Exhibit 99.1

Con f i d e n t i a l Redefining Central Nervous System Therapies Investor Presentation March 2021 Alex Zwyer, CEO Redefining Central Nervous System Therapies Investor Presentation April 2021 Alexander Zwyer, CEO

Forward Looking Statements 2 This presentation contains express or implied forward - looking statements within the Private Securities Litigation Reform Act of 1995 and other U . S . Federal securities laws . For example, we are using forward - looking statements when we discuss the expected timing of our clinical trials, the receipt of the results from clinical trials and obtaining regulatory approval for Quilience® ; clinical data readout ; proposed trials that may occur in the future ; our ability to generate revenue from licensing agreements or in compassionate use programs ; compounds or product candidates that we may seek to develop or add to our pipeline ; and the potential benefits and impact our products could have on improving patient health care . These forward - looking statements and their implications are based on the current expectations of our management only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements . The following factors, among others, could cause actual results to differ materially from those described in the forward - looking statements : our ability to obtain additional capital ; a global pandemic, such as COVID - 19 , or economic downturn and any resulting government actions therefrom ; changes in technology and market requirements ; we may encounter delays or obstacles in launching and/or successfully completing our clinical trials ; our products may not be approved by regulatory agencies, our technology may not be validated as we progress further and our methods may not be accepted by the scientific community ; we may be unable to retain or attract key employees whose knowledge is essential to the development of our products ; unforeseen scientific difficulties may develop with our process ; our products may wind up being more expensive than we anticipate ; results in the laboratory may not translate to equally good results in real clinical settings ; results of preclinical studies may not correlate with the results of human clinical trials ; our patents may not be sufficient ; our products may harm recipients ; changes in legislation ; inability to timely develop and introduce new technologies, products and applications ; loss of market share and pressure on pricing resulting from competition, which could cause our actual results or performance to differ materially from those contemplated in such forward - looking statements . Except as otherwise required by law, we undertake no obligation to publicly release any revisions to these forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events . For a more detailed description of the risks and uncertainties affecting us, reference is made to our reports filed from time to time with the Securities and Exchange Commission . Our logo and some of our trademarks and tradenames are used or incorporated by reference in this presentation . This presentation also includes trademarks, tradenames and service marks that are the property of other organizations . Solely for convenience, trademarks, tradenames and service marks referred to in this presentation may appear without the ®, TM and SM symbols, but those references are not intended to indicate in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensor to these trademarks, tradenames and service marks . We obtained the statistical data, market data and other industry data and forecasts described by reference in this presentation from market research, publicly available information and industry publications . Industry publications generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy and completeness of the information . Similarly, while we believe that the statistical data, industry data and forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of the information . As used in this presentation, “the Company,” “we” and “our” refer to NLS Pharmaceutics Ltd .
