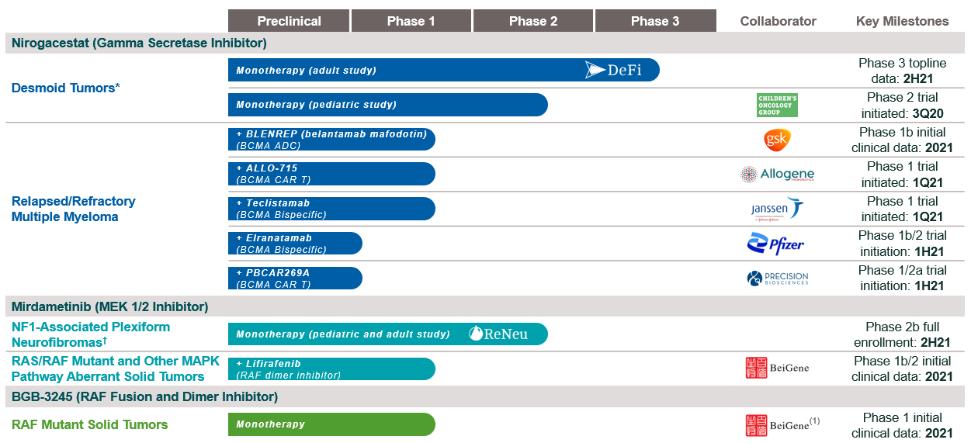
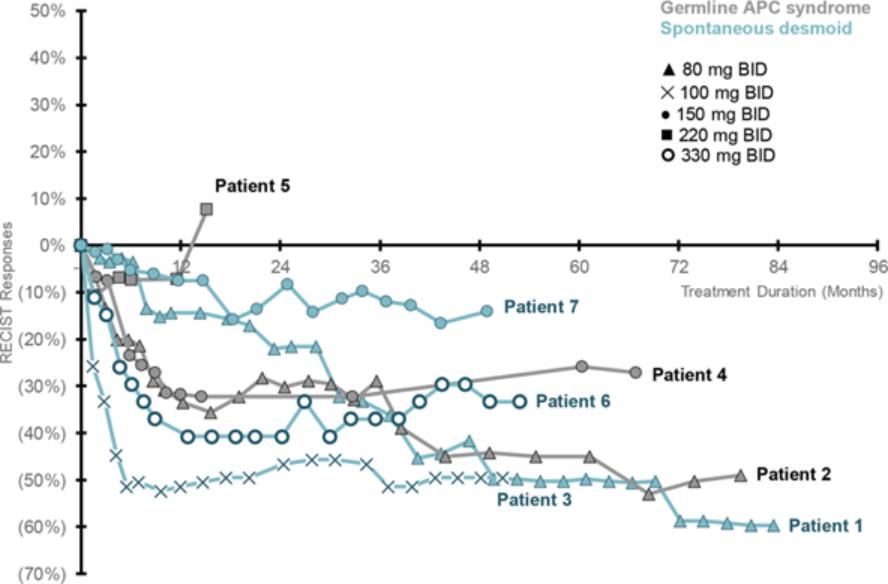
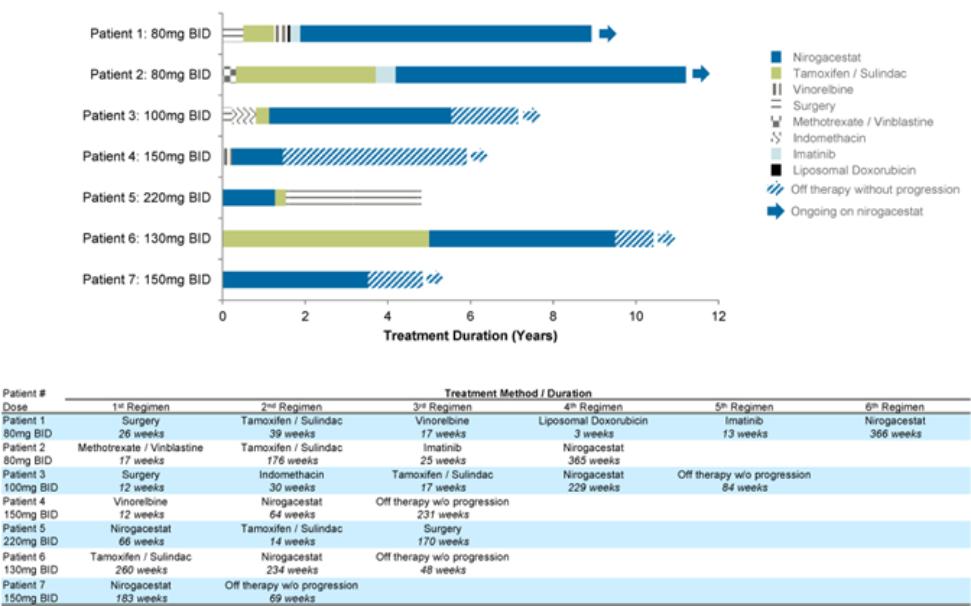
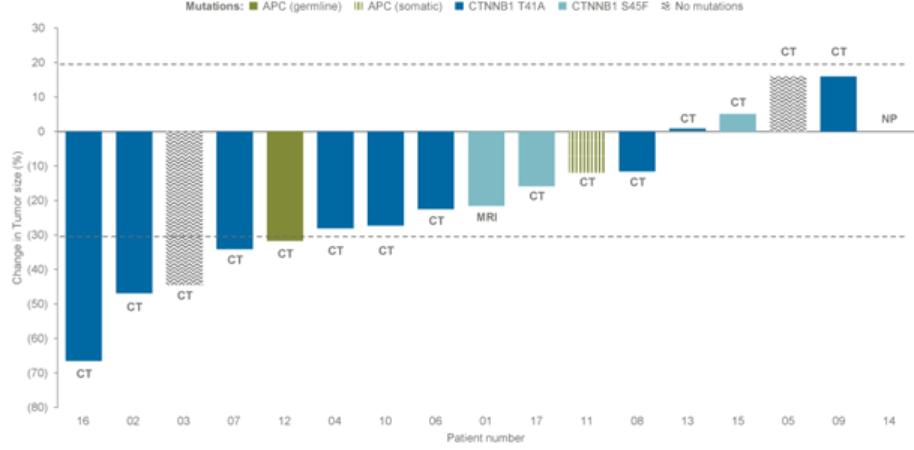
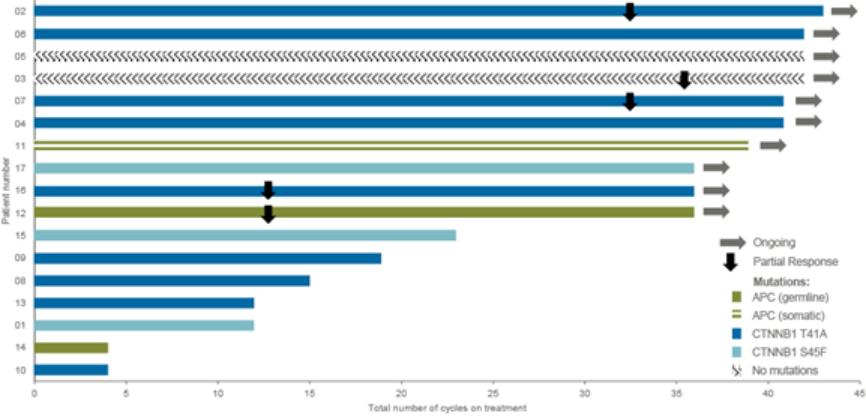
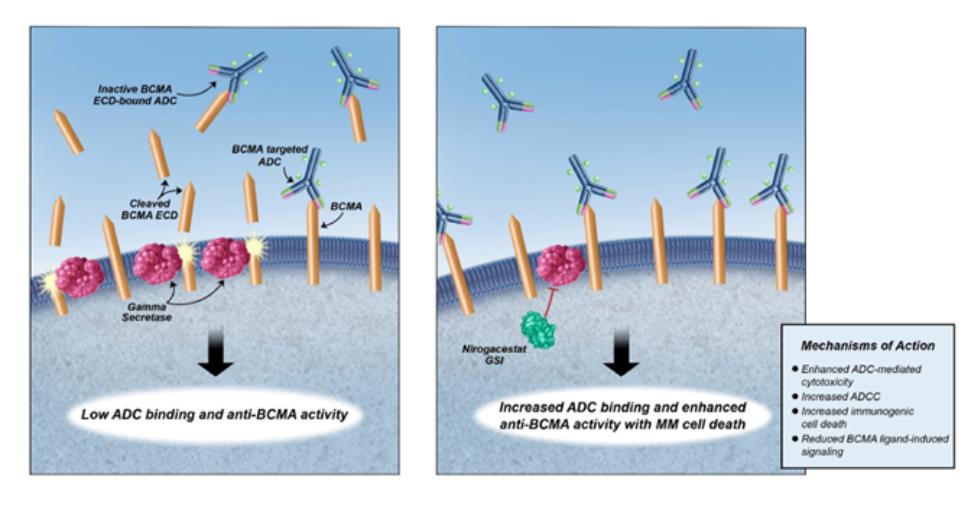
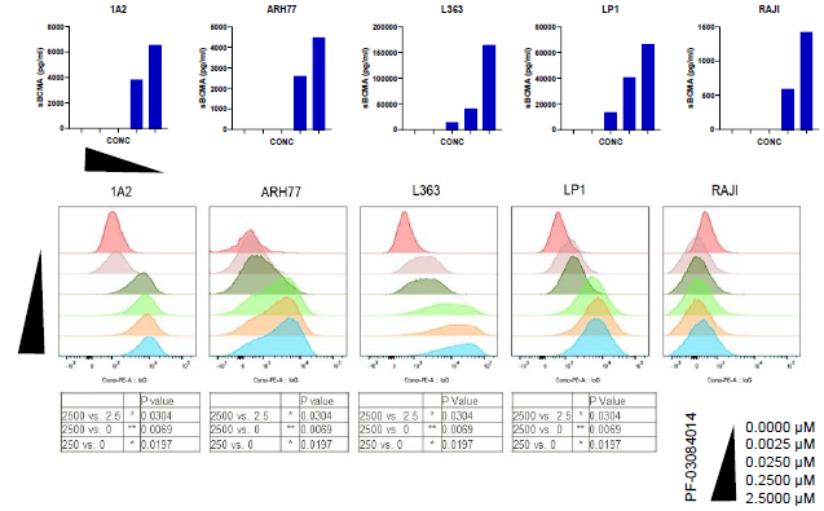
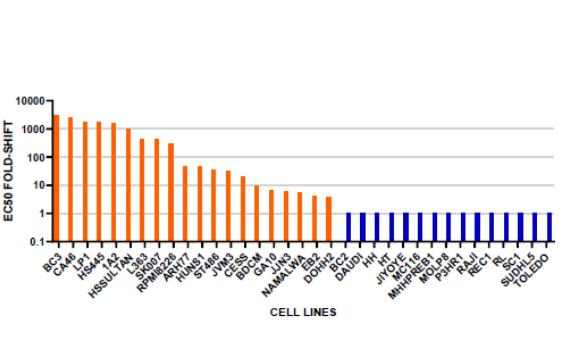
therapies, some of which are generic, that are used off-label for the treatment of NF1-PN. These therapies include radiotherapy and various systemic treatments, such as chemotherapy and immunotherapy.
For our targeted oncology portfolio, we are aware that other oncology focused companies are or may be developing products for the treatment of solid tumors with RAS mutations, RAF mutations and other MAPK aberrations, including Amgen Inc., AstraZeneca PLC, Basilea Pharmaceutica Ltd., Black Diamond Therapeutics, Inc., Boehringer Ingelheim International GmbH, Chugai Pharmaceutical Co Ltd, Daiichi Sankyo Co., Ltd., Day One Biopharmaceuticals, Inc., Eli Lilly and Company, F. Hoffmann-La Roche Ltd., Hanmi Pharmaceutical Co., Ltd., Kinnate Biopharma Inc., Merck & Co., Inc., Mirati Therapeutics, Inc., Moderna Inc., Novartis International AG, Pfizer, Revolution Medicines, Inc., TheRas, Inc. and Wellspring Biosciences, Inc. There may be additional companies with programs suitable for addressing these patient populations that could be competitive with our efforts but that have not yet disclosed specific clinical development plans. In addition we are aware that other oncology focused companies are or may be developing products targeting BCMA for the treatment of multiple myeloma patients, including AbbVie Inc., Amgen Inc, AstraZeneca PLC, Autolus Therapeutics plc, bluebird bio, Inc., Bristol-Myers Squibb, Cartesian Therapeutics, Inc., CRISPR Therapeutics AG, Heidelberg Pharma GmbH, Legend Biotech Corporation, Novartis International AG, Poseida Therapeutics, Inc., Regeneron Pharmaceuticals and Seagen.
Smaller or early-stage companies, including oncology-focused therapeutics companies, may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies may also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites, enrolling patients in clinical trials and acquiring technologies complementary to, or necessary for, our programs.
The availability of reimbursement from government and private payors will also significantly impact the pricing and competitiveness of our products. Our competitors may obtain FDA or other regulatory approvals for their products more rapidly than we may obtain approvals for our product candidates, which could result in our competitors establishing a strong market position before we are able to commercialize our product candidates.
Intellectual property
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, manufacturing and process discoveries and other know-how, to operate without infringing the proprietary rights of others, and to prevent others from infringing our proprietary rights. We plan to protect our proprietary position using a variety of methods, which include pursuit of U.S. and foreign patent applications related to proprietary technology, inventions and improvements, such as compositions of matter and methods-of-use, that we determine are important to the development and implementation of our business. For example, we, our licensors, or our collaborators currently have, or are pursuing, patents covering the composition of matter for our product candidates and we plan to generally pursue patent protection covering methods-of-use for one or more clinical programs. We also rely on trade secrets, trademarks, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our proprietary position.
Patents
At the time we were formed in August 2017, we entered into license agreements with Pfizer for our lead product candidates, pursuant to which we acquired exclusive worldwide rights under Pfizer patents and know-how to develop, manufacture and commercialize our lead product candidates.
We have exclusive licenses under the Nirogacestat License Agreement to patent rights in the U.S. and numerous foreign jurisdictions relating to nirogacestat. The patent rights in-licensed under the Nirogacestat License Agreement include five granted patents in the U.S. (with the agreement originally covering three such patents, and two additional patents having been subsequently granted based on work conducted by us and with Pfizer’s consent) and more than 25 patents granted in foreign jurisdictions including Australia, Canada, China, France, Germany, Spain, United Kingdom and Japan. A U.S. patent covering nirogacestat as a composition of matter has a statutory expiration date in 2025 and a U.S. composition of matter patent that covers the polymorphic form of nirogacestat that is currently in clinical development