this and other aspects of our organizational structure may adversely impact the future trading market for the Class A common stock.
In certain cases, payments under the TRA to the members may be accelerated or significantly exceed the actual benefits we realize in respect of the tax attributes subject to the TRA.
The TRA provides that upon certain mergers, asset sales, other forms of business combinations or other changes of control or if, at any time, we elect an early termination of the TRA, then our obligations, or our successor’s obligations, under the TRA to make payments thereunder would be based on certain assumptions, including an assumption that we would have sufficient taxable income to fully utilize all potential future tax benefits that are subject to the TRA.
As a result of the foregoing, (i) we could be required to make payments under the TRA that are greater than the specified percentage of the actual benefits we ultimately realize in respect of the tax benefits that are subject to the TRA, and (ii) if we elect to terminate the TRA early, we would be required to make an immediate cash payment equal to the present value of the anticipated future tax benefits that are the subject of the TRA, which payment may be made significantly in advance of the actual realization, if any, of such future tax benefits. In these situations, our obligations under the TRA could have a substantial negative impact on our liquidity and could have the effect of delaying, deferring or preventing certain mergers, asset sales, other forms of business combinations or other changes of control. There can be no assurance that we will be able to fund or finance our obligations under the TRA.
We will not be reimbursed for any payments made to the members under the TRA in the event that any tax benefits are disallowed.
Payments under the TRA will be based on the tax reporting positions that we determine, and the IRS or another tax authority may challenge all or part of the tax basis increases, as well as other related tax positions we take, and a court could sustain such challenge. If the outcome of any such challenge would reasonably be expected to materially affect a recipient’s payments under the TRA, then we will not be permitted to settle or fail to contest such challenge without the consent (not to be unreasonably withheld or delayed) of each member that directly or indirectly owns at least 10% of the outstanding Common Units. We will not be reimbursed for any cash payments previously made to the members under the TRA in the event that any tax benefits initially claimed by us and for which payment has been made to a member are subsequently challenged by a taxing authority and are ultimately disallowed. Instead, any excess cash payments made by us to a member will be netted against any future cash payments that we might otherwise be required to make to such member under the terms of the TRA. However, we might not determine that we have effectively made an excess cash payment to a member for a number of years following the initial time of such payment and, if any of our tax reporting positions are challenged by a taxing authority, we will not be permitted to reduce any future cash payments under the TRA until any such challenge is finally settled or determined. As a result, payments could be made under the TRA in excess of the tax savings that we realize in respect of the tax attributes with respect to a member that are the subject of the TRA.
Fluctuations in our tax obligations and effective tax rate and realization of our deferred tax assets may result in volatility of our operating results.
We are subject to taxes by the U.S. federal, state, local and foreign tax authorities, and our tax liabilities will be affected by the allocation of expenses to differing jurisdictions. We record tax expense based on our estimates of future earnings, which may include reserves for uncertain tax positions in multiple tax jurisdictions, and valuation allowances related to certain net deferred tax assets. At any one time, many tax years may be subject to audit by various taxing jurisdictions. The results of these audits and negotiations with taxing authorities may affect the ultimate settlement of these matters. We expect
that throughout the year there could be ongoing variability in our quarterly tax rates as events occur and exposures are evaluated. Our future effective tax rates could be subject to volatility or adversely affected by a number of factors, including:
•changes in the valuation of our deferred tax assets and liabilities;
•expected timing and amount of the release of any tax valuation allowances;
•tax effects of stock-based compensation;
•changes in tax laws, regulations or interpretations thereof; or
•future earnings being lower than anticipated in countries where we have lower statutory tax rates and higher than anticipated earnings in countries where we have higher statutory tax rates.
In addition, our effective tax rate in a given financial statement period may be materially impacted by a variety of factors including but not limited to changes in the mix and level of earnings, varying tax rates in the different jurisdictions in which we operate, fluctuations in valuation allowances, deductibility of certain items, or by changes to existing accounting rules or regulations. Further, tax legislation may be enacted in the future which could negatively impact our current or future tax structure and effective tax rates. We may be subject to audits of our income, sales, and other transaction taxes by U.S. federal, state, local, and foreign taxing authorities. Outcomes from these audits could have an adverse effect on our operating results and financial condition.
If we were deemed to be an investment company under the U.S. Investment Company Act of 1940, as amended (the “1940 Act”), as a result of our ownership of the Operating Company, applicable restrictions could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business.
Under Sections 3(a)(1)(A) and (C) of the 1940 Act, a company generally will be deemed to be an “investment company” for purposes of the 1940 Act if (i) it is, or holds itself out as being, engaged primarily, or proposes to engage primarily, in the business of investing, reinvesting or trading in securities or (ii) it engages, or proposes to engage, in the business of investing, reinvesting, owning, holding or trading in securities and it owns or proposes to acquire investment securities having a value exceeding 40% of the value of its total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis. We do not believe that we are an “investment company,” as such term is defined in either of those sections of the 1940 Act.
As the sole manager of the Operating Company, we control and operate the Operating Company. On that basis, we believe that our interest in the Operating Company is not an “investment security” as that term is used in the 1940 Act. However, if we were to cease participation in the management of the Operating Company, our interest in The Operating Company could be deemed an “investment security” for purposes of the 1940 Act.
We and the Operating Company intend to continue to conduct our operations so that we will not be deemed an investment company. However, if we were to be deemed an investment company, restrictions imposed by the 1940 Act, including limitations on our capital structure and our ability to transact with affiliates, could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business.
We are a controlled company within the meaning of the Nasdaq Marketplace Rules, and, as a result, qualify for, and may avail ourselves of, exemptions from certain corporate governance requirements that provide protection to stockholders of other companies. You will not have the same protections afforded to stockholders of companies that are subject to such requirements.
The founder members control more than 50% of our combined voting power. As a result, we qualify as a “controlled company” within the meaning of the Nasdaq Marketplace Rules.
As a controlled company, we are exempt from certain Nasdaq Marketplace Rules, including those that would otherwise require our board of directors to have a majority of independent directors and require that we either establish a Compensation and Nominating and Corporate Governance Committees, each comprised entirely of independent directors, or otherwise ensure that the compensation of our executive officers and nominees for directors are determined or recommended to the board of directors by the independent members of the board of directors. Accordingly, holders of our Class A common stock will not have the same protections afforded to stockholders of companies that are subject to all of the Nasdaq Marketplace Rules. As of the date of this filing, we have not availed ourselves of the controlled company exemptions.
Our failure to meet the continued listing requirements of Nasdaq could result in a de-listing of our common stock.
If we fail to continue to satisfy the continued listing requirements of Nasdaq, such as the corporate governance requirements or the minimum closing bid price requirement, Nasdaq may take steps to de-list our Class A common stock. As a result of several factors, including the expanding outbreak of COVID-19, the per share price of our Class A common stock has declined below the minimum bid price threshold required for continued listing. Such a de-listing would likely have a negative effect on the price of our Class A common stock and would impair your ability to sell or purchase our Class A common stock when you wish to do so. In the event of a de-listing, we would take actions to restore our compliance with Nasdaq Marketplace Rules, but we can provide no assurances that the listing of our Class A common stock would be restored, that our Class A common stock will remain above the Nasdaq minimum bid price requirement or that we otherwise will remain in compliance with the Nasdaq Marketplace Rules.
Risks Related to Ownership of Our Class A Common Stock
The market price of our Class A common stock has been volatile and has declined significantly since our initial public offering and may face more volatility and price declines in the future. As a result, you may not be able to resell your shares at or above the price at which you have acquired or will acquire shares of our Class A common stock.
The market price of our Class A common stock has been volatile and has declined significantly since our initial public offering and could face more volatility and price declines in the future as a result of a number of factors, many of which are beyond our control. Furthermore, volatility in our stock price may occur regardless of our operating performance. As a result, you may not be able to sell your shares at or above the price you paid and you could lose a substantial part or all of your investment in our Class A common stock. The following factors could affect our stock price:
•general market conditions, including conditions that are outside of our control, such as actions or proposed actions of the new U.S. Presidential administration, international trade disputes that disrupt our supply chain and the impact of health and safety concerns, such as the current COVID-19 outbreak;
•novel and unforeseen market volatility and trading strategies, such as the massive short squeeze rally caused by retail investors on retail trading platforms;
•our operating and financial performance and the performance of other similar companies;
•the market perception of our industry;
•the impact, or perceived impact, of new regulations applicable to us, our suppliers or our customers;
•quarterly variations in the rate of growth of our financial indicators, such as net income, net income per share, net sales and adjusted EBITDA;
•our ability to successfully execute our merger and acquisition strategy;
•significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving us or our competitors;
•strategic actions by our competitors or our suppliers;
•product recalls or product liability claims;
•changes in revenue or earnings estimates, or changes in recommendations or withdrawal of research coverage, by equity research analysts;
•liquidity and activity in the market for our Class A common stock;
•speculation in the press or investment community;
•sales of our Class A common stock by us or other stockholders, or the perception that such sales may occur;
•the issuance of Class A common stock upon redemption of Common Units by members in the Operating Company;
•the future incurrence of debt;
•changes in accounting principles;
•additions or departures of key management personnel;
•news reports relating to trends, concerns or competitive developments, regulatory changes and other related issues in our industry or target markets, including, but not limited to, EVALI;
•investors’ general perception of us and the public’s reaction to our press releases, our other public announcements and our filings with the SEC;
•actions by our stockholders; and
•domestic and international economic, legal and regulatory factors.
The stock markets in general have experienced extreme volatility, particularly recently, that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our Class A common stock.
The requirements of being a public company may strain our resources and distract our management, which could make it difficult to manage our business, particularly after we are no longer an “emerging growth company.”
As a public company, we are required to comply with various regulatory and reporting requirements, including those required by the SEC. Complying with these reporting and other regulatory requirements is time-consuming and expensive and could have a negative effect on our business, results of operations and financial condition. As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the requirements of the Sarbanes-Oxley Act of 2002 (“SOX”). The cost of complying with these requirements may place a strain on our systems and resources. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. SOX requires that we maintain effective disclosure controls and procedures and internal controls over financial reporting. To maintain and improve the effectiveness of our disclosure controls and procedures, we must commit significant resources, may be required to hire additional staff and need to continue to provide effective management oversight. Sustaining our growth also will require us to commit additional management, operational and financial resources to identify new professionals to join our company and to maintain appropriate operational and financial systems to adequately support expansion. These activities may divert management’s attention from other business concerns, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
In connection with becoming a public company, we obtained Side A directors’ and officers’ insurance coverage, which increased our annual insurance costs. In the future, it may be more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members to our board of directors in the future, particularly to serve on our audit committee, and qualified executive officers.
As an “emerging growth company” as defined in the JOBS Act, we may take advantage of certain temporary exemptions from various reporting requirements, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404(b) of SOX and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements.
When these exemptions cease to apply, we expect to incur additional expenses and devote increased management effort toward ensuring compliance with them. We will remain an “emerging growth company” for up to five years, although we may cease to be an “emerging growth company” earlier under certain circumstances. We cannot predict or estimate the amount of additional costs we may incur as a result of becoming a public company or the timing of such costs.
As a public reporting company, we are subject to rules and regulations established from time to time by the SEC regarding our internal control over financial reporting. In connection with our assessment of the effectiveness of our disclosure controls and procedures, we identified certain material weaknesses in our internal control over financial reporting, which caused our Chief Executive Officer and Chief Financial Officer to determine that our internal control over financial reporting, as well well our disclosure controls and procedures, were not effective as of December 31, 2020.
As a public reporting company, we are subject to the rules and regulations established from time to time by the SEC. These rules and regulations require that, among other things, we establish and periodically evaluate procedures with respect to our internal control over financial reporting. Reporting obligations as a public company are likely to place a considerable strain on our financial and management systems, processes and controls, as well as on our personnel.
Our management, including our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act). Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2020. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2020, the Company has not maintained effective internal control over financial reporting as a result of the existence of material weaknesses. Consequently, management, with the participation of our Chief Executive Officer and Chief Financial Officer, also concluded that our disclosure controls and procedures were not effective as of December 31, 2020 to provide reasonable assurance that information required to be disclosed by the Company in the reports filed or submitted by it
under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC's rules and forms, and to provide reasonable assurance that information required to be disclosed by the Company in such reports is accumulated and communicated to the Company’s management, including, our Chief Executive Officer and our Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
A “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis. Although we are implementing measures to remediate the material weaknesses, we cannot give any assurances that the identified material weaknesses will be remediated on a timely basis or at all or that additional material weaknesses will not be identified in the future in connection with our compliance with the provisions of Section 404 of SOX. Our management may be required to devote significant time and expense to remediate these material weaknesses and any other material weaknesses that may be discovered in the future and may not be able to remediate such material weaknesses in a timely manner. The existence of any future material weakness in our internal control over financial reporting could also result in errors in our financial statements that could require us to restate our financial statements, cause us to fail to meet our reporting obligations, and cause investors to lose confidence in our reported financial information, any of which could lead to a decline in the per share trading price of our common stock.
Because we are an "emerging growth company" under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting for so long as we are an emerging growth company. Our independent registered public accounting firm will be engaged to provide an attestation report on the effectiveness of our internal control over financial reporting at such time as we cease to be an ‘‘emerging growth company,’’ as defined in the JOBS Act.
We have not paid dividends in the past and have no current plans to pay dividends in the future, and any return on investment may be limited to the value of our common stock.
We do not anticipate paying cash dividends in the foreseeable future. The payment of dividends will depend on our earnings, capital requirements, financial condition, prospects and other factors our board of directors may deem relevant. If we do not pay dividends, our stock may be less valuable because a return on your investment will only occur if you sell our Class A common stock after our stock price appreciates above the price at which you acquired such shares.
Future sales of our Class A common stock in the public market, or the perception that such sales may occur, could reduce our stock price, and any additional capital raised by us through the sale of equity or convertible securities may dilute your ownership in us.
Subject to certain limitations and exceptions, the members of the Operating Company may redeem their Common Units for shares of Class A common stock (in which case, their shares of Class B common stock or Class C common stock, as the case may be, will be cancelled on a one-to-one basis in the case of Class B common stock or three-to-one basis in the case of Class C common stock upon any such issuance), and then sell those shares of Class A common stock. Additionally, we may issue additional shares of Class A common stock or convertible securities in subsequent public or private offerings.
We cannot predict the timing of any redemption of Common Units or the size of future issuances of our Class A common stock or securities convertible into Class A common stock or the effect, if any, that future issuances and sales of shares of our Class A common stock will have on the market price of our Class A common stock. Sales of substantial amounts of our Class A common stock (including shares issued in connection with an acquisition), or the perception that such sales could occur, may adversely affect prevailing market price of our Class A common stock.
If securities analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our stock depends in part on the research and reports that securities or industry analysts publish about us or our industry. While there are currently securities analysts covering us, we can provide no assurances that the analysts will continue to publish report or that other securities analysts will initiate coverage. If no securities analysts cover our company, the trading price for our stock could be negatively impacted. In addition, if one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price could decline as a result. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our Class A stock could decrease, which might cause the market price and trading volume of our Class A common stock to decline.
Anti-takeover provisions in our certificate of incorporation and amended and restated bylaws and Delaware law could discourage a takeover.
Our amended and restated certificate of incorporation and our amended and restated bylaws contain provisions that might enable our management to resist a takeover. These provisions include:
•authorizing the issuance of “blank check” preferred stock that could be issued by our board of directors to increase the number of outstanding shares and thwart a takeover attempt;
•advance notice requirements applicable to stockholders for matters to be brought before a meeting of stockholders and requirements as to the form and content of a stockholder’s notice;
•restrictions on the transfer of our outstanding shares of Class B common stock and Class C common stock;
•a supermajority stockholder vote requirement for amending certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws;
•the inability of our stockholders to act by written consent;
•a requirement that the authorized number of directors may be changed only by resolution of the board of directors;
•allowing all vacancies, including newly created directorships, to be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum, except as otherwise required by law;
•limiting the forum for certain litigation against us to Delaware; and
•limiting the persons that can call special meetings of our stockholders to our board of directors or the chairperson of our board of directors.
These provisions might discourage, delay or prevent a change in control of our company or a change in our board of directors or management. The existence of these provisions could adversely affect the voting power of holders of Class A common stock and limit the price that investors might be willing to pay in the future for shares of our Class A common stock. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any “interested” stockholder for a period of three years following the date on which the stockholder became an “interested” stockholder.
We may issue shares of preferred stock in the future, which could make it difficult for another company to acquire us or could otherwise adversely affect holders of our Class A common stock, which could depress the market price of our Class A common stock.
Our amended and restated certificate of incorporation authorizes us to issue one or more series of preferred stock. Our board of directors has the authority to determine the preferences, limitations and relative rights of the shares of preferred stock and to fix the number of shares constituting any series and the designation of such series, without any further vote or action by our stockholders. Our preferred stock can be issued with voting, liquidation, dividend and other rights superior to the rights of our Class A common stock. The potential issuance of preferred stock may delay or prevent a change in control of us, discourage bids for our Class A common stock at a premium to the market price, and materially and adversely affect the market price and the voting and other rights of the holders of our Class A common stock.
Our amended and restated certificate of incorporation and bylaws provide that the Court of Chancery of the State of Delaware is the sole and exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation and our amended and restated bylaws provide that, unless we consent to the selection of an alternative forum, the Court of Chancery of the State of Delaware is the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, other than any action or proceeding that, under applicable law, may only be commenced or prosecuted in another forum, (ii) any action asserting a claim of breach of fiduciary duty owed by any of our directors, officers or other employees to us or to our stockholders, (iii) any action asserting a claim arising pursuant to the Delaware General Corporation Law or our amended and restated certificate of incorporation or bylaws (iv) any action to interpret, apply, enforce or determine the validity of our amended and restated certificate of incorporation.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We own our headquarters in Boca Raton, Florida with approximately 50,000 square feet of office space, which includes office space leased to third-party tenants. We have also entered into leases for distribution centers in the United States and Europe, administrative office locations in the United States and Europe, and retail stores in the United States and Europe. We believe that our facilities are adequate for our current global operational needs and we are capable of acquiring or leasing additional space as necessary.
ITEM 3. LEGAL PROCEEDINGS
For information regarding legal proceedings as of December 31, 2020, see "Note 7—Commitments and Contingencies" of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our Class A common stock has been listed on the Nasdaq Global Select Market under the symbol "GNLN" since April 18, 2019. Prior to that time, there was no public market for our stock. Our Class B common stock and Class C common stock are neither listed nor traded.
Holders
As of December 31, 2020, there were approximately 21 stockholders of record of our Class A common stock. Since certain of our shares of Class A common stock are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders. As of December 31, 2020, there were approximately 17 and 3 stockholders of record of our Class B common stock and Class C common stock, respectively.
Dividends
We have never declared or paid any cash dividend on our Class A common stock. Holders of our Class B common stock and our Class C common stock are not entitled to participate in any dividends declared by our board of directors. We intend to retain any future earnings and do not expect to pay cash dividends in the foreseeable future.
Unregistered Sales of Equity Securities
In August 2020, we issued an aggregate of 15,000 shares of Class A common stock as compensation for certain executive officers. These shares were issued in reliance of an exemption from registration pursuant to Section 4(a)(2) of the Securities Act of 1933.
During the fourth quarter of 2020, we issued an aggregate of 100,000 shares of Class A common stock in exchange for an equivalent number of shares of Class B common stock and Common Units of the Operating Company pursuant to the terms of our Amended and Restated Certificate of Incorporation and the Operating Company's Third Amended and Restated Operating Agreement. These shares were issued in reliance on an exemption from registration pursuant to Section 4(a)(2) of the Securities Act of 1933.
During the fourth quarter of 2020, we issued an aggregate of 150,000 shares of Class A common stock in exchange for 450,000 shares of Class C common stock and 150,000 Common Units of the Operating Company pursuant to the terms of our Amended and Restated Certificate of Incorporation and the Operating Company's Third Amended and Restated Operating Agreement. These shares were issued in reliance on an exemption from registration pursuant to Section 4(a)(2) of the Securities Act of 1933.
Use of Proceeds from Registered Securities
On April 23, 2019, we completed our IPO of 6,000,000 shares of Class A common stock, which was comprised of 5,250,000 shares of Class A common stock sold by Greenlane and 750,000 shares sold by certain selling stockholders, in each case at a public offering price of $17.00 per share. On April 29, 2019, the underwriters purchased an additional 450,000 shares of Class A common stock from selling stockholders pursuant to the partial exercise of their option to purchase additional shares in the IPO. We received aggregate net proceeds of approximately $79.5 million, after deducting the underwriting discounts and commissions and offering expenses. We used approximately $3.1 million of the proceeds from the IPO to fund a portion of the purchase price of the Conscious Wholesale business acquisition. Effective March 2, 2021, we acquired the assets of Eyce LLC, for which we used approximately $2.5 million of the proceeds from the IPO to fund a portion of the purchase price. We have used and intend to continue using the remainder of the net proceeds for working capital and general corporate purposes, including to fund seller financing and potential contingent payments due under the asset purchase agreement with Eyce LLC, possible investments in, and acquisitions of, complementary companies or their assets, businesses, partnerships, minority
investments, products or technologies. Other than as described above, we currently have no other commitments or agreements regarding any such acquisitions or investments. All shares were sold pursuant to a registration statement on Form S-1, as amended (File No. 333-230405), which was declared effective by the SEC on April 17, 2019. Cowen and Company, LLC and Canaccord Genuity LLC served as representatives of the several underwriters in the offering.
ITEM 6. SELECTED FINANCIAL DATA
Not required.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are one of the largest global sellers of premium cannabis accessories and liquid nicotine products in the world. We operate as a powerful house of brands, third party brand accelerator and distribution platform for consumption devices and lifestyle brands. We have expanded our global reach, serving the global cannabis, hemp-derived CBD, and liquid nicotine markets with an expansive customer base, generating an average 6 orders per minute from over 1,100 licensed cannabis dispensaries, and 4,100 smoke and vape shops around the world.
Over the course of 2020, we shifted away from a high-volume and low-margin sales mix to a lower-volume and higher-margin mix, with a focus on our Greenlane Brands. As evidence of this shift, sales from nicotine products decreased to 9.5% of total net sales from 39.9%, while Greenlane Brand sales increased to 16.5% of total net sales as for the year ended December 31, 2020, from 8.3% of total net sales for the year ended December 31, 2019. When including 2020 Eyce product sales, which were integrated into our Greenlane Brand products effective March 2, 2021, our Greenlane Brand sales would have represented 19.7% of total net sales for the year ended December 31, 2020. The increased focus on Greenlane Brand sales resulted in a year-over-year growth rate of approximately 49.2% for brands including Vibes Rolling Papers, Marley Natural, K. Haring Glass Collection and Higher Standards, and resulted in an increase in total Greenlane Brand sales of approximately $7.5 million. The significant growth rate was especially evident in the fourth quarter of 2020, wherein:
•Greenlane Brand sales reached a record $6.3 million for the fourth quarter of 2020, or approximately 17.5% of total net sales for the fourth quarter of 2020; when including Eyce product sales, Greenlane Brands reached a record $7.8 million, or approximately 21.4% of total net sales for the fourth quarter of 2020, and
•Vibes Rolling Papers, Marley Natural, K. Haring Glass Collection, and Aerospaced posted record quarterly sales figures with quarter-over-quarter growth of 53.6%, 68.3%, 73.1% and 24.5%, respectively.
Given the emphasis on increased international market penetration, including the marketing of Vibes Rolling Papers in Europe, our recent acquisition of Eyce LLC, and an increasingly diverse product mix including the introduction of new house brands and product lines in the coming years, we believe our brands have the ability to reach customers across multiple markets and demographics. Coupled with recent strategic business arrangements, such as the launch of Canada.Vapor.com and the expansion of Vibes Rolling papers, we expect the growth trend to continue over the coming years.
We have also restructured our commercial departments to allow us to have a more structured and focused approach as we prepare for category maturation. We have made significant investments into our management team by adding seasoned additions to the Sales, Marketing and E-Commerce departments at the Vice President level. Additionally, we have added a field sales department in both the United States and Canada. We feel that this approach will ensure a stronger relationship and insights with our growing brick and mortar account base, as well the ability to develop merchandising solutions and final consumer engagement.
In December 2019, a novel strain of coronavirus known as COVID-19 was reported in Wuhan, China. In March 2020, the World Health Organization declared the outbreak of COVID-19 a pandemic. Since the outbreak of COVID-19, we have closely monitored developments and operated with the health and safety of our employees as the Company's top priority. As of the current date listed on this filing, all of our retail locations are open (although they were closed for portions of 2020), and our B2B revenues have not seen any additional setbacks since the quarter ended June 30, 2020, with the third and fourth quarters reporting sales increases of 29.8% and 27.1% over the second quarter, respectively. Our corporate headquarters in Boca Raton, FL remains open; we implemented social distancing guidelines and continue to adhere to CDC guidelines and recommendations.
Although the impact of the COVID-19 pandemic has not had a significant adverse impact on our operations, we cannot reasonably estimate the length or severity of this pandemic on the macroeconomic environment which we operate in. Accordingly, the extent to which the COVID-19 pandemic will impact our financial condition or results of operations will depend on future developments that of the date of this Form 10-K, include but not limited to the following:
•the duration and intensity of the pandemic, as well as the timing and effectiveness of COVID-19 vaccines and treatments;
•the impact on our customers, including their ability to remain in business and make payments to us in the ordinary course;
•the impact on end-user demand for our products, including whether any scientific findings demonstrate smoking or vaping negatively impact health outcomes of individuals who contract COVID-19;
•our ability to hold and attend employee and industry events;
•our ability to operate our retail stores;
•our employees' ability to work effectively in a remote work environment;
•our ability to continue operating our distribution centers;
•our ability to capitalize on any new consumer trends resulting from the pandemic; and
•the pandemic's effect on our vendors.
