Table of Contents
| ☐ | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES ACT OF 1934 |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| ☐ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Title of each class | Trading Symbol | Name of each exchange on which registered | ||
American depositary shares, each representing one Class A ordinary share | BNR | The Nasdaq Stock Market LLC (The Nasdaq Global Market) | ||
Class A ordinary share, par value US$0.0002 per share* | The Nasdaq Stock Market LLC (The Nasdaq Global Market) | |||
American depositary shares, each representing one Class A ordinary share | BNR | London Stock Exchange (The Main Market) ) |
| * | Not for trading, but only in connection with the listing of the Nasdaq Global Market of American depositary shares. |
| Large accelerated filer | ☐ | Accelerated filer | ☒ | Non-accelerated filer | ☐ | |||||
| Emerging growth company | ☐ | |||||||||
| † | The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012. |
| U.S. GAAP ☒ | International Financial Reporting Standards as issued by the International Accounting Standards Board ☐ | Other ☐ |
Table of Contents
TABLE OF CONTENTS
| 1 | ||||
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS | 1 | |||
| 1 | ||||
| 1 | ||||
| 53 | ||||
| 86 | ||||
| 86 | ||||
| 101 | ||||
| 111 | ||||
| 112 | ||||
| 113 | ||||
| 113 | ||||
ITEM 11. QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK | 128 | |||
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES | 129 | |||
| 132 | ||||
| 132 | ||||
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS | 132 | |||
| 132 | ||||
| 133 | ||||
| 133 | ||||
| 133 | ||||
ITEM 15D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES | 133 | |||
ITEM 15E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS | 134 | |||
| 134 | ||||
| 134 | ||||
| 134 | ||||
ITEM 15I. DISCLOSURE REGARDING FOREIGN JURISDICTION THAT PREVENT INSPECTIONS | 134 | |||
| 134 | ||||
| 135 | ||||
| 135 | ||||
| 135 | ||||
| 135 | ||||
| 136 |
i
Table of Contents
CONVENTIONS THAT APPLY TO THIS ANNUAL REPORT ON FORM 20-F
Unless otherwise indicated and except where the context otherwise requires, references in this annual report on Form 20-F to:
| • | “ADSs” refer to American depositary shares, each of which represents one Class A ordinary share; |
| • | “Burning Rock,” “we,” “us,” “our company” and “our” refer to Burning Rock Biotech Limited, a Cayman Islands exempted company, and its subsidiaries and consolidated affiliated entities; |
| • | “China” or “the PRC” refers to the People’s Republic of China, including Hong Kong and Macau; the only instances in which “China” or “the PRC” do not include Hong Kong or Macau are when used in the case of laws and regulations adopted by the People’s Republic of China; the legal and operational risks associated with operating in China also apply to our operations in Hong Kong; |
| • | “liquid biopsy” refers to a test done on a blood sample that enables the access to the molecular information, by looking for cancer cells from a tumor that are circulating in the blood or for pieces of DNA from tumor cells that are in the blood, throughout all stages of cancer; |
| • | “MRD” refers to minimal residual disease, a small number of cancer cells left in the body after treatment; |
| • | “NGS” refers to next-generation sequencing, a DNA sequencing technology used to determine the nucleotide sequence of an individual’s genome; |
| • | “RMB” or “Renminbi” refers to the legal currency of China; |
| • | “sensitivity” refers to the percentage of people who test positive for a specific disease or condition among people who actually have the disease or condition; |
| • | “shares” or “ordinary shares” refer to our Class A and Class B ordinary shares, par value US$0.0002 per share; |
| • | “specificity” refers to the percentage of people who test negative for a specific disease or condition among people who do not have the disease or condition; |
| • | “U.S. GAAP” refers to accounting principles generally accepted in the U.S.; |
| • | “US$,” “U.S. dollars,” “$,” and “dollars” refer to the legal currency of the U.S; and |
| • | “the VIE” refers to our PRC variable interest entity, Burning Rock (Beijing) Biotechnology Co. Ltd. |
Our reporting currency is the Renminbi. This annual report also contains translations of certain foreign currency amounts into U.S. dollars for the convenience of the reader. Unless otherwise stated, all translations from Renminbi to U.S. dollars were made at a rate of RMB7.0999 to US$1.00, the exchange rate set forth in the H.10 statistical release of the Board of Governors of the Federal Reserve System on December 29, 2023. We make no representation that any Renminbi or U.S. dollar amounts referred to in this annual report could have been or could be converted into U.S. dollars or Renminbi, as the case may be, at any particular rate, or at all. On April 19, 2024, the exchange rate set forth in the H.10 statistical release of the Federal Reserve Board was RMB7.2403 to US$1.00.
All of our share related numbers contained in this annual report, including but not limited to the numbers of authorized, issued and outstanding shares, have retroactively reflected the 2-for-1 reverse share split that we effected in January 2020.
ii
Table of Contents
FORWARD-LOOKING STATEMENTS
This annual report on Form 20-F contains statements of a forward-looking nature. All statements other than statements of current or historical facts are forward-looking statements. These forward-looking statements are made under the “safe harbor” provision under Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and as defined in the Private Securities Litigation Reform Act of 1995. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “likely to” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include, but are not limited to, statements about:
| • | our mission and strategies; |
| • | trends and competition in China’s cancer genotyping industry; |
| • | our expectations regarding demand for and market acceptance of our NGS-based products and services and our ability to expand our customer base; |
| • | our ability to obtain and maintain intellectual property protections for our cancer therapy selection technologies and our continued research and development to keep pace with technology developments; |
| • | our ability to obtain and maintain regulatory approvals from China’s National Medical Products Administration (“NMPA”), the NCCL and have our laboratory certified or accredited by authorities including the CLIA and the CAP; |
| • | our future business development, financial condition and results of operations; |
| • | our ability to obtain financing cost-effectively; |
| • | potential changes of government regulations, regardless of whether they are directly related to our industry; |
| • | our ability to hire and maintain key personnel; |
| • | global or national health concerns, including the outbreak of pandemic or contagious diseases such as the pandemic of COVID-19; |
| • | our relationship with our major business partners and customers; and |
| • | general economic and business conditions in China and elsewhere. |
You should read these statements in conjunction with the risks disclosed in “Item 3. Key Information—D. Risk Factors” of this annual report and other risks outlined in our other filings with the Securities and Exchange Commission, or the SEC. Moreover, we operate in an emerging and evolving environment. New risks may emerge from time to time, and it is not possible for our management to predict all risks, nor can we assess the impact of such risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ materially from those contained in any forward-looking statements. The forward-looking statements made in this annual report relate only to events or information as of the date on which the statements are made in this annual report. Except as required by law, we undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this annual report and the documents that we have referred to in this annual report, completely and with the understanding that our actual future results may be materially different from what we expect.
iii
Table of Contents
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
Risks Associated with Being Based in or Having the Majority of the Operations in China
As we mainly conduct our business in China, we may be subject to PRC laws relating to, among others, data security and restrictions over foreign investments in scientific research and technical services and other industry sectors set out in the Special Administrative Measures (Negative List) for the Access of Foreign Investment (2021 Edition), or the Negative List (2021 Edition). Specifically, we may be subject to PRC laws relating to the collection, use, sharing, retention security, and transfer of confidential and private information, such as personal information and other data. These PRC laws apply not only to third-party transactions, but also to transfers of information between us and our wholly foreign-owned enterprises in China, and other parties with which we have commercial relations. These PRC laws and their interpretations and enforcement continue to develop and are subject to change, and the PRC government may adopt other rules and restrictions in the future.
We are exposed to legal and operational risks associated with our operations in China. The PRC government has significant authority to exert influence on the ability of a company with operations in China, including us, to conduct its business. Changes in China’s economic, political or social conditions or government policies could materially and adversely affect our business and results of operations. We are subject to risks due to the uncertainty of the interpretation and the application of the PRC laws and regulations, including but not limited to the risks of uncertainty about any future actions of the PRC government on U.S. listed companies. We may also be subject to sanctions imposed by PRC regulatory agencies, including the China Securities Regulatory Commission (the “CSRC”), if we fail to comply with their rules and regulations. Any actions by the PRC government to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in companies having operations in China, including us, could significantly limit or completely hinder our ability to offer or continue to offer securities to investors, and cause the value of our securities to significantly decline or become worthless. These China-related risks could result in a material change in our operations and/or the value of our securities, or could significantly limit or completely hinder our ability to offer securities to investors in the future and cause the value of such securities to significantly decline or become worthless.
The PRC government may exert, at any time, substantial intervention and influence over the manner of our operations. Recently, the PRC government initiated a series of regulatory actions and statements to regulate business operations in China with little advance notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas, adopting new measures to extend the scope of cybersecurity reviews and new laws and regulations related to data security, and expanding the efforts in anti-monopoly enforcement.
On December 28, 2021, the CAC, and 12 other departments jointly promulgated the newly revised Measures for Cybersecurity Review with effect from February 15, 2022 (“Measures”), which provides that (i) a critical information infrastructure operator (“CIIO”) which intends to purchase network products and services shall prejudge the possible risks to national security that may arise after the products and services are put into use and where national security will or may be affected, the operator shall apply with the Cybersecurity Review Office for cybersecurity review, and (ii) a network platform operator (“NPO”) that possesses more than one million users’ personal information must apply for cybersecurity review before listing in a foreign country.
On November 14, 2021, the CAC publicly solicited opinions on the Regulations on the Administration of Cyber Data Security (Draft for Comments) which expanded the scope of application of cybersecurity review, established the data classified and categorized protection system, and defined the relevant rules for cross-border security management of data. It provides that data processors carrying out the following activities shall apply for cybersecurity review: (i) merger, reorganization or division of Internet platform operators that gather and possess a large number of data resources having bearing on the national security, economic development or public interests, which affects or may affect national security; (ii) listing in a foreign country of a data processor that processes the personal information of more than one million persons; (iii) listing in Hong Kong of a data processor, which affects or may affect national security; and (iv) other data processing activities that affect or may affect national security.
According to the above provisions, we will be subject to cybersecurity review if we are identified as a CIIO that procures network products or services which affect or may affect national security after being put into use or NPO that conducts data processing activities which affect or may affect national security, or we have more than one million users’ personal information and plans to be listed abroad.
