
Transforming Potential into Reality I-Mab Biopharma August 28, 2024

Legal Disclaimer. This presentation has been prepared by I-Mab (the “Company”) solely for informational purposes. Certain of the information included herein was obtained from various sources, including certain third parties, and has not been independently verified by the Company. By viewing or accessing the information contained in this presentation, you hereby acknowledge and agree that no representations, warranties, or undertakings, express or implied, are made by the Company or any of its directors, shareholders, employees, agents, affiliates, advisors, or representatives as to, and no reliance should be placed on the truth, accuracy, fairness, completeness, or reasonableness of the information or opinions presented or contained in, and omission from, this presentation. Neither the Company nor any of its directors, employees, agents, affiliates, advisors, or representatives shall be responsible or liable whatsoever (in negligence or otherwise) for any loss, howsoever arising from any information presented or contained in this presentation or otherwise arising in connection with the presentation, except to the extent required by applicable law. The information presented or contained in this presentation speaks only as of the date hereof and is subject to change without notice. No Offer or Solicitation. This presentation does not constitute an offer to buy or sell or a solicitation of an offer to buy or sell any securities or instrument of the Company or to participate in any investment activity or trading strategy, nor may it or any part of it form the basis of or to be relied on in connection with any contract or commitment whatsoever. NOTHING HEREIN CONSTITUTES AN OFFER TO SELL OR THE SOLICITATION OF AN OFFER TO BUY ANY SECURITIES OR INSTRUMENT IN ANY STATE OR JURISDICTION. This presentation does not purport to and does not contain all relevant information relating to the Company or its securities, particularly with respect to the risks and special considerations involved with an investment in the securities of the Company. Nothing contained in this presentation shall be relied upon as a promise or representation as to the past or future performance of the Company. Past performance does not guarantee or predict future performance. You acknowledge that any assessment of the Company that may be made by you will be independent of this presentation and that you will be solely responsible for your own assessment of the market and the market position of the Company, and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of the business of the Company. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties, and our own estimates of potential market opportunities. All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research, and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. Forward Looking Statements. This presentation contains forward-looking statements. These statements are made under the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as “future”, “promising”, “may”, “plans”, “potential”, “will”, “could position”, “promise”, “advance”, “target”, “design”, “strategy”, “pipeline”, and “project”, and similar terms or the negative thereof. Statements that are not historical facts, including statements about I-Mab's beliefs and expectations, are forward-looking statements. The forward-looking statements in this presentation include, without limitation, statements regarding the following: the Company’s pipeline