NLS discovers and develops drug treatments for rare and complex central nervous system (CNS) disorders Lead product candidate, Quilience ® , entering Phase 2 for narcolepsy treatment, a $ 2 . 4 billion annual market and growing** Mazindol, the active ingredient in Quilience ® was used extensively off - label and in compassionate use programs to treat narcolepsy Pipeline of additional compounds in research and development to address CNS disorders with high unmet need 3 Quilience ® is a proprietary formulation of mazindol, designed for once - daily dosing . Orphan Drug Designation granted in both the US and Europe Patent applications filed – expiry in 2037 if granted Phase 2 clinical trial planned to begin mid - year with topline results expected in Q4 2021 NLS Pharmaceutics Overview NASDAQ: NLSP, NLSPW Market Cap*: 48.6M Shares Out: 11.8M ADTV: 4.4M *Market data as of April 1, 2021 **ResearchAndMarkets.com press release (Sept. 25, 2019 )

Management: Deep Expertise in Clinical Development & Drug Repurposing (1) Currently engaged as consultant / strategic advisor Carlos Camozzi, MD, PhD interim Medical Director (1) Experienced Chief Medical and Corporate Governance Officer in the Biopharmaceuticals field with over 30 years of C - level expertise and experience in orphan drugs and other areas . As CMO for several European Biotech companies, he generated innovative solutions for the design and execution of non - clinical, translational, and clinical development projects for several orphan drugs, paediatric, and oncology clinical trials . He successfully led several clinical development programmes, regulatory interactions, consultations, submissions and approvals of products with Orphan Drug Designations (ODD), Paediatric Investigational Plans (PIP) and Marketing Authorisations Applications (MAA) with both the European Medicines Agency (EMA) and the U . S . Food and Drug Administration (FDA) . He received his medical doctorate and PhD from the National University of Buenos Aires, Argentina . Eric Konofal, MD, PhD, interim Chief Scientific Officer (1) IP expert and co - founder of NLS Pharmaceutics AG . Active for more than 27 years in the field of sleep research, including narcolepsy and hypersomnia, as a clinician, scientific researcher, and drug hunter . He is a senior medical consultant for the Pediatric Sleep Disorders Center and the Child and Adolescent Psychiatry Department at Robert Debré Hospital (APHP) . His research interests are focused on brain - and iron - dopamine interactions in subjects with neurological sleep disorders (RLS, PLMS), and ADHD . He received his medical doctorate and Ph . D . from the University Pierre – Marie Curie, Paris / France . Hervé Girsault, MBA, Head of Business Dev./Licensing (1) Experienced C - level pharma/biotech executive, former positions include : Global Head M&A/BD Novartis Consumer Health, Head of Novartis Pharma Out - Licensing & Partnering ; CBO of PIQUR Therapeutics AG (Basel) ; CEO, Synarc Inc . (now BioClinica Inc . ) (San Francisco, CA) ; CEO Novartis Consumer Health, Benelux ; CEO Gerber Products Company (France) . Alexander Zwyer, MBA, Chief Executive Officer A co - founder of the company with extensive operational, C - level pharmaceutical experience as well as a serial entrepreneur and strong leader with a proven track - record . Served as Chief Operating Officer at Viforpharma AG, a specialty pharmaceutical company focused on Nephrology where he was globally responsible for marketing and sales, business development, and regulatory and medical affairs . He was named ‘Healthcare CEO of the Year, Switzerland’ in 2018 by Business Worldwide . Holds two MBAs incl . from the State University of New York, Albany and is a veteran Tank Captain of the Swiss Army . Silvia Panigone, PhD, Chief Operating Officer Track record in complex project management ; former positions include : Global Project Manager in Quintiles for phase II/III trials in the US/EU and Asia, managing large teams including Regulatory, CMC and pharmacology, with full P&L responsibilities . Executive position in R&D in Bracco Diagnostics, Managing Director at I - Bankers Direct, Investment Director at BSI Healthcapital, a VC firm focused on life sciences . Holds a Ph . D . in Molecular Oncology from National Cancer Institute, Milan