1
Table of Contents
We and our PRC legal counsel, Tian Yuan Law Firm, are of the view that, as of the date of this annual report, the possibility that we become identified as a CIIO or NPO and accordingly would be subject to the cybersecurity review pursuant to the relevant regulations and policies that have been issued by the CAC is relatively low, due to the following reasons:
| (i). | we have not received any CIIO identification notice as of the date of this annual report, which is required to be issued in a timely manner by competent departments responsible for the security protection work of critical information infrastructures after they have organized the CIIO identification in the industry in accordance with the Regulations on the Security Protection of Critical Information Infrastructures; |
| (ii). | NPO is not defined in the Measures and even if a company has been identified as NPO, whether such a company needs to be subject to cybersecurity review depends on whether its data processing activities will “affect or may affect national security.” As of the date of this annual report, we have not experienced any major information security incident in relation to the theft, leakage, damage, illegal use or illegal export of data or personal information. In addition, all the user data collected by us in business operation are stored in mainland China; |
| (iii). | We process no more than one million users’ personal information; and |
| (iv). | However, according to article 16 of the Measures, the member unit of the cybersecurity review work mechanism (the “Cybersecurity Member Unit”) has the right to initiate review on network products and services and data processing activities that it deems as “affect or may affect national security” at its own discretion. If the Cybersecurity Member Unit decides to take a cybersecurity review on us and we fail such review, it could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline or become worthless. |
Currently, the cybersecurity laws and regulations have not directly affected our business and operations, but in anticipation of the strengthened implementation of cybersecurity laws and regulations and the expansion of our business, we face potential risks if we are deemed as a CIIO under applicable laws. In such case, we must fulfill certain obligations as required under the Cybersecurity Law and other applicable laws, including, among others, storing personal information and important data collected and produced within the PRC territory during our operations in China, which we are already doing in our business, and we may be subject to review when purchasing internet products and services. As the amended Measures for Cybersecurity Review took effect in February 2022, we may be subject to review when conducting data processing activities, and may face challenges in addressing its requirements and make necessary changes to our internal policies and practices in data processing. As of the date of this annual report, we have not been involved in any investigations on cybersecurity review made by the CAC on such basis, and we have not received any inquiry, notice, warning, or sanctions in such respect. Based on the foregoing, we and our PRC legal counsel, Tian Yuan Law Firm, do not expect that, as of the date of this annual report, the current applicable PRC laws on cybersecurity would have a material adverse impact on our business. After consulting with our PRC legal counsel, Tian Yuan Law Firm, we believe that we are in compliance with regulations or policies that have been issued by the CAC as of the date of this annual report in all material aspects, on the following bases: (i) we have set up internal cybersecurity regulations, including data backup and recovery measures and disaster recovery measures; (ii) we have completed the Grade III information security protection filing for major software as required by the relevant regulations and policies issued by relevant authorities; (iii) we inform our users and obtain their consent before collecting their personal information; (iv) we store relevant information in our own servers within the PRC; (v) we have not been investigated or received any request from any CAC authorities as of the date of this annual report; (vi) we have not been subject to any administrative penalties regarding cybersecurity or data security issues as of the date of this annual report; and (vii) we have not been a party to any litigation or arbitration regarding with cybersecurity or data security issues as of the date of this annual report. As advised by Tian Yuan Law Firm, our PRC counsel, as of the date of this annual report, we are not subject to any cybersecurity review under the cybersecurity laws and regulations.
On September 1, 2021, the PRC Data Security Law became effective, which imposes data security and privacy obligations on entities and individuals conducting data-related activities, and introduces a data classification and hierarchical protection system based on the importance of data in economic and social development, as well as the degree of harm it will cause to national security, public interests, or legitimate rights and interests of individuals or organizations when such data is tampered with, destroyed, leaked, or illegally acquired or used. As of the date of this annual report, we have not been involved in any investigations on data security compliance made in connection with the PRC Data Security Law, and we have not received any inquiry, notice, warning, or sanctions in such respect. Based on the foregoing, we do not expect that, as of the date of this annual report, the PRC Data Security Law would have a material adverse impact on our business.
On July 6, 2021, the relevant PRC governmental authorities published the Opinions on Strictly Cracking Down Illegal Securities Activities in Accordance with the Law. These opinions emphasized the need to strengthen the administration over illegal securities activities and the supervision on overseas listings by China-based companies and proposed to take effective measures, such as promoting the construction of relevant regulatory systems to deal with the risks and incidents faced by China-based overseas-listed companies. As these opinions were recently issued, official guidance and related implementation rules have not been issued yet and the interpretation of these opinions remains unclear at this stage. As of the date of this annual report, we have not received any inquiry, notice, warning, or sanctions from the CSRC or any other PRC government authorities. Based on the foregoing and the currently effective PRC laws, we and our PRC legal counsel, Tian Yuan Law Firm, are of the view that, as of the date of this annual report, these opinions do not have a material adverse impact on our business.
2
Table of Contents
On February 17, 2023, the CSRC promulgated the Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies and five related guidelines, collectively, the Overseas Listing Trial Measures, which became effective on March 31, 2023. The Overseas Listing Trial Measures regulate both direct and indirect overseas offering and listing of PRC domestic companies’ securities by adopting a filing-based regulatory regime. Pursuant to the Overseas Listing Trial Measures, initial public offering or listings in overseas markets shall be filed with the CSRC within three working days after the relevant application is submitted overseas. Furthermore, it is stipulated, among others, that an overseas offering and listing shall be prohibited under any of the following circumstances: (i) where such securities offering and listing is explicitly prohibited by provisions in laws, administrative regulations and relevant state rules; (ii) where the intended securities offering and listing may endanger national security as reviewed and determined by competent authorities under the State Council in accordance with law; (iii) where the PRC domestic company intending to make the securities offering and listing, or its controlling shareholders and the actual controller, have committed crimes such as corruption, bribery, embezzlement, misappropriation or property or undermining the order of the socialist market economy during the last three years; (iv) where the PRC domestic company intending to make the securities offering and listing is suspected of committing crimes or major violations of laws and regulations, and is under investigation according to law, and no conclusion has been made thereof; (v) where there are material ownership disputes over equity held by the PRC domestic company’s controlling shareholder or by other shareholders that are controlled by the controlling shareholder and/or actual controller. The Overseas Listing Trial Measures also provide that any overseas offering and listing made by an issuer that meets both the following conditions will be determined as indirect overseas offering and listing of PRC domestic companies: (i) 50% or more of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent accounting year is accounted for by PRC domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in mainland China, or its main places of business are located mainland China, or the senior management personal in charge of its business operation and management are mostly Chinses citizens or domiciled in mainland China. On February 17, 2023, the CSRC issued the Notice on the Administrative Arrangements for the Filing of Overseas Listings by Domestic Enterprises. The CSRC clarified that on March 31, 2023, the effective date of the Overseas Listing Trial Measures, the PRC domestic companies that have been listed overseas by March 31, 2023 are not required to file immediately, and filing should be made as required if they involve refinancing and other filing matters. As the requirements under Overseas Listing Trial Measures are new and evolving, there remains substantial uncertainties as to their interpretation and implementation and how they may impact our ability to raise or utilize fund and business operation. In particular, there are uncertainties as to the form and substance of regulatory requirements and we cannot assure you that, when required, we can complete the requisite filing with such form and content satisfactory to the relevant regulators in a timely manner. Any delays in completing the filing requirements may adversely affect our ability to complete our capital raising activities from the capital markets in the future.
On February 24, 2023, the CSRC, the Ministry of Finance, the National Administration of State Secrets Protection and the National Archives Administration jointly issued the Provisions on Strengthening Confidentiality and Archives Administration of Overseas Securities Offering and Listing by Domestic Companies, or the Confidentiality and Archives Provisions, which will take effective from March 31, 2023. The Confidentiality and Archives Provisions specify that during the overseas issuance of securities and listing activities of domestic enterprises, domestic enterprises and securities companies and securities service institutions that provide relevant securities services shall, by strictly abiding by the relevant laws and regulations of the PRC and the requirements therein, establish sound confidentiality and archives management systems, take necessary measures to implement confidentiality and archives management responsibilities, and shall not leak national secrets, work secrets of governmental agencies and undermine national and public interests. Work manuscripts generated in the PRC by securities companies and securities service institutions that provide relevant securities services for overseas issuance and listing of securities by domestic enterprises shall be kept in the PRC. Without the approval of relevant competent authorities, it shall not be transferred overseas. Where archives or copies need to be transferred outside of the PRC, it shall be subject to the approval procedures in accordance with relevant PRC regulations.
Since these statements and regulatory actions are new, it is highly uncertain how soon legislative or administrative regulation making bodies will respond and what existing or new laws or regulations or detailed implementations and interpretations will be modified or promulgated, if any, and the potential impact such modified or new laws and regulations will have on our daily business operation, our ability to accept foreign investments and conduct follow-on offerings, and listing or continuing listing on a U.S. or other foreign exchanges. In addition, the PRC government has recently published new policies that significantly affected certain industries such as the education and internet industries, and we cannot rule out the possibility that it will in the future release regulations or policies regarding any other industry including the industry in which we operate, which could adversely affect our business, financial condition and results of operations.
3
Table of Contents
Risks Associated with Our Corporate Structure
Burning Rock Biotech Limited, our ultimate Cayman Islands holding company, does not have any substantive operations other than directly controlling Beijing Burning Rock Biotech Limited, our wholly foreign owned entity, or WFOE, and indirectly Burning Rock (Beijing) Biotechnology Co., Ltd., the variable interest entity, or VIE, through certain contractual arrangements. These contractual arrangements were amended and restated in October 2019. See “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements.” What the ADSs investors purchased are equity securities of our ultimate Cayman Islands holding company rather than equity securities of the VIE. We conduct our business operations through both the consolidated subsidiaries, and the VIE and the VIE’s subsidiaries. We, together with the VIE and its subsidiaries, are subject to PRC laws relating to, among others, restrictions over foreign investments in distribution of online information and other value-added telecommunication services set out in the Negative List (2021 Edition) promulgated by the Ministry of Commerce, or MOFCOM, and the National Development and Reform Commission, or NDRC. The VIE structure is used to replicate foreign investment in China-based companies where the PRC law prohibits direct foreign investment in the operating companies. Neither we nor our subsidiaries own any share in the VIE or any of its subsidiaries. Instead, we control and receive the economic benefits of the business operation of the VIE or any of its subsidiaries through a series of contractual agreements with the VIE. The contractual agreements with the VIE are designed to provide the WFOE with the power, rights, and obligations equivalent in all material respects to those it would possess as the principal equity holder of the VIE, including absolute control rights and the rights to the assets, property, and revenue of the VIE and its subsidiaries. As a result of our direct ownership in the WFOE and the contractual agreements with the VIE, we are regarded as the primary beneficiary of the VIE and its subsidiaries. Because of our corporate structure, we are subject to risks due to uncertainty of the interpretation and the application of the PRC laws and regulations, including but not limited to limitation on foreign ownership of internet technology companies, and regulatory review of oversea listing of PRC companies through a special purpose vehicle, and the validity and enforcement of the contractual agreements. We are also subject to the risks of uncertainty about any future actions of the PRC government in this regard. Our contractual agreements may not be effective in providing control over the VIE and its subsidiaries. We may also subject to sanctions imposed by PRC regulatory agencies including CSRC if we fail to comply with their rules and regulations.