and capital strategy; the potential benefits, advantages, promise, attributes, and target usage of uliledlimab; the projected advancement of the Company’s portfolio and anticipated milestones and related timing; the market opportunity and I-Mab’s potential next steps (including the potential expansion, differentiation, or commercialization) for uliledlimab, givastomig and ragistomig; the Company’s expectations regarding the impact of data from ongoing and future trials; the timing of the settlement of remaining redemption obligations; the benefits of the Company’s collaboration with development partners; the Company’s ability to continue to comply with certain audit requirements; the timing and progress of studies (including with respect to patient enrollment and dosing); the availability of data and information from ongoing studies; and the Company’s expectations regarding its cash runway. These forward-looking statements involve inherent risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such forward-looking statements. These risks and uncertainties include, but are not limited to, the following: I-Mab’s ability to demonstrate the safety and efficacy of its drug candidates; the clinical results for its drug candidates, which may or may not support further development or new drug application/biologics license application approval; the content and timing of decisions made by the relevant regulatory authorities regarding regulatory approval of I-Mab’s drug candidates; I-Mab’s ability to achieve commercial success for its drug candidates, if approved; I-Mab’s ability to obtain and maintain protection of intellectual property for its technology and drugs; I-Mab’s reliance on third parties to conduct drug development, manufacturing and other services; I-Mab’s limited operating history and I-Mab’s ability to obtain additional funding for operations and to complete the development and commercialization of its drug candidates; and discussions of potential risks, uncertainties, and other important factors in I-Mab’s most recent annual report on Form 20-F and I-Mab’s subsequent filings with the U.S. Securities and Exchange Commission (the “SEC”). I-Mab may also make written or oral forward-looking statements in its periodic reports to the SEC, in its annual report to shareholders, in press releases and other written materials, and in oral statements made by its officers, directors, or employees to third parties. All forward-looking statements are based on information currently available to I-Mab. I-Mab undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law. Disclaimer
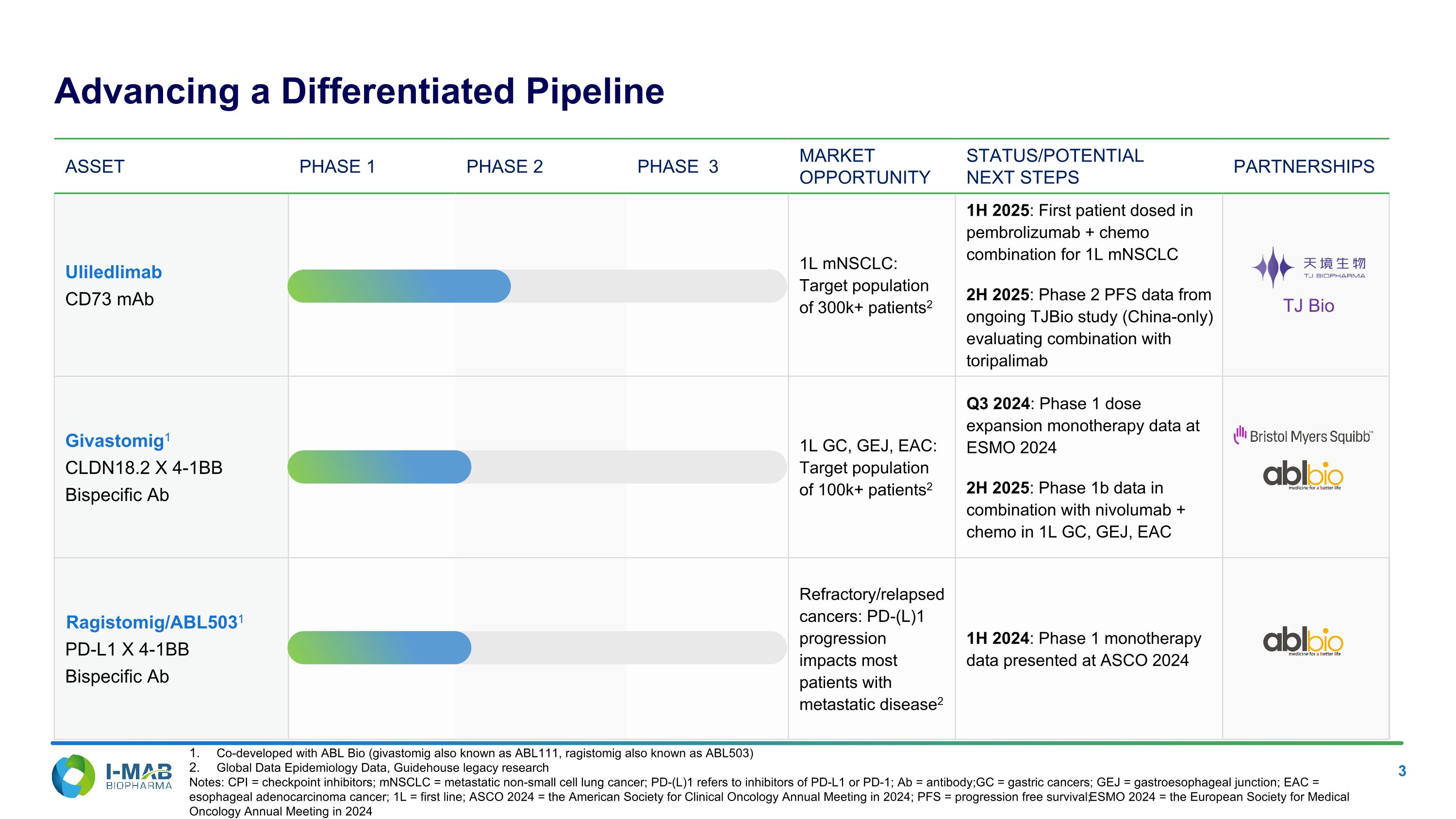
Asset PHASE 1 PHASE 2 PHASE 3 MARKET OPPORTUNITY STATUS/POTENTIAL NEXT STEPS PARTNERSHIPS Uliledlimab CD73 mAb 1L mNSCLC: Target population of 300k+ patients2 1H 2025: First patient dosed in pembrolizumab + chemo combination for 1L mNSCLC 2H 2025: Phase 2 PFS data from ongoing TJBio study (China-only) evaluating combination with toripalimab Givastomig1 CLDN18.2 X 4-1BB Bispecific Ab 1L GC, GEJ, EAC: Target population of 100k+ patients2 Q3 2024: Phase 1 dose expansion monotherapy data at ESMO 2024 2H 2025: Phase 1b data in combination with nivolumab + chemo in 1L GC, GEJ, EAC Ragistomig/ABL5031 PD-L1 X 4-1BB Bispecific Ab Refractory/relapsed cancers: PD-(L)1 progression impacts most patients with metastatic disease2 1H 2024: Phase 1 monotherapy data presented at ASCO 2024 Co-developed with ABL Bio (givastomig also known as ABL111, ragistomig also known as ABL503) Global Data Epidemiology Data, Guidehouse legacy research Notes: CPI = checkpoint inhibitors; mNSCLC = metastatic non-small cell lung cancer; PD-(L)1 refers to inhibitors of PD-L1 or PD-1; Ab = antibody; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma cancer; 1L = first line; ASCO 2024 = the American Society for Clinical Oncology Annual Meeting in 2024; PFS = progression free survival; ESMO 2024 = the European Society for Medical Oncology Annual Meeting in 2024 Advancing a Differentiated Pipeline TJ Bio