and a Masters in Finance - Management of Economical Resources from SDA Bocconi, Milan, Italy . Subhasis Roy, MBA, interim Chief Financial Officer (1) Over 25 years of healthcare industry experience as a healthcare banker/advisor and more recently as a biopharmaceutical company leader . Former top executive of Novaremed, a clinical stage Swiss biopharma company and Managing Partner and co - founder of Sirius Healthcare Partners, a healthcare advisory boutique . Previously, he was a healthcare banker at bulge bracket investments Banks (UBS, HSBC, DKB), where he executed numerous financing and M&A transactions . Holds an MBA in Finance and General Management from Fuqua School of Business at Duke University, and Masters and Bachelors degrees in Commerce from University of Mumbai, India . . 4
• Second mazindol - based product candidate with strong clinical evidence • Build on success of Nolazol ® Phase 2 clinical trial • Ability to capture income from licensing deal with Eurofarma in Latin America • Large market, need for more - effective treatments remains high • Strong validation with decades of off - label use of mazindol in narcolepsy • Novel mechanism of action addressing the root cause of the disease • Given prior approval for obesity, relatively modest development costs anticipated to achieve regulatory approval in the US and Europe • Several value inflection points expected in the 2021 - 2023 timeframe • Leverage current portfolio of compounds and core competency to identify compelling drug candidates through potential in - house innovation and in - house licensing and partnering/out - licensing deals • Several product candidate leads in early research and development Focused on Rare and Complex CNS Disorders Develop Quilience ® (mazindol controlled - release) for the treatment of narcolepsy in both the US and EU As a follow - on program, pursue development or licensure of Nolazol ® (mazindol CR) to treat ADHD Advance development of research and development stage product candidates/compounds Strategic Priorities Rationale 5
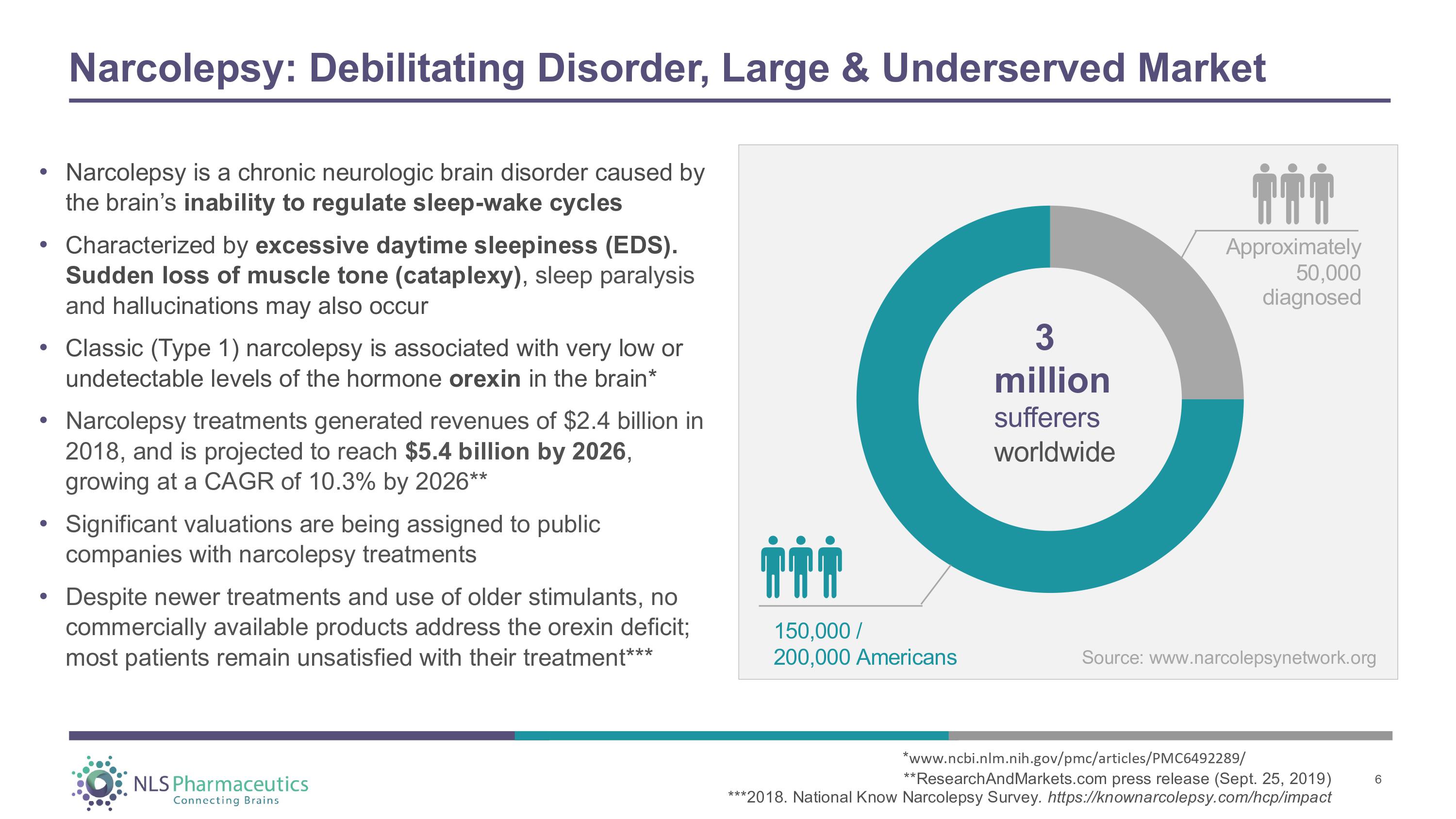
Narcolepsy: Debilitating Disorder, Large & Underserved Market • Narcolepsy is a chronic neurologic brain disorder caused by the brain’s inability to regulate sleep - wake cycles • Characterized by excessive daytime sleepiness (EDS). Sudden loss of muscle tone (cataplexy) , sleep paralysis and hallucinations may also occur • Classic (Type 1) narcolepsy is associated with very low or undetectable levels of the hormone orexin in the brain* • Narcolepsy treatments generated revenues of $2.4 billion in 2018, and is projected to reach $5.4 billion by 2026 , growing at a CAGR of 10.3% by 2026** • Significant valuations are being assigned to public companies with narcolepsy treatments • Despite newer treatments and use of older stimulants, no commercially available products address the orexin deficit; most patients remain unsatisfied with their treatment*** Approximately 50,000 diagnosed 3 million sufferers w o r l d w i d e 150 , 00 0 / 200 , 00 0 A m e r i c ans * www.ncbi.nlm.nih.gov/pmc/articles/PMC6492289/ **ResearchAndMarkets.com press release (Sept. 25, 2019) ***2018. National Know Narcolepsy Survey . https://knownarcolepsy.com/hcp/impact 6 Source: www.narcolepsynetwork.org
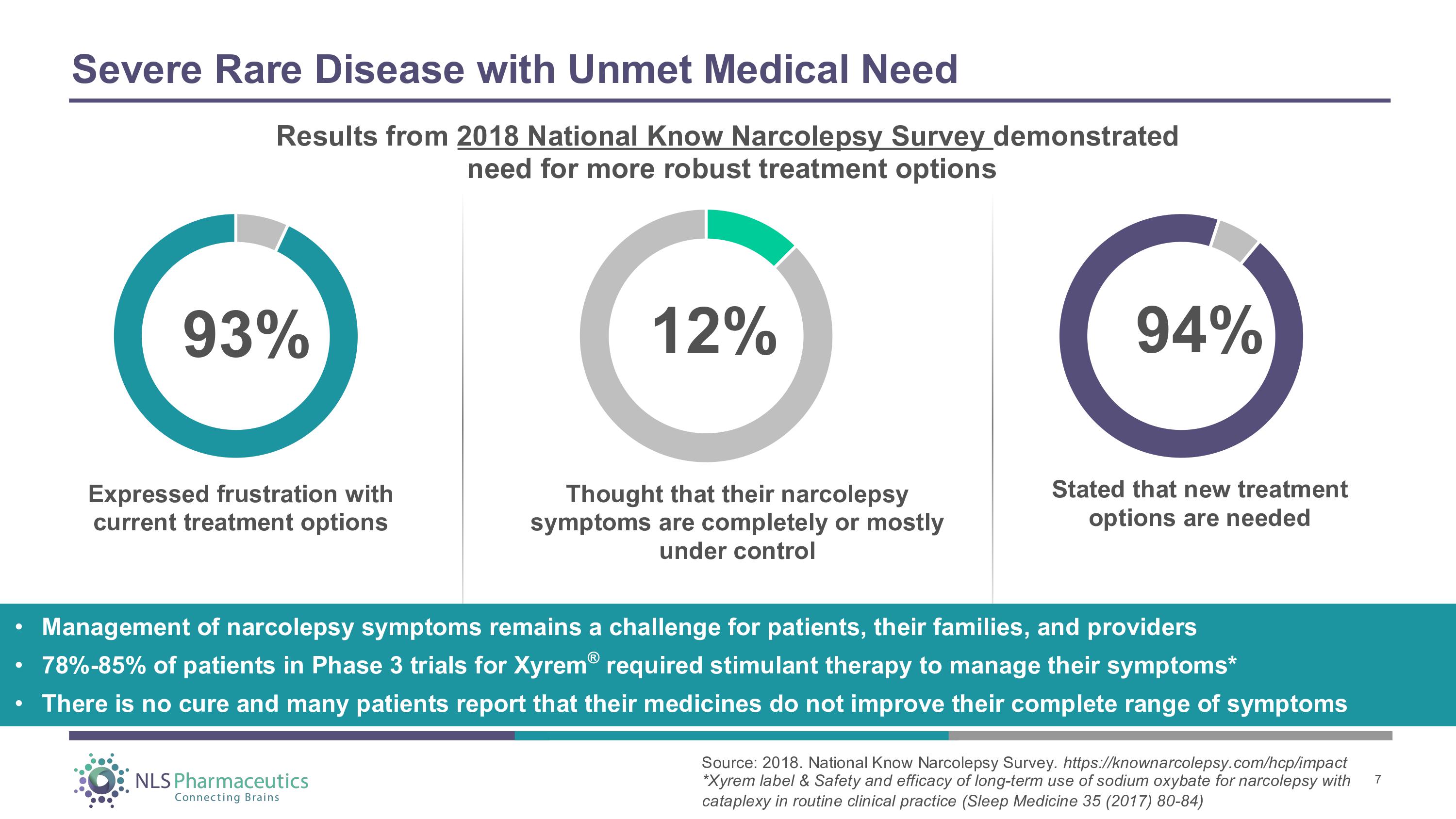
Severe Rare Disease with Unmet Medical Need Expressed frustration with current treatment options Thought that their narcolepsy symptoms are completely or mostly under control Stated that new treatment options are needed 93% 12 % 94% • Management of narcolepsy symptoms remains a challenge for patients, their families, and providers • 78% - 85% of patients in Phase 3 trials for Xyrem ® required stimulant therapy to manage their symptoms* • There is no cure and many patients report that their medicines do not improve their complete range of symptoms Results from 2018 National Know Narcolepsy Survey demonstrated need for more robust treatment options Source: 2018. National Know Narcolepsy Survey . https://knownarcolepsy.com/hcp/impact *Xyrem label & Safety and efficacy of long - term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice (Sleep Medicine 35 (2017) 80 - 84) 7