We and the VIE and its subsidiaries face various legal and operational risks and uncertainties related to being based in and having significant operations in China. The PRC government has significant authority to exert influence on the ability of a China-based company, such as us and the VIE and its subsidiaries, to conduct its business, accept foreign investments or list on U.S. or other foreign exchanges. For example, we and the VIE and its subsidiaries face risks associated with regulatory approvals of offshore offerings, oversight on cybersecurity and data privacy. Such risks could result in a material change in our operations and/or the value of the ADSs or could significantly limit or completely hinder our ability to offer ADSs and/or other securities to investors and cause the value of such securities to significantly decline or be worthless. The PRC government also has significant discretion over the conduct of the business of us and the VIE and its subsidiaries and may intervene with or influence our operations as it deems appropriate to further regulatory, political and societal goals. Furthermore, the PRC government has recently indicated an intent to exert more oversight and control over overseas securities offerings and foreign investment in China-based companies like us. Any such action, once taken by the PRC government, could significantly limit or completely hinder our ability to offer securities to investors and cause the value of such securities to significantly decline or in extreme cases, become worthless.
As used in this annual report, “we,” “us,” “our company,” “our,” or “the Company” refers to Burning Rock Biotech Limited and its subsidiaries, “the VIE” refers to our PRC variable interest entity, Burning Rock (Beijing) Biotechnology Co. Ltd..
For more information on risks related to our corporate structure, see “—D. Risk Factors—Risks Relating to Our Corporate Structure.” For more information on the requisite permissions and approvals for our business and the consequences in relation to failure to obtain the same, see “—D. Risk Factors—Risks Relating to Government Regulations.”
Permissions Required from the PRC Authorities
We conduct our business in China primarily through our subsidiaries in China and the VIE and its subsidiaries. Our operation and the operation of the VIE and its subsidiaries are governed by PRC laws and regulations. Save as otherwise disclosed in “—D. Risk Factors—Risks Relating to Government Regulations”, as of the date of this annual report, our PRC subsidiaries and the VIE and its subsidiaries have obtained the requisite licenses and permits from the PRC government authorities that are material for their business operations in China. If we, the VIE, or any of its subsidiaries fail to obtain or maintain such licenses or permits, the relevant PRC regulatory authorities would have broad discretion in dealing with such violations or failures, including imposing fines, confiscating our incomes and products that are deemed to have been obtained through illegal operations, and discontinuing or restricting our operations. As of the date of this annual report, none of us, the VIE, or any of its subsidiaries have been subject to any penalties from the relevant authorities for failure to obtain or maintain any required licenses or permits. Given the uncertainties of interpretation and implementation of relevant laws and regulations and the enforcement practice by government authorities, we cannot assure you that we are able to maintain our existing licenses and permits or obtain additional licenses, permits, filings or approvals for providing our products and services in the future. See “—D. Risk Factors—Risks Relating to Our Business and Industry—We are subject to extensive legal and regulatory requirements in China for our NGS-based products and services. Any lack of requisite certificates, licenses or permits applicable to our business may have an adverse impact on our business, financial condition and results of operations.”
4
Table of Contents
Risks Associated with the Holding Foreign Companies Accountable Act
The United States adopted the Holding Foreign Companies Accountable Act on December 18, 2020, and it was amended by the Consolidated Appropriations Act, 2023 on December 17, 2022 (the amended act, the “HFCA Act”). The HFCA Act states if the SEC determines that we have filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for two consecutive years, the SEC shall prohibit our securities, including our ADSs, from being traded on a U.S. national securities exchange, including the Nasdaq, or in the over-the-counter trading market in the U.S. On December 16, 2021, the PCAOB issued a report to notify the SEC of its determination that the PCAOB was unable to inspect or investigate completely registered public accounting firms headquartered in mainland China and Hong Kong, including our auditor. In May 2022, the SEC conclusively listed us as a Commission-Identified Issuer under the HFCA Act following the filing of our annual report on Form 20-F for the fiscal year ended December 31, 2021. On December 15, 2022, the PCAOB issued a report that vacated its previous determination issued on December 16, 2021 and removed mainland China and Hong Kong from the list of jurisdictions where it is unable to inspect or investigate completely registered public accounting firms. For this reason, we do not expect to be identified as a Commission Identified Issuer under the HFCA Act after we file this annual report on Form 20-F.
Pursuant to amendments made to the HFCA Act, the PCAOB may determine that it is unable to inspect or investigate completely registered public accounting firms in any foreign jurisdictions because of positions taken by any foreign authority, rather than an authority in the location in which the firms are headquartered or in which they have a branch or office, as was the case under the original version of the Act. If the PCAOB determines in the future that it no longer has full access to inspect and investigate completely accounting firms in mainland China and Hong Kong and we use an accounting firm headquartered in one of these jurisdictions to issue an audit report on our financial statements filed with the SEC, we would again be identified as a Commission Identified Issuer following the filing of the annual report on Form 20-F for the relevant fiscal year. There can be no assurance that we would not be identified as a Commission Identified Issuer for any future fiscal year, and if we were so identified for two consecutive years, we would become subject to the prohibition on trading under the HFCA Act. See “Item 3.D. Key Information—Risk Factors—Risks Related to Doing Business in the PRC—The PCAOB had historically been unable to inspect our auditor in relation to their audit work performed for our financial statements and the inability of the PCAOB to conduct inspections of our auditor in the past has deprived our investors with the benefits of such inspections” and “—Our ADSs may be prohibited from trading in the United States under the HFCA Act in the future if the PCAOB is unable to inspect or investigate completely our current auditor. The delisting of the ADSs, or the threat of their being delisted, may materially and adversely affect the value of your investment.”
Results of Operations, Financial Position and Cash Flows of the VIE and Its Subsidiaries
The tables below set forth the results of operations of the VIE and subsidiaries of the VIE included in our consolidated statements of comprehensive loss for 2021, 2022 and 2023:
| For the year ended December 31, 2021 | ||||||||||||||||
| Non-VIE entities | VIE and VIE’s subsidiaries | Eliminations | Consolidated Total | |||||||||||||
| RMB | RMB | RMB | RMB | |||||||||||||
Revenues | 77,234 | 526,071 | (95,443 | ) | 507,862 | |||||||||||
Net loss | (277,034 | ) | (508,803 | ) | (10,860 | ) | (796,697 | ) | ||||||||
| For the year ended December 31, 2022 | ||||||||||||||||
| Non-VIE entities | VIE and VIE’s subsidiaries | Eliminations | Consolidated Total | |||||||||||||
| RMB | RMB | RMB | RMB | |||||||||||||
Revenues | 186,658 | 557,667 | (181,087 | ) | 563,238 | |||||||||||
Net loss | (370,725 | ) | (605,934 | ) | 5,426 | (971,233 | ) | |||||||||
| For the year ended December 31, 2023 | ||||||||||||||||||||
| Non-VIE entities | VIE and VIE’s subsidiaries | Eliminations | Consolidated Total | Consolidated Total | ||||||||||||||||
| RMB | RMB | RMB | RMB | US$ | ||||||||||||||||
Revenues | 87,759 | 537,502 | (87,826 | ) | 537,435 | 75,696 | ||||||||||||||
Net loss | (318,484 | ) | (340,575 | ) | 5,370 | (653,689 | ) | (92,070 | ) | |||||||||||
5
Table of Contents
The tables below set forth the condensed consolidating schedule of financial position of the VIE and subsidiaries of the VIE as of the dates indicated:
| As of December 31, 2022 | ||||||||||||||||
| Non-VIE entities | VIE and VIE’s subsidiaries | Eliminations | Consolidated Total | |||||||||||||
| RMB | RMB | RMB | RMB | |||||||||||||
Cash and cash equivalents | 528,716 | 376,735 | — | 905,451 | ||||||||||||
Restricted cash | 9,540 | 10,277 | — | 19,817 | ||||||||||||
Inter-company receivables | 1,601,116 | 245,391 | (1,846,507 | ) | — | |||||||||||
Total current assets | 2,187,606 | 917,663 | (1,846,507 | ) | 1,258,762 | |||||||||||
Total non-current assets | 230,109 | 98,596 | — | 328,705 | ||||||||||||
Total assets | 2,417,715 | 1,016,259 | (1,846,507 | ) | 1,587,467 | |||||||||||
Inter-company payables | 1,127,041 | 1,601,116 | (2,728,157 | ) | — | |||||||||||
Total liabilities | 1,259,374 | 1,897,909 | (2,728,157 | ) | 429,126 | |||||||||||
Total shareholders’ equity (deficit) | 1,158,341 | (881,650 | ) | 881,650 | 1,158,341 | |||||||||||
Total liabilities and shareholders’ equity (deficit) | 2,417,715 | 1,016,259 | (1,846,507 | ) | 1,587,467 | |||||||||||
| As of December 31, 2023 | ||||||||||||||||||||
| Non-VIE entities | VIE and VIE’s subsidiaries | Eliminations | Consolidated Total | Consolidated Total | ||||||||||||||||
| RMB | RMB | RMB | RMB | US$ | ||||||||||||||||
Cash and cash equivalents | 300,372 | 314,724 | — | 615,096 | 86,634 | |||||||||||||||
Restricted cash | 100 | 20 | — | 120 | 17 | |||||||||||||||
Inter-company receivables | 1,693,577 | 343,342 | (2,036,919 | ) | — | — | ||||||||||||||
Total current assets | 2,035,306 | 885,709 | (2,036,919 | ) | 884,096 | 124,523 | ||||||||||||||
Total non-current assets | 103,412 | 52,493 | — | 155,905 | 21,959 | |||||||||||||||
Total assets | 2,138,718 | 938,202 | (2,036,919 | ) | 1,040,001 | 146,482 | ||||||||||||||
Inter-company payables | 1,317,825 | 1,693,577 | (3,011,402 | ) | — | — | ||||||||||||||
Total liabilities | 1,370,308 | 1,912,685 | (3,011,402 | ) | 271,591 | 38,253 | ||||||||||||||
Total shareholders’ equity (deficit) | 768,410 | (974,483 | ) | 974,483 | 768,410 | 108,229 | ||||||||||||||
Total liabilities and shareholders’ equity (deficit) | 2,138,718 | 938,202 | (2,036,919 | ) | 1,040,001 | 146,482 | ||||||||||||||
The tables below set forth the cash flows of the VIE and subsidiaries of the VIE included in our consolidated statements of cash flows for 2021, 2022 and 2023:
| For the year ended December 31, 2021 | ||||||||||||||||
| Non-VIE entities | VIE and VIE’s subsidiaries | Eliminations | Consolidated Total | |||||||||||||
| RMB | RMB | RMB | RMB | |||||||||||||
Net cash used in operating activities | (220,380 | ) | (257,506 | ) | — | (477,886 | ) | |||||||||
Net cash (used in) generated from investing activities | (222,038 | ) | (11,265 | ) | 315,000 | 81,697 | ||||||||||
Net cash (used in) generated from financing activities | (42,522 | ) | 304,623 | (315,000 | ) | (52,899 | ) | |||||||||
| For the year ended December 31, 2022 | ||||||||||||||||
| Non-VIE entities | VIE and VIE’s subsidiaries | Eliminations | Consolidated Total | |||||||||||||
| RMB | RMB | RMB | RMB | |||||||||||||
Net cash used in operating activities | (317,427 | ) | (139,381 | ) | — | (456,808 | ) | |||||||||
Net cash generated from (used in) investing activities | 29,625 | (37,088 | ) | — | (7,463 | ) | ||||||||||
Net cash (used in) generated from financing activities | (83,869 | ) | 377,630 | (380,000 | ) | (86,239 | ) | |||||||||
| For the year ended December 31, 2023 | ||||||||||||||||||||
| Non-VIE entities | VIE and VIE’s subsidiaries | Eliminations | Consolidated Total | Consolidated Total | ||||||||||||||||
| RMB | RMB | RMB | RMB | US$ | ||||||||||||||||
Net cash used in operating activities | (253,015 | ) | (2,768 | ) | — | (255,783 | ) | (36,026 | ) | |||||||||||
Net cash used in investing activities | (8,694 | ) | (606 | ) | — | (9,300 | ) | (1,310 | ) | |||||||||||
Net cash (used in) generated from financing activities | (48,832 | ) | (68,894 | ) | 68,894 | (48,832 | ) | (6,878 | ) | |||||||||||
6
Table of Contents
The typical structure of cash flows through our organization is as follows: (i) we transfer funds to our WFOE, Beijing Burning Rock Biotech Limited, through either capital contributions or loans from our Hong Kong subsidiary, BR Hong Kong Limited; (ii) our WFOE makes loans to the VIE, Burning Rock (Beijing) Biotechnology Co. Ltd.; (iii) the VIE and its subsidiaries receive funds generated from sales of products and/or services to third party customers; and (iv) when the VIE intends to settle any amounts owed to us under a series of contractual arrangement (the “VIE Agreements”), the VIE will pay service fees to our WFOE pursuant to the Exclusive Business Cooperation Agreement, and our WFOE will transfer funds to BR Hong Kong Limited, which in turn will transfer funds to us, all through distributions, dividends or repayment of shareholder loans. As of the date of this annual report, none of our PRC subsidiaries nor VIE has declared or paid any dividends or made any distributions to their respective holding companies, including Burning Rock Biotech Limited, nor does any of them have intention to do so. As of the date of this annual report, Burning Rock Biotech Limited has not declared any dividend and does not have a plan to declare a dividend to its shareholders. Nevertheless, cash transfers have been made to date between Burning Rock Biotech Limited, our subsidiaries and the VIE and its subsidiaries and such cash transfers have been made both in the direction to the VIE and from the VIE to our WFOE as of the date of annual report. We currently do not have cash management policies that dictate when or how funds are transferred between us, our subsidiaries and the VIE and its subsidiaries. In practice, we estimate and allocate funds to our WFOE and the VIE and the VIE’s subsidiaries based on their respective available cash balances and forecasted cash requirements.