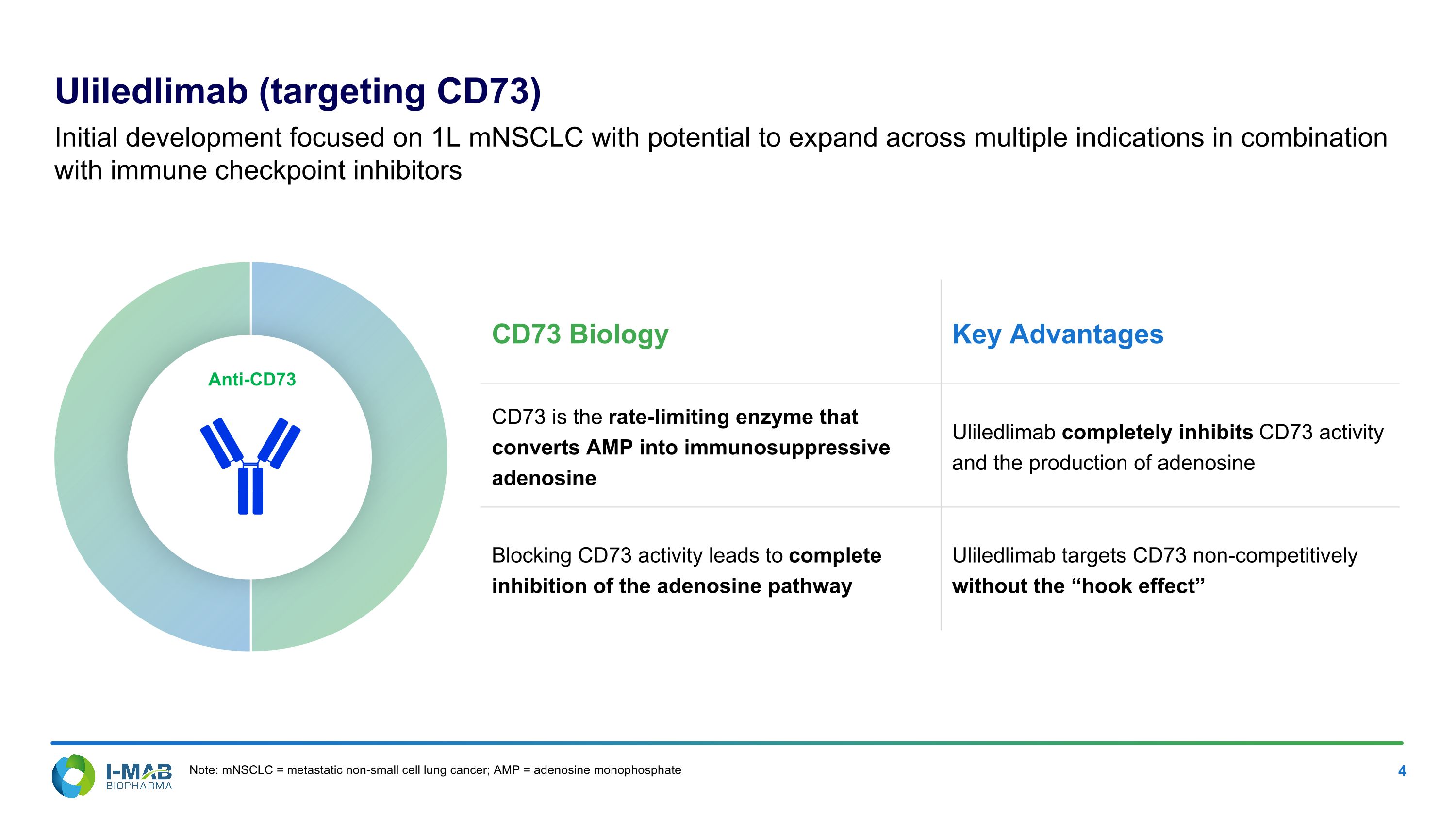
Uliledlimab (targeting CD73) Initial development focused on 1L mNSCLC with potential to expand across multiple indications in combination with immune checkpoint inhibitors Anti-CD73 CD73 Biology Key Advantages CD73 is the rate-limiting enzyme that converts AMP into immunosuppressive adenosine Uliledlimab completely inhibits CD73 activity and the production of adenosine Blocking CD73 activity leads to complete inhibition of the adenosine pathway Uliledlimab targets CD73 non-competitively without the “hook effect” Note: mNSCLC = metastatic non-small cell lung cancer; AMP = adenosine monophosphate
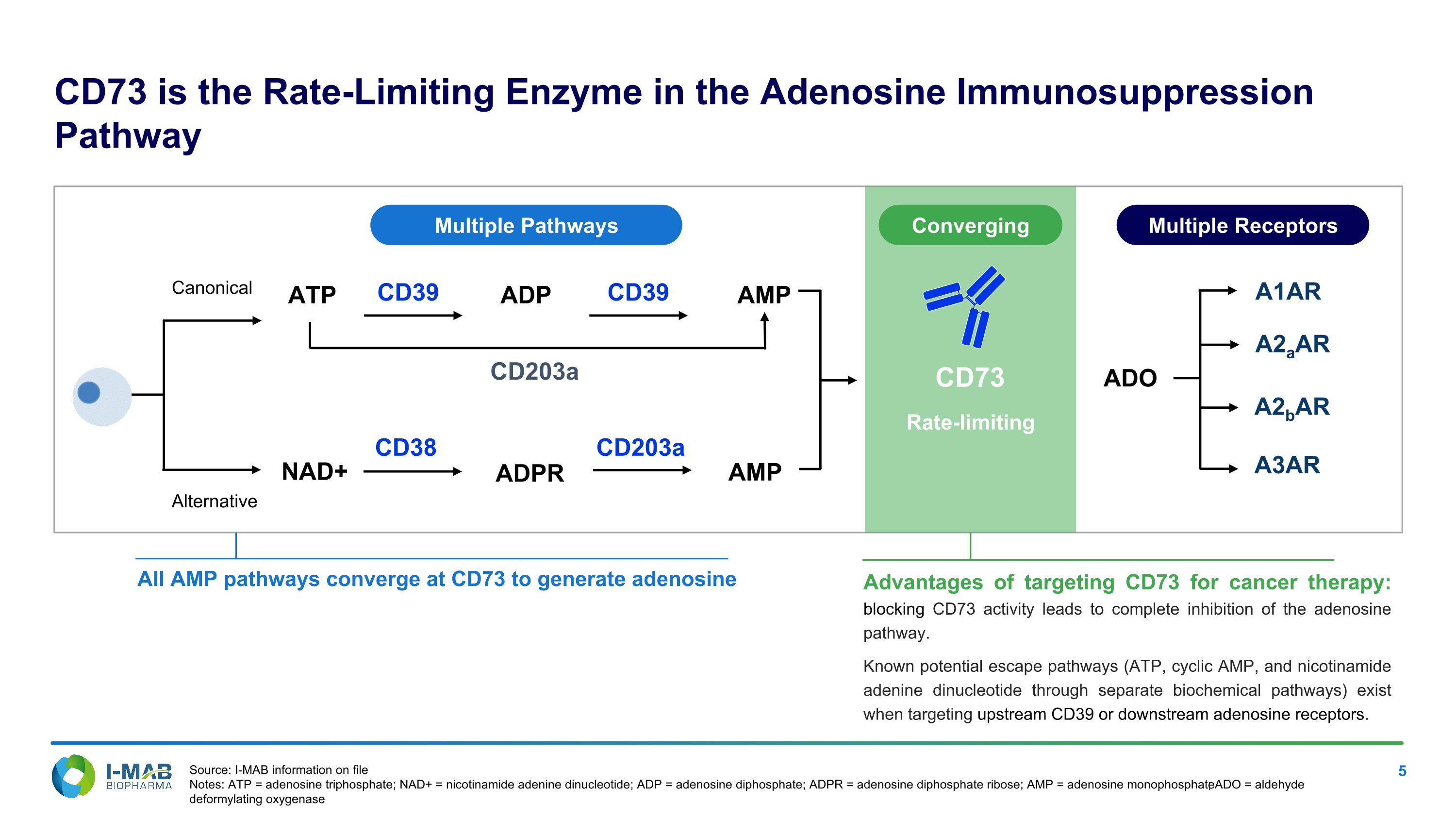
CD73 is the Rate-Limiting Enzyme in the Adenosine Immunosuppression Pathway All AMP pathways converge at CD73 to generate adenosine Advantages of targeting CD73 for cancer therapy: blocking CD73 activity leads to complete inhibition of the adenosine pathway. Known potential escape pathways (ATP, cyclic AMP, and nicotinamide adenine dinucleotide through separate biochemical pathways) exist when targeting upstream CD39 or downstream adenosine receptors. NAD+ ATP CD39 CD203a ADP CD38 ADPR CD39 AMP CD203a AMP ADO A1AR A2aAR A2bAR A3AR Canonical Alternative Multiple Pathways Multiple Receptors CD73 Rate-limiting Converging Source: I-MAB information on file Notes: ATP = adenosine triphosphate; NAD+ = nicotinamide adenine dinucleotide; ADP = adenosine diphosphate; ADPR = adenosine diphosphate ribose; AMP = adenosine monophosphate; ADO = aldehyde deformylating oxygenase
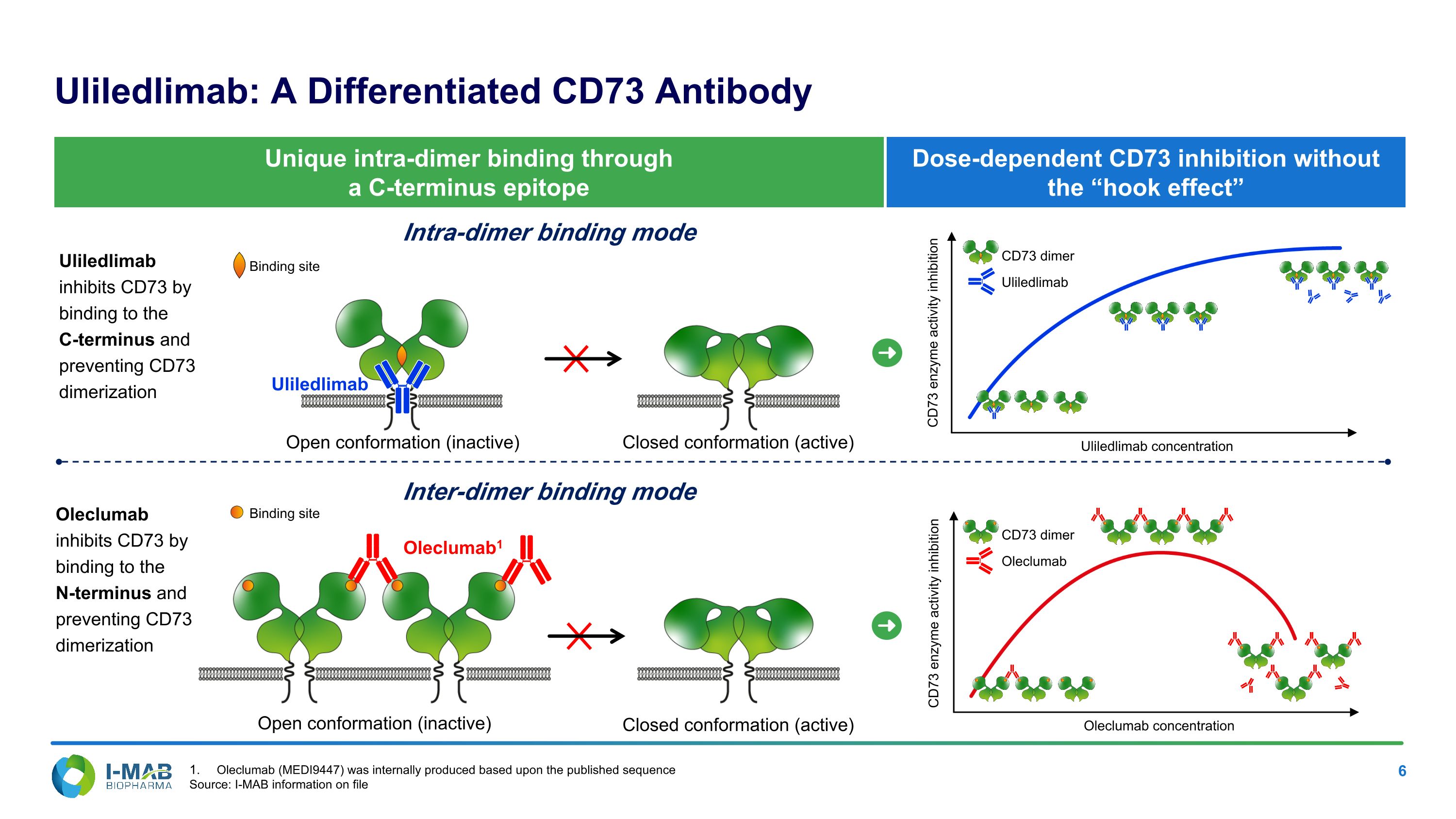
CD73 enzyme activity inhibition Dose-dependent CD73 inhibition without the “hook effect” Uliledlimab: A Differentiated CD73 Antibody Open conformation (inactive) Closed conformation (active) Oleclumab1 Intra-dimer binding mode Inter-dimer binding mode Open conformation (inactive) Closed conformation (active) Unique intra-dimer binding through a C-terminus epitope Uliledlimab inhibits CD73 by binding to the C-terminus and preventing CD73 dimerization Oleclumab inhibits CD73 by binding to the N-terminus and preventing CD73 dimerization Uliledlimab CD73 enzyme activity inhibition Uliledlimab concentration Oleclumab concentration Uliledlimab CD73 dimer Oleclumab CD73 dimer Binding site Binding site Oleclumab (MEDI9447) was internally produced based upon the published sequence Source: I-MAB information on file
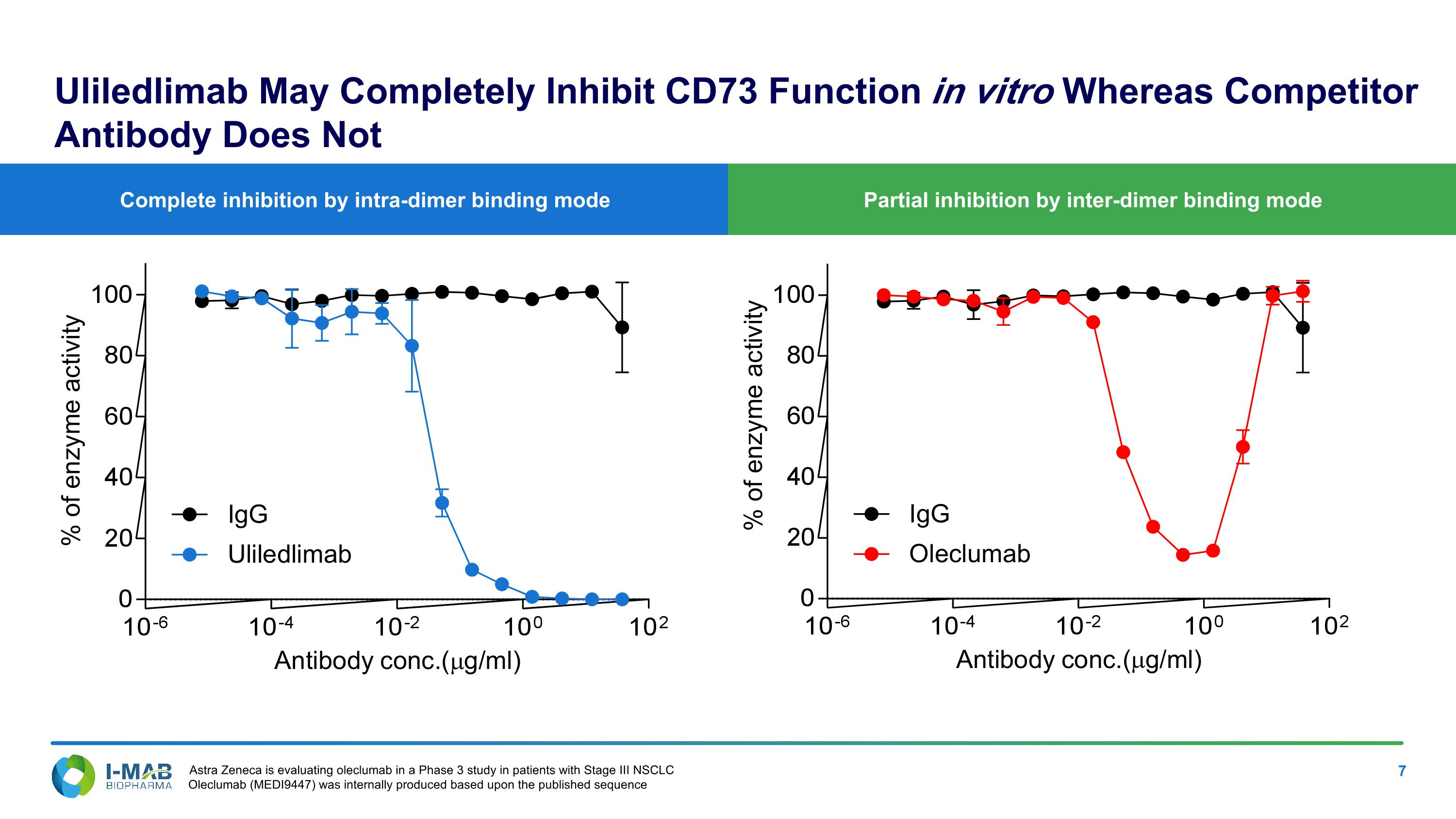
Partial inhibition by inter-dimer binding mode Complete inhibition by intra-dimer binding mode Uliledlimab May Completely Inhibit CD73 Function in vitro Whereas Competitor Antibody Does Not Astra Zeneca is evaluating oleclumab in a Phase 3 study in patients with Stage III NSCLC Oleclumab (MEDI9447) was internally produced based upon the published sequence
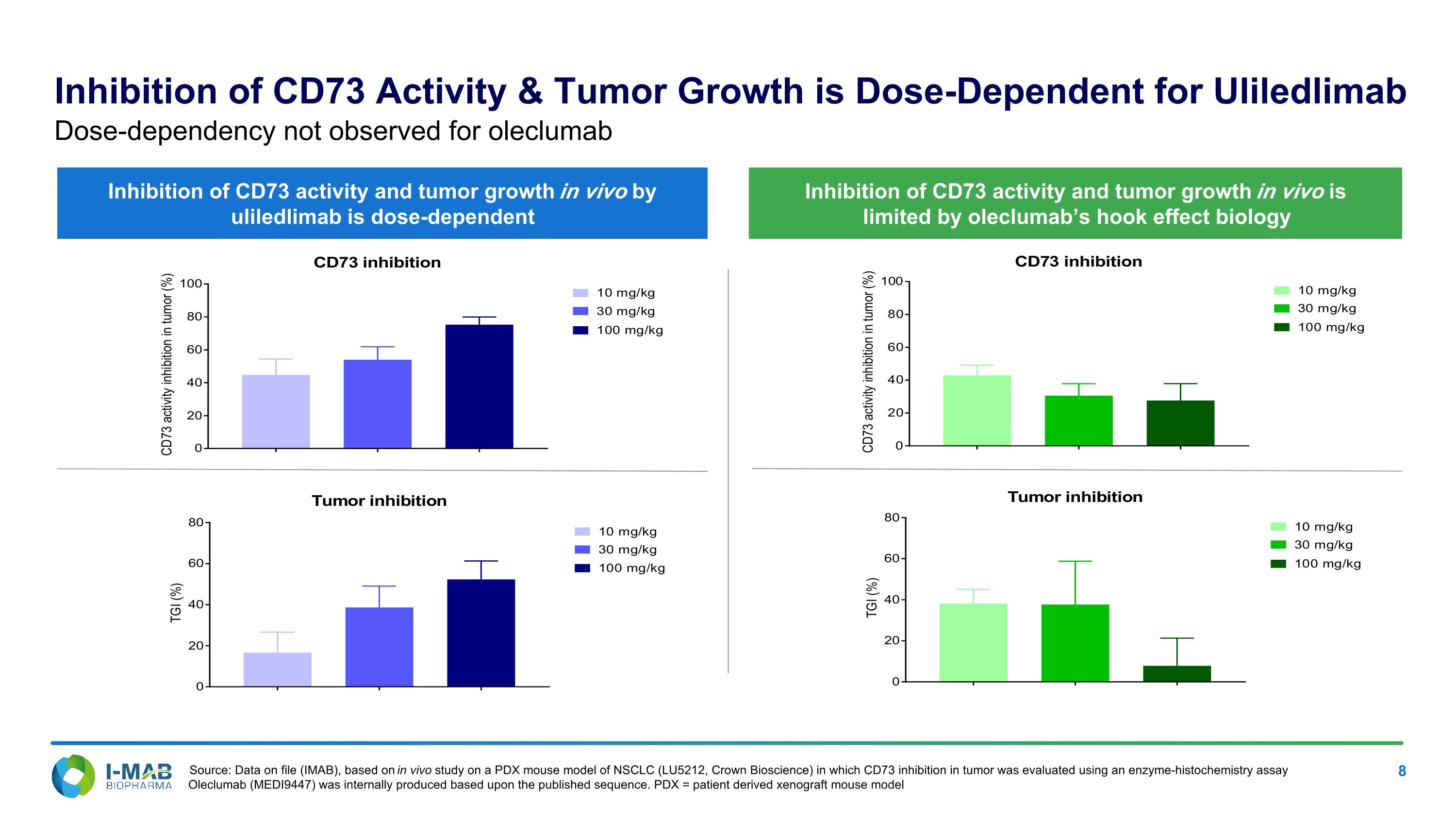
