Failure to comply with existing or future laws and regulations related to the management of human genetic resources in China could lead to government enforcement actions, which could include civil or criminal fines or penalties, private litigation, other liabilities, and/or adverse publicity. Compliance or the failure to comply with such laws could increase the costs of, limit and cause significant delay in our clinical studies and research and development activities, and could otherwise materially and adversely affect our operating results, business and prospects.
Laws and regulations related to the management of human genetic resources in China are rapidly evolving and the enforcement thereof is likely to remain uncertain for the foreseeable future. On June 10, 1998, the Ministry of Science and Technology, or MOST, and the Ministry of Health jointly established the rules for protecting and utilizing human genetic resources, or HGR, in China. From 2006 to 2016, MOST and other regulatory agencies in China have been focused on HGR legislation, and proactively sought opinions from the public on draft regulations. In 2015, MOST issued a Guideline on HGR and reinforced its legislative efforts in HGR administration. In May 2019, the Regulation on Human Genetic Resources Management, or the HGR Regulation, was put in place. The State Council promulgated the HGR Regulation on May 28, 2019 and it became effective on July 1, 2019.
The HGR Regulation prohibits foreign entities or individuals or such entities established or actually controlled thereby, or “Foreign Persons,” from collecting or preserving China HGR in China, or providing China HGR abroad, whereas activities of collection and preservation of organs, tissues and cells for purposes of clinical diagnosis and treatment, service of blood collection and provision, investigation of illegal activities, doping test and funeral service, are required to be conducted in accordance with other relevant laws and regulations. The HGR Regulation permits Foreign Persons’ limited use of China HGR “to carry out scientific research activities,” which must be conducted through collaboration with Chinese scientific research institutions, higher education institutions, medical institutions, or enterprises, collectively, the “Chinese Entities.” Such activities must be approved by MOST, and the application for approval must be filed jointly by the Foreign Person and the relevant Chinese Entity. The only exception to the approval requirement is “international collaboration in clinical trials” that do not involve the outbound transfer of China HGR materials such as organs, tissues, or cells comprising the human genome, genes, or other genetic substances, collectively, China HGR Materials. Such clinical trial collaboration, however, must still be pre-registered with MOST. There remain significant uncertainties as to how provisions of the HGR Regulation might be interpreted and implemented. A VIE entity actually controlled by a foreign entity through contractual agreements would be deemed as a Foreign Person under the HGR Regulation. Short-term storage of samples of laboratory testing by foreign laboratories or foreign-invested laboratories may also be interpreted as preserving China HGR, thus being subjected to MOST application, approval or pre-registration processes.
On October 17, 2020, the Standing Committee of the NPC promulgated the Biosecurity Law of the PRC which became effective on April 15, 2021. The new law, among other things, restates relevant approval or pre-registration requirements of HGR collection, preservation, utilization and external provision, as provided in the HGR Regulation. Moreover, the promulgation of the new law, which takes the form of national law, further demonstrates the commitments of protecting China HGR and safeguarding state biosecurity by the PRC government.
As a company with a VIE structure since our inception, we are deemed as a Foreign Person under the HGR Regulation. As a result, when conducting or participating in research and clinical studies that involve any of our products, performing clinical studies for any of our pipeline products that are under development (including our early detection products), or providing companion diagnostics services to pharmaceutical companies, we are required to seek approval of or make pre-registration with MOST with respect to our collaborations with Chinese Entities under the HGR Regulation. These procedures could be lengthy and require additional expenses, and there is no assurance that we can complete these pre-registrations, or obtain such approvals, in a timely manner, or at all. As a result, our clinical studies and research and development activities of any of our products or pipeline products that are under development (including our early detection products), and our companion diagnostics development services to pharmaceutical companies may suffer significant delay, experience suspension, rejections, cancellations and other obstacles. As a result, our business, financial conditions, results of operations and prospects could be materially adversely affected.