Mazindol Controlled - Release (CR) - for the Treatment of Excessive Daytime Sleepiness and Cataplexy in Narcolepsy ® • Long history of active ingredient (mazindol) used to treat narcolepsy (“compassionate use” programs) • Novel mechanism of action (MOA) with partial orexin - 2 receptor activation • Anticipated Phase 2 trial initiation mid - year, top - line results expected Q4 2021 • Potential expedited development: intent to leverage 505(b)(2) and/or accelerated approval pathways (Breakthrough, FastTrack, PRIME designations) 8
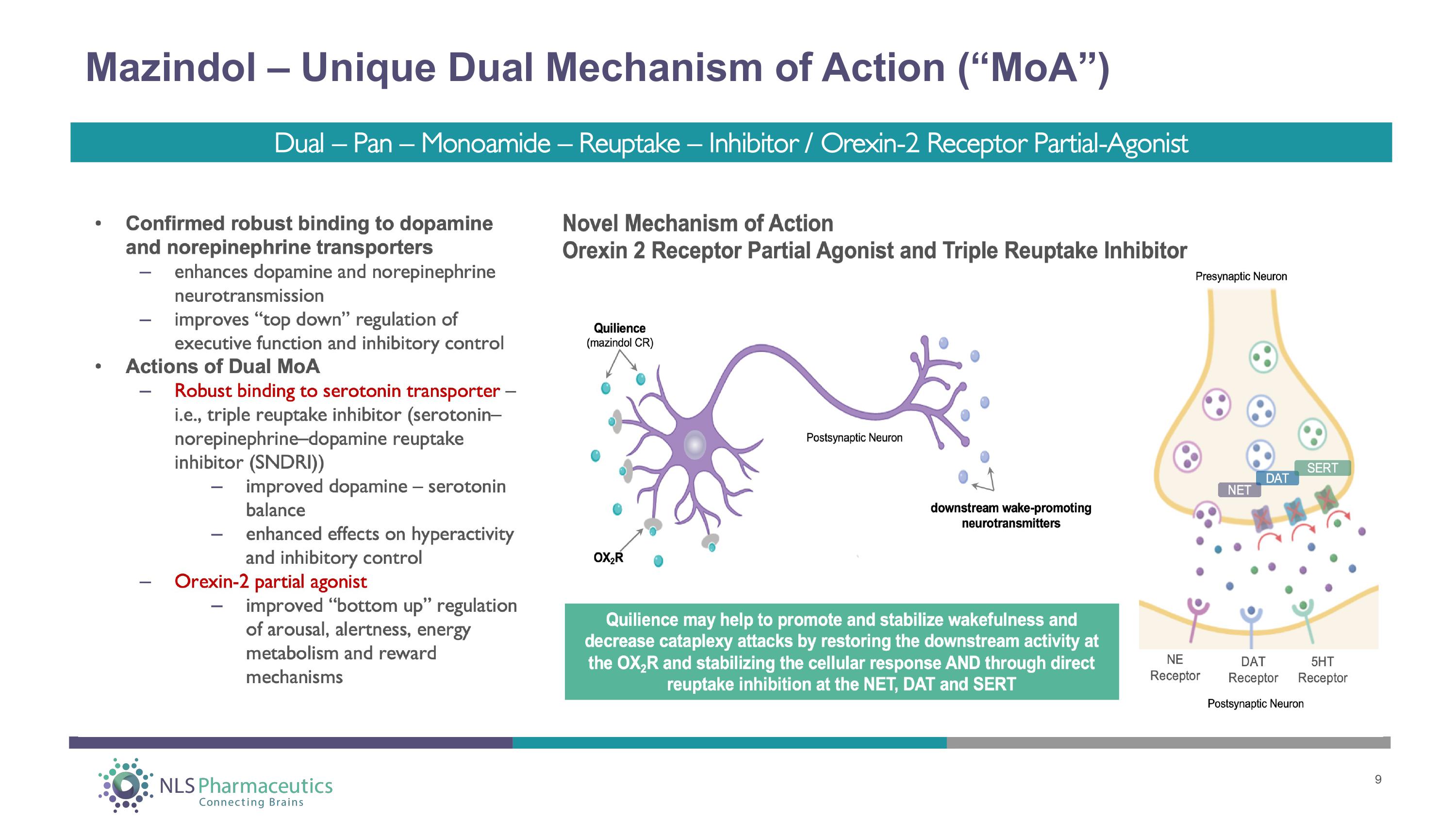
Mazindol – Unique Dual Mechanism of Action (“MoA”) 9 Dual – Pan – Monoamide – Reuptake – Inhibitor / Orexin - 2 Receptor Partial - Agonist
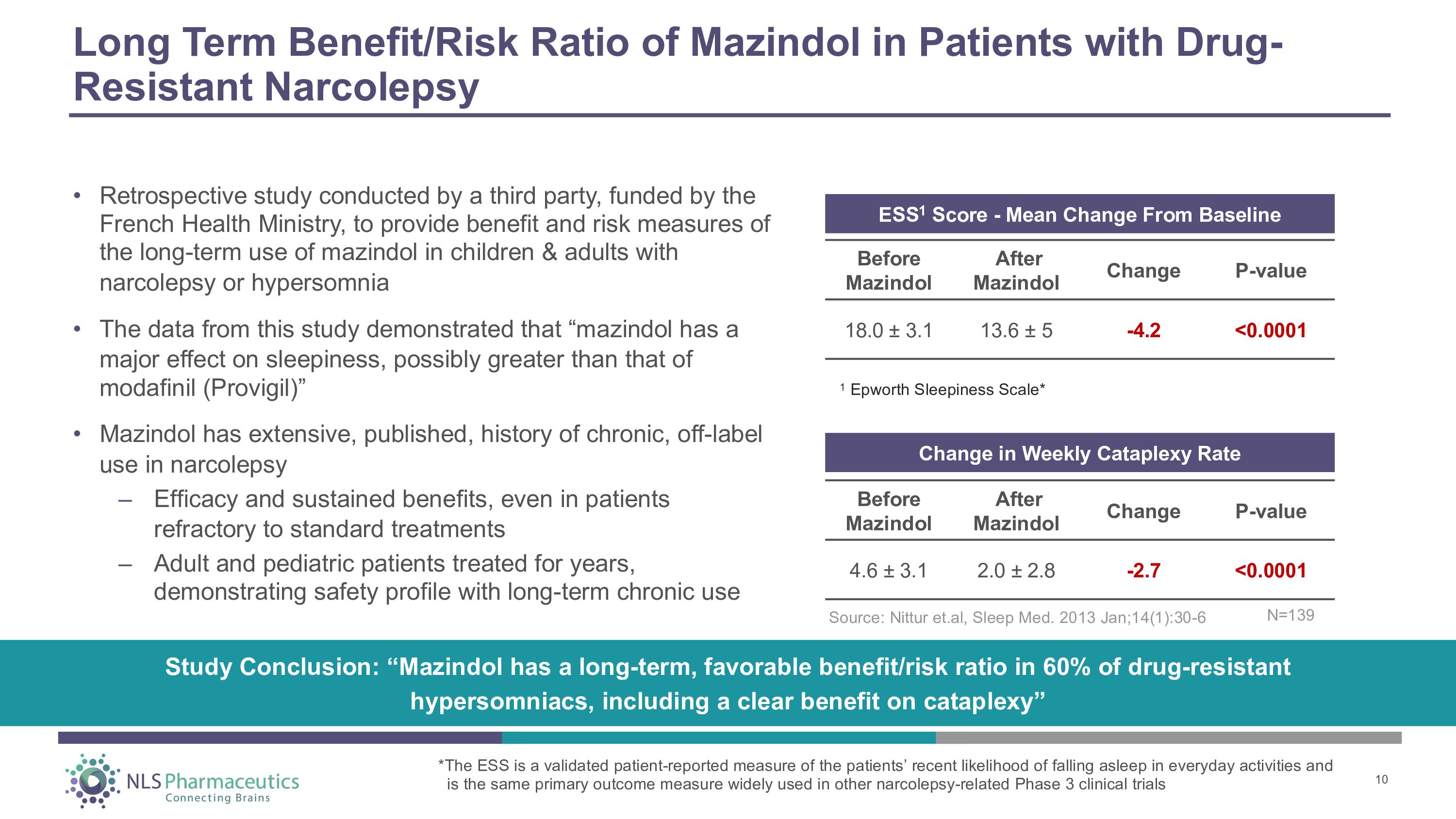
Long Term Benefit/Risk Ratio of Mazindol in Patients with Drug - Resistant Narcolepsy • Retrospective study conducted by a third party, funded by the French Health Ministry, to provide benefit and risk measures of the long - term use of mazindol in children & adults with narcolepsy or hypersomnia • The data from this study demonstrated that “mazindol has a major effect on sleepiness, possibly greater than that of modafinil (Provigil)” • Mazindol has extensive, published, history of chronic, off - label use in narcolepsy – Efficacy and sustained benefits, even in patients refractory to standard treatments – Adult and pediatric patients treated for years, demonstrating safety profile with long - term chronic use Before M a z i ndol After M a z i ndol Ch a nge P - va l ue 4.6 “ 3.1 2.0 “ 2.8 - 2.7 <0.0001 Change in Weekly Cataplexy Rate ESS 1 Score - Mean Change From Baseline Before M a z i ndol After M a z i ndol Ch a nge P - va l ue - 4.2 <0.0001 18.0 “ 3.1 13.6 “ 5 1 Epworth Sleepiness Scale* Study Conclusion: “Mazindol has a long - term, favorable benefit/risk ratio in 60% of drug - resistant hypersomniacs, including a clear benefit on cataplexy” Source: Nittur et.al, Sleep Med. 2013 Jan;14(1):30 - 6 10 *The ESS is a validated patient - reported measure of the patients’ recent likelihood of falling asleep in everyday activities and is the same primary outcome measure widely used in other narcolepsy - related Phase 3 clinical trials N = 13 9
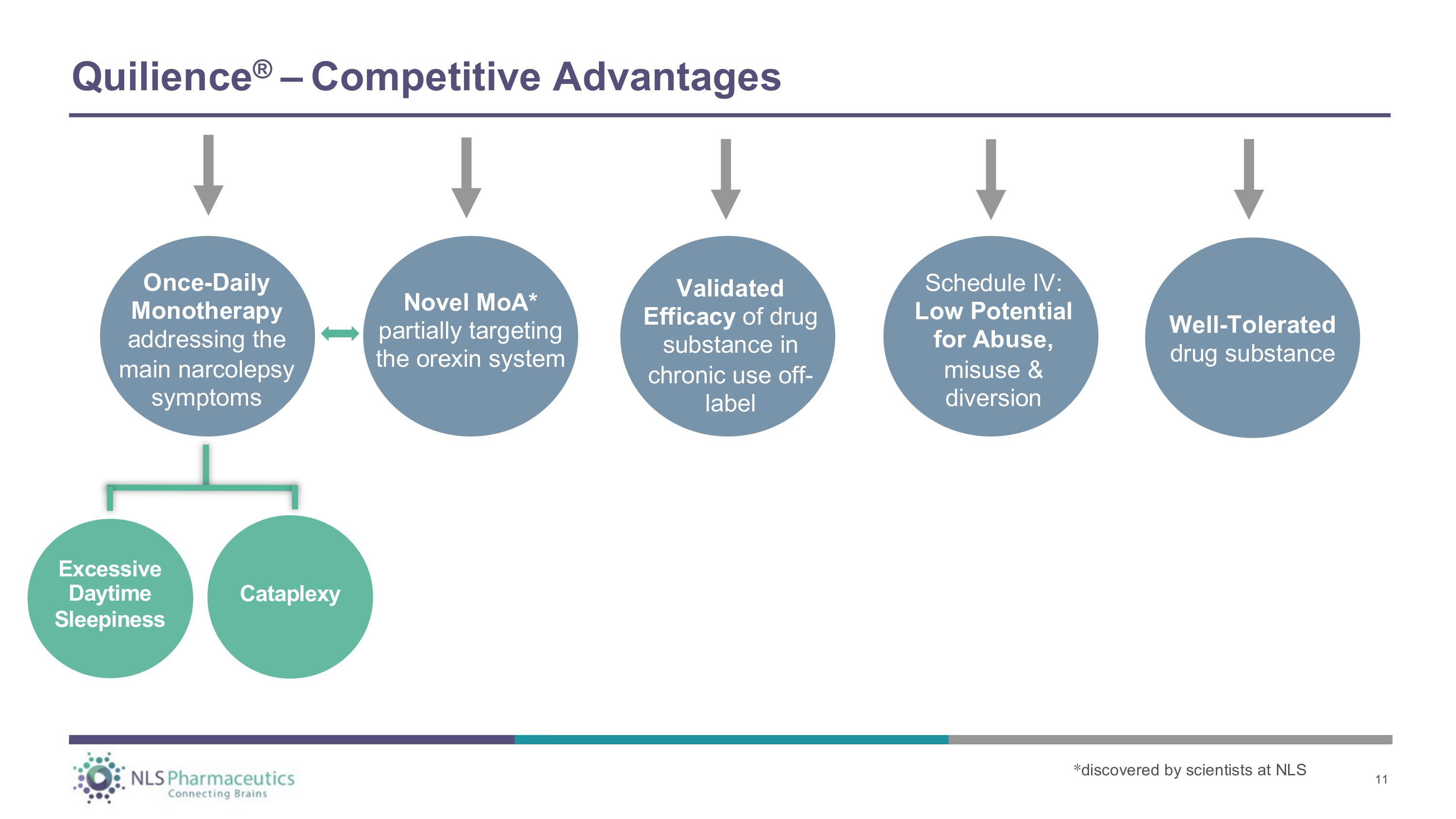
Novel MoA* partially targeting the orexin system Once - Daily Monotherap y addressing the main narcolepsy symptoms C a t a p l ex y Validated Efficacy of drug substance in chronic use off - label Well - Tolerated drug substance Excessive Daytime S l ee p i n es s Q u ili en ce ® – C o m p et i t i v e A d va n ta g e s Schedule IV: Low Potential for Abuse, misuse & diversion * discovered by scientists at NLS 11
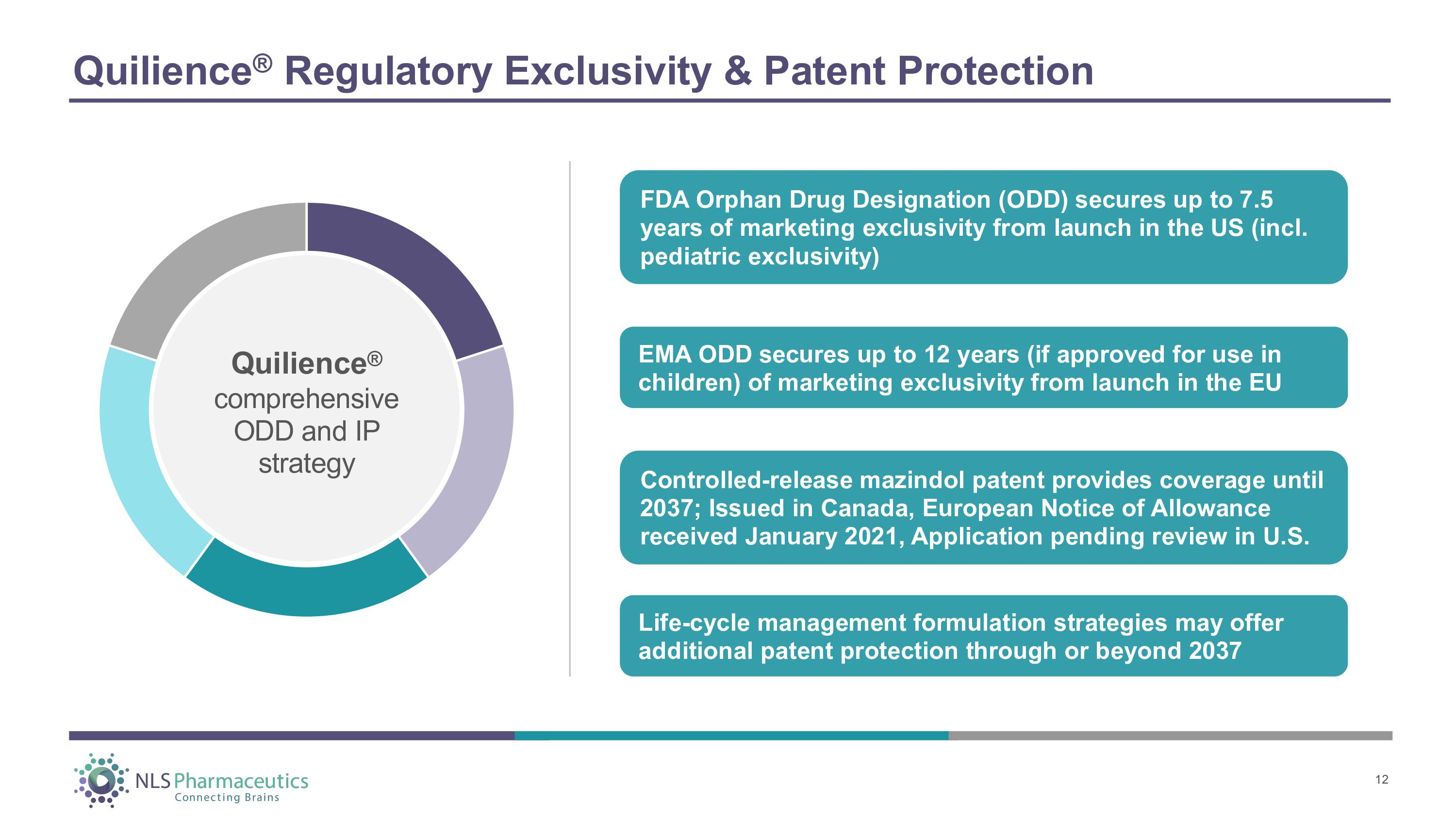
Quilience ® Regulatory Exclusivity & Patent Protection Quilience ® co m p r ehens i ve ODD and IP strategy FDA Orphan Drug Designation (ODD) secures up to 7.5 years of marketing exclusivity from launch in the US (incl. pediatric exclusivity) Controlled - release mazindol patent provides coverage until 2037; Issued in Canada, European Notice of Allowance received January 2021, Application pending review in U.S. EMA ODD secures up to 12 years (if approved for use in children) of marketing exclusivity from launch in the EU Life - cycle management formulation strategies may offer additional patent protection through or beyond 2037 12
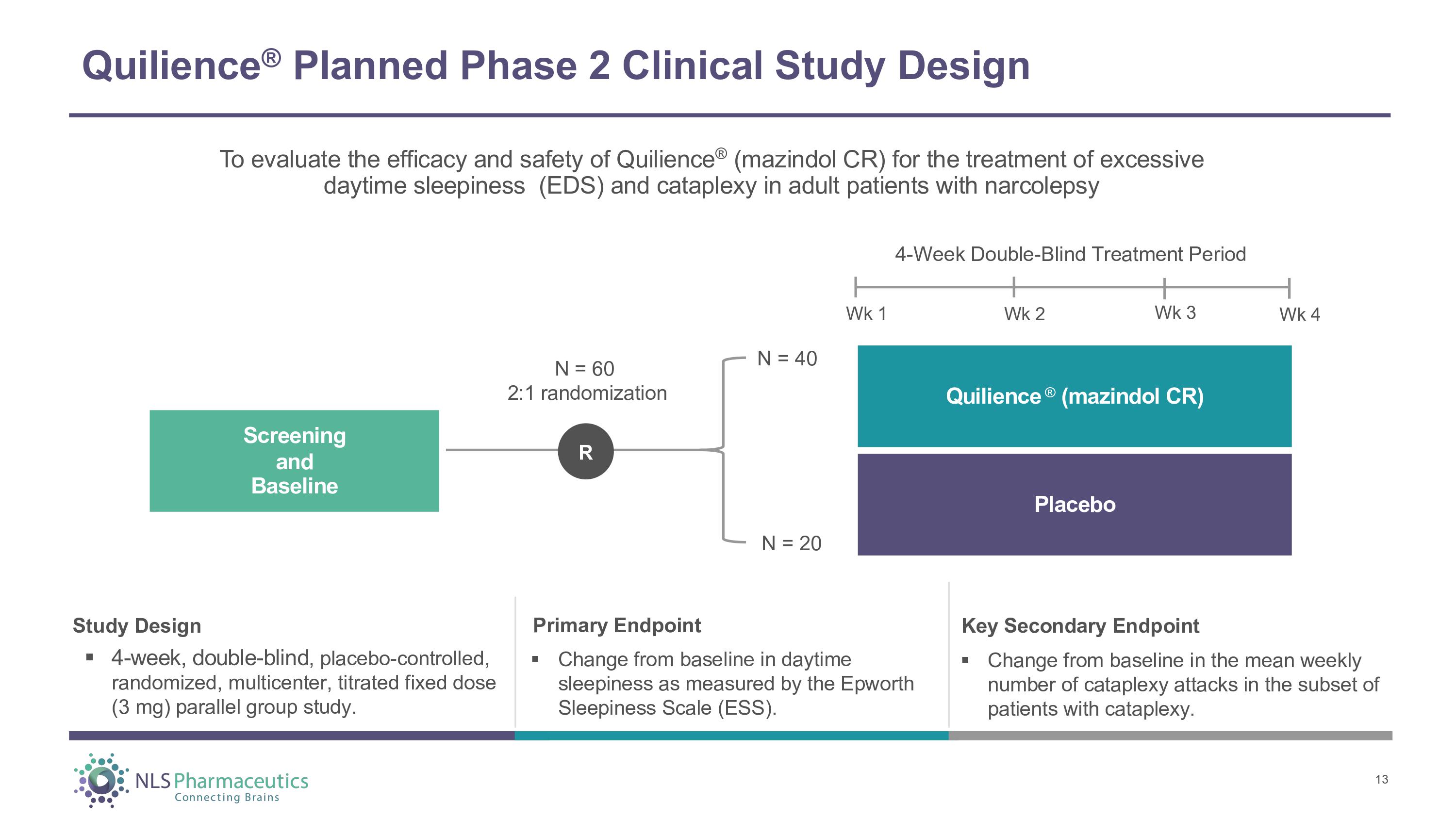
Quilience ® Planned Phase 2 Clinical Study Design Study Design ▪ 4 - week, double - blind , placebo - controlled, randomized, multicenter, titrated fixed dose (3 mg) parallel group study. Primary Endpoint ▪ Change from baseline in daytime sleepiness as measured by the Epworth Sleepiness Scale (ESS). To evaluate the efficacy and safety of Quilience ® (mazindol CR) for the treatment of excessive daytime sleepiness (EDS) and cataplexy in adult patients with narcolepsy 4 - Week Double - Blind Treatment Period Wk 1 Wk 2 Wk 3 Wk 4 Q u ili e n c e ® ( m a z i ndo l CR ) Placebo S c r ee n i n g and Baseline N = 60 2:1 randomization N = 40 N = 20 R 13 Key Secondary Endpoint ▪ Change from baseline in the mean weekly number of cataplexy attacks in the subset of patients with cataplexy.