Inhibition of CD73 Activity & Tumor Growth is Dose-Dependent for Uliledlimab Inhibition of CD73 activity and tumor growth in vivo is limited by oleclumab’s hook effect biology Inhibition of CD73 activity and tumor growth in vivo by uliledlimab is dose-dependent Dose-dependency not observed for oleclumab Source: Data on file (IMAB), based on in vivo study on a PDX mouse model of NSCLC (LU5212, Crown Bioscience) in which CD73 inhibition in tumor was evaluated using an enzyme-histochemistry assay Oleclumab (MEDI9447) was internally produced based upon the published sequence. PDX = patient derived xenograft mouse model
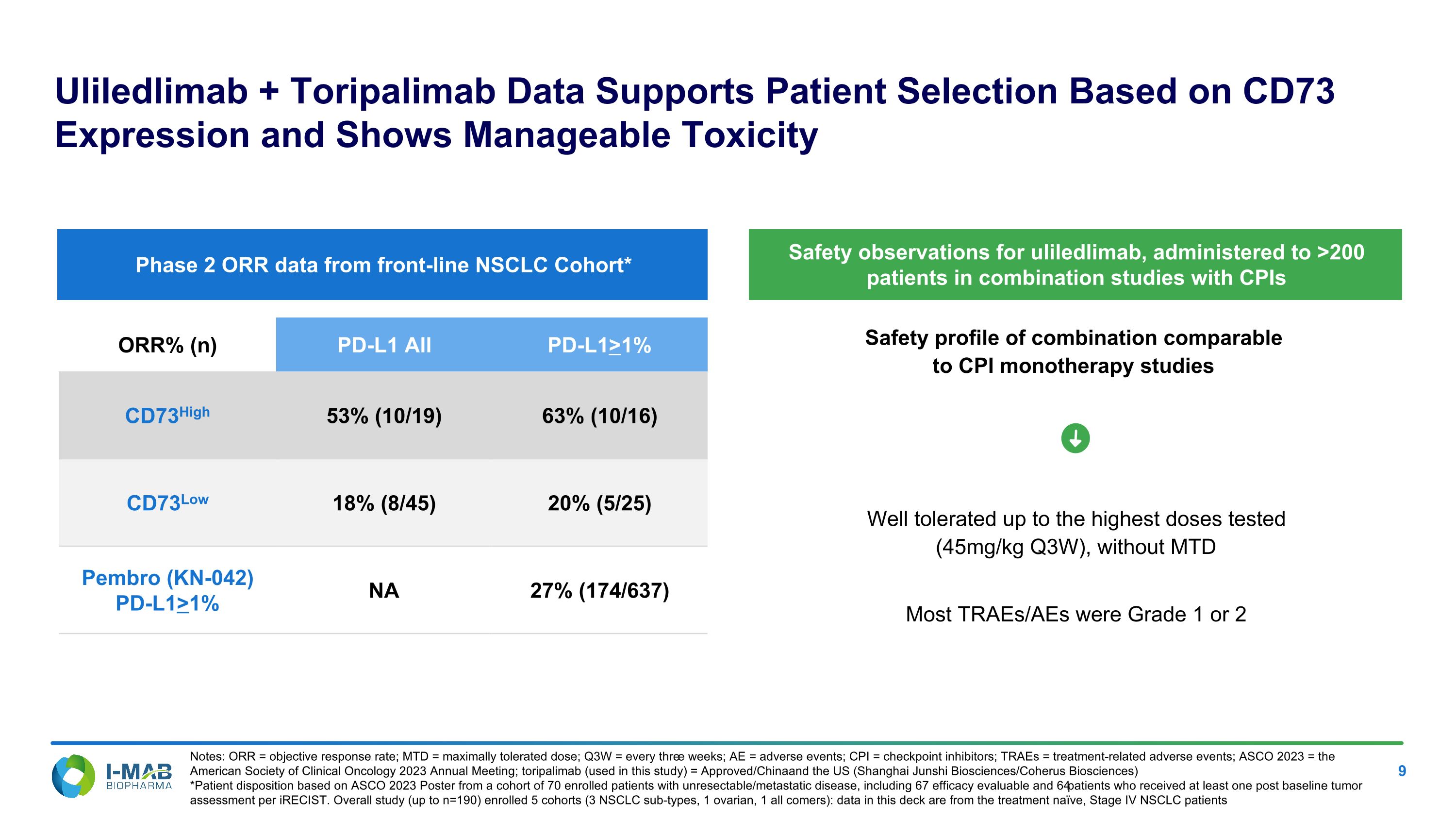
Safety profile of combination comparable �to CPI monotherapy studies Uliledlimab + Toripalimab Data Supports Patient Selection Based on CD73 Expression and Shows Manageable Toxicity Notes: ORR = objective response rate; MTD = maximally tolerated dose; Q3W = every three weeks; AE = adverse events; CPI = checkpoint inhibitors; TRAEs = treatment-related adverse events; ASCO 2023 = the American Society of Clinical Oncology 2023 Annual Meeting; toripalimab (used in this study) = Approved/China and the US (Shanghai Junshi Biosciences/Coherus Biosciences) *Patient disposition based on ASCO 2023 Poster from a cohort of 70 enrolled patients with unresectable/metastatic disease, including 67 efficacy evaluable and 64 patients who received at least one post baseline tumor assessment per iRECIST. Overall study (up to n=190) enrolled 5 cohorts (3 NSCLC sub-types, 1 ovarian, 1 all comers): data in this deck are from the treatment naïve, Stage IV NSCLC patients Well tolerated up to the highest doses tested �(45mg/kg Q3W), without MTD Most TRAEs/AEs were Grade 1 or 2 ORR% (n) PD-L1 All PD-L1>1% CD73High 53% (10/19) 63% (10/16) CD73Low 18% (8/45) 20% (5/25) Pembro (KN-042) PD-L1>1% NA 27% (174/637) Phase 2 ORR data from front-line NSCLC Cohort* Safety observations for uliledlimab, administered to >200 patients in combination studies with CPIs
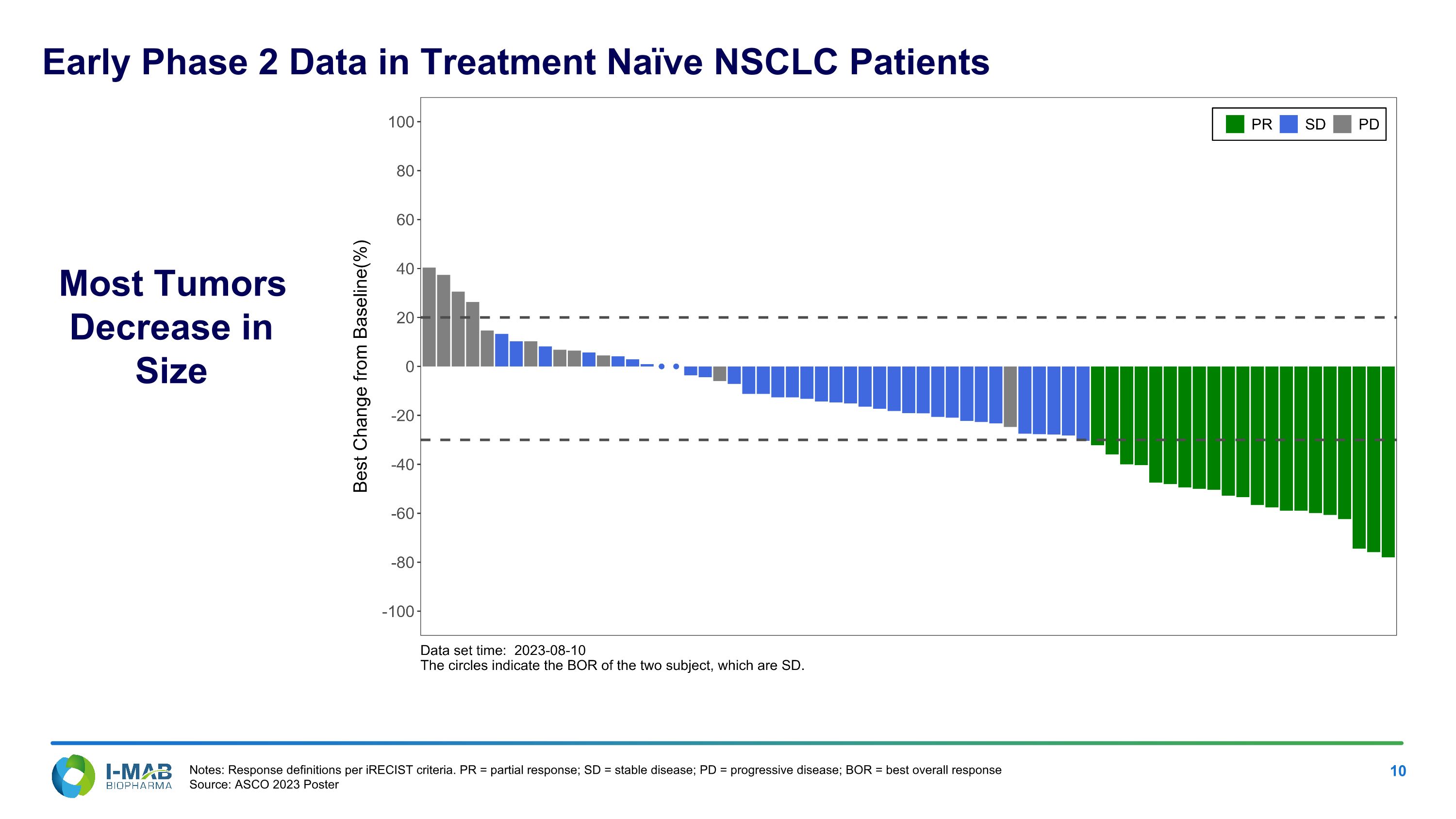
Most Tumors Decrease in Size Notes: Response definitions per iRECIST criteria. PR = partial response; SD = stable disease; PD = progressive disease; BOR = best overall response Source: ASCO 2023 Poster Early Phase 2 Data in Treatment Naïve NSCLC Patients
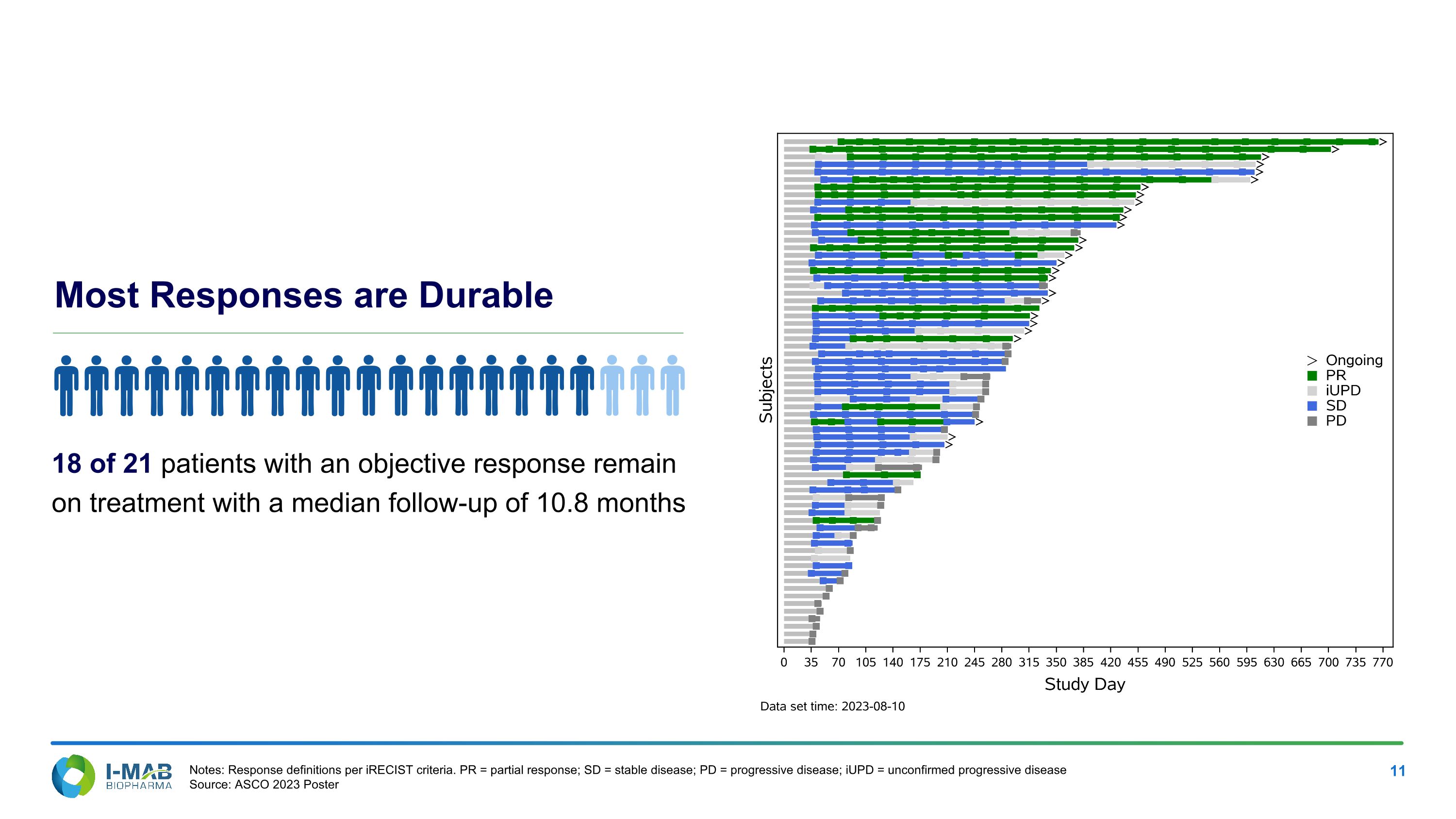
Most Responses are Durable 18 of 21 patients with an objective response remain on treatment with a median follow-up of 10.8 months Notes: Response definitions per iRECIST criteria. PR = partial response; SD = stable disease; PD = progressive disease; iUPD = unconfirmed progressive disease Source: ASCO 2023 Poster
Rationale to Support Uliledlimab + Pembro + Chemotherapy in 1L mNSCLC Samanta D, Park Y, Ni XH, Semenza G. 2017. Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. PNAS Vol. 115, No 6. Notes: mNSCLC = metastatic non-small cell lung cancer; IO = Immuno-oncology The addition of chemotherapy to IO monotherapy extends the benefit of IO to lower levels of PD-L1 expression Uliledlimab has a favorable toxicity profile in combination with IO agents Chemotherapy induces CD73 expression suggesting additional benefit by combining uliledlimab with pembrolizumab + chemotherapy1 Based on this rationale I-Mab plans to dose the first patient with uliledlimab in combination with pembrolizumab + chemotherapy in newly diagnosed patients with mNSCLC in 1H 2025
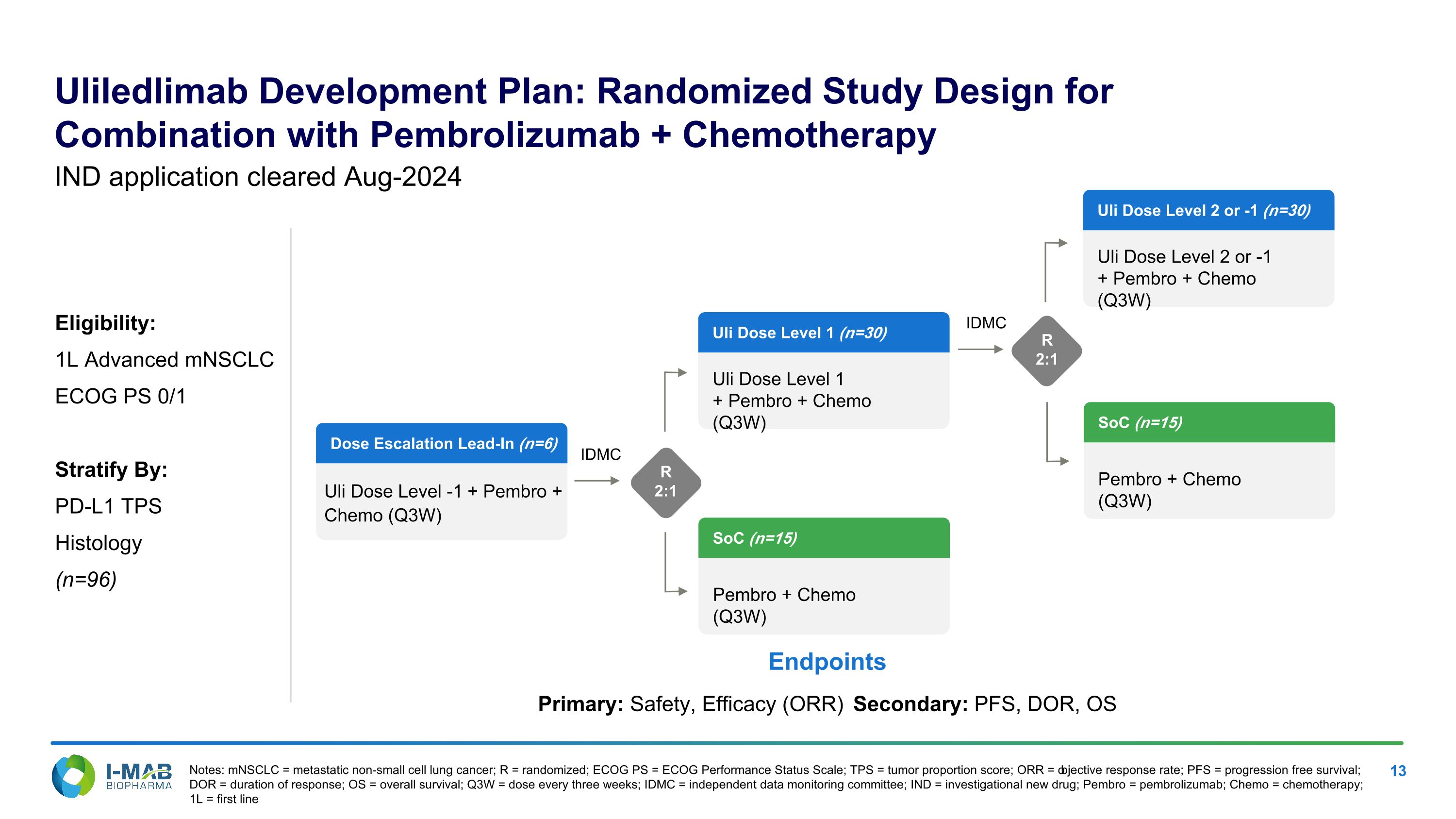
