We own various trademark registrations and applications, and unregistered trademarks, including our name and our corporate logo. All other trade names, trademarks and service marks of other companies appearing in this report are the property of their respective holders. Solely for convenience, the trademarks and trade names in this report may be referred to without the ®,™ or © symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend to use or display other companies' trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Company-Owned Intellectual Property
As of December 31, 2021, our patent portfolio included four patent families directed to novel scaffolds, 15 patent families directed to our platform technology, 88 patent families directed to bicyclic peptides and related conjugates, and 15 patent families directed to methods of making or using certain bicyclic peptide conjugates for treating various indications. In total, as of December 31, 2021, we owned about 88 patents in the United States and in foreign jurisdictions, such as Australia, Canada, China, Europe, Hong Kong, Japan, New Zealand, Russia and Singapore, with terms expiring at various dates in February 2029 to February 2039 exclusive of potential patent term adjustment and/or patent term extension.
In addition, as of December 31, 2021, we had about 402 patent applications pending in the United States and in foreign jurisdictions, such as Argentina, Australia, Brazil, Canada, China, Europe, Hong Kong, India, Japan, Korea, New Zealand, Russia, Singapore, Taiwan, and Venezuela, as well as pending international applications under the Patent Cooperation Treaty, or PCT, and any patents that may be issued from these patent applications are generally expected to have terms that will expire at various dates in February 2029 to December 2042, subject to possible patent term extensions and/or patent term adjustments.
Trade Secret Protection
Finally, we may rely, in some circumstances, on trade secrets to protect our technology. We anticipate relying on trade secrets to protect the know-how behind our Bicycle platform. However, trade secrets can be difficult to protect. We seek to protect our technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants, contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. For further information, please see “Risk Factors — Risks Related to Our Intellectual Property.”
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technologies, knowledge, experience and scientific resources provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
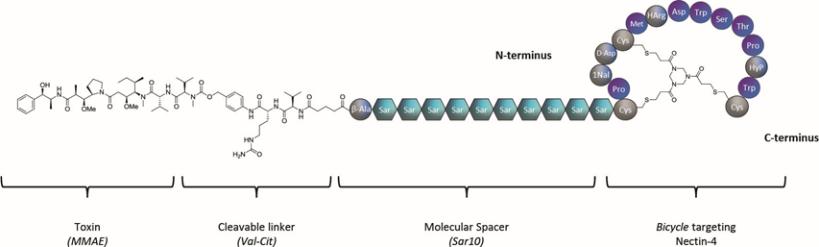
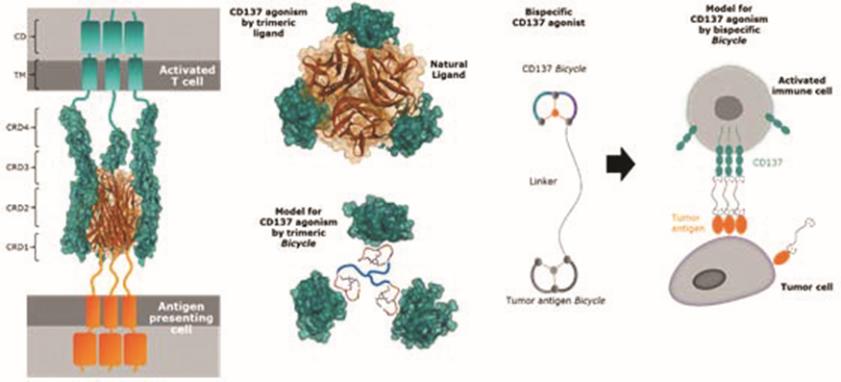
There are a number of currently marketed products and product candidates in preclinical research and clinical development by third parties for the various oncology applications that we are targeting. For example, a number of multinational companies as well as large biotechnology companies, are developing programs for the targets that we are exploring for our BTC programs, including Seagen, which has a marketed Nectin-4 antibody-drug conjugate. Furthermore, many companies are developing programs for CD137 or CD137 bi-specific antibodies. In addition, we are