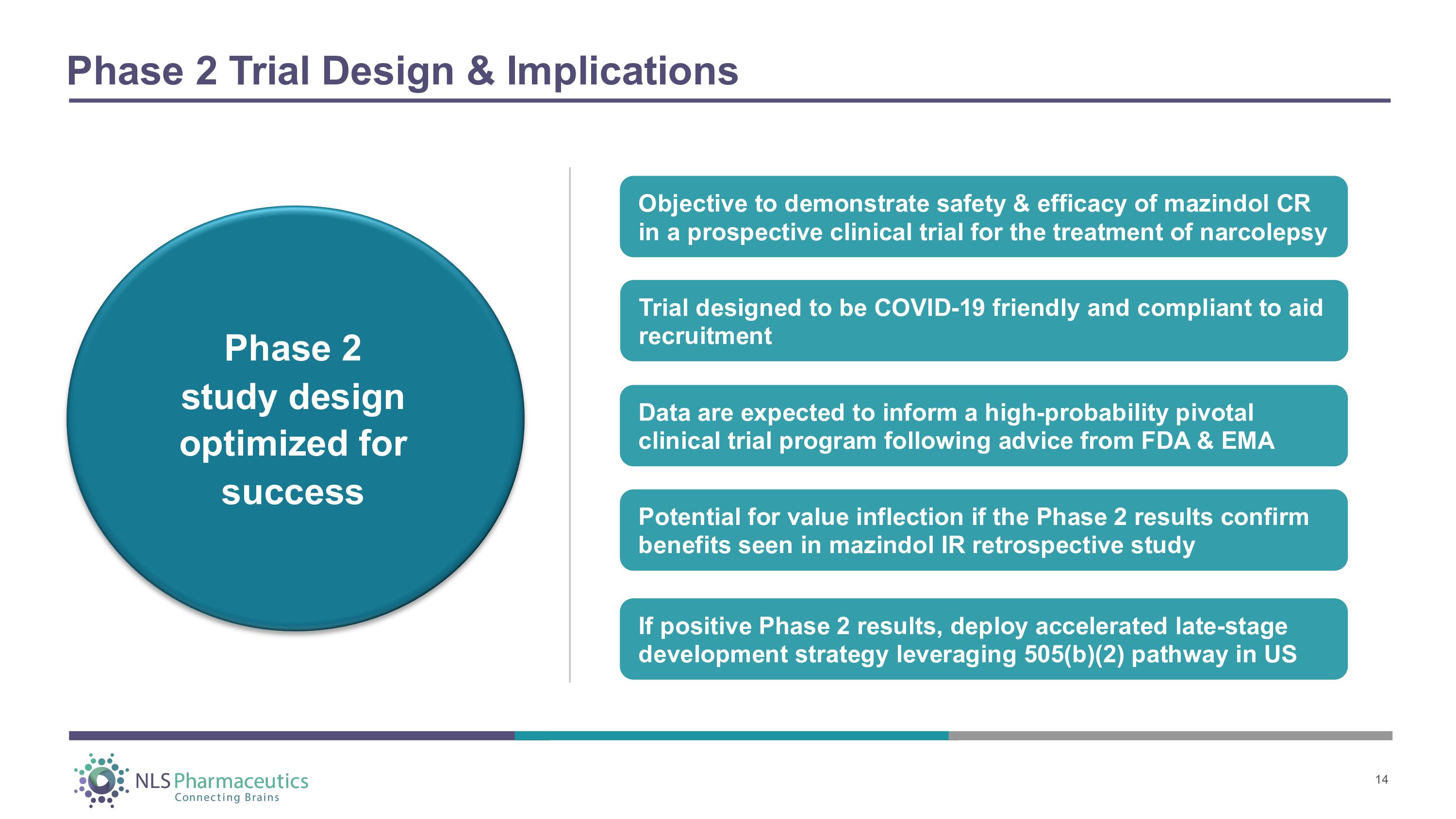
Phase 2 Trial Design & Implications Objective to demonstrate safety & efficacy of mazindol CR in a prospective clinical trial for the treatment of narcolepsy Data are expected to inform a high - probability pivotal clinical trial program following advice from FDA & EMA Potential for value inflection if the Phase 2 results confirm benefits seen in mazindol IR retrospective study If positive Phase 2 results, deploy accelerated late - stage development strategy leveraging 505(b)(2) pathway in US Phase 2 study design optimized for success Trial designed to be COVID - 19 friendly and compliant to aid recruitment 14
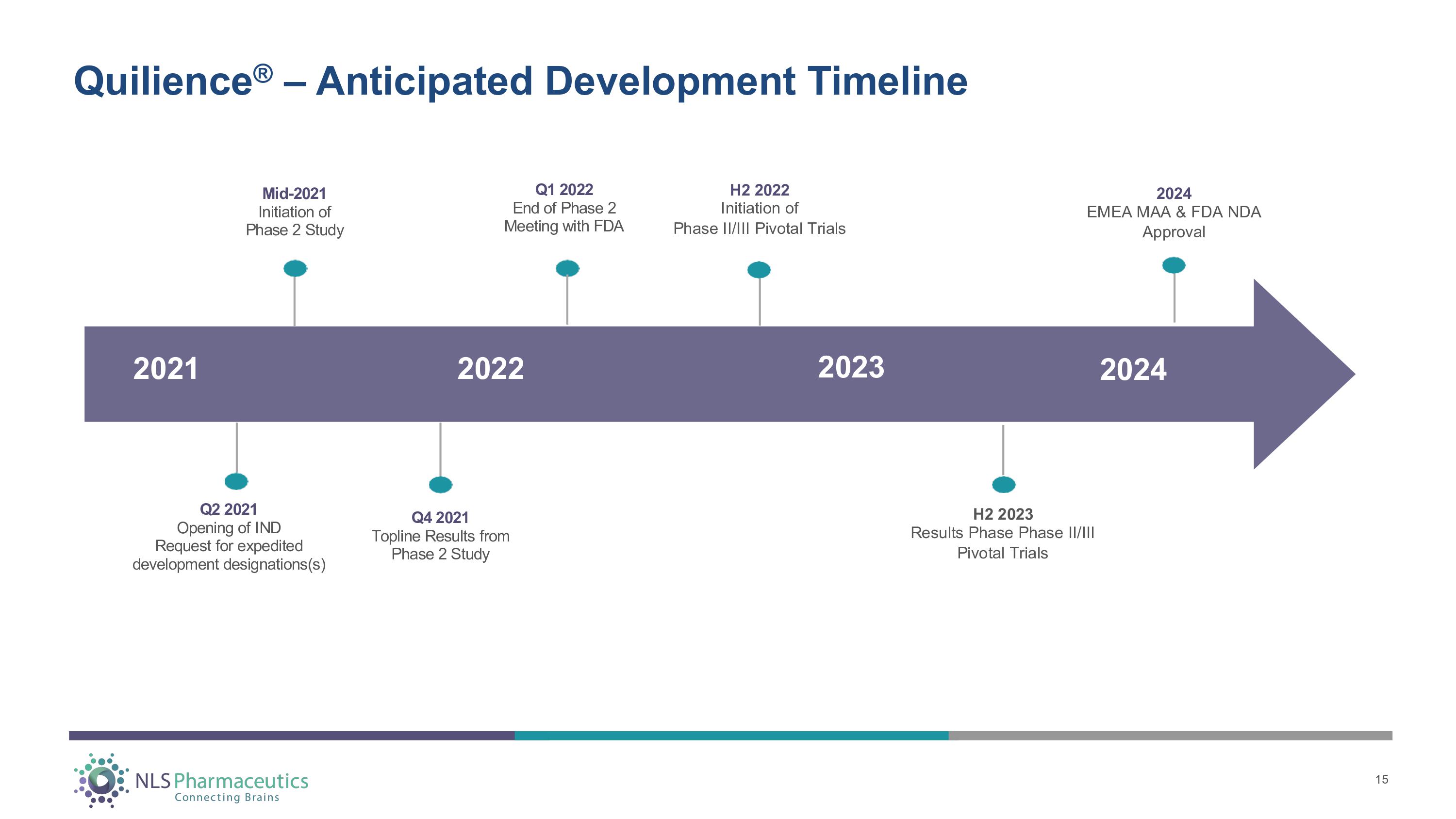
Quilience ® – Anticipated Development Timeline 202 4 202 3 202 2 202 1 H2 2022 Initiation of Phase II/III Pivotal Trials H2 2023 Results Phase Phase II/III Pivotal Trials 2024 EMEA MAA & FDA NDA Approval Q 2 202 1 Open i n g o f I N D R eque s t f o r e x ped i t e d development designations(s) Q 4 202 1 T op li n e R e s u l t s f r o m Pha s e 2 S t ud y Mid - 2021 I n i t i a t i o n o f Pha s e 2 S t ud y Q 1 202 2 En d o f Pha s e 2 M ee t i n g w i t h F D A 15

PROF. Dr. G.J. LAMMERS I know some narcolepsy patients who had a very good response on mazindol, and never reached a comparable level with any other pharmacological treatment including the most recently approved substances. Prof. of Neurology at Leiden University, current Chair of the Dutch Society for Sleep Medicine and co - founder of the European Narcolepsy Network (EU - NN) and served as President from 2007 - 2014. 16
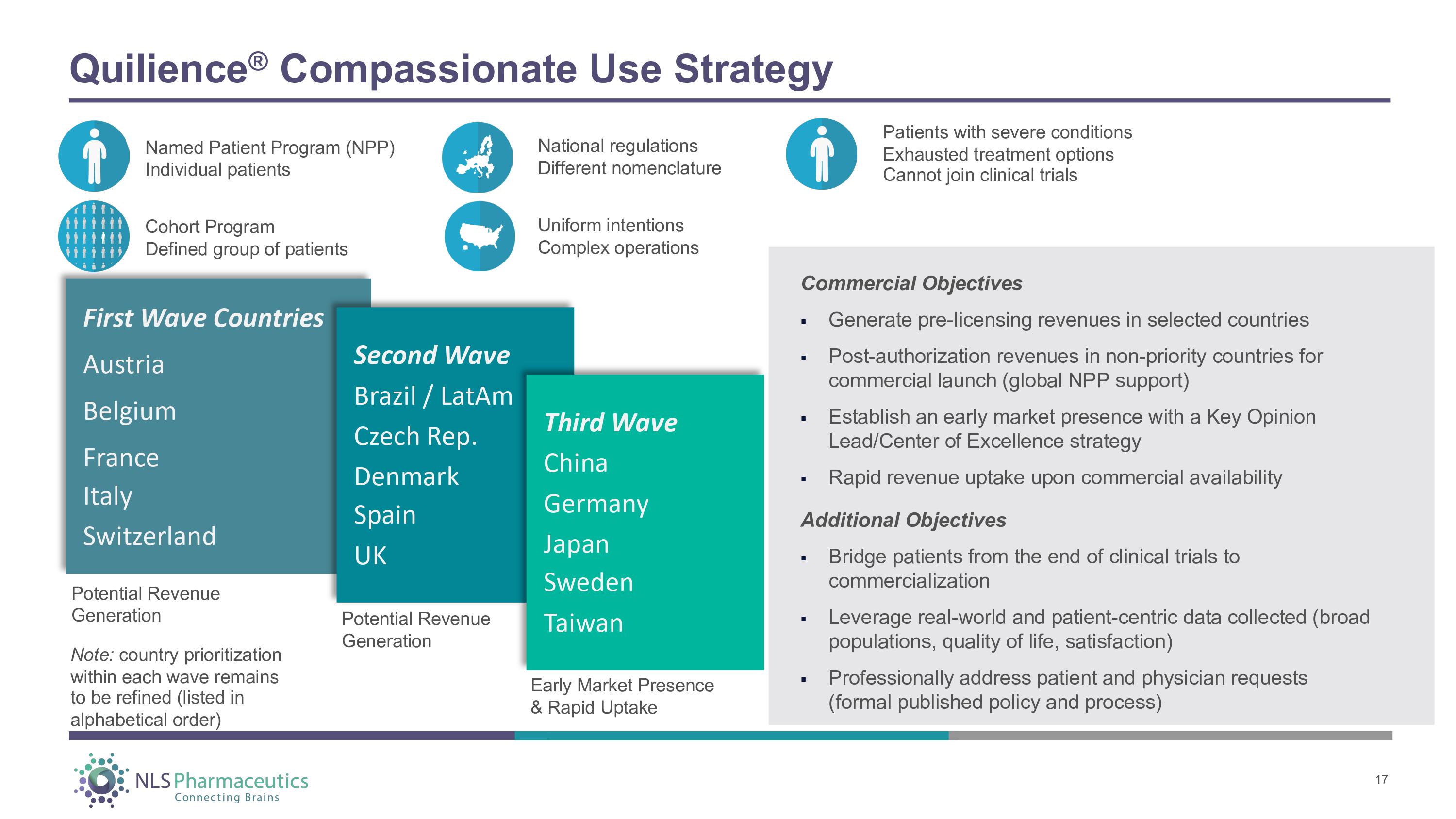
Quilience ® Compassionate Use Strategy Named Patient Program (NPP) Individual patients Cohort Program Defined group of patients National regulations Different nomenclature Patients with severe conditions Exhausted treatment options Cannot join clinical trials First Wave Countries Austria Belgium F r an ce Italy Switzerland Second Wave Brazil / LatAm Czech Rep. Denmark S pain UK Third Wave China Germany J a pan Sweden Taiwan Potential Revenue Generation Potential Revenue Generation Early Market Presence & Rapid Uptake Co mm e r c i a l O b j ec t i ve s 17 ▪ Generate pre - licensing revenues in selected countries ▪ Post - authorization revenues in non - priority countries for commercial launch (global NPP support) ▪ Establish an early market presence with a Key Opinion Lead/Center of Excellence strategy ▪ Rapid revenue uptake upon commercial availability Additional Objectives ▪ Bridge patients from the end of clinical trials to commercialization ▪ Leverage real - world and patient - centric data collected (broad populations, quality of life, satisfaction) ▪ Professionally address patient and physician requests (formal published policy and process) Uniform intentions Complex operations Note: country prioritization within each wave remains to be refined (listed in alphabetical order)