Uliledlimab Development Plan: Randomized Study Design for Combination with Pembrolizumab + Chemotherapy Notes: mNSCLC = metastatic non-small cell lung cancer; R = randomized; ECOG PS = ECOG Performance Status Scale; TPS = tumor proportion score; ORR = objective response rate; PFS = progression free survival; DOR = duration of response; OS = overall survival; Q3W = dose every three weeks; IDMC = independent data monitoring committee; IND = investigational new drug; Pembro = pembrolizumab; Chemo = chemotherapy; 1L = first line IND application cleared Aug-2024 Eligibility: 1L Advanced mNSCLC ECOG PS 0/1 Stratify By: PD-L1 TPS Histology (n=96) Endpoints Primary: Safety, Efficacy (ORR) Secondary: PFS, DOR, OS IDMC Dose Escalation Lead-In (n=6) Uli Dose Level -1 + Pembro + Chemo (Q3W) SoC (n=15) Pembro + Chemo (Q3W) Uli Dose Level 1 (n=30) Uli Dose Level 1 + Pembro + Chemo (Q3W) R 2:1 IDMC R 2:1 Uli Dose Level 2 or -1 (n=30) Uli Dose Level 2 or -1 + Pembro + Chemo (Q3W) SoC (n=15) Pembro + Chemo (Q3W)
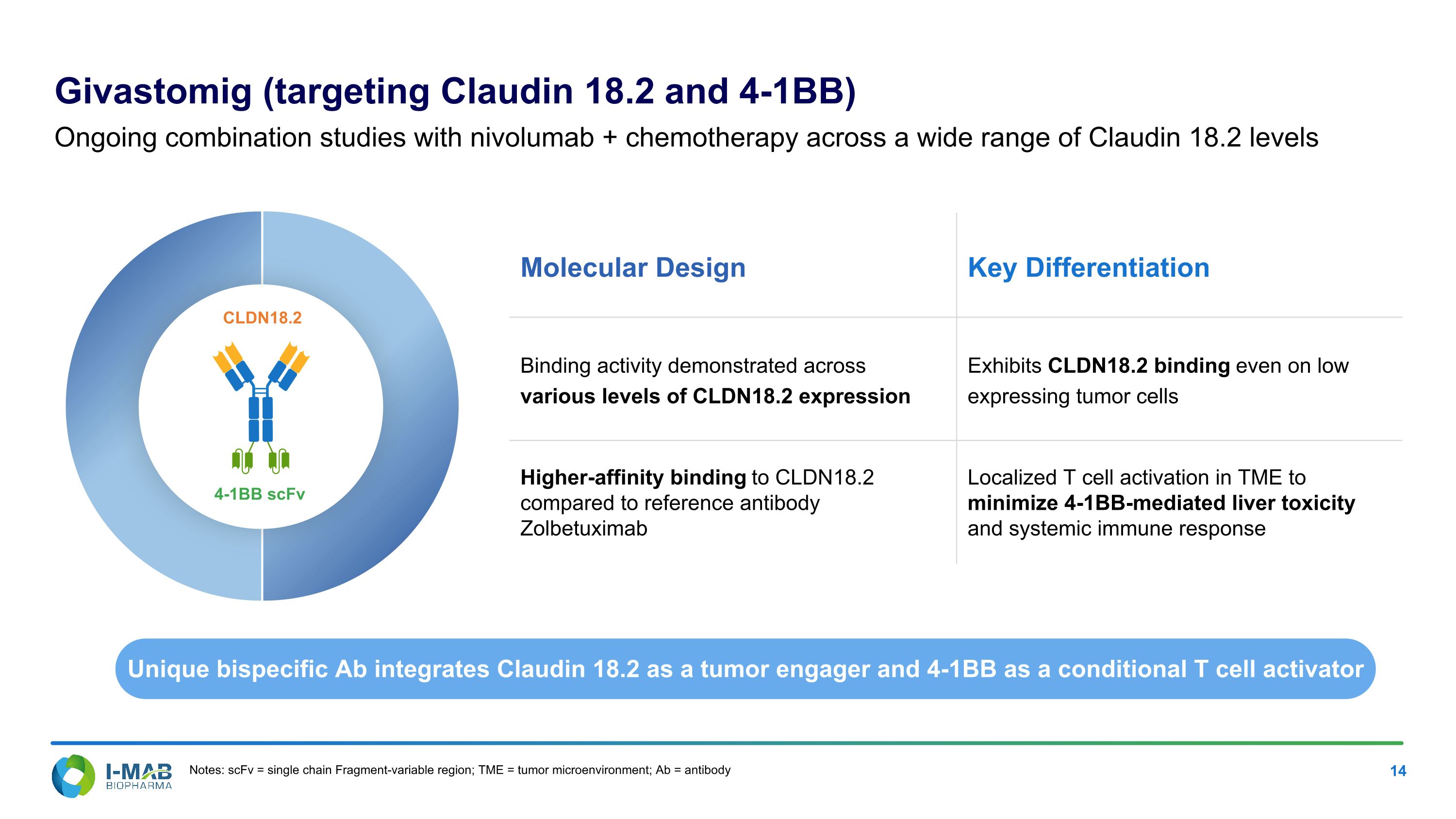
Molecular Design Key Differentiation Binding activity demonstrated across various levels of CLDN18.2 expression Exhibits CLDN18.2 binding even on low expressing tumor cells Higher-affinity binding to CLDN18.2 compared to reference antibody Zolbetuximab Localized T cell activation in TME to minimize 4-1BB-mediated liver toxicity and systemic immune response Givastomig (targeting Claudin 18.2 and 4-1BB) Ongoing combination studies with nivolumab + chemotherapy across a wide range of Claudin 18.2 levels Unique bispecific Ab integrates Claudin 18.2 as a tumor engager and 4-1BB as a conditional T cell activator 4-1BB scFv CLDN18.2 Notes: scFv = single chain Fragment-variable region; TME = tumor microenvironment; Ab = antibody
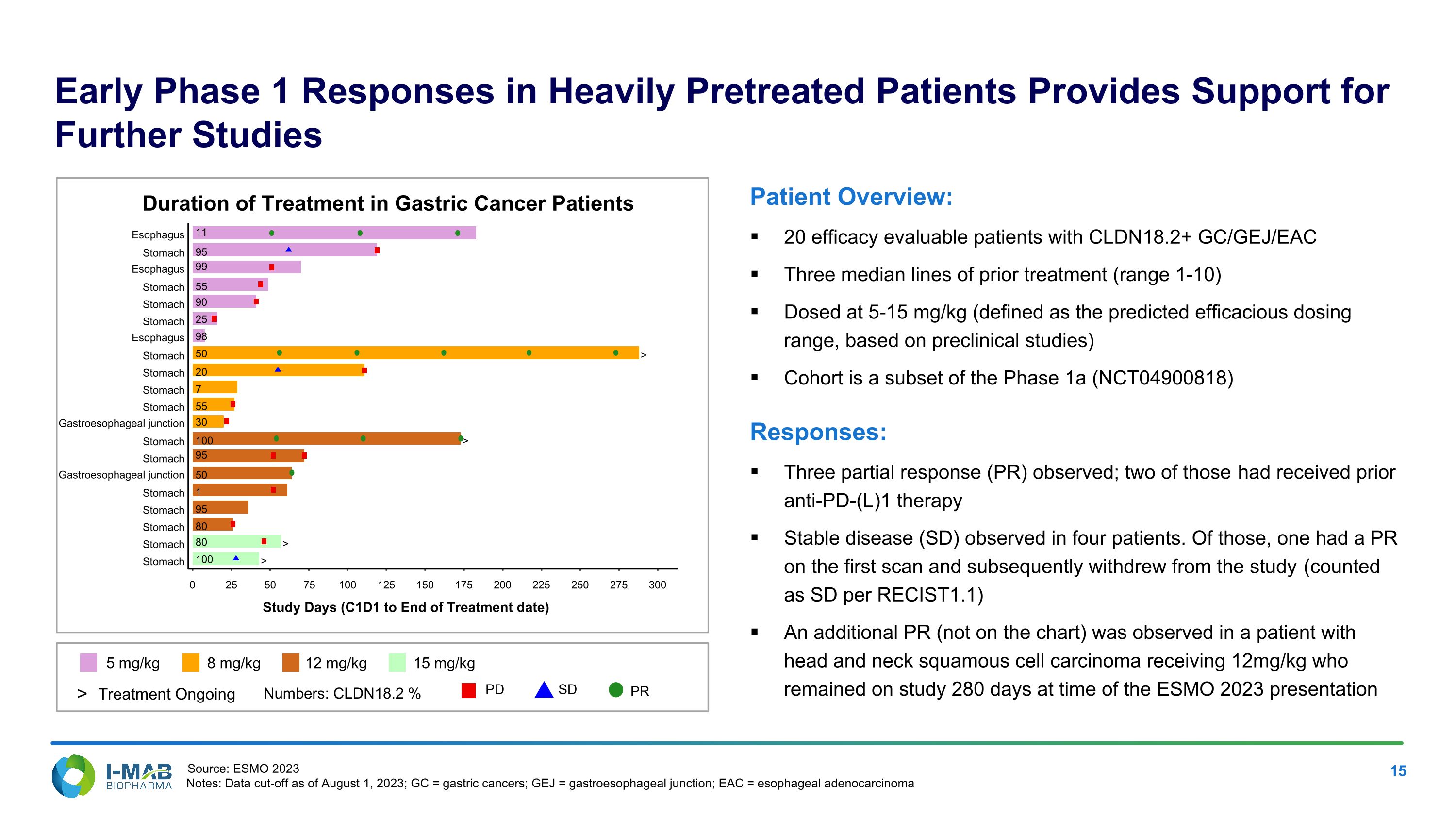
Early Phase 1 Responses in Heavily Pretreated Patients Provides Support for Further Studies 5 mg/kg 8 mg/kg 12 mg/kg 15 mg/kg Numbers: CLDN18.2 % > Treatment Ongoing PD SD PR Patient Overview: 20 efficacy evaluable patients with CLDN18.2+ GC/GEJ/EAC Three median lines of prior treatment (range 1-10) Dosed at 5-15 mg/kg (defined as the predicted efficacious dosing range, based on preclinical studies) Cohort is a subset of the Phase 1a (NCT04900818) Responses: Three partial response (PR) observed; two of those had received prior anti-PD-(L)1 therapy Stable disease (SD) observed in four patients. Of those, one had a PR on the first scan and subsequently withdrew from the study (counted as SD per RECIST1.1) An additional PR (not on the chart) was observed in a patient with head and neck squamous cell carcinoma receiving 12mg/kg who remained on study 280 days at time of the ESMO 2023 presentation Duration of Treatment in Gastric Cancer Patients > > > > 100 80 80 95 1 50 95 100 30 55 7 20 50 98 25 90 55 99 95 11 Stomach Stomach Stomach Stomach Stomach Gastroesophageal junction Stomach Stomach Gastroesophageal junction Stomach Stomach Stomach Stomach Esophagus Stomach Stomach Stomach Esophagus Stomach Esophagus 0 25 50 75 100 125 150 175 200 225 250 275 300 Study Days (C1D1 to End of Treatment date) Source: ESMO 2023 Notes: Data cut-off as of August 1, 2023; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma
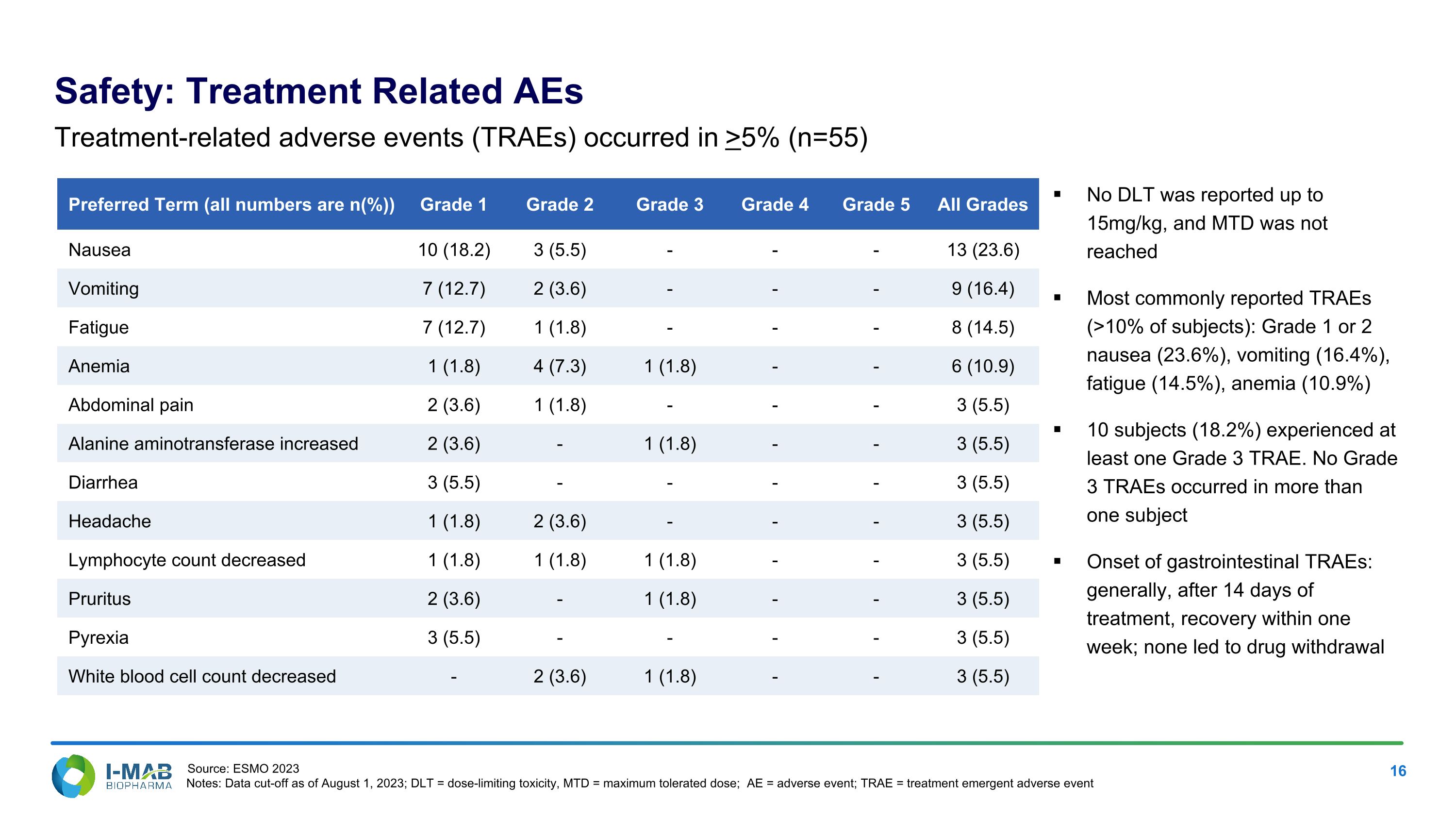
Safety: Treatment Related AEs Preferred Term (all numbers are n(%)) Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 All Grades Nausea 10 (18.2) 3 (5.5) - - - 13 (23.6) Vomiting 7 (12.7) 2 (3.6) - - - 9 (16.4) Fatigue 7 (12.7) 1 (1.8) - - - 8 (14.5) Anemia 1 (1.8) 4 (7.3) 1 (1.8) - - 6 (10.9) Abdominal pain 2 (3.6) 1 (1.8) - - - 3 (5.5) Alanine aminotransferase increased 2 (3.6) - 1 (1.8) - - 3 (5.5) Diarrhea 3 (5.5) - - - - 3 (5.5) Headache 1 (1.8) 2 (3.6) - - - 3 (5.5) Lymphocyte count decreased 1 (1.8) 1 (1.8) 1 (1.8) - - 3 (5.5) Pruritus 2 (3.6) - 