There are limitations on foreign exchange and our ability to transfer cash among us, our subsidiaries (including our WFOE) and the VIE and the VIE’s subsidies, and to transfer funds across borders and to the U.S. investors. There is no assurance that the PRC government will not intervene or impose restrictions on the ability of us, our subsidiaries and the VIE and its subsidiaries to transfer cash. Most of our cash is in Renminbi, and the PRC government could prevent the cash maintained from leaving the PRC, restrict deployment of the cash into the business of our Company, our subsidiaries, the VIE and the VIE’s subsidiaries and restrict the ability to pay dividends to their respective shareholders, including our U.S. shareholders. Such restrictions are primarily related to the following aspects:
| (1) | regulations in China currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. PRC regulations also require our PRC subsidiaries to set aside at least 10% of their after-tax profits each year to fund the statutory reserve and restrict dividend and shareholder distributions until the statutory reserve reach 50% of their respective registered capital. Our PRC subsidiaries may at their discretion allocate a portion of their after-tax profits to staff welfare and bonus funds in accordance with relevant PRC rules and regulations. These reserve funds and staff welfare and bonus funds cannot be distributed as cash dividends. As a result, our PRC subsidiaries are restricted in their ability to transfer a portion of their net assets to us in the form of dividends. Moreover, if the PRC subsidiaries incur debt on their own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make other distributions to us; |
| (2) | if any of our PRC subsidiaries incurs debt on its own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us; and |
| (3) | our PRC subsidiaries generate primarily all of their revenue in Renminbi, which is not freely convertible into other currencies. As a result, any restriction on currency exchange may limit the ability of our PRC subsidiaries to use their Renminbi revenues to pay dividends to us. Under existing PRC foreign exchange regulations, payments of current account items, such as profit distributions and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval from SAFE, by complying with certain procedural requirements. Therefore, our PRC subsidiaries are able to pay dividends in foreign currencies to us without prior approval from SAFE, subject to the condition that the remittance of such dividends outside of the PRC complies with certain procedures under PRC foreign exchange regulations. However, approval from or registration with appropriate governmental authorities or commercial banks authorized by such authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses, such as the repayment of loans denominated in foreign currencies. |
Historically, in response to the persistent capital outflow and the Renminbi’s depreciation against the U.S. dollar in 2016, the People’s Bank of China, or the PBOC, and the State Administration of Foreign Exchange, or SAFE, have implemented a series of capital control measures, including stricter vetting procedures for China-based companies to remit foreign currency for overseas acquisitions, dividend payments and shareholder loan repayments. The PRC government may continue to strengthen its capital controls and our PRC subsidiary’s dividends and other distributions may be subjected to tighter scrutiny. Furthermore, as the interpretation and implementation of these foreign exchange regulations has been constantly evolving, it is unclear how these regulations, and any future regulations concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant government authorities. For example, we may be subject to a more stringent review and approval process with respect to our foreign exchange activities, such as remittance of dividends and foreign-currency-denominated borrowings, which may adversely affect our financial condition and results of operations. In addition, if we decide to acquire a PRC domestic company, we cannot assure you that we or the owners of such company will be able to obtain the necessary approvals or complete the necessary filings and registrations required by the foreign exchange regulations. This may restrict our ability to implement our acquisition strategy and could adversely affect our business and prospects.
7
Table of Contents
In addition, there are limitations on our ability to settle amounts owed by the VIE under the relevant VIE agreements to us. We are entitled to receive substantially all of the economic benefits of the VIE in consideration for the services provided by our WFOE, according to the VIE Agreements. However, the VIE agreements are not equivalent to equity ownership. For example, the contractually bound nominee shareholders of the VIE could potentially breach their contractual agreements with us by failing to fulfill their contractual obligations, failing to act in our interest, or acting to the detriment of our interest. Moreover, as these nominee shareholders, rather than our WFOE and us, are the actual shareholders of the VIE, we are unable to independently exercise any rights as a shareholder of the VIE and force the VIE to distribute its earnings to us. In addition, the legality or enforceability of the VIE agreements have never been tested in a court of law in China. If any relevant contractual provisions were to ultimately be held unenforceable by the PRC courts or other governmental authorities, such uncertainty could result in us facing a reduced ability or complete inability to receive the economic benefits of the business operations of the VIE and its subsidiaries. These restrictions and limitations could limit our ability to settle amounts owed under the VIE agreements and our subsidiaries’ ability to pay dividends.
The cash flows that have occurred between our Company, our subsidiaries and the VIE and its subsidiaries are summarized as the following: the VIE, Burning Rock (Beijing) Biotechnology Co. Ltd., generates and retains cash generated from operating activities and re-invests it in the business activities conducted by the VIE and its subsidiaries. Unrelated to those services as stipulated under the Exclusive Business Cooperation Agreement, the agreement that allows the Company to receive economic benefits from the VIE, our WFOE, Beijing Burning Rock Biotech Limited, charges service fee to the VIE for certain operating expenses that it bears on behalf of the VIE for the business operations of the VIE and its subsidiaries. The service fee is determined at an amount subject to mutual negotiation and agreement between the WFOE and the VIE, and if both parties fail to reach such an agreement, our WFOE has the sole discretion to make the final determination. Our WFOE charged service fees of RMB72.7 million, RMB191.6 million and RMB77.2 million (US$10.9 million) to the VIE and received RMB88.7 million, RMB398.5 million and RMB284.5 million (US$40.1 million) of service fees from the VIE in 2021, 2022 and 2023, respectively. We transferred nil, RMB55.8 million and RMB109.5 million (US$15.4 million) of financing proceeds to our WFOE, which was then transferred to the VIE as advance payments during the same periods. Additionally, we transferred RMB315.0 million and RMB380.0 million of financing proceeds to our WFOE and our Hong Kong subsidiary, which was then transferred to the VIE, in 2021 and 2022, respectively. Additionally, the VIE repaid RMB68.9 million (US$9.7 million) of financing to our WFOE and our Hong Kong subsidiary in 2023.
We may transfer cash proceeds raised from future overseas financing activities through our holding company, to our WFOE through capital contributions and shareholder loans. Our WFOE is expected to then transfer funds to the VIE and its subsidiaries to meet their capital needs.
The contractual arrangements may not be as effective as direct ownership in providing us with control over the VIE, and we may incur substantial costs to enforce the terms of the arrangements. The VIE, its subsidiaries or shareholders could breach their contractual arrangements with us in ways including failing to fulfill their contractual obligations or taking other actions that are detrimental to our interests. If we had direct ownership of the VIE and its subsidiaries, we would be able to exercise our rights as a shareholder to effect changes in the board of directors of the VIE, which in turn could implement changes, subject to any applicable fiduciary obligations, at the management and operational level. However, under the current contractual arrangements, we rely on the performance by the VIE, its subsidiaries and its shareholders of their obligations under the contracts to exercise any control over the VIE. The VIE’s shareholders may have actual or potential conflicts of interest with us, and may not act in the best interests of our company. These shareholders may refuse to sign or breach, or cause the VIE to breach, or refuse to renew, the existing contractual arrangements we have with them and the VIE. If any dispute relating to these contracts arises, we will have to enforce our rights under these contracts through the operations of PRC law and arbitration, litigation and other legal proceedings and, therefore, will be subject to uncertainties in the PRC legal system. Therefore, our contractual arrangements may not be as effective in ensuring our control over the relevant portion of our business operations as direct ownership would be.
