to manufacture and develop novel cell or viral therapy products. Additional regulatory protection may also be afforded through data exclusivity, market exclusivity and patent term extensions where available.
As of May 2, 2023, we own or exclusively license 14 issued U.S. patents and 96 issued foreign patents in Australia, Austria, Belgium, Brazil, Canada, China, France, Germany, Great Britain, Hong Kong, India, Ireland, Israel, Italy, Japan, Lebanon, Luxembourg, Mexico, Netherlands, Russia, and Spain. We currently own or exclusively license 13 pending U.S. patent applications, eight U.S. provisional applications, and 121 pending foreign patent applications in Algeria, Argentina, Australia, Brazil, Canada, Chile, China, Colombia, Egypt, Europe, Gulf Coast Cooperation, Hong Kong, India, Israel, Japan, Korea, Malaysia, Mexico, New Zealand, Peru, Philippines, Singapore, South Africa, Thailand, Ukraine and Vietnam.
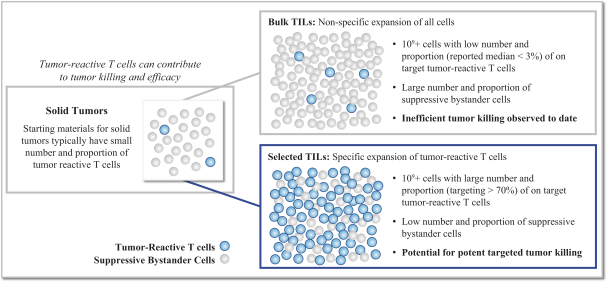
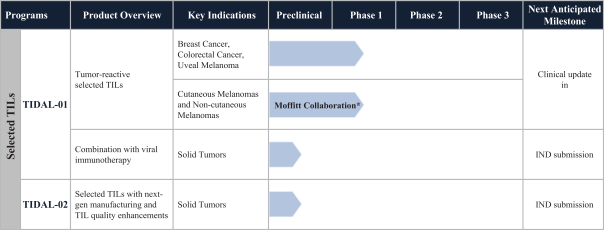
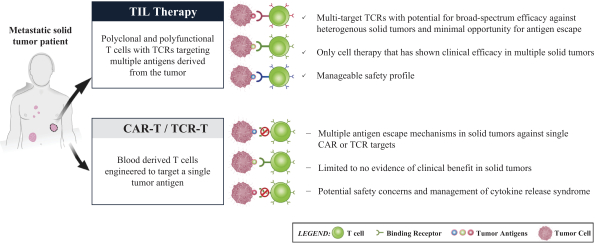
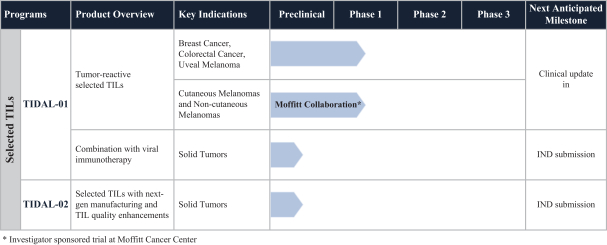
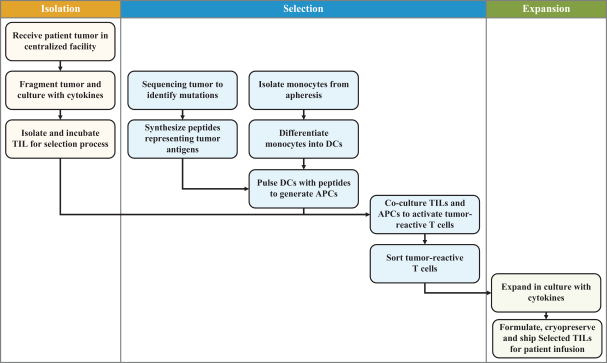
TIL Therapy, Including TIDAL-01
We own four patent families related to TIL therapy that are filed worldwide. The first TIL-001, includes 12 patent applications pending in Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Korea, Mexico, New Zealand and the United States. The TIL-001 patent applications are directed to a processing method for producing autologous T cells for the treatment of cancer and resulting cell therapy compositions, which, if issued, are expected to expire in 2040, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
The second family, TIL-002, includes 13 patent applications pending in Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Japan, Korea, Mexico, New Zealand and the United States. The TIL-002 patent applications are related to further aspects of processes for producing a TIL therapy and related compositions and methods, and patents that issue from this family, if any, are expected to expire in 2040, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
The third family, TIL-003, includes 10 patent applications pending in Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Korea, New Zealand and the United States. The TIL-003 patent applications are directed to methods of producing tumor-reactive T cell compositions using modulatory agents, and patents that issue from this family are expected to expire in 2040, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
The fourth family, TIL-004, includes 12 patent applications pending in Australia, Brazil, Canada, China, Europe, Israel, India, Japan, Korea, Mexico, New Zealand and the United States. The TIL-004 patent applications are directed to methods for ex vivo enrichment and expansion of tumor-reactive T cells and related compositions, and any patents that issue from this family are expected to expire in 2041, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
We own four provisional application families, in which, if patents from applications claiming priority to these provisional applications issue, the patents are expected to expire in 2043 or 2044, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees. One provisional application family is directed to methods of producing tumor-reactive T cell compositions using multi-specific binding agents. We have two provisional application families directed to particular TIL compositions and related methods, and a further provisional application family directed to combination of TILS and viral immunotherapy.
Additional Miscellaneous Virus IP
We have 10 pending patent families covering oncolytic Maraba rhabdoviruses, including 109 issued patents and 17 pending applications. If patents issue from each pending family, the patents are expected to expire from 2027 to 2039, without taking into account any possible patent term adjustment or extension and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
143