
Transforming Potential into Reality I-Mab Biopharma 1H 2024 Results August 28, 2024

Disclaimer Legal Disclaimer. This presentation has been prepared by I-Mab (the “Company”) solely for informational purposes. Certain of the information included herein was obtained from various sources, including certain third parties, and has not been independently verified by the Company. By viewing or accessing the information contained in this presentation, you hereby acknowledge and agree that no representations, warranties, or undertakings, express or implied, are made by the Company or any of its directors, shareholders, employees, agents, affiliates, advisors, or representatives as to, and no reliance should be placed on the truth, accuracy, fairness, completeness, or reasonableness of the information or opinions presented or contained in, and omission from, this presentation. Neither the Company nor any of its directors, employees, agents, affiliates, advisors, or representatives shall be responsible or liable whatsoever (in negligence or otherwise) for any loss, howsoever arising from any information presented or contained in this presentation or otherwise arising in connection with the presentation, except to the extent required by applicable law. The information presented or contained in this presentation speaks only as of the date hereof and is subject to change without notice. No Offer or Solicitation. This presentation does not constitute an offer to buy or sell or a solicitation of an offer to buy or sell any securities or instrument of the Company or to participate in any investment activity or trading strategy, nor may it or any part of it form the basis of or to be relied on in connection with any contract or commitment whatsoever. NOTHING HEREIN CONSTITUTES AN OFFER TO SELL OR THE SOLICITATION OF AN OFFER TO BUY ANY SECURITIES OR INSTRUMENT IN ANY STATE OR JURISDICTION. This presentation does not purport to and does not contain all relevant information relating to the Company or its securities, particularly with respect to the risks and special considerations involved with an investment in the securities of the Company. Nothing contained in this presentation shall be relied upon as a promise or representation as to the past or future performance of the Company. Past performance does not guarantee or predict future performance. You acknowledge that any assessment of the Company that may be made by you will be independent of this presentation and that you will be solely responsible for your own assessment of the market and the market position of the Company, and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of the business of the Company. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties, and our own estimates of potential market opportunities. All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research, and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. Forward Looking Statements. This presentation contains forward-looking statements. These statements are made under the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as “future”, “promising”, “may”, “plans”, “potential”, “will”, “could position”, “promise”, “advance”, “target”, “design”, “strategy”, “pipeline”, and “project”, and similar terms or the negative thereof. Statements that are not historical facts, including statements about