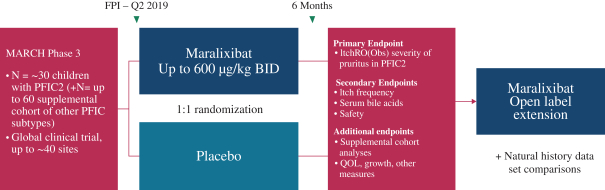
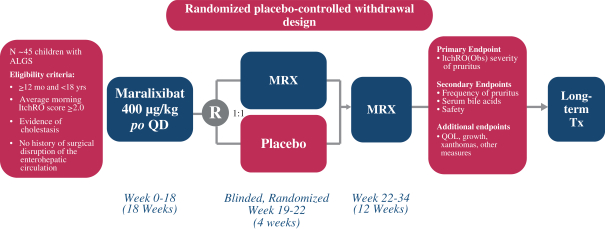
TGR5 Technology, which percentage is in thelow-fifties and is subject to adjustment in certain circumstances. This payment will not exceed an amount that isone-half of our low single-digit royalty obligation to Satiogen.
We may unilaterally terminate the Satiogen Agreement for any reason or no reason upon 90 days’ written notice to Satiogen. If we cease all research, development and commercialization efforts with respect to all licensed products related to the ASBTi Technology or the TGR5 Technology for over one year, or we determine to cease all such efforts, Satiogen may elect to terminate the Satiogen Agreement with respect to the license under the ASBTi Technology or the TGR5 Technology, respectively. Either party may terminate the Satiogen Agreement for the other party’s material breach of the Satiogen Agreement which remains uncured after 90 days of receiving written notice of such breach. Absent early termination, the Satiogen Agreement will automatically terminate upon the expiration of our royalty obligations.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates and other discoveries, inventions, trade secrets andknow-how that are critical to our business operations. Our success also depends in part on our ability to operate without infringing the proprietary rights of others, and in part, on our ability to prevent others from infringing our proprietary rights. A comprehensive discussion on risks relating to intellectual property is provided under “Risk Factors” under the subsection “Risks Related to Our Intellectual Property.”
We have developed and continue to develop patent portfolios around our product candidates, maralixibat and volixibat. We have pending patent applications in the United States, Europe, South Korea, Israel, Brazil, Canada, Hong Kong and Singapore covering the methods of treating cholestasis using ASBT inhibitors that have limited systemic exposure, which, if issued, would expire in October 2032, absent any patent term adjustments or extensions. We have granted and/or issued patents in Australia, China, Japan, Mexico, Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Russia, Tajikistan, Turkmenistan, South Africa and Macau covering the methods of treating cholestasis using ASBT inhibitors that have limited systemic exposure, which expire in October 2032. We also have pending patent applications in Brazil, Canada, South Korea, Mexico, Hong Kong, Singapore and South Africa, covering pediatric formulations of ASBT inhibitors that have limited systemic exposure, which, if issued, would expire in October 2032, absent any patent term adjustments or extensions. We have granted and/or issued patents in Australia, China, Israel, Japan, Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, France, Germany, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Slovak Republic, Spain, Sweden, Switzerland, United Kingdom, Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Russia, Tajikistan, Macau and Turkmenistan covering pediatric formulations of ASBT inhibitors that have limited systemic exposure, which expire in October 2032. We have licensed patent applications in the United States and Europe from Satiogen covering therapeutic uses of ASBT inhibitors that have limited systemic exposure for treating inflammatory intestinal conditions, which, if issued, would expire in May 2031, absent any patent term adjustments or extensions. One of these Satiogen applications recently issued as United States Patent No. 10,251,880. We have licensed an issued United States patent, as well as issued foreign counterparts in Argentina, Austria, Australia, Belgium, Canada, Switzerland, China, Germany, Denmark, Spain, Finland, France, United Kingdom, Greece, Hong Kong, Ireland, Israel, India, Italy, Japan, South Korea, Liechtenstein, Mexico, Malaysia, Netherlands, Norway, Portugal, Russia, Sweden, Singapore, Taiwan and Turkey, and a pending counterpart in Brazil from Sanofi, that cover the composition and methods of making volixibat and salts thereof, expiring in December 2027. Patents related to maralixibat and volixibat may be eligible for patent term extensions in certain jurisdictions, including the United States and Europe, upon approval of a commercial use of the corresponding product by a regulatory agency in the jurisdiction where the patent was granted and/or issued.
We do not have patents or patent applications covering maralixibat as a composition of matter. Therefore, the primary patent-based intellectual property protection for our maralixibat program will be any patents granted on the pendingmethod-of-use and formulation patent applications.
116