8
Table of Contents
In addition, the Company and its investors may never directly hold equity interests in the businesses that are conducted by the VIE and its subsidiaries. Uncertainties in the PRC legal system could limit our ability to enforce these contractual arrangements, and these contractual arrangements have not been tested in a court of law. The legal environment in the PRC is not as developed as in other jurisdictions, such as the United States. As a result, uncertainties in the PRC legal system could limit our ability, as a Cayman holding company, to enforce these contractual arrangements and doing so may be quite costly. There are also substantial uncertainties regarding the interpretation and application of current and future PRC laws, regulations and rules regarding the status of the rights of our Cayman Islands holding company with respect to its contractual arrangements with the VIE, its founders and owners. It is uncertain whether any new PRC laws or regulations relating to the VIE structures will be adopted or if adopted, what they would provide. If we, the VIE or its subsidiaries are found to be in violation of any existing or future PRC laws or regulations, or fail to obtain or maintain any of the required permits or approvals, the relevant PRC regulatory authorities would have broad discretion to take action in dealing with such violations or failures. In addition, Mr. Yusheng Han, our founder, chairman of the board of directors and chief executive officer holds 45.9% of the equity interests in the VIE as of March 31, 2024. Mr. Han also has 55.0% of the aggregate voting power of our issued and outstanding share capital as of March 31, 2024 due to the disparate voting powers associated with our dual-class share structure. Accordingly, the enforceability of the various contracts described above by our company against the VIE is substantially dependent upon Mr. Han. If he fails to perform his obligations under the contractual arrangements, we could be unable to enforce the contractual arrangements that enable us to consolidate the VIE’s operations and financial results in our financial statements in accordance with U.S. GAAP as the primary beneficiary. If this happens, we would need to deconsolidate the VIE and its subsidiaries. The majority of our assets, including the necessary licenses to conduct business in China are held by the VIE and its subsidiaries. A significant part of our revenues is generated by the VIE and its subsidiaries. An event that results in the deconsolidation of the VIE would have a material effect on our operations and result in the value of the securities diminish substantially or even become worthless. For a detailed description of the risks associated with our corporate structure, please refer to risks disclosed under “Item 3.D. Key Information—Risk Factors—Risks Related to Our Corporate Structure” in this annual report.
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Our business, financial condition and results of operations are subject to various changing business, competitive, economic, political and social conditions in China and worldwide. In addition to the factors discussed elsewhere in this annual report, the following are some of the important factors that could adversely affect our operating results, financial condition and business prospects, and cause our actual results to differ materially from those projected in any forward-looking statements.
Risk Factor Summary
Risks Relating to Our Business and Industry
| • | We are a cancer diagnostics company with a limited operating history, which may make it difficult to evaluate our current business and predict our future performance. |
| • | We have incurred net losses historically, and may not be able to achieve and maintain profitability. |
| • | Failure to maintain significant commercial market acceptance for our cancer therapy selection products and services, or any future products and services may harm our business and results of operations. |
| • | We may be unable to develop and commercialize our early cancer detection products, MRD products or new cancer therapy selection products on a timely basis, or at all. |
| • | If we fail to keep up with industry and technology developments in a timely and cost-effective manner, we may be unable to compete effectively and our business and prospects could suffer. |
9
Table of Contents
| • | If our products or services do not perform as expected, our operating results, reputation and business could suffer. |
| • | If we were to be sued for product liability or professional liability, we could face substantial liabilities that exceed our resources. |
| • | If we cannot maintain or develop relationships with hospitals and physicians, our results of operations and prospects could be adversely affected. |
| • | We require substantial funding for our operations. If we cannot raise sufficient additional capital on acceptable terms, our business, financial condition and prospects may be adversely affected. |
| • | We face risks related to natural disasters, health epidemics, civil and social disruption and other outbreaks, which could significantly disrupt our operations. |
| • | If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenue or achieve and sustain profitability. |
| • | Failure to manage our growth or execute our strategies effectively may adversely affect our business and prospects. |
Risks Relating to Government Regulations
| • | We are subject to extensive legal and regulatory requirements in China for our NGS-based products and services. Any lack of requisite certificates, licenses or permits applicable to our business may have an adverse impact on our business, financial condition and results of operations. |
| • | Failure to comply with existing or future laws and regulations related to the management of human genetic resources in China could lead to government enforcement actions, which could include civil or criminal fines or penalties, private litigation, other liabilities, and/or adverse publicity. Compliance or the failure to comply with such laws could increase the costs of, limit and cause significant delay in our clinical studies and research and development activities, and could otherwise materially and adversely affect our operating results, business and prospects. |
Risks Relating to Our Corporate Structure
| • | If the PRC government finds that the agreements that establish the structure for operating our businesses in China do not comply with applicable PRC laws and regulations, or if these regulations or their interpretations change, we could be subject to severe penalties or be forced to relinquish our interests in those operations. |
| • | Our contractual arrangements with the VIE and its shareholders may not be as effective in providing operational control or enabling us to derive economic benefits as a direct ownership of a controlling equity interest would be. |
| • | We may lose the ability to use and enjoy assets held by the VIE that are critical to the operation of our business if the VIE declares bankruptcy or becomes subject to a dissolution or liquidation proceeding. |
Risks Relating to Doing Business in the PRC
| • | Recent regulatory developments in China may subject us to additional regulatory review and disclosure requirements, expose us to government interference, or otherwise restrict or completely hinder our ability to offer securities and raise capitals outside China, all of which could materially and adversely affect our business, and cause the value of our securities to significantly decline or become worthless. |
| • | We are subject to many of the economic and political risks associated with emerging markets due to our operation in China. Adverse changes in the Chinese or global economic, political and social conditions as well as government policies could adversely affect our business and prospects. |
| • | Geopolitical tensions have led to a worsening relationship between China and the United States and this adverse trend may continue to deteriorate, which could negatively affect our business and results of operations. |
10
Table of Contents
| • | Uncertainties in the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and us. |
| • | The PCAOB had historically been unable to inspect our auditor in relation to their audit work performed for our financial statements and the inability of the PCAOB to conduct inspections of our auditor in the past has deprived our investors with the benefits of such inspections. |
| • | Our ADSs may be prohibited from trading in the United States under the HFCAA in the future if the PCAOB is unable to inspect or investigate completely our current auditor. The delisting of the ADSs, or the threat of their being delisted, may materially and adversely affect the value of your investment. |
| • | Proceedings instituted by the SEC against the Big Four PRC-based accounting firms, including our independent registered public accounting firm, could result in financial statements being determined to not be in compliance with the requirements of the Exchange Act. |
Risks Relating to Hong Kong
| • | There may be political risks associated with having business connection with Hong Kong. |
Risks Relating to The ADSs
| • | The trading price of ADSs has been and may continue to be volatile, which could result in substantial losses to investors. |
| • | If we fail to meet the applicable listing requirements, Nasdaq or the London Stock Exchange, as applicable, may delist our ADSs from trading on its respective exchange in which case the liquidity and market price of our ADSs could decline and our ability to raise additional capital would be adversely affected. |
| • | If securities or industry analysts do not publish research or reports about our business, or if they adversely change their recommendations regarding the ADSs, the market price for the ADSs and trading volume could decline. |
| • | The sale or availability for sale of substantial amounts of ADSs could adversely affect their market price. |
| • | Our dual-class share structure with different voting rights will limit your ability to influence corporate matters and could discourage others from pursuing any change of control transactions that holders of our Class A ordinary shares and the ADSs may view as beneficial. |
Risks Relating to Our Business and Industry
We are a cancer diagnostics company with a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
We commercially launched our first cancer therapy selection test in 2014 and started generating revenue in 2014. We launched our first MRD product, brPROPHET™, in March 2022. Our limited operating history may make it difficult to evaluate our current business and predict our future performance. Any assessment of our profitability or prediction about our future success or viability is subject to significant uncertainty.
China’s NGS-based cancer therapy selection market is still in its early stage of development and rapidly evolving, and companies operating in this industry face a variety of risks. We may not have sufficient experience or resources to address risks frequently encountered in this industry, which include, among other things, our potential failure to:
| • | acquire and retain customers and increase adoption of our cancer therapy selection products and services by hospitals, physicians, patients, pharmaceutical companies and others in the medical community; |
| • | timely respond to changing market conditions and keep up with evolving industry and technological standards and regulatory developments; |
| • | obtain and maintain the regulatory approvals required for us to further market and sell our cancer therapy selection products and services and commercialize our early cancer detection products and services; |
11
Table of Contents
| • | manage our relationships with our suppliers, customers and research partners; |
| • | protect proprietary technologies and intellectual property rights; and |
| • | attract, train, motivate and retain research and development and other qualified personnel. |
If we are unsuccessful in addressing any one or more of these risks, our business, financial condition and results of operations could be adversely affected.
We have incurred net losses historically, and may not be able to achieve and maintain profitability.
Although our revenue grew rapidly in recent years, we have historically incurred net losses. In 2021, 2022 and 2023, we incurred net loss of RMB796.7 million, RMB971.2 million and RMB653.7 million (US$92.1 million), respectively. To date, we have financed our operations principally from revenue generated from operations, proceeds from our initial public offering and concurrent private placement and equity contributions from our shareholders.
We have invested and expect to continue to invest significantly in the research, development, and sales and marketing of our products. As such, we may continue to incur losses in the future. We cannot predict the extent of these future losses, or when we may achieve profitability, if at all. If we are unable to generate sufficient revenue from our business and control our costs and expenses to achieve and maintain profitability, the value of your investment in us could be negatively affected.
Failure to maintain significant commercial market acceptance for our cancer therapy selection products and services, or any future products and services may harm our business and results of operations.
Our cancer therapy selection products and services contributed substantially all of our revenue for 2021, 2022 and 2023. Although we are in the process of developing and commercializing MRD and early cancer detection products, our cancer therapy selection tests will continue to account for a significant portion of our revenue in the foreseeable future. Our ability to execute our growth strategy and become profitable will therefore depend upon the continued and further adoption of our cancer therapy selection products and services by hospitals and patients. Continued adoption and use of these products and services will depend on several factors, including, among others:
| • | our ability to demonstrate among the medical community the clinical utility, superiority and the benefits of our cancer therapy selection products and services; |
| • | our ability to further validate our cancer therapy selection technologies through clinical research and accompanying publications; |
| • | the timing and scope of approval by the NMPA for our additional cancer therapy selection products; |
| • | the prices we charge for our cancer therapy selection products and services; |
| • | our ability to maintain our laboratory certification, accreditation and regulatory approvals, including the NCCL PCR clinical test laboratory certificate, the NCCL NGS laboratory certificate, the CAP accreditation, the CLIA certification, and complete required inspections; and |
| • | the impact of negative publicity regarding our or our competitors’ tests and technologies resulting from defects or errors. |
We cannot assure you that our cancer therapy selection products and services will continue to maintain or gain market acceptance, and any failure to do so would harm our business and results of operations.
We may be unable to develop and commercialize our early cancer detection products, MRD products or new cancer therapy selection products on a timely basis, or at all.