As of the date of this annual report, we, same as our peer companies in the healthcare industry in China, have received and may continue receiving notices from the relevant governmental authorities requiring us to share HGR-related information with competent government agencies from time to time, and have complied with all such requests. As of the same date, we have not been subject to any penalties from the competent governmental authorities for our business operations or clinical studies involving the use of China HGR. However, regulatory agencies in China may change their enforcement practices. Therefore, prior enforcement activity, or lack of enforcement activity, is not necessarily predictive of future actions. Failure to comply with existing or future HGR laws and regulations, including the HGR Regulation and the Biosecurity Law, may subject us to penalties, including fines, suspension of related activities and confiscation of related HGR and gains generated from conducting these activities.
The evolving government regulations may place additional burdens on our efforts to commercialize our products and services.
The PRC government has introduced various reforms to the Chinese healthcare system in recent years and may continue to do so, with an overall objective of expanding basic medical insurance coverage and improve the quality and reliability of healthcare services. The specific regulatory changes under the reforms still remain uncertain. The implementing measures to be issued may not be sufficiently effective to achieve the stated goals, and as a result, we may not be able to benefit from these reforms to the level we expect, if at all. Moreover, the reforms could give rise to regulatory developments, such as more burdensome administrative procedures, which may have an adverse effect on our business and prospects.
23
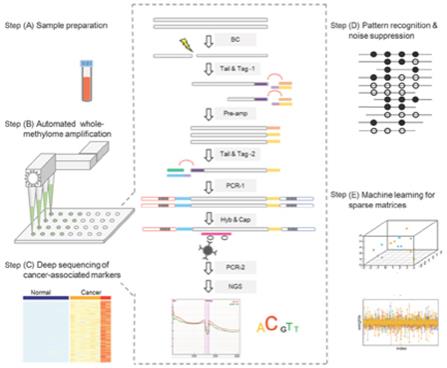
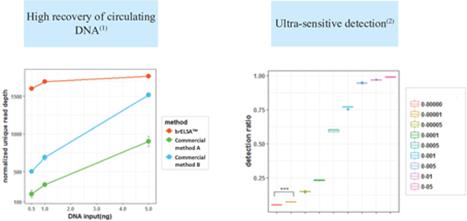
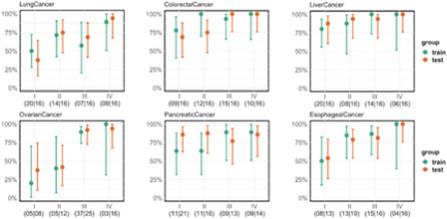
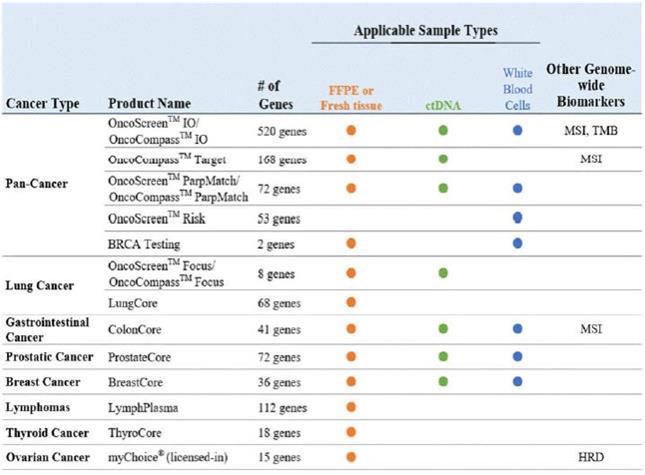
 ”, “BURNING ROCK DX”, “
”, “BURNING ROCK DX”, “  ” and product and service names, and 107 trademark applications pending in China. We had registered six trademarks, including “BURNING ROCK DX”, “ONCOSCREEN” AND “ONCOCOMPASS” in European Union, the U.K. and Australia, and thirteen trademark applications pending in the US, European Union, the U.K., Japan, Australia and Brazil. We also own four registered domain names, including our official website.
” and product and service names, and 107 trademark applications pending in China. We had registered six trademarks, including “BURNING ROCK DX”, “ONCOSCREEN” AND “ONCOCOMPASS” in European Union, the U.K. and Australia, and thirteen trademark applications pending in the US, European Union, the U.K., Japan, Australia and Brazil. We also own four registered domain names, including our official website.