Mazindol CR - for the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) ® 18
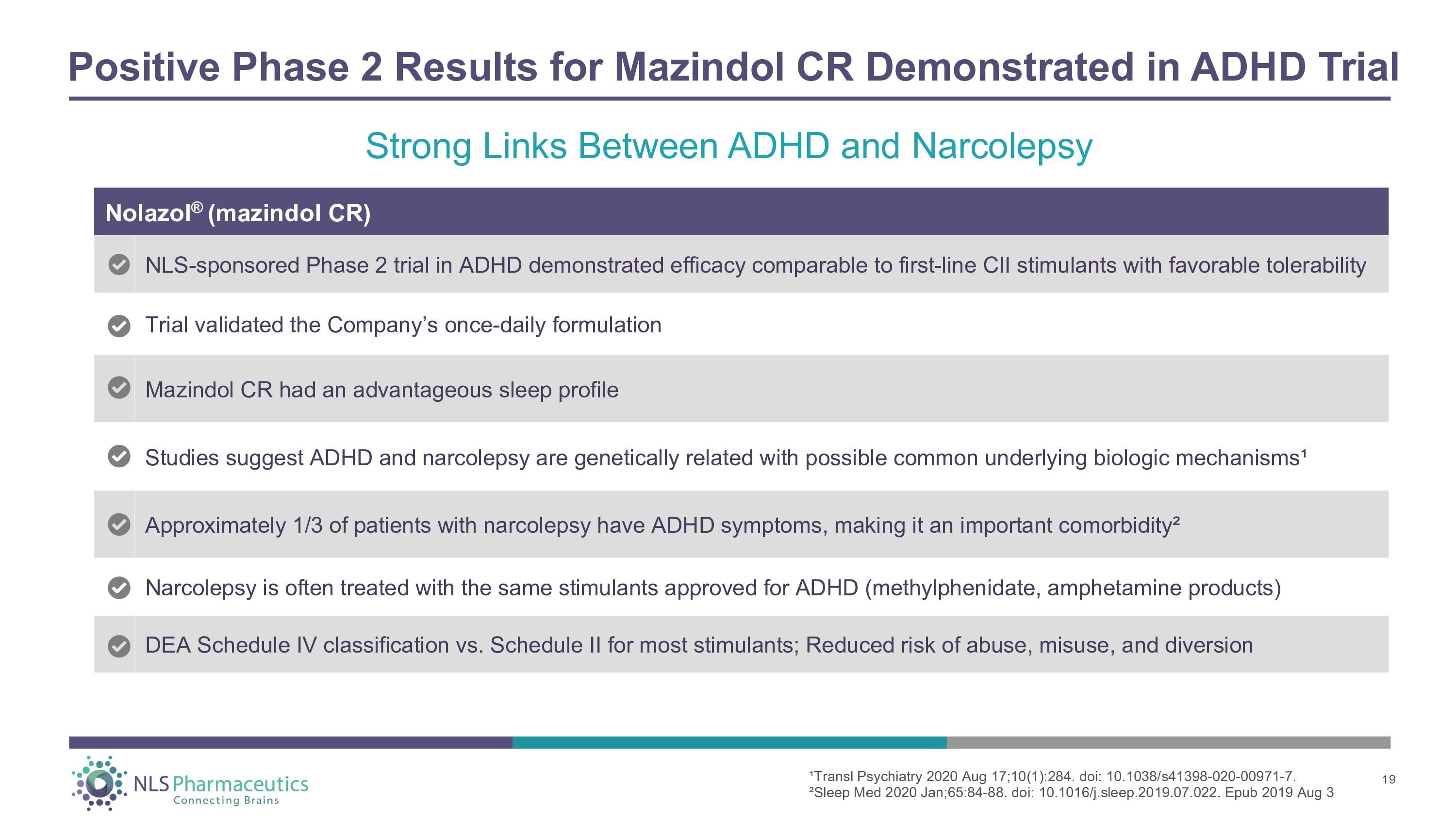
Positive Phase 2 Results for Mazindol CR Demonstrated in ADHD Trial Nolazol ® (mazindol CR) NLS - sponsored Phase 2 trial in ADHD demonstrated efficacy comparable to first - line CII stimulants with favorable tolerability Trial validated the Company’s once - daily formulation Mazindol CR had an advantageous sleep profile Studies suggest ADHD and narcolepsy are genetically related with possible common underlying biologic mechanisms¹ Approximately 1/3 of patients with narcolepsy have ADHD symptoms, making it an important comorbidity² Narcolepsy is often treated with the same stimulants approved for ADHD (methylphenidate, amphetamine products) DEA Schedule IV classification vs. Schedule II for most stimulants; Reduced risk of abuse, misuse, and diversion 19 1 Strong Links Between ADHD and Narcolepsy ¹Transl Psychiatry 2020 Aug 17;10(1):284. doi: 10.1038/s41398 - 020 - 00971 - 7. ²Sleep Med 2020 Jan;65:84 - 88. doi: 10.1016/j.sleep.2019.07.022. Epub 2019 Aug 3

Phase 2 Trial Primary Endpoint Results – Effect Size Greater Than or Equal to C - II Stimulant Levels Adult patients receiving Nolazol ® (mazindol CR) showed effect size greater than current ADHD best - in - class drugs Phase 2 clinical trial at seven sites in US Outpatient, multicenter, randomized, double - blind, placebo - controlled trial to assess efficacy, safety, tolerability, and pharmacokinetics (N=85) Flexible dose (1mg, 2mg, 3mg) Mean change from baseline in the ADHD - RS - DSM5 score at Day 42 of - 18.9 (and - 5.7 for placebo) was statistically significant in favor of Nolazol ® (mazindol CR) (P<0.001) AD HD - RS Effect size G o l d s t a nd a r d e ndpo i n t i n t h e f i e l d f o r de sc r i b i n g t h e d i ff e r en c e be t w ee n d r u g an d p l a c eb o c a l c u l a t e d a s t h e m ean d i ff e r en c e i n ADH D - R S sc o r e be t w ee n a r m s fr o m ba s e li n e t o Day 42 / pooled standard deviation at Day 42 ADHD - R S sc o r e – sca l e fr o m 0 - 54 ADHD - RS - DSM5 Least Squares Mean Scores *P = 0 . 005 ; **P < 0 . 001 . Effect size: 1.09 Effect Size >1 indicating high efficacy of Nolazol ® (mazindol CR) - low efficacy would be <0.4; moderate: 0.6; high: 0.8 Least Squares Mean improvements: Mean difference: - 18.9 (mazindol CR) and - 5.7 (placebo) - 13.2 ( - 18.7, - 7.6) Source: Wigal, T. L., Newcorn, J. H., Handal, N., Wigal, S. B., Mulligan, I., Schmith, V., & Konofal, E. (2018). A Double - Blind, Placebo - Controlled, Phase II Study to Determine the Efficacy, Safety, Tolerability and Pharmacokinetics of a Controlled Release (CR) Formulation of Mazindol in Adults with DSM - 5 Attention - Deficit/Hyperactivity Disorder (ADHD). CNS Drugs, 32(3), 289 - 301. doi:10.1007/s40263 - 018 - 0503 - y 20
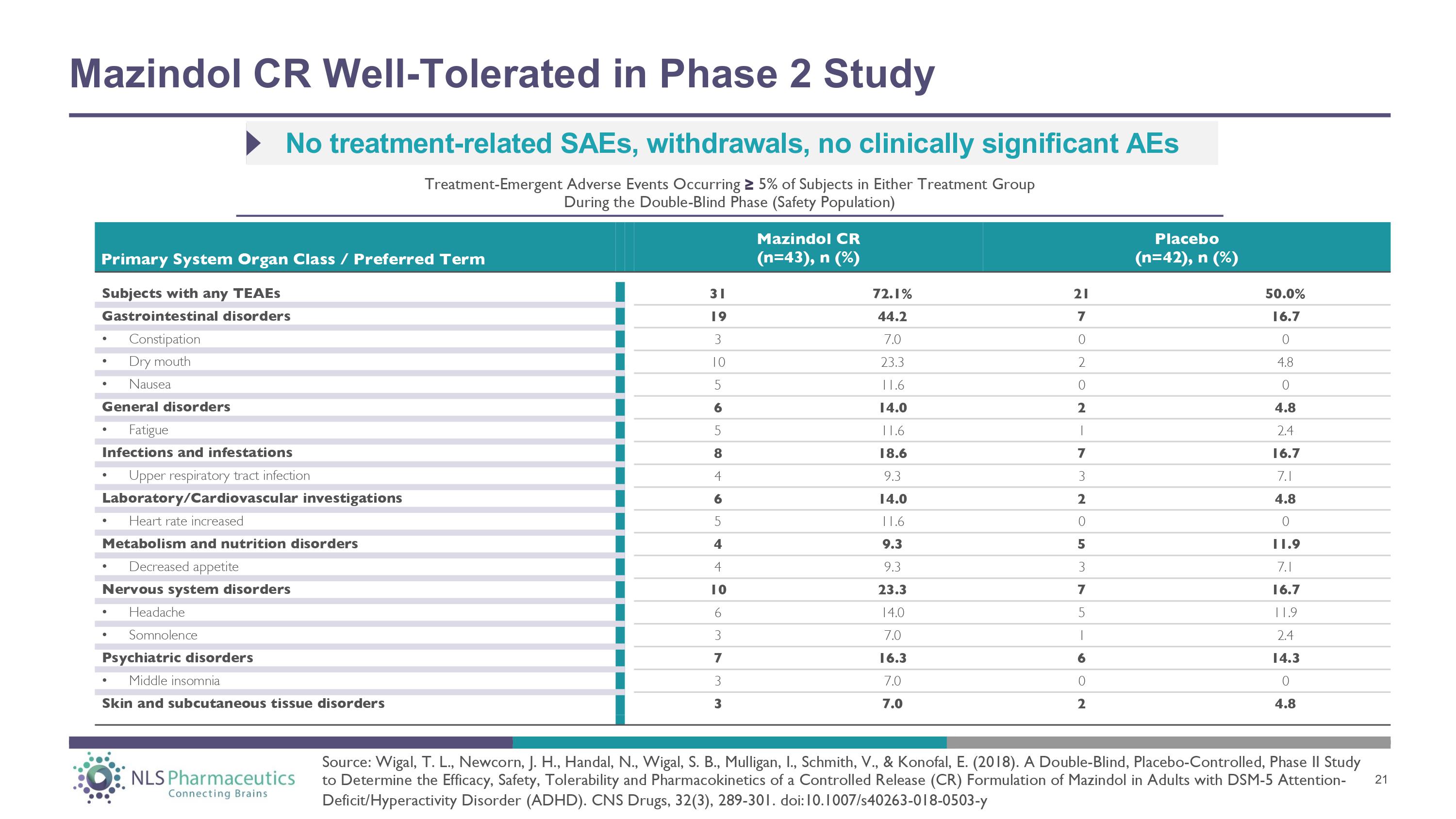
