Exhibit 99.2
Description of the Acquired Tenet Business
Background
On June 27, 2024, Eliem completed its acquisition of Tenet Medicines, Inc. (“Tenet”), in accordance with an Agreement and Plan of Merger and Reorganization, dated as of April 10, 2024 (the “Acquisition Agreement”), by and among Eliem, Tango Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of Eliem (“Transitory Subsidiary”), Tenet, and, solely in his capacity as Tenet equityholder representative, Stephen Thomas, providing for the acquisition of Tenet by Eliem through the merger of Transitory Subsidiary into Tenet, with Tenet surviving as a wholly owned subsidiary of Eliem (the “Acquisition”).
Following the closing of the Acquisition, Tenet became a wholly owned subsidiary of Eliem and Eliem’s business included the business conducted by Tenet immediately prior to the Acquisition, including the advancement of TNT119, and Tenet’s agreements and arrangements effectively became agreements and arrangements of Eliem.
Below is the description of Tenet’s business from the definitive proxy statement on Schedule 14A, filed by Eliem with the Securities and Exchange Commission (the “SEC”) on June 4, 2024, which was supplemented by supplements filed with the SEC on June 12, 2024 and June 14, 2024. Unless the context indicates otherwise, all references in the following description of the acquired Tenet business to “Eliem,” “our,” “us” or “we” refer to Eliem Therapeutics, Inc. and its wholly owned subsidiaries after the effective time of the Acquisition, and all references to Tenet refer to Tenet Medicines, Inc. prior to the effective time of the Acquisition.
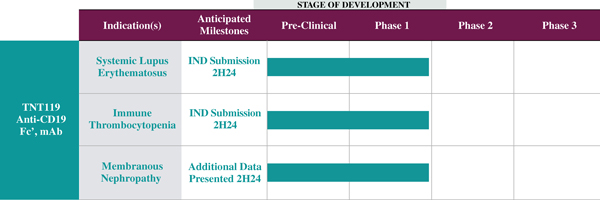
Following the Acquisition, Eliem plans to focus primarily on advancing TNT119, an anti-CD19 antibody, designed for a broad range of autoimmune diseases, including systemic lupus erythematosus, immune thrombocytopenia and membranous nephropathy. A previously disclosed, the Eliem board of directors appointed Aoife Brennan as President and Chief Executive Officer of Eliem effective upon the closing of the Acquisition. It is expected that the management team of Eliem, led by Dr. Brennan, will evaluate and consider revisions to the company’s development plan on a go-forward basis, which updates Eliem will disclose in its future filings with the SEC.
Overview and Corporate History of Tenet
Tenet is a clinical stage biotechnology company dedicated to developing its product candidate, TNT119. Also known as budoprutug, TNT119 is an anti-CD19 monoclonal antibody (“mAb”) designed for a broad range of autoimmune diseases, including systemic lupus erythematosus (“SLE”), immune thrombocytopenia (“ITP”) and membranous nephropathy (“MN”). Tenet was founded in November 2023 and entered into an asset purchase agreement with Acelyrin, Inc. (“Acelyrin”) in January 2024, which granted Tenet worldwide licenses to develop, manufacture, use and commercialize TNT119 for any non-oncology indication. Prior to the Acquisition, approximately 81% of Tenet’s equity interests were held by Sera Medicines, LLC (“Sera Medicines”), which is majority owned by RA Capital Management L.P., and approximately 19% of its equity interests were held by Tenet’s management.
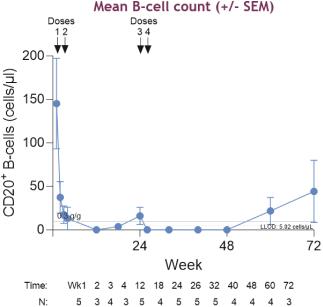
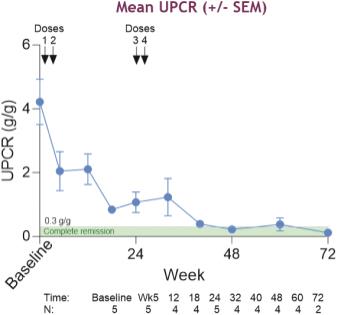
TNT119 is an anti-CD19 mAb with a fragmented crystallizable region engineered to achieve effector function through low-fucosylation (“Fc+”). CD19 is expressed on B-lineage cells and plays a key role in B cell autoimmune diseases. TNT119, an anti-CD19 mAb, is designed to deplete CD19-positive B cells, including antibody secreting cells, in order to directly reduce pathogenic autoantibodies. This reduction of autoantibodies has the potential to be disease modifying in autoantibody driven diseases, such as SLE, ITP and MN. In a Phase 1b clinical trial of TNT119 in MN, 3 out of 5 (or 60%) of patients that received four doses of TNT119 achieved a complete remission of proteinuria, a primary symptom of MN.
In SLE, one of TNT119’s lead indications, the underlying pathology involves production of autoantibodies by autoreactive B cells that contribute to inflammation and tissue damage. CD19 is a protein expressed on the surface of these B-cells and plays a key role in B cell activation. Because TNT119 is designed to target and deplete CD19-expressing B cells known to produce autoantibodies, Tenet believes TNT119 has the potential to treat SLE. In ITP, Tenet believes targeting plasmablasts and plasma cells is likely to decrease the production of autoantibodies, increase platelet count and ameliorate disease. B-cell depletion with anti-CD20 targeting mAbs, whose expression