Merger with KushCo
On March 31, 2020, we announced the entry into an Agreement and Plan of Merger with KushCo with respect to the KushCo Transaction. For more information, see "Note 13—Subsequent Events" of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K.
Critical Accounting Policies and Estimates
We prepare our consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. We evaluate our estimates and assumptions on an ongoing basis. We base our estimates on historical experience, outside advice from parties believed to be experts in such matters, and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Judgments and uncertainties affecting the application of those policies may result in materially different amounts being reported under different conditions or using different assumptions. See "Note 2—Summary of Significant Accounting Policies" of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K for a description the significant accounting policies and methods used in the preparation of our consolidated financial statements.
Inventories
Inventories, consisting of finished products, are primarily accounted for using the weighted-average method, and are valued at the lower of cost and net realizable value. This valuation requires us to make judgments, based on currently available information, about the likely method of disposition, such as through sales to customers or liquidations. Assumptions about the future disposition of inventory are inherently uncertain and changes in our estimates and assumptions may cause us to realize material write-downs in the future.
Valuation of Goodwill
Assets acquired and liabilities assumed in business combinations are generally recognized at the date of acquisition at their respective fair values. Any excess of the purchase price over the estimated fair values of the net assets acquired is recognized as goodwill. Subsequent to acquisition, goodwill is tested at least annually for impairment, or when events or changes in circumstances indicate it is more likely than not that the carrying amount of goodwill may not be recoverable. During the first quarter of 2020, we determined that the estimated fair value of the United States reporting unit was below its carrying value, and we recorded a goodwill impairment charge of approximately $9.0 million, which is included in the consolidated statement of operations and comprehensive loss for the year ended December 31, 2020.
Our annual assessment may consist of a qualitative or quantitative analysis to determine whether it is more likely than not that fair value exceeds the carrying value. When performing a qualitative analysis, the factors we consider include our share price, our projected financial performance, long-term financial plans, macroeconomic, industry and market conditions as well as the results of our most recently completed annual impairment test.
When performing a quantitative analysis, we use a combination of an income approach, using discounted cash flow techniques, and market valuation methods, using the guideline public company method, and may weigh the outcomes of valuation approaches when estimating the fair value of each reporting unit. We then compare the fair value to its carrying amount to determine the amount of impairment, if any. If a reporting unit’s fair value is less than its carrying amount, we record
an impairment charge based on that difference, up to the amount of goodwill allocated to that reporting unit. Inputs and assumptions used to determine fair value are determined from a market participant view, which might be different than our specific views. The valuation process is complex and requires significant input and judgment. Market approaches depend on the availability of guideline companies and representative transactions. When using the income approach, complex and judgmental matters applicable to the valuation process include projections of future revenues, which are estimated after considering many factors such as historical results, market opportunity, pricing, and sales trajectories.
The estimated fair value of a reporting unit is highly sensitive to changes in projections and assumptions; therefore, in some instances, changes in these assumptions could potentially lead to impairment. Ultimately, future potential changes in these assumptions may impact the estimated fair value of a reporting unit and cause the fair value of the reporting unit to be below its carrying value. We believe that our estimates are consistent with the assumptions that market participants would use in their fair value determination.
Income Taxes and TRA Liability
We are subject to U.S. federal, state and foreign income taxes with respect to our allocable share of any taxable income or loss of Greenlane Holdings, LLC and will be taxed at the prevailing corporate tax rates on such income. Significant judgment is required in determining our provision or benefit for income taxes and in evaluating uncertain tax positions. We account for income taxes under the asset and liability method, which requires the recognition of deferred tax assets or deferred tax liabilities for the expected future tax consequences of events included in our financial statements.
Greenlane Holdings, LLC is a limited liability company and is treated as a partnership for U.S. federal and most applicable state and local income tax purposes. As a result, we are not liable for U.S. federal or state and local income taxes in most jurisdictions in which we operate, and the income, expenses, gains and losses are reported on the returns of our members. Greenlane Holdings, LLC is subject to Canadian, Dutch, and U.S. state and local income tax in certain jurisdictions in which it is not treated as a partnership for income tax purposes, and in which jurisdictions it pays an immaterial amount of taxes.
In addition to tax expenses, we may incur expenses related to our operations and may be required to make payments under the Tax Receivable Agreement (the "TRA"), which could be significant. Pursuant to the Greenlane Operating Agreement, Greenlane Holdings, LLC will generally make pro rata tax distributions to its members in an amount sufficient to fund all or part of their tax obligations with respect to the taxable income of Greenlane Holdings, LLC that is allocated to them and possibly in excess of such amount.
Legal Contingencies
In the ordinary course of business, we are involved in legal proceedings involving a variety of matters. Certain of these matters include speculative claims for substantial or indeterminate amounts of damages. We evaluate the associated developments on a regular basis and accrue a liability when we believe that it is both probable that a loss has been incurred and the amount can be reasonably estimated. If we determine there is a reasonable possibility that we may incur a loss and the loss or range of loss can be estimated, we disclose the possible loss in the accompanying notes to the consolidated financial statements to the extent material.
We review the developments in our contingencies that could affect the amount of the provisions that have been previously recorded, and the matters and related reasonably possible losses disclosed. We make adjustments to our provisions and changes to our disclosures accordingly to reflect the impact of negotiations, settlements, rulings, advice of legal counsel, and updated information. Significant judgment is required to determine both the probability of loss and the estimated amount of loss.
The outcome of these matters is inherently uncertain. Therefore, if one or more of these matters were resolved against us for amounts in excess of management's expectations, our results of operations and financial condition, including in a particular reporting period in which any such outcome becomes probable and estimable, could be materially adversely affected. See "Note 7—Commitments and Contingencies" of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K for additional information regarding these contingencies.
Recent Accounting Pronouncements
See "Note 2—Summary of Significant Accounting Policies" of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K.
Results of Operations
The following table presents operating results as a percentage of total net sales:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31, | | | | |
| | | | | % of Net sales | | Change |
| 2020 | | 2019 | | 2020 | | 2019 | | $ | | % |
| Net sales: | | | | | | | | | | | |
| United States | $ | 112,543 | | | $ | 160,243 | | | 81.3 | % | | 86.6 | % | | $ | (47,700) | | | (29.8) | % |
| Canada | 15,457 | | | 22,120 | | | 11.2 | % | | 12.0 | % | | (6,663) | | | (30.1) | % |
| Europe | 10,304 | | | 2,643 | | | 7.5 | % | | 1.4 | % | | 7,661 | | | 289.9 | % |
| Total net sales | 138,304 | | | 185,006 | | | 100.0 | % | | 100.0 | % | | (46,702) | | | (25.2) | % |
| Cost of sales | 115,539 | | | 153,916 | | | 83.5 | % | | 83.2 | % | | (38,377) | | | (24.9) | % |
| Gross profit | 22,765 | | | 31,090 | | | 16.5 | % | | 16.8 | % | | (8,325) | | | (26.8) | % |
| | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | |
| Salaries, benefits and payroll taxes | 24,909 | | | 29,716 | | | 18.0 | % | | 16.1 | % | | (4,807) | | | (16.2) | % |
| General and administrative | 35,315 | | | 23,593 | | | 25.5 | % | | 12.8 | % | | 11,722 | | | 49.7 | % |
| Goodwill impairment charge | 8,996 | | | — | | | 6.5 | % | | — | % | | 8,996 | | | 100.0 | % |
| Depreciation and amortization | 2,520 | | | 2,705 | | | 1.9 | % | | 1.5 | % | | (185) | | | (6.8) | % |
| Total operating expenses | 71,740 | | | 56,014 | | | 51.9 | % | | 30.4 | % | | 15,726 | | | 28.1 | % |
| Loss from operations | (48,975) | | | (24,924) | | | (35.4) | % | | (13.6) | % | | (24,051) | | | 96.5 | % |
| | | | | | | | | | | |
| Other income (expense), net: | | | | | | | | | | | |
| Change in fair value of convertible notes | — | | | (12,063) | | | — | % | | (6.5) | % | | 12,063 | | | (100.0) | % |
| Interest expense | (437) | | | (975) | | | (0.3) | % | | (0.5) | % | | 538 | | | (55.2) | % |
| Other income, net | 1,902 | | | 9,073 | | | 1.4 | % | | 4.9 | % | | (7,171) | | | (79.0) | % |
| Total other expense, net | 1,465 | | | (3,965) | | | 1.1 | % | | (2.1) | % | | 5,430 | | | * |
| Loss before income taxes | (47,510) | | | (28,889) | | | (34.3) | % | | (15.7) | % | | (18,621) | | | 64.5 | % |
| Provision for income taxes | 194 | | | 10,935 | | | 0.1 | % | | 5.9 | % | | (10,741) | | | (98.2) | % |
| Net loss | (47,704) | | | (39,824) | | | (34.4) | % | | (21.6) | % | | (7,880) | | | 19.8 | % |
| Net loss attributable to non-controlling interest | (33,187) | | | (11,008) | | | (24.0) | % | | (6.0) | % | | (22,179) | | | 201.5 | % |
| Net loss attributable to Greenlane Holdings, Inc. | $ | (14,517) | | | $ | (28,816) | | | (10.4) | % | | (15.6) | % | | $ | 14,299 | | | (49.6) | % |
*Not meaningful
Net Sales
United States
For the year ended December 31, 2020, our United States operating segment reported net sales of approximately $112.5 million, compared to approximately $160.2 million for the year ended December 31, 2019, representing a decrease of $47.7 million, or 29.8%. The year-over-year decrease was primarily due to a $54.3 million dollar decrease in nicotine product revenue as a result of the January 2, 2020 U.S. Food and Drug Administration's ban on flavored vape pods, which adversely impacted our B2B wholesale revenue channel, coupled with our shift away from a high-volume, low-margin sales mix. This decrease in B2B wholesale revenue was partially offset by an increase in the United States e-commerce net sales of $6.5 million, or an 86.7% year-over-year growth. This $6.5 million increase was primarily driven by an increase in Vapor.com's net sales of approximately $2.6 million, or 87.9%, compared to the prior year, and an aggregate increase in net sales our other websites, such as Higherstandards.com, Marleynatural.com and Gpen.com, of approximately $3.9 million, or 84.6%.
Canada
For the year ended December 31, 2020, our Canadian operating segment reported net sales of approximately $15.5 million, compared to approximately $22.1 million for the year ended December 31, 2019, representing a decrease of $6.7 million, or 30.1%, primarily due to regulatory restrictions on the sale of flavored vape pods. Similar to the results in our United States operating segment, the decrease in net sales in our Canadian operating segment was entirely observed in our B2B
wholesale operations, which reported a net sales decrease of approximately $6.7 million, or 34.8%. These impacts were the result of the aforementioned regulatory restrictions, paired with temporary customer store closures resulting from the onset of COVID-19, and the revocation of our PAX exclusivity agreement. On October 27, 2020, we launched Canada.Vapor.com, with the expectation of expanding the results seen from Vapor.com in the United States. We believe that expanding our E-commerce business will provide our Canadian operating segment with higher-margin sales, as we are able to sell products at full retail pricing. We expect to see long-term sustained growth in the customer base; juxtaposing the explosive growth of its United States counterpart, Vapor.com, we expect gradual but healthy and organic growth in Canada.Vapor.com.
Europe
For the year ended December 31, 2020, after completing the first full year of operations since our acquisition of Conscious Wholesale on September 30, 2019, our European operations reported net sales of approximately $10.3 million. Although our European operations endured significant challenges over the course of the year, including a change in leadership personnel in Europe and significant COVID-19 restrictions that adversely impacted retail and supply chain operations, our European operating segment still produced promising results, with record fourth quarter revenue and growth rates. Specifically, net sales for the quarter ended December 31, 2020 were approximately $3.1 million, representing growth of approximately $0.8 million, or 32.4%, over the third quarter of 2020. Additionally, total net sales for our European segment for the last two quarters of 2020 increased by approximately $0.5 million, or 10.9%, compared to the first two quarters of 2020.
Cost of Sales and Gross Margin
For the year ended December 31, 2020, cost of sales decreased by $38.4 million, or 24.9%, as compared to the year ended December 31, 2019. The decrease in cost of sales was primarily due to an overall sales volume decrease of $47.7 million. Refer to Item 7 - "Net Sales" above for additional information on sales volume. This decrease was partially offset by an approximated $11.3 million, or 176.1%, increase in non-merchandise cost of sales for the year ended December 31, 2020, compared to the same period in 2019, primarily due to an increase in damaged and obsolete inventory of approximately $3.2 million in the third quarter of 2020. These charges were related to write-offs and lower of cost or net realizable value adjustments, which were made by management as part of a strategic initiative to free-up warehouse space for products with higher margins and higher marketability, and to increase inventory turnover for certain slow-moving products.
United States
For the year ended December 31, 2020, gross margin was materially impacted by significant damaged and obsolete inventory adjustments during the third quarter of 2020 driven by management's strategic initiatives, mitigating margin improvements from our shift to a lower-volume but higher-margin sales mix. Accordingly, year-over-year gross margin for our United States operating segment decreased to 15.7% for the year ended December 31, 2020, down from approximately 16.7% for the year ended December 31, 2019, representing a $9.1 million decrease in gross profit. Excluding the aforementioned strategic inventory adjustments of $3.2 million during the year ended December 31, 2020, gross profit margin would have increased to 18.5% for the year ended December 31, 2020. There were no equivalent adjustments during the year ended December 31, 2019.
Canada
For the year ended December 31, 2020, gross margin decreased to 15.2% in 2020 from 15.9% for the year ended December 31, 2019, representing a $1.2 million decrease in gross profit. Unlike our United States operating segment, our Canadian operating segment's gross profit margin was not significantly impacted by damaged and obsolete inventory adjustments. However, the gross margin for our Canadian operating segment was hindered by a reduction sales from regulatory restrictions of sales of nicotine products, which accounted for 42.0% of our Canadian operating segment's total revenue for the year ended December 31, 2020 compared to 52.2% of total revenue for the year ended December 31, 2019. The market for these nicotine products has changed drastically, with competitive pricing and discounts driven by the fear of both adverse market conditions resulting from COVID-19 and increased regulation. We expect future shifts in Canadian market away from nicotine products similar to the shifts observed in the United States market during 2020, which will lead to substitution of these low-margin, high-velocity products to high-margin, low-velocity products.
Europe
Through the acquisition of Conscious Wholesale during the third-quarter of 2019, we began operations in the Netherlands and expanded our reach to other European countries. For the year ended December 31, 2020, our European operating segment's gross margin of 26.8% contributed to approximately $2.8 million in gross profit, representing approximately 12.1% of consolidated gross profit. For the quarter ended December 31, 2020, our European operating segment's gross margin of 31.4% was relatively consistent with the prior quarter ended December 31, 2019, in which we reported a gross margin of approximately 31.6%. Our European operating segment's 2020 gross margin was driven by its B2C sales, specifically e-commerce, which accounted for approximately $4.4 million, or 42.4%, of our European operating segment's net sales, compared to B2B revenues, which represented $4.2 million, or 41.0%, of net sales. This shift was primarily influenced by the
impact of the COVID-19 pandemic; however, we expect to continue capitalizing on the increase in our E-commerce sales, as these offer our business access to retail pricing and the ability to double margins compared to those of B2B wholesale transactions.
Operating Expenses
Salaries, Benefits and Payroll Taxes
Salaries, benefits and payroll taxes expenses decreased by approximately $4.8 million, or 16.2%, for the year ended December 31, 2020, compared to the same period in 2019, primarily due to a decrease in stock compensation expense of approximately $7.1 million. Specifically, we recognized $0.9 million in stock compensation expense during the year ended December 31, 2020, which was significantly reduced due to actual forfeitures of unvested equity awards held by former officers, as compared to approximately $8.0 million of stock compensation expense during the same period for 2019. This decrease in stock compensation expense was offset by increases of approximately $1.4 million in employee wages, primarily due to securing talent in several senior and executive level roles during the year ended December 31, 2020.
As part of our transformation initiative, we reduced our workforce by an aggregate of 93 employees during the year ended December 31, 2020, primarily in Q1 2020 and Q3 2020. The impact of these reductions in force resulted in a reduction in our salaries, benefits and payroll tax expenses of approximately $3.5 million for the year ended December 31, 2020. These personnel reduction efforts will eliminate approximately $5.8 million of recurring salaries and benefits charges annually.
As we continue to closely monitor the evolving business landscape, including the impacts of COVID-19 on our customers, vendors, and overall business performance, we remain focused on identifying cost-saving opportunities while delivering on our strategy to recruit, train, promote and retain the most talented and success-driven personnel in the industry. Management is continuing to explore opportunities in 2021 to further reduce salary expenses and other operating expenses.
General and Administrative Expenses
General and administrative expenses increased by approximately $11.7 million, or 49.7%, for the year ended December 31, 2020, compared to the same period in 2019. This increase was primarily due to an increase of approximately $2.0 million in subcontractor fees related to our enterprise resource planning ("ERP") system implementation and additional labor associated with the closing and consolidation of our distribution centers; an increase of $2.5 million in third party logistics costs, directly incurred as part of the transition of our distribution centers to our new 3PL facilities in Kentucky and Canada; a loss of approximately $4.5 million related to an indemnification asset which was not probable of recovery; approximately $1.3 million in additional accounting fees, driven by a combination of incremental fees from the change in auditors in the third quarter of 2019, whose fees were substantially higher than the predecessor, and due diligence related to acquisition targets in late 2019 and early 2020; an increase of approximately $1.2 million in severance related costs associated with our restructuring plan during the period; and impairment charges of approximately $0.4 million recorded during the year ended December 31, 2020, related to assets classified as held-for-sale during the year. These increases were offset by a decrease in marketing expenses of approximately $1.0 million, primarily driven by the decrease in trade show activity in fiscal 2020 in direct response to COVID-19 lockdown and social distancing protocols during the period.
Impairment Charge
Due to market conditions and estimated adverse impacts from the COVID-19 pandemic, management concluded that a triggering event occurred in the first quarter of 2020, requiring a quantitative impairment test of our goodwill for our United States and Europe reporting units. Based on this assessment, we concluded that the fair value of our Europe reporting unit exceeded its carrying value and no impairment charge was required. However, the estimated fair value of the United States reporting unit was determined to be below its carrying value, which resulted in a $9.0 million goodwill impairment charge, recorded in the first quarter of 2020. We did not recognize incremental impairment charges to goodwill as a result of our annual impairment assessment as of December 31, 2020.
Depreciation and Amortization Expenses
Depreciation and amortization expense remained relatively consistent for the year ended December 31, 2020, compared to the same period in 2019, only slightly decreasing due to the disposition of fixed assets in connection with our distribution center consolidation initiative in 2020.
Other Income (Expense), Net
Change in fair value of convertible notes.
We accounted for the convertible notes issued in December 2018 and January 2019 at fair value with changes in the fair value recognized in the consolidated statement of operations and comprehensive loss as a component of other income
(expense), net for the year ended December 31, 2019. The convertible notes were converted to shares of Class A common stock in conjunction with the completion of the IPO in April 2019. There were no changes in fair value of convertible notes recognized during the year ended December 31, 2020.
Interest expense.
Interest expense consists of interest incurred on our Real Estate Note, line of credit and other debt obligations, as well as debt issuance costs related to the convertible notes issued in December 2018 and January 2019.
Other income, net.
Other income (expense), net, increased by approximately $5.4 million for the year ended December 31, 2020 compared to the same period in 2019, primarily due a change in the fair value of our convertible notes payable during the year ended December 31, 2019, which resulted in an expense of approximately $12.1 million, with no corresponding expense in 2020. This increase in 2019 was offset by a gain of approximately $7.2 million recognized in the year ended December 31, 2019, resulting from the reversal of the TRA liability, as well as an unrealized gain of $1.5 million recognized on our equity securities investment in Airgraft Inc., in the same period. Additionally, we recognized a gain from the fair value adjustment of contingent consideration of approximately $0.7 million during the year ended December 31, 2020, which was largely attributed to changes in forecasted revenues and gross profits for our European operating segment over the remainder of 2020, driven primarily by the impacts of the COVID-19 pandemic. We also experienced a reduction of interest expense of approximately $0.5 million during the year ended December 31, 2020, due to the absence of debt issuance costs that were reflected in the year ended December 31, 2019.
Provision for Income Taxes
As a result of the IPO and the related transactions (defined in "Note 1—Business Operations and Organizations" of the Notes to Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K), we own a portion of the Common Units of the Operating Company, which is treated as a partnership for U.S. federal and most applicable state and local income tax purposes. As a partnership, the Operating Company is not subject to U.S. federal and certain state and local income taxes. Any taxable income or loss generated by the Operating Company is passed through to, and included in the taxable income or loss of, its members, including us, in accordance with the terms of the Operating Agreement. We are subject to federal income taxes, in addition to state and local income taxes with respect to our allocable share of the Operating Company’s taxable income or loss.
As discussed above, prior to the consummation of the IPO, the provision for income taxes included only income taxes on income from the Operating Company’s Canadian subsidiary, based upon an estimated annual effective tax rate of approximately 15.0%. After the consummation of the IPO, Greenlane became subject to U.S. federal, state and local income taxes with respect to Greenlane’s allocable share of the Operating Company’s taxable income or loss. Furthermore, after completing the Conscious Wholesale acquisition in September 2019, the Operating Company became subject to Dutch income taxes on income from its Netherlands-based subsidiary, based upon an estimated effective tax rate of approximately 25.0%.
During the third quarter of 2019, management performed an assessment of our ability to realize our deferred tax assets based upon which management determined that it is not more likely than not that our results of operations will generate sufficient taxable income to realize portions of the net operating loss benefits. Consequently, we established a full valuation allowance against our deferred tax assets, thus reducing the carrying balance to $0. In the event that management determines that we would be able to realize our deferred tax assets in the future in excess of their net recorded amount, an adjustment to the valuation allowance will be made which would reduce the provision for income taxes.
Key Metrics and Non-GAAP Financial Measures
We monitor the following key metrics to help us measure and evaluate the effectiveness of our operations, develop financial forecasts, and make strategic decisions:
| | | | | | | | | | | | | | |
| | For the year ended December 31, |
| ($ in thousands) | | 2020 | | 2019 |
| Net sales | | $ | 138,304 | | | $ | 185,006 | |
| Period-over-period change | | (25.2) | % | | 3.4 | % |
| Net cash used in operations | | $ | (12,302) | | | $ | (36,903) | |
Adjusted net loss (1) | | $ | (25,863) | | | $ | (18,544) | |
Adjusted EBITDA (1) | | $ | (24,352) | | | $ | (13,424) | |
(1) Adjusted Net Loss and Adjusted EBITDA are non-GAAP financial measures. For the definitions and reconciliation of Adjusted Net Loss and Adjusted EBITDA to net loss, see “ Non-GAAP Financial Measures.”
Non-GAAP Financial Measures
Adjusted Net Income (Loss) is defined as net loss before equity-based compensation expense, changes in the fair value of our convertible notes, debt placement costs for the convertible notes, and non-recurring expenses primarily related to our transition to being a public company. The debt placement costs related to the convertible notes issued in January 2019 are reported within the "interest expense" line item in our consolidated statement of operations and comprehensive loss for the years ended December 31, 2020 and 2019. Non-recurring expenses related to our transition to being a public company, which are reported within "general and administrative expenses" in our consolidated statements of operations and comprehensive loss, represent fees and expenses primarily attributable to consulting fees and incremental audit and legal fees.
Adjusted EBITDA is defined as net loss before interest expense, income tax expense, depreciation and amortization expense, equity-based compensation expense, other income, net (which includes a gain recognized on an equity investment and a gain due to the adjustment of our TRA liability), changes in fair value of our convertible notes, and non-recurring expenses primarily related to our transition to being a public company. These non-recurring expenses, which are reported within general and administrative expenses in our consolidated statements of operations and comprehensive loss, represent fees and expenses primarily attributable to consulting fees and incremental audit and legal fees.
We disclose Adjusted Net Income (Loss) and Adjusted EBITDA, which are non-GAAP performance measures, because management believes these metrics assist investors and analysts in assessing our overall operating performance and evaluating how well we are executing our business strategies. You should not consider Adjusted Net Income (Loss) or Adjusted EBITDA as alternatives to net loss, as determined in accordance with U.S. GAAP, as indicators of our operating performance. Adjusted Net Income (Loss) and Adjusted EBITDA have limitations as an analytical tool. Some of these limitations are:
•Although depreciation and amortization are non-cash charges, the assets being depreciated and amortized may have to be replaced in the future and adjusted EBITDA does not reflect capital expenditure requirements for such replacements or for new capital expenditures;
•Adjusted EBITDA does not include interest expense, which has been a necessary element of our costs;
•Adjusted EBITDA does not reflect income tax payments we may be required to make;
•Adjusted EBITDA and Adjusted Net Loss do not reflect equity-based compensation;
•Adjusted EBITDA and Adjusted Net Loss do not reflect transaction and other costs which are generally incremental costs that result from an actual or planned transaction;
•Other companies, including companies in our industry, may calculate adjusted EBITDA differently, which reduces its usefulness as a comparative measure.
