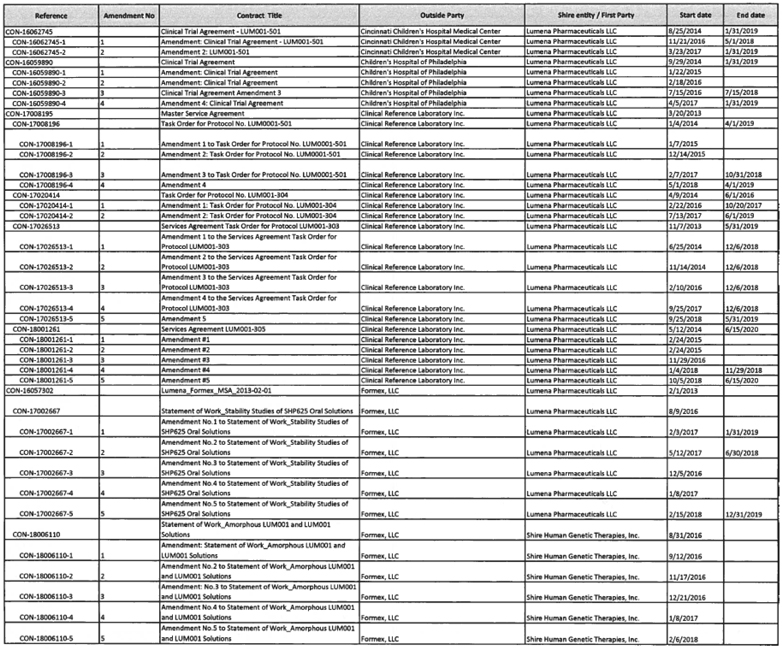
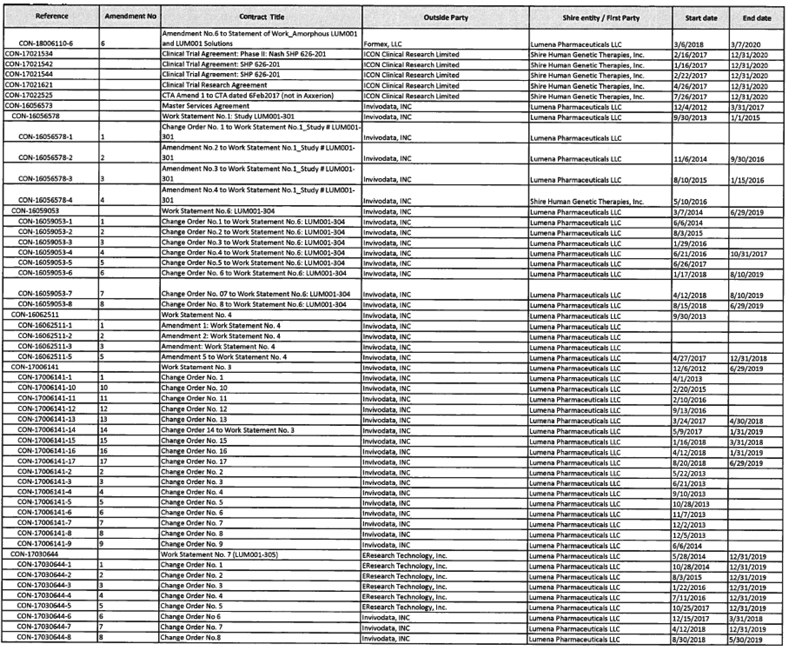
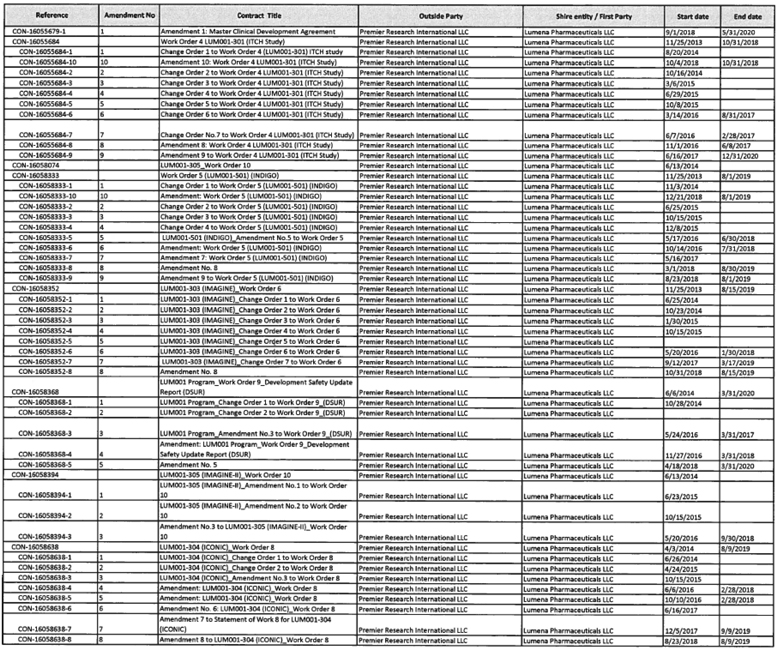
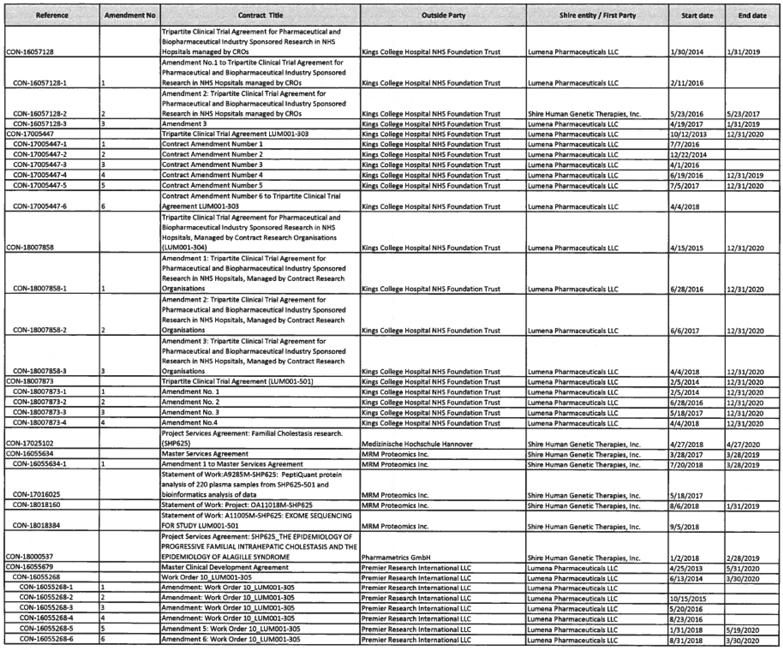
To the extent provided for in Section 5.2(d) of the Assignment and License Agreement, those services identified in Exhibit A with an asterisk in the “TSA #” column will be provided at no additional cost to Mirum.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
TSA features | | Duration | | Number of FTEs/ contractors | | | | | | | | | | | | |
TSA# | | Shire TSA
coordinator | | Service Type | | TSA description | | KPI’s | | Country
providing
service | | Country
receiving
service | | Target Start
Date | | Target
Completion
Date | | Exit Plan | | Name of Shire’s
resource providing
TSA | | Shire
systems
required | | Data
transfer
TSA? | | Notes / Comments | | TSA duration/
comment | | TSA
duration
(in months,
starting
from Day
1—for cost
calculation) | | # of
High
Cost
FTEs | | # of
Medium
Cost
FTEs | | # of
Low
Cost
FTEs | | # of
High Cost
Contractors | | # of
Medium
Cost
Contractors | | # of
Low Cost
Contractors | | Entity
invoiced | | Monthly
Cost per
TSA |
| REG-1 | | Erik Bjornson | | SHP625: Regulatory management of IND and CTAs, and transfer of dossiers | | Shire will perform ongoing regulatory management of all regulatory dossiers (39 total: 24 CTAs, 4 active INDs, 8 ODAs, 3 PIPs) ) for up to 3 monthsending with submission of formal notifications of transfer of sponsorship. Shire will provide additional signed documentation as needed to support the transfers of sponsorship for 1 month after the submissions have been made. Assumptions:
• Buyer will make best efforts to complete acceptance of sponsorship in a timely fashion once data transfer is complete and all requisite conditions have been met.
• Buyer will provide Shire with timely confirmation (confirmations within 10 business days or less) that the regulatory authorities have acknowledged the transfers of sponsorship. | | Submission of transfer and acceptance of ownership documentation to IND and CTAs. | | US | | US | | 11/5/2018 | | 2/15/2019 | | Transfer of sponsorships complete and signed documentation provided (as needed) following transfer submissions. | | Global Regulatory Lead | | | | Yes | | | | 3.5 months | | 3.5 | | 0.017 | | 0.133 | | 0.004 | | | | | | | | | | $ 2,853 |
| | | | | | | | | | | | | | | | | | | | | | | |
| REG-2 | | Sunil Kadam | | SHP626: Regulatory management of IND and CTAs, and transfer of dossiers | | Maintenance of open regulatory dossiers (US IND, CTAs in Canada and UK for 201 study) including CSR submission andend-of-trial notifications as required.
Preparation and submission of IND transfer letter and delivery to buyer for inclusion in their submission. | | Submission of transfer and acceptance of ownership documentation to IND and CTAs. | | US | | US | | 11/5/2018 | | 1/31/2019 | | Transfer of sponsorships complete and signed documentation provided (as needed) following transfer submissions. | | Global Regulatory Lead | | | | Yes | | | | 3 months | | 3 | | 0.004 | | 0.013 | | | | | | | | | | | | $ 355 |
| | | | | | | | | | | | | | | | | | | | | | | |
| REG-3 | | Erik Bjornson, Sunil Kadam | | SHP625/SHP626: Maintenance | | Ad-hoc regulatory consulting as requested by Buyer for either SHP625 or SHP626. This includes (but is not limited to) support of regulatory agency meeting preparation, review of Buyer-prepared documents for regulatory submissions, or participation in strategic discussions. This task includes up to 12 hours over a3-month period after deal close. | | Provide consultation to buyer for the first 3months | | | | US | | 11/5/2018 | | 1/31/2019 | | Transfer of sponsorships complete and signed documentation provided (as needed) following transfer submissions. | | Global Regulatory Lead | | | | | | | | 3 months | | 3 | | 0.025 | | | | | | | | | | | | | | $ 864 |
| | | | | | | | | | | | | | | | | | | | | | | |
| REG-4* | | Shakira Baez | | SHP625/SHP626: Data transfer | | Shire will transfer all Output Submissions folder; Correspondence, Components, Administrative files and other relevant Regulatory documents that are located in Shire EDMS, and other repository such as Share drive. | | | | | | | | | | 1/31/2019 | | | | | | | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 3 months | | 3 | | | | 0.5 | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| GDS-1 | | Tom Organ | | SHP626: Prepare and submit DSUR | | Shire will prepare and submit the Development Safety Update Report (DSUR), covering the period of 19 Dec 2017 to 18 Dec 2018, according to Shire SOPs and processes. The final report will be submitted to the IND no later than 60 days after the end of the reporting period. Buyer will receive a copy of the final DSUR. | | Submission of final DSUR and delivery of copy to buyer | | | | | | 11/5/2018 | | 2/28/2019 | | Complete DSUR and submit to IND; provide copy to buyer | | | | | | | | | | 4 months | | 4 | | 0.12 | | 0.24 | | | | | | | | | | | | $ 8,172 |
| | | | | | | | | | | | | | | | | | | | | | | |
| GDS-2 | | Nancy Blanchard | | SHP625/SHP626: Signal detection | | Shire to perform signal detection according to Shire’s procedures and provide Buyer with the results. Work will end when all INDs have been transferred to Buyer. | | Quarterly meetings for review and agreement on signal assessment | | US | | WW | | 11/5/2018 | | 2/28/2019 | | INDs transferred to buyer | | | | | | | | | | 4 months | | 4 | | 0.0025 | | 0.005 | | | | | | | | | | | | $ 171 |
| | | | | | | | | | | | | | | | | | | | | | | |
| DD-1* | | Kelly Spencer/Melissa Griffin/Arthur Van Leerberghe | | SHP625: Data Transfer | | Transfer all relevant electronic (i.e ncl completed/on going BBD specific clinical and nonclinical databases) and/or paper related to SHP625 (i.e. Trial Master File) R&D data that is reasonably available and in Sellers’ possession as of the Closing Date, within 6 monthsof the Closing Date to Service Recipient. To the extent CRO data included in the Transferred Assets is not reasonably practical to be delivered in that time period, Service Provider will reassigned all such data to the Buyer. (SHP625) | | Electronic and/or physical transfer (as appropriate) of all materials related to SHP625- Est 7 Nov018. As applicable, to include Letters of formal transfer (N.Rzucidlo) to be completed 1 month after Day 1. As applicable, consolidation of records from Shire’s EPL archive facility (N.Rzucidlo) to be completed in < 6 months. | | US | | US | | | | 4/30/2019 | | N/A | | R&D Scientific Documentation | | N/A | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 6 months | | 6 | | | | 0.5 | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| DD-2* | | Kelly Spencer/Melissa Griffin | | SHP625: Materials Transfer (nonclinical) | | Transfer all material that is reasonably available and in Sellers’ possession as of the Closing Date, within 6 monthsof the Closing Date to Service Recipient. To the extent any biomaterials (including study tissues and slides, drug substance and drug product, and related records, reagents and standards) included in the Acquired Assets not delivered at the Closing, Service Provider will deliver all such biomaterials to Service Recipient after the Closing. Service Provider shall be deemed to have complied with its obligation to deliver such Acquired Assets to Service Recipient if it has made such Acquired Assets available forpick-up by Service Recipient.In the event any biomaterials included in the Acquired Assets are held at third party vendors, Service Provider will be deemed to have transferred such biomaterials to Service Recipient at the Closing. | | Electronic and/or physical transfer (as appropriate) of all materials related to SHP625- Est 7Nov2018. As applicable, to include Letters of formal transfer (N.Rzucidlo) to be completed 1 month after Day 1. • As applicable, consolidation of records from Shire’s EPL archive facility (N.Rzucidlo) to be completed in < 6 months. | | US | | US | | | | 4/30/2019 | | N/A | | R&D Scientific Documentation | | N/A | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 6 months(3m?) | | 6 | | | | 0.5 | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| DD-3 | | Kelly Spencer / John McNulty | | SHP625: Preclinical Program Support | | Make introduction to and transfer ownership of work (transfer of completed / ongoing reports and any related materials to the buyer)with the CRO responsible for providing Toxicology support to the SHP625 program, within 4 monthsof closing date to recipient (more detail in KPI) . The Shire’s activity and support of these studies relate to, but not exclusively theon-going bioanalytical reports, toxicology reports. | | (i) pass of oversight of ongoing juvenile DRF study (CRL); pass of protocol (agency reviewed/commented) for pivotal juvenile toxicity study (CRL/Ashland) -final report November. Propose Buyer establish a MSA with CRL in order to perform the pivotal juvenile study in support of the ongoing PIP (ii) pass of ongoing DRF transgenic mouse study (Covance) and information of planned path forward-option to review/finalziation by Buyer (Shire to complete Feb2019). Propose Buyer establish a MSA with Covance to continue pivotal transgeneic mouse study (iii) pass of DRF early development information for2-year carcinogenicity studies(timing-PMC) 1 monthsFrom Day 1, Buyer and Shire to agree scedules for the regular transfer of knowledge, the activity of which is completed by the duration of the TSA, or before. | | US | | US | | 11/5/2018 | | 2/28/2019 | | N/A | | NCD-Toxicology | | N/A | | | | | | 4 months | | 4 | | | | 0.1 | | | | | | | | | | | | $ 1,350 |
| | | | | | | | | | | | | | | | | | | | | | | |
| DD-4 | | Kelly Spencer / Marita Larsson Cohen | | SHP625: Clinical Program Support | | Vendor Oversight—Provide Bioanalytical and Biomarker Development (BBD) support to the SHP625 program.
To ensure business continuity and established partnering in support of SHP625, Shire will oversee the CROs performing biomarker and bioanalytical work, and will provide bioanalytical support to the Buyer during the transition period. Shire will work with the Buyer and CROs to ensure transfer of ownership of completed work and transfer of knowledge for ongoing activities.
- additional details on activities reviewed with Mirum on Jan 7—presentation Shire-Mirum BBD activities 07Jan2019_to share.pptx | | Propose Buyer establish a MSA with following vendors to enable SOW transfer, as desired: BioAglytix, Prometheus, EGL Genetics/Eurofins, Envigo, Frontage, Q2 Solutions.Propose buyer to obtain full contract transfer of Cinnciniatti Children’s Hospital (CCH) and MRM Proteomics i. Assume contracts for ongoing LUM001 (SHP625) Phase II Clinical Trials LUM001-301, finishing LUM001-303, ongoing study LUM001-304, ongoing study LUM001-305, ongoing study LUM001-501, ongoing study a. Cincinnati Children’s Hospital (CCH), all studies b. Envigo CRS, Inc., all studies c. Bioagilytix Labs, LLC, all studies d. MRM Proteomics,LUM001-501 Exome sequencing and bioinformatics of protein analysis e. Q Squared Solutions Expressions Analysis, genomic biomarkers ii. Assume work for LUM001 (SHP625) Phase III Clinical Trials (none started) a. Envigo CRS, Inc, methods validated, to move from CCH b. Frontage Laboratories, methods validated, alternate site to move from CCH,SHP625-306 work proposed but not started c. Prometheus Laboratories, Inc.: proposed SHP625 Ph III, not started iii. Assume work for LUM002 (SHP626), work suspended a. Envigo CRS, Inc., wrapping upSHP626-201 study | | US | | US | | 11/5/2018 | | 1/31/2019 | | N/A | | NCD-BBD | | N/A | | | | | | 3 months | | 3 | | | | 0.75 | | | | | | | | | | | | $ 12,659 |
| | | | | | | | | | | | | | | | | | | | | | | |
| DD-5* | | Kelly Spencer/Melissa Griffin/Holly Oakley | | SHP626: Data Transfer | | Transfer all relevant electronic (i.e ncl completed/on going BBD specific clinical and nonclinical databases) and/or paper (i.e. Trial Master File) R&D data that is reasonably available and in Sellers’ possession as of the Closing Date, within 6 monthsof the Closing Date to Service Recipient. To the extent CRO data included in the Transferred Assets is not reasonably practical to be delivered in that time period, Service Provider will reassigned all such data to the Buyer. (SHP626) | | Electronic and/or physical transfer (as appropriate) of all materials related to SHP626- Est 21 Nov018. As applicable, to include Letters of formal transfer (N.Rzucidlo) to be completed 1 month after Day 1. As applicable, consolidation of records from Shire’s EPL archive facility (N.Rzucidlo) to be completed in < 6 months. | | US | | US | | | | 4/30/2019 | | N/A | | R&D Scientific Documentation | | N/A | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 6 months | | 6 | | | | 0.5 | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| DD-6* | | Kelly Spencer/Melissa Griffin | | SHP626: Materials Transfer | | Transfer all material that is reasonably available and in Sellers’ possession as of the Closing Date, within 6 monthsof the Closing Date to Service Recipient.
To the extent any biomaterials (including study tissues and slides, drug substance and drug product, and related records, reagents and standards) included in the Acquired Assets not delivered at the Closing, Service Provider will deliver all such biomaterials to Service Recipient after the Closing. Service Provider shall be deemed to have complied with its obligation to deliver such Acquired Assets to Service Recipient if it has made such Acquired Assets available forpick-up by Service Recipient. In the event any biomaterials included in the Acquired Assets are held at third party vendors, Service Provider will be deemed to have transferred such biomaterials to Service Recipient at the Closing. | | Electronic and/or physical transfer (as appropriate) of all materials related to SHP626- Est 21Nov2018. As applicable, to include Letters of formal transfer (N.Rzucidlo) to be completed 1 month after Day 1. • As applicable, consolidation of records from Shire’s EPL archive facility (N.Rzucidlo) to be completed in < 6 months. | | US | | US | | | | 4/30/2019 | | N/A | | R&D Scientific Documentation | | N/A | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 6 months | | 6 | | | | 0.5 | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| DD-7 | | Melissa Griffin | | SHP625/SHP626: Archiving | | Continued GxP archiving of paper/physical R&D materials (beyond 31 Jan 2019) at pass-through costs until buyer’s establishment of own GxP archiving contract(s). | | | | | | | | 11/5/2018 | | 4/30/2019 | | Buyer’s establishment of their own contracts for GxP archiving | | | | | | | | | | 6 months | | 6 | | | | | | 0.006 | | | | | | | | | | $ 40 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-1 | | Jeremy Chadwick | | SHP625: Clinical Program Management | | Oversight of project management activities for the 4 ongoing Phase 2 studies(SHP625-303, 304, 305, 501) until knowledge transfer completed. | | Complete knowledge transfer to manage and oversee the clinical study activities of the Phase 2 studies, as acknowledged by buyer. | | USA | | USA | | 11/5/2018 | | 1/31/2019 | | Buyer’s counterpart acknowledging complete transition | | GCOL | | Systems as defined in data transfer forms | | | | Contingent on Transfer of FTEs/Contractors | | 3 months | | 3 | | 0.5 | | | | | | | | | | | | | | $ 17,285 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CD-1 | | Thomas Jaecklin | | SHP625: Clinical Medical oversight and management | | Clinical Medical oversight and management of the 4 ongoing Phase 2 studies(SHP625-303, 304, 305, 501) until knowledge transfer completed. | | Complete knowledge transfer to manage and oversee the clinical medical aspects of the Phase 2 studies, as acknowledged by buyer. | | Switzerland | | USA | | 11/5/2018 | | 1/31/2019 | | Buyer-Shire agree transfer is complete | | Global Clinical Development Lead | | Systems as defined in data transfer forms | | | | Contingent on Transfer of FTEs/Contractors | | 3 months | | 3 | | 1.0 | | | | | | | | | | | | | | $ 34,569 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-4 | | Jeremy Chadwick | | SHP625: Clinical Program Management | | Clinical Project Management and oversight of study conduct, oversight and management of vendors and CRO for the 4 ongoing Phase 2 studies(SHP625-303, 304, 305, 501) until knowledge transfer completed. | | Complete knowledge transfer to manage and oversee the clinical study activities of the Phase 2 studies, as acknowledged by buyer. | | USA | | USA | | 11/5/2018 | | 2/28/2019 | | Buyer’s counterpart acknowledging complete transition | | Sr Clinical Project Manager | | Systems as defined in data transfer forms | | | | Contingent on Transfer of FTEs/Contractors | | 4 months | | 4 | | | | 1.0 | | | | | | | | | | | | $ 16,879 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-5 | | Courtney Bryant | | SHP625: Clinical Program Management | | Clinical Administrative tasks (meeting organization, agendas, minutes, tracker and system review and completion), until knowledge transfer completed. | | Complete knowledge transfer to manage and oversee the admin clinical study activities of the Phase 2 studies, as acknowledged by buyer. | | USA | | USA | | 11/5/2018 | | 2/28/2019 | | Buyer’s counterpart acknowledging complete transition | | CRA | | Systems as defined in data transfer forms | | | | Contingent on Transfer of FTEs/Contractors | | 4 months | | 4 | | | | | | 0.3 | | | | | | | | | | $ 1,924 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-6 | | Michael Hoffman | | SHP625: Clinical Scientific Writing | | Clinical Scientific Writing management and oversight of required clinical documentation for the 4 ongoing Phase 2 studies(SHP625-303, 304, 305, 501), as well as delivery of existing finalized, published reports, until knowledge transfer completed. | | Completed knowledge transfer for SHP625 clinical documentation, as acknowledged by buyer. | | USA | | USA | | 11/5/2018 | | 12/31/2018 | | Buyer-Shire agree transfer is complete | | clinical scientific writer | | Systems as defined in data transfer forms | | Yes | | | | 2 months | | 2 | | | | 0.2 | | | | | | | | | | | | $ 3,376 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-7* | | Wendy Beeby | | SHP625: Data Transfer | | Delivery of all product TMF documentation - mixed media and paper | | All TMF assets delivered successfully to buyer - buyer assumes all responsibility for TMF maintenance upon confirmation | | USA | | USA | | | | 2/28/2019 | | Complete transfer of all documentation | | Clinical Document Mgr | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.3 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-8* | | Mike Hale | | SHP625: Data Transfer | | Data transfer of all Biostats assets and Biostats knowledge transfer to buyer - provide point in time copy - when sponsorship is transferred then final Db will be transferred - this TSA represents the Final data delivery | | All data assets successfully transferred to buyer with buyer acceptance | | USA | | USA | | | | 2/28/2019 | | Completion of transfer of all documentation and knowledge | | Biostats Project Lead/ Programming Project Lead | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.5 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-9* | | Deb Jameson | | SHP625: Data Transfer for 306 | | StudySHP625-306: Transfer all IMP Supply Chain Maps, Packaging, Labeling, Distribution info, and Inventory data if applicable | | Quarterly meetings for review and agreement on signal assessment | | USA | | USA | | | | 2/28/2019 | | Completion of transfer of all documentation | | Clinical Supplies Team lead | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.1 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-10* | | Deb Jameson | | SHP625: Data Transfer for Phase 2 studies | | SHP625 Phase 2 studies: Transfer all IMP Supply Chain Maps, Packaging, Labeling, Distribution info, and Inventory data if applicable | | Transfer all relevant electronic (i.e inventory records) and/or paper (i.e. Batch Records) R&D data that is reasonably available and in Sellers’ possession as of the Closing Date, within 4 monthsof the Closing Date to Service Recipient. | | USA | | USA | | | | 2/28/2019 | | Completion of transfer of all documentation | | Clinical Supplies Team lead | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.1 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-11 | | Mike Hale | | SHP625: Ongoing study activities | | Biostatistics support: SAP review, DRM, SRT, meetings, etc until knowledge transfer completed | | Buyer’s counterpart acknowledging complete knowledge transfer | | USA | | USA | | 11/5/2018 | | 2/28/2019 | | Transfer complete | | Biostats Project Lead/ Programming Project Lead | | Systems as defined in data transfer forms | | | | | | 4 months | | 4 | | | | 0.35 | | | | | | | | | | | | $ 5,908 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-12 | | Jeremy Chadwick | | SHP626: Clinical trial management | | Shire will complete knowledge transfer for all 626 studies
Shire will complete all activities for ongoing studySHP626-201, including:
• Completeclose-out visit reports
• Complete study drug accountability
• Complete TMF and transfer
• Complete TFLs
• Complete abbreviated CSR
• Vendor close-outs
• Clinical project management
• biostatistical analysis
• final published CSR | | Completion of activities as noted. | | USA | | USA | | 11/5/2018 | | 1/31/2019 | | Complete knowledge transfer and complete study(SHP626-201) activities. | | Clinical Programs Lead | | Systems as defined in data transfer forms | | Yes | | | | 3 months | | 3 | | 1.87 | | 1.50 | | 0.50 | | 0.50 | | 0.50 | | | | | | $ 118,721 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-13* | | Diane Piper | | SHP626: Data Transfer | | Data transfer of all Data Management assets. Knowledge transfer to buyer - provide point in time copy - when sponsorship is transferred then final Db will be transferred - this TSA represents the Final data delivery. | | All Data management assets successfully transferred to buyer with buyer acceptance | | USA | | USA | | | | 2/28/2019 | | Completion of transfer of all documentation and knowledge | | Lead Clinical data mgr | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.5 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-14* | | Wendy Beeby | | SHP626: Data Transfer | | Delivery of all product TMF documentation - mixed media and paper | | All TMF assets delivered successfully to buyer - buyer assumes all responsibility for TMF maintenance upon confirmation | | USA | | USA | | | | 2/28/2019 | | Completion of transfer of all documentation and knowledge | | Clinical Document Mgr | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.3 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-15* | | Mike Hale | | SHP626: Data Transfer | | Data transfer of all Biostats assets - Biostats knowledge transfer to buyer - provide point in time copy - when sponsorship is transferred then final Db will be transferred - this TSA represents the Final data delivery | | All data assets successfully transferred to buyer with buyer acceptance | | USA | | USA | | | | 2/28/2019 | | Completion of transfer of all documentation and knowledge | | Biostats Project Lead/ Programming Project Lead | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.5 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-16* | | Diane Piper | | SHP626: Data Transfer | | Data transfer of all Data Management assets. Knowledge transfer to buyer. | | All Data management assets successfully transferred to buyer with buyer acceptance | | USA | | USA | | | | 2/28/2019 | | Completion of transfer of all documentation and knowledge | | Lead Clinical data mgr | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.3 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| CDO-17* | | Deb Jameson | | SHP626: Data Transfer for 201 | | StudySHP626-201: Transfer all IMP Supply Chain Maps, Packaging, Labeling, Distribution info, and Inventory data if applicable | | Transfer all relevant electronic (i.e inventory records) and/or paper (i.e. Batch Records) R&D data that is reasonably available and in Sellers’ possession as of the Closing Date, within 4 monthsof the Closing Date to Service Recipient. | | USA | | USA | | | | 2/28/2019 | | Completion of transfer of all documentation | | Clinical Supplies Team lead | | Systems as defined in data transfer forms | | Yes | | TSA billed at zero cost. FTE allocation is for internal purposes only. | | 4 months | | 4 | | 0.3 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| QAC-1 | | Christine Sahagian | | SHP625: Quality Assurance and Compliance | | Provide QA&C oversight on ongoing studies and compliance support (typical QA&C involvement in an ongoing clinical program - e.g. available for advice / guidance as needed) needed until complete transfer | | Buyer’s counterpart acknowledging complete knowledge transfer | | USA | | USA | | 11/5/2018 | | 2/28/2019 | | Transfer complete | | QA&C Lead | | Systems as defined in data transfer forms | | | | | | 4 months | | 4 | | | | 0.2 | | | | | | | | | | | | $ 3,376 |
| | | | | | | | | | | | | | | | | | | | | | | |
| QAC-2 | | Christine Sahagian | | SHP626: Quality Assurance and Compliance | | Provide QA&C oversight on ongoing studies and compliance support (typical QA&C involvement in an ongoing clinical program - e.g. available for advice / guidance as needed) needed until complete transfer | | Buyer’s counterpart acknowledging complete knowledge transfer | | USA | | USA | | 11/5/2018 | | 2/28/2019 | | Transfer complete | | QA&C Lead | | Systems as defined in data transfer forms | | | | | | 4 months | | 4 | | | | 0.1 | | | | | | | | | | | | $ 1,688 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CD-2 | | Mary Corcoran | | SHP625: Compassionate Use | | Shire will provide the required review/approval of the application and IMP resupply (if approved) for the current PFIC patient receiving SHP625 through a compassionate use/named patient program at Pr. Jankowska’s site in Poland.
- Two agreements in scope between Shire/Investigator and Shire/Hospital (no existing agreements with 3rd party) | | Application review andre-supply provided. | | USA | | USA | | 11/5/2018 | | 1/31/2019 | | Completion of application review and resupply (if approved) | | Head of Development Planning and Compassionate Use | | | | | | | | 3 months | | 3 | | 0.1 | | | | | | | | | | | | | | $ 2,593 |
| | | | | | | | | | | | | | | | | | | | | | | |
| CD-3 | | Melissa Palmer | | SHP626: Publications | | In order to comply with clinical trial transparency requirements, Shire will support publication of theSHP626-201 study results. Shire will submit an abstract of the study results for presentation at the European Association for the Study of the Liver (EASL) 2019 Annual Meeting. In conjunction with the publications vendor (Oxford PharmaGenesis), Shire will prepare the full manuscript for the study results, submit the manuscript to a peer-reviewed open access journal for publication, and make any necessary revisions and resubmissions. Direct costs for submission fees and open access fees will be passed through to Mirum. | | Abstract submission. Manuscript submission and resubmission (if needed). | | USA | | USA | | 11/5/2018 | | 4/30/2019 | | Completion of submissions | | Global Development Lead | | | | | | Shire has agreed not to pass through costs for Pharmagenesis to Mirum. Given additional workload for the Shire team, the FTE estimate was revised | | 6 months | | 6 | | 0.05 | | 0.02 | | | | | | 0.13 | | | | | | $ 4,346 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | $237,130 |
Any “Target Start Date” that occurred in the past was the actual start date and will be billed accordingly.
Out of pocket costs are not accounted for and will be billed separately in accordance with the Agreement.