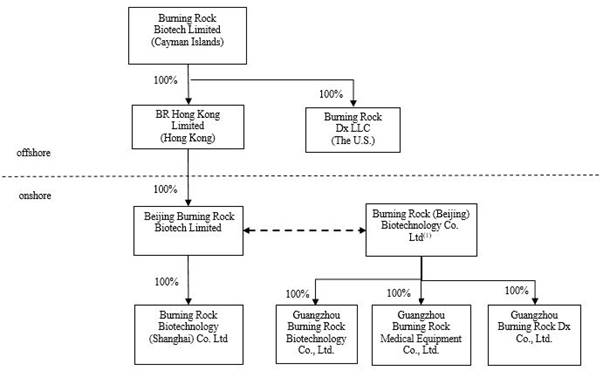
We conduct
our NGS-based cancer
therapy selection business primarily through the
wholly-owned
subsidiaries of the VIE, Guangzhou Burning Rock Dx Co., Ltd. and Guangzhou Burning Rock Medical Equipment Co., Ltd., which were established in March 2014 and January 2015, respectively.
On June 12, 2020, our ADSs commenced trading on NASDAQ Global Market under the symbol “BNR.” We raised from our initial public offering US$234.9 million net proceeds, after the underwriters exercised in full their option to purchase additional ADSs. Concurrently with our initial public offering, we raised US$25 million from Lake Bleu Prime Healthcare Master, in a private placement.
On December 8, 2020, we completed a registered
follow-on
public offering by certain selling shareholders of 2,243,000 ADSs at a public offering price of US$25.75 per ADS. We did not receive any proceeds from the
follow-on
public offering.
On May 27, 2021, we were included in the MSCI China Index. We were the only
NGS-based
precision oncology company from China that has been selected in the current MSCI
semi-annual
index review.
Our principal executive offices are located at No. 5, Xingdao Ring Road North, International Bio Island, Guangzhou, the People’s Republic of China. Our telephone number at this address is
+86 020-3403 7871.
Our registered office in the Cayman Islands is located at the offices of Maples Corporate Services Limited at PO Box 309, Ugland House, Grand
Cayman, KY1-1104, Cayman
Islands.
Investors should submit any inquiries to the address and telephone number of our principal executive offices. Our main website is http://www.brbiotech.com. The information contained on our website is not a part of this annual report.
SEC maintains an internet site (http://www.sec.gov), which contains reports, proxy and information statements, and other information regarding us that file electronically with the SEC.
We aim to transform precision oncology and early cancer detection. We are China’s
leading NGS-based cancer
therapy selection company. Our cancer therapy selection platform is built upon our advanced proprietary technologies, comprehensive portfolio of products and
a two-pronged market-driven
commercial infrastructure addressing both larger hospitals through
our in-hospital model
and smaller hospitals through our central laboratory model.
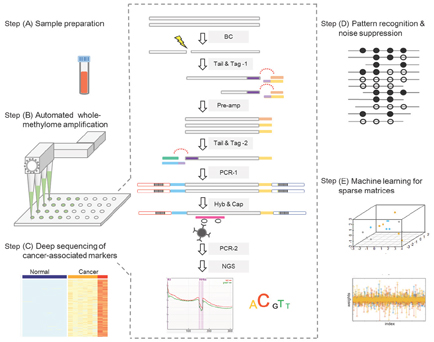
Our advanced technology platform integrates
cutting-edge
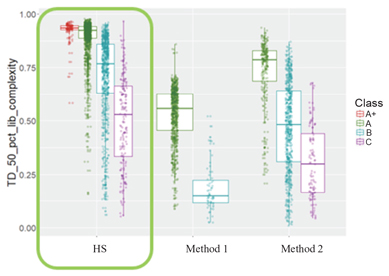
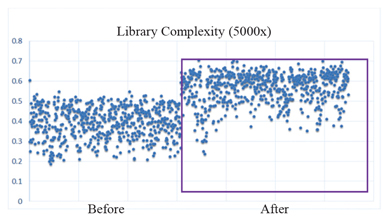
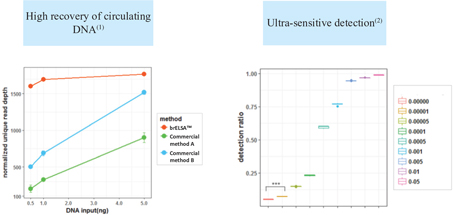
proprietary cancer therapy selection technologies using both tissue and liquid biopsies, including assay biochemistry, bioinformatics and a patented laboratory information management system. Our proprietary HS library preparation technology allows us to work with poor quality and limited volume samples and enables enhanced sensitivity—capabilities that are critical to effectively
deploying NGS-based cancer
therapy selection, especially in China.
Our in-depth cancer
genomics insights, accumulated from the large number of tests we have performed since our inception, enable us to process and accurately analyze genomic information and achieve a median turnaround time of 6 days.
Our NGS-based cancer
therapy selection test products are used to assist physicians in selecting the most effective therapy for cancer patients. We primarily offer
15 NGS-based cancer
therapy selection and prognosis prediction tests applicable to a broad range of cancer types, including lung cancer, gastrointestinal cancer, prostate cancer, breast cancer, lymphomas, thyroid cancer, colorectal cancer, ovarian cancer, pancreatic cancer, and bladder cancer, using both tissue and liquid biopsy samples. Our core products, including OncoCompass
™
IO, OncoScreen
™
IO and OncoCompass
™
Target,
perform on par with those of our global peers. We launched our minimal residual disease (MRD) product, brPROPHET
™
, in March 2022, which has demonstrated superior sensitivity and specificity to fixed panel in
pre-operative
ctDNA detection and
post-operative
MRD calling among relapsed patients. We are the clear leader in the lung cancer segment of
China’s NGS-based cancer
therapy selection market. We believe we offer the
best NGS-based cancer
therapy selection products and services in China, and we have won the trust of pharmaceutical companies, physicians, hospitals and patients with our high quality standards, superior product performance and strong service support. Our products are recognized by the medical, pharmaceutical and scientific communities, as evidenced by (i) the use of our products by oncology key opinion leaders in clinical trials and research studies they initiate, and (ii) our collaborations on clinical trials and research studies with leading pharmaceutical companies including AstraZeneca (NYSE: AZN), Bayer (ETR: BAYN), Johnson & Johnson (NYSE: JNJ), CStone (HKEX: 2616), BeiGene (HKEX: 6160), Abbisko Therapeutics (HKEX: 2256), IMPACT Therapeutics and Merck KGaA (ETR: MRK), primarily by providing central laboratory services and companion diagnostics development services to these pharmaceutical companies. The results of these clinical trials and research studies have been published in over 200
peer-reviewed
articles, and the results of research studies using our products have been published in numerous
peer-reviewed
articles.