Because Adjusted Net Income (Loss) and Adjusted EBITDA do not account for these items, these measures have material limitations as indicators of operating performance. Accordingly, management does not view Adjusted Net Income (Loss) or Adjusted EBITDA in isolation or as substitutes for measures calculated in accordance with U.S. GAAP.
The reconciliation of our net loss to Adjusted Net Income (Loss) is as follows:
| | | | | | | | | | | | | | | |
| Year ended December 31, | | |
| (in thousands) | 2020 | | 2019 | | |
| Net loss | $ | (47,704) | | | $ | (39,824) | | | |
Debt placement costs for convertible notes (1) | — | | | 422 | | | |
Transition to being a public company (2) | — | | | 775 | | | |
| Equity-based compensation expense | 853 | | | 8,020 | | | |
Initial consulting costs related to ERP system implementation (3) | 215 | | | — | | | |
Restructuring expenses (4) | 1,229 | | | — | | | |
| Due diligence costs related to acquisition target | 903 | | | — | | | |
| Goodwill impairment charge | 8,996 | | | — | | | |
| Adjustments related to the product rationalization to increase inventory turnover of slow-selling products | 3,222 | | | — | | | |
Obsolete inventory charges related to management's strategic initiative (5) | 1,137 | | | — | | | |
Allowances for uncollectible vendor deposits incurred in connection with management's strategic initiative (5) | 822 | | | — | | | |
| Loss related to indemnification asset not probable of recovery | 4,464 | | | — | | | |
| Change in fair value of convertible notes | — | | | 12,063 | | | |
| Adjusted net loss | $ | (25,863) | | | $ | (18,544) | | | |
(1)Debt placement costs related to the issuance of convertible notes in January 2019.
(2)Includes certain non-recurring fees and expenses primarily attributable to consulting fees and incremental audit and legal fees incurred in connection with our IPO.
(3)Includes non-recurring expenses related to the initial project design for our planned ERP system implementation.
(4)Includes primarily severance payments for employees terminated as part of our transformation plan.
(5)Includes certain non-recurring charges related to management's strategic initiative. These adjustments were incurred liquidate inventory on hand and on order, rationalize product offerings, improve inventory turnover of slow-selling products and vacate warehouse space for products with higher margin and marketability.
The reconciliation of our net loss to Adjusted EBITDA is as follows:
| | | | | | | | | | | |
| Year ended December 31, |
| (in thousands) | 2020 | | 2019 |
| Net loss | $ | (47,704) | | | $ | (39,824) | |
Other income, net (1) | (1,902) | | | (9,073) | |
Transition to being a public company (2) | — | | | 775 | |
| Interest expense | 437 | | | 975 | |
| Provision for (benefit from) income taxes | 194 | | | 10,935 | |
| Depreciation and amortization | 2,520 | | | 2,705 | |
| Equity-based compensation expense | 853 | | | 8,020 | |
Initial consulting costs related to ERP system implementation (3) | 215 | | | — | |
Restructuring expenses (4) | 1,229 | | | — | |
| Due diligence costs related to acquisition target | 903 | | | — | |
Adjustments related to product rationalization to increase inventory turnover of slow-selling products (5) | 3,222 | | | — | |
| One-time early termination fee on operating lease in connection with moving to a centralized distribution center model | 262 | | | — | |
| Goodwill impairment charge | 8,996 | | | — | |
Inventory charges related to management's strategic initiative(5) | 1,137 | | | — | |
Allowances for uncollectible vendor deposits incurred in connection with management's strategic initiative (5) | 822 | | | — | |
| Loss related to indemnification asset not probable of recovery | 4,464 | | | — | |
| Change in fair value of convertible notes | — | | | 12,063 | |
| Adjusted EBITDA | $ | (24,352) | | | $ | (13,424) | |
(1)Includes rental and interest income, changes in the fair value of contingent consideration, and other miscellaneous income.
(2)Includes certain non-recurring fees and expenses primarily attributable to consulting fees and incremental audit and legal fees incurred in connection with our IPO.
(3)Includes non-recurring expenses related to the initial project design for our planned ERP system implementation.
(4)Includes primarily severance payments for employees terminated as part of our transformation plan.
(5)Includes certain non-recurring charges related to management's strategic initiative. These adjustments were incurred liquidate inventory on hand and on order, rationalize product offerings, improve inventory turnover of slow-selling products and vacate warehouse space for products with higher margin and marketability.
Liquidity and Capital Resources
Our primary requirements for liquidity and capital are working capital, debt service and general corporate needs. Historically, these cash requirements have been met through cash provided by operating activities and borrowings under our revolving line of credit.
As of December 31, 2020, we had approximately $30.4 million of cash, of which $2.3 million was held in foreign bank accounts, and approximately $58.2 million of working capital, which is calculated as current assets minus current liabilities, compared with approximately $47.8 million of cash, of which $0.9 million was held in foreign bank accounts, and approximately $88.7 million of working capital as of December 31, 2019. The repatriation of cash balances from our foreign subsidiaries could have adverse tax impacts or be subject to capital controls; however, these balances are generally available to fund the ordinary business operations of our foreign subsidiaries without legal or other restrictions.
On October 1, 2018, one of the Operating Company’s wholly-owned subsidiaries closed on the purchase of a building for $10.0 million, which serves as our corporate headquarters. The purchase was financed through a real estate term note (the “Real Estate Note”) in the principal amount of $8.5 million, with one of the Operating Company’s wholly-owned subsidiaries as the borrower and Fifth Third Bank as the lender. Principal amounts plus any accrued interest at a rate of LIBOR plus 2.39% are due monthly. Our obligations under the Real Estate Note are secured by a mortgage on the property.
Our future liquidity needs may also include payments in respect of the redemption rights of the Common Units held by its members that may be exercised from time to time (in the event we elect to exchange such Common Units for a cash payment in lieu of shares of Class A common stock), payments under the TRA and state and federal taxes to the extent not sheltered by our tax assets, including those arising as a result of purchases, redemptions or exchanges of Common Units for Class A common stock. Although the actual timing and amount of any payments that may be made under the TRA will vary, the payments that we will be required to make to the members may be significant. Any payments made by us to the members under the TRA will generally reduce the amount of overall cash flow that might have otherwise been available to us or to the Operating Company and, to the extent that we are unable to make payments under the TRA for any reason, the unpaid amounts
generally will be deferred and will accrue interest until paid by us; provided, however, that nonpayment for a specified period may constitute a material breach of a material obligation under the TRA and therefore may accelerate payments due under the TRA.
Despite decreases in gross profit for the year ended December 31, 2020 and the uncertainty around the ongoing COVID-19 pandemic, we believe that our cash on hand will be sufficient to fund our working capital and capital expenditure requirements, as well as our debt repayments and other liquidity requirements associated with our existing operations, for at least the the next 12 months.
In addition, we may choose to raise additional funds at any time through equity or debt financing arrangements, which may or may not be needed for additional working capital, capital expenditures or other strategic investments. Our opinions concerning liquidity are based on currently available information. To the extent this information proves to be inaccurate, or if circumstances change, future availability of trade credit or other sources of financing may be reduced and our liquidity could be adversely affected. Our future capital requirements and the adequacy of available funds will depend on many factors, including those described in the section titled “Risk Factors” in Item 1A of this Form 10-K. Depending on the severity and direct impact of these factors on us, we may be unable to secure additional financing to meet our operating requirements on terms favorable to us, or at all.
Cash Flows
The following summary of cash flows for the periods indicated has been derived from our consolidated financial statements included in Part II, Item 8 of this Form 10-K:
| | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands) | 2020 | | 2019 |
| Net cash used in operating activities | $ | (12,302) | | | $ | (36,903) | |
| Net cash used in investing activities | (4,144) | | | (3,732) | |
| Net cash (used in) provided by financing activities | (1,063) | | | 80,979 | |
Net Cash Used in Operating Activities
During 2020, net cash used in operating activities of approximately $12.3 million was a result of a net loss of $47.7 million offset by non-cash adjustments to net loss of $17.7 million, and a $17.7 million increase in cash provided by working capital primarily driven by increases in our accrued expenses and accounts payable, and decreases in inventories offset by higher other current assets.
During 2019, net cash used in operating activities of approximately $36.9 million was a result of a net loss of $39.8 million offset by non-cash adjustments to net loss of $28.0 million, and a $25.1 million increase in cash consumed by working capital primarily driven by an increase in our vendor deposits, inventories, and other current assets, and a decrease in accounts payable offset by higher accrued expenses.
Net Cash Used in Investing Activities
During 2020, we used approximately $4.1 million of cash for capital expenditures, including computer hardware and software to support our growth and development, and warehouse supplies and equipment, including the build-out of our two retail locations, and the purchase of a domain name and VIBES trademarks in Europe.
During the year ended December 31, 2019, we completed the Pollen Gear LLC and Conscious Wholesale business acquisitions, for which we paid cash consideration of $2.1 million offset by net cash acquired of $0.9 million, which resulted in net cash used of approximately $1.2 million. We also made an investment in equity securities of an entity for approximately $0.5 million, which represents a 1.49% ownership interest in the entity.
Net Cash (Used in) Provided by Financing Activities
During the year ended December 31, 2020, net cash used in financing activities primarily consisted of approximately $1.1 million in payments on other long-term liabilities, notes payable and finance lease obligations.
During 2019, cash provided by financing activities was primarily attributable to net proceeds of approximately $79.5 million from the sale of Class A common stock in the IPO, and proceeds from the issuance of convertible notes of approximately
Off-Balance Sheet Arrangements
As of December 31, 2020, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K, that have or are reasonably likely to have a current or future effect on our financial condition, changes in our
financial condition, revenues, or expenses, results of operations, liquidity, capital expenditures, or capital resources that are material to investors.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
Our primary exposure to interest rate risk relates to the amount of interest we must pay on borrowed funds under our Real Estate Note with Fifth Third Bank. As of December 31, 2020, we had approximately $7.8 million outstanding under the Real Estate Note, which is hedged by an interest rate swap agreement in which we pay a fixed annual rate of 2.0775% and receive variable interest payments monthly through maturity based on the one-month LIBOR rate. We do not believe our interest rate risk is material given the low volatility of interest rates in recent years and the current effectiveness of our interest rate swap.
Foreign Currency Risk
Our primary exposure to foreign currency risk relates to our operations in Canada and Europe through our foreign subsidiaries. Through these subsidiaries, our results of operations and cash flows are subject to fluctuations due to changes in foreign currency exchange rates, principally the Canadian dollar and the Euro. As we grow and expand the geographic reach of our operations, our exposure to foreign currency risk could become more significant; however, we believe the exposure to foreign currency fluctuations is immaterial at this time.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
| | | | | | | | |
| Index to Consolidated Financial Statements | | Page |
| | |
| | |
| | |
| | |
| | |
| | |
Report of Independent Registered Public Accounting Firm
To the shareholders and the Board of Directors of Greenlane Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Greenlane Holdings, Inc. and subsidiaries (the "Company") as of December 31, 2020 and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for the year ended December 31, 2020, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Change in Accounting Principle
As discussed in Note 2 to the financial statements, the Company changed its method of accounting for leases in 2019 due to the adoption of ASC 842, Leases.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Deloitte & Touche LLP
Boca Raton, Florida
March 31, 2021
We have served as the Company’s auditor since 2019.
GREENLANE HOLDINGS, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except par value per share amounts)
| | | | | | | | | | | |
| December 31,
2020 | | December 31,
2019 |
| ASSETS | | | |
| Current assets | | | |
| Cash | $ | 30,435 | | | $ | 47,773 | |
| Accounts receivable, net of allowance of $1,084 and $936 at December 31, 2020 and 2019, respectively | 6,330 | | | 8,091 | |
| Inventories, net | 36,064 | | | 43,060 | |
| Vendor deposits | 11,289 | | | 11,120 | |
| Assets held for sale | 1,073 | | | 0 | |
| Other current assets (Note 8) | 10,892 | | | 4,924 | |
| Total current assets | 96,083 | | | 114,968 | |
| | | |
| Property and equipment, net | 12,201 | | | 13,165 | |
| Intangible assets, net | 5,945 | | | 6,301 | |
| Goodwill | 3,280 | | | 11,982 | |
| Operating lease right-of-use assets | 3,104 | | | 4,695 | |
| Other assets | 2,037 | | | 2,091 | |
| Total assets | $ | 122,650 | | | $ | 153,202 | |
| | | |
| LIABILITIES | | | |
| Current liabilities | | | |
| Accounts payable | $ | 18,405 | | | $ | 11,310 | |
| Accrued expenses and other current liabilities (Note 8) | 19,390 | | | 10,422 | |
| Customer deposits | 2,729 | | | 3,152 | |
| Current portion of notes payable | 182 | | | 178 | |
| Current portion of operating leases | 966 | | | 1,084 | |
| Current portion of finance leases | 184 | | | 116 | |
| Total current liabilities | 41,856 | | | 26,262 | |
| | | |
| Notes payable, less current portion and debt issuance costs, net | 7,844 | | | 8,018 | |
| Operating leases, less current portion | 2,524 | | | 3,844 | |
| Finance leases, less current portion | 205 | | | 194 | |
| Other liabilities | 964 | | | 620 | |
| Total long-term liabilities | 11,537 | | | 12,676 | |
| Total liabilities | 53,393 | | | 38,938 | |
| | | |
| Commitments and contingencies (Note 7) | 0 | | 0 |
| | | |
| STOCKHOLDERS’ EQUITY | | | |
| Preferred stock, $0.0001 par value, 10,000 shares authorized, NaN issued and outstanding | 0 | | | 0 | |
| Class A common stock, $0.01 par value per share, 125,000 shares authorized; 13,322 shares issued and outstanding as of December 31, 2020; 9,999 shares issued and 9,812 shares outstanding as of December 31, 2019 | 133 | | | 98 | |
| Class B common stock, $0.0001 par value per share, 10,000 shares authorized; 3,491 shares issued and outstanding as of December 31, 2020; 5,975 shares issued and outstanding as of December 31, 2019 | 1 | | | 1 | |
| Class C Common stock, $0.0001 par value per share, 100,000 shares authorized; 76,039 shares issued and outstanding as of December 31, 2020; 77,791 shares issued and outstanding as of December 31, 2019 | 8 | | | 8 | |
| Additional paid-in capital | 39,742 | | | 32,108 | |
| Accumulated deficit | (24,848) | | | (9,727) | |
| Accumulated other comprehensive income (loss) | 29 | | | (72) | |
| Total stockholders’ equity attributable to Greenlane Holdings, Inc. | 15,065 | | | 22,416 | |
| Non-controlling interest | 54,192 | | | 91,848 | |
| Total stockholders’ equity | 69,257 | | | 114,264 | |
| Total liabilities and stockholders’ equity | $ | 122,650 | | | $ | 153,202 | |
| | | |
The accompanying notes are an integral part of these consolidated financial statements.
61
GREENLANE HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except per share amounts)
| | | | | | | | | | | |
| For the year ended December 31, |
| 2020 | | 2019 |
| Net sales | $ | 138,304 | | | $ | 185,006 | |
| Cost of sales | 115,539 | | | 153,916 | |
| Gross profit | 22,765 | | | 31,090 | |
| | | |
| Operating expenses: | | | |
| Salaries, benefits and payroll taxes | 24,909 | | | 29,716 | |
| General and administrative | 35,315 | | | 23,593 | |
| Goodwill impairment charge | 8,996 | | | 0 | |
| Depreciation and amortization | 2,520 | | | 2,705 | |
| Total operating expenses | 71,740 | | | 56,014 | |
| Loss from operations | (48,975) | | | (24,924) | |
| | | |
| Other income (expense), net: | | | |
| Change in fair value of convertible notes | 0 | | | (12,063) | |
| Interest expense | (437) | | | (975) | |
| Other income, net | 1,902 | | | 9,073 | |
| Total other income (expense), net | 1,465 | | | (3,965) | |
| Loss before income taxes | (47,510) | | | (28,889) | |
| Provision for income taxes | 194 | | | 10,935 | |
| Net loss | (47,704) | | | (39,824) | |
Less: Net loss attributable to non-controlling interest | (33,187) | | | (11,008) | |
| Net loss attributable to Greenlane Holdings, Inc. | $ | (14,517) | | | $ | (28,816) | |
| | | |
| Net loss attributable to Class A common stock per share - basic and diluted (Note 9) | $ | (1.22) | | | $ | (0.96) | |
| Weighted-average shares of Class A common stock outstanding - basic and diluted (Note 9) | 11,947 | | | 10,145 | |
| | | |
| Other comprehensive income (loss): | | | |
| Foreign currency translation adjustments | 654 | | | 193 | |
| Unrealized loss on derivative instrument | (459) | | | (206) | |
| Comprehensive loss | (47,509) | | | (39,837) | |
| Less: Comprehensive loss attributable to non-controlling interest | (33,092) | | | (11,033) | |
| Comprehensive loss attributable to Greenlane Holdings, Inc. | $ | (14,417) | | | $ | (28,804) | |
The accompanying notes are an integral part of these consolidated financial statements.
62
GREENLANE HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Redeemable
Class B
Units | | | Members’
Deficit | | Class A
Common Stock | | Class B
Common Stock | | Class C
Common Stock | | Additional
Paid-In
Capital | | Accumulated
Deficit | | Accumulated
Other
Comprehensive
Income (Loss) | | Non-
Controlling
Interest | | Total
Stockholders’
Equity /
Members’
Deficit |
| Shares | | Amount | Shares | | Amount | Shares | | Amount | |
| Balance, December 31, 2018 | $ | 10,033 | | | | $ | (10,773) | | | 0 | | | $ | 0 | | | 0 | | | $ | 0 | | | 0 | | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | (286) | | | $ | 0 | | | $ | (11,059) | |
| Activity prior to IPO and related organizational transactions: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of redeemable Class B units, net of issuance costs | 6,514 | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Member distributions | (76) | | | | (822) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (822) | |
| Redemption of Class A and redeemable Class B units | (416) | | | | (2,602) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (2,602) | |
| Equity-based compensation | 2,417 | | | | 328 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 328 | |
| Net loss recognized prior to the organizational transactions | (3,291) | | | | (15,798) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (15,798) | |
| Other comprehensive income | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 20 | | | — | | | 20 | |
| Effects of IPO and related organizational transactions: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Effects of the organizational transactions | (15,181) | | | | 29,667 | | | — | | | — | | | — | | | — | | | — | | | — | | | (114,094) | | | — | | | 203 | | | 99,404 | | | 15,180 | |
| Issuance of Class A common stock in the IPO, net of underwriting discount | — | | | | — | | | 5,250 | | | 53 | | | — | | | — | | | — | | | — | | | 82,950 | | | — | | | — | | | — | | | 83,003 | |
| Issuance of Class A common stock to convertible notes holders | — | | | | — | | | 3,548 | | | 35 | | | — | | | — | | | — | | | — | | | 60,277 | | | — | | | — | | | — | | | 60,312 | |
| Issuance of Class A common to stock selling stockholders | — | | | | — | | | 750 | | | 8 | | | (106) | | | — | | | (1,935) | | | — | | | (7) | | | — | | | — | | | — | | | 1 | |
| Issuance of Class A common stock to underwriter upon exercise of overallotment option | — | | | | — | | | 450 | | | 4 | | | (63) | | | — | | | (1,161) | | | — | | | (4) | | | — | | | — | | | — | | | 0 | |
| Issuance of Class B common stock | — | | | | — | | | — | | | — | | | 6,157 | | | 1 | | | — | | | — | | | (1) | | | — | | | — | | | — | | | — | |
| Issuance of Class C common stock | — | | | | — | | | — | | | — | | | — | | | — | | | 80,887 | | | 8 | | | (8) | | | — | | | — | | | — | | | — | |
| Issuance costs charged against the gross proceeds of the IPO | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (3,523) | | | — | | | — | | | — | | | (3,523) | |
| Establishment of liabilities under tax receivable agreement and related changes to deferred tax assets associated with increases in tax basis | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 5,173 | | | — | | | — | | | — | | | 5,173 | |
| Joint venture consolidation | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 60 | | | 60 | |
| Activity subsequent to IPO and related organizational transactions: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (9,727) | | | — | | | (11,008) | | | (20,735) | |
| Equity-based compensation | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,532 | | | — | | | — | | | 3,743 | | | 5,275 | |
| Other comprehensive loss | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (9) | | | (25) | | | (34) | |
| Reclassification of effects of the organizational transactions | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 297 | | | — | | | — | | | (297) | | | — | |
| Repurchases of Class A common stock, constructively retired | — | | | | — | | | (187) | | | (2) | | | — | | | — | | | — | | | — | | | (513) | | | — | | | — | | | — | | | (515) | |
| Exchanges of noncontrolling interest for Class A common stock | — | | | | — | | | 1 | | | — | | | (1) | | | — | | | — | | | — | | | 2 | | | — | | | — | | | (2) | | | — | |
| Cancellation of Class B common stock due to forfeitures (Note 10) | — | | | | — | | | — | | | — | | | (12) | | | — | | | — | | | — | | | 27 | | | — | | | — | | | (27) | | | — | |
| Balance December 31, 2019 | 0 | | | | 0 | | | 9,812 | | | 98 | | | 5,975 | | | 1 | | | 77,791 | | | 8 | | | 32,108 | | | (9,727) | | | (72) | | | 91,848 | | | 114,264 | |
| Net loss | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (14,517) | | | — | | | (33,187) | | | (47,704) | |
| Equity-based compensation | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 192 | | | — | | | — | | | 661 | | | 853 | |
| Other comprehensive income (loss) | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 101 | | | 95 | | | 196 | |
| Member distribution | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (604) | | | — | | | — | | | (604) | |
| Joint venture consolidation | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 189 | | | 189 | |
| Issuance of Class A common stock | — | | | | — | | | 686 | | | 7 | | | — | | | — | | | — | | | — | | | 2,056 | | | — | | | — | | | — | | | 2,063 | |
| Exchanges of noncontrolling interest for Class A common stock | — | | | | — | | | 2,824 | | | 28 | | | (2,240) | | | — | | | (1,752) | | | — | | | 4,934 | | | — | | | — | | | (4,962) | | | — | |
| Cancellation of Class B common stock due to forfeitures (Note 10) | — | | | | — | | | — | | | — | | | (244) | | | — | | | — | | | — | | | 452 | | | — | | | — | | | (452) | | | — | |
| Balance December 31, 2020 | $ | 0 | | | | $ | 0 | | | 13,322 | | | $ | 133 | | | 3,491 | | | $ | 1 | | | 76,039 | | | $ | 8 | | | $ | 39,742 | | | $ | (24,848) | | | $ | 29 | | | $ | 54,192 | | | $ | 69,257 | |
The accompanying notes are an integral part of these consolidated financial statements.
63
GREENLANE HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | |
| For the year ended December 31 |
| 2020 | | 2019 |
| Cash flows from operating activities: | | | |
| Net loss (including amounts attributable to non-controlling interest) | $ | (47,704) | | | $ | (39,824) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | |
| Depreciation and amortization | 2,520 | | | 2,705 | |
| Reversal of tax receivable agreement liability | 0 | | | (5,721) | |
| Change in deferred tax asset, net | 0 | | | 10,894 | |
| Equity-based compensation expense | 853 | | | 8,020 | |
| Unrealized gain on equity investment | 0 | | | (1,537) | |
| Goodwill impairment charge | 8,996 | | | 0 | |
| Change in fair value of contingent consideration | (719) | | | 0 | |
| Change in fair value of convertible notes | 0 | | | 12,063 | |
| Change in provision for doubtful accounts | 576 | | | 352 | |
| Loss on disposal of assets | 579 | | | 0 | |
| Loss related to indemnification asset not probable of recovery | 4,464 | | | 0 | |
| Impairment of held-for-sale assets | 376 | | | 0 | |
| Other | 75 | | | 32 | |
| Changes in operating assets and liabilities, net of the effects of acquisitions: | | | |
| Decrease in accounts receivable | 1,186 | | | 635 | |
| Decrease (increase) in inventories | 6,996 | | | (11,739) | |
| Decrease (increase) in vendor deposits | 29 | | | (1,503) | |
| Decrease (increase) in deferred offering costs | 0 | | | (1,238) | |
| (Increase) in other current assets | (10,194) | | | (1,993) | |
| Increase (decrease) in accounts payable | 7,095 | | | (11,261) | |
| Increase in accrued expenses | 13,104 | | | 3,132 | |
| (Decrease) increase in customer deposits | (534) | | | 80 | |
| Net cash used in operating activities | (12,302) | | | (36,903) | |
| Cash flows from investing activities: | | | |
| Purchase consideration paid for acquisitions, net of cash acquired | (1,841) | | | (1,159) | |
| Purchases of property and equipment, net | (1,788) | | | (2,020) | |
| Purchase of intangible assets | (515) | | | (53) | |
| Investment in equity securities | 0 | | | (500) | |
| | | |
| Net cash used in investing activities | (4,144) | | | (3,732) | |
| Cash flows from financing activities: | | | |
| Proceeds from issuance of convertible notes | 0 | | | 8,050 | |
| Proceeds from issuance of Class A common stock sold in initial public offering, net of underwriting costs | 0 | | | 83,003 | |
| Payment of debt issuance costs - convertible notes | 0 | | | (1,734) | |
| Deferred offering costs paid | 0 | | | (3,523) | |
| | | |
| | | |
| | | |
| Redemption of Class A and Class B units of Greenlane Holdings, LLC | 0 | | | (3,018) | |
| Member distributions | (604) | | | (898) | |
| Other | (459) | | | (901) | |
| Net cash (used in) provided by financing activities | (1,063) | | | 80,979 | |
| Effects of exchange rate changes on cash | 171 | | | 88 | |
| Net (decrease) increase in cash | (17,338) | | | 40,432 | |
| Cash, as of beginning of the period | 47,773 | | | 7,341 | |
| Cash, as of end of the period | $ | 30,435 | | | $ | 47,773 | |
| | | |
| Supplemental disclosures of cash flow information | | | |
| Cash paid during the period for interest | $ | 437 | | | $ | 975 | |
| Cash paid during the period for income taxes | $ | 192 | | | $ | 498 | |
| Cash paid for amounts included in the measurement of lease liabilities | $ | 1,252 | | | $ | 1,119 | |
| Lease liabilities arising from obtaining finance lease assets | $ | 272 | | | $ | 86 | |
| Lease liabilities arising from obtaining operating lease right-of-use assets | $ | 793 | | | $ | 5,573 | |
| | | |
| Non-cash investing and financing activities: | | | |
| Conversion of convertible debt to Class A common stock | $ | 0 | | | $ | 60,313 | |
| Redeemable Class B Units issued for acquisition of a subsidiary, net of issuance costs | $ | 0 | | | $ | 6,514 | |
| Shares of Class A common stock issued for acquisition of Conscious Wholesale | $ | 1,988 | | | $ | 0 | |
| Contingent consideration for the Conscious Wholesale acquisition included in "Accrued expenses and other current liabilities" | $ | 0 | | | $ | 1,609 | |
| Purchase consideration for the Conscious Wholesale acquisition included in "Accrued expenses and other current liabilities" | $ | 0 | | | $ | 3,029 | |
| Purchases of property, plant, and equipment with unpaid costs accrued in "Other liabilities" | $ | 98 | | | $ | 414 | |
| Exchanges of non-controlling interest for Class A common stock | $ | (4,962) | | | $ | 0 | |
The accompanying notes are an integral part of these consolidated financial statements.