1 (1.8) - - 3 (5.5) Pyrexia 3 (5.5) - - - - 3 (5.5) White blood cell count decreased - 2 (3.6) 1 (1.8) - - 3 (5.5) Treatment-related adverse events (TRAEs) occurred in >5% (n=55) No DLT was reported up to 15mg/kg, and MTD was not reached Most commonly reported TRAEs (>10% of subjects): Grade 1 or 2 nausea (23.6%), vomiting (16.4%), fatigue (14.5%), anemia (10.9%) 10 subjects (18.2%) experienced at least one Grade 3 TRAE. No Grade 3 TRAEs occurred in more than one subject Onset of gastrointestinal TRAEs: generally, after 14 days of treatment, recovery within one week; none led to drug withdrawal Source: ESMO 2023 Notes: Data cut-off as of August 1, 2023; DLT = dose-limiting toxicity, MTD = maximum tolerated dose; AE = adverse event; TRAE = treatment emergent adverse event
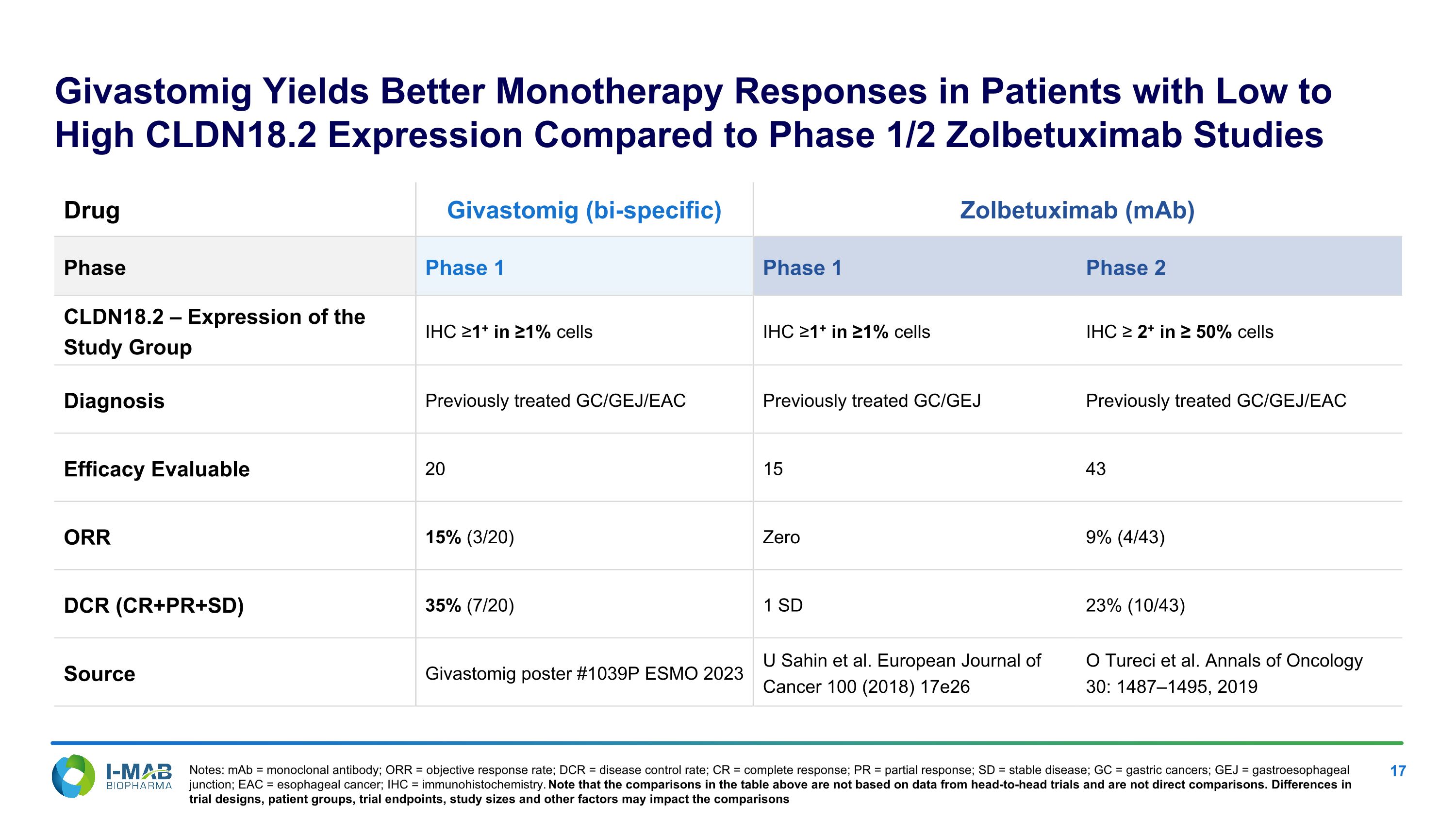
Givastomig Yields Better Monotherapy Responses in Patients with Low to High CLDN18.2 Expression Compared to Phase 1/2 Zolbetuximab Studies Drug Givastomig (bi-specific) Zolbetuximab (mAb) Phase Phase 1 Phase 1 Phase 2 CLDN18.2 – Expression of the Study Group IHC ≥1+ in ≥1% cells IHC ≥1+ in ≥1% cells IHC ≥ 2+ in ≥ 50% cells Diagnosis Previously treated GC/GEJ/EAC Previously treated GC/GEJ Previously treated GC/GEJ/EAC Efficacy Evaluable 20 15 43 ORR 15% (3/20) Zero 9% (4/43) DCR (CR+PR+SD) 35% (7/20) 1 SD 23% (10/43) Source Givastomig poster #1039P ESMO 2023 U Sahin et al. European Journal of Cancer 100 (2018) 17e26 O Tureci et al. Annals of Oncology �30: 1487–1495, 2019 Notes: mAb = monoclonal antibody; ORR = objective response rate; DCR = disease control rate; CR = complete response; PR = partial response; SD = stable disease; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal cancer; IHC = immunohistochemistry. Note that the comparisons in the table above are not based on data from head-to-head trials and are not direct comparisons. Differences in trial designs, patient groups, trial endpoints, study sizes and other factors may impact the comparisons
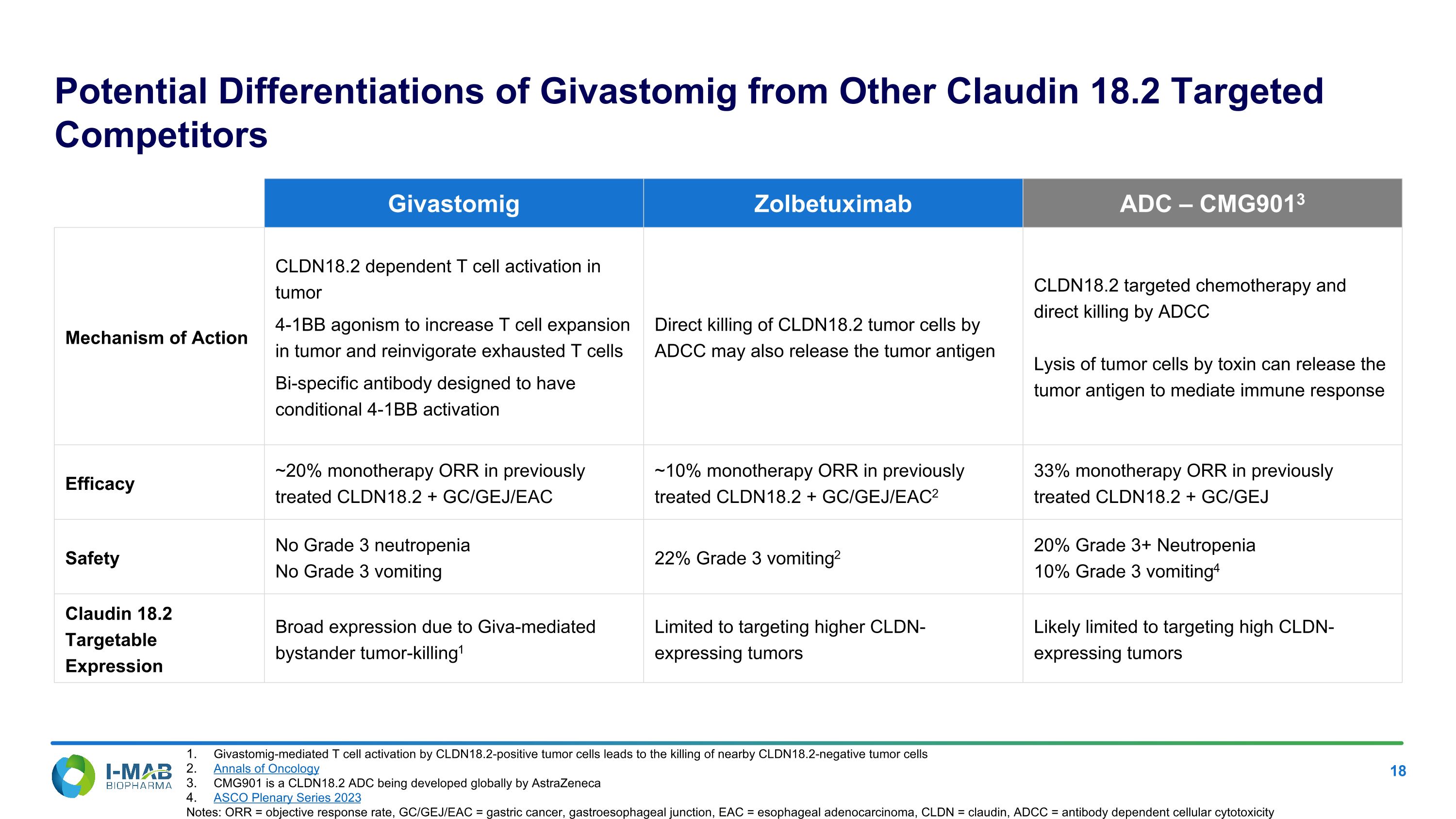
Potential Differentiations of Givastomig from Other Claudin 18.2 Targeted Competitors Givastomig Zolbetuximab ADC – CMG9013 Mechanism of Action CLDN18.2 dependent T cell activation in tumor 4-1BB agonism to increase T cell expansion in tumor and reinvigorate exhausted T cells Bi-specific antibody designed to have conditional 4-1BB activation Direct killing of CLDN18.2 tumor cells by ADCC may also release the tumor antigen CLDN18.2 targeted chemotherapy and direct killing by ADCC Lysis of tumor cells by toxin can release the tumor antigen to mediate immune response Efficacy ~20% monotherapy ORR in previously treated CLDN18.2 + GC/GEJ/EAC ~10% monotherapy ORR in previously treated CLDN18.2 + GC/GEJ/EAC2 33% monotherapy ORR in previously treated CLDN18.2 + GC/GEJ Safety No Grade 3 neutropenia No Grade 3 vomiting 22% Grade 3 vomiting2 20% Grade 3+ Neutropenia 10% Grade 3 vomiting4 Claudin 18.2 Targetable Expression Broad expression due to Giva-mediated bystander tumor-killing1 Limited to targeting higher CLDN-expressing tumors Likely limited to targeting high CLDN-expressing tumors Givastomig-mediated T cell activation by CLDN18.2-positive tumor cells leads to the killing of nearby CLDN18.2-negative tumor cells Annals of Oncology CMG901 is a CLDN18.2 ADC being developed globally by AstraZeneca ASCO Plenary Series 2023 Notes: ORR = objective response rate, GC/GEJ/EAC = gastric cancer, gastroesophageal junction, EAC = esophageal adenocarcinoma, CLDN = claudin, ADCC = antibody dependent cellular cytotoxicity