We are developing and commercializing early cancer detection products and MRD products and may develop and commercialize new cancer therapy selection products from time to time in the future. Developing early cancer detection, MRD and new cancer diagnostics products is a lengthy and complex process. New products may take time to commercialize, and their launch could be delayed or may not be successful.
12
Table of Contents
Our product development process involves various risks, and we may not be able to develop and commercialize new early cancer detection products, MRD products or cancer therapy selection products on a timely basis, or at all. A product candidate that appears promising in the early phases of development may fail to reach the market for a number of reasons. For example:
| • | our product candidates may fail to demonstrate clinical utility, or the development process may produce negative or inconclusive results, and we may decide, or regulators may require us to conduct additional clinical trials or we may decide to abandon our development programs; |
| • | our employees, or third-party clinical investigators, medical institutions and contract research organizations, may fail to comply with their contractual duties or obligations or meet expected deadlines, and if the quality, completeness or accuracy of the clinical data they obtain are compromised due to any failure to adhere to our clinical protocols or for other reasons, our clinical trials may have to be extended, delayed or terminated; |
| • | we may fail to obtain approvals for our product candidates from relevant regulatory authorities; and |
| • | failure to generate additional data and insights from our existing products to advance the research and development of new products as quickly, or at all. |
In addition, our competitors may develop and commercialize competing products faster than we are able to, in which case our results of operations could be adversely affected.
If we fail to keep up with industry and technology developments in a timely and cost-effective manner, we may be unable to compete effectively and our business and prospects could suffer.
China’s NGS-based cancer therapy selection market is characterized by rapid changes, including technological and scientific breakthroughs, increasing amounts of data, frequent introductions of new tests, constant emergence of alternative diagnostic methods, and evolving industry standards. If we are not able to keep pace with these advances and increased customer expectations as a result of these advances and capture new market opportunities that develop as a result of these advances, our proprietary technologies could be rendered obsolete, our existing products and services and products and services we are developing could be rendered less clinically effective, and our future operations and prospects could suffer. To remain competitive, we must continuously upgrade our existing products and services and launch new products and services, to keep pace with these developments. We cannot assure you that these efforts will be successful.
In addition, we must expend significant resources in order to continuously upgrade our existing products and services or launch new ones to keep pace with industry and technological advances. We may never realize a return on investment on these efforts, especially if the improved or new products and services fail to perform as expected, in which case our business, financial condition and results of operations could be adversely affected.
If our products or services do not perform as expected, our operating results, reputation and business could suffer.
Our success depends on the market confidence that we can provide reliable, high-quality products and services, including those for cancer therapy selection, MRD and early cancer detection, that will provide physicians with real-time clinically actionable diagnostic information. However, there is no assurance that our current and future products and services, including our early cancer detection tests currently under development, will consistently perform as expected, if at all. Our tests may fail to accurately detect gene variants or incompletely or incorrectly identify the significance of genomic alterations, or contain other errors or mistakes due to a variety of reasons (such as malfunction of our laboratory equipment and degraded liquid biopsy or tissue samples provided by our delivery service providers), which could either delay treatments or incur unnecessary medical expenses to people on whom the tests are performed. In addition, inaccurate results or misunderstandings of, or inappropriate reliance on, the diagnostic information our current and future tests provide could lead to, or be associated with, side effects or adverse events in patients who use our tests, including treatment- related death, and could lead to termination of our services or claims against us. Any such inaccurate diagnostic results, or perception thereof, could further subject us to claims or lawsuits brought by people taking our tests and their families. Any product defects or other failure of our existing products and products currently under development may result in adverse or negative publicity, lost revenue, rising insurance premium, and significant warranty and other expenses and could have a material adverse impact on our operation, business prospects, financial condition and results of operations.
13
Table of Contents
If we were to be sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
We could face product liability claims should someone allege that our products or services identified inaccurate or incomplete information regarding the genomic alteration of the tumor or malignancy analyzed, reported inaccurate or incomplete information concerning the available therapies for a certain type of cancer or otherwise failed to perform as designed. A claimant could allege that our test results caused unnecessary treatment or other costs or resulted in the patient missing the best opportunity or timing for treatment. A patient could also allege other mental or physical injury or that our tests provided inaccurate or misleading information concerning the diagnosis, prognosis or recurrence of, or available therapies for, his or her cancer. We may also be subject to liability for errors in, a misunderstanding of or inappropriate reliance upon, the diagnostic information our tests provided. The tense physician-patient relationship in China could also expose us to an increased risk of potential liability claims. A product liability or professional liability claim could result in substantial damages and be costly and time-consuming for us to defend and could divert our management’s attention.
Similar to other Chinese companies, we do not carry product liability or professional liability insurance. As the insurance industry in China is at a relatively preliminary stage of development compared to more developed markets such as the United States, insurance companies in China generally offer a limited selection of product liability and professional liability insurance policies and it is often difficult to secure suitable product liability and professional liability insurance coverage at reasonable rates in China. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage. Additionally, any product liability or professional liability lawsuit could damage our reputation, or cause our business partners to terminate existing agreements with us and seek other business partners, or cause us to lose our current or potential customers. Any of these developments could adversely impact our results of operations and business prospects.
If we cannot maintain or develop relationships with hospitals and physicians, our results of operations and prospects could be adversely affected.
We collaborate with hospitals and physicians across China in many aspects of our business, and our success in part depends on our ability to maintain our relationships with our existing partner hospitals and physicians and continue to build new relationships with additional hospitals and physicians.
Central laboratory collaboration. Currently, we primarily collaborate with hospitals and physicians under the central laboratory model, where the cancer patients’ treating physicians order our tests for the patients during the diagnostic process, have the patients’ liquid biopsy or tissue samples shipped to our laboratories for testing and then design treatment plans based on our test results. Since our inception, over 6,700 physicians from 829 hospitals across China had ordered our tests. To generate demand, we will need to continue to educate physicians at an increasing number of hospitals on the clinical utility, benefits and value of our tests through clinical trials, published papers, presentations at scientific conferences and one-on-one education by our in-house sales force. We may need to hire additional sales and marketing, research and development and other personnel to support this process. If the physicians currently using our tests services stop ordering our tests or order fewer tests from us for any reason, or if we fail to convince physicians at new hospitals to order our tests, we will likely be unable to generate demand for our tests in sufficient volume for us to achieve profitability.
In-hospital collaboration. We are also actively expanding our collaboration with hospitals under the in-hospital model. Under this model, we partner with hospitals to establish in-hospital laboratories so that the partner hospitals can conduct cancer therapy selection tests on their own using our reagent kits. As of December 31, 2023, we had partnered with 87 hospitals under the in-hospital model. Any deterioration or termination of our relationships with these partner hospitals could result in temporary or permanent loss of our revenue.
In addition, we will need to continue to advocate the clinical utility, benefits and value of our tests in order to enter into collaboration with additional hospitals under the in-hospital model. Even if we have convinced the new hospitals to partner with us, establishing in-hospital laboratories with hospitals in China involves a lengthy and costly process, including going through tender procedures, the outcome of which is subject to uncertainties, and complying with the respective hospitals’ operating protocols. If we fail to enter into collaboration with additional hospitals under the in-hospital model in a timely and cost-effective manner, or if due to regulatory change or any other reasons, our current partner hospitals terminate their current collaborations with us, our business and prospects could be adversely affected.
Furthermore, depending on our partner hospitals’ clinical needs and budgets for cancer therapy selection products and services, our revenues from in-hospital business have fluctuated, and may continue to fluctuate from quarter to quarter.
14
Table of Contents
Clinical collaboration. We have obtained the NMPA approval for two of our NGS reagent kits and in the future we may from time to time seek the NMPA approval for additional products. The NMPA approval is valid for five years and we are in the process of renewing the NMPA approval for one of our NMPA-approve products. In addition, we have one early cancer detection product, OverC™ Multi-Cancer Detection Blood Test, that has been granted Breakthrough Device Designation by both the FDA and the NMPA. The NMPA approval and FDA approval involve, among other things, successful completion of clinical trials for these products. We may rely on our partner hospitals to obtain sufficient data and samples to cost-effectively and timely perform these clinical trials. If we fail to establish or maintain clinical collaboration with our partner hospitals, our business and results of operations may be harmed. If we fail to renew or otherwise maintain the NMPA approval for our products, our sale and use of these medical devices in China may be restricted, and our business and results of operation may be adversely affected.
We require substantial funding for our operations. If we cannot raise sufficient additional capital on acceptable terms, our business, financial condition and prospects may be adversely affected.
We require substantial capital to fund our existing operations, commercialize new products, expand our business and pursue strategic investments. In particular, we require substantial capital to:
| • | advance our early cancer detection technologies and develop early cancer detection product candidates; |
| • | increase our sales and marketing efforts to drive market adoption of our products and services and address competitive developments; |
| • | seek regulatory and marketing approvals for our tests; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | hire and retain additional personnel, such as scientists and sales and marketing personnel; |
| • | develop, acquire and improve operational, financial and management information systems; |
| • | add equipment and physical infrastructure to support our research and development programs; |
| • | finance general and administrative expenses; and |
| • | operate as a public company. |
Based on our current business plan, we believe our cash and cash equivalents, together with our cash generated from financing activities will be sufficient to meet our current and anticipated needs for general corporate purposes for at least the next 12 months. If our available cash balances and current and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements, in particular, for the development and commercialization of our products, we may seek to obtain further funding through public or private equity offerings, debt financings or other sources.
Further financing may not be available to us on acceptable terms, or at all. If we fail to raise capital as and when needed it would have a negative impact on our financial condition and our ability to pursue our business strategy. In addition, if we raise funds by issuing debt securities or incurring additional borrowings, the terms of debt securities issued or borrowings could impose significant restrictions on our operations, and we may be unable to repay the indebtedness when due. If we raise funds by issuing equity securities, your investment in our company could be diluted.
We depend on third-party suppliers and service providers for different aspects of our business. If these suppliers and service providers can no longer provide satisfactory products or services to us on commercially reasonable terms, our business and results of operations could be adversely affected.
We depend on third parties for different aspects of our business, such as supplying sequencers, reagents and other laboratory equipment and materials, and collecting and delivering samples for our tests. Selecting, managing and supervising these third-party suppliers and service providers requires significant resources and expertise. Poor performance by these third parties, including their failure to provide services or products according to applicable legal and regulatory requirements, the terms of our contracts or otherwise below standard, could significantly and negatively affect the quality of our cancer therapy selection tests and damage our reputation. For example, we rely on third-party delivery service providers to transport liquid biopsy and tissue samples to our laboratory. Disruptions in such delivery services, whether due to labor disruptions, bad weather, natural disaster, terrorist acts or threats or for other reasons could adversely affect specimen integrity and our ability to process samples and conduct tests in a timely manner and to service our customers satisfactorily, and ultimately our reputation and our business. In addition, if we are unable to continue to obtain expedited delivery services on commercially reasonable terms, our operating results may be adversely affected.