Mazindol CR Well - Tolerated in Phase 2 Study Subjects with any TEAEs 31 72.1% 21 50.0% Gastrointestinal disorders 19 44.2 7 16.7 • Constipation 3 7.0 0 0 • Dry mouth 10 23.3 2 4.8 • Nausea 5 11.6 0 0 General disorders 6 14.0 2 4.8 • Fatigue 5 11.6 1 2.4 Infections and infestations 8 18.6 7 16.7 • Upper respiratory tract infection 4 9.3 3 7.1 Laboratory/Cardiovascular investigations 6 14.0 2 4.8 • Heart rate increased 5 11.6 0 0 Metabolism and nutrition disorders 4 9.3 5 11.9 • Decreased appetite 4 9.3 3 7.1 Nervous system disorders 10 23.3 7 16.7 • Headache 6 14.0 5 11.9 • Somnolence 3 7.0 1 2.4 Psychiatric disorders 7 16.3 6 14.3 • Middle insomnia 3 7.0 0 0 Skin and subcutaneous tissue disorders 3 7.0 2 4.8 Primary System Organ Class / Preferred Term Mazindol CR (n=43), n (%) Placebo (n=42), n (%) No treatment - related SAEs, withdrawals, no clinically significant AEs Treatment - Emergent Adverse Events Occurring ≥ 5% of Subjects in Either Treatment Group During the Double - Blind Phase (Safety Population) Source: Wigal, T. L., Newcorn, J. H., Handal, N., Wigal, S. B., Mulligan, I., Schmith, V., & Konofal, E. (2018). A Double - Blind, Placebo - Controlled, Phase II Study to Determine the Efficacy, Safety, Tolerability and Pharmacokinetics of a Controlled Release (CR) Formulation of Mazindol in Adults with DSM - 5 Attention - Deficit/Hyperactivity Disorder (ADHD). CNS Drugs, 32(3), 289 - 301. doi:10.1007/s40263 - 018 - 0503 - y 21
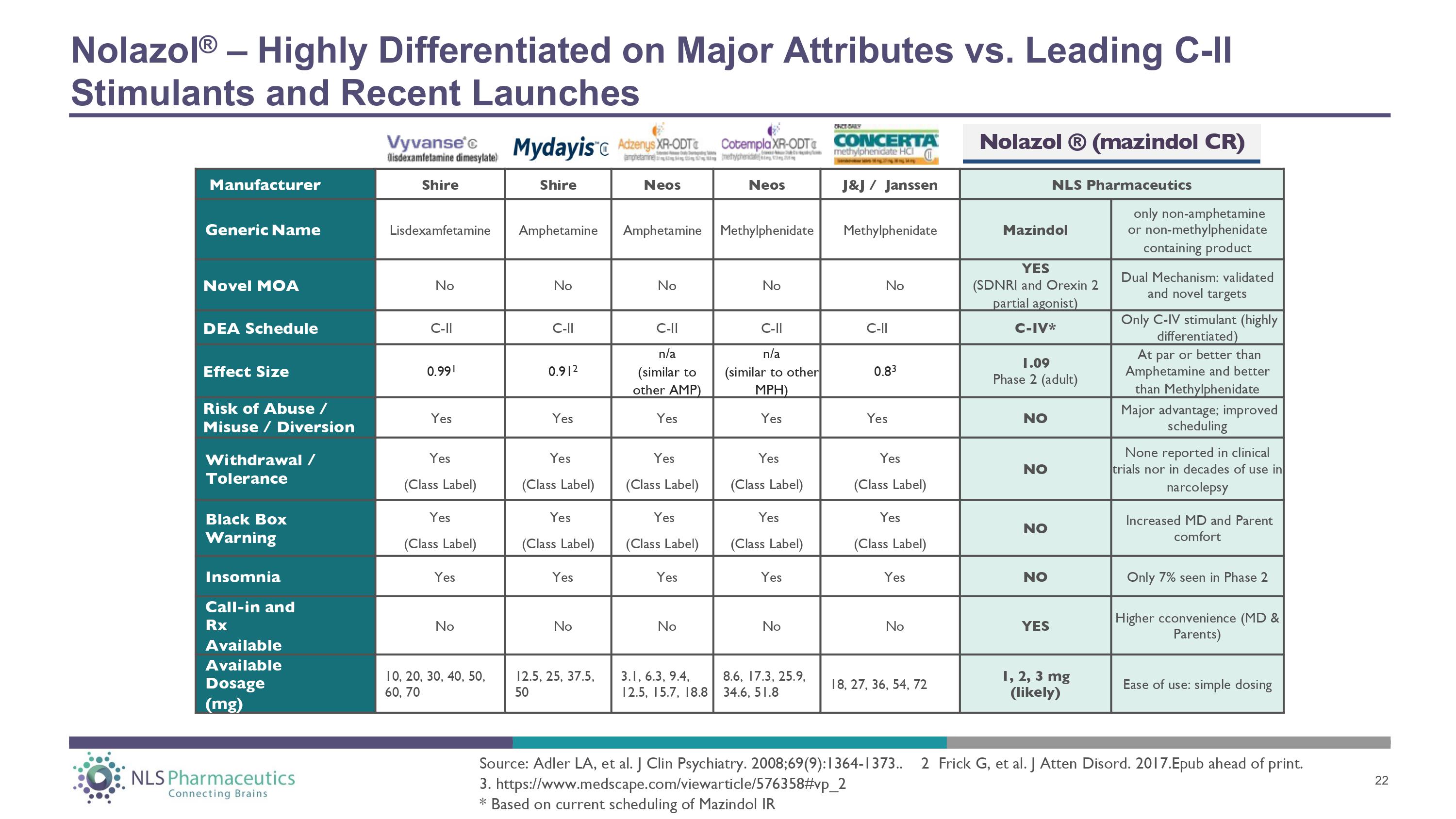
Manufacturer Shire Shire Neos Neos J&J / Janssen NLS Pharmaceutics Ge n e ric N a m e Lisdexamfetamine Amphetamine Amphetamine Methylphenidate Methylphenidate Mazindol only non - amphetamine or non - methylphenidate containing product Novel MOA No No No No No YES (SDNRI and Orexin 2 partial agonist) Dual Mechanism: validated and novel targets DEA Schedule C - II C - II C - II C - II C - II C - IV* Only C - IV stimulant (highly differentiated) Effect Size 0.99 1 0.91 2 n/a (similar to other A M P ) n/a (similar to other MPH) 0.8 3 1.09 Phase 2 (adult) At par or better than Amphetamine and better than Methylphenidate Risk of Abuse / Misuse / Diversion Yes Yes Yes Yes Yes NO Major advantage; improved scheduling Withdrawal / Tolerance Yes (Class Label) Yes (Class Label) Yes (Class Label) Yes (Class Label) Yes (Class Label) NO None reported in clinical trials nor in decades of use in narcolepsy B la c k Box Warning Yes (Class Label) Yes (Class Label) Yes (Class Label) Yes (Class Label) Yes (Class Label) NO Increased MD and Parent comfort Insomnia Yes Yes Yes Yes Yes NO Only 7% seen in Phase 2 Call - in and Rx Available No No No No No YES Higher cconvenience (MD & Parents) Av a il a ble Dosage (mg) 10, 20, 30, 40, 50, 60, 70 12.5, 25, 37.5, 50 3.1, 6.3, 9.4, 12.5, 15.7, 18.8 8.6, 17.3, 25.9, 34.6, 51.8 18, 27, 36, 54, 72 1, 2, 3 mg (likely) Ease of use: simple dosing Nolazol ® – Highly Differentiated on Major Attributes vs. Leading C - II Stimulants and Recent Launches Source: Adler LA, et al. J Clin Psychiatry. 2008;69(9):1364 - 1373.. 2 Frick G, et al. J Atten Disord. 2017.Epub ahead of print. 3. https:// www.medscape.com/viewarticle/576358#vp_2 * Based on current scheduling of Mazindol IR Nolazol ® (mazindol CR) 22