64
GREENLANE HOLDINGS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. BUSINESS OPERATIONS AND ORGANIZATION
Organization
Greenlane Holdings, Inc. (“Greenlane” and, collectively with the Operating Company (as defined below) and its consolidated subsidiaries, the “Company”, "we", "us" and "our") was formed as a Delaware corporation on May 2, 2018. We are a holding company that was formed for the purpose of completing an underwritten initial public offering (“IPO”) of shares of our Class A common stock (as defined below) and other related Transactions (as defined below) in order to carry on the business of Greenlane Holdings, LLC (the “Operating Company”). The Operating Company was organized under the laws of the state of Delaware on September 1, 2015, and is based in Boca Raton, Florida. Unless the context otherwise requires, references to the “Company” refer to us, and our consolidated subsidiaries, including the Operating Company. Our authorized shares consist of (i) Class A common stock, par value $0.01 per share (the “Class A common stock”); (ii) shares of Class B common stock, par value $0.0001 per share (the “Class B common stock"); (iii) shares of Class C common stock, par value $0.0001 per share (the “Class C common stock",and together with the Class A common stock and the Class B common stock, the “Common Stock”); and (iv) shares of preferred stock, par value $0.0001 per share.
As a result of the IPO and the Transactions described below, we became the sole manager of the Operating Company and our principal asset is Common Units of the Operating Company. As the sole manager of the Operating Company, we operate and control all of the business and affairs of the Operating Company, and we conduct our business through the Operating Company and its subsidiaries. We have a board of directors and executive officers, but no employees. All of our assets are held and all of our employees, are employed by the Operating Company.
We merchandise vaporizers and other products in the United States, Canada and Europe and we distribute to retailers through wholesale operations and to consumers through e-commerce activities. We operate four distribution centers in the United States, two distribution centers in Canada, and one distribution center in Europe.
Although we have a minority economic interest in the Operating Company, we have the sole voting interest in, and control the management of, the Operating Company, and we have the obligation to absorb losses of, and receive benefits from, the Operating Company, that could be significant. We determined that, as a result of the Transactions described below, the Operating Company is a variable interest entity (“VIE”) and that we are the primary beneficiary of the Operating Company. Accordingly, pursuant to the VIE accounting model, beginning in the fiscal quarter ended June 30, 2019, we consolidated the Operating Company in our consolidated financial statements and reported a non-controlling interest related to the Common Units held by the members of the Operating Company (other than the Common Units held by us) on our consolidated financial statements.
The Operating Company has been determined to be our predecessor for accounting purposes and, accordingly, the consolidated financial statements for periods prior to the IPO and the related Transactions have been adjusted to combine the previously separate entities for presentation purposes. Amounts for the period from January 1, 2019 through April 22, 2019 presented in the consolidated financial statements and notes to the consolidated financial statements represent the historical operations of the Operating Company. Amounts for the period from April 23, 2019 through December 31, 2020 reflect our consolidated operations.
Initial Public Offering and Organizational Transactions
On April 23, 2019, we completed our IPO of 6,000,000 shares of Class A common stock, which was comprised of 5,250,000 shares of Class A common stock sold by us and 750,000 shares sold by certain selling stockholders (comprised of Aaron LoCascio, Greenlane’s Chief Executive Officer, Adam Schoenfeld, Greenlane’s Chief Strategy Officer, and Jacoby & Co. Inc., an affiliated entity of Messrs. LoCascio and Schoenfeld), in each case at a public offering price of $17.00 per share. In addition, we issued 3,547,776 shares of our Class A common stock to the holders of convertible notes upon conversion of such convertible notes at a settlement price equal to 80% of the IPO price. On April 29, 2019, the underwriters purchased an additional 450,000 shares of our Class A common stock from selling stockholders pursuant to the exercise of their option to purchase additional shares in the IPO. We did not receive any proceeds from the sale of our Class A common stock by the selling stockholders. Our sale of Class A common stock generated aggregate net proceeds, after deducting the underwriting discounts and commissions and offering expenses we paid, of approximately $79.5 million. We contributed all of the net proceeds to the Operating Company in exchange for a number of common units of the Operating Company (“Common Units”) equal to the number of shares of our Class A common stock sold by us in the IPO at a price per Common Unit equal to the IPO price per share of Class A common stock. After giving effect to the IPO and the related Transactions and the use of the net proceeds from the IPO, we owned approximately 23.9% of the Operating Company’s outstanding Common Units. As a result of the IPO, Mr. Schoenfeld and Jacoby & Co. Inc. collectively controlled approximately 83.0% of the combined voting power
of our common stock as a result of their ownership of our Class C common stock, which are issued on a three-to-one basis with the number of Common Units owned and each share of common stock is entitled to one vote all matters submitted to a vote of our stockholders.
In connection with the closing of the IPO, Greenlane and the Operating Company consummated the following organizational transactions (collectively, the “Transactions”):
● The Operating Company adopted and approved the Third Amended and Restated Operating Agreement of the Operating Company (the “Operating Agreement”), which converted each member’s existing membership interests in the Operating Company into Common Units, including unvested membership interests and profits interests into unvested Common Units, and appointed Greenlane as the sole manager of the Operating Company;
● We amended and restated our certificate of incorporation to, among other things, provide for Class A common stock, Class B common stock and Class C common stock;
● We issued, for nominal consideration, 1 share of our Class B common stock to our non-founder members for each Common Unit they owned and issued, for nominal consideration, 3 shares of Class C common stock to our founder members for each Common Unit they owned;
● We issued and sold 3,547,776 shares of our Class A common stock upon conversion of the convertible notes at a settlement price equal to 80% of the IPO price;
● We issued and sold 1,200,000 shares of our Class A common stock to our members upon exchange of an equal number of Common Units, which shares were sold by the members as selling stockholders in the IPO, including 450,000 shares issued pursuant to the partial exercise of the underwriters’ option to purchase additional shares;
● We issued and sold 5,250,000 shares of our Class A common stock to the purchasers in the IPO, and used all of the net proceeds received from the IPO to acquire Common Units from the Operating Company at a purchase price per Common Unit equal to the IPO price per share of our Class A common stock, less underwriting discounts and commissions, which Common Units, when added to the Common Units received from the selling stockholders, collectively represented approximately 15.4% of the Operating Company’s outstanding Common Units after the IPO;
● The members of the Operating Company continue to own their Common Units not exchanged for the shares of our Class A common stock sold by them as selling stockholders in the IPO. Common Units are redeemable, subject to contractual restrictions, at the election of such members for newly-issued shares of our Class A common stock on a 1-to-one basis (and their shares of our Class B common stock or our Class C common stock, as the case may be, will be canceled on a 1-to-one basis in the case of our Class B common stock or 3-to-one basis in the case of our Class C common stock upon any such issuance). We have the option to instead make a cash payment equal to a volume weighted average market price of one share of our Class A common stock for each Common Unit redeemed (subject to customary adjustments, including for stock splits, stock dividends and reclassifications) in accordance with the terms of the Operating Agreement. Our decision to make a cash payment upon a member’s redemption election will be made by our independent directors (within the meaning of the Nasdaq Marketplace Rules) who are disinterested in such proposed redemption; and
● We entered into (i) a Tax Receivable Agreement (the “TRA”) with the Operating Company and the Operating Company’s members and (ii) a Registration Rights (the “Registration Rights Agreement”) with the Operating Company’s members.
Our corporate structure following the IPO, as described above, is commonly referred to as an “Up-C” structure, which is often used by partnerships and limited liability companies when they undertake an initial public offering of their business. The Up-C structure allows the members of the Operating Company to continue to realize tax benefits associated with owning interests in an entity that is treated as a partnership, or “pass-through” entity, for income tax purposes following the IPO. One of these benefits is that future taxable income of the Operating Company that is allocated to its members will be taxed on a flow-through basis and therefore will not be subject to corporate taxes at the Operating Company entity level. Additionally, because the members may redeem their Common Units for shares of our Class A common stock on a 1-for-one basis, or at our option, for cash, the Up-C structure also provides the members with potential liquidity that holders of non-publicly traded limited liability companies are not typically afforded.
We will receive the same benefits as the Operating Company's members because of our ownership of Common Units in an entity treated as a partnership, or “pass-through” entity, for income tax purposes. As additional Common Units from the Operating Company’s members are redeemed under the mechanism described above, we will obtain a step-up in tax basis in our share of the Operating Company’s assets. This step-up in tax basis will provide us with certain tax benefits, such as future depreciation and amortization deductions that can reduce the taxable income allocable to us. We entered into the TRA with the Operating Company and each of the Operating Company’s members, which provides for the payment by us to the Operating Company’s members of 85% of the amount of tax benefits, if any, that we actually realize (or in some cases, are deemed to realize) as a result of (i) increases in tax basis resulting from the redemption of Common Units and (ii) certain other tax benefits attributable to payments made under the TRA.
As a result of the completion of the Transactions, including the IPO, our amended and restated certificate of incorporation and the Operating Agreement require that (i) we at all times maintains a ratio of one Common Unit owned by us for each share of our Class A common stock issued by us (subject to certain exceptions for treasury shares and shares underlying certain convertible or exchangeable securities), and (ii) the Operating Company at all times maintains (x) a one-to-one ratio between the number of shares of our Class A common stock issued by us and the number of Common Units owned by us, (y) a one-to-
one ratio between the number of shares of our Class B common stock owned by the non-founder members of the Operating Company and the number of Common Units owned by the non-founder members of the Operating Company, and (z) a three-to-one ratio between the number of shares of our Class C common stock owned by the founder members of the Operating Company and their affiliates and the number of Common Units owned by the founder members of the Operating Company and their affiliates.
The following table sets forth the economic and voting interests of holders of our Common Stock as of the date of this Form 10-K:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Class of Common Stock (ownership) | | Total Shares Outstanding (1) | | Class A Shares (as converted) (2) | | Economic Interest in the Operating Company (3) | | Voting Interest in Greenlane (4) | | Economic Interest in Greenlane (5) |
| Class A | | 13,322,416 | | 13,322,416 | | 31.6 | % | | 14.3 | % | | 100.0 | % |
| Class B (non-founder members) | | 3,490,909 | | 3,490,909 | | 8.3 | % | | 3.8 | % | | 0 | % |
| Class C (founder members) | | 76,039,218 | | 25,346,406 | | 60.1 | % | | 81.9 | % | | 0 | % |
| Total | | 92,852,543 | | 42,159,731 | | 100.0 | % | | 100.0 | % | | 100.0 | % |
| | | | |
| | |
| (1) Represents the total number of outstanding shares for each class of common stock as of December, 31, 2020. |
| (2) Represents the number of shares of Class A common stock that would be outstanding assuming the exchange of all outstanding shares of Class B common stock and Class C common stock upon redemption of all related Common Units. Shares of Class B common stock and Class C common stock, as the case may be, would be canceled, without consideration, on a one-to-one basis in the case of Class B common stock and a three-to-one basis in the case of Class C common stock, pursuant to the terms and subject to the conditions of the Operating Agreement. |
| (3) Represents the indirect economic interest in the Operating Company through the holders' ownership of common stock. |
| (4) Represents the aggregate voting interest in us through the holders' ownership of Common Stock. Each share of Class A common stock, Class B common stock and Class C common stock entitles its holder to one vote per share on all matters submitted to a vote of our stockholders. |
| (5) Represents the aggregate economic interest in us through the holders' ownership of Class A common stock. |
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
Our audited consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and with the instructions to Form 10-K and Article 8 of Regulation S-X.
Principles of Consolidation
Our consolidated financial statements include our accounts, the accounts of the Operating Company, and the accounts of the Operating Company's consolidated subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
Conformity with U.S. GAAP requires the use of estimates and judgments that affect the reported amounts in our consolidated financial statements and accompanying notes. These estimates form the basis for judgments we make about the carrying values of our assets and liabilities, which are not readily apparent from other sources. We base our estimates and judgments on historical information and on various other assumptions that we believe are reasonable under the circumstances. U.S. GAAP requires us to make estimates and judgments in several areas. Such areas include, but are not limited to: the collectability of accounts receivable; the allowance for slow-moving or obsolete inventory; the realizability of deferred tax assets; the fair value of goodwill; the fair value of contingent consideration arrangements; the useful lives of intangible assets and property and equipment; the calculation of our VAT taxes receivable and VAT taxes, fines, and penalties payable; our loss contingencies, including our TRA liability; and the valuation and assumptions underlying equity-based compensation. These estimates are based on management's knowledge about current events and expectations about actions we may undertake in the future. Actual results could differ materially from those estimates.
In March 2020, the World Health Organization declared the novel coronavirus ("COVID-19") a global pandemic. We expect uncertainties around our key accounting estimates to continue to evolve depending on the duration and degree of impact associated with the COVID-19 pandemic. Our estimates may change as new events occur and additional information emerges, and such changes are recognized or disclosed in our consolidated financial statements.
Segment Reporting
Beginning with the quarter ended March 31, 2019, we had a change in reportable segments as our Canadian operating segment met certain quantitative thresholds based upon which its separate disclosure was required. Our Canadian operating segment consists of the Operating Company’s wholly-owned, Canada-based, subsidiary. We completed our acquisition of ARI Logistics B.V. and Shavita B.V. (collectively, "Conscious Wholesale") based in Amsterdam, the Netherlands, on September 30, 2019. During the fourth quarter of 2019, we assigned goodwill from the Conscious Wholesale acquisition to our new European operating segment, which was also established as a reportable segment during the fourth quarter of 2019. Our United States operating segment is comprised of all other operating subsidiaries. Our United States, Canada, and Europe reportable segments have been identified based on how our Chief Operating Decision Makers ("CODMS"), a committee comprised of our Chief Executive Officer ("CEO") and out Chief Financial Officer ("CFO"), manage the business, make resource allocation and operating decisions, and evaluate operating performance. See “Note 12—Segment Reporting.”
Business Combinations
Our business combinations are accounted for under the acquisition method of accounting in accordance with ASC Topic 805, Business Combinations (“ASC 805”). Under the acquisition method, we recognize 100% of the assets we acquire and liabilities we assume, regardless of the percentage we own, at their estimated fair values as of the date of acquisition. Any excess of the purchase price over the fair value of the net assets and other identifiable intangible assets we acquire is recorded as goodwill. To the extent the fair value of the net assets we acquire, including other identifiable assets, exceeds the purchase price, a bargain purchase gain is recognized. The assets we acquire, and liabilities we assume from contingencies, are recognized at fair value if we can readily determine the fair value during the measurement period. The operating results of businesses we acquire are included in our consolidated statement of operations from the date of acquisition. Acquisition-related costs are expensed as incurred. See “Note 3— Business Acquisitions.”
Equity-Based Compensation
We account for equity-based compensation grants of equity awards to employees in accordance with ASC Topic 718, Compensation — Stock Compensation. This standard requires us to measure compensation expense based on the estimated fair value of share-based awards on the grant date and recognize as expense over the requisite service period, which is generally the vesting period. We estimate the fair value of stock options using the Black-Scholes model on the grant date. The Black-Scholes model requires us to use several variables to estimate the grant-date fair value of our equity-based compensation awards including expected term, expected volatility and risk-free interest rates. Our equity-based compensation costs are recognized using a graded vesting schedule. For liability-classified awards, we record fair value adjustments up to and including the settlement date. Changes in the fair value of our equity-based compensation liability that occur during the requisite service period are recognized as compensation cost over the vesting period. Changes in the fair value of the equity-based compensation liability that occur after the end of the requisite service period but before settlement, are recognized as compensation cost of the period in which the change occurs. We account for forfeitures as they occur. See “Note 10—Compensation Plans.”
Fair Value Measurements
We apply the provisions of ASC Topic 820, Fair Value Measurements, which defines fair value, establishes a framework for its measurement and expands disclosures about fair value measurements. Fair value is defined as the exchange price we would receive for an asset or an exit price we would pay to transfer a liability in the principal, or most advantageous, market for our asset or liability in an orderly transaction with a market participant on the measurement date. We determine the fair market values of our financial instruments based on the fair value hierarchy, which requires us to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The following three levels of inputs may be used to measure fair value:
Level 1 Observable inputs such as unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date.
Level 2 Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The carrying amounts of our financial instruments, including cash, accounts receivable, accounts payable, accrued expenses and short-term debt, are carried at historical cost basis, which approximates their fair values because of their short-term nature. The fair value of our long-term debt is the estimated amount we would have to pay to repurchase the debt, inclusive of any premium or discount attributable to the difference between the stated interest rate and market rate of interest at each balance sheet date. As of December 31, 2020 and 2019, the carrying amount of our long-term debt approximated its fair value. On a recurring
basis, we measure and record contingent consideration and our interest-rate swap arrangement using fair value measurements in the accompanying consolidated financial statements. See “Note 4—Fair Value of Financial Instruments.”
We also own equity securities of a private entity, which do not have readily determinable fair values. We elected to measure these equity securities at cost minus impairment, if any. At each reporting period, we make a qualitative assessment considering impairment indicators to evaluate whether our investment is impaired. The equity securities are adjusted to fair value when an observable price change can be identified. See “Investments” further below in Note 2.
Cash
For purposes of reporting cash flows, we consider cash on hand, checking accounts, and savings accounts to be cash. We also consider all highly-liquid investments with original maturities of three months or less from the date of purchase to be cash equivalents. We place our cash with high credit quality financial institutions, which provide insurance through the Federal Deposit Insurance Company. At times, the balance in our accounts may exceed federal insured limits. We perform periodic evaluations of the relative credit standing of these institutions and do not expect any losses related to such concentrations. As of December 31, 2020, and 2019, approximately $2.3 million and $0.9 million, respectively, of our cash balances were in foreign bank accounts and uninsured. As of December 31, 2020 and 2019, we had no cash equivalents.
Accounts Receivable, net
Accounts receivable represent amounts due from customers for merchandise sales and are recorded when revenue is earned and are carried at the original invoiced amount less an allowance for any potentially uncollectible amounts. An account is considered past due when payment has not been rendered by its due date based upon the terms of the sale. Generally, accounts receivable are due 30 days after the billing date. We maintain an allowance for doubtful accounts to reserve for potentially uncollectible receivable amounts. In evaluating our ability to collect outstanding receivable balances, we consider various factors including the age of the balance, the creditworthiness of the customer, the customer's current financial condition, current economic conditions, and other factors that may affect our ability to collect from customers. We write off accounts as uncollectible on a case-by-case basis. We pledge accounts receivable as collateral for our line of credit. See “Note 6—Long Term Debt.”
Inventories, net
Inventories consist of finished goods that we value at the lower of cost or net realizable value on a weighted average cost basis. We established an allowance for slow-moving or obsolete inventory based upon assumptions about future demands and market conditions. At December 31, 2020 and 2019, the reserve for obsolescence was approximately $1.6 million and $1.3 million, respectively. We pledge inventory as collateral for our line of credit. See “Note 6—Long Term Debt.”
Assets Held for Sale
We generally consider assets to be held for sale when (i) we commit to a plan to sell the assets, (ii) the assets are available for immediate sale in their present condition, (iii) we have initiated an active program to locate a buyer and other actions required to complete the plan to sell the assets, (iv) consummation of the planned sale transaction is probable, (v) the assets are being actively marketed for sale at a price that is reasonable in relation to their current fair value, (vi) the transaction is expected to qualify for recognition as a completed sale, within one year, and (vii) significant changes to or withdrawal of the plan is unlikely. Following the classification of any depreciable assets within a disposal group as held for sale, we discontinue depreciating the asset and write down the asset to the lower of carrying value or fair market value less cost to sell, if needed. As described in "Note 5—Leases" and "Note 8—Supplemental Financial Statement Information," we have taken actions that have caused certain property and equipment and right-of-use assets to meet the relevant criteria for classification and reporting as held for sale. We recognized an impairment charge of approximately $0.4 million during the year ended December 31, 2020 related to assets classified as held for sale.
Deferred Financing Costs
Costs incurred in obtaining certain debt financing are deferred and amortized over the respective terms of the related debt instruments using the interest method for term debt and the straight-line method for revolving debt. The debt issuance costs related to our revolving line of credit are presented as an asset in our consolidated balance sheets while the debt issuance costs related to our real estate note are presented net against the long-term debt in our consolidated balance sheets.
We account for costs of issuing equity instruments to effect business combinations as a reduction of the otherwise determined fair value of the equity instruments we issue. We expense any fees not associated with arranging equity or debt financing as incurred.
Property and Equipment, net
We state property and equipment at cost or, if acquired through a business combination, fair value at the date of acquisition. We calculate depreciation and amortization using the straight-line method over the estimated useful lives of the assets, except for our leasehold improvements, which are depreciated over the shorter of their estimated useful lives or their related lease term. Upon the sale or retirement of assets, the cost and related accumulated depreciation are removed from our accounts and the resulting gain or loss is credited or charged to income. We expense costs for repairs and maintenance when incurred. Property and equipment includes assets recorded under finance leases, see “Note 5—Leases.” We pledge property and equipment as collateral for our line of credit. See “Note 6—Long Term Debt.”
Impairment of Long-Lived Assets
We assess the recoverability of the carrying amount of our long lived-assets, including property and equipment and finite-lived intangibles, whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. An impairment loss would be assessed when estimated undiscounted future cash flows from the operation and disposition of the asset group are less than the carrying amount of the asset group. Asset groups have identifiable cash flows and are largely independent of other asset groups. Measurement of an impairment loss is based on the excess of the carrying amount of the asset group over its fair value. Other than the impairment charge recognized on our assets held for sale as noted above, we did not recognized any other impairment charges for long-lived assets during the years ended December 31, 2020 and 2019.
Intangible Assets, net
Our intangible assets consist of domain names, intellectual property, distribution agreements, proprietary technology, trademarks and tradenames, customer relationships, and other rights. We amortize intangible assets with finite lives over their estimated useful lives on a straight-line basis. The straight-line method of amortization represents our best estimate of the distribution of the economic value of the identifiable intangible assets. We carry intangible assets at cost less accumulated amortization. We assess the recoverability of finite-lived intangible assets in the same manner we do for property and equipment, as described above. We recognized 0 impairment charges for intangible assets during the years ended December 31, 2020 and 2019. See "Note 8—Supplemental Financial Statement Information."
Goodwill
Goodwill represents the excess of the price we paid over the fair value of the net identifiable assets we acquired in business combinations. In accordance with ASC Topic 350, Intangibles—Goodwill and Other, we review goodwill for impairment at the reporting unit level annually or, when events or circumstances dictate, more frequently. The impairment review for goodwill consists of a qualitative assessment of whether it is more-likely-than-not that a reporting unit's fair value is less than its carrying amount, and if necessary, a quantitative goodwill impairment test. Factors to consider when performing the qualitative assessment include general economic conditions, limitations on accessing capital, changes in forecasted operating results and fluctuations in foreign exchange rates. If the qualitative assessment demonstrates that it is more-likely-than-not that the estimated fair value of the reporting unit exceeds its carrying value, it is not necessary to measure and record impairment loss. We may elect to bypass the qualitative assessment and proceed directly to the quantitative assessment, for any reporting unit, in any period. We can resume the qualitative assessment for any reporting unit in any subsequent period.
When we perform a quantitative impairment test, we use a combination of an income approach, a discounted cash flow valuation approach, and a market approach, using the guideline public company method, to determine the fair value of each reporting unit, and then compare the fair value to its carrying amount to determine the amount of impairment, if any. If a reporting unit's fair value is less than its carrying amount, we record an impairment charge based on that difference, up to the amount of goodwill allocated to that reporting unit.