Unique Design to Enable Potential Wide Use Plus Favorable Initial Safety Profile Encouraging Responses in Previously Treated Patients, Including Those with Low CLDN18.2 Expression Levels Dose Expansion Data and New Nivolumab + Chemotherapy Combo Study Ongoing Unique Bispecific Design Properties and Monotherapy Data in Gastric Cancers May Position Givastomig as Best-in-Class Claudin 18.2 bispecific Objective responses seen in patients with gastric and esophageal cancer who had received multiple lines of prior treatment, including PD-(L)1, and had low CLDN18.2 levels Response rate and tolerability supports combination in 1L SOC regimens New dose expansion in combination with nivolumab + chemotherapy cohort study began in 1H 2024 in treatment naïve patients with gastric cancers Updated monotherapy dose expansion data in CLDN18.2+ patients with gastric cancers whose disease has progressed after previous treatment to be presented at ESMO 2024 Bispecific design results in CLDN18.2 conditional 4-1BB and T cell activation, potentially limiting toxicity and inducing long-lasting immune memory response Phase 1 dose escalation reached highest planned dose without encountering DLT or liver toxicity signals Notes: Gastric cancers = gastric, gastroesophageal junction and esophageal cancer; ESMO 2024 = the European Society for Medical Oncology Annual Meeting in 2024; SOC = standard of care; DLT = dose limiting toxicity
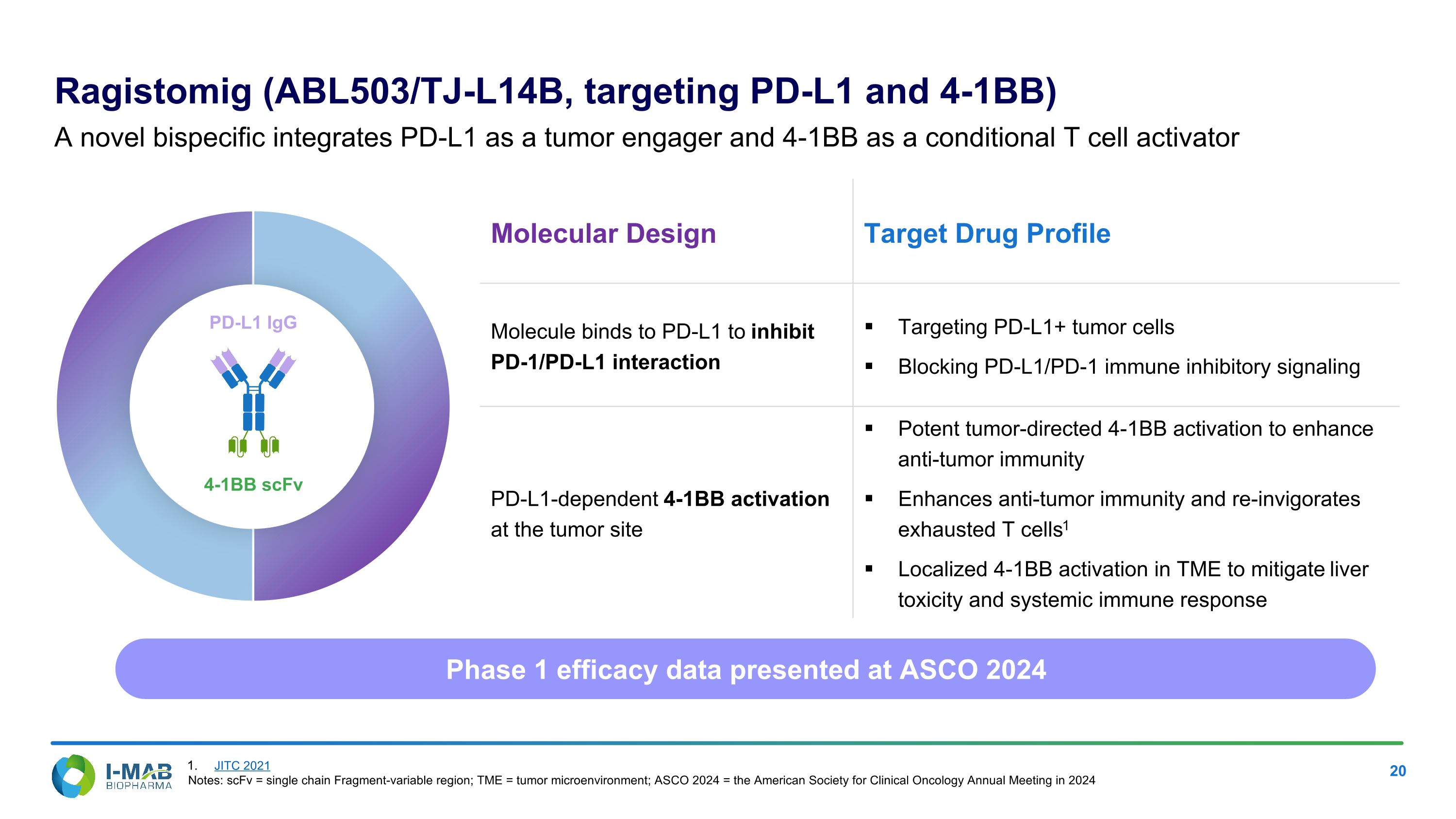
Molecular Design Target Drug Profile Molecule binds to PD-L1 to inhibit PD-1/PD-L1 interaction Targeting PD-L1+ tumor cells Blocking PD-L1/PD-1 immune inhibitory signaling PD-L1-dependent 4-1BB activation at the tumor site Potent tumor-directed 4-1BB activation to enhance anti-tumor immunity Enhances anti-tumor immunity and re-invigorates exhausted T cells1 Localized 4-1BB activation in TME to mitigate liver toxicity and systemic immune response Ragistomig (ABL503/TJ-L14B, targeting PD-L1 and 4-1BB) A novel bispecific integrates PD-L1 as a tumor engager and 4-1BB as a conditional T cell activator 4-1BB scFv PD-L1 IgG Phase 1 efficacy data presented at ASCO 2024 JITC 2021 Notes: scFv = single chain Fragment-variable region; TME = tumor microenvironment; ASCO 2024 = the American Society for Clinical Oncology Annual Meeting in 2024
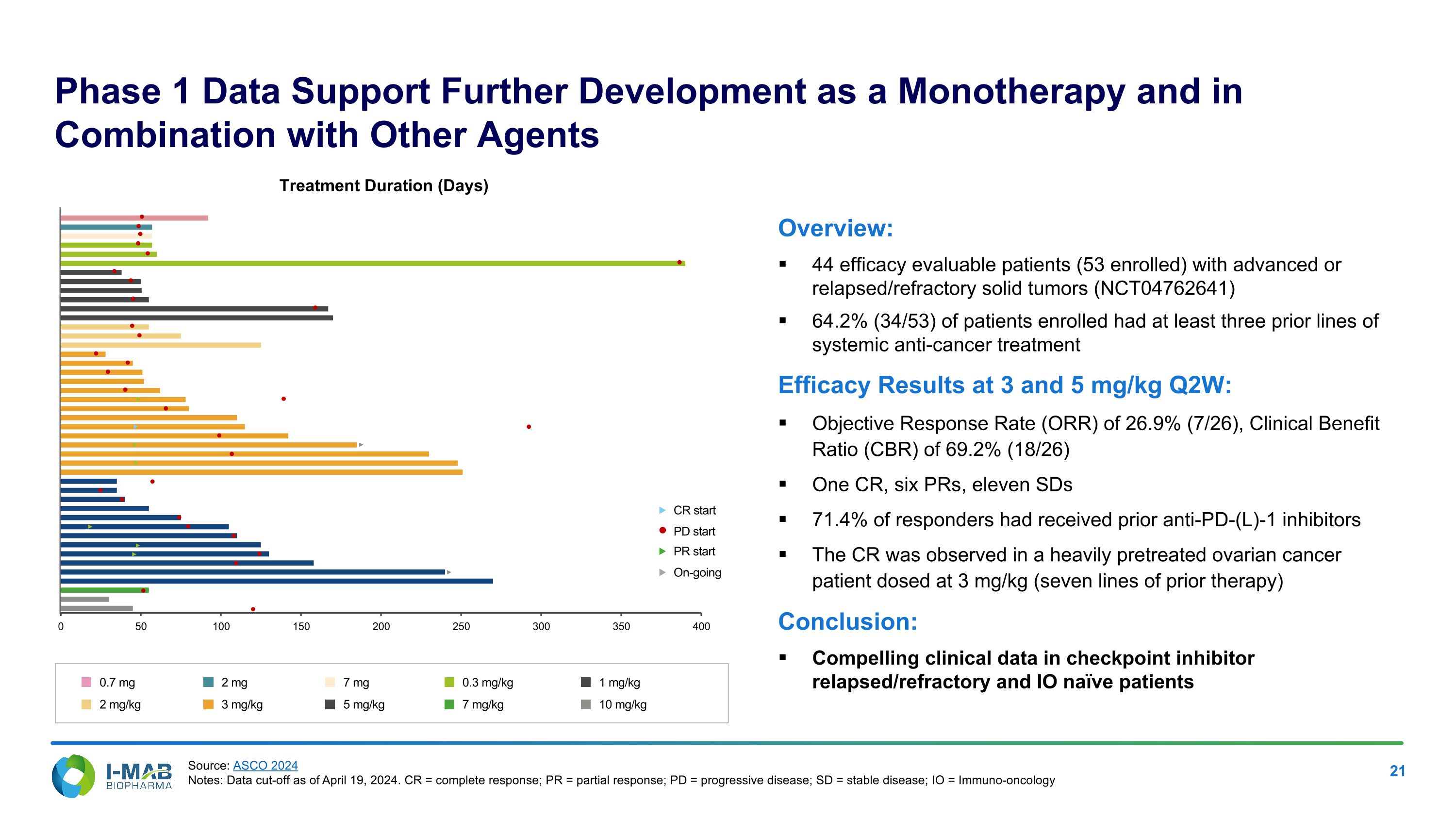
Phase 1 Data Support Further Development as a Monotherapy and in Combination with Other Agents Overview: 44 efficacy evaluable patients (53 enrolled) with advanced or relapsed/refractory solid tumors (NCT04762641) 64.2% (34/53) of patients enrolled had at least three prior lines of systemic anti-cancer treatment Efficacy Results at 3 and 5 mg/kg Q2W: Objective Response Rate (ORR) of 26.9% (7/26), Clinical Benefit Ratio (CBR) of 69.2% (18/26) One CR, six PRs, eleven SDs 71.4% of responders had received prior anti-PD-(L)-1 inhibitors The CR was observed in a heavily pretreated ovarian cancer patient dosed at 3 mg/kg (seven lines of prior therapy) Conclusion: Compelling clinical data in checkpoint inhibitor relapsed/refractory and IO naïve patients Treatment Duration (Days) CR start PR start On-going PD start 0.7 mg 2 mg/kg 2 mg 3 mg/kg 7 mg 5 mg/kg 0.3 mg/kg 7 mg/kg 1 mg/kg 10 mg/kg Source: ASCO 2024 Notes: Data cut-off as of April 19, 2024. CR = complete response; PR = partial response; PD = progressive disease; SD = stable disease; IO = Immuno-oncology
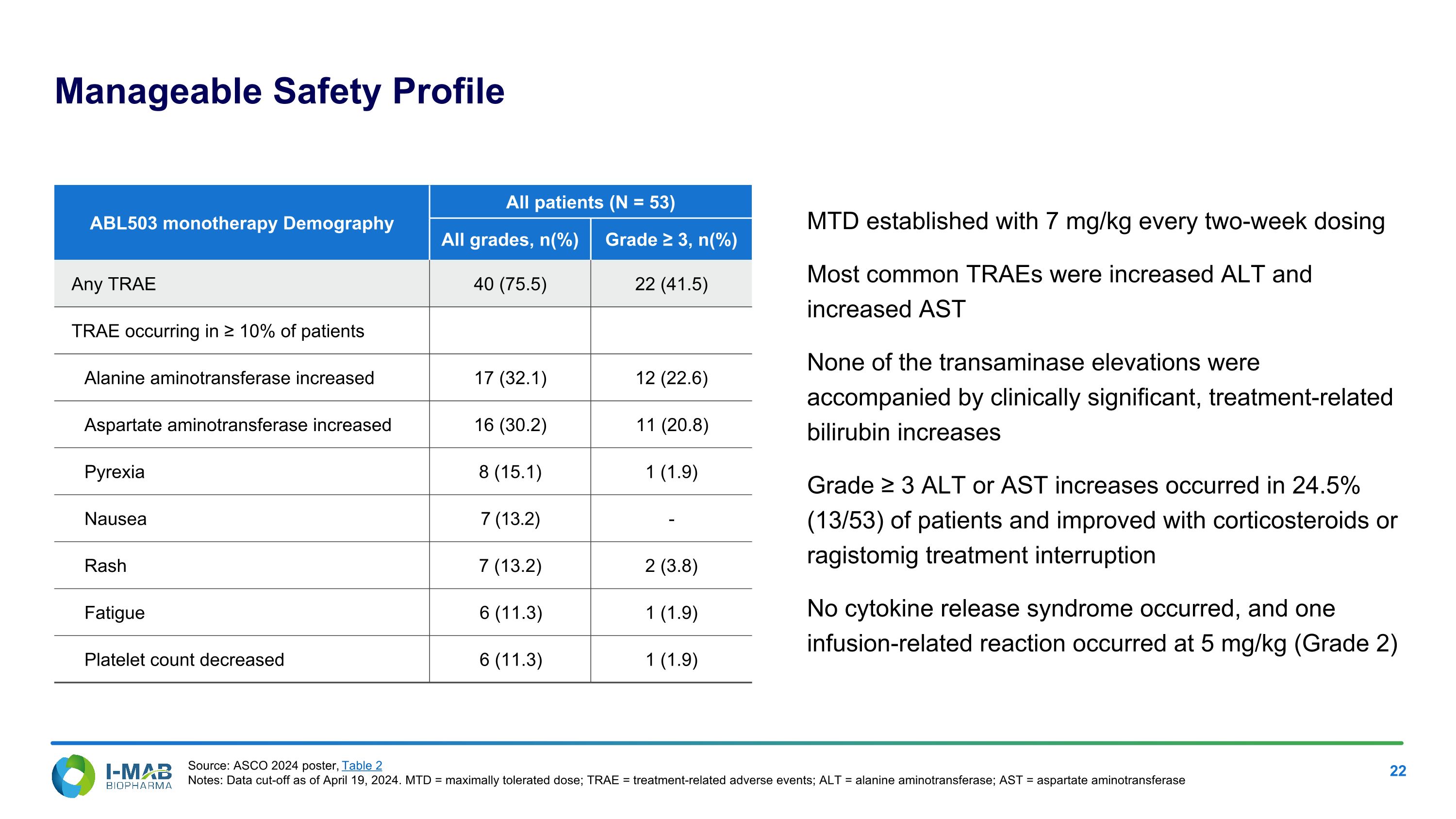