15
Table of Contents
In addition, the service or cooperative agreements we have with third-party suppliers and service providers are generally not on an exclusive basis. If these third parties do not continue to maintain or expand their cooperation with us, we would be required to seek new substitutes for these third-party material or service providers, which could disrupt our operations and adversely affect our results of operations.
If we cannot maintain or develop relationships with our research partners, the market adoption and endorsement of our products and services could suffer, which could in turn reduce our revenue prospects.
Currently, we have wide academic collaborations with oncology key opinion leaders, who conducted clinical trials and research studies on early cancer detection and cancer targeted therapies and immunotherapies using our products. We believe our relationships with oncology key opinion leaders, as well as the resulting peer-to-peer interaction they generated, have been instrumental in raising the awareness of our technology platform, endorsing the high quality of our R&D and product development capabilities and driving adoption of our products. In addition, we collaborate with pharmaceutical companies who employ cancer therapy selection using our products and services to help develop new drugs for targeted therapies and immunotherapies on various types of cancers. We believe their rigorous standards for the consistency and accuracy of cancer therapy selection results provide validation and endorsement for our technology platform and our products.
Our future success depends in part on our ability to maintain these relationships and to establish new relationships. Many factors have the potential to impact such collaborations, with both key opinion leaders and pharmaceutical companies, including the type of biomarker support required and our ability to deliver it, pharmaceutical companies’ satisfaction with our products or services, and our ability to pass the periodic or random inspections of our pharmaceutical company partners, and other factors that may be beyond our control. Furthermore, our research partners may decide to decrease or discontinue their use of our products and services due to changes in their research focus; pharmaceutical companies may decide to cease or change their new drugs development plans due to various reasons, such as failures in their clinical trials, financial constraints, or utilization of internal testing resources or tests performed by other parties, or other circumstances outside of our control. We cannot assure you that such existing relationships will continue, or if we establish new relationships with other key opinion leaders and pharmaceutical companies, the resulting relationship will be successful or that academic research and clinical studies conducted as part of the collaborations will produce successful outcomes.
We rely on a limited number of suppliers for some of our laboratory equipment and supplies and may not be able to find replacements or immediately transition to alternative suppliers.
We source sequencers, reagents and certain other laboratory supplies used in our laboratory operations from a limited number of suppliers. Our suppliers are typically trading companies that procure laboratory supplies from a variety of manufacturers. Our laboratory operations may be interrupted if we encounter delays or difficulties in securing these supplies, or if we become unable to procure supplies from any of these suppliers due to their lack of required licenses, permits or certifications. If we cannot timely obtain an acceptable substitute, our business, financial condition, results of operations and reputation could be adversely affected.
We believe that there are a number of replacement suppliers that are capable of supplying all of the sequencers, reagents and other laboratory supplies necessary for our laboratory operations. However, the use of laboratory equipment and supplies furnished by any replacement suppliers may require us to alter our laboratory operations. Transitioning to a new supplier may be time consuming and expensive, result in interruptions in our laboratory operations or require that we revalidate our cancer therapy selection test products and services. There can be no assurance that we will be able to bring the equipment and supplies supplied by these replacement suppliers online and revalidate them without experiencing interruptions in our workflow. In addition, there can be no assurance that replacement suppliers will meet our quality control and performance requirements. If we encounter delays or difficulties in securing, reconfiguring or revalidating the laboratory equipment and supplies we require for our laboratory operations, our business, financial condition, results of operations and reputation could be adversely affected.
If we are unable to support the demand for our current or future products and services, including ensuring that we have adequate capacity to meet increased demand, our business could suffer.
Since our inception, we have experienced rapid growth, and we anticipate further growth in our business operations. Our growth could strain our organizational, administrative and operational infrastructure. As the sales volume of our products and services grows, we will face increased demands on our capacity and efficiency for sample intake, testing results analysis and other laboratory operations, quality control, customer service, and general workflow management processes.
16
Table of Contents
To maintain the quality and expected turnaround time of our tests and effectively meet increased demand, we must continue to improve our operational, financial and management controls and hire, train and manage additional qualified scientists, laboratory personnel and sales and marketing personnel. Failure to do so could adversely affect our business, financial condition and results of operations. For example, if we encounter difficulties in scaling our operations as a result of quality control and quality assurance issues, we will likely experience reduced sales of our cancer therapy selection tests, increased repair or re-engineering costs and increased expenses due to switching to alternate suppliers, any of which would adversely affect our results of operations.
We face risks related to natural disasters, health epidemics, civil and social disruption and other outbreaks, which could significantly disrupt our operations.
We are vulnerable to social and natural catastrophic events that are beyond our control, such as natural disasters, health epidemics, and other catastrophes, which may materially and adversely affect our business. For example, between 2020 and 2022, China and many other countries were affected by the widespread of a novel strain of coronavirus, or COVID-19 and its variants. In an effort to control the spread this virus, the Chinese government imposed various restrictive measures, such as quarantines, travel restrictions and home office policies. In response to this pandemic, hospitals and physicians across China also focused their efforts on treating COVID-19 patients and prioritized resources toward containing the virus, resulting in many diagnostic procedures and cancer therapy selection testing being deferred. As a result, the demand for our products and services under both our central laboratory model and in-hospital model decreased and fluctuated from quarter to quarter, which adversely affected our business operations and financial performance in various periods between 2020 and 2022. Our business growth resumed as the COVID-19 impact started to lessen and the Chinese government significantly loosened its zero-COVID policy in December 2022. However, there is no assurance that the Chinese economy and the pace of its growth can return to pre-COVID level in a short period of time, and our business operation and financial performance may accordingly be affected by the post-COVID recovery of China’s overall economy.
In general, there is also no assurance that a pandemic of similar severity or scale will not affect China in the future. The extent to which similar widespread epidemic in the future can impact our business, results of operations and financial condition will depend on many factors beyond our control, including the extent of future outbreak and resurgences of any infectious disease and its variants, vaccine distribution and other actions in response to such virus or to contain its impact. Our business, results of operations, financial conditions and prospects could be materially adversely affected to the extent similar epidemic harms the Chinese and global economy in general, and the trading price of our ADSs may be adversely affected. To the extent the outbreak of such health epidemics adversely affect our business, results of operations, financial conditions and prospects, they may also have the effect of heightening many of the other risks described in this section.
If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenue or achieve and sustain profitability.
With the development of NGS and cancer genotyping, China’s NGS-based cancer therapy selection market has become increasingly competitive, and we expect this competition to intensify further in the future. Our principal competition comes from other NGS-based cancer therapy selection companies in China. As we gradually commercialize our MRD products and cancer early detection products, we also expect to face competition from players in the relevant fields. Some of our existing and potential future competitors may have longer operating histories, larger customer bases, more expansive brand recognition and deeper market penetration, substantially greater financial, technological and research and development resources and selling and marketing capabilities, and more favorable terms from suppliers. As a result, they may be able to respond more quickly to changes in customer requirements or preferences, develop faster, better and more expansive advancements for their technologies and tests, create and implement more successful strategies for the promotion and sale of their tests, adopt more aggressive pricing policies for their tests, secure supplies from vendors on more favorable terms or devote substantially more resources to infrastructure and system development. In addition, competitors may be acquired by, receive investments from or enter into other commercial relationships with larger, well-established and well-financed companies as the use of cancer therapy selection increases. In addition, if we expand into international markets in the future, we could face competition from NGS-based cancer therapy selection companies in these markets.
If we are unable to compete successfully with current and future competitors for these or any other reasons, we may be unable to increase market acceptance and sales volume of our tests, which could prevent us from maintaining or increasing our revenue levels or achieving or sustaining profitability or could otherwise negatively affect our performance.
17
Table of Contents
Failure to manage our growth or execute our strategies effectively may adversely affect our business and prospects.
We have achieved rapid growth in the past few years. If we are not successful in managing our growth or executing our strategies effectively, our business, results of operations, financial condition and future growth may be adversely affected. For example, as part of our growth strategies, we plan to continue our research and development in early cancer detection, which is technically challenging. In addition, we will continue to implement the strategy to expand our collaboration with partner hospitals under the in-hospital model. As China is a large and diverse market, industry trends and clinical demands may vary significantly by regions. Our experience in collaborations with partner hospitals in major cities under the in-hospital model may not be applicable in hospitals located in other cities. As a result, we may not be able to leverage our experience to expand into local or regional hospitals in other parts of China. Any failure to effectively manage our growth or execute our strategies, including our early cancer detection research and development and our collaboration with hospitals under the in-hospital model, could have an adverse impact on our business and prospects.
Our future success depends on our ability to promote our brand and protect our reputation. If we are unable to effectively promote our brand, our business may be adversely affected.
We believe that enhancing and maintaining awareness of our “Burning Rock” brand is critical to achieving widespread acceptance of our NGS- based products, gaining trust for our testing services and attracting new customers. Successful promotion of our brand depends largely on the quality of the products and services we offer and the effectiveness of our branding and marketing efforts. Currently, we rely primarily on our own sales and marketing team to promote our brand and our NGS-based products and testing services. We expect that our branding and marketing efforts will require us to incur significant expenses and devote substantial resources. We cannot guarantee that our sales and marketing efforts will be successful. Brand promotion activities may not lead to increased revenue in the near term, and, even if they do, any revenue increases may not offset the expenses we incur to promote our brand. Our failure to establish and promote our brand and any damage to our reputation will hinder our growth. In addition, our reputation may be undermined as a result of the negative publicity about our company or our industry in general. If NGS-based products or services provided by us or our competitors do not perform to customers’ expectations, it may result in lower confidence in cancer therapy selection in general, which may in turn impair our operating results and our reputation.
Failure to attract and retain our senior management and other key employees could adversely affect our business.
Our future success is significantly dependent upon the continued service of our senior management, such as Mr. Yusheng Han, our chairman of the board of directors and chief executive officer. If we lose their services, we may not be able to locate suitable or qualified replacements, and we may incur additional expenses to recruit new senior management team members, which could severely disrupt our business and growth. In addition, if these personnel join our competitors or form a competing business, our business and prospects could be adversely affected.
Our research and development activities and laboratory operations depend upon our ability to attract and retain highly skilled scientists and technicians. We are also in strong need of sales and marketing personnel with the relevant technology background and industry expertise in order to effectively conduct our sales and marketing activities and increase our hospital network. We face intense competition for qualified individuals from numerous biotechnology and pharmaceutical companies, universities, governmental entities and other research institutions. We may be unable to attract and retain suitably qualified individuals, and our failure to do so could adversely affect our business.
Misconduct of our employees, consultants or other business partners could engage in misconduct that adversely affects our business.