The quantitative impairment test requires the application of a number of significant assumptions, including estimated projections of future revenue growth rates, EBITDA margins, terminal value growth rates, market multiples, discount rates, and foreign currency exchange rates. The projections of future cash flows used to assess the fair value of the reporting units are based on the internal operation plans reviewed by management. The market multiples are based on comparable public company multiples. The discount rates are based on the risk-free rate of interest and estimated risk premiums for the reporting units at the time the impairment analysis is prepared. The projections of future exchange rates are based on the current exchange rates at the time the projections are prepared. if the fair value of the reporting unit exceeds its carrying value, no further analysis or write-down of goodwill is required. If the fair value of the reporting unit is less than the carrying value of its net assets, the implied fair value value of the reporting unit is allocated to all its underlying assets and liabilities, including both recognized and unrecognized tangible and intangible assets, based on their fair value. If necessary, goodwill is then written down to its implied fair value.
We recognized approximately $9.0 million in impairment charges during the year ended December 31, 2020. We recognized 0 goodwill impairment charges during the year ended December 31, 2019. See "Note 8—Supplemental Financial Statement Information."
Investments
Our investments in equity securities consist of a 1.49% ownership interest in Airgraft Inc. We determined that our ownership does not provide us with significant influence over the operations of this investee. Accordingly, we account for our investment in this entity as equity securities. Airgraft Inc. is a private entity and its equity securities do not have a readily determinable fair value. We elected to measure this security at cost minus impairment, if any. The security is adjusted to fair value when an observable price change can be identified. At December 31, 2020, the carrying value of this investment was approximately $2.0 million, which included an upward adjustment of $1.5 million based on an observable price change recognized during the year ended December 31, 2019. The adjustment was determined based on Airgraft Inc.’s price per share sold in connection with a new financing round during the third quarter of 2019, for shares which were determined to be similar to the equity securities held by us. This adjustment in the carrying value of our investment in equity securities was recorded as an unrealized gain of approximately $1.5 million within “Other income (expense), net” in our consolidated statements of operations for the year ended December 31, 2019. There were no observable price changes in our equity securities for the year ended December 31, 2020.
Vendor Deposits
Vendor deposits represent prepayments we make to vendors for inventory purchases. A significant number of vendors require us to prepay for inventory purchases.
Deferred Offering Costs
We capitalized certain legal, accounting, and other third-party fees that were directly attributable to our IPO. Following the successful consummation of the IPO in April 2019, deferred offering costs of approximately $3.5 million were recorded in stockholders’ equity as a reduction of our additional paid-in capital.
Vendor Incentives and Rebates
Sales incentives we receive in the form of payments from vendors solely to reimburse us for acting as the vendors' agent in redeeming a sales incentive that is between our vendor and our customers and end consumers are included in net sales in the consolidated statements of operations and comprehensive loss.
We also have agreements with certain vendors to receive volume rebates which are dependent upon reaching minimum purchase thresholds. When volume rebates can be reasonably estimated and it is probable that minimum purchase thresholds will be met, we record a portion of the rebate when or as we make progress towards the purchase threshold. Amounts received from vendors relating to volume rebates are considered a reduction of the carrying value of our inventory and, therefore, such amounts are ultimately recorded as a reduction of cost of goods sold in the consolidated statements of operations and comprehensive loss.
Foreign Currency Translation
Our consolidated financial statements are presented in United States (U.S.) dollars. The functional currency of one of the Operating Company’s wholly-owned, Canada-based, subsidiaries is the Canadian dollar. The functional currency of the Operating Company’s wholly-owned, Netherlands-based subsidiary is the Euro. The assets and liabilities of these subsidiaries are translated into U.S. dollars at current exchange rate at each balance sheet date for assets and liabilities and an appropriate average exchange rate for each applicable period within our consolidated statements of operations and comprehensive loss. Capital accounts are translated at their historical exchange rates when the capital transactions occurred. The foreign currency translation adjustments are included in accumulated other comprehensive loss, a separate component of members’/stockholders’ deficit in our consolidated balance sheets. Other exchange gains and losses are reported within our consolidated statements of operations and comprehensive loss.
Comprehensive (Loss) Income
Comprehensive (loss) income includes net (loss) income as currently reported by us, adjusted for other comprehensive items. Other comprehensive items consist of foreign currency translation gains and losses and unrealized gains and losses on derivative financial instruments that qualify as hedges.
Advertising
We expense advertising costs as incurred and include them in general and administrative expenses in our consolidated statements of operations and comprehensive loss. Advertising costs were approximately $3.6 million and $4.6 million for the years ended December 31, 2020 and 2019, respectively.
Income Taxes
We are a corporation subject to income taxes in the United States. Certain subsidiaries of the Operating Company are taxable separately from us. Our proportional share of the Operating Company’s subsidiaries’ provisions are included in our consolidated financial statements.
Our deferred income tax assets and liabilities are computed for differences between the tax basis and financial statement amounts that will result in taxable or deductible amounts in the future. We compute deferred balances based on enacted tax laws and applicable rates for the periods in which the differences are expected to affect taxable income. A valuation allowance is recognized for deferred tax assets if it is more likely than not that some portion or all of the net deferred tax assets will not be realized. In making such a determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. If we determine we would be able to realize our deferred tax assets for which a valuation allowance had been recorded, then we would adjust the deferred tax asset valuation allowance, which would reduce our provision for income taxes.
We evaluate the tax positions taken on income tax returns that remain open and positions expected to be taken on the current year tax returns to identify uncertain tax positions. Unrecognized tax benefits on uncertain tax positions are recorded on the basis of a two-step process in which (1) we determine whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold, the largest amount of tax benefit that is more than 50 percent likely to be realized is recognized. Interest and penalties related to unrecognized tax benefits are recorded in income tax benefit. We have no uncertain tax positions that qualify for inclusion in our consolidated financial statements. See “Note 11—Income Taxes.”
Tax Receivable Agreement (TRA)
We entered into the TRA with the Operating Company and each of the members of the Operating Company that provides for the payment by the Operating Company to the members of 85% of the amount of tax benefits, if any, that we may actually realize (or in some circumstances are deemed to realize) as a result of (i) increases in tax basis resulting from any future redemptions that are funded by us or exchanges of Common Units as described above in “Note 1—Business Operations and Organization” and (ii) certain other tax benefits attributable to payments made under the TRA.
We compute annual tax benefits by calculating the income taxes due, including such tax benefits, and the income taxes due without such benefits. The Operating Company expects to benefit from the remaining 15% of any tax benefits that it may actually realize. The TRA payments are not conditioned upon any continued ownership interest in the Operating Company. The rights of each noncontrolling interest holder under the TRA are assignable to transferees of its interest in the Operating Company. The timing and amount of aggregate payments due under the TRA may vary based on a number of factors, including the amount and timing of the taxable income the Operating Company generates each year and the applicable tax rate.
We periodically evaluate the realizability of the deferred tax assets resulting from the exchange of Common Units for our Class A common stock. If the deferred tax assets are determined to be realizable, we then assess whether payment of amounts under the TRA have become probable. If so, we record a TRA liability equal to 85% of such deferred tax assets. In subsequent periods, we assess the realizability of all of deferred tax assets subject to the TRA. If we determine that a deferred tax asset with a valuation allowance is realizable in a subsequent period, the related valuation allowance will be released and consideration of a corresponding TRA liability will be assessed. The realizability of deferred tax assets, including those subject to the TRA, is dependent upon the generation of future taxable income during the periods in which those deferred tax assets become deductible and consideration of prudent and feasible tax-planning strategies.
The measurement of the TRA is accounted for as a contingent liability. Therefore, once we determine that a payment to a member of the Operating Company has become probable and can be estimated, the estimated payment will be accrued. See “Note 11—Income Taxes.”
Revenue Recognition
Revenue is recognized when customers obtain control of goods and services promised by us. Revenue is measured based on the amount of consideration that we expect to receive in exchange for those goods or services, reduced by promotional discounts and estimates for return allowances and refunds. Taxes collected from customers for remittance to governmental authorities are excluded from net sales.
We generate revenue primarily from the sale of finished products to customers, whereby each product unit represents a single performance obligation. We recognize revenue from product sales when the customer has obtained control of the products, which is either at point of sale or delivery to the customer, depending upon the specific terms and conditions of the arrangement, or at the point of sale for our retail store sales. We provide no warranty on products sold. Product warranty is provided by the manufacturers.
Our performance obligations for services are satisfied when the services are rendered within the arranged service period. Total service revenue is not material and accounted for less than 0.1% of revenues for the years ended December 31, 2020 and 2019.
Beginning with the first quarter of 2020, we entered into a limited number of bill-and-hold arrangements. Each bill-and-hold arrangement is reviewed and revenue is recognized only when certain criteria have been met: (i) the customer has requested delayed delivery and storage of the products by us, in exchange for a storage fee, because they want to secure a supply of the products but lack storage space, (ii) the risk of ownership has passed to the customer, (iii) the products are segregated from our other inventory items held for sale, (iv) the products are ready for shipment to the customer, and (v) the products are customized and thus we do not have the ability to use the products or direct them to another customer. During the year ended December 31, 2020, we recorded approximately $1.7 million of revenue under bill-and-hold arrangements. We did not recognize any revenue under bill-and-hold arrangements during the year ended December 31, 2019. Storage fees charged to customers for bill-and-hold arrangements are recognized as invoiced. Such fees were not significant for the year ended December 31, 2020.
We act as the principal in relation to our contracts with customers and recognize revenue on a gross basis as we (i) are the primary entity responsible for fulfilling the promise to provide the specified products in the arrangement with the customer and we provide the primary customer service for all products sold, (ii) have discretion in establishing the price for the specified products sold and selecting our suppliers, as applicable, and (iii) we maintain inventory risk upon accepting returns.
For certain product offerings such as premium, patented, child-resistant packaging, closed-system vaporization solutions and custom-branded retail products, we generally receive a deposit from the customer (generally 50% of the total order cost, but the amount can vary by customer contract), when an order is placed by a customer. We typically complete these orders within six weeks to three months from the date of order, depending on the complexity of the customization and the size of the order. See “Note 8—Supplemental Financial Statement Information” for a summary of changes to our customer deposit liability balance during the years ended December 31, 2020 and 2019.
We estimate product returns based on historical experience and record them as a refund liability that reduces the net sales for the period. We analyze actual historical returns, current economic trends and changes in order volume when evaluating the adequacy of our sales returns allowance in any reporting period. Our liability for returns is included within "Accrued expenses and other current liabilities" in our consolidated balance sheets and was approximately $0.8 million and $0.6 million at December 31, 2020 and 2019, respectively. The recoverable cost of merchandise estimated to be returned by customers is included within "Other current assets" in our consolidated balance sheets and was approximately $0.2 million and $0.3 million as of December 31, 2020 and 2019, respectively.
We elected to account for shipping and handling expenses that occur after the customer has obtained control of products as a fulfillment activity in cost of sales. Shipping and handling fees charged to customers are included in net sales upon completion of our performance obligations. We apply the practical expedient provided for by ASC 606 by not adjusting the transaction price for significant financing components for periods less than one year. We also apply the practical expedient provided for by ASC 606 based upon which we generally expense sales commissions when incurred because the amortization period is one year or less. Sales commissions are recorded within "Salaries, benefits and payroll tax expenses" in the consolidated statements of operations and comprehensive loss.
No single customer represented more than 10% of our net sales for the years ended December 31, 2020 and 2019, respectively. As of December 31, 2020 and 2019, no single customer represented more than 10% of our accounts receivable balance.
Federal Drug Administration's ENDS Enforcement Guidance and Premarket Tobacco Product Applications
In January 2020, the FDA issued ENDS Enforcement Guidance, which outlines the FDA's intent to prioritize enforcement against flavored, cartridge-based ENDS products (except tobacco or menthol flavored products), all other ENDS products for which the manufacturer has failed to take adequate measures to prevent access to minors, and any ENDS products targeted to minors or whose marketing is likely to promote usage by minors. Additionally, the deadline for ENDS manufacturers to submit Premarket Tobacco Product Applications ("PMTA") was September 9, 2020. The FDA has indicated its intent to prioritize enforcement against ENDS products offered for sale after September 9, 2020 for which the manufacturer has not submitted a PMTA. The FDA is not necessarily bound by these enforcement priorities, and it has recently taken actions against other products and may take additional actions against other products as warranted by circumstances.
The ENDS Enforcement Guidance had the effect of prohibiting the sale of certain products in the United States, including mint-flavored products from JUUL Labs and other flavored ENDS, starting February 2020. Products impacted by the ENDS Enforcement Guidance represented less than 0.1% of our net sales for the year ended December 31, 2020, and approximately 17.8% of our net sales for the year ended December 31, 2019.
During the years ended December 31, 2020 and 2019, we sold products for which manufacturers have not submitted a PMTA to the FDA by September 9, 2020. Sales of these products represented approximately 0.4% and 1.0% of our net sales for the years ended December 31, 2020 and 2019, respectively.
While we have been compliant with and expect to remain in compliance with the ENDS Enforcement Guidance, further actions and developments of FDA's guidance could adversely affect our sales of ENDS products and may have a material adverse effect on our business, results of operations and financial condition.
Value Added Taxes
During the year ended December 31, 2020, as part of a global tax strategy review, we determined that our European subsidiaries based in the Netherlands, which we acquired on September 30, 2019, had historically collected and remitted value added tax ("VAT") payments, which related to direct-to-consumer sales to other European Union ("EU") member states, directly to the Dutch tax authorities. In connection with our subsidiaries' payment of VAT to Dutch tax authorities rather than other EU member states, the German government has commenced a criminal investigation, which could result in penalties; other jurisdictions could commence such investigations as well. We have performed an analysis of the VAT overpayments to the Dutch tax authorities, which we expect will be refunded to us, and VAT payable to other EU member states, including potential fines and penalties. Based on this analysis, we recorded a VAT payable of approximately $9.9 million within "Accrued expenses and other current liabilities" and VAT receivable of approximately $4.4 million within "Other current assets" in our consolidated balance sheet as of December 31, 2020.
Pursuant to the purchase and sale agreement by which we acquired our European subsidiaries, the sellers are required to indemnify us against certain specified matters and losses, including any and all liabilities, claims, penalties and costs incurred or sustained by us in connection with non-compliance with tax laws in relation to activities of the sellers. The indemnity is limited to an amount equal to the purchase price under the purchase and sale agreement. Furthermore, we are the beneficiary to a bank guarantee in the amount of approximately $0.9 million for claims for which we are entitled to indemnification under the purchase and sale agreement. The bank guarantee has an expiration date of October 1, 2021. Accordingly, as of December 31, 2020, we recognized an indemnification asset of approximately $0.9 million within "Other current assets" using the loss recovery model, as management believes that amounts covered by the bank guarantee are probable of recovery.
Management intends to pursue recovery of all additional losses from the sellers to the full extent of the indemnification provisions of the purchase and sale agreement, however, the collectability of such additional indemnification amounts may be subject to litigation and may be affected by the credit risk of indemnifying parties, and are therefore subject to significant uncertainties as to the amount and timing of recovery. Therefore, during the year ended December 31, 2020, we recognized a charge of approximately $4.5 million within "general and administrative expenses" in our consolidated statements of operations and comprehensive loss, which represents the difference between the VAT payable and the VAT receivable and indemnification asset recorded as of December 31, 2020.
We establish VAT receivables in jurisdictions where VAT paid exceeds VAT collected and are recoverable through the filing of refund claims. Our VAT receivable balance as of December 31, 2020 relates to refund claims with the Dutch tax authorities. We intend to voluntarily disclose VAT owed to the relevant tax authorities in the EU member states and believe in doing so we will reduce our liability for penalties and interest. Nonetheless, we may incur expenses in future periods related to such matters, including litigation costs and other expenses to defend our position. The outcome of such matters is inherently unpredictable and subject to significant uncertainties.
Refer to "Note 7—Commitments and Contingencies" for additional discussion regarding our contingencies.
Treasury Stock
When Class A common stock is acquired for purposes other than formal or constructive retirement, the purchase price of the acquired stock is recorded in a separate treasury stock account, which is separately reported as a reduction of stockholders' equity.
When Class A common stock is retired or purchased for formal or constructive retirement, the purchase price is initially recorded as a reduction to the par value of the shares repurchased, with any excess purchase price over par value recorded as a reduction to additional paid-in capital related to the series of shares repurchased and any remainder excess purchase price recorded as a reduction to retained earnings. If the purchase price exceeds the amounts allocated to par value and additional paid-in capital related to the series of shares repurchased and retained earnings, the remainder is allocated to additional paid-in capital related to other series of shares.
Net Loss Per Share
Basic net income (loss) per share is computed by dividing net income (loss) attributable to us by the weighted average number of shares outstanding during the period. Diluted net income (loss) per share is computed by giving effect to all potential weighted average dilutive shares including stock options, restricted Common Units granted as equity-based compensation, and Common Units exchangeable for shares of our Class A common stock for the periods after the closing of the IPO. The dilutive effect of outstanding awards, if any, is reflected in diluted earnings per share by application of the treasury stock method or if-converted method, as applicable. See “Note 9—Stockholders' Equity - Net Loss Per Share.”
Recently Adopted Accounting Guidance
In February 2016, the Financial Accounting (“FASB”) issued Accounting Standard Update (“ASU”) No. 2016-02, Leases (Topic 842), which, among other things, requires lessees to recognize substantially all leases on their balance sheets and disclose key information about leasing arrangements. The new standard establishes a right of use (“ROU”) model that requires a lessee to recognize a ROU asset and liability on the balance sheet for all leases with a term longer than 12 months. Leases are classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the statement of operations. The new standard became effective for the Company on January 1, 2019.
We adopted Topic 842 utilizing the modified retrospective adoption method with an effective date of January 1, 2019. We made the election to not apply the recognition requirements in Topic 842 to short-term leases (i.e., leases of 12 months or less) for all classes of underlying assets. Instead, we recognize lease payments in profit or loss on a straight-line basis over the lease term. In addition, in accordance with Topic 842, variable lease payments in the period in which the obligation for those payments is incurred are not included in the recognition of a lease liability or right-of-use asset. We elected to not separate non-lease components from the associated lease component for all underlying classes of assets with lease and non-lease components. The adoption of Topic 842 resulted in the recognition of operating lease liabilities of approximately $2.6 million and operating ROU assets of $2.4 million, primarily related to warehouses, retail stores, regional offices, and machinery and equipment. There was no cumulative effect adjustment to beginning Members' Deficit on the consolidated balance sheet. The accounting for our finance leases remained substantially unchanged, as finance lease liabilities and their corresponding ROU assets were already recorded on the consolidated balance sheets under the previous guidance. The adoption of Topic 842 did not have a significant effect on our results from operations or cash flows. See “Note 5—Leases” for additional disclosures required by Topic 842.
In June 2018, the FASB issued ASU No. 2018-07, Compensation - Stock Compensation: Improvements to Nonemployee Share Based Payment Accounting. ASU 2018-07 provides guidance on accounting for equity-based awards issued to nonemployees. The standard was effective for annual and interim periods beginning after December 15, 2018. We adopted this standard beginning January 1, 2019. Adoption of the new standard did not impact our consolidated financial statements as we did not have any outstanding equity-based compensation awards granted to non-employees prior to the adoption of this ASU.
In August 2017, the FASB issued ASU No. 2017-12, Derivatives and Hedging (Topic 815): Targeted Improvements to Accounting for Hedging Activities. The ASU expands and enhances hedge accounting to become more closely aligned with an entity’s risk management activities through hedging strategies. The ASU provides changes to both the designation and measurement guidance for qualifying hedging relationships and the presentation of hedge results in the financial statements and creates more transparency and makes the economic results presented in the financial statements easier to understand. In addition, the new guidance makes certain targeted improvements to ease the application of accounting guidance relative to hedge effectiveness. The standard was effective for annual and interim periods beginning after December 15, 2018. We adopted this ASU prospectively beginning July 1, 2019 and applied the guidance provided by the ASU to the derivative instrument discussed in "Note 4—Fair Value of Financial Instruments”. Adoption of the new standard did not impact our consolidated financial statements as we did not hold any derivative instruments to which this new ASU was applicable in earlier reporting periods.
In August 2018, the FASB issued ASU 2018-15, Intangibles-Goodwill and Other-Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement, which aligns the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. We adopted this standard prospectively beginning January 1, 2020. Adoption of this standard did not have a material impact on our consolidated financial statements.
Recently Issued Accounting Guidance Not Yet Adopted
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments - Credit Losses. The standard requires the use of an “expected loss” model on certain types of financial instruments. The standard also amends the impairment model for available-for-sale securities and requires estimated credit losses to be recorded as allowances rather than as reductions to the amortized cost of the securities. This standard is effective for fiscal years, and interim periods within those years, beginning after December 15, 2022 for filers that are eligible to be smaller reporting companies under the SEC's definition. Early adoption is permitted. We do not expect the adoption of this new guidance will have a material impact on our consolidated financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which removes certain exceptions to the general principles in Topic 740 and also clarifies and amends existing guidance to improve consistent application. This update will be effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. We will adopt this guidance effective January 1, 2021, and do not expect the adoption of this guidance to have a material impact on our consolidated financial statements.
In January 2020, the FASB issued ASU No. 2020-01, Investments—Equity Securities (Topic 321), Investments—Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815), which clarifies the interaction of accounting for equity securities under Topic 321, the accounting for equity investments in Topic 323, and the accounting for certain forward contracts and purchased options in Topic 815. We will adopt this guidance effective January 1, 2021 and do not expect the adoption of this guidance to have a material impact on our consolidated financial statements.
In March 2020, the FASB issued ASU No. 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting. This ASU provides optional expedient an exceptions for applying generally accepted accounting principles to contracts, hedging relationships, and other transactions affected by reference rate reform if certain criteria are met. In response to the concerns about structural risks of interbank offered rates and, particularly, the risk of cessation of the London Interbank Offered Rate ("LIBOR"), regulators in several jurisdictions around the world have undertaken reference rate reform initiatives to identify alternative reference rates that are more observable or transaction-based and less susceptible to manipulation. The ASU provides companies with optional guidance to ease the potential accounting burden associated with transitioning away from reference rates that are expected to be discontinued. In January 2021, the FASB issued ASU No. 2021-01, Reference Rate Reform (Topic 848): Scope, which clarifies certain provisions in Topic 848, if elected by an entity, apply to derivative instruments that use an interest rate for margining, discounting, or contract price alignment that is modified as a result of reference rate reform. The amendments in these ASUs are effective for all entities as of March 12, 2020 through December 31, 2022. We are still evaluating the effect of adopting this guidance.
NOTE 3. BUSINESS ACQUISITIONS
Pollen Gear LLC
Effective January 14, 2019, the Operating Company acquired a 100% interest in Pollen Gear LLC (“Pollen Gear”) in exchange for an aggregate four percent (4.0%) equity interest in the Operating Company. As consideration for the transaction, the Operating Company issued its Class B units, which, as described below in “Note 9—Stockholders’ Equity/Members’ Deficit,” were contingently redeemable by the holder. The Pollen Gear acquisition was accounted for as a business combination under the acquisition method under ASC Topic 805, Business Combinations. Pollen Gear has been consolidated in our consolidated financial statements commencing on January 14, 2019, the date of acquisition. Pollen Gear's operating activities have been integrated with an existing subsidiary of the Operating Company, and as such it is impracticable to identify post-acquisition revenues and earnings included within "net sales" and "net loss" in our consolidated statement of operations and comprehensive loss for the years ended December 31, 2020 and 2019. The following table summarizes the purchase price allocation and the estimated fair value of the net assets acquired and liabilities assumed at the date of acquisition.
| | | | | | | | |
| Pollen Gear LLC (in thousands) | | January 14, 2019 |
| Cash | | $ | 91 | |
| Accounts receivable | | 546 | |
| Vendor deposits | | 1,700 | |
| Other deposits | | 18 | |
| Property and equipment, net | | 342 | |
| Trade name | | 918 | |
| Design libraries | | 1,677 | |
| Goodwill | | 3,550 | |
| Net liabilities | | (2,178) | |
| Total purchase price | | $ | 6,664 | |
At January 14, 2019, the Operating Company had accounts payable to Pollen Gear of approximately $0.6 million and Pollen Gear had accounts receivable for the corresponding amount from the Operating Company. Furthermore, at the date of acquisition, the Operating Company had vendor deposits with Pollen Gear of approximately $1.7 million, and Pollen Gear had customer deposits for the corresponding amount due to the Operating Company. Both the vendor deposits and accounts payable recorded by the Operating Company and the corresponding customer deposits and accounts receivable recorded by Pollen Gear approximated fair value. As a result of the business acquisition, the preexisting relationship between the Operating Company and Pollen Gear was effectively settled. No gain or loss was recognized on this settlement.