PROF. Dr. J. NEWCORN Associate Professor of Psychiatry and Pediatrics and Director of the Division of Child and Adolescent Psychiatry at Mount Sinai Medical Center in New York We have not seen any potential treatment for ADHD that had effects as robust as we saw in the Phase 2 adult study, except for C - II stimulants. The prospect of a medication with efficacy as high as is seen with the C - II stimulants that would be a DEA Schedule IV drug would be most welcome and no doubt very well received. This would especially be true for prescribers who have not had a track record of using C - II stimulants - and who would prefer not to, all things being equal. Dr. Newcorn is a founding member of the board of directors of the American Professional Society for ADHD and Related Disorders (APSARD), and has served as the Chair of the APSARD Program Committee for the past three years. He is also Chair of the Advisory Board of the Klingenstein Third Generation Foundation and Head of the SAB for NLS in ADHD. 23
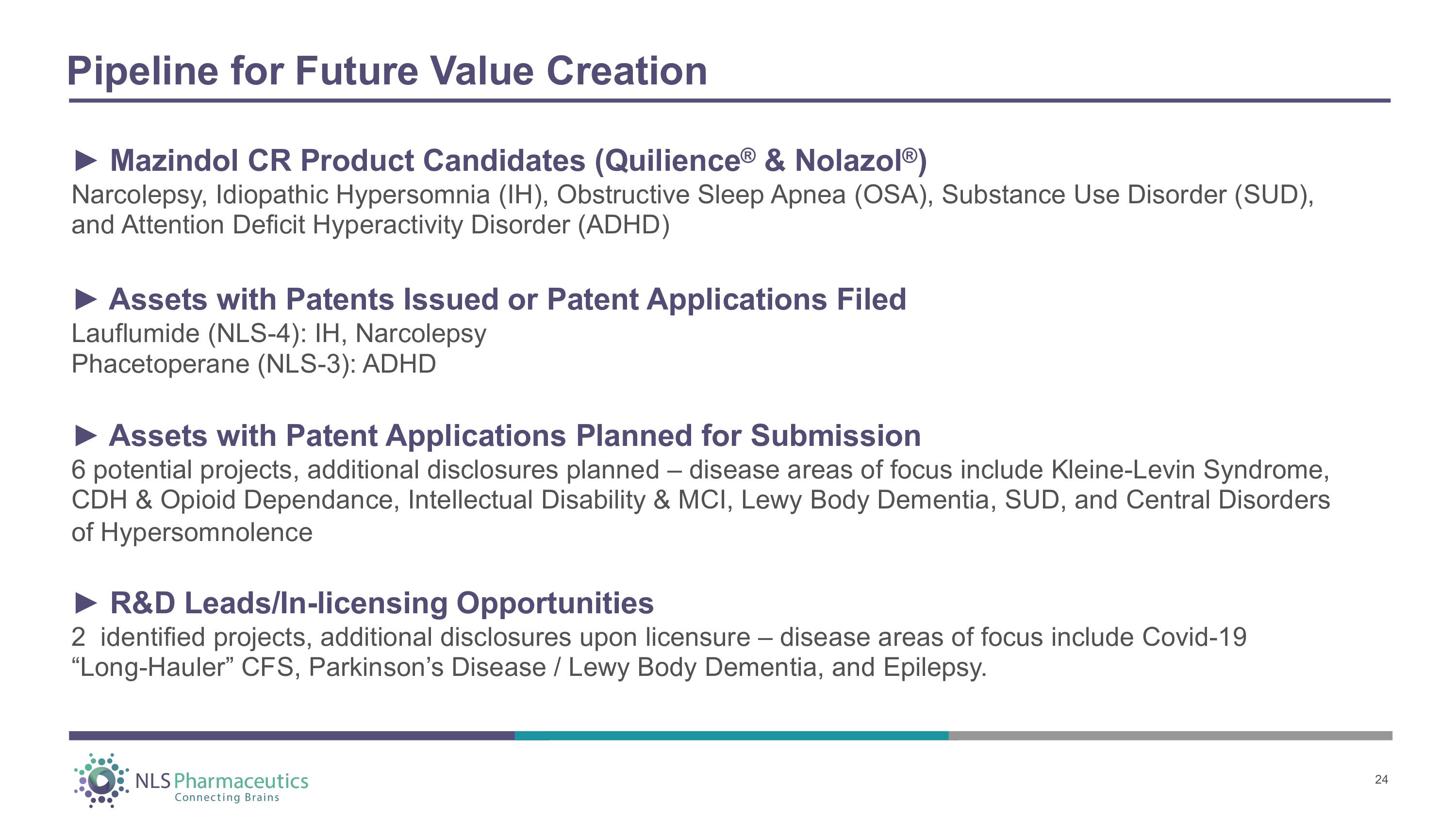
Pipeline for Future Value Creation 24 ► Mazindol CR Product Candidates (Quilience ® & Nolazol ® ) Narcolepsy, Idiopathic Hypersomnia (IH), Obstructive Sleep Apnea (OSA), Substance Use Disorder (SUD), and Attention Deficit Hyperactivity Disorder (ADHD) ► Assets with Patents Issued or Patent Applications Filed Lauflumide (NLS - 4): IH, Narcolepsy Phacetoperane (NLS - 3): ADHD ► Assets with Patent Applications Planned for Submission 6 potential projects, additional disclosures planned – disease areas of focus include Kleine - Levin Syndrome, CDH & Opioid Dependance, Intellectual Disability & MCI, Lewy Body Dementia, SUD, and Central Disorders of Hypersomnolence ► R&D Leads/In - licensing Opportunities 2 identified projects, additional disclosures upon licensure – disease areas of focus include Covid - 19 “Long - Hauler” CFS, Parkinson’s Disease / Lewy Body Dementia, and Epilepsy.
File IND in U . S . to initiate Quilience ® Phase 2 trial in patients with narcolepsy ; potential for accelerated regulatory pathway designations Initiate compassionate use programs outside the U.S. with potential for revenue generation Complete Phase 2 trial for Quilience ® ; results anticipated in late 2021 Potential for business development activity including license agreements or partnerships with other drug companies Patent applications for mazindol controlled - release formulation anticipated to mature in the U . S . and Europe Disclosures and updates for the Company’s CNS drug pipeline 25 Anticipated Milestones & Corporate Priorities

26

