I-Mab's beliefs and expectations, are forward-looking statements. The forward-looking statements in this presentation include, without limitation, statements regarding the following: the Company’s pipeline and capital strategy; the potential benefits, advantages, promise, attributes, and target usage of uliledlimab; the projected advancement of the Company’s portfolio and anticipated milestones and related timing; the market opportunity and I-Mab’s potential next steps (including the potential expansion, differentiation, or commercialization) for uliledlimab, givastomig and ragistomig; the Company’s expectations regarding the impact of data from ongoing and future trials; the timing of the settlement of remaining redemption obligations; the benefits of the Company’s collaboration with development partners; the Company’s ability to continue to comply with certain audit requirements; the timing and progress of studies (including with respect to patient enrollment and dosing); the availability of data and information from ongoing studies; and the Company’s expectations regarding its cash runway. These forward-looking statements involve inherent risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such forward-looking statements. These risks and uncertainties include, but are not limited to, the following: I-Mab’s ability to demonstrate the safety and efficacy of its drug candidates; the clinical results for its drug candidates, which may or may not support further development or new drug application/biologics license application approval; the content and timing of decisions made by the relevant regulatory authorities regarding regulatory approval of I-Mab’s drug candidates; I-Mab’s ability to achieve commercial success for its drug candidates, if approved; I-Mab’s ability to obtain and maintain protection of intellectual property for its technology and drugs; I-Mab’s reliance on third parties to conduct drug development, manufacturing and other services; I-Mab’s limited operating history and I-Mab’s ability to obtain additional funding for operations and to complete the development and commercialization of its drug candidates; and discussions of potential risks, uncertainties, and other important factors in I-Mab’s most recent annual report on Form 20-F and I-Mab’s subsequent filings with the U.S. Securities and Exchange Commission (the “SEC”). I-Mab may also make written or oral forward-looking statements in its periodic reports to the SEC, in its annual report to shareholders, in press releases and other written materials, and in oral statements made by its officers, directors, or employees to third parties. All forward-looking statements are based on information currently available to I-Mab. I-Mab undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law.
Agenda CEO Remarks and Introduction, Sean Fu R&D Strategy, Phillip Dennis Closing Remarks, Sean Fu Finance and Transition Update, Joseph Skelton