Manageable Safety Profile MTD established with 7 mg/kg every two-week dosing Most common TRAEs were increased ALT and increased AST None of the transaminase elevations were accompanied by clinically significant, treatment-related bilirubin increases Grade ≥ 3 ALT or AST increases occurred in 24.5% (13/53) of patients and improved with corticosteroids or ragistomig treatment interruption No cytokine release syndrome occurred, and one infusion-related reaction occurred at 5 mg/kg (Grade 2) ABL503 monotherapy Demography All patients (N = 53) All grades, n(%) Grade ≥ 3, n(%) Any TRAE 40 (75.5) 22 (41.5) TRAE occurring in ≥ 10% of patients Alanine aminotransferase increased 17 (32.1) 12 (22.6) Aspartate aminotransferase increased 16 (30.2) 11 (20.8) Pyrexia 8 (15.1) 1 (1.9) Nausea 7 (13.2) - Rash 7 (13.2) 2 (3.8) Fatigue 6 (11.3) 1 (1.9) Platelet count decreased 6 (11.3) 1 (1.9) Source: ASCO 2024 poster, Table 2 Notes: Data cut-off as of April 19, 2024. MTD = maximally tolerated dose; TRAE = treatment-related adverse events; ALT = alanine aminotransferase; AST = aspartate aminotransferase
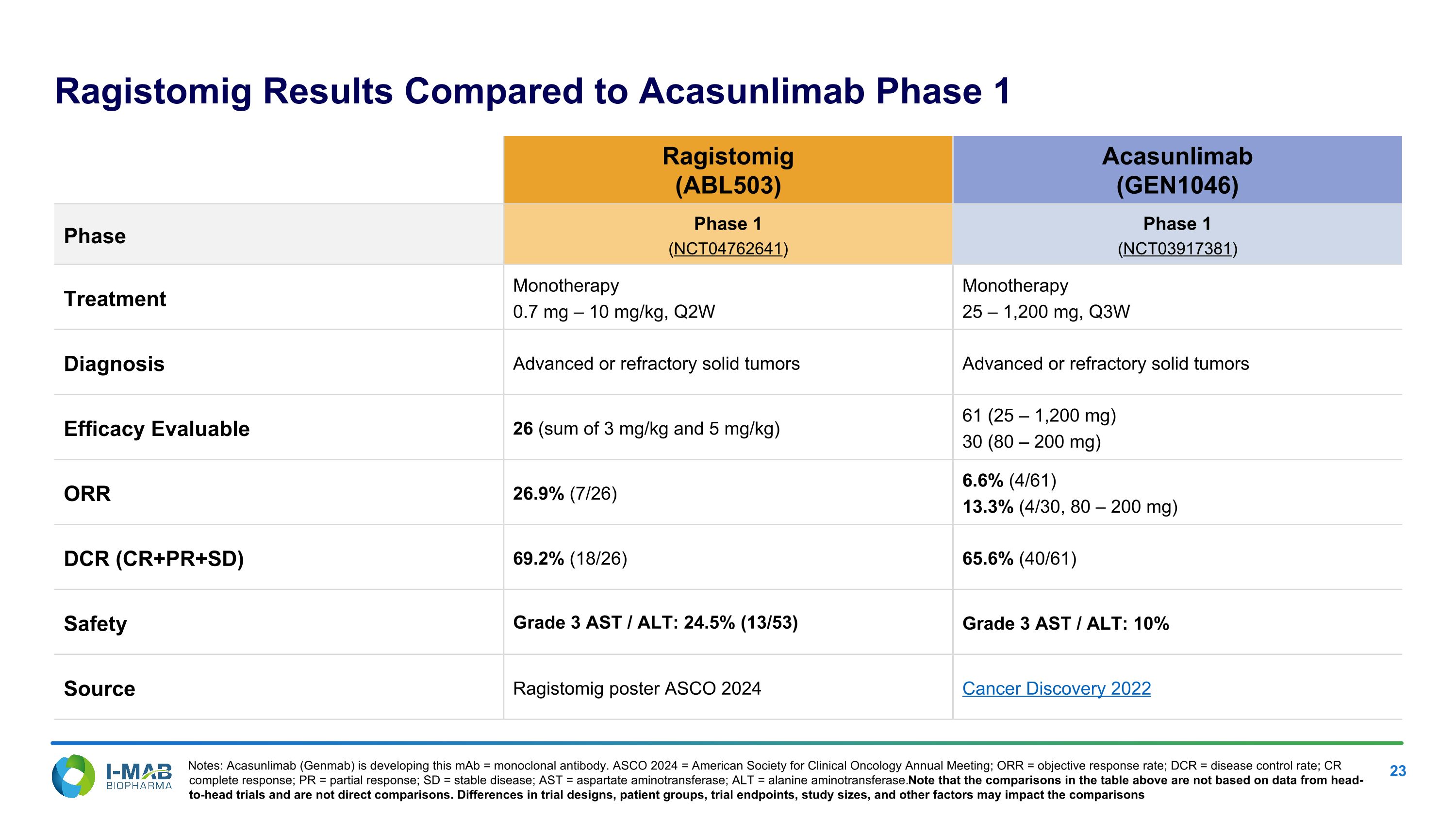
Ragistomig Results Compared to Acasunlimab Phase 1 Ragistomig (ABL503) Acasunlimab (GEN1046) Phase Phase 1 (NCT04762641) Phase 1 (NCT03917381) Treatment Monotherapy 0.7 mg – 10 mg/kg, Q2W Monotherapy 25 – 1,200 mg, Q3W Diagnosis Advanced or refractory solid tumors Advanced or refractory solid tumors Efficacy Evaluable 26 (sum of 3 mg/kg and 5 mg/kg) 61 (25 – 1,200 mg) 30 (80 – 200 mg) ORR 26.9% (7/26) 6.6% (4/61) 13.3% (4/30, 80 – 200 mg) DCR (CR+PR+SD) 69.2% (18/26) 65.6% (40/61) Safety Grade 3 AST / ALT: 24.5% (13/53) Grade 3 AST / ALT: 10% Source Ragistomig poster ASCO 2024 Cancer Discovery 2022 Notes: Acasunlimab (Genmab) is developing this mAb = monoclonal antibody. ASCO 2024 = American Society for Clinical Oncology Annual Meeting; ORR = objective response rate; DCR = disease control rate; CR complete response; PR = partial response; SD = stable disease; AST = aspartate aminotransferase; ALT = alanine aminotransferase. Note that the comparisons in the table above are not based on data from head-to-head trials and are not direct comparisons. Differences in trial designs, patient groups, trial endpoints, study sizes, and other factors may impact the comparisons
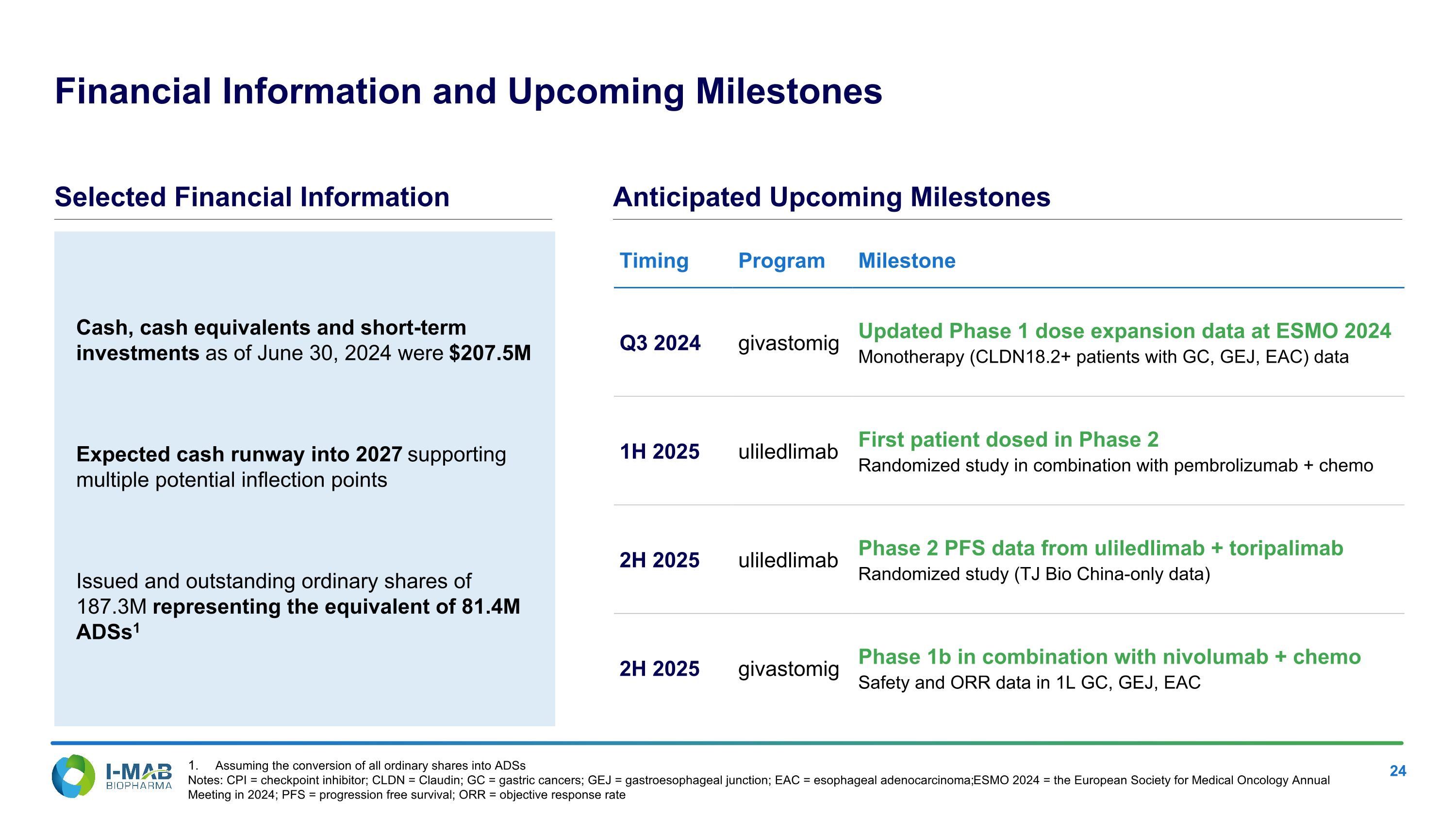
Cash, cash equivalents and short-term investments as of June 30, 2024 were $207.5M Expected cash runway into 2027 supporting multiple potential inflection points Issued and outstanding ordinary shares of 187.3M representing the equivalent of 81.4M ADSs1 Financial Information and Upcoming Milestones Timing Program Milestone Q3 2024 givastomig Updated Phase 1 dose expansion data at ESMO 2024 Monotherapy (CLDN18.2+ patients with GC, GEJ, EAC) data 1H 2025 uliledlimab First patient dosed in Phase 2 Randomized study in combination with pembrolizumab + chemo 2H 2025 uliledlimab Phase 2 PFS data from uliledlimab + toripalimab Randomized study (TJ Bio China-only data) 2H 2025 givastomig Phase 1b in combination with nivolumab + chemo Safety and ORR data in 1L GC, GEJ, EAC Selected Financial Information Anticipated Upcoming Milestones Assuming the conversion of all ordinary shares into ADSs Notes: CPI = checkpoint inhibitor; CLDN = Claudin; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma; ESMO 2024 = the European Society for Medical Oncology Annual Meeting in 2024; PFS = progression free survival; ORR = objective response rate

Stay connected I-Mab Biopharma IR Contact Tyler Ehler Sr. Director, Investor Relations IR@imabbio.com
