Conscious Wholesale
Effective September 30, 2019, we acquired a 100% interest in Conscious Wholesale, a leading European wholesaler and retailer of consumption accessories, vaporizers, and other high-quality products. As consideration for the transaction, we paid $6.7 million, which consisted of $5.1 million in a combination of cash and our Class A common stock and $1.6 million of contingent consideration, payable in a combination of cash and our Class A common stock. The contingent consideration arrangement requires us to make contingent payments based on the achievement of certain operational and financial
performance targets for the year ended December 31, 2020, as set forth in the acquisition agreement. We estimated the fair value of the contingent consideration by using a Monte Carlo simulation that includes significant unobservable inputs such as the risk-free rate, risk-adjusted discount rate, the volatility of the underlying financial metrics and projected financial forecast of the acquired business over the earn-out period. Conscious Wholesale has been consolidated in our consolidated financial statements commencing on September 30, 2019, the date of acquisition. "Net sales" and "net loss" in the consolidated statements of operations and comprehensive loss for the years ended December 31, 2020 and 2019 includes revenue and net loss of Conscious Wholesale from the date of acquisition through December 31, 2019 of approximately $2.6 million and $0.3 million, respectively, and revenue and net loss of Conscious Wholesale for the year ended December 31, 2020 of approximately $10.3 million and $3.3 million, respectively.
We accounted for the Conscious Wholesale acquisition as a business combination under the acquisition method under ASC Topic 805, Business Combinations. The purchase price for the Conscious Wholesale acquisition was allocated based on estimates of the fair value of assets acquired and liabilities assumed at the acquisition date, with the excess allocated to goodwill. As a result of additional information obtained about facts and circumstances that existed as of the acquisition date, we calculated the working capital adjustment to the purchase price and recorded measurement period adjustments during the fourth quarter of 2019. The following table summarizes (in thousands) the purchase price allocation and the estimated fair value of the net assets acquired and liabilities assumed at the date of acquisition.
| | | | | | | | | | | |
| Conscious Wholesale | Estimated Fair Value
as of Acquisition Date
(as Previously Reported) | Measurement
Period Adjustments | Estimated Fair Value as of Acquisition Date (as Adjusted) |
| Cash | $ | 812 | | $ | 0 | | $ | 812 | |
| Accounts receivable | 313 | | 0 | | 313 | |
| Inventory, net | 1,820 | | 0 | | 1,820 | |
| Other assets | 955 | | 184 | | 1,139 | |
| Trade names | 153 | | 0 | | 153 | |
| Customer relationships | 1,044 | | 175 | | 1,219 | |
| Goodwill | 2,264 | | 657 | | 2,921 | |
| Net liabilities | (1,494) | | (184) | | (1,678) | |
| Total purchase price | $ | 5,867 | | $ | 832 | | $ | 6,699 | |
Unaudited Pro Forma Financial Information
The following unaudited pro forma financial information represents the combined results for us, Pollen Gear, and Conscious Wholesale for the years ended December 31, 2020 and 2019 as if Pollen Gear and Conscious Wholesale had been acquired by us on January 1, 2019, and their results had been included in our consolidated results beginning on that date (in thousands):
| | | | | |
| Year Ended December 31, |
| 2019 |
| (Unaudited) |
| Net Sales | $ | 193,351 | |
| Cost of Goods Sold | 159,252 | |
| Gross Profit | 34,099 | |
| Net Loss | $ | (39,621) | |
The pro forma amounts have been calculated after applying our accounting policies to the financial statements of Pollen Gear and Conscious Wholesale and adjusting the combined results of us, Pollen Gear, and Conscious Wholesale (a) to remove Pollen Gear and Conscious Wholesale product sales to us and to remove the cost incurred by us related to products purchased from Pollen Gear and Conscious Wholesale prior to the acquisition, (b) to reflect the increased amortization expense that would have been charged assuming intangible assets identified in the acquisition of Pollen Gear and Conscious Wholesale had been recorded on January 1, 2019, and (c) to remove the transaction costs incurred by us related to the acquisition of Conscious Wholesale.
The impact of the Pollen Gear and Conscious Wholesale acquisitions on the actual results reported by us in subsequent periods may differ significantly from that reflected in this pro forma information for a number of reasons, including but not limited to, non-achievement of the expected synergies from these combinations and changes in the regulatory environment. As a result, the pro forma information is not necessarily indicative of what our financial condition or results of operations would have been had the acquisitions been completed on the applicable dates of this pro forma financial information. In addition, the pro forma financial information does not purport to project our future financial condition and results of operations.
NOTE 4. FAIR VALUE OF FINANCIAL INSTRUMENTS
The carrying amounts of certain financial instruments we have including cash, accounts receivable, accounts payable and certain accrued expenses and other assets and liabilities approximate fair value due to the short-term nature of these instruments. Our financial liabilities measured at fair value on a recurring basis were as follows at December 31, 2020 and 2019:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Consolidated
Balance Sheet Caption | | Fair Value at December 31, 2020 |
| (in thousands) | | | Level 1 | | Level 2 | | Level 3 | | Total |
| Liabilities: | | | | | | | | | | |
| Interest rate swap contract | | Other long-term liabilities | | $ | 0 | | | $ | 665 | | | $ | 0 | | | $ | 665 | |
| Total Liabilities | | | | $ | 0 | | | $ | 665 | | | $ | 0 | | | $ | 665 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Consolidated
Balance Sheet Caption | | Fair Value at December 31, 2019 |
| (in thousands) | | | Level 1 | | Level 2 | | Level 3 | | Total |
| Liabilities: | | | | | | | | | | |
| Interest rate swap contract | | Other long-term liabilities | | $ | 0 | | | $ | 206 | | | $ | 0 | | | $ | 206 | |
| Contingent consideration | | Accrued expenses and other current liabilities | | 0 | | | 0 | | | 1,568 | | | 1,568 | |
| Total Liabilities | | | | $ | 0 | | | $ | 206 | | | $ | 1,568 | | | $ | 1,774 | |
There have been no transfers between Level 1 and Level 2 and no transfers to or from Level 3 of the fair value hierarchy.
Derivative Instrument and Hedging Activity
On July 11, 2019, we entered into an interest rate swap contract to manage our risk associated with the interest rate fluctuations on the Company's floating rate Real Estate Note. The counterparty to this instrument is a reputable financial institution. The interest rate swap contract is entered into for periods consistent with the related underlying exposure and does not constitute a position independent of this exposure. Our interest rate swap contract was designated as a cash flow hedge at the inception date, and is reflected at its fair value in our consolidated balance sheet.
The fair value of our interest rate swap liability is determined based on the present value of expected future cash flows. Since our interest rate swap value is based on the LIBOR forward curve and credit default swap rates, which are observable at commonly quoted intervals for the full term of the swap, it is considered a Level 2 measurement.
Details of the outstanding swap contract as of December 31, 2020, which is a pay fixed and receive floating contract, is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Swap Maturity | | Notional Value
(in thousands) | | Pay Fixed Rate | | Receive Floating Rate | | Floating Rate
Reset Terms |
| October 1, 2025 | | $ | 8,125 | | | 2.0775 | % | | One-Month LIBOR | | Monthly |
We performed an initial qualitative assessment of hedge effectiveness using the hypothetical derivative method in the period in which the hedging transaction was entered, as the critical terms of the hypothetical derivative and the hedging instrument were the same. On a quarterly basis, we perform a qualitative analysis for quarterly prospective and retrospective assessments of hedge effectiveness. The unrealized loss on the derivative instrument is included within "Other comprehensive income (loss)" in our consolidated statement of operations and comprehensive loss for the years ended December 31, 2020 and 2019. There was no measure of hedge ineffectiveness and no reclassifications from other comprehensive loss into interest expense for the years ended December 31, 2020 and 2019.
Contingent Consideration
Each period we revalue our contingent consideration obligations associated with business acquisitions to their fair value. The estimate of the fair value of contingent consideration is determined by applying a risk-neutral framework using a Monte Carlo Simulation, which includes inputs not observable in the market, such as the risk-free rate, risk-adjusted discount rate, the volatility of the underlying financial metrics and projected financial forecast of the acquired business over the earn-out period, and therefore represents a Level 3 measurement. Significant increases or decreases in these inputs could result in a significantly lower (higher) fair value measurement of the contingent consideration liability. During the year ended December 31, 2020, we recognized a gain from the fair value adjustment of contingent consideration of approximately $0.7 million. The fair value adjustment was largely attributed to changes in forecasted revenues and gross profits for our European operating segment over
the remainder of 2020 driven primarily by the impacts of the COVID-19 pandemic. Changes in the fair value of our contingent consideration from business combinations are included within "Other income, net" in our consolidated statements of operations and comprehensive loss. As of December 31, 2020, we did not have any contingent consideration obligations outstanding.
A reconciliation of our liabilities that are measured and recorded at fair value on a recurring basis using significant unobservable inputs (Level 3) for the years ended December 31, 2020 and 2019 is as follows:
| | | | | | | | | | | |
| (in thousands) | Conscious Wholesale Contingent Consideration | | Convertible Notes |
| Balance at December 31, 2018 | $ | 0 | | | $ | 40,200 | |
| Convertible notes issued in January 2019 | — | | | 8,050 | |
| Contingent consideration issued in September 2019 | 1,609 | | | — | |
| Total (gains) losses in fair value included in results of operations | (41) | | | 12,063 | |
| Conversion of convertible debt to Class A common stock | — | | | (60,313) | |
| Balance at December 31, 2019 | 1,568 | | | 0 | |
| Foreign currency translation adjustments | (14) | | | — | |
| Payment for contingent consideration | (835) | | | — | |
| Total gains in fair value included in results of operations | (719) | | | — | |
| Balance at December 31, 2020 | $ | 0 | | | $ | 0 | |
NOTE 5. LEASES
Greenlane as a Lessee
As of December 31, 2020, we had 14 facilities financed under operating leases consisting of warehouses, offices, and retail stores, with lease term expirations between 2021 and 2026. Lease terms are generally three to five years for warehouses, office space and retail store locations. Our lease agreements do not contain any material residual value guarantees or material restrictive covenants.
During the year ended December 31, 2020, we took steps to optimize our distribution network, transitioning to a more streamlined network with fewer, centrally-located, highly automated facilities. Accordingly, we entered into service agreements with third-party logistics ("3PL") companies in the United States and Canada to handle the bulk of the North American supply chain needs, and entered into an agreement for a California-based facility. As of December 31, 2020, we have successfully transferred, subleased or terminated leases for our Jacksonville, FL, Torrance, CA, Visalia, CA, and B.C Canada distribution centers. With regard to our retail locations, we entered into a new operating lease agreement for a new retail store location in Barcelona, Spain, and we permanently closed our Ponce City Market retail location.
During the year ended December 31, 2020, we recorded approximately $1.7 million in charges related to the closures above, comprised of $1.3 million related to right-of-use asset impairments, $0.1 million related to impairments of leasehold improvements, and a lease cancellation fee of approximately $0.3 million. These charges were offset by the derecognition of the associated operating lease liabilities of approximately $1.4 million, recorded within "general and administrative expenses" in our consolidated statement of operations and comprehensive loss.
In August 2020, we initiated the process of seeking a third-party to assume our Jacksonville, FL leases. In November 2020, we transferred the right-of-use asset and corresponding operating lease liability of one lease for our Jacksonville, FL distribution center. In December 2020, we initiated the process of seeking a third-party to assume our Visalia, CA lease. As of December 31, 2020, our United States operating segment recorded approximately $0.2 million of right-of-use assets held for sale within "assets held for sale" and approximately $0.2 million of liabilities held for sale within "accrued expenses and other current liabilities" in our consolidated balance sheet as of December 31, 2020. We expect to transfer the right-of-use assets and corresponding operating lease liabilities by the third quarter of 2021.
The following table provides details of our future minimum lease payments under finance and operating lease liabilities recorded in our condensed consolidated balance sheet as of December 31, 2020. The table below does not include commitments that are contingent on events or other factors that are currently uncertain or unknown.
| | | | | | | | | | | | | | | | | |
| (in thousands) | Finance
Leases | | Operating Leases | | Total |
| 2021 | $ | 195 | | | $ | 1,102 | | | $ | 1,297 | |
| 2022 | 134 | | | 949 | | | 1,083 | |
| 2023 | 69 | | | 922 | | | 991 | |
| 2024 | 0 | | | 611 | | | 611 | |
| 2025 | 0 | | | 124 | | | 124 | |
| Thereafter | 0 | | | 126 | | | 126 | |
| Total minimum lease payments | 398 | | | 3,834 | | | 4,232 | |
| Less: imputed interest | 9 | | | 344 | | | 353 | |
| Present value of minimum lease payments | 389 | | | 3,490 | | | 3,879 | |
| Less: current portion | 184 | | | 966 | | | 1,150 | |
| Long-term portion | $ | 205 | | | $ | 2,524 | | | $ | 2,728 | |
Rent expense under operating leases was approximately $1.6 million and $1.3 million for the years ended December 31, 2020 and 2019, respectively.
The majority of our finance lease obligations relate to leased warehouse equipment. Payments under our finance lease agreements are fixed for terms ranging from three to five years. We recorded approximately $0.4 million and $0.3 million of finance lease assets, net within "property and equipment, net" as of December 31, 2020, and 2019, respectively, and the related liabilities within "current portion of finance leases" and "finance leases, less current portion" in our consolidated balance sheets.
The following expenses related to our finance and operating leases were included in "general and administrative expenses" within our consolidated statements of operations and comprehensive loss for the years ended December 31, 2020 and 2019:
| | | | | | | | |
| (in thousands) | December 31,
2020 | December 31, 2019 |
| Finance lease cost | | |
| Amortization of leased assets | $ | 142 | | $ | 130 | |
| Interest of lease liabilities | 18 | | 24 | |
| Operating lease costs | | |
| Operating lease cost | 1,383 | | 919 | |
| Variable lease cost | 255 | | 378 | |
| Total lease cost | $ | 1,798 | | $ | 1,451 | |
The table below presents lease-related terms and discount rates as of December 31, 2020:
| | | | | |
| December 31,
2020 |
| Weighted average remaining lease terms | |
| Operating leases | 3.6 years |
| Finance leases | 2.0 years |
| Weighted average discount rate | |
| Operating leases | 4.8 | % |
| Finance leases | 6.6 | % |
Greenlane as a Lessor
We have five operating leases for office space leased to third-party tenants in our corporate headquarters building in Boca Raton, Florida. For the years ended December 31, 2020 and 2019, we recorded approximately $0.6 million and $0.7 million in rental income related to these operating leases, respectively, which we include within “Other income, net” in our consolidated statements of operations and comprehensive loss.
The following table represents the maturity analysis of undiscounted cash flows related to lease payments, which we expect to receive from our existing operating lease agreements with tenants:
| | | | | | |
| Rental Income | | (in thousands) |
| 2021 | | $ | 711 | |
| 2022 | | 199 | |
| 2023 | | 99 | |
| 2024 | | 77 | |
| 2025 | | 53 | |
| Total | | $ | 1,139 | |
NOTE 6. LONG TERM DEBT
Our long-term debt, excluding operating lease liabilities and finance lease liabilities, consisted of the following amounts at the dates indicated:
| | | | | | | | | | | |
| (in thousands) | December 31, 2020 | | December 31, 2019 |
3.0% note payable for a four-year loan for the purchase of a truck | $ | 0 | | | $ | 18 | |
| Real Estate Note | 8,125 | | | 8,297 | |
| 8,125 | | | 8,315 | |
| Less unamortized debt issuance costs | (99) | | | (119) | |
| Less current portion of long-term debt | (182) | | | (178) | |
| Long-term debt, net, excluding operating leases and finance leases | $ | 7,844 | | | $ | 8,018 | |
Line of Credit
On October 1, 2018, the Operating Company, as the borrower, entered into an amended and restated revolving credit note (the “line of credit”) with Fifth Third Bank, for a $15 million revolving credit loan with a maturity date of August 23, 2020. On April 5, 2019, the Operating Company, as the borrower, entered into a second amendment to the first amended and restated credit agreement, dated October 1, 2018 (the “line of credit”) with Fifth Third Bank, for a $15.0 million revolving credit loan with a maturity date of August 23, 2020. In August 2020, the maturity date of the line of credit was further extended to November 30, 2020. This line of credit was not renewed on November 30, 2020. There were no borrowings outstanding on the line of credit at December 31, 2020 and 2019.
Real Estate Note
On October 1, 2018, one of the Operating Company’s wholly-owned subsidiaries closed on the purchase of a building for $10 million, which serves as our corporate headquarters. The purchase was financed through a real estate term note (the “Real Estate Note”) in the principal amount of $8.5 million, with one of the Operating Company’s wholly-owned subsidiaries as the borrower and Fifth Third Bank as the lender. Principal amounts plus any accrued interest at a rate of LIBOR plus 2.39% are due monthly. The Real Estate Note contains customary covenants and restrictions, including, without limitation, covenants that require us to comply with laws, restrictions on our ability to incur additional indebtedness, and various customary remedies for the lender following an event of default, including the acceleration of repayment of outstanding amounts under the Real Estate Note and execution upon the collateral securing obligations under the Real Estate Note. Our obligations under the Real Estate Note are secured by a mortgage on the property. Our Real Estate Note is subject to an interest rate swap contract. See “Note 4—Fair Value of Financial Instruments.”
LIBOR is expected to be discontinued and replaced after 2021 and the credit facility has a maturity date beyond that time. There can be no assurances as to what the alternative base rate will be in the event that LIBOR is discontinued, and we can provide no assurances whether that base rate will be more or less favorable than LIBOR. We intend to monitor the developments with respect to the phasing out of LIBOR after 2021 and work with our lenders to ensure that any transition away from LIBOR will have minimal impact on our financial condition but can provide no assurances regarding the impact of LIBOR discontinuation.
Convertible Notes
In December 2018, the Operating Company issued an aggregate of $40.2 million in convertible promissory notes (the “convertible notes”) and received net cash proceeds of $38.9 million. In January 2019, the Operating Company issued an additional $8.1 million in convertible notes and received net cash proceeds of $6.5 million. During the three months ended March 31, 2019, we recognized debt issuance costs of $0.4 million associated with the issuance of January 2019 convertible notes within "interest expense," and we also recognized an expense related to the change in fair value of the convertible notes of $12.1 million within "other income (expense), net" in our consolidated statement of operations and comprehensive loss. The
convertible notes did not accrue interest. In April 2019, in connection with the closing of our IPO, we issued 3,547,776 shares of our Class A common stock to the holders of the convertible notes upon conversion of the convertible notes of the Operating Company at a settlement price equal to 80% of the IPO price per share. There were 0 convertible notes outstanding at December 30, 2020 or December 31, 2019.
NOTE 7. COMMITMENTS AND CONTINGENCIES
Contingencies
In the ordinary course of business, we are involved in various legal proceedings involving a variety of matters. We do not believe there are any pending legal proceedings that will have a material adverse effect on our business, consolidated financial position, results of operations, or cash flows. However, the outcome of such legal matters is inherently unpredictable and subject to significant uncertainties.
On August 2, 2019, a purported stockholder of the Company filed a purported class action lawsuit against the Company, officers and directors of the Company, and the underwriters for related to the Company’s initial public offering. The complaint alleges, among other things, that the Company’s registration statement related to its initial public offering contained untrue statements of material fact and, or omitted to state material facts necessary to make the statements in the registration statement not misleading, in violation of Sections 11, 12 and 15 of the Securities Act of 1933, as amended. Since August 2, 2019, four additional purported class action lawsuits have been filed making substantially similar allegations.
Two of the complaints alleging violations of securities laws as described above were filed against the Company in the United States District Court for the Southern District of Florida. These cases have been consolidated under the caption In re Greenlane Holdings, Inc. Securities Litigation (Case No. 19-CV-81259). The plaintiffs filed an amended complaint on March 6, 2020 and the Company filed a motion to dismiss on March 20, 2020. On January 6, 2021, the United States District Court for the Southern District of Florida granted the Company's motion to dismiss, and dismissed the case with prejudice.
Three of the complaints alleging violations of securities laws as described above were filed against the Company in the Circuit Court of the Fifteenth Judicial Circuit for Palm Beach County, Florida. These cases have been consolidated under the caption In re Greenlane Holdings, Inc. Securities Litigation (Case No. 50-2019-CA-010026). The plaintiffs filed an amended complaint on December 9, 2019 and the Company filed a motion to dismiss on February 7, 2020. On February 5, 2021, The Circuit Court of the Fifteenth Judicial Circuit for Palm Beach County, Florida granted the Company's motion to dismiss.
As a result of the rulings mentioned above, there are currently no securities lawsuits pending against the Company.
See “Note 5—Leases” for details of our future minimum lease payments under finance lease liabilities and operating lease liabilities. See "Note 11—Income Taxes" for information regarding income tax contingencies.
Other Contingencies
We are potentially subject to claims related to various non-income taxes (such as sales, value added, consumption, and similar taxes) from various tax authorities, including in jurisdictions in which we already collect and remit such taxes. If the relevant taxing authorities were successfully to pursue these claims, we could be subject to significant additional tax liabilities.
NOTE 8. SUPPLEMENTAL FINANCIAL STATEMENT INFORMATION
Assets Held for Sale
During the year ended December 31, 2020, we performed a review of our property and equipment held at our distribution centers, corporate headquarters, and retail locations for disposal or sale in connection with our transformation plan. As a result of this review, we made the decision to commit to a formal plan to sell machinery that was used by our United States operating segment. Accordingly, we determined that this machinery met the criteria to be reclassified as held for sale as of December 31, 2020.
An asset group classified as held for sale is reflected at the lower of its carrying amount or estimated fair value less cost to sell. If the carrying amount of the assets exceeds its estimated fair value, a loss is recognized. Due to the reclassification as held-for-sale of this machinery, we recognized impairment charges of approximately $0.3 million for the year ended December 31, 2020, which was included within "general and administrative expenses" in our consolidated statement of operations and comprehensive loss. We recorded approximately $0.9 million of machinery held for sale within "Assets Held for Sale" in our consolidated balance sheet as of December 31, 2020. We are actively seeking a buyer and expect to complete the sale of the machinery by the third quarter of 2021.
Other Current Assets
The following table summarizes the composition of other current assets as of the dates indicated:
| | | | | | | | | | | |
| (in thousands) | December 31, 2020 | | December 31, 2019 |
| Other current assets: | | | |
| VAT refund receivable | $ | 4,391 | | | $ | 0 | |
| Prepaid expenses | 1,542 | | | 2,850 | |
| Other | 4,959 | | | 2,074 | |
| $ | 10,892 | | | $ | 4,924 | |
Property and Equipment, Net
The following is a summary of our property and equipment, at costs less accumulated depreciation and amortization:
| | | | | | | | | | | | | | | | | |
| As of December 31, | | Estimated useful life |
| (in thousands) | 2020 | | 2019 | |
| Furniture, equipment and software (includes $0.6 million and $0.5 million under finance leases as of December 31, 2020 and 2019, respectively) | $ | 2,978 | | | $ | 3,130 | | | 3 - 7 years |
| Personal property | 1,130 | | | 1,105 | | | 5 years |
| Leasehold improvements | 844 | | | 1,077 | | | Lesser of lease term or 5 years |
| Land improvements | 601 | | | 601 | | | 15 years |
| Building | 8,088 | | | 8,064 | | | 39 years |
| Land | 691 | | | 691 | | | |
| Work in process | 633 | | | 712 | | | |
| 14,965 | | | 15,380 | | | |
| Less: accumulated depreciation (includes $0.2 million under finance leases as of December 31, 2020 and 2019) | 2,764 | | | 2,215 | | | |
| Property and equipment, net | $ | 12,201 | | | $ | 13,165 | | | |
Depreciation expense for property and equipment (excluding assets recorded under finance leases) for the years ended December 31, 2020 and 2019 was approximately $1.1 million and $1.2 million, respectively.
Intangible Assets, Net
Identified intangible assets consisted of the following at the dates indicated below:
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 |
| (in thousands) | Gross carrying
amount | | Accumulated
amortization | | Carrying value | | Estimated useful life |
| Design libraries | $ | 1,677 | | | $ | (214) | | | $ | 1,463 | | | 15 years |
| Trademarks and tradenames | 3,617 | | | (1,572) | | | 2,045 | | | 1 - 15 years |
| Customer relationships | 2,565 | | | (796) | | | 1,769 | | | 5 - 15 years |
| Other intangibles | $ | 2,045 | | | (1,377) | | | 668 | | | 2 - 15 years |
| $ | 9,904 | | | $ | (3,959) | | | $ | 5,945 | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2019 |
| (in thousands) | Gross carrying
amount | | Accumulated
amortization | | Carrying value | | Estimated useful life |
| Design libraries | $ | 1,677 | | | $ | (103) | | | $ | 1,574 | | | 15 years |
| Trademarks and tradenames | 3,388 | | | (962) | | | 2,426 | | | 1 - 15 years |
| Customer relationships | 2,446 | | | (473) | | | 1,973 | | | 5 - 15 years |
| Other intangibles | 2,089 | | | (1,761) | | | 328 | | | 2 - 15 years |
| $ | 9,600 | | | $ | (3,299) | | | $ | 6,301 | | | |
The changes in the gross carrying amounts of our trademarks and tradenames, customer relationships, and other intangibles is primarily driven by the acquisition of certain trademarks and domain names during the year ended December 31, 2020, accompanied by fluctuations in foreign currency exchange rates. The weighted-average amortization period for intangible assets
we acquired during the year ended December 31, 2020 was approximately 12.3 years. The weighted-average amortization period for trademarks and tradenames and other intangibles we acquired during the year ended December 31, 2020 was approximately 5 years and 15 years, respectively.
Amortization expense for intangible assets was approximately $1.3 million and $1.4 million during the years ended December 31, 2020 and 2019, respectively. Total estimated amortization expense for our intangible assets for the years 2021 through 2025 are as follows:
| | | | | |
| Amortization Expense | (in thousands) |
| 2021 | $ | 1,004 | |
| 2022 | $ | 1,003 | |
| 2023 | $ | 505 | |
| 2024 | $ | 410 | |
| 2025 | $ | 394 | |
Goodwill
Due to recent market conditions and estimated adverse impacts from the COVID-19 pandemic, management concluded that a triggering event occurred in the first quarter of 2020, requiring a quantitative impairment test of our goodwill for our United States and Europe reporting units. Based on this assessment, we concluded that the fair value of our Europe reporting unit exceeded its carrying value and no impairment charge was required. However, the estimated fair value of the United States reporting unit was determined to be below its carrying value, which resulted in a $9.0 million goodwill impairment charge. The impairment charge resulted from the impacts of COVID-19 on our current and forecasted wholesale revenues and the restrictions on certain products we sell imposed by the Federal Drug Administration's Enforcement Priorities for Electronic Nicotine Delivery Systems and Other Deemed products on the Market Without Premarket Authorization, which resulted in changes to our estimates and assumptions of the expected future cash flows of the United States reporting unit.
During the fourth quarter of 2020, we performed a quantitative assessment for our Europe reporting unit. Based on this assessment, we concluded that the fair value of our Europe reporting unit exceeded its carrying value and no impairment charge was required. The estimated fair value of our reporting unit is highly sensitive to changes in the underlying projections and assumptions; therefore, in some instances, changes in these assumptions could potentially lead to impairment. Specifically, conditions brought on by the COVID-19 pandemic may have material impacts on the assumptions used in determining the fair value of our reporting unit. Should the business environment worsen from impacts of the COVID-19 pandemic, the fair value of our reporting unit may decrease below its carrying value and result in an impairment charge to goodwill in future periods.
Changes in the carrying amount of our goodwill by reporting unit for the year ended December 31, 2020 were as follows:
| | | | | | | | | | | | | | |
| (in thousands) | U.S. | Canada | Europe | Total |
| Balance at December 31, 2019 | $ | 8,996 | | $ | 0 | | $ | 2,986 | | $ | 11,982 | |
| Impairment expense | (8,996) | | 0 | | 0 | | (8,996) | |
| Foreign currency translation adjustment | 0 | | 0 | | 294 | | 294 | |
| Balance at December 31, 2020 | $ | 0 | | $ | 0 | | $ | 3,280 | | $ | 3,280 | |
Accrued Expenses and Other Current Liabilities
The following table summarizes the composition of accrued expenses and other current liabilities as of the dates indicated:
| | | | | | | | | | | |
| (in thousands) | December 31, 2020 | | December 31, 2019 |
| Accrued expenses and other current liabilities: | | | |
| VAT payable | $ | 9,882 | | | $ | 0 | |
| Payroll-related including bonus | 2,361 | | | 1,314 | |
| Accrued professional fees | 1,750 | | | 305 | |
| Accrued third-party logistics fees | 1,295 | | | 0 | |
| Liabilities held for sale | 226 | | | 0 | |
| Accrued taxes, state and income | 211 | | | 1,423 | |
| Accrued purchase price consideration for business acquisition | 0 | | | 3,029 | |
| Contingent consideration payable | 0 | | | 1,568 | |
| Other | 3,665 | | | 2,783 | |
| $ | 19,390 | | | $ | 10,422 | |
Customer Deposits
We established a supply chain for premium, patented, child-resistant packaging, closed-system vaporization solutions and custom-branded retail products. For these product offerings, we generally receive a deposit from the customer (generally 50% of the total order cost, but the amount can vary by customer contract), when an order is placed by a customer. Changes in our customer deposits during the year ended December 31, 2020 were as follows:
| | | | | |
| (in thousands) | Customer Deposits |
| Balance as of December 31, 2019 | $ | 3,152 | |
| Increases due to deposits received, net of other adjustments | 9,164 | |
| Revenue recognized | (9,587) | |
| Balance as of December 31, 2020 | $ | 2,729 | |
We typically complete orders related to customer deposits within six weeks to three months from the date of order, depending on the complexity of the customization and the size of the order.
Accumulated Other Comprehensive Loss
For the years ended December 31, 2020 and 2019, changes in accumulated other comprehensive loss were as follows:
| | | | | | | | | | | | | | | | | |
| (in thousands) | Foreign Currency Translation | | Unrealized Loss on Derivative Instrument | | Total |
| Balance at December 31, 2018 | $ | (286) | | | $ | 0 | | | $ | (286) | |
| Other comprehensive income (loss) | 193 | | | (206) | | | (13) | |
| Effects of the reorganization transactions | 203 | | | 0 | | | 203 | |
| Less: Other comprehensive (income) loss attributable to non-controlling interest | (132) | | | 156 | | | 24 | |
| Balance at December 31, 2019 | (22) | | | (50) | | | (72) | |
| Other comprehensive income (loss) | 654 | | | (459) | | | 195 | |
| Less: Other comprehensive (income) loss attributable to non-controlling interest | (449) | | | 355 | | | (94) | |
| Balance at December 31, 2020 | $ | 183 | | | $ | (154) | | | $ | 29 | |
Supplier Concentration
We have four major vendors whose products accounted for an aggregate of approximately 49.5% of our total net sales and 41.6% of our total purchases for the year ended December 31, 2020, and an aggregate of approximately 63.4% of our total net sales and 52.9% of our total purchases for the year ended December 31, 2019. We expect to maintain our existing relationships with these vendors.
NOTE 9. STOCKHOLDERS’ EQUITY
On April 17, 2019, in connection with the IPO and the Transactions, we amended and restated our certificate of incorporation. After giving effect to the amendment and restatement of our certificate of incorporation, the total number of shares of all classes
of stock that we are authorized to issue is two hundred forty-five million (245,000,000), consisting of (i) one hundred twenty-five million (125,000,000) shares of our Class A common stock; (ii) ten million (10,000,000) shares of our Class B common stock; and (iii) one hundred million (100,000,000) shares of our Class C common stock; and (iv) ten million (10,000,000) shares of our preferred stock, par value $0.0001 per share. Pursuant to the amended and restated certificate of incorporation, the two hundred (200) shares of our common stock, par value $0.01 per share, issued and outstanding prior to the effective time were canceled without further action by, or consideration to, the holders thereof.
Shares of our Class A common stock have both voting interests and economic interests (i.e., the right to receive distributions or dividends, whether cash or stock, and proceeds upon dissolution, winding up or liquidation), while shares of our Class B common stock and Class C common stock have voting interests but no economic interests. Each share of our Class A common stock, Class B common stock and Class C common stock entitles the record holder thereof to one vote on all matters on which stockholders generally are entitled to vote, and except as otherwise required in the amended and restated certificate of incorporation, the holders of Common Stock will vote together as a single class on all matters (or, if any holders of our preferred stock are entitled to vote together with the holders of Common Stock, as a single class with such holders of preferred stock).
Redeemable Class B Units
The Operating Company issued Class B units as consideration for its recent business acquisitions, as well as in form of equity-based compensation to certain of the Operating Company’s executive employees. The Operating Company’s Class B units are non-voting and contained a put right whereby, at any time after the third anniversary of February 20, 2018 (in each case prior to an effective IPO or capital event), each of the holders of Class B units had the right to require that the Operating Company purchase all, but not less than all, of its Class B units at an aggregate price equal to the fair market value of the Class B units as of the date of the put notice (as defined), in the form of a cash payment. The Class B units did not contain any mandatory redemption provisions.
The Operating Company classified the Class B units outside of members’ deficit as of December 31, 2018 as the units contained contingent redemption features that were not solely within the Operating Company’s control. The initial carrying value of the amount classified in temporary equity for the Class B units issued as consideration for business acquisitions was based on the issuance date fair value of the redeemable Class B units, net of issuance costs.
As discussed in “Note 1—Business Operations and Organization,” we completed our IPO of 6,000,000 shares of our Class A common stock (which was comprised of 5,250,000 shares of our Class A common stock sold by us and 750,000 shares of our Class A common stock sold by certain selling stockholders, comprised of Messrs. LoCascio and Schoenfeld and an affiliated entity of Messrs. LoCascio and Schoenfeld) at a public offering price of $17.00 per share on April 23, 2019 and became the sole manager of the Operating Company. As part of the Transactions, the Class B units were converted to Common Units of the Operating Company and the put right was eliminated. There were no redeemable Class B units outstanding at December 31, 2020 or 2019.
Class A Common Stock Repurchases
In November 2019, our Board of Directors approved a stock repurchase program authorizing up to $5.0 million in repurchases of our outstanding shares of Class A common stock. Under the program, we may repurchase shares in accordance with all applicable securities laws and regulations, including Rule 10b-18 of the Securities Exchange Act of 1934, as amended. We may periodically repurchase shares in open market transactions, directly or indirectly, in block purchases and in privately negotiated transactions or otherwise. The timing, pricing, and amount of any repurchases under the share repurchase program will be determined by management at its discretion based on a variety of factors, including, but not limited to, trading volume and market price of our Class A common stock, corporate considerations, our working capital and investment requirements, general market and economic conditions, and legal requirements. The share repurchase program does not obligate us to repurchase any common stock and may be modified, discontinued, or suspended at any time. Shares of Class A common stock repurchased under the program are subsequently retired. There were no share repurchases under the program during the year ended December 31, 2020.
Non-Controlling Interests
As discussed in “Note 1—Business Operations and Organization,” we consolidate the financial results of the Operating Company and report a non-controlling interest related to the Common Units held by non-controlling interest holders on our consolidated financial statements. As of December 31, 2020, we owned 31.6% of the economic interests in the Operating Company, with the remaining 68.4% of the economic interests owned by non-controlling interest holders. The non-controlling interest on the accompanying consolidated statements of operations and comprehensive loss represents the portion of the loss attributable to the economic interest in the Operating Company held by the non-controlling holders of Common Units calculated based on the weighted average non-controlling interests’ ownership during the periods presented.
Net Loss Per Share
Basic net loss per share of our Class A common stock is computed by dividing net loss attributable to us by the weighted-average number of shares of our Class A common stock outstanding during the period. Diluted net loss per share of our Class A common stock is computed by dividing net loss attributable to us by the weighted-average number of shares of our Class A common stock outstanding adjusted to give effect to potentially dilutive elements.
Prior to the amendment and restatement of the Operating Company's LLC Agreement on April 17, 2019 in connection with the IPO, the Operating Company's membership interests were defined solely as percentage interests as the LLC Agreement did not define a number of membership units outstanding or authorized. As a result, the basic and diluted net loss per share for the year ended December 31, 2019 includes only the period from the IPO on April 23, 2019 through December 31, 2019.
A reconciliation of the numerator and denominator used in the calculation of basic and diluted net loss per share of our Class A common stock is as follows (in thousands):
| | | | | | | | | | | |
| For the year ended December 31, 2020 | | For the year ended December 31, 2019 |
| Numerator: | | | |
| Net loss | $ | (47,704) | | | $ | (20,735) | |
| Less: Net loss attributable to non-controlling interests | (33,187) | | | (11,008) | |
| Net loss attributable to Class A common stockholders | $ | (14,517) | | | $ | (9,727) | |
| Denominator: | | | |
| Weighted average shares of Class A common stock outstanding | 11,947 | | | 10,145 | |
| Net loss per share of Class A common stock - basic and diluted | $ | (1.22) | | | $ | (0.96) | |
For the year ended December 31, 2020, 3,490,909 shares of our Class B common stock, 76,039,218 shares of our Class C common stock and 1,373,972 stock options were excluded from the weighted-average in the computation of diluted net loss per share of our Class A common stock because the effect would have been anti-dilutive.
For the year ended December 31, 2019, 5,975,477 shares of our Class B common stock, 77,791,218 shares of our Class C common stock and 629,773 stock options were excluded from the weighted-average in the computation of diluted net loss per share of our Class A common stock because the effect would have been anti-dilutive.
Shares of our Class B common stock and Class C common stock do not share in our earnings or losses and are therefore not participating securities. As such, separate calculations of basic and diluted net loss per share for each of our Class B common stock and Class C common stock under the two-class method have not been presented.
NOTE 10. COMPENSATION PLANS
2019 Equity Incentive Plan
On April 17, 2019, we adopted the 2019 Equity Incentive Plan (the “2019 Plan”). The 2019 Plan provides eligible participants with compensation opportunities in the form of cash and equity incentive awards. The 2019 Plan is designed to enhance our ability to attract, retain and motivate our employees, directors, and executive officers, and incentivizes them to increase our long-term growth and equity value in alignment with the interests of our stockholders. Under the 2019 Plan, we may grant up to 5,000,000 stock options and other equity-based awards to employees, directors and executive officers.
During the year ended December 31, 2020, we granted an aggregate of 949,126 options to our directors and certain employees. The stock options were granted with exercise prices ranging from $2.00 per share to $6.14 per share, and vesting periods ranging from six months to four years. During the year ended December 31, 2019, we granted an aggregate of 654,284 options to our directors and certain employees. The stock options were granted with exercise prices ranging from $6.42 per share to $17.00 per share, and vesting periods ranging from zero to ten years.
We recorded stock compensation expense of approximately $1.6 million and $1.1 million related to stock options during the year ended December 31, 2020 and 2019, respectively, which was included within "salaries, benefits and payroll taxes" in our consolidated statements of operations and comprehensive loss. As of December 31, 2020, total unrecognized compensation expenses related to unvested stock options was approximately $3.0 million, which we expect to recognize over a weighted-average period of 3.1 years.
The fair value of the stock option awards granted during the years ended December 31, 2020 and 2019 was determined on the grant date using the Black-Scholes valuation model based on the following ranges of weighted-average assumptions:
| | | | | | | | |
| December 31, 2020 | December 31, 2019 |
Expected volatility (1) | 96% - 103% | 85% |
Expected dividend yield (2) | 0 | 0 |
Expected term (3) | 5.15 - 6.25 years | 2.5 - 7.75 years |
Risk-free interest rate (4) | 0.23% - 1.72% | 1.49% - 2.49% |
(1)Expected volatility is based on the historical volatility of a selected peer group over a period equivalent to the expected term.
(2)We assumed a dividend yield of 0 as management has no plans to declare dividends in the foreseeable future.
(3)Expected term represents the estimated period of time until an award is exercised and was determined using the simplified method.
(4)The risk-free rate is an interpolation of yields on U.S. Treasury securities with maturities equivalent to the expected term.
A summary of stock option activity for the years ended December 31, 2020 and 2019 is as follows:
| | | | | | | | | | | |
| Stock Options |
| Number of Options | | Weighted-Average
Exercise Price |
| Outstanding as of December 31, 2018 | 0 | | | $ | 0 | |
| Granted | 654,284 | | | 9.28 | |
| Exercised | 0 | | | 0 | |
| Forfeited | (24,511) | | | 17.00 | |
| Outstanding as of December 31, 2019 | 629,773 | | | 8.98 | |
| Granted | 949,126 | | | 3.72 | |
| Exercised | 0 | | | 0 | |
| Forfeited | (204,927) | | | 6.94 | |
| Outstanding as of December 31, 2020 | 1,373,972 | | | $ | 5.47 | |
The weighted-average grant date fair value of options granted for the years ended December 31, 2020 and 2019 was $2.95 and $6.70, respectively. The total fair value of stock options vested during the years ended December 31, 2020 and 2019 was approximately $1.4 million and $0.1 million , respectively.
During the year ended December 31, 2020, we issued 15,000 restricted shares of our Class A common stock to certain executive officers under the 2019 Plan. Compensation expense related to these restricted shares was de minimis for the year ended December 31, 2020.
Common Units of the Operating Company Granted as Equity-Based Compensation
In connection with the closing of the IPO, we consummated certain organizational transactions with the Operating Company, as described in further detail in "Note 1—Business Operations and Organization," among which, the Operating Company reclassified unvested Class B membership interests and profits interests which had been granted as equity-based compensation into Common Units of the Operating Company.
During the year ended December 31, 2020, we recorded a net reversal compensation expense related to Common Units of approximately $0.8 million, which was comprised of compensation expense of approximately $1.9 million offset by a reversal of compensation expense for actual forfeitures that occurred during the period of approximately $2.7 million, which is included within "salaries, benefits and payroll taxes" in our consolidated statement of operations and comprehensive loss. During the year ended December 31, 2019, we recorded compensation expense of approximately $6.9 million related to Common Units. As of December 31, 2020, total unrecognized compensation expense related to unvested Common Units was approximately $0.8 million, which we expect to recognize over a weighted-average period of 1.9 years.
The following table provides a summary of the unvested Common Units outstanding and related transactions:
| | | | | |
| Common Units
Subject to Vesting |
| Unvested Common Units as of December 31, 2018 | 775,979 | |
| Granted | 288,444 | |
| Vested | (235,756) | |
| Forfeited | (12,008) | |
| Unvested Common Units as of December 31, 2019 | 816,659 | |
| Granted | 0 | |
| Vested | (368,489) | |
| Forfeited | (244,266) | |
| Unvested Common Units as of December 31, 2020 | 203,904 | |
401(k) Plan
We have a 401(k) retirement savings plan. Eligible employees must be at least 18 years of age and have completed six months of service. Participants are eligible to receive a matching contribution from us up to the first 3% of compensation plus 50% of participant contributions between 3% and 5% of compensation. Matching contributions, other than safe-harbor contributions, vest 33% per year and are 100% vested after three years of service. Safe-harbor matching contributions are 100% vested as of the date of the contribution. Our matching contributions to the plan were approximately $0.5 million and $0.3 million for the years ended December 31, 2020 and 2019, respectively.
NOTE 11. INCOME TAXES
As a result of the IPO and the Transactions completed in April 2019, we own a portion of the Common Units of the Operating Company, which is treated as a partnership for U.S. federal and most applicable state and local income tax purposes. As a partnership, the Operating Company is generally not subject to U.S. federal and certain state and local income taxes. Any taxable income or loss generated by the Operating Company is passed through to and included in the taxable income or loss of its members, including Greenlane, on a pro-rata basis, in accordance with the terms of the Operating Agreement. The Operating Company is also subject to taxes in foreign jurisdictions. We are a corporation subject to U.S. federal income taxes, in additional to state and local income taxes, based on our share of the Operating Company’s pass-through taxable income.
Income Tax Expense
A reconciliation of the income tax benefit computed at the U.S. federal statutory income tax rate to the income tax expense recognized is as follows:
| | | | | | | | |
| (in thousands) | December 31, 2020 | December 31, 2019 |
| Expected federal income tax (benefit) expense at statutory rate | $ | (9,977) | | $ | (6,067) | |
| State tax expense, net of federal benefit | (652) | | 108 | |
| Loss attributable to non-controlling interests | 5,628 | | 6,264 | |
| Valuation allowance | 5,290 | | 10,041 | |
| Other, net | (95) | | 589 | |
| Income tax expense | $ | 194 | | $ | 10,935 | |
Deferred Tax Assets and Liabilities
The components of deferred tax assets and liabilities were as follows:
| | | | | | | | |
| (in thousands) | December 31, 2020 | December 31, 2019 |
| Deferred tax assets: | | |
| Intangible assets | $ | 9,197 | | $ | 9,144 | |
| Basis difference in investment in the Operating Company | 742 | | 0 | |
| Net operating loss carryforwards | 5,129 | | 1,120 | |
| Other | 43 | | 0 | |
| Total deferred tax assets | 15,111 | | 10,264 | |
| Valuation allowance | (15,111) | | (10,041) | |
| Net deferred tax assets | 0 | | 223 | |
| Deferred tax liability: | | |
| Basis difference in investment in the Operating Company | 0 | | (223) | |
| Net deferred tax assets and liabilities | $ | 0 | | $ | 0 | |
We had approximately $12.8 million of Federal net operating loss carryforwards not subject to expiration. Their utilization is limited to 80% of our future taxable income. We also had approximately $10.1 million of State net operating loss carryforwards that begin expiring in 2039 and $7.6 million of Dutch net operating loss carryforwards that begin expiring in 2026. Their utilization is limited to our future taxable income.
The Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”), which was enacted on March 27, 2020, made tax law changes to provide financial relief to companies as a result of the business impacts of COVID-19. Key income tax provisions of the CARES Act include changes in net operating loss carryback and carryforward rules, acceleration of alternative minimum tax credit recovery, increase in the net interest expense deduction limit and charitable contribution limit, and immediate write-off of qualified improvement property. The changes are not expected to have a significant impact on us.
During the years ended December 31, 2020 and December 31, 2019, management performed an assessment of the realizability of our deferred tax assets based upon which management determined that it is not more likely than not that the results of operations will generate sufficient taxable income to realize portions of the net operating loss benefits. Consequently, we established a full valuation allowance against our deferred tax assets, and reflected a carrying balance of $0 as of December 31, 2020 and December 31, 2019, respectively. In the event that management determines that we would be able to realize our deferred tax assets in the future in excess of their net recorded amount, an adjustment to the valuation allowance will be made, which would reduce the provision for income taxes. The provision for income taxes for the year ended December 31, 2020 and 2019, respectively, relates to taxes in foreign jurisdictions, including Canada and the Netherlands.
Uncertain Tax Positions
For the year ended December 31, 2020, we did not have any unrecognized tax benefits as a result of tax positions taken during a prior period or during the current period. NaN interest or penalties have been recorded as a result of tax uncertainties.
Tax Receivable Agreement (TRA)
We entered into the TRA with the Operating Company and each of the members that provides for the payment by the Operating Company to the members of 85% of the amount of tax benefits, if any, that we may actually realize (or in some circumstances are deemed to realize) as a result of (i) increases in tax basis resulting from any future redemptions of Common Units as described in “Note 1—Business Operations and Organization” and (ii) certain other tax benefits attributable to payments made under the TRA.
The annual tax benefits are computed by calculating the income taxes due, including such tax benefits, and the income taxes due without such benefits. The Operating Company expects to benefit from the remaining 15% of any tax benefits that it may actually realize. The TRA payments are not conditioned upon any continued ownership interest in the Operating Company. The rights of each noncontrolling interest holder under the TRA are assignable to transferees of its interest in the Operating Company. The timing and amount of aggregate payments due under the TRA may vary based on a number of factors, including the amount and timing of the taxable income the Operating Company generates each year and the applicable tax rate.
As noted above, we evaluated the realizability of the deferred tax assets resulting from the IPO and the Transactions completed in April 2019 and established a full valuation allowance against those benefits. As a result, we determined that the amount or timing of payments to noncontrolling interest holders under the TRA are no longer probable or reasonably estimable. Based on this assessment, our TRA liability was $0 as of December 31, 2020 and December 31, 2019.
If utilization of the deferred tax assets subject to the TRA becomes more likely than not in the future, we will record a liability related to the TRA, which would be recognized as expense within our condensed consolidated statements of operations and comprehensive (loss) income.
During the years ended December 31, 2020 and 2019, we did not make any payments, inclusive of interest, to members of the Operating Company pursuant to the TRA.
NOTE 12. SEGMENT REPORTING
We merchandise vaporizers and other products in the United States, Canada and Europe and we distribute to retailers through our wholesale operations and to consumers through e-commerce activities. We define our segments as those operations whose results our CODMs regularly review to analyze performance and allocate resources. Therefore, segment information is prepared on the same basis that management reviews financial information for operational decision-making purposes.
The reportable segments identified are our business activities for which discrete financial information is available and for which operating results are regularly reviewed by our CODMs. As of December 31, 2020, we have 3 reportable segments: (1) United States, (2) Canada and (3) Europe. The United States operating segment is comprised of our United States operations, the Canadian operating segment is comprised of our Canadian operations, and the European operating segment is comprised of our European operations, currently based in the Netherlands. Corporate and other activities which are not allocated to our reportable segments consist primarily of equity-based compensation expenses, unrealized gains on equity securities, and other corporate overhead items. We sell similar products in each of our segments. Assets related to our corporate headquarters as well as our cash proceeds from the IPO are not allocated to any of our reportable segments. We sell similar individuals products and services in each of our segments.
The table below provides information on revenues from external customers, intersegment revenues, segment operating income (loss), and depreciation and amortization by reportable segment for the years ended December 31, 2020 and 2019. We eliminate intersegment revenues in consolidation.
| | | | | | | | | | | |
| For the Year Ended December 31, |
| (in thousands) | 2020 | | 2019 |
| Revenue from external customers: | | | |
| United States | $ | 112,543 | | | $ | 160,243 | |
| Canada | 15,457 | | | 22,120 | |
| Europe | 10,304 | | | 2,643 | |
| $ | 138,304 | | | $ | 185,006 | |
| Intercompany revenues: | | | |
| United States | $ | 11,945 | | | $ | 5,624 | |
| Canada | 74 | | | 143 | |
| Europe | 2,497 | | | 284 | |
| $ | 14,516 | | | $ | 6,051 | |
| Income (loss) before income taxes: | | | |
| United States | $ | (32,525) | | | $ | (10,417) | |
| Canada | 743 | | | 74 | |
| Europe | (3,361) | | | (278) | |
| Corporate and other | (12,367) | | | (18,268) | |
| $ | (47,510) | | | $ | (28,889) | |
| Depreciation and amortization: | | | |
| United States | $ | 1,758 | | | $ | 2,117 | |
| Canada | 32 | | | 43 | |
| Europe | 233 | | | 65 | |
| Corporate and other | 497 | | | 480 | |
| $ | 2,520 | | | $ | 2,705 | |
The table below provides information on total goodwill and intangibles and total assets by reportable segment as of December 31, 2020 and 2019.
| | | | | | | | | | | |
| As of December 31, |
| (in thousands) | 2020 | | 2019 |
| Total goodwill and intangibles: | | | |
| United States | $ | 4,258 | | | $ | 13,625 | |
| Canada | 0 | | | 0 | |
| Europe | 4,685 | | | 4,328 | |
| Corporate and other | 282 | | | 330 | |
| $ | 9,225 | | | $ | 18,283 | |
| Total assets: | | | |
| United States | $ | 64,037 | | | $ | 77,034 | |
| Canada | 8,341 | | | 10,768 | |
| Europe | 15,543 | | | 8,809 | |
| Corporate and other | 34,729 | | | 56,591 | |
| $ | 122,650 | | | $ | 153,202 | |
The table below provides information on our revenue by product type:
| | | | | | | | | | | |
| For the Year Ended December 31, |
| (in thousands) | 2020 | | 2019 |
| Revenues: | | | |
| Vaporizers and components | $ | 93,567 | | | $ | 144,192 | |
| Customized products and packaging | 10,520 | | | 12,107 | |
| Functional glass | 5,162 | | | 6,040 | |
| Tools and appliances | 6,838 | | | 3,640 | |
| Hemp-derived CBD products | 2,455 | | | 3,364 | |
| Closed system | 3,697 | | | 4,094 | |
| Grinders | 5,769 | | | 3,351 | |
| Papers and wraps | 7,219 | | | 4,086 | |
| Other | 3,077 | | | 4,132 | |
| $ | 138,304 | | | $ | 185,006 | |
The tables below provides information on our revenue and long-lived assets by geographical area. Our long-lived assets are primarily comprised of property and equipment, net and operating lease ROU assets.
| | | | | | | | | | | |
| For the Year Ended December 31, |
| (in thousands) | 2020 | | 2019 |
| Revenues: | | | |
| United States | $ | 109,660 | | | $ | 155,002 | |
| Canada | 15,094 | | | 22,840 | |
| Europe | 10,833 | | | 3,628 | |
| Other | 2,717 | | | 3,536 | |
| $ | 138,304 | | | $ | 185,006 | |
| | | | | | | | | | | |
| As of December 31, |
| (in thousands) | 2020 | | 2019 |
| Long-lived assets: | | | |
| United States | $ | 14,308 | | | $ | 17,162 | |
| Canada | 248 | | | 519 | |
| Europe | 750 | | | 179 | |
| $ | 15,306 | | | $ | 17,860 | |
NOTE 13. SUBSEQUENT EVENTS
Redemptions of Common Units of the Operating Company
During the first quarter of 2021, the Operating Company received redemption notices for an aggregate of 1,325,000 Common Units. Based upon these redemption notices, pursuant to the terms of the Operating Agreement, we issued shares of Class A common stock in the first quarter of fiscal 2021 to the redeeming members of the Operating Company on a 1-to-one basis to the number of Common Units redeemed, and we also cancelled a number of Class C common stock held by the redeeming members equal to three times the number of Common Units redeemed for no consideration.
During the first quarter of 2021, the Operating Company received redemption notices for an aggregate of 1,042,326 Common Units. Based upon these redemptions notices, pursuant to the terms of the Operating Agreement, we issued shares of Class A common stock in the first quarter of fiscal 2021 to the redeeming members of the Operating Company on a 1-to-one basis to the number of Common Units redeemed, and we also cancelled an equivalent number of Class B common stock held by the redeeming members for no consideration.
Acquisition of Eyce, LLC
Effective March 2, 2021, we acquired substantially all the assets of Eyce, LLC ("Eyce"), a designer and manufacturer of pipes, bubblers, rigs and other smoking and vaporization related accessories and merchandise, for consideration of $11.1 million, which consisted of cash and our Class A common stock, contingent consideration, and a promissory note payable to Eyce. We acquired Eyce to bolster our quality product offerings and accelerate growth in our Greenlane Brands portfolio.
Consolidated Appropriations Act, 2021
On December 27, 2020, the Consolidated Appropriations Act, 2021 was signed into law, which contains provisions that became effective in March 2021, which prohibit the mailing of electronic nicotine delivery systems ("ENDS") through the United States Postal Service ("USPS") and places certain regulatory requirements on shipment of ENDS through other carriers. Certain private carriers, including UPS and FedEx, also have policies restricting or prohibiting the shipment of certain vaporization products. These restrictions apply to nicotine vaporizers we sell, but it remains unclear if the restrictions apply to other vaporizer products. If the products we carry cannot be shipped by USPS or private carriers, or we must comply with burdensome policies and regulations, our shipping costs could be adversely and materially impacted, and we could lose our ability to deliver products to customers in a timely and economical manner. We are unable to determine the extent of the impact to the business until further guidance and clarification is issued.
Proposed Merger with KushCo Holdings, Inc.
On March 31, 2021, Greenlane, Merger Sub Gotham 1, LLC, a wholly owned subsidiary of the Company (“Merger Sub 1”), and Merger Sub Gotham 2, LLC, a wholly owned subsidiary of the Company (“Merger Sub 2” and, together with the Company and Merger Sub I, the “Greenlane Parties”), entered into an Agreement and Plan of Merger (the “Merger Agreement”) with KushCo Holdings, Inc. (“KushCo”). The Merger Agreement, the Mergers (as defined below) and the other transactions contemplated by the Merger Agreement were unanimously approved by a special committee of the Company’s Board of Directors consisting entirely of the Company’s independent and disinterested directors (the “Special Committee”) and the Company’s Board of Directors.
Merger Agreement
Pursuant to the terms of the Merger Agreement, subject to the satisfaction or waiver of certain conditions set forth in the Merger Agreement:
•Merger Sub 1 will be merged with and into KushCo with KushCo as the surviving corporation and a wholly -owned subsidiary of Parent (“Initial Surviving Corporation”) (“Merger 1”); and
•the Initial Surviving Corporation will then be merged with and into Merger Sub 2 with Merger Sub 2 as the surviving limited liability company (“Merger 2,” and together with Merger 1, the “Mergers”).
Under the terms of the Merger Agreement, KushCo’s stockholders will receive approximately 0.2546 shares of the Company’s Class A common stock for each share of KushCo common stock (the “Base Exchange Ratio”), subject to adjustment as described in the Merger Agreement (the Base Exchange Ratio, as adjusted, the “Exchange Ratio”). The Base Exchange Ratio is expected to result in KushCo stockholders owning approximately 49.9% of the Company’s common stock and existing stockholders of the Company owning approximately 50.1% of the Company’s common stock.
The Merger Agreement permits the Company to continue to pursue opportunistic and strategic priorities prior to the closing of the Mergers, including engaging in certain contemplated acquisitions and capital raising transactions. If the Company issues additional securities prior to the closing of the Transaction in connection with any acquisitions or capital raising transactions the Base Exchange Ratio will be adjusted such that the Company’s existing stockholders maintain an aggregate interest of at least 50.1%, and not more than 51.9%, in the Company following the completion of the Mergers.
At or immediately prior to the effective time of Merger 1, subject to the approval of the Company’s, stockholders, the Company’s Amended and Restated Certificate of Incorporation will be amended and restated (the “Charter Amendment”) in order to (i) effect a conversion of each outstanding share of Class C common stock for three shares of Class B common stock (the “Class C Conversion”), an increase the number of authorized shares of Class B common stock from 10,000,000 shares to 30,000,000 shares and (ii) increase the number of authorized shares of Class A common stock from 125,000,000 million shares to 600,000,000 shares).
The Mergers are subject to customary closing conditions including, among other things, (1) the approval of the Merger Agreement by holders of a majority of the outstanding shares of KushCo’s common stock (the “Requisite KushCo Approval”), (2) the repayment of certain KushCo indebtedness and release of related liens, (3) approval of the Merger Agreement by holders of a majority of the voting power of the outstanding shares of the Company’s common stock held by stockholders other than (i) Jacoby & Co. LLC, an entity controlled by the Company’s co-founders, and its affiliates and (ii) the chief executive officer, chief financial officer, chief operating officer, and general counsel of the Company, (4) the approval of the Charter Amendment by holders of a majority of the voting power of the outstanding shares of the Company’s common stock, (5) the approval of the issuance of shares of the Company’s Class A common stock in connection with Merger 1 by the affirmative vote of a majority of the votes cast by stockholders of the Company entitled to vote on the matter (the items numbered (3) through (5) are referred to herein as the “Requisite Greenlane Approvals”), (6) the approval for the Nasdaq listing of the shares of the Company’s Class A common stock to be issued in Merger 1, (7) the absence of certain legal impediments, (8) the accuracy of the representations and warranties made by the parties (subject to customary materiality qualifications), (9) the effectiveness of a Registration Statement on Form S-4 registering the issuance of the shares of Class A common stock to be issued by the Company in Merger 1, (10) the performance by the parties in all material respects of their covenants, obligations and agreements under the Merger Agreement, (11) the expiration or termination of the required waiting period (and any extensions thereof) under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, (12) the delivery of tax opinions that the Company Merger will qualify as a reorganization within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended and (13) no occurrence of a material adverse effect (which exclude COVID-19 related effects) on the Company or KushCo.
Treatment of Equity Awards
Immediately prior to the effective time of Merger 1, each KushCo stock option (whether or not vested or exercisable) will be converted into an option to purchase, on the same terms and conditions that apply to such option, that number of shares of the Company’s Class A common stock multiplied by the Exchange Ratio at an exercise price determined by dividing the per share exercise price covered by the Company option immediately prior to Merger 1 by the Exchange Ratio.
Immediately prior to Merger 1, each KushCo restricted stock unit will vest in full and be settled and treated as a share of the KushCo’s common stock in Merger 1.
Immediately prior to Merger 1, all unvested Greenlane equity awards other than those held by non-employee directors will become fully vested.
No Shop
Effective as of the signing of the Merger Agreement, the Company and KushCo are prohibited from soliciting, initiating, seeking, encouraging, facilitating (including by furnishing non-pubic information), continuing, or engaging in discussions or negotiations regarding, a proposal or inquiry that constitutes or could reasonably be expected to lead to a proposal to acquire 20% or more of their respective assets or capital stock (an “Acquisition Proposal”). However, if prior to obtaining the Requisite Greenlane Approvals or the Requisite KushCo Approval, as applicable, Greenlane or KushCo receives a bona fide, unsolicited, written Acquisition Proposal that Greenlane’s Board of Directors or KushCo’s Board of Directors determines to be, or could reasonably be expected to lead to, a “superior proposal,” and the Greenlane Board of Directors (or the Special Committee) or the KushCo Board of Directors, as applicable, reasonably determines that failure to take the following actions would be inconsistent with its fiduciary duties, then the party that received the Acquisition Proposal may provide to the person who made
the Acquisition Proposal non-public information and engage in discussions and negotiations, under an acceptable confidentiality agreement. Within 48 hours, the party that received the Acquisition Proposal is required to notify the other party to the Merger Agreement regarding any Acquisition Proposal and provide the identity of the party submitting the proposal and a copy of the proposal or a summary of the material terms of the proposal, and must keep the other party to the Merger Agreement reasonably apprised of material developments.
If, prior to obtaining the Requisite Greenlane Approvals or the Requisite KushCo Approval, as applicable, a superior proposal is received or an certain intervening events occur, Greenlane’s Board of Directors (or the Special Committee) or KushCo’s Board of Directors, as applicable, may change its recommendation with respect to the Merger Agreement if it reasonably determines that failure to do so would be inconsistent with its fiduciary duties.
Termination
The Merger Agreement may be terminated under certain circumstances, including by mutual consent or by the Company or KushCo if (1) if the Mergers have not been completed on or before December 30, 2021, subject to one thirty-day (30) extension, (2) if there is in effect an order of a governmental entity restraining or enjoining the Mergers (whether temporary, preliminary or permanent) (3) upon failure of either party to obtain the requisite stockholder approval, (4) upon a material breach by the other party that would result in the failure of a closing condition to be capable of being satisfied before the earlier of 30 days after written notice of the breach and December 31, 2020 or (5) if a material adverse effect (which exclude COVID-19 related effects) occurs with respect to the other party. Additionally, each of the Company and KushCo may terminate the Merger Agreement in order to enter into an alternative transaction that is considered a superior proposal, following a prescribed process described above under “No Shop” above. In connection with the termination of the Merger Agreement for such reason and under other specified circumstances set forth in the Merger Agreement, the terminating party will be required to pay a termination fee equal to four percent of its equity value as of the date of the signing of the Merger Agreement. Specifically, the Merger Agreement provides for a termination fee payable by us of 4% of our equity value as of March 31, 2021 if the Merger Agreement is terminated under certain circumstances, including as a result of a material breach by us of any covenant or agreement under the Merger Agreement.
Voting Agreement
On March 31, 2021, in connection with the execution of the Merger Agreement, Jacoby & Co. Inc., a stockholder of the Company that is controlled by Aaron LoCascio, the Company’s Chief Executive Officer and Adam Schoenfeld, the Company’s Chief Strategy Officer, (the “Voting Agreement Stockholder”), entered into a voting agreement (the “Voting Agreement”) with the Company and KushCo.
Pursuant to the Voting Agreement, the Voting Agreement Stockholder has agreed, among other things, to vote or cause to be voted any issued and outstanding shares of the Company’s common stock beneficially owned by the Voting Agreement Stockholder, or that may otherwise become beneficially owned by the Voting Agreement Stockholder, during the term of the Voting Agreement, (i) in favor of all proposals presented at the special meeting of stockholders to be held by the Company in connection with the Mergers and related transactions other than the proposal to adopt the Merger Agreement and the transactions contemplated by the Merger Agreement, which it is prohibited from voting upon, (ii) against any action or agreement that would result in a breach of any covenant, representation or warranty or any other obligation of the Company contained in the Merger Agreement or of the Voting Agreement Stockholder contained in the Voting Agreement, and (iii) against any Acquisition Proposal or any other action, agreement or transaction that is intended, or could reasonably be expected, to materially impede, interfere or be inconsistent with, delay, postpone, discourage or materially and adversely affect the consummation of the transactions contemplated by the Merger Agreement or the Voting Agreement. The Voting Agreement Stockholder is permitted to transfer its shares by sale in the open market through a broker dealer.
The Voting Agreement will automatically terminate upon the earliest of (i) mutual written agreement of the Voting Agreement Stockholder and the Company, (ii) the consummation of the Mergers, (iii) any change in recommendation by the Company’s Board of Directors and (iv) a termination of the Merger Agreement in accordance with its terms.
Increase to Shares Available Under the Equity Plan
At the special meeting relating to the approval of the Merger Agreement and the other matters described above, the Company will seek stockholder approval of an amendment to the Company’s 2019 Equity Incentive Plan in order to increase of the number shares of common stock available under the Plan to an amount equal to 7.5% of the aggregate number of shares of Class A and Class B common stock to be outstanding after completion of the Mergers and the Class C Conversion.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation (pursuant to Rule 13a-15(b) of the Exchange Act) of the effectiveness of our disclosure controls and procedures, as defined in Rule 13a-15(e) under the Exchange Act as of December 31, 2020.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include controls and procedures designed to ensure that information required to be disclosed in our company’s reports filed under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure.
Based on the evaluation of our disclosure controls and procedures, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were ineffective as of December 31, 2020 due to the material weaknesses identified and described below.
Management's Report on Internal Control Over Financial Reporting
Our management, including our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act). Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2020, based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) (2013 framework). Based on this evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2020, the Company has not maintained effective internal control over financial reporting due to the material weaknesses identified and described below.
Because we are an "emerging growth company" under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting for so long as we are an emerging growth company.
Material Weaknesses
As previously described in Item 9A of our Annual Report on Form 10-K for the year ended December 31, 2019, we began implementing a remediation plan to address the material weaknesses identified in the fourth quarter of 2019, and our management continues to be actively engaged in the remediation efforts. Management concluded that certain previously identified material weaknesses, individually and in the aggregate, were not remediated as of December 31, 2020. Consequently, additional material weaknesses were identified relating to our control environment and information and communication, as described below.
Among the previously reported design and operating deficiencies which contributed to material weaknesses in our control activities, management noted ineffective user access controls over certain IT systems to appropriately segregate duties and adequately restrict user access to financial applications and data to the appropriate personnel. While certain compensating control activities have been designed and implemented to mitigate the risks related to ineffective user access controls, these compensating control activities are not expected to operate at a level of precision that would prevent or detect a misstatement that could be material.
Control Environment
We did not maintain an effective control environment to enable the identification and mitigation of risks of material accounting errors and ensure corrective activities were appropriately applied, prioritized, and implemented in a timely manner.
Risk Assessment
As part of our remediation efforts related to the material weaknesses identified in the prior year, we undertook efforts during 2020 to begin designing an effective risk assessment, which was not completed or fully implemented in order to identify and mitigate key business and financial reporting risks to the organization. Control deficiencies were identified which constitute material weaknesses relating to: (i) identifying, assessing, and communicating appropriate objectives, (ii) identifying and analyzing risks to achieve these objectives, (iii) considering the potential for fraud in assessing risks to the achievement of objectives, and (iv) identifying and assessing changes that could significantly impact the system of internal controls.
Control Activities
As part of our remediation efforts related to the material weaknesses identified in the prior year, we undertook efforts during 2020 to design and implement control activities, however, design efforts relating to control activities were not fully implemented. Control deficiencies were identified associated with control activities. Specifically, these control deficiencies constitute material weaknesses, either individually or in the aggregate, relating to: (i) selecting and developing control activities that contribute to the mitigation of risks and support achievement of objectives, (ii) selecting and developing general control activities over technology to support the achievement of objectives, and (iii) deploying control activities through policies that establish what is expected and procedures that put policies into action.
The following design and operating deficiencies, individually and in the aggregate, contributed to material weaknesses in our control activities, including:
•Lack of direct and precise journal entry review and account reconciliation controls over certain account balances
•Ineffective controls over inventory counts and recording of inventory reserves
•Ineffective user access controls over certain IT systems to appropriately segregate duties and adequately restrict user access to financial applications and data to the appropriate personnel, including systems and data used in financial close and reporting
•Ineffective controls over significant non-recurring transactions
Information and Communication
We did not design and implement effective information and communication control activities. A control deficiency was identified which constitutes a material weakness relating to information technology controls, which includes information security, systems change management and computer operations for systems and applications that are critical to processing financial transactions and capturing and reporting information in the financial reporting process. These ineffective information technology controls contributed to ineffective data validation of spreadsheets and system-generated reports utilized in the preparation of the financial statements and disclosures.
Monitoring
We did not design and implement effective monitoring activities. Control deficiencies were identified which constitute material weaknesses, individually and in the aggregate, relating to: (i) selecting, developing, and performing ongoing evaluation to ascertain whether the components of internal controls are present and functioning, and (ii) evaluating and communicating internal control deficiencies in a timely manner to those parties responsible for taking corrective action.
Remediation Status
As previously disclosed, in 2020, we began a multi-year implementation of a new enterprise resource planning (“ERP”) system, which will replace our existing core financial systems, and which we expect will be completed during 2021. Management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures, based upon which management expects to focus its allocation of organizational resources to ensure the successful implementation of the new ERP system, including as it relates to designing and implementing effective control activities. Conversely, management expects that additional efforts related to re-designing user access roles and permissions in the existing ERP system, which is expected to be decommissioned in 2021, will be limited. Based on these considerations, and subject to management’s ongoing assessment, we cannot provide assurances that the previously reported material weaknesses related to ineffective user access controls, which also have a pervasive impact on the other components of our internal control over financial reporting structure, will be considered remediated until we complete the implementation of our new ERP system. Additionally, we are taking, and expect to continue to take the following remediation actions:
•the implementation of additional review procedures designed to enhance the control owner’s execution of control activities, including entity level controls, through the implementation of improved documentation standards evidencing execution of these controls, oversight, and training;
•improvement of the control activities and procedures associated with certain accounting areas, including proper segregation of duties and assigning personnel with the appropriate experience as preparers and reviewers over analyses relating to such accounting areas;
•educating and re-training control owners regarding internal control processes to mitigate identified risks and maintaining adequate documentation to evidence the effective design and operation of such processes; and
•implementing enhanced controls to monitor the effectiveness of the underlying business process controls that are dependent on the data and financial reports generated from the relevant information systems.
The material weaknesses identified will not be considered remediated until the applicable controls operate for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively.
Changes in Internal Control Over Financial Reporting
During the second quarter of 2020, we entered into service agreements with two third-party logistics facilities located in Hebron, Kentucky and Delta, B.C., Canada, both of which serve as replacement facilities to the distribution centers we have closed. In conjunction with this transition, we have adjusted our processes and designed and implemented controls related to our inventory management and order fulfillment. During the quarter ended September 30, 2020, we designed and implemented additional control activities related to inventory counts at the third-party logistics facilities. These changes in our internal control over financial reporting that occurred during the quarters ended June 30, 2020 and September 30, 2020 have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. As noted above, we are continuing our remediation efforts related to the material weaknesses in our internal control over financial reporting, including as it relates to controls over inventory counts and recording of inventory reserves. Any changes to our internal control of our internal reporting related to our transition to the third-party logistics, and the related controls, will be evaluated once the applicable controls operate for a sufficient period of time and management can conclude, through testing, that these controls are operating effectively.
Inherent Limitations on Effectiveness of Controls
Management recognizes that a control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud or error, if any, have been detected. These inherent limitations include the realities that judgments in decision making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item is incorporated by reference to our Proxy Statement for the 2021 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the fiscal year ended December 31, 2020.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item is incorporated by reference to our Proxy Statement for the 2021 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the fiscal year ended December 31, 2020.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated by reference to our Proxy Statement for the 2021 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the fiscal year ended December 31, 2020.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item is incorporated by reference to our Proxy Statement for the 2021 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the fiscal year ended December 31, 2020.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item is incorporated by reference to our Proxy Statement for the 2021 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the fiscal year ended December 31, 2020.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
We have filed the following documents as part of this Form 10-K:
(1) Consolidated Financial Statements
| | | | | | | | |
| Index to Consolidated Financial Statements | | Page |
| | |
| | |
| | |
| | |
| | |
| | |
(2) Financial Statement Schedules
All financial statement schedules are omitted since they are not required or are not applicable, or the required information is included in the consolidated financial statements and accompanying notes included in this Form 10-K.
(3) Exhibits Required by Item 601 of Regulation S-K
| | | | | | | | | | | |
| Exhibit Number | | Description |
| | |
| 3.1 | | |
| 3.2 | | |
| 4.1 | | |
| 4.2 | | |
| 4.3* | | |
| 10.1 | | |
| 10.2 | | |
| 10.3 | | |
| 10.4 | | |
| 10.5 | | |
| 10.6 | | Omnibus Amendment No. 1 to Credit Agreement, Guarantees, and Security Agreements, dated as of August 23, 2018, by and among Greenlane Holdings, LLC, Jacoby & Co. Inc., the other Borrower Parties listed on the signature page thereto and Fifth Third Bank (Incorporated by reference to Exhibit 10.7 to Greenlane Holdings, Inc.’s Registration Statement on Form S-1, filed on March 20, 2019). |
| 10.7 | | |
| 10.8 | | |
| 10.9 | | |
| | | | | | | | | | | |
| 10.10 | | |
| 10.11 | | Contribution Agreement, dated as of February 20, 2018, by and among Greenlane Holdings, LLC (f/k/a Jacoby Holdings LLC), the Sellers named therein and Better Life Products, Inc., as Seller Representative (Incorporated by reference to Exhibit 10.10 to Greenlane Holdings, Inc.’s Registration Statement on Form S-1, filed on March 20, 2019). |
| 10.12 | | |
| 10.13 | | |
| 10.14 | | |
| 10.15 | | |
| 10.16 | | |
| 10.17 | | |
| 10.18 | | |
| 10.19 | | |
| 10.20 | | |
| 21.1* | | |
| 23.1* | | |
| 31.1* | | |
| 31.2* | | |
| 32.1* | | |
| 101.INS | | XBRL Instance Document* |
| 101.SCH | | XBRL Taxonomy Extension Schema Document* |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document* |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document* |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document* |
______________________________________________
* Filed herewith.
# Furnished herewith.
† Indicates a management contract or compensatory plan or arrangement.
ITEM 16. FORM 10-K SUMMARY
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| GREENLANE HOLDINGS, INC. |
| | |
| Date: Date: March 31, 2021 | By: | /s/ Aaron LoCascio |
| | Aaron LoCascio
Chief Executive Officer
(Principal Executive Officer) |
| | | | | | | | |
| Date: Date: March 31, 2021 | By: | /s/ William Mote |
| | William Mote
Chief Financial Officer
(Principal Financial and Accounting Officer) |
Pursuant to the requirements of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| /s/ Aaron LoCascio | | Chairperson, Director and Chief Executive Officer
(Principal Executive Officer) | | March 31, 2021 |
| Aaron LoCascio | | | | |
| | | | |
| /s/ William Mote | | Chief Financial Officer
(Principal Financial and Accounting Officer) | | March 31, 2021 |
| William Mote | | | | |
| | | | |
| /s/ Adam Schoenfeld | | Chief Strategy Officer and Director | | March 31, 2021 |
| Adam Schoenfeld | | | | |
| | | | |
| /s/ Neil Closner | | Director | | March 31, 2021 |
| Neil Closner | | | | |
| | | | |
| /s/ Richard Taney | | Director | | March 31, 2021 |
| Richard Taney | | | | |
| | | | |
| /s/ Jeff Uttz | | Director | | March 31, 2021 |
| Jeff Uttz | | | | |
| | | | |