Significant Progress Towards Becoming a U.S.-Based Biotech Completed divestiture of China operations; closed on 04/02/2024 Extinguished ~$200M of redemption obligations and significantly reduced operating expenses; remaining ~$15M of redemption obligations to be settled in Sept-20241 New streamlined organization with U.S. leadership team: key hires in 1H24 included CEO, CFO, and CMO; optimized workforce of 34 FTEs2 Completed transition to U.S.-based auditors, appointed PwC U.S. effective 08/07/2024 I-Mab well positioned to execute on existing pipeline In response to arbitration commenced against I-Mab Biopharma Hong Kong Limited and included in divestiture related expenses, I-Mab placed $17.5M into escrow for future settlement of redemption obligations. As of the date of this presentation, $17.3M of redemption obligations have been settled from funds placed into escrow leaving the remaining obligations to be settled of ~$15M Full-time employees as of 06/30/2024 Strategically evaluating assets with best-in-class potential
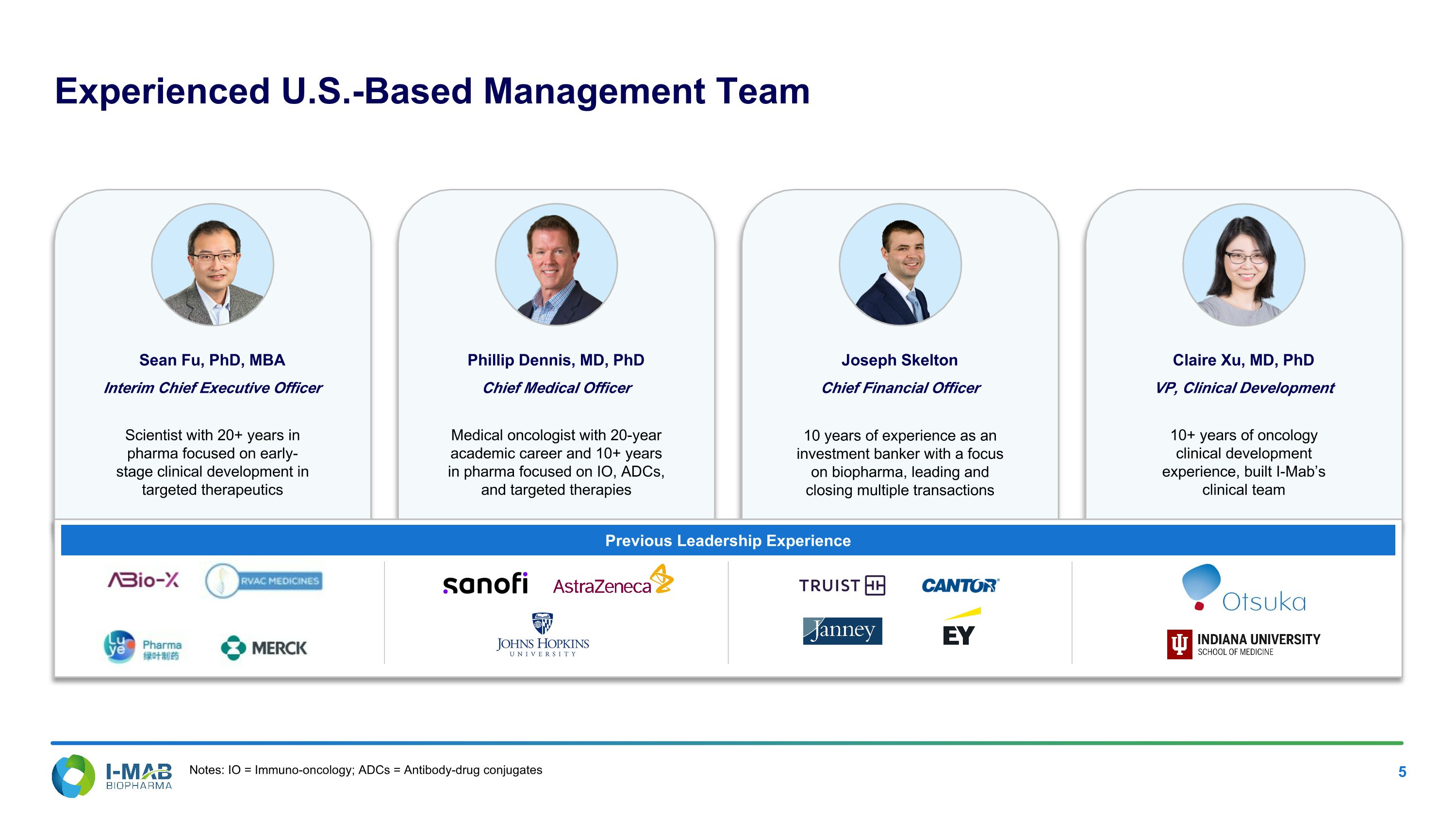
Experienced U.S.-Based Management Team Sean Fu, PhD, MBA Interim Chief Executive Officer Scientist with 20+ years in pharma focused on early-stage clinical development in targeted therapeutics Previous Leadership Experience Phillip Dennis, MD, PhD Chief Medical Officer Medical oncologist with 20-year academic career and 10+ years in pharma focused on IO, ADCs, and targeted therapies Joseph Skelton Chief Financial Officer 10 years of experience as an investment banker with a focus on biopharma, leading and closing multiple transactions Claire Xu, MD, PhD VP, Clinical Development 10+ years of oncology clinical development experience, built I-Mab’s clinical team Notes: IO = Immuno-oncology; ADCs = Antibody-drug conjugates
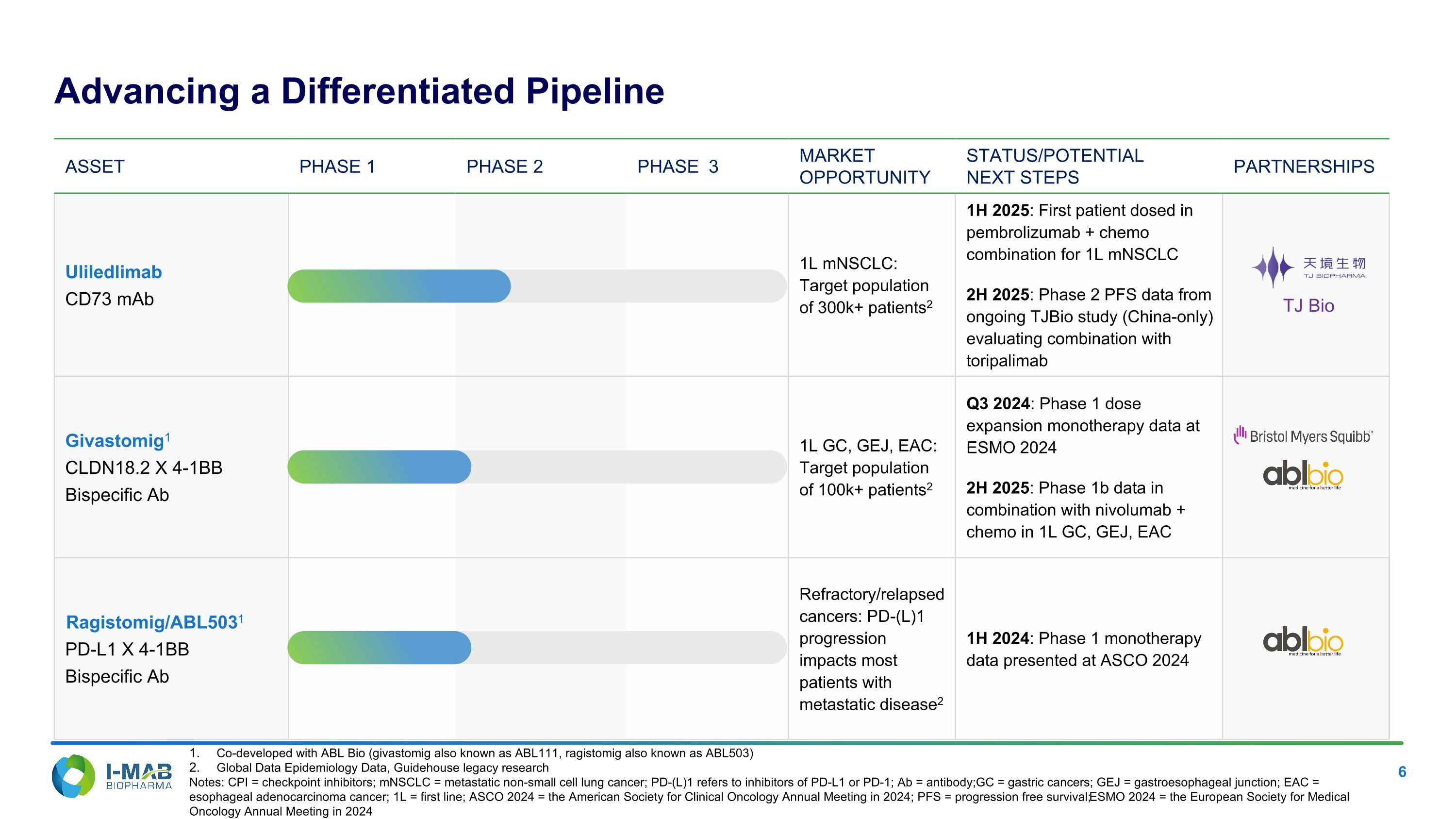
Asset PHASE 1 PHASE 2 PHASE 3 MARKET OPPORTUNITY STATUS/POTENTIAL NEXT STEPS PARTNERSHIPS Uliledlimab CD73 mAb 1L mNSCLC: Target population of 300k+ patients2 1H 2025: First patient dosed in pembrolizumab + chemo combination for 1L mNSCLC 2H 2025: Phase 2 PFS data from ongoing TJBio study (China-only) evaluating combination with toripalimab Givastomig1 CLDN18.2 X 4-1BB Bispecific Ab 1L GC, GEJ, EAC: Target population of 100k+ patients2 Q3 2024: Phase 1 dose expansion monotherapy data at ESMO 2024 2H 2025: Phase 1b data in combination with nivolumab + chemo in 1L GC, GEJ, EAC Ragistomig/ABL5031 PD-L1 X 4-1BB Bispecific Ab Refractory/relapsed cancers: PD-(L)1 progression impacts most patients with metastatic disease2 1H 2024: Phase 1 monotherapy data presented at ASCO 2024 Co-developed with ABL Bio (givastomig also known as ABL111, ragistomig also known as ABL503) Global Data Epidemiology Data, Guidehouse legacy research Notes: CPI = checkpoint inhibitors; mNSCLC = metastatic non-small cell lung cancer; PD-(L)1 refers to inhibitors of PD-L1 or PD-1; Ab = antibody; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma cancer; 1L = first line; ASCO 2024 = the American Society for Clinical Oncology Annual Meeting in 2024; PFS = progression free survival; ESMO 2024 = the European Society for Medical Oncology Annual Meeting in 2024 Advancing a Differentiated Pipeline TJ Bio
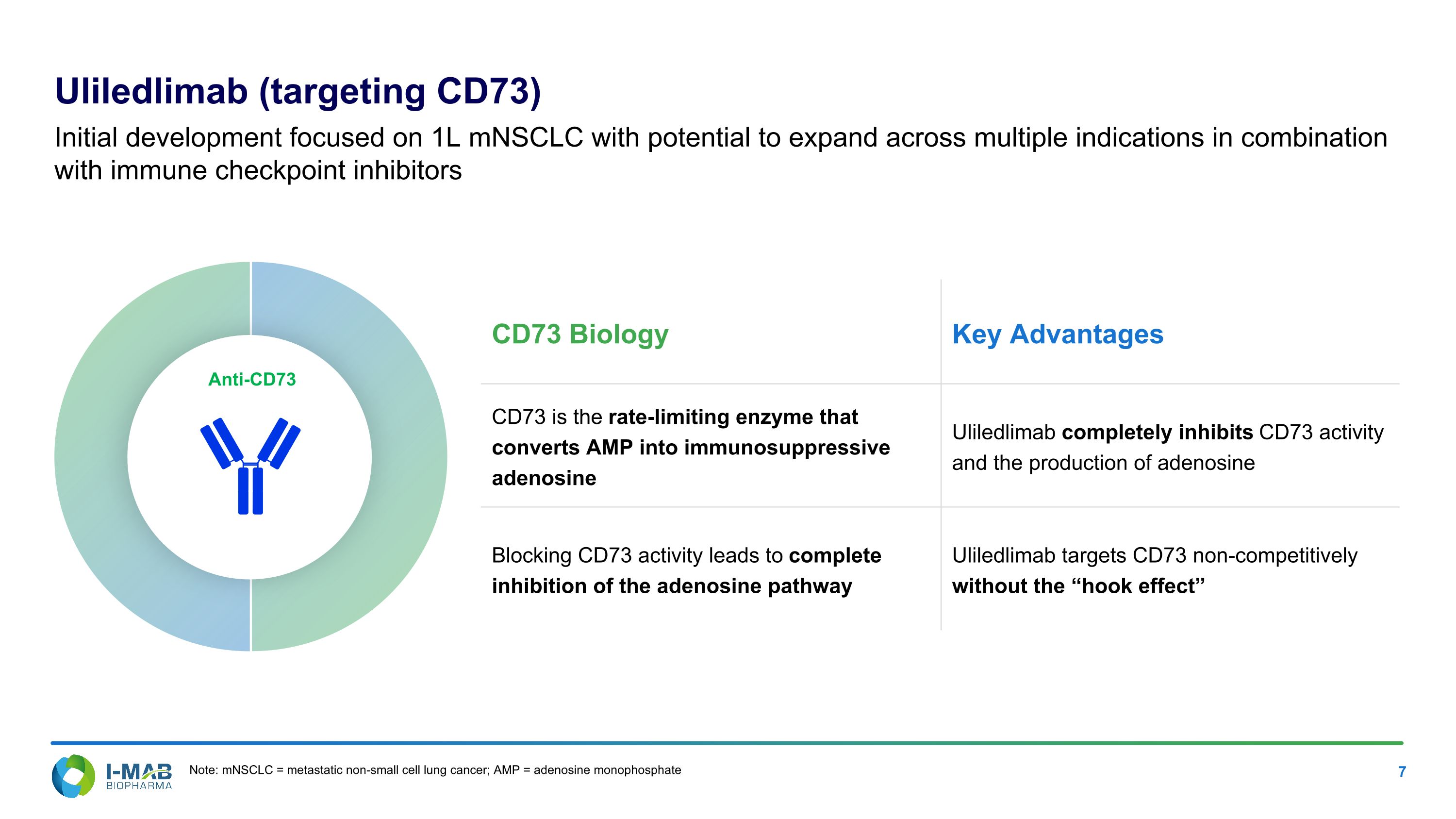
Uliledlimab (targeting CD73) Initial development focused on 1L mNSCLC with potential to expand across multiple indications in combination with immune checkpoint inhibitors Anti-CD73 CD73 Biology Key Advantages CD73 is the rate-limiting enzyme that converts AMP into immunosuppressive adenosine Uliledlimab completely inhibits CD73 activity and the production of adenosine Blocking CD73 activity leads to complete inhibition of the adenosine pathway Uliledlimab targets CD73 non-competitively without the “hook effect” Note: mNSCLC = metastatic non-small cell lung cancer; AMP = adenosine monophosphate
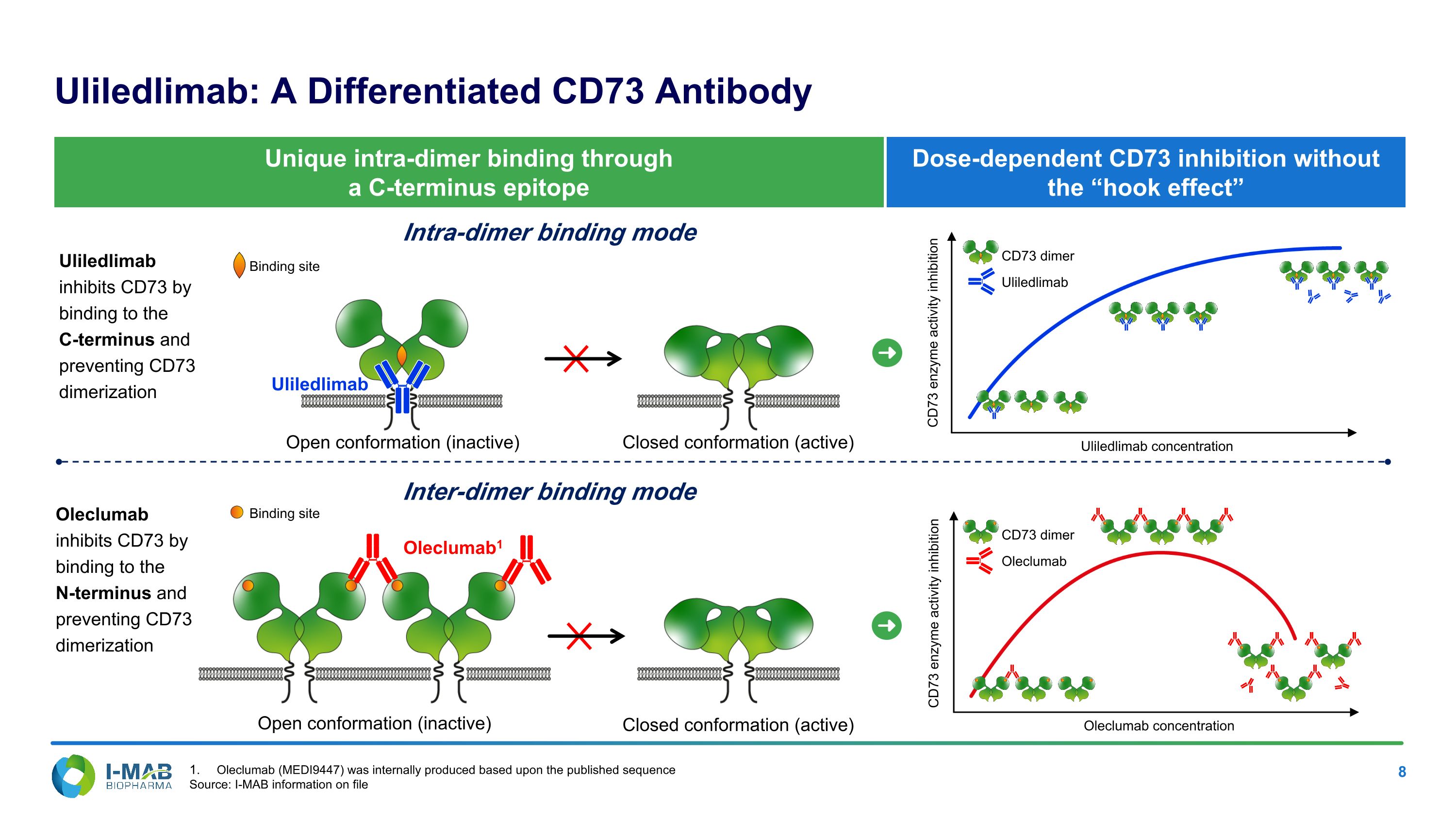
CD73 enzyme activity inhibition Dose-dependent CD73 inhibition without the “hook effect” Uliledlimab: A Differentiated CD73 Antibody Open conformation (inactive) Closed conformation (active) Oleclumab1 Intra-dimer binding mode Inter-dimer binding mode Open conformation (inactive) Closed conformation (active) Unique intra-dimer binding through a C-terminus epitope Uliledlimab inhibits CD73 by binding to the C-terminus and preventing CD73 dimerization Oleclumab inhibits CD73 by binding to the N-terminus and preventing CD73 dimerization Uliledlimab CD73 enzyme activity inhibition Uliledlimab concentration Oleclumab concentration Uliledlimab CD73 dimer Oleclumab CD73 dimer Binding site Binding site Oleclumab (MEDI9447) was internally produced based upon the published sequence Source: I-MAB information on file
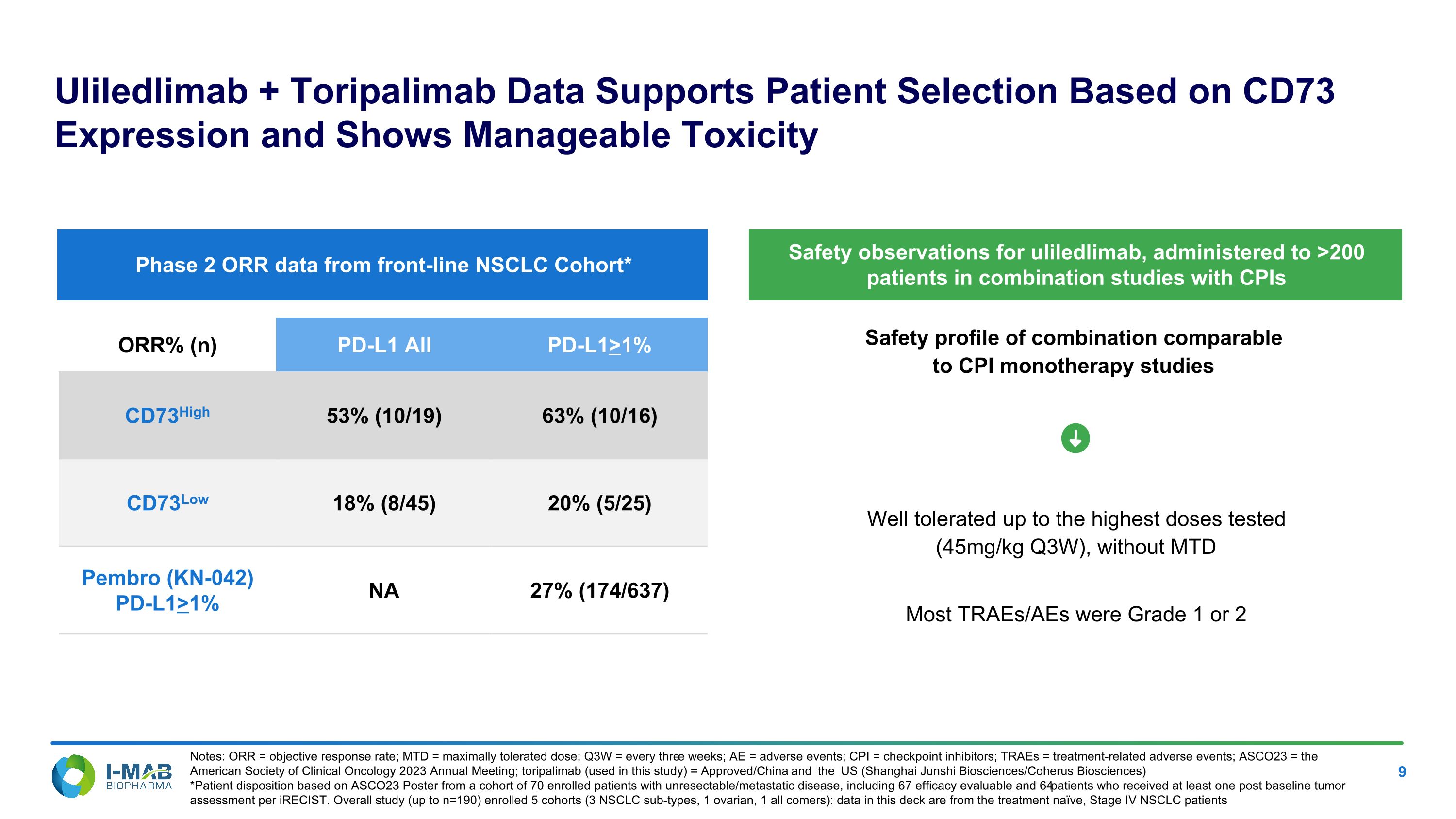
Safety profile of combination comparable �to CPI monotherapy studies Uliledlimab + Toripalimab Data Supports Patient Selection Based on CD73 Expression and Shows Manageable Toxicity Notes: ORR = objective response rate; MTD = maximally tolerated dose; Q3W = every three weeks; AE = adverse events; CPI = checkpoint inhibitors; TRAEs = treatment-related adverse events; ASCO23 = the American Society of Clinical Oncology 2023 Annual Meeting; toripalimab (used in this study) = Approved/China and the US (Shanghai Junshi Biosciences/Coherus Biosciences) *Patient disposition based on ASCO23 Poster from a cohort of 70 enrolled patients with unresectable/metastatic disease, including 67 efficacy evaluable and 64 patients who received at least one post baseline tumor assessment per iRECIST. Overall study (up to n=190) enrolled 5 cohorts (3 NSCLC sub-types, 1 ovarian, 1 all comers): data in this deck are from the treatment naïve, Stage IV NSCLC patients Well tolerated up to the highest doses tested �(45mg/kg Q3W), without MTD Most TRAEs/AEs were Grade 1 or 2 ORR% (n) PD-L1 All PD-L1>1% CD73High 53% (10/19) 63% (10/16) CD73Low 18% (8/45) 20% (5/25) Pembro (KN-042) PD-L1>1% NA 27% (174/637) Phase 2 ORR data from front-line NSCLC Cohort* Safety observations for uliledlimab, administered to >200 patients in combination studies with CPIs
Rationale to Support Uliledlimab + Pembro + Chemotherapy in 1L mNSCLC Samanta D, Park Y, Ni XH, Semenza G. 2017. Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. PNAS Vol. 115, No 6. Notes: mNSCLC = metastatic non-small cell lung cancer; IO = Immuno-oncology The addition of chemotherapy to IO monotherapy extends the benefit of IO to lower levels of PD-L1 expression Uliledlimab has a favorable toxicity profile in combination with IO agents Chemotherapy induces CD73 expression suggesting additional benefit by combining uliledlimab with pembrolizumab + chemotherapy1 Based on this rationale I-Mab plans to dose the first patient with uliledlimab in combination with pembrolizumab + chemotherapy in newly diagnosed patients with mNSCLC in 1H 2025
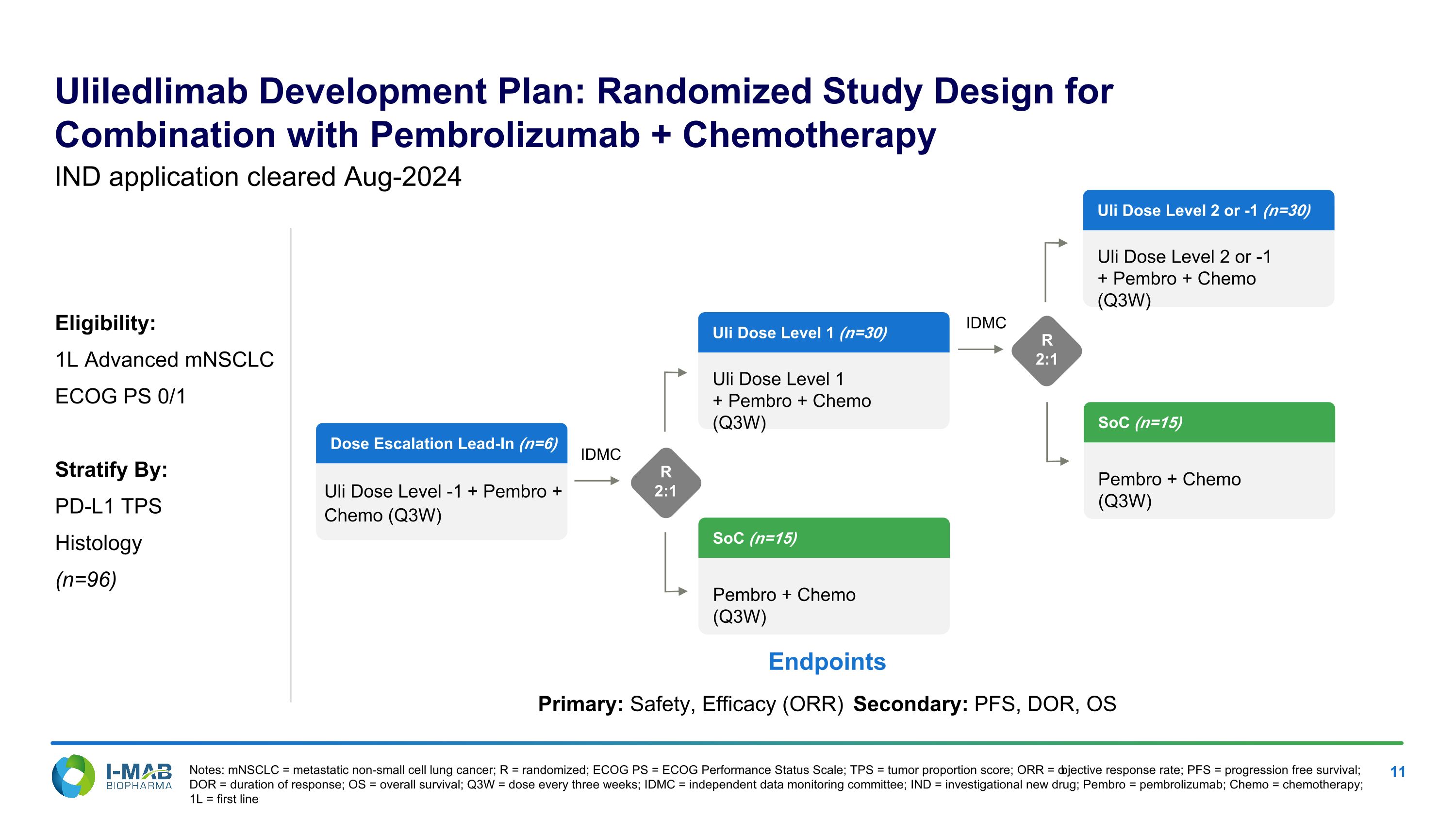