Our employees, consultants and other business partners could engage in misconduct that adversely affects our business. For example, we are subject to a number of obligations and standards arising from our business relationships with our customers. The violation of these obligations and standards by any of our employees, consultants and other business partners would adversely affect our relationship with our customers. The misconduct of our employees, consultants and other business partners, including the improper use or disclosure of confidential information and improper speech and behavior in the public domain (especially when they identify us in such speech or behavior), could cause harm to our reputation, financial position and current and future business relationships. Detecting or deterring employee misconduct is not always possible, and the extensive precautions we take to detect and prevent this activity may not be effective in all cases. If our employees, consultants and other business partners were to engage in misconduct or were to be accused of such misconduct, our business and our reputation could be adversely affected.
18
Table of Contents
If our central laboratory fails to comply with applicable laboratory licensing requirements, or become damaged or inoperable, our ability to perform tests may be jeopardized.
We currently derive a substantial part of our revenue from tests performed at our central laboratory located in Guangzhou, Guangdong Province, China.
Currently, our central laboratory holds an NGS laboratory certificate issued by Guangdong Branch of the NCCL in May 2018. This certificate is valid for five years and its renewal is conditioned upon additional inspection on a regular and irregular basis. In May 2021, our central laboratory successfully renewed our clinical PCR testing laboratory certificate issued by Guangdong Branch of the NCCL, which is valid for five years. If our central laboratory loses these certificates or fails to renew any certificate in a timely manner, or at all, whether as a result of revocation, suspension, limitation or any other external factors beyond our control, we would no longer be able to perform our tests, which could have an adverse effect on our business, financial condition and results of operations. In addition, in November 2022, we successfully renewed the certification that we voluntarily obtained from the CLIA to perform laboratory examinations and procedures on human specimens. In January 2024, we also successfully renewed the accreditation that we voluntarily obtained from the CAP for our central laboratory, which has been extended until January 2026. As a condition of the CLIA certification and the CAP accreditation, our central laboratory is subject to survey and inspection every other year, in addition to being subject to additional random inspections. There is no assurance that we could maintain or successfully renew the CLIA certification and the CAP accreditation. Loss of, or failure to renew, our CLIA certificate or CAP accreditation may have an adverse effect on our business and reputation.
In addition, our laboratory facilities and equipment could be harmed or rendered inoperable by natural or man-made disasters, including war, fire, earthquake, power loss, communications failure or terrorism, which may render it difficult or impossible for us to perform tests for some period of time. We do not carry any insurance for damage to our property and the disruption of our business. Damages to, or interruptions in the operations of, our laboratory and other facilities could have an adverse impact on our results of operations and financial condition.
Furthermore, our laboratory facilities and equipment could be unavailable or costly and time-consuming to repair or replace. It would be difficult, time-consuming and expensive to rebuild our laboratory facilities, to locate and qualify a new facility or license or transfer our proprietary technology to a third-party to conduct our tests at their facilities, particularly in light of licensure and accreditation requirements. Even in the unlikely event we are able to find a third party with such qualifications to enable us to conduct our tests, we may be unable to negotiate commercially reasonable terms.
We depend on our information technology systems, and any failure of these systems could harm our business.
We depend on information technology systems for significant elements of our operations. We have also installed a number of enterprise software systems that affect a broad range of business processes and functional areas, including for example, systems handling human resources, financial controls and reporting, contract management, regulatory compliance, and other infrastructure operations. These information technology systems support a variety of functions, including laboratory operations, test validation, sample tracking, quality control, customer service support, billing and reimbursement, research and development activities, scientific and medical curation, and general administrative activities.
Information technology systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses, and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our information technology and telecommunications systems, failures or significant downtime of our information technology or telecommunications systems or those used by third-party service providers could prevent us from conducting our daily operations. Any disruption or loss of information technology systems on which critical aspects of our operations depend could have an adverse effect on our business.
Security threats to our information technology infrastructure and unauthorized use of data by third parties could expose us to liability or damage our reputation and business.
Our information technology systems store and process a variety of sensitive data, including our proprietary business information, as well as patients’ personal data such as health information and personally identifiable information.
It is essential that our information technology infrastructure remains secure and is perceived by hospitals, patients and our research partners to be secure. Despite our security measures, we may face cyber-attacks that attempt to penetrate our network security, sabotage or otherwise disable our research, tests and services, misappropriate our proprietary business information or cause interruptions of our internal systems and services. Any cyber- attacks could negatively affect our reputation, damage our network infrastructure and our ability to deploy our products and services, harm our relationship with customers and research partners, and expose us to significant financial liabilities.
19
Table of Contents
Moreover, we may not be able to prevent third parties from illegally obtaining and misappropriating personal data of the tested patients that we collect. Concerns about data leakage or unauthorized use of data by third parties, even if unfounded, could damage our reputation and negatively affect our results of operations.
If we are unable to effectively protect our intellectual property, our business and competitive position would be harmed.
We rely on patents, software copyrights, trademarks, trade secrets and other intellectual property rights protection and contractual restrictions to protect our products, services and technologies. We have registered a number of patents and trademarks in China and have applied for additional patent registrations in China, Hong Kong, the U.S., the European Union and Japan. However, such protection is limited and may not adequately protect our rights. For example, some of the trademark applications for the labels we use in our products have been rejected by the Trademark Office of National Intellectual Property Administration (the “Trademark Office”) as they have been preemptively registered by an independent third party. In July 2022, we filed a request for invalidation against these preemptively registered trademarks. The relevant authority ruled in our favor and invalidated the preemptively registered trademarks in January 2024 and November 2023, respectively. The decisions have been deemed final as there was no appeals made by the independent third party. In 2023, we filed requests of a review of our trademark applications that had been previously rejected by the Trademark Office due to two preemptively registered trademarks or other grounds. As a result of our efforts, the Trademark Office reversed the refusal and granted preliminary approval to the trademarks in March 2024.
In addition, competitors could purchase our products and attempt to replicate and/or improve some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, and design their devices and tests around our protected technologies or develop their own competitive technologies that fall outside of our intellectual property rights.
Monitoring unauthorized disclosure and uses of our trade secrets is difficult, and we do not know whether the steps we have taken to prevent such disclosure and uses are, or will be, adequate. If we resort to litigation to enforce or protect our intellectual property rights, such litigation could result in substantial costs and a diversion of our managerial and financial resources, while the outcome would be unpredictable and any remedy may be inadequate. Our contractual agreements may be breached by our counterparties, and there may not be adequate remedies available to us for any such breach. In addition, our trade secrets may be leaked or otherwise become available to, or be independently discovered by, our competitors, and we would have no right to prevent others from using them. Moreover, if a party having an agreement with us has an overlapping or conflicting obligation to a third party, our rights in and to certain intellectual property could be undermined.
If we fail to effectively protect our intellectual property, our competitive position and prospects could be adversely affected.
We may be subject to intellectual property infringement or misappropriation claims by third parties, which may force us to incur substantial legal expenses and, if determined adversely against us, could disrupt our business.
The validity, enforceability and scope of intellectual property rights protection in China are uncertain and still evolving. We cannot be certain that our products, tests and technologies do not or will not infringe patents, software copyrights, trademarks or other intellectual property rights held by third parties. From time to time, we may be subject to legal proceedings and claims alleging infringement of patents, trademarks or copyrights, or misappropriation of creative ideas or formats, or other infringement of proprietary intellectual property rights. Any such proceedings and claims could result in significant costs to us and divert the time and attention of our management and technical personnel from the operation of our business. These types of claims could also potentially adversely impact our reputation and our ability to conduct business and raise capital, even if we are ultimately absolved of all liability. Moreover, third parties making claims against us may be able to obtain injunctive relief against us, which could block our ability to offer one or more devices or tests and could result in a substantial award of damages against us. In addition, since we sometimes indemnify our customers or collaboration partners, we may have additional liability in connection with any infringement or alleged infringement of third party intellectual property. Intellectual property litigation can be very expensive, and we may not have the financial means to defend ourselves or our customers or collaboration partners.
Because patent applications can take many years to issue, there may be pending applications, some of which are unknown to us, that may result in issued patents upon which our products, tests or proprietary technologies may infringe. Moreover, we may fail to identify issued patents of relevance or incorrectly conclude that an issued patent is invalid or not infringed by our technology or any of our devices or tests. There is a substantial amount of litigation involving patents and other intellectual property rights in our industry. If a third-party claims that we infringe upon a third-party’s intellectual property rights, we may have to:
| • | seek to obtain licenses that may not be available on commercially reasonable terms, if at all; |
| • | abandon any product alleged or held to infringe, or redesign our products or processes to avoid potential assertion of infringement; |
20
Table of Contents
| • | pay substantial damages including, in exceptional cases, treble damages and attorneys’ fees, if a court decides that the device, test or proprietary technology at issue infringes upon or violates the third-party’s rights; |
| • | pay substantial royalties or fees or grant cross-licenses to our technology; and |
| • | defend litigation or administrative proceedings that may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
Ethical, legal and social concerns related to the use of genomic information could reduce demand for our products and services.
Cancer therapy selection and cancer early detection, especially cancer genotyping, has raised ethical, legal and social issues regarding privacy and the appropriate uses of the resulting information. Government authorities could, for social or other purposes, limit or regulate the use of genomic information or prohibit testing for genomic predisposition to certain conditions, particularly for those that have no known cure. Similarly, these concerns may cause patients to refuse to use, or physicians to be reluctant to order, cancer therapy selection and cancer early detection tests such as ours, even if permissible. These and other ethical, legal and social concerns may limit market acceptance and adoption of our tests or reduce the potential markets for our tests, any of which could have an adverse effect on our business, financial condition and results of operations.
If we fail to maintain an effective system of internal controls, we may be unable to accurately report our results of operations or prevent fraud, and investor confidence and the market price of our ADSs may be materially and adversely affected.
We are subject to the reporting obligations under the U.S. and U.K. securities laws. The SEC, as required under Section 404 of the Sarbanes-Oxley
Act of 2002, has adopted rules requiring a public company to include a report by management on the effectiveness of the Company’s internal control over financial reporting in its annual report on Form 20-F. In addition, an independent registered public accounting firm must issue an attestation report on the effectiveness of our internal control over financial reporting. As required by Section 404 of the Sarbanes-Oxley Act of 2002 and related rules promulgated by SEC, our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023 using criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, our management concluded that our internal control over financial reporting was effective as of December 31, 2023. In addition, our independent registered public accounting firm attested to the effectiveness of our internal control and reported that our internal control over financial reporting was effective as of December 31, 2023.
If we fail to maintain an effective internal control environment for our financial reporting, we may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with the Sarbanes-Oxley Act of 2002. We have incurred and will continue to incur additional costs and use additional management and other resources to comply with Section 404 of the Sarbanes-Oxley Act of 2002 and other requirements going forward. Moreover, effective internal control over financial reporting is necessary for us to produce reