Topline Preliminary Week 96 Results From Phase 2b SYMMETRY Study in Patients with Compensated Cirrhosis (F4) Due to MASH January 27, 2025 Restoring balance. Renewing Life. Exhibit 99.2

Safe Harbor and Legal Disclaimers This presentation may contain “forward-looking statements” of Akero Therapeutics, Inc. (“we,” “us,” “our,” “Akero” or the “Company”) within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to current express or implied beliefs, expectations, and assumptions regarding: the future of our business; future plans and strategies, including our expectations around the therapeutic potential and clinical benefits of Efruxifermin (“EFX”); our development plans for EFX, including our belief in the unique potential of EFX as a foundational MASH therapy; our preclinical and clinical results, including the week 96 results from our Phase 2b SYMMETRY study, which are preliminary, topline results that are subject to audit and verification procedures and additional data that could result in material changes in the final data; the SYNCHRONY Phase 3 program, including the SYNCHRONY Histology, SYNCHRONY Real-World, and SYNCHRONY Outcomes studies, their respective trial designs; and risks related to the competitive landscape. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should,” and “could,” and similar expressions or words, identify forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in our most recent annual report on Form 10-K and quarterly report on Form 10-Q filed with the Securities and Exchange Commission (the “SEC”), as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the SEC. All information in this presentation is as of the date hereof, and we undertake no duty to update this information unless required by law. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. ©2025 AKERO THERAPEUTICS.
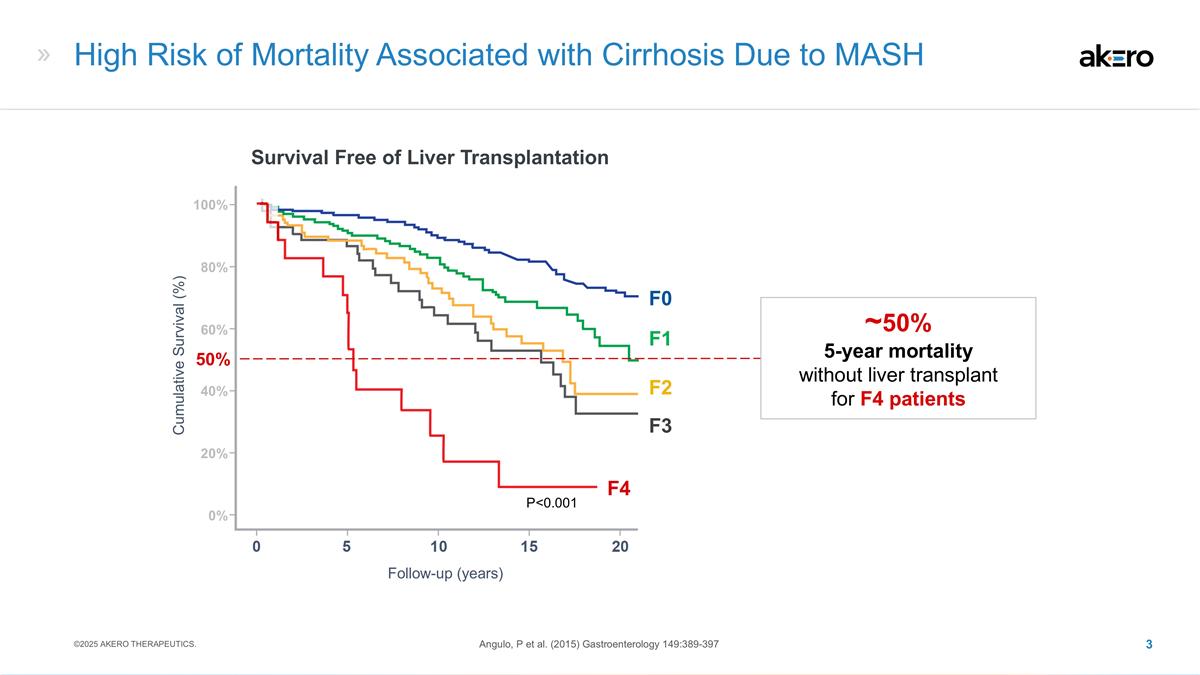
High Risk of Mortality Associated with Cirrhosis Due to MASH Survival Free of Liver Transplantation Follow-up (years) Cumulative Survival (%) Angulo, P et al. (2015) Gastroenterology 149:389-397 P<0.001 F4 50% ~50% 5-year mortality without liver transplant for F4 patients 40% 20% 0% 100% 80% 60% ©2025 AKERO THERAPEUTICS. F3 F2 F1 F0
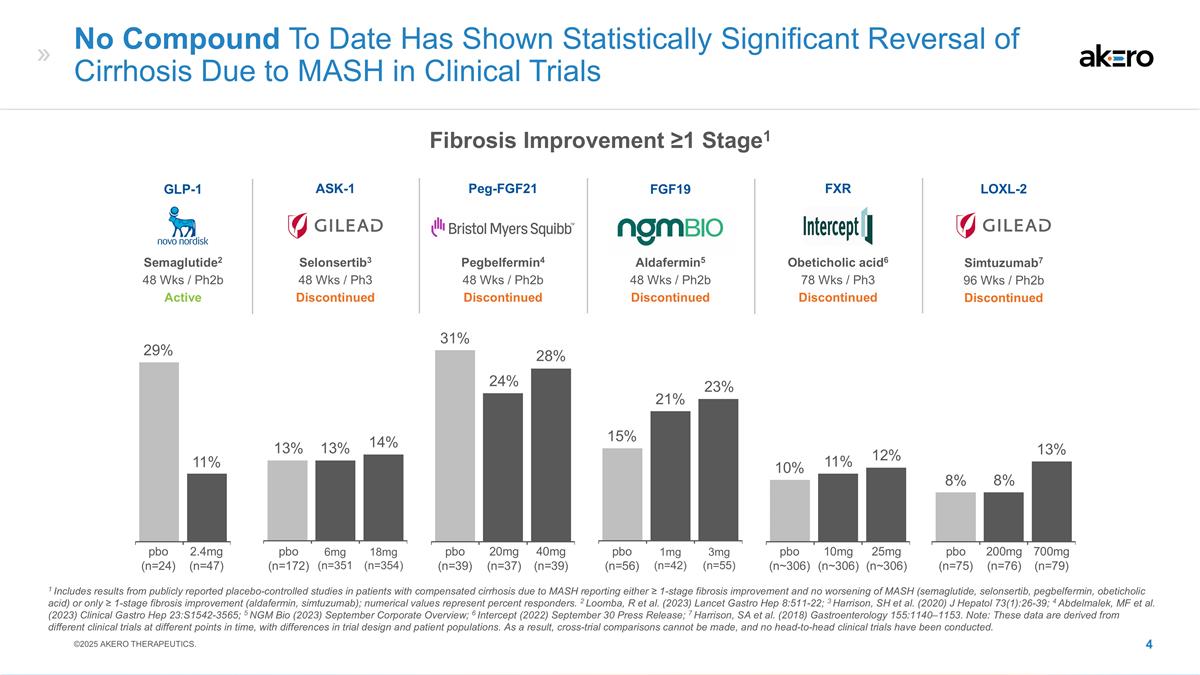
1mg (n=42) 3mg (n=55) 21% 15% No Compound To Date Has Shown Statistically Significant Reversal of Cirrhosis Due to MASH in Clinical Trials 6mg (n=351 18mg (n=354) 20mg (n=37) 40mg (n=39) 14% 13% 31% 24% 25mg (n~306) 10mg (n~306) 10% 12% 2.4mg (n=47) 11% 200mg (n=76) 700mg (n=79) 8% 8% Aldafermin5 48 Wks / Ph2b Discontinued FGF19 Selonsertib3 48 Wks / Ph3 Discontinued ASK-1 Peg-FGF21 Pegbelfermin4 48 Wks / Ph2b Discontinued FXR Obeticholic acid6 78 Wks / Ph3 Discontinued Semaglutide2 48 Wks / Ph2b Active GLP-1 Simtuzumab7 96 Wks / Ph2b Discontinued LOXL-2 1 Includes results from publicly reported placebo-controlled studies in patients with compensated cirrhosis due to MASH reporting either ≥ 1-stage fibrosis improvement and no worsening of MASH (semaglutide, selonsertib, pegbelfermin, obeticholic acid) or only ≥ 1-stage fibrosis improvement (aldafermin, simtuzumab); numerical values represent percent responders. 2 Loomba, R et al. (2023) Lancet Gastro Hep 8:511-22; 3 Harrison, SH et al. (2020) J Hepatol 73(1):26-39; 4 Abdelmalek, MF et al. (2023) Clinical Gastro Hep 23:S1542-3565; 5 NGM Bio (2023) September Corporate Overview; 6 Intercept (2022) September 30 Press Release; 7 Harrison, SA et al. (2018) Gastroenterology 155:1140–1153. Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. 29% 13% 28% 23% 11% 13% pbo (n=24) pbo (n=172) pbo (n=39) pbo (n=56) pbo (n~306) pbo (n=75) ©2025 AKERO THERAPEUTICS. Fibrosis Improvement ≥1 Stage1
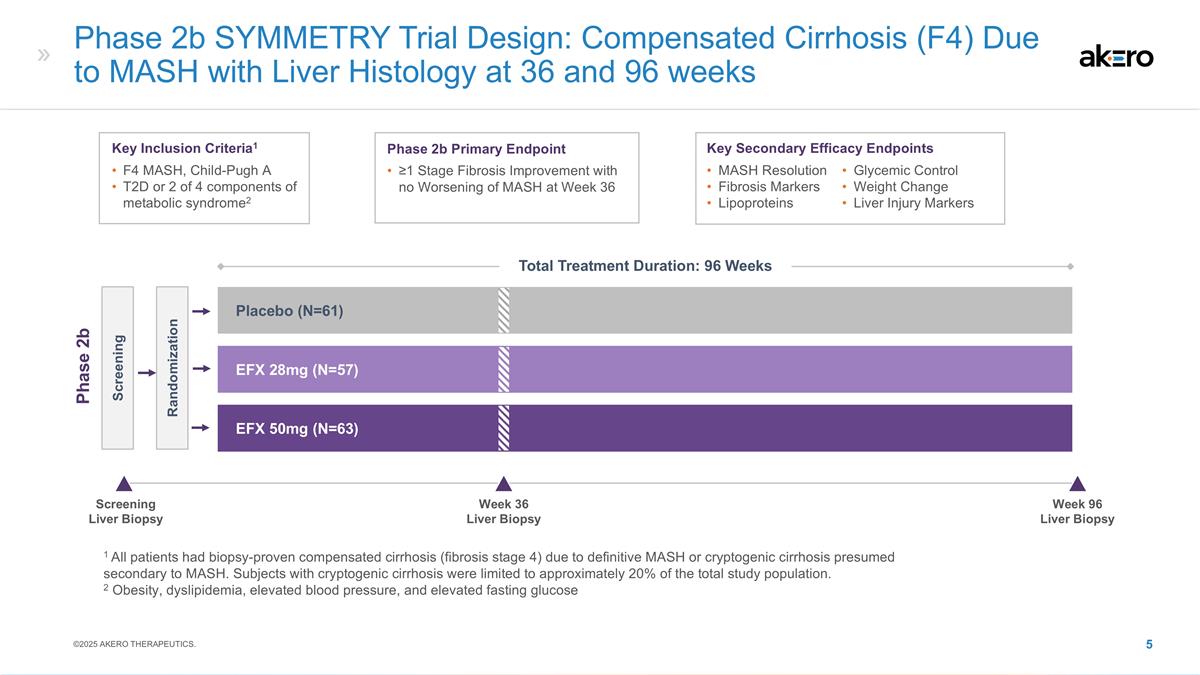
Phase 2b SYMMETRY Trial Design: Compensated Cirrhosis (F4) Due to MASH with Liver Histology at 36 and 96 weeks EFX 28mg (N=57) Placebo (N=61) EFX 50mg (N=63) Screening Randomization Phase 2b Key Inclusion Criteria1 F4 MASH, Child-Pugh A T2D or 2 of 4 components of metabolic syndrome2 Phase 2b Primary Endpoint ≥1 Stage Fibrosis Improvement with no Worsening of MASH at Week 36 Key Secondary Efficacy Endpoints MASH Resolution Fibrosis Markers Lipoproteins Glycemic Control Weight Change Liver Injury Markers 1 All patients had biopsy-proven compensated cirrhosis (fibrosis stage 4) due to definitive MASH or cryptogenic cirrhosis presumed secondary to MASH. Subjects with cryptogenic cirrhosis were limited to approximately 20% of the total study population. 2 Obesity, dyslipidemia, elevated blood pressure, and elevated fasting glucose Screening Liver Biopsy Week 36 Liver Biopsy Week 96 Liver Biopsy ©2025 AKERO THERAPEUTICS. Total Treatment Duration: 96 Weeks
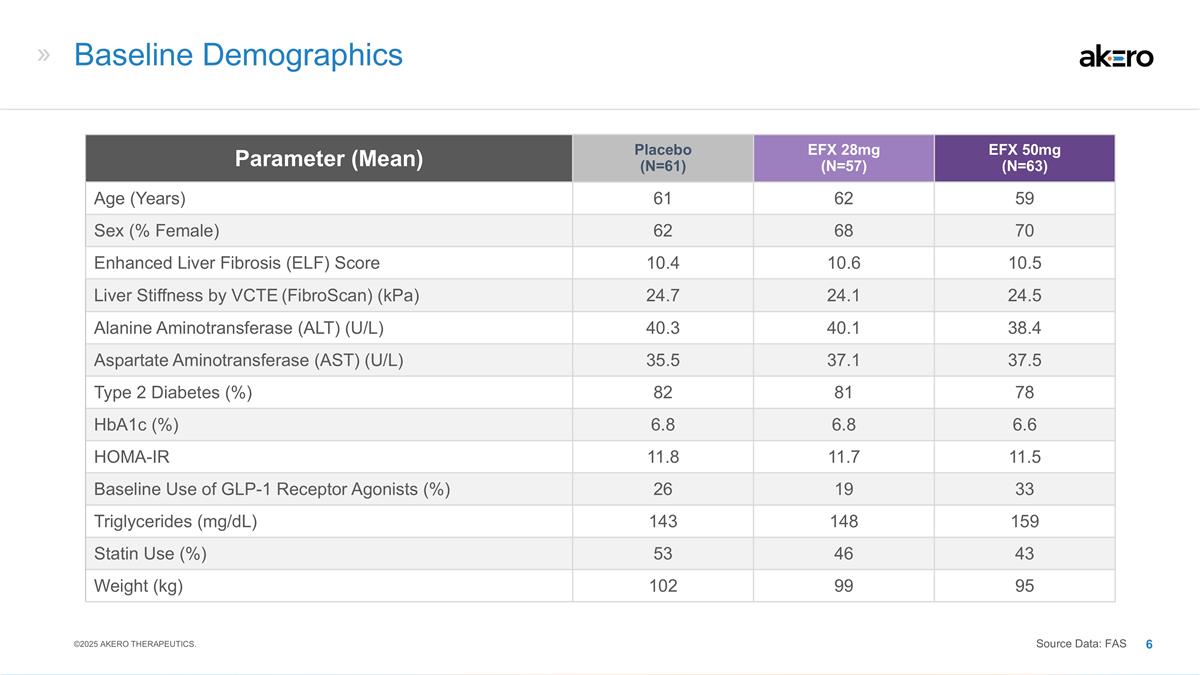
Baseline Demographics Parameter (Mean) Placebo (N=61) EFX 28mg (N=57) EFX 50mg (N=63) Age (Years) 61 62 59 Sex (% Female) 62 68 70 Enhanced Liver Fibrosis (ELF) Score 10.4 10.6 10.5 Liver Stiffness by VCTE (FibroScan) (kPa) 24.7 24.1 24.5 Alanine Aminotransferase (ALT) (U/L) 40.3 40.1 38.4 Aspartate Aminotransferase (AST) (U/L) 35.5 37.1 37.5 Type 2 Diabetes (%) 82 81 78 HbA1c (%) 6.8 6.8 6.6 HOMA-IR 11.8 11.7 11.5 Baseline Use of GLP-1 Receptor Agonists (%) 26 19 33 Triglycerides (mg/dL) 143 148 159 Statin Use (%) 53 46 43 Weight (kg) 102 99 95 ©2025 AKERO THERAPEUTICS. Source Data: FAS
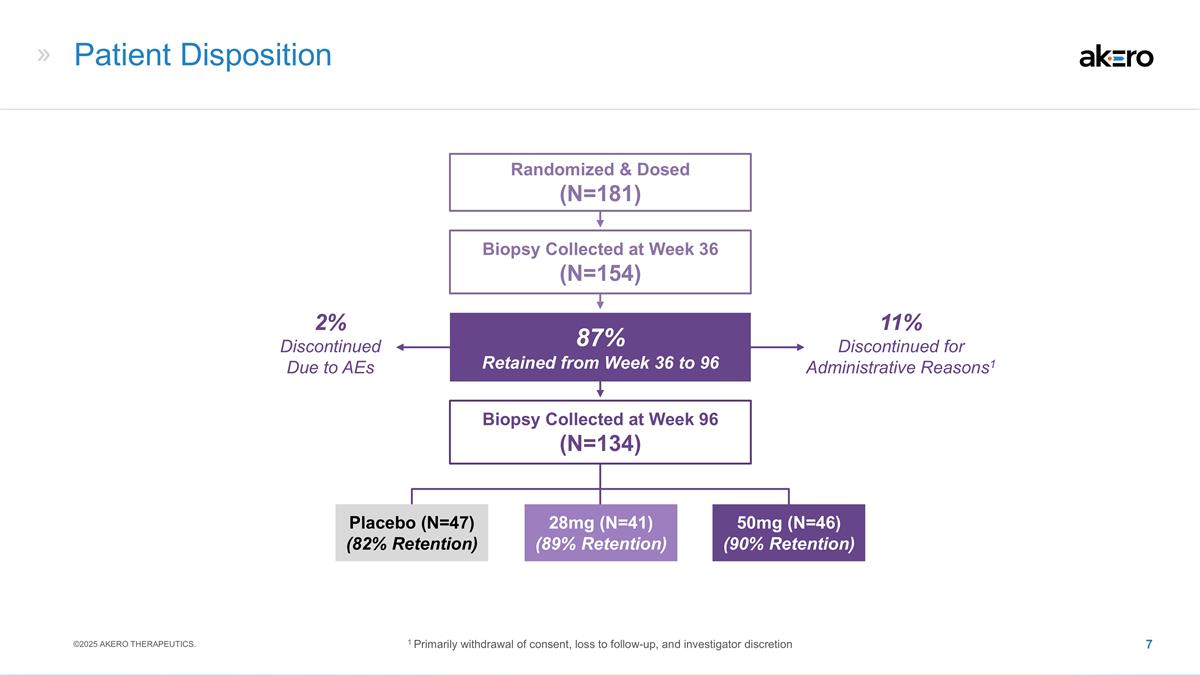
11% Discontinued for Administrative Reasons1 Patient Disposition 28mg (N=41) (89% Retention) 50mg (N=46) (90% Retention) Placebo (N=47) (82% Retention) Biopsy Collected at Week 36 (N=154) Randomized & Dosed (N=181) Biopsy Collected at Week 96 (N=134) 87% Retained from Week 36 to 96 2% Discontinued Due to AEs ©2025 AKERO THERAPEUTICS. 1 Primarily withdrawal of consent, loss to follow-up, and investigator discretion
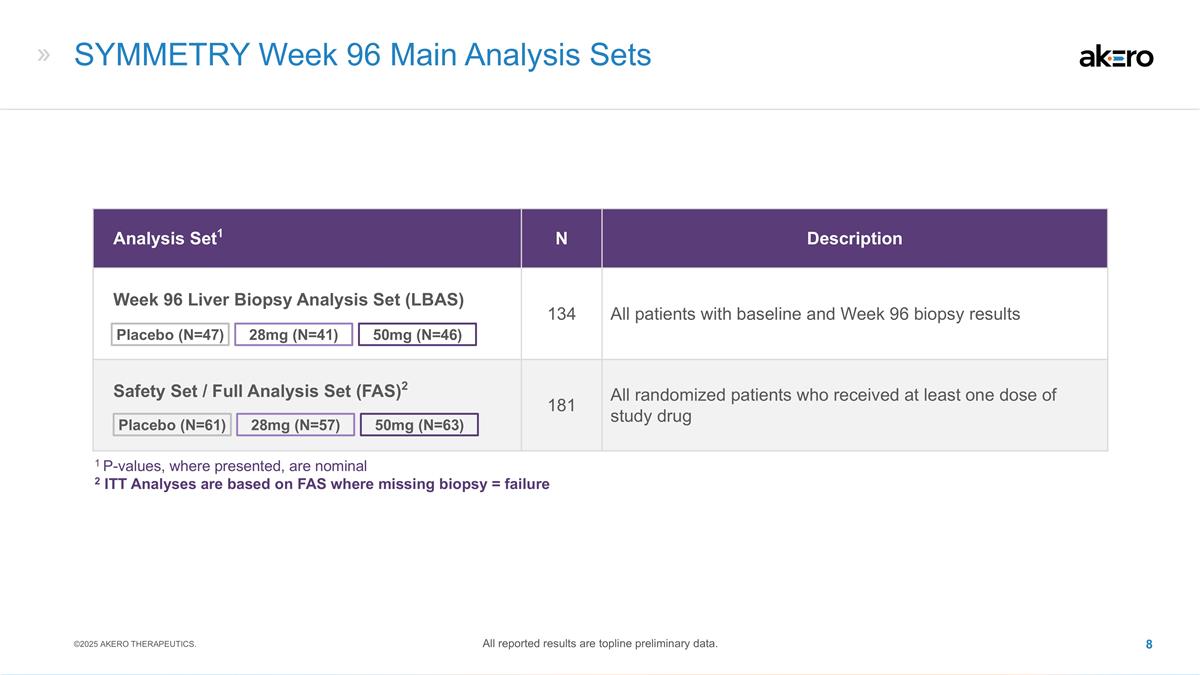
SYMMETRY Week 96 Main Analysis Sets Analysis Set1 N Description Week 96 Liver Biopsy Analysis Set (LBAS) 134 All patients with baseline and Week 96 biopsy results Safety Set / Full Analysis Set (FAS)2 181 All randomized patients who received at least one dose of study drug 28mg (N=41) 50mg (N=46) Placebo (N=47) 50mg (N=63) 28mg (N=57) Placebo (N=61) 1 P-values, where presented, are nominal 2 ITT Analyses are based on FAS where missing biopsy = failure ©2025 AKERO THERAPEUTICS. All reported results are topline preliminary data.
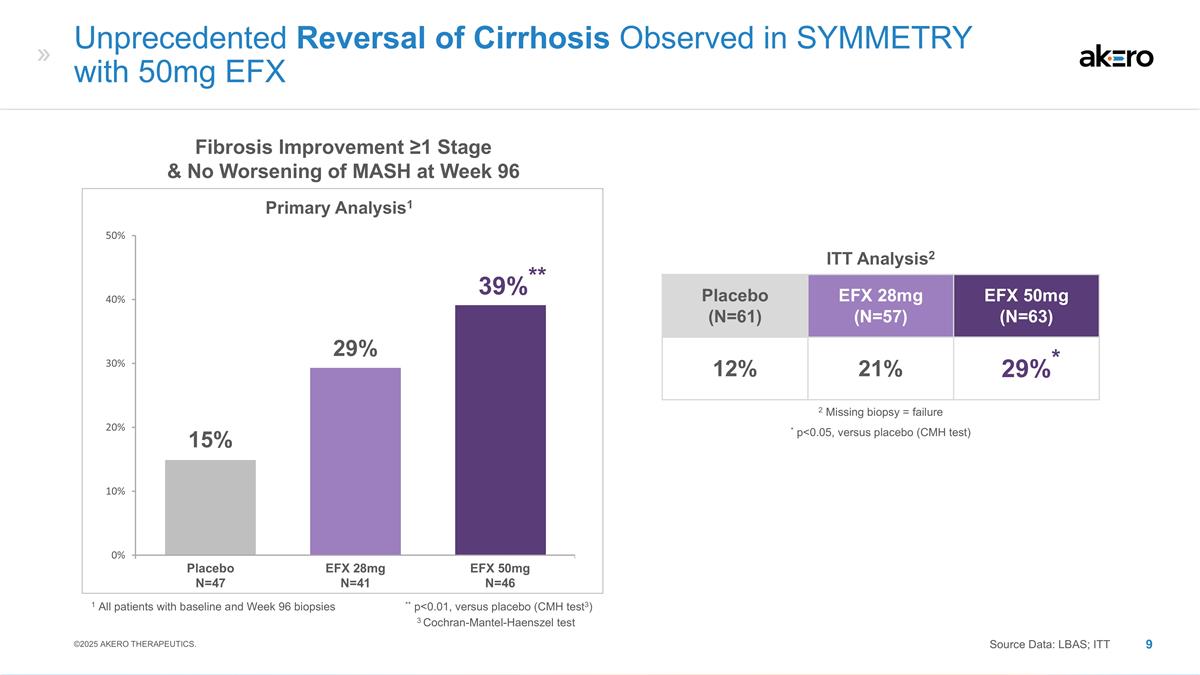
Placebo (N=61) EFX 28mg (N=57) EFX 50mg (N=63) 12% 21% *29%* EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 Primary Analysis1 1 All patients with baseline and Week 96 biopsies ** p<0.01, versus placebo (CMH test3) ** 39%** 29% 15% Unprecedented Reversal of Cirrhosis Observed in SYMMETRY with 50mg EFX ITT Analysis2 * p<0.05, versus placebo (CMH test) 2 Missing biopsy = failure Source Data: LBAS; ITT ©2025 AKERO THERAPEUTICS. 3 Cochran-Mantel-Haenszel test
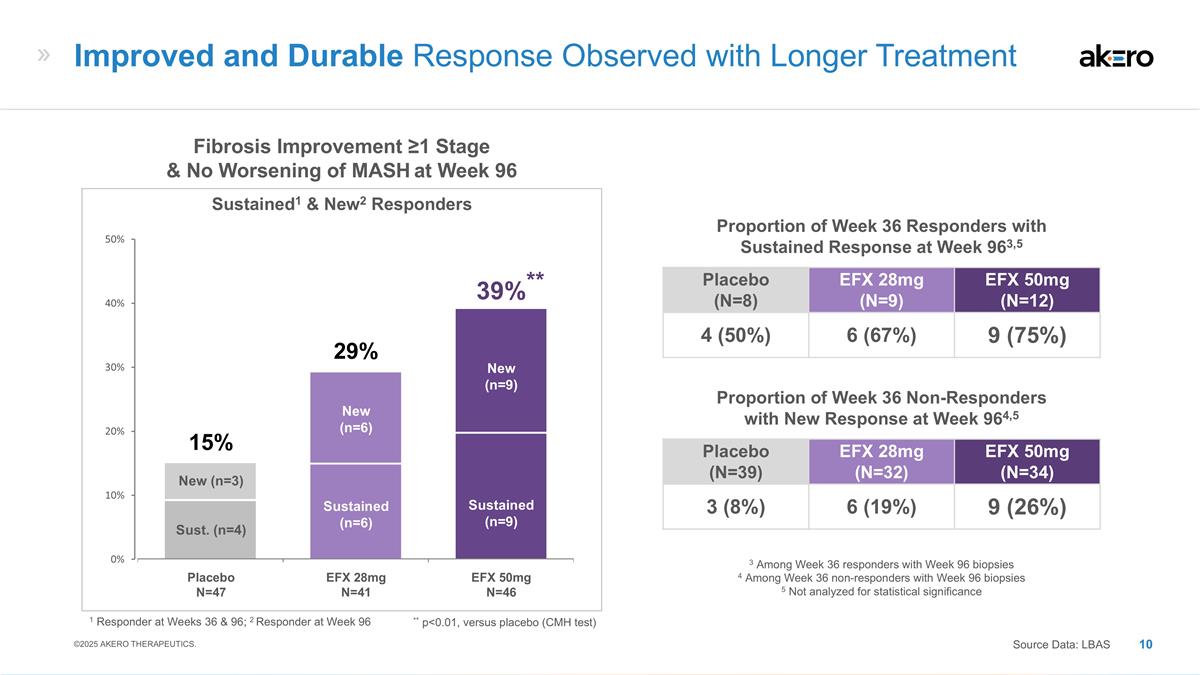
Sustained (n=6) New (n=6) Sustained (n=9) New (n=9) Sust. (n=4) New (n=3) **39%** EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 29% 15% Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 Sustained1 & New2 Responders Placebo (N=8) EFX 28mg (N=9) EFX 50mg (N=12) 4 (50%) 6 (67%) 9 (75%) Proportion of Week 36 Responders with Sustained Response at Week 963,5 Placebo (N=39) EFX 28mg (N=32) EFX 50mg (N=34) 3 (8%) 6 (19%) 9 (26%) Proportion of Week 36 Non-Responders with New Response at Week 964,5 3 Among Week 36 responders with Week 96 biopsies 4 Among Week 36 non-responders with Week 96 biopsies 5 Not analyzed for statistical significance Improved and Durable Response Observed with Longer Treatment Source Data: LBAS ©2025 AKERO THERAPEUTICS. 1 Responder at Weeks 36 & 96; 2 Responder at Week 96 ** p<0.01, versus placebo (CMH test)
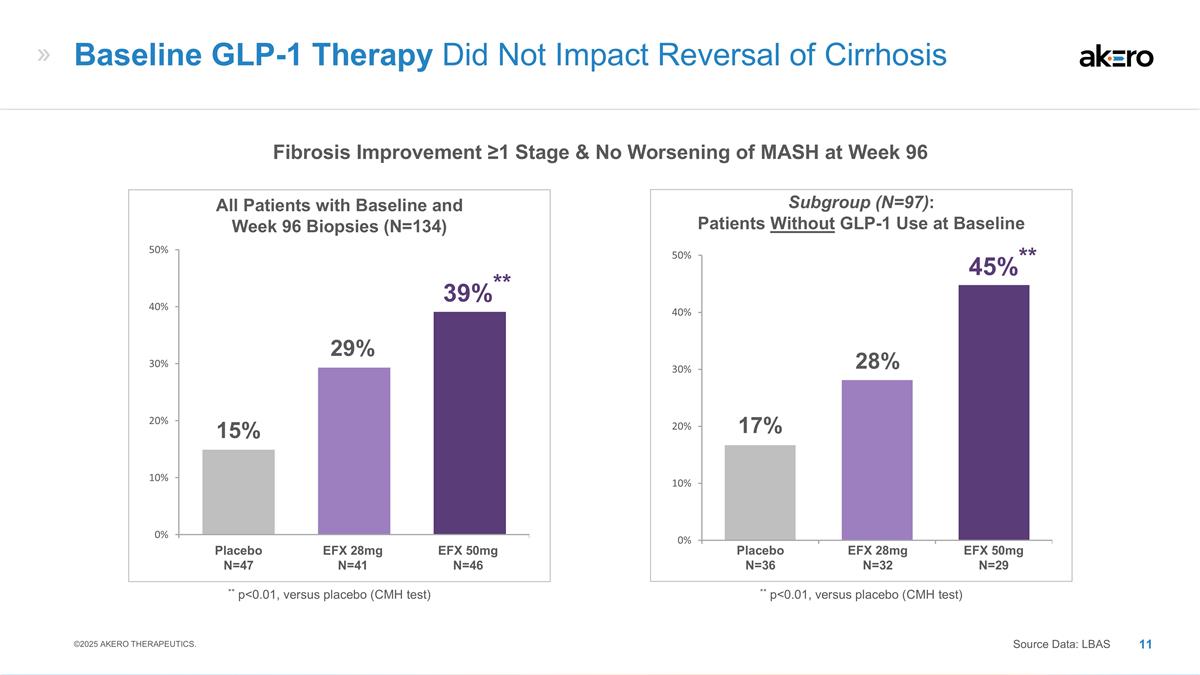
EFX 28mg N=32 EFX 50mg N=29 Placebo N=36 **45%** 28% 17% Subgroup (N=97): Patients Without GLP-1 Use at Baseline EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 ** p<0.01, versus placebo (CMH test) **39%** 29% 15% All Patients with Baseline and Week 96 Biopsies (N=134) Baseline GLP-1 Therapy Did Not Impact Reversal of Cirrhosis Source Data: LBAS ** p<0.01, versus placebo (CMH test) Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 ©2025 AKERO THERAPEUTICS.
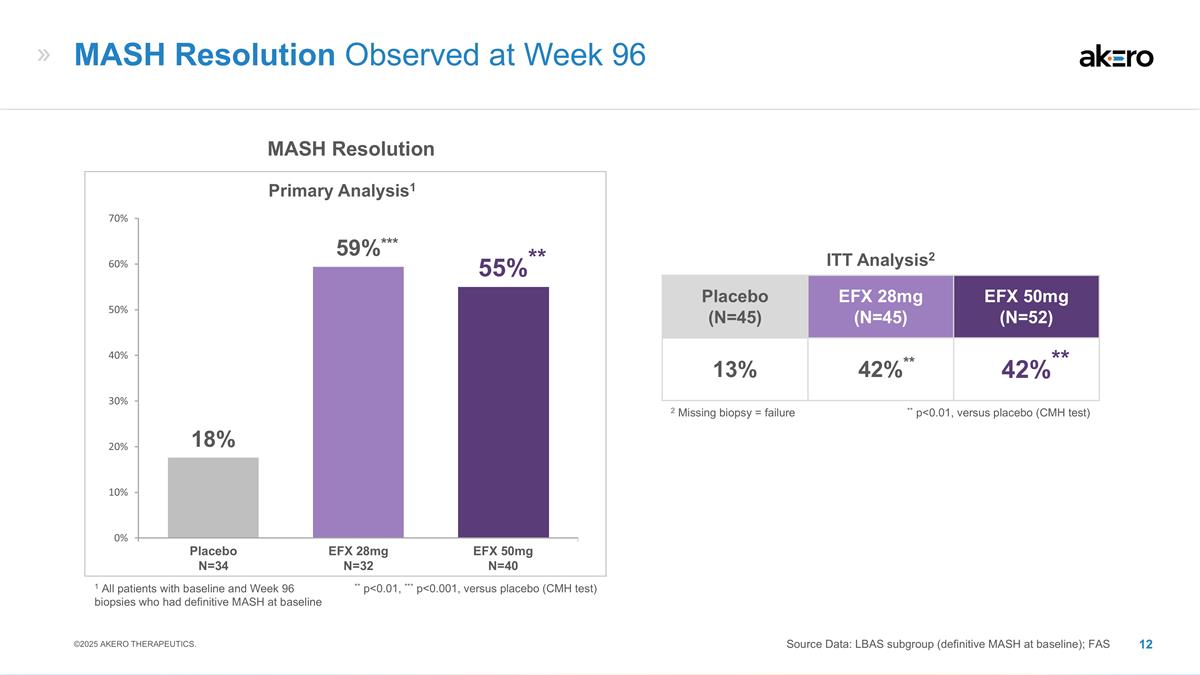
EFX 28mg N=32 EFX 50mg N=40 Placebo N=34 MASH Resolution Primary Analysis1 ** p<0.01, *** p<0.001, versus placebo (CMH test) **55%** ***59%*** 18% MASH Resolution Observed at Week 96 Source Data: LBAS subgroup (definitive MASH at baseline); FAS ©2025 AKERO THERAPEUTICS. Placebo (N=45) EFX 28mg (N=45) EFX 50mg (N=52) 13% **42%** **42%** ITT Analysis2 ** p<0.01, versus placebo (CMH test) 2 Missing biopsy = failure 1 All patients with baseline and Week 96 biopsies who had definitive MASH at baseline

Treatment-Emergent Adverse Events (TEAEs): Cumulative from Baseline through Week 96 TEAE Overview Placebo (N=61) EFX 28mg (N=57) EFX 50mg (N=63) TEAEs Leading to Death a 1 (2%) a 0 (0%) 0 (0%) Treatment-Emergent Serious Adverse Events (SAEs) 11 (18%) 15 (26%) 15 (24%) Drug-Related SAEs 0 0 0 TEAEs Leading to Discontinuation of Treatment 2 (3%) 6 (11%) 11 (18%) Weeks 37-96: Cirrhosis-Related Clinical Events 0 b 1 b c 1 c Weeks 37-96: Other Adverse Events 0 0 d 1 d Most Frequent (≥15%) Drug-Related TEAEs Placebo (N=61) EFX 28mg (N=57) EFX 50mg (N=63) Diarrhea, n (%) 10 (16%) 11 (19%) 19 (30%) Nausea, n (%) 8 (13%) 11 (19%) 18 (29%) Increased Appetite, n (%) 3 (5%) 7 (12%) 18 (29%) Injection Site Erythema, n (%) 5 (8%) 10 (18%) 14 (22%) a Pneumonia (prior to week 36); b Ascites; c Hepatic encephalopathy; d Diarrhea ©2024 AKERO THERAPEUTICS. Source Data: Safety Set

Additional Safety Observations for 50mg EFX (Phase 3 Dose) Versus Placebo at Week 96 Bone Mineral Density 43% of 50mg EFX and 41% of placebo patients had osteopenia1 at baseline, but only 3% of 50mg EFX and 7% of placebo patients were treated with bisphosphonates Placebo-adjusted, significant relative reductions in bone mineral density (5%) for spine and hip were observed for 50mg EFX, or about 2-3% per year For context, treatment with a diabetic dose of GLP-1 was recently reported to be associated with about a 2% reduction in bone mineral density over one year.2 Equal number of fractures (4 each) observed for 50mg EFX and placebo Markers of Hemostasis and Liver Function Markers of hemostasis and liver function, including bilirubin, MELD score, INR, platelet count, albumin and Child-Pugh score, remained stable or trended toward improvement for 50mg EFX Electrocardiograms (ECGs), Vital Signs & Body Weight No statistically significant changes in ECGs, heart rate, or blood pressure were observed for 50mg EFX versus placebo Weight reduction of ~2% was observed for 50mg EFX and placebo ©2025 AKERO THERAPEUTICS. 1 T-score < -1.0 by DXA 2 M. S. Hansen et al., eClinicalMedicine. 72, 102624 (2024)
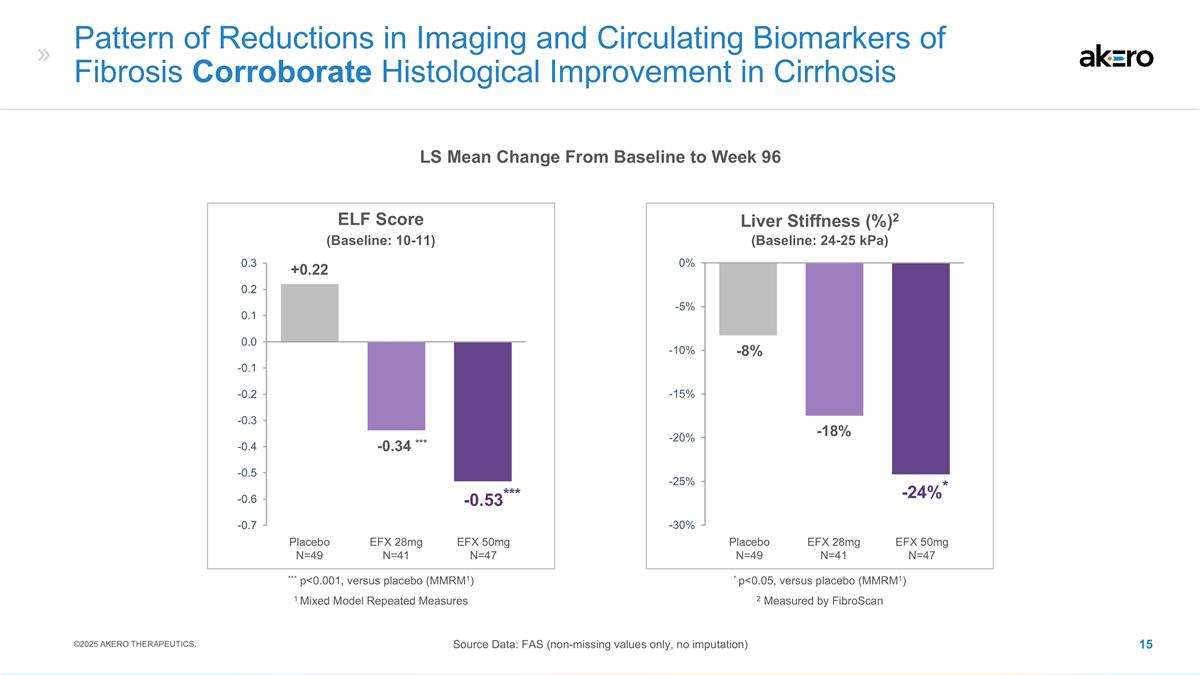
Pattern of Reductions in Imaging and Circulating Biomarkers of Fibrosis Corroborate Histological Improvement in Cirrhosis LS Mean Change From Baseline to Week 96 Source Data: FAS (non-missing values only, no imputation) ©2025 AKERO THERAPEUTICS. Liver Stiffness (%)2 -18% -8% EFX 50mg N=47 Placebo N=49 EFX 28mg N=41 *-24%* 2 Measured by FibroScan * p<0.05, versus placebo (MMRM1) (Baseline: 24-25 kPa) ELF Score EFX 50mg N=47 Placebo N=49 EFX 28mg N=41 ***-0.34 *** +0.22 ***-0.53*** *** p<0.001, versus placebo (MMRM1) (Baseline: 10-11) 1 Mixed Model Repeated Measures
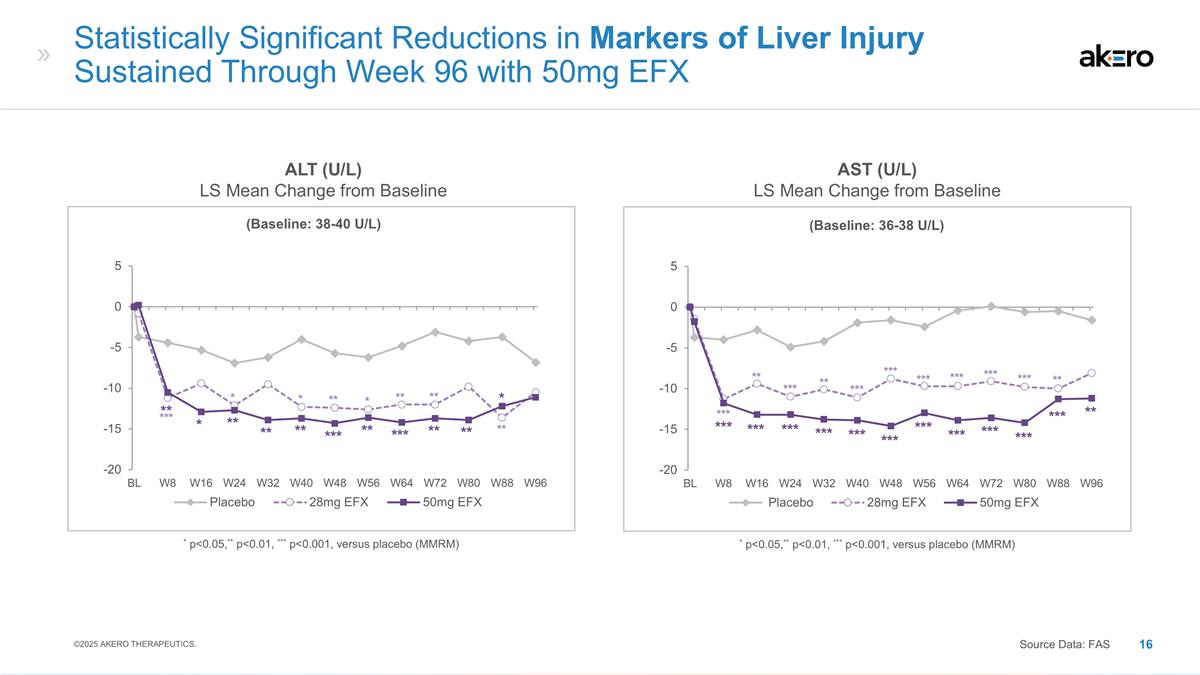
(Baseline: 38-40 U/L) * p<0.05,** p<0.01, *** p<0.001, versus placebo (MMRM) Statistically Significant Reductions in Markers of Liver Injury Sustained Through Week 96 with 50mg EFX ALT (U/L) LS Mean Change from Baseline AST (U/L) LS Mean Change from Baseline *** * * ** * ** ** ** ** * ** ** ** *** ** *** ** ** * ©2025 AKERO THERAPEUTICS. Source Data: FAS (Baseline: 36-38 U/L) * p<0.05,** p<0.01, *** p<0.001, versus placebo (MMRM) *** *** *** *** *** *** *** *** *** *** *** ** ** ** *** *** *** *** *** ** *** *** ***
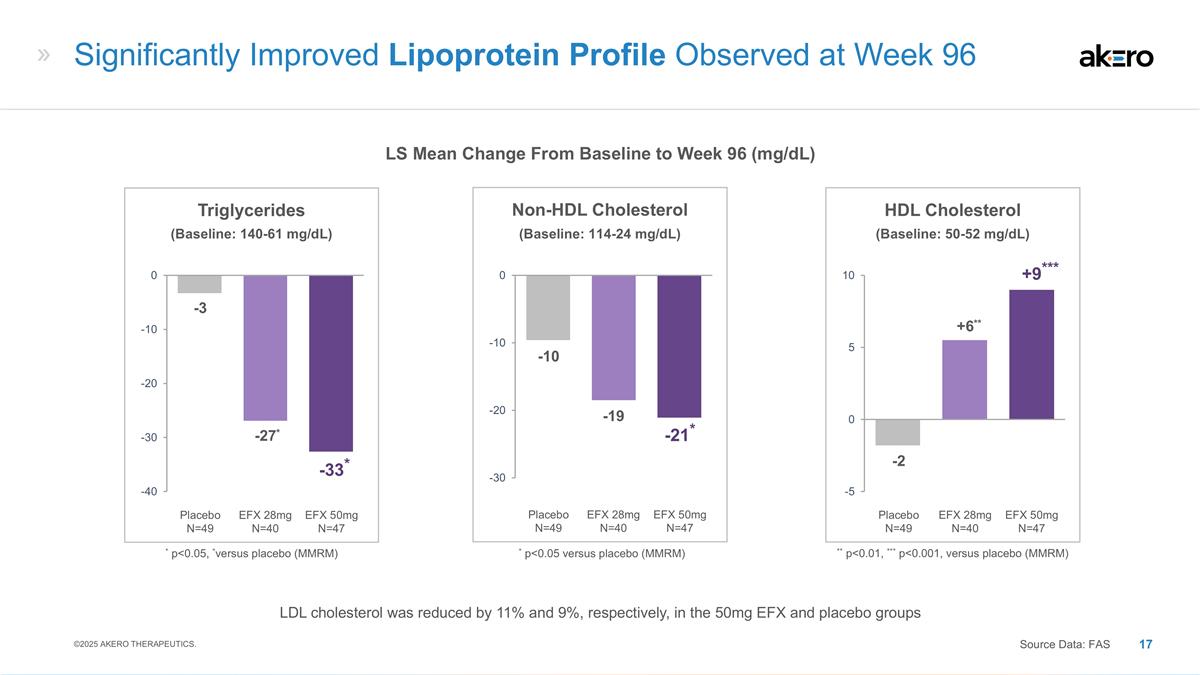
EFX 50mg N=47 Placebo N=49 EFX 28mg N=40 HDL Cholesterol **+6** -2 ***+9*** ** p<0.01, *** p<0.001, versus placebo (MMRM) EFX 50mg N=47 Placebo N=49 EFX 28mg N=40 Non-HDL Cholesterol -19 -10 -21* EFX 50mg N=47 Placebo N=49 EFX 28mg N=40 Triglycerides *-27* -3 *-33* * p<0.05, *versus placebo (MMRM) Significantly Improved Lipoprotein Profile Observed at Week 96 LS Mean Change From Baseline to Week 96 (mg/dL) ©2025 AKERO THERAPEUTICS. Source Data: FAS * p<0.05 versus placebo (MMRM) (Baseline: 114-24 mg/dL) (Baseline: 50-52 mg/dL) (Baseline: 140-61 mg/dL) LDL cholesterol was reduced by 11% and 9%, respectively, in the 50mg EFX and placebo groups
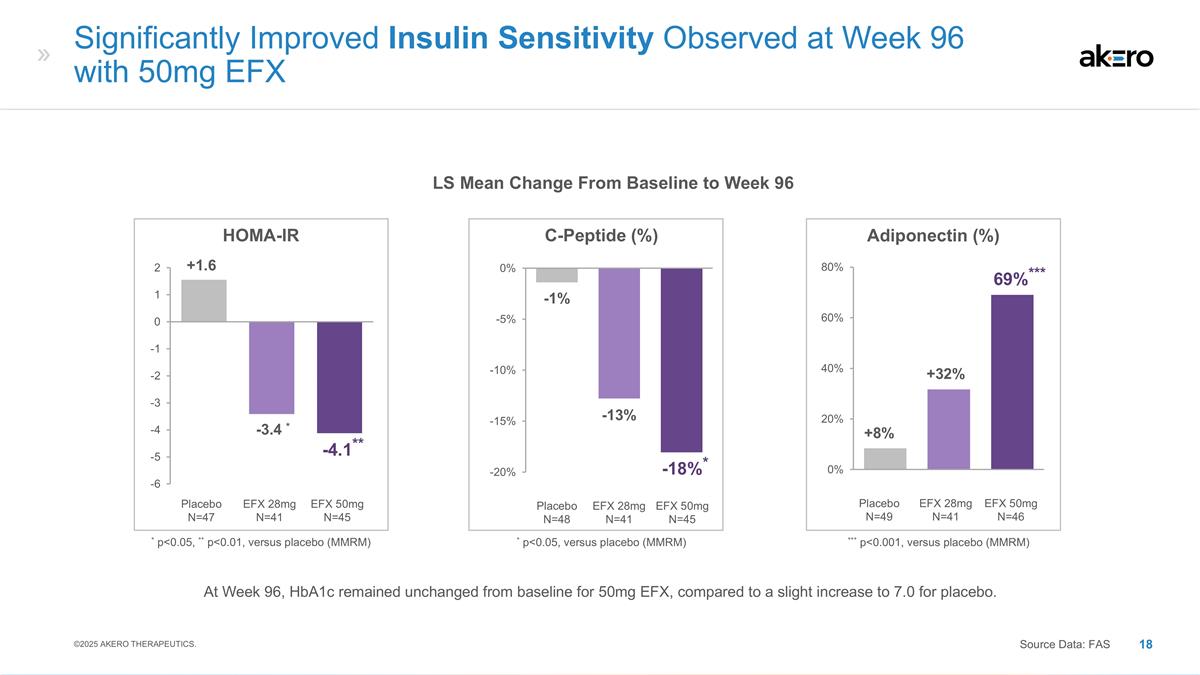
EFX 50mg N=45 Placebo N=48 EFX 28mg N=41 C-Peptide (%) * p<0.05, versus placebo (MMRM) -13% -1% *-18%* EFX 50mg N=46 Placebo N=49 EFX 28mg N=41 Adiponectin (%) +32% +8% ***69%*** *** p<0.001, versus placebo (MMRM) Significantly Improved Insulin Sensitivity Observed at Week 96 with 50mg EFX Source Data: FAS LS Mean Change From Baseline to Week 96 EFX 50mg N=45 Placebo N=47 EFX 28mg N=41 HOMA-IR * -3.4 * +1.6 **-4.1** * p<0.05, ** p<0.01, versus placebo (MMRM) ©2025 AKERO THERAPEUTICS. At Week 96, HbA1c remained unchanged from baseline for 50mg EFX, compared to a slight increase to 7.0 for placebo.
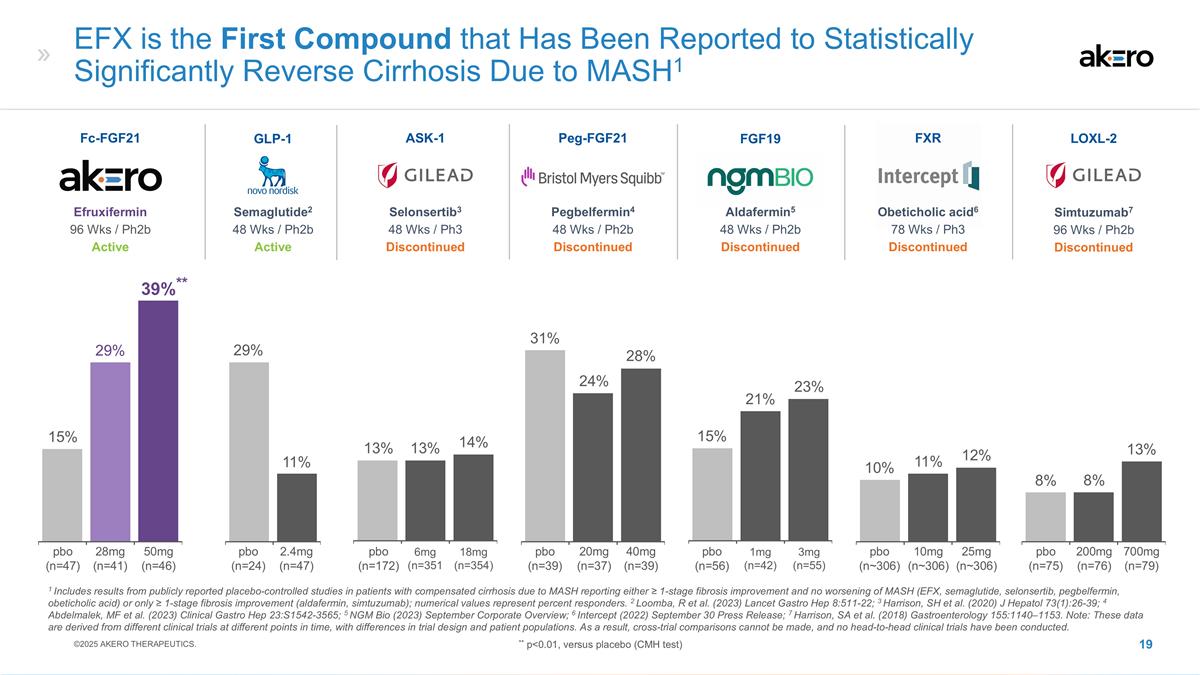
Aldafermin5 48 Wks / Ph2b Discontinued FGF19 1mg (n=42) 3mg (n=55) 21% 15% EFX is the First Compound that Has Been Reported to Statistically Significantly Reverse Cirrhosis Due to MASH1 Selonsertib3 48 Wks / Ph3 Discontinued ASK-1 Peg-FGF21 Pegbelfermin4 48 Wks / Ph2b Discontinued 6mg (n=351 18mg (n=354) 20mg (n=37) 40mg (n=39) 14% 13% 31% 24% FXR Obeticholic acid6 78 Wks / Ph3 Discontinued 25mg (n~306) 10mg (n~306) 10% 12% Semaglutide2 48 Wks / Ph2b Active GLP-1 2.4mg (n=47) 11% Simtuzumab7 96 Wks / Ph2b Discontinued LOXL-2 200mg (n=76) 700mg (n=79) 8% 8% 1 Includes results from publicly reported placebo-controlled studies in patients with compensated cirrhosis due to MASH reporting either ≥ 1-stage fibrosis improvement and no worsening of MASH (EFX, semaglutide, selonsertib, pegbelfermin, obeticholic acid) or only ≥ 1-stage fibrosis improvement (aldafermin, simtuzumab); numerical values represent percent responders. 2 Loomba, R et al. (2023) Lancet Gastro Hep 8:511-22; 3 Harrison, SH et al. (2020) J Hepatol 73(1):26-39; 4 Abdelmalek, MF et al. (2023) Clinical Gastro Hep 23:S1542-3565; 5 NGM Bio (2023) September Corporate Overview; 6 Intercept (2022) September 30 Press Release; 7 Harrison, SA et al. (2018) Gastroenterology 155:1140–1153. Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. 29% 13% 28% 23% 11% 13% Efruxifermin 96 Wks / Ph2b Active Fc-FGF21 50mg (n=46) 28mg (n=41) **39%** 29% 15% pbo (n=47) pbo (n=24) pbo (n=172) pbo (n=39) pbo (n=56) pbo (n~306) pbo (n=75) ©2025 AKERO THERAPEUTICS. ** p<0.01, versus placebo (CMH test)
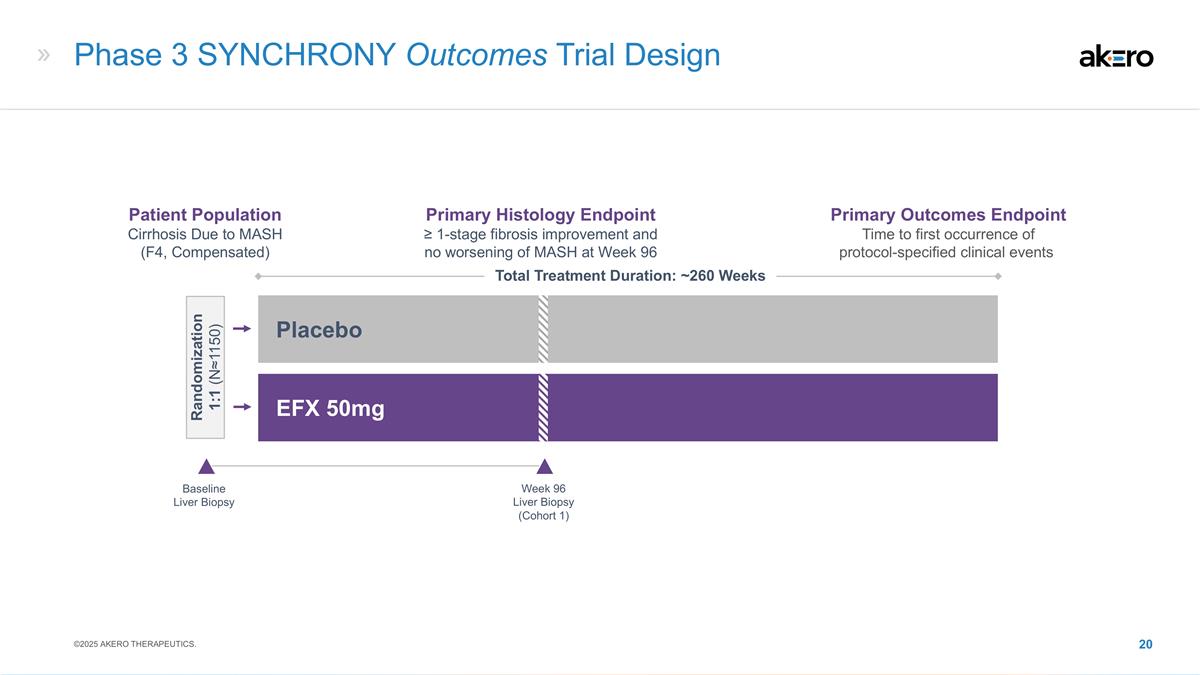
Phase 3 SYNCHRONY Outcomes Trial Design Baseline Liver Biopsy EFX 50mg Placebo Total Treatment Duration: ~260 Weeks Week 96 Liver Biopsy (Cohort 1) Patient Population Cirrhosis Due to MASH (F4, Compensated) Primary Histology Endpoint ≥ 1-stage fibrosis improvement and no worsening of MASH at Week 96 Primary Outcomes Endpoint Time to first occurrence of protocol-specified clinical events Randomization 1:1 (N≈1150) ©2025 AKERO THERAPEUTICS.
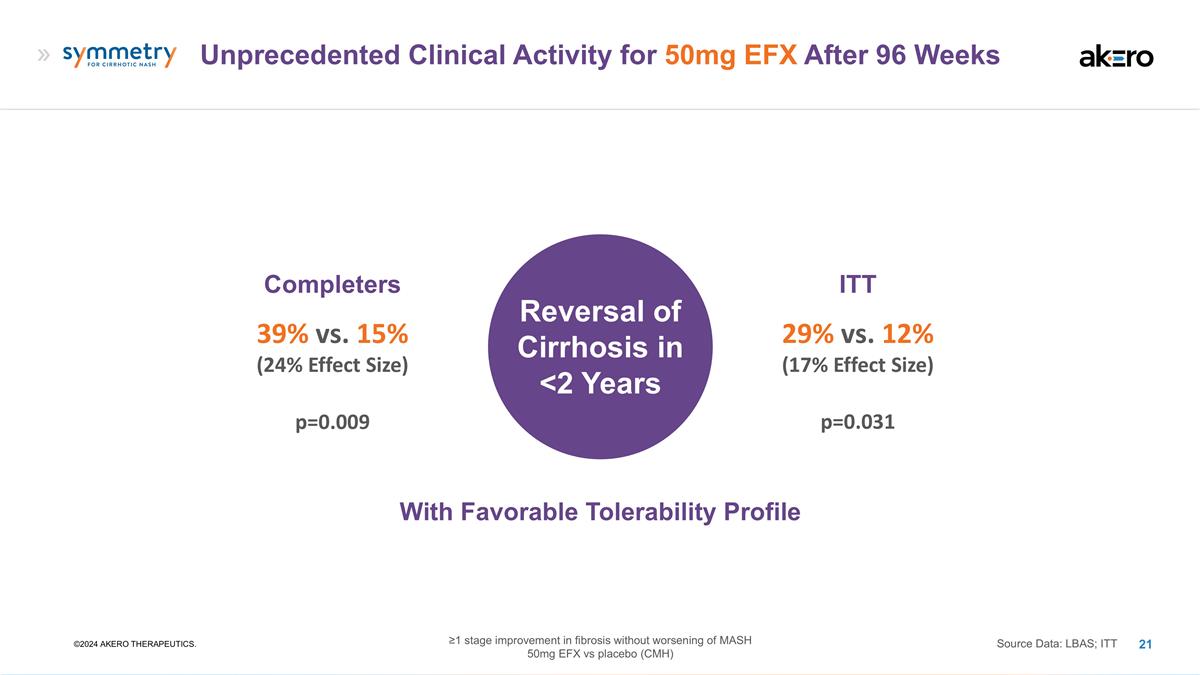
Unprecedented Clinical Activity for 50mg EFX After 96 Weeks ©2024 AKERO THERAPEUTICS. Completers 39% vs. 15% (24% Effect Size) Source Data: LBAS; ITT ITT 29% vs. 12% (17% Effect Size) Reversal of Cirrhosis in <2 Years p=0.009 p=0.031 With Favorable Tolerability Profile ≥1 stage improvement in fibrosis without worsening of MASH 50mg EFX vs placebo (CMH)

AKERO THERAPEUTICS 601 Gateway Boulevard Suite 350 South San Francisco, CA 94080 Nasdaq: AKRO





