Uliledlimab Development Plan: Randomized Study Design for Combination with Pembrolizumab + Chemotherapy Notes: mNSCLC = metastatic non-small cell lung cancer; R = randomized; ECOG PS = ECOG Performance Status Scale; TPS = tumor proportion score; ORR = objective response rate; PFS = progression free survival; DOR = duration of response; OS = overall survival; Q3W = dose every three weeks; IDMC = independent data monitoring committee; IND = investigational new drug; Pembro = pembrolizumab; Chemo = chemotherapy; 1L = first line IND application cleared Aug-2024 Eligibility: 1L Advanced mNSCLC ECOG PS 0/1 Stratify By: PD-L1 TPS Histology (n=96) Endpoints Primary: Safety, Efficacy (ORR) Secondary: PFS, DOR, OS IDMC Dose Escalation Lead-In (n=6) Uli Dose Level -1 + Pembro + Chemo (Q3W) SoC (n=15) Pembro + Chemo (Q3W) Uli Dose Level 1 (n=30) Uli Dose Level 1 + Pembro + Chemo (Q3W) R 2:1 IDMC R 2:1 Uli Dose Level 2 or -1 (n=30) Uli Dose Level 2 or -1 + Pembro + Chemo (Q3W) SoC (n=15) Pembro + Chemo (Q3W)
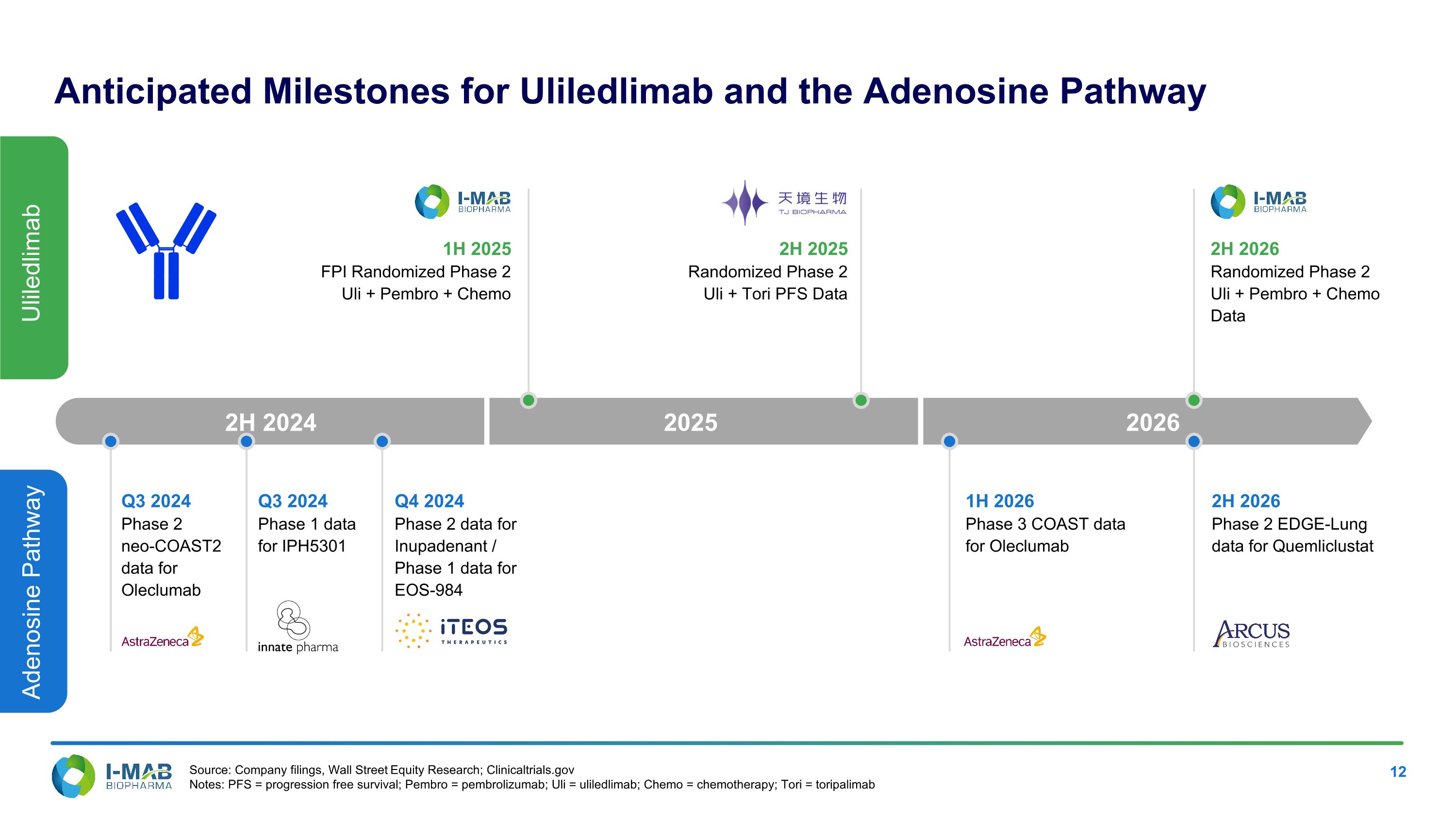
Anticipated Milestones for Uliledlimab and the Adenosine Pathway Source: Company filings, Wall Street Equity Research; Clinicaltrials.gov Notes: PFS = progression free survival; Pembro = pembrolizumab; Uli = uliledlimab; Chemo = chemotherapy; Tori = toripalimab Uliledlimab Adenosine Pathway 2H 2025 Randomized Phase 2 Uli + Tori PFS Data 2H 2026 Randomized Phase 2 Uli + Pembro + Chemo Data 1H 2026 Phase 3 COAST data for Oleclumab 2H 2026 Phase 2 EDGE-Lung data for Quemliclustat 2H 2024 2025 2026 Q4 2024 Phase 2 data for Inupadenant / Phase 1 data for EOS-984 1H 2025 FPI Randomized Phase 2 Uli + Pembro + Chemo Q3 2024 Phase 1 data for IPH5301 Q3 2024 Phase 2 neo-COAST2 data for Oleclumab
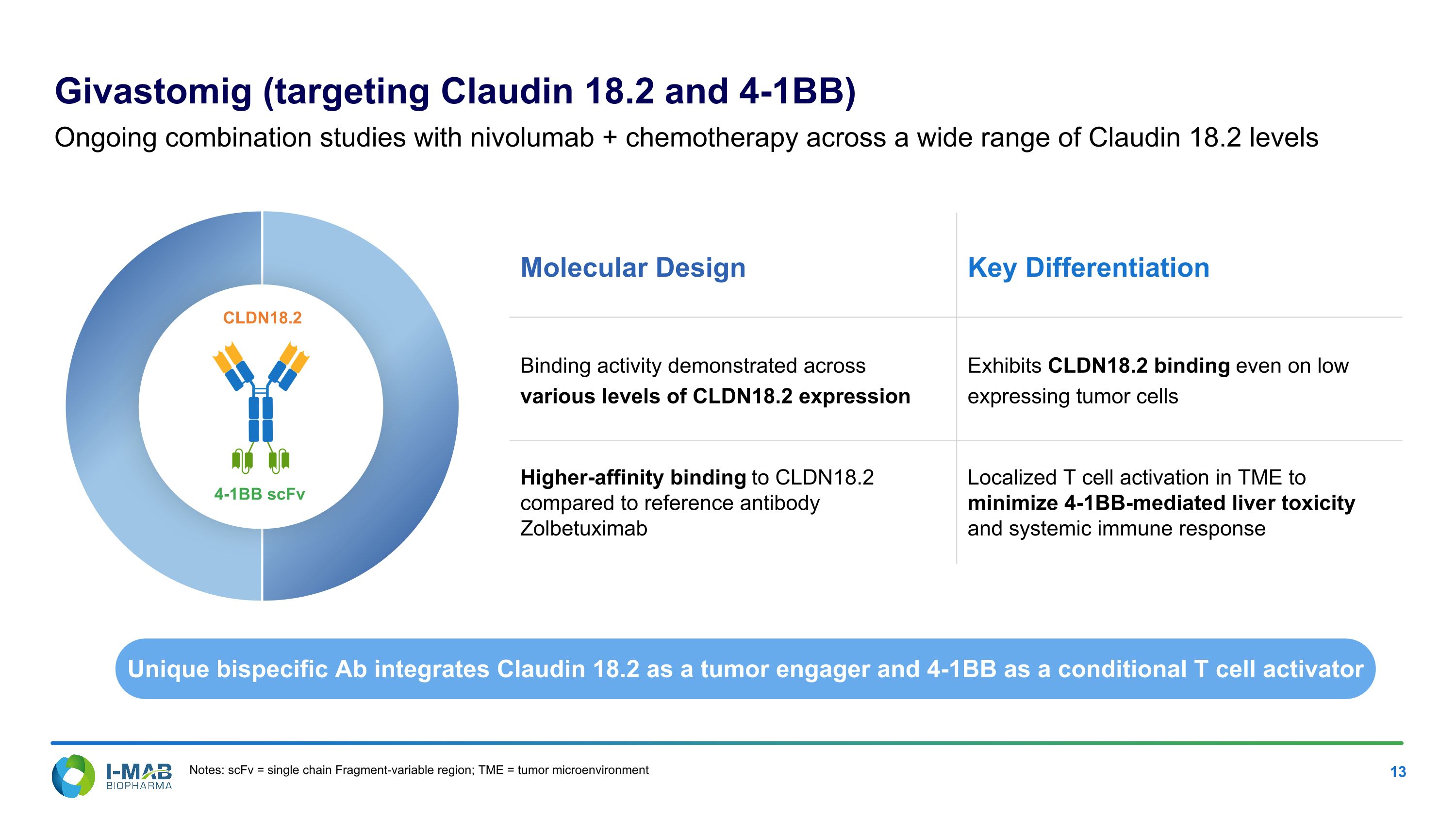
Molecular Design Key Differentiation Binding activity demonstrated across various levels of CLDN18.2 expression Exhibits CLDN18.2 binding even on low expressing tumor cells Higher-affinity binding to CLDN18.2 compared to reference antibody Zolbetuximab Localized T cell activation in TME to minimize 4-1BB-mediated liver toxicity and systemic immune response Givastomig (targeting Claudin 18.2 and 4-1BB) Ongoing combination studies with nivolumab + chemotherapy across a wide range of Claudin 18.2 levels Unique bispecific Ab integrates Claudin 18.2 as a tumor engager and 4-1BB as a conditional T cell activator 4-1BB scFv CLDN18.2 Notes: scFv = single chain Fragment-variable region; TME = tumor microenvironment
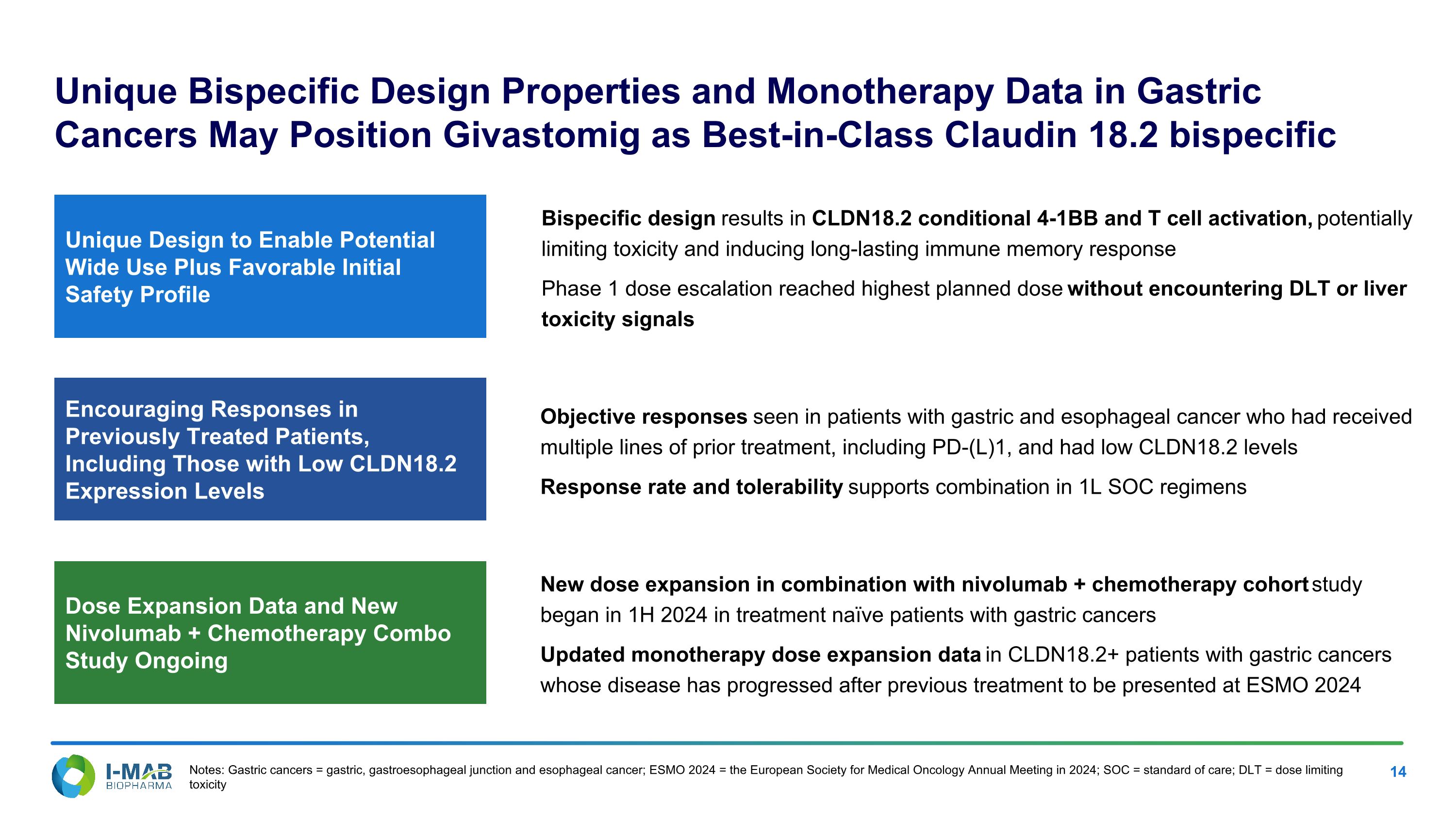
Unique Design to Enable Potential Wide Use Plus Favorable Initial Safety Profile Encouraging Responses in Previously Treated Patients, Including Those with Low CLDN18.2 Expression Levels Dose Expansion Data and New Nivolumab + Chemotherapy Combo Study Ongoing Unique Bispecific Design Properties and Monotherapy Data in Gastric Cancers May Position Givastomig as Best-in-Class Claudin 18.2 bispecific Objective responses seen in patients with gastric and esophageal cancer who had received multiple lines of prior treatment, including PD-(L)1, and had low CLDN18.2 levels Response rate and tolerability supports combination in 1L SOC regimens New dose expansion in combination with nivolumab + chemotherapy cohort study began in 1H 2024 in treatment naïve patients with gastric cancers Updated monotherapy dose expansion data in CLDN18.2+ patients with gastric cancers whose disease has progressed after previous treatment to be presented at ESMO 2024 Bispecific design results in CLDN18.2 conditional 4-1BB and T cell activation, potentially limiting toxicity and inducing long-lasting immune memory response Phase 1 dose escalation reached highest planned dose without encountering DLT or liver toxicity signals Notes: Gastric cancers = gastric, gastroesophageal junction and esophageal cancer; ESMO 2024 = the European Society for Medical Oncology Annual Meeting in 2024; SOC = standard of care; DLT = dose limiting toxicity
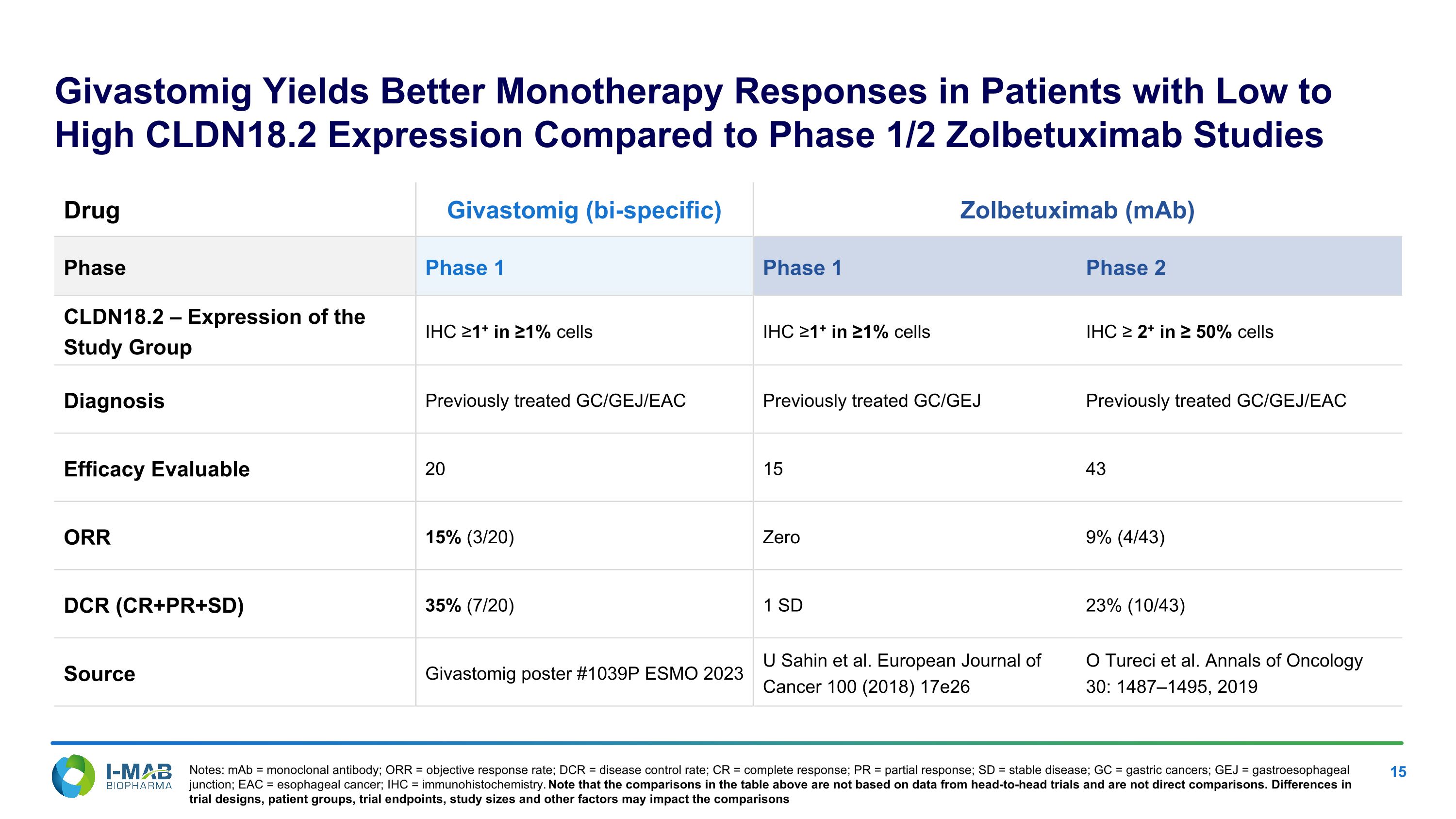
Givastomig Yields Better Monotherapy Responses in Patients with Low to High CLDN18.2 Expression Compared to Phase 1/2 Zolbetuximab Studies Drug Givastomig (bi-specific) Zolbetuximab (mAb) Phase Phase 1 Phase 1 Phase 2 CLDN18.2 – Expression of the Study Group IHC ≥1+ in ≥1% cells IHC ≥1+ in ≥1% cells IHC ≥ 2+ in ≥ 50% cells Diagnosis Previously treated GC/GEJ/EAC Previously treated GC/GEJ Previously treated GC/GEJ/EAC Efficacy Evaluable 20 15 43 ORR 15% (3/20) Zero 9% (4/43) DCR (CR+PR+SD) 35% (7/20) 1 SD 23% (10/43) Source Givastomig poster #1039P ESMO 2023 U Sahin et al. European Journal of Cancer 100 (2018) 17e26 O Tureci et al. Annals of Oncology �30: 1487–1495, 2019 Notes: mAb = monoclonal antibody; ORR = objective response rate; DCR = disease control rate; CR = complete response; PR = partial response; SD = stable disease; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal cancer; IHC = immunohistochemistry. Note that the comparisons in the table above are not based on data from head-to-head trials and are not direct comparisons. Differences in trial designs, patient groups, trial endpoints, study sizes and other factors may impact the comparisons
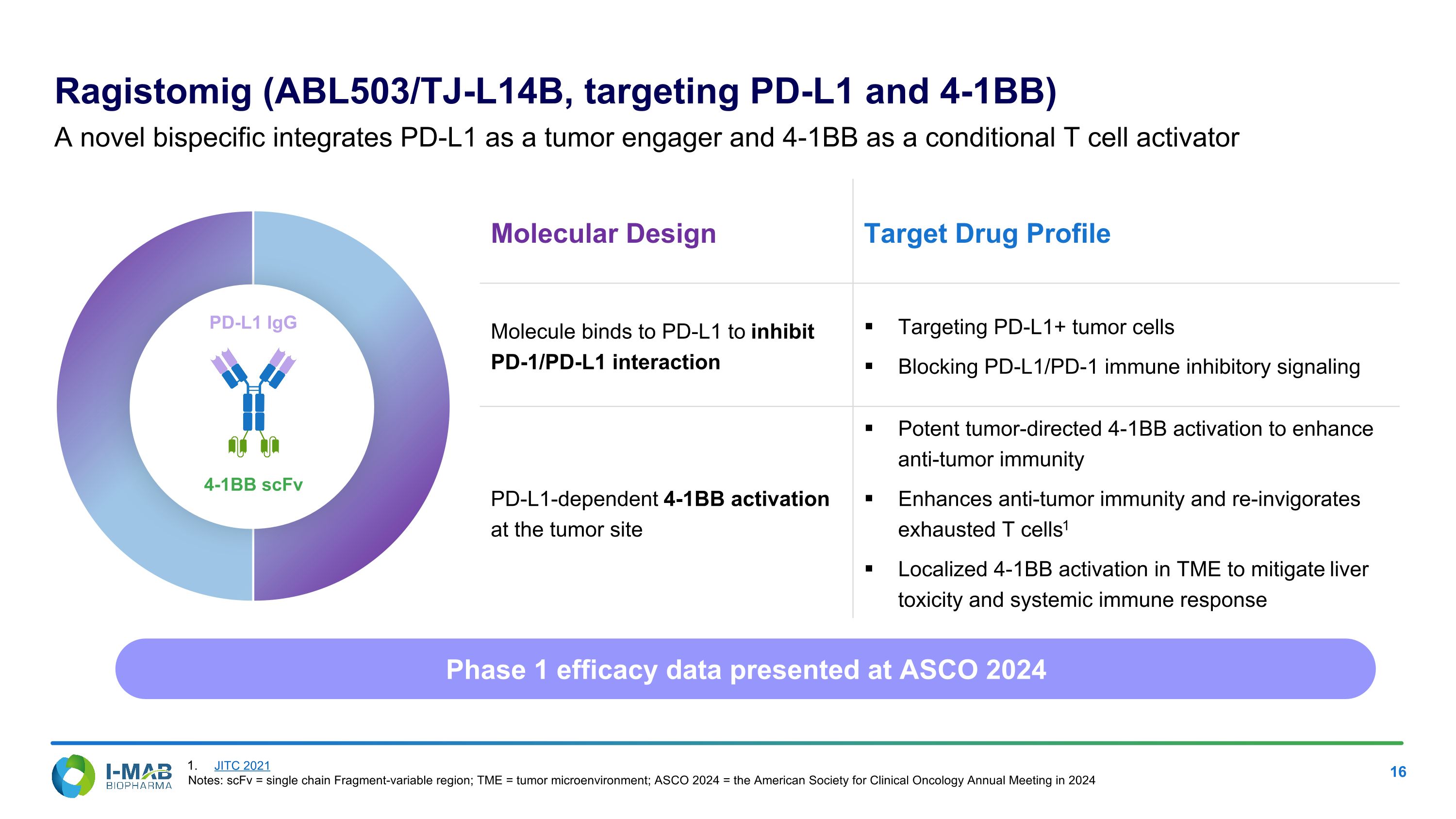
Molecular Design Target Drug Profile Molecule binds to PD-L1 to inhibit PD-1/PD-L1 interaction Targeting PD-L1+ tumor cells Blocking PD-L1/PD-1 immune inhibitory signaling PD-L1-dependent 4-1BB activation at the tumor site Potent tumor-directed 4-1BB activation to enhance anti-tumor immunity Enhances anti-tumor immunity and re-invigorates exhausted T cells1 Localized 4-1BB activation in TME to mitigate liver toxicity and systemic immune response Ragistomig (ABL503/TJ-L14B, targeting PD-L1 and 4-1BB) A novel bispecific integrates PD-L1 as a tumor engager and 4-1BB as a conditional T cell activator 4-1BB scFv PD-L1 IgG Phase 1 efficacy data presented at ASCO 2024 JITC 2021 Notes: scFv = single chain Fragment-variable region; TME = tumor microenvironment; ASCO 2024 = the American Society for Clinical Oncology Annual Meeting in 2024
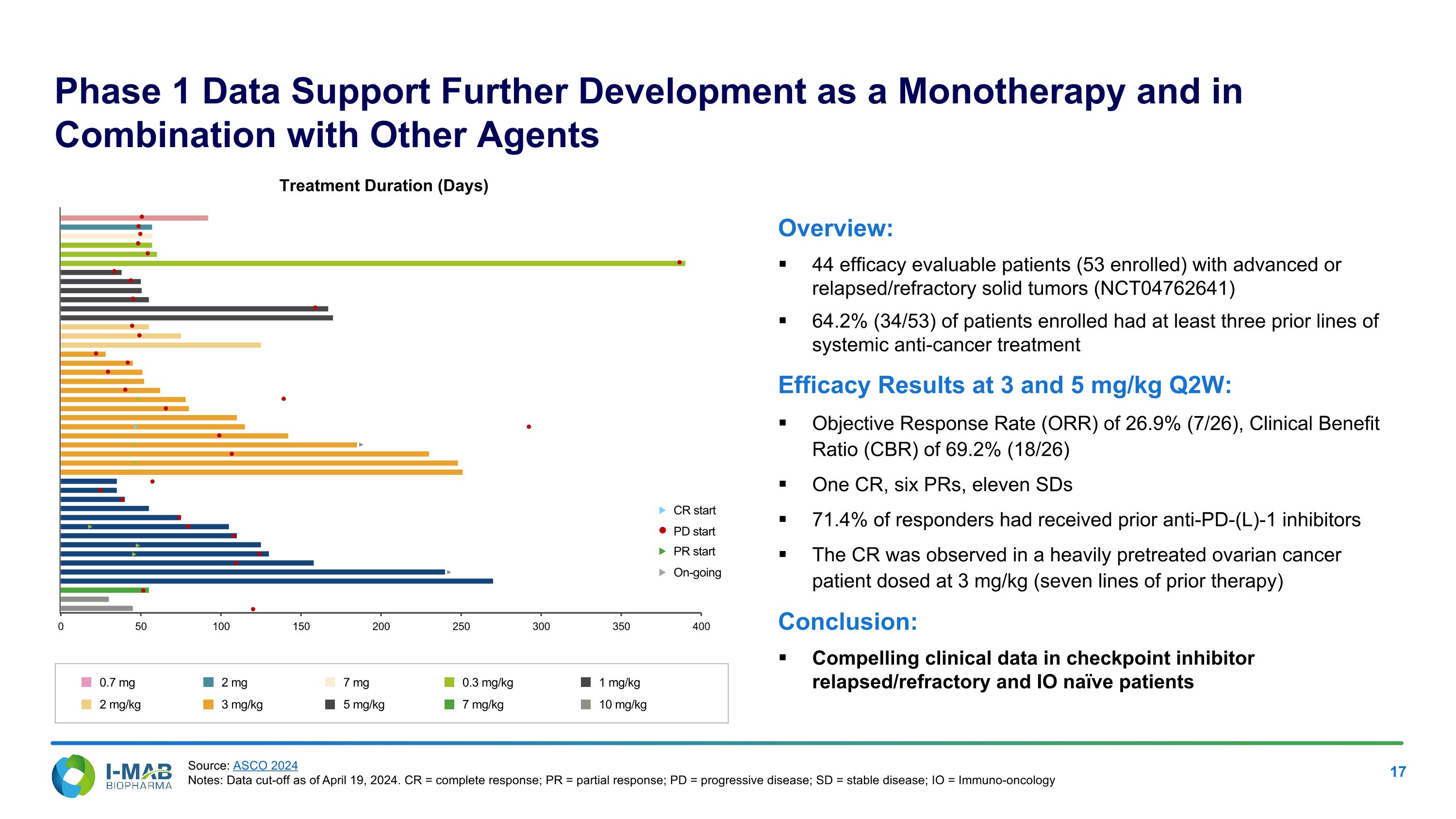
Phase 1 Data Support Further Development as a Monotherapy and in Combination with Other Agents Overview: 44 efficacy evaluable patients (53 enrolled) with advanced or relapsed/refractory solid tumors (NCT04762641) 64.2% (34/53) of patients enrolled had at least three prior lines of systemic anti-cancer treatment Efficacy Results at 3 and 5 mg/kg Q2W: Objective Response Rate (ORR) of 26.9% (7/26), Clinical Benefit Ratio (CBR) of 69.2% (18/26) One CR, six PRs, eleven SDs 71.4% of responders had received prior anti-PD-(L)-1 inhibitors The CR was observed in a heavily pretreated ovarian cancer patient dosed at 3 mg/kg (seven lines of prior therapy) Conclusion: Compelling clinical data in checkpoint inhibitor relapsed/refractory and IO naïve patients Treatment Duration (Days) CR start PR start On-going PD start 0.7 mg 2 mg/kg 2 mg 3 mg/kg 7 mg 5 mg/kg 0.3 mg/kg 7 mg/kg 1 mg/kg 10 mg/kg Source: ASCO 2024 Notes: Data cut-off as of April 19, 2024. CR = complete response; PR = partial response; PD = progressive disease; SD = stable disease; IO = Immuno-oncology
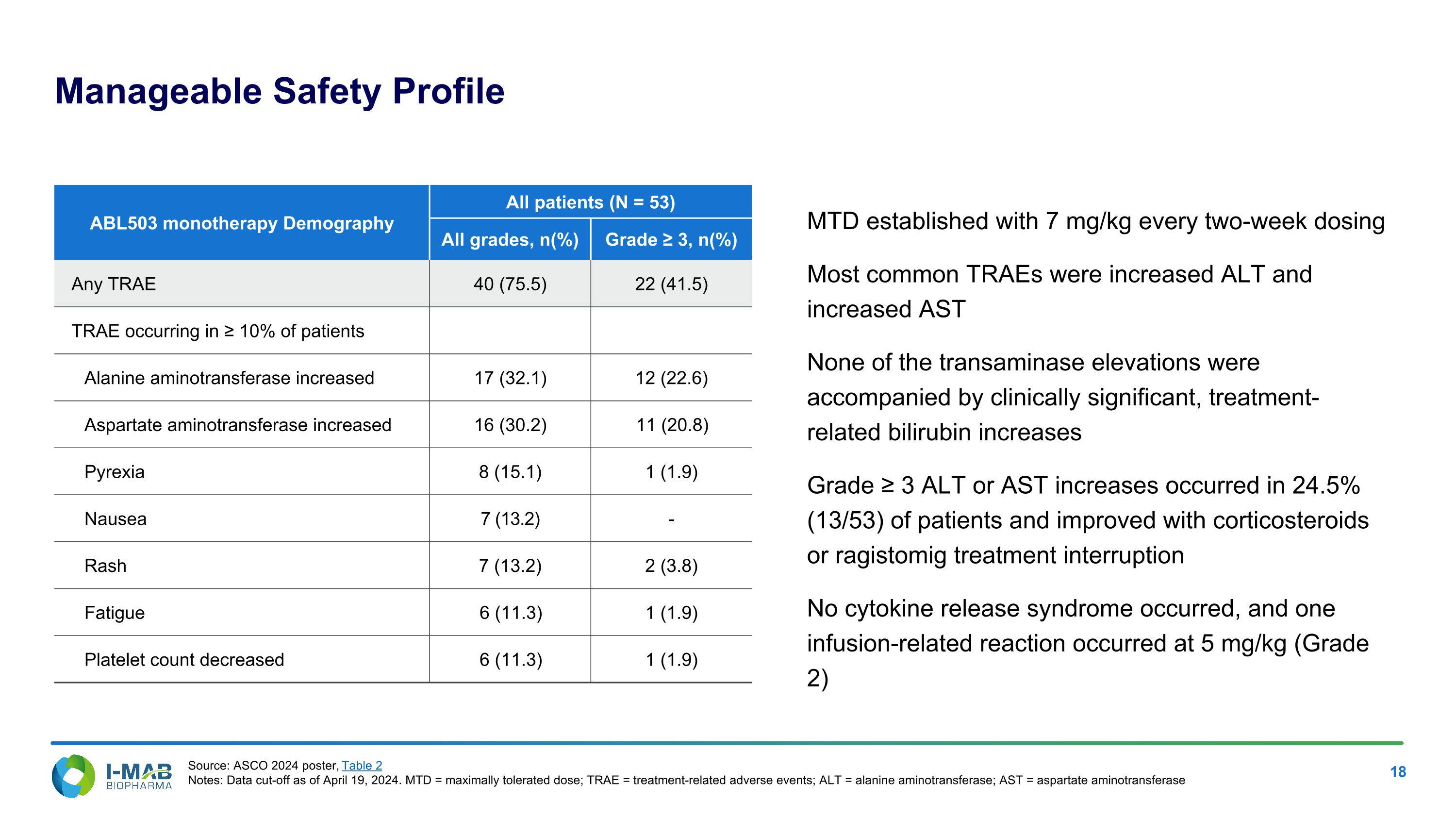
Manageable Safety Profile MTD established with 7 mg/kg every two-week dosing Most common TRAEs were increased ALT and increased AST None of the transaminase elevations were accompanied by clinically significant, treatment-related bilirubin increases Grade ≥ 3 ALT or AST increases occurred in 24.5% (13/53) of patients and improved with corticosteroids or ragistomig treatment interruption No cytokine release syndrome occurred, and one infusion-related reaction occurred at 5 mg/kg (Grade 2) ABL503 monotherapy Demography All patients (N = 53) All grades, n(%) Grade ≥ 3, n(%) Any TRAE 40 (75.5) 22 (41.5) TRAE occurring in ≥ 10% of patients Alanine aminotransferase increased 17 (32.1) 12 (22.6) Aspartate aminotransferase increased 16 (30.2) 11 (20.8) Pyrexia 8 (15.1) 1 (1.9) Nausea 7 (13.2) - Rash 7 (13.2) 2 (3.8) Fatigue 6 (11.3) 1 (1.9) Platelet count decreased 6 (11.3) 1 (1.9) Source: ASCO 2024 poster, Table 2 Notes: Data cut-off as of April 19, 2024. MTD = maximally tolerated dose; TRAE = treatment-related adverse events; ALT = alanine aminotransferase; AST = aspartate aminotransferase
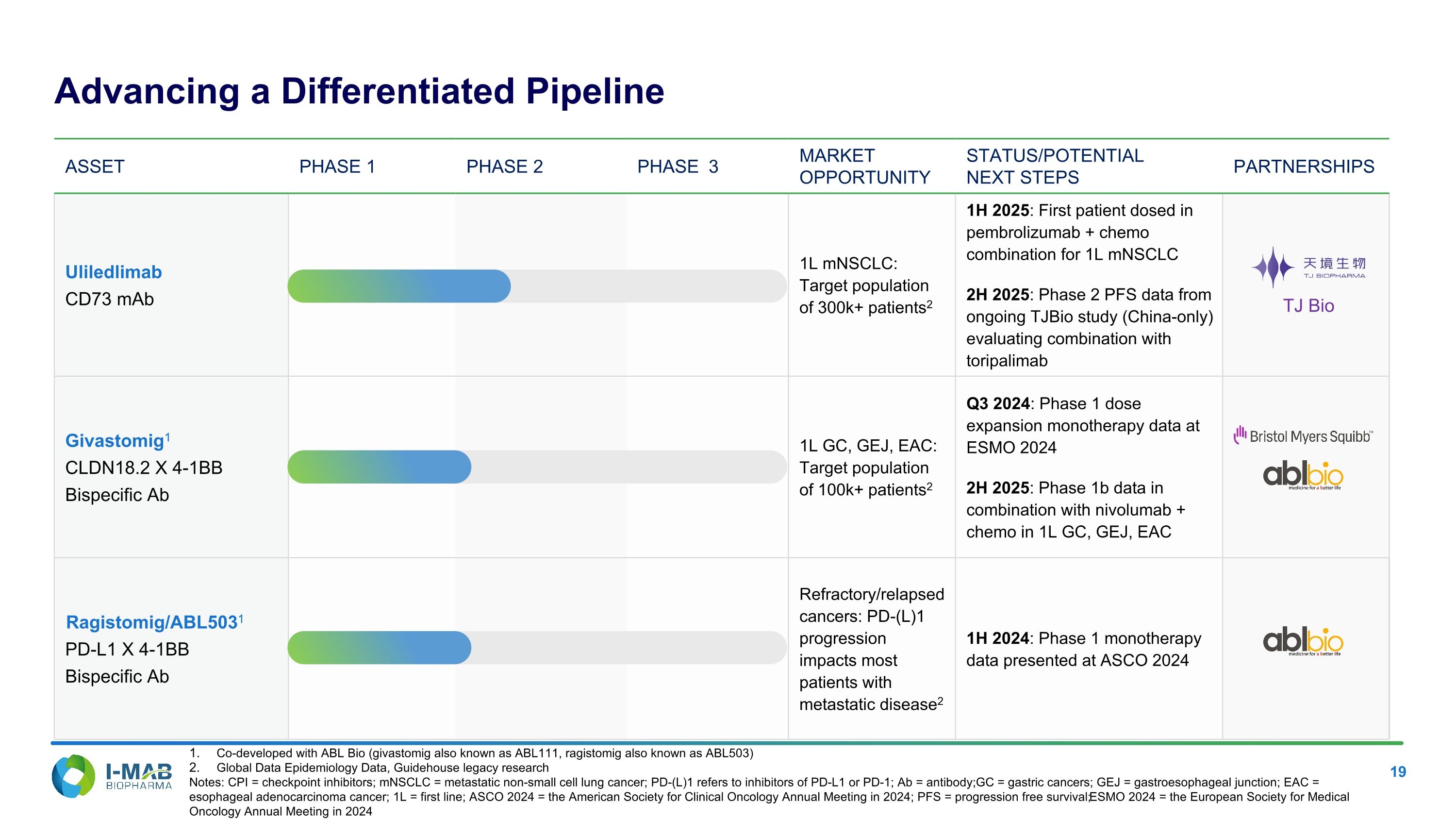
Asset PHASE 1 PHASE 2 PHASE 3 MARKET OPPORTUNITY STATUS/POTENTIAL NEXT STEPS PARTNERSHIPS Uliledlimab CD73 mAb 1L mNSCLC: Target population of 300k+ patients2 1H 2025: First patient dosed in pembrolizumab + chemo combination for 1L mNSCLC 2H 2025: Phase 2 PFS data from ongoing TJBio study (China-only) evaluating combination with toripalimab Givastomig1 CLDN18.2 X 4-1BB Bispecific Ab 1L GC, GEJ, EAC: Target population of 100k+ patients2 Q3 2024: Phase 1 dose expansion monotherapy data at ESMO 2024 2H 2025: Phase 1b data in combination with nivolumab + chemo in 1L GC, GEJ, EAC Ragistomig/ABL5031 PD-L1 X 4-1BB Bispecific Ab Refractory/relapsed cancers: PD-(L)1 progression impacts most patients with metastatic disease2 1H 2024: Phase 1 monotherapy data presented at ASCO 2024 Co-developed with ABL Bio (givastomig also known as ABL111, ragistomig also known as ABL503) Global Data Epidemiology Data, Guidehouse legacy research Notes: CPI = checkpoint inhibitors; mNSCLC = metastatic non-small cell lung cancer; PD-(L)1 refers to inhibitors of PD-L1 or PD-1; Ab = antibody; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma cancer; 1L = first line; ASCO 2024 = the American Society for Clinical Oncology Annual Meeting in 2024; PFS = progression free survival; ESMO 2024 = the European Society for Medical Oncology Annual Meeting in 2024 Advancing a Differentiated Pipeline TJ Bio
Strong Corporate Development Progress and Reduction in Expenditures Full-time employees as of 12/31/2023 per the 20-F Full-time employees as of 06/30/2024 Entered into clinical collaboration with Bristol Myers Squibb to evaluate Claudin 18.2 x 4-1BB bispecific antibody givastomig in combination with nivolumab + chemotherapy for the treatment of gastric and esophageal cancer Under the terms of the agreement, the study will be a multi-national Phase 1b study conducted by I-Mab. Bristol Myers Squibb will supply nivolumab Strengthened Study and Secured Drug Supply Engaged PwC as U.S. auditors Enables I-Mab to continue to comply with the audit requirements of the Holding Foreign Companies Accountable Act Engaged U.S. Auditors Streamlined workforce from 220 FTEs1 to 34 FTEs2 Senior leadership team all based in the U.S. Workforce primarily based in the U.S. Optimized Workforce Focused on advancing uliledlimab into Phase 2 in the U.S. and continuing advancement of givastomig through its Phase 1b Divestiture removed two ongoing Phase 3 trials in China (felzartamab and eftansomatropin alfa) Streamlined Pipeline Extinguished ~$200M of potential ~$215M redemption obligations Expect to settle the remaining redemption obligations of ~$15M in Sep-2024 Extinguishment of Redemption Obligations
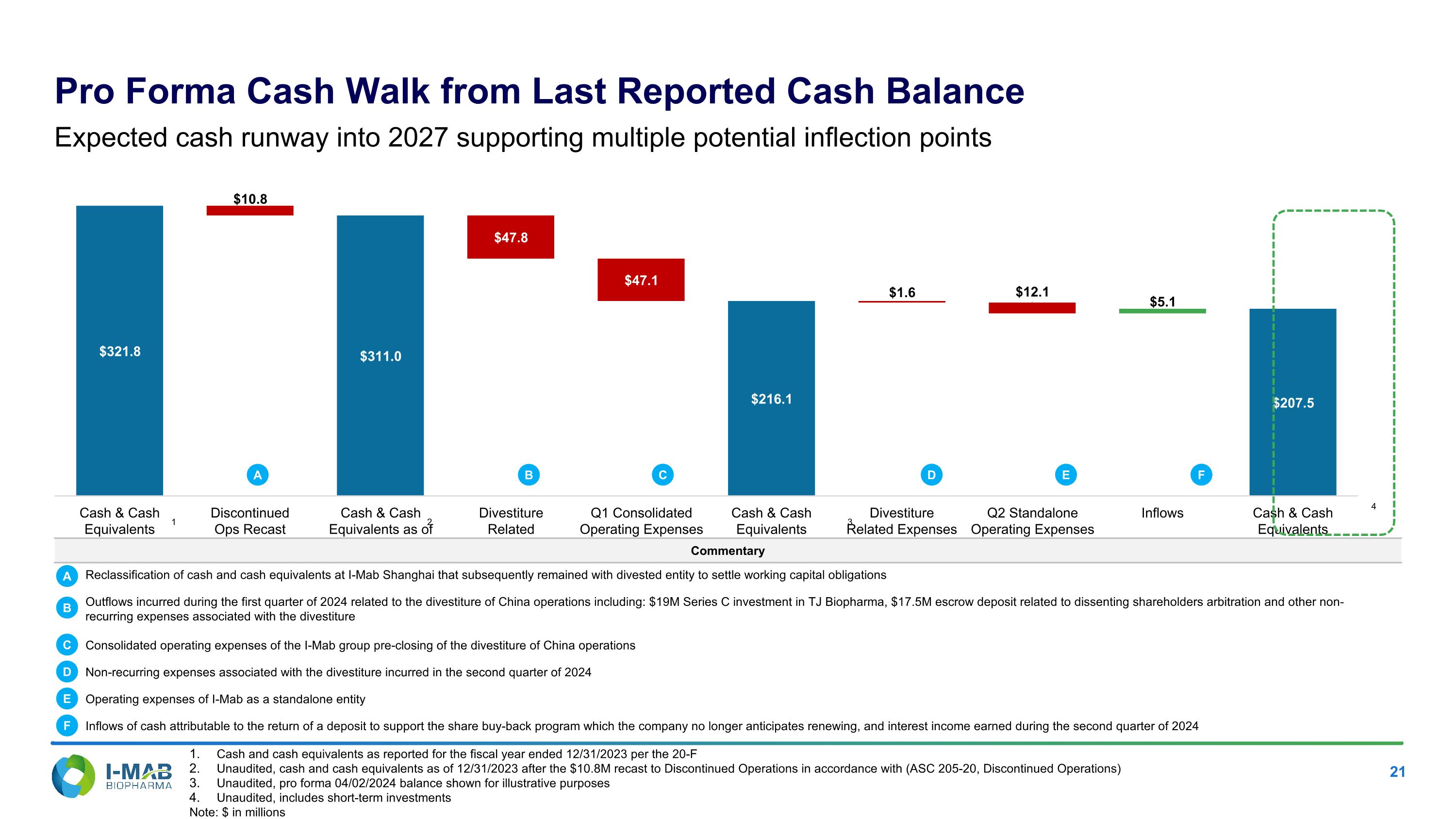
Reclassification of cash and cash equivalents at I-Mab Shanghai that subsequently remained with divested entity to settle working capital obligations Outflows incurred during the first quarter of 2024 related to the divestiture of China operations including: $19M Series C investment in TJ Biopharma, $17.5M escrow deposit related to dissenting shareholders arbitration and other non-recurring expenses associated with the divestiture Consolidated operating expenses of the I-Mab group pre-closing of the divestiture of China operations Non-recurring expenses associated with the divestiture incurred in the second quarter of 2024 Operating expenses of I-Mab as a standalone entity Inflows of cash attributable to the return of a deposit to support the share buy-back program which the company no longer anticipates renewing, and interest income earned during the second quarter of 2024 Pro Forma Cash Walk from Last Reported Cash Balance Cash and cash equivalents as reported for the fiscal year ended 12/31/2023 per the 20-F Unaudited, cash and cash equivalents as of 12/31/2023 after the $10.8M recast to Discontinued Operations in accordance with (ASC 205-20, Discontinued Operations) Unaudited, pro forma 04/02/2024 balance shown for illustrative purposes Unaudited, includes short-term investments Note: $ in millions 1 Commentary A A B C D E F B C D E F 2 4 Expected cash runway into 2027 supporting multiple potential inflection points 3
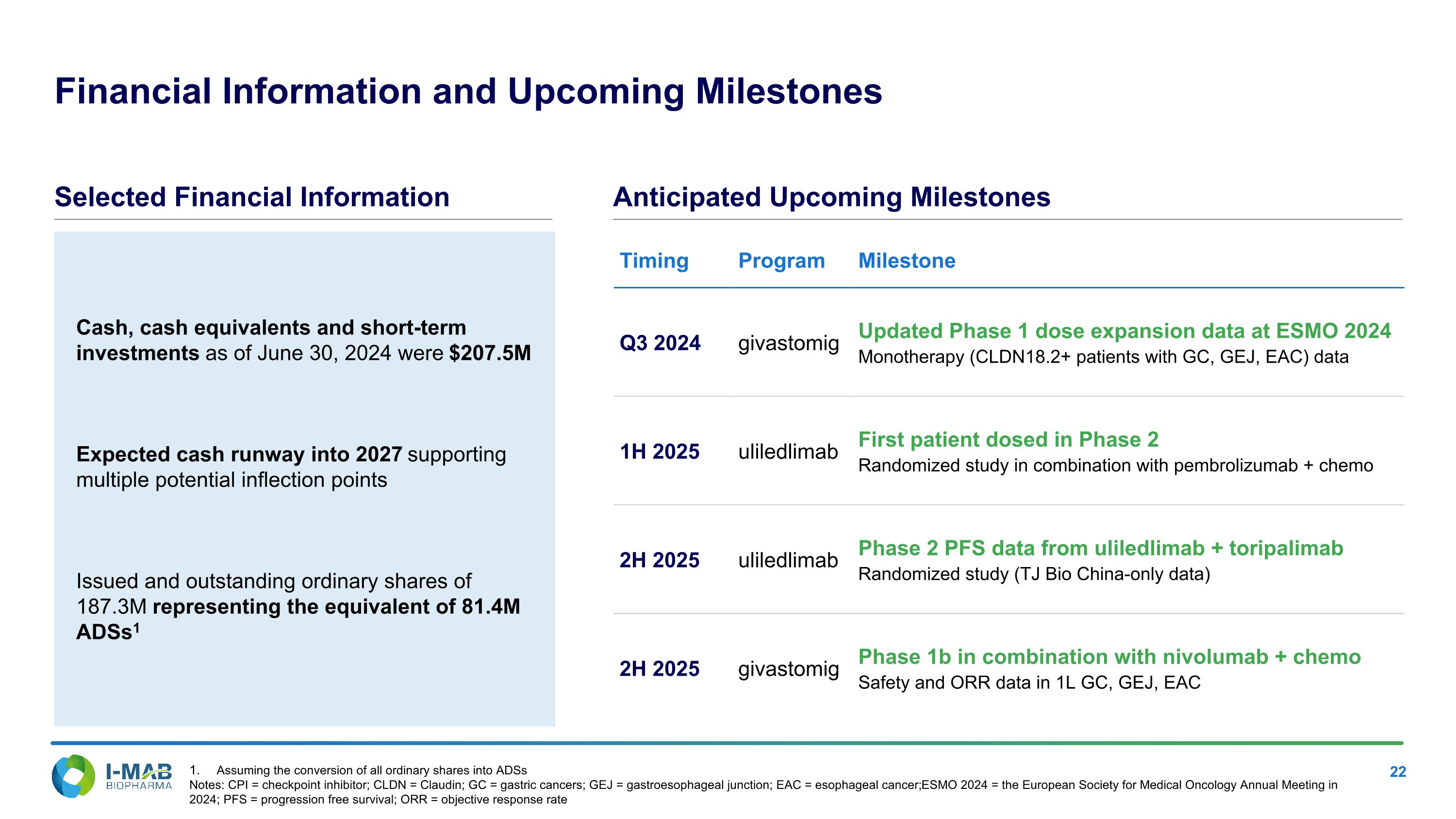
Assuming the conversion of all ordinary shares into ADSs Notes: CPI = checkpoint inhibitor; CLDN = Claudin; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal cancer; ESMO 2024 = the European Society for Medical Oncology Annual Meeting in 2024; PFS = progression free survival; ORR = objective response rate Cash, cash equivalents and short-term investments as of June 30, 2024 were $207.5M Expected cash runway into 2027 supporting multiple potential inflection points Issued and outstanding ordinary shares of 187.3M representing the equivalent of 81.4M ADSs1 Financial Information and Upcoming Milestones Timing Program Milestone Q3 2024 givastomig Updated Phase 1 dose expansion data at ESMO 2024 Monotherapy (CLDN18.2+ patients with GC, GEJ, EAC) data 1H 2025 uliledlimab First patient dosed in Phase 2 Randomized study in combination with pembrolizumab + chemo 2H 2025 uliledlimab Phase 2 PFS data from uliledlimab + toripalimab Randomized study (TJ Bio China-only data) 2H 2025 givastomig Phase 1b in combination with nivolumab + chemo Safety and ORR data in 1L GC, GEJ, EAC Selected Financial Information Anticipated Upcoming Milestones

Stay connected Q&A IR@imabbio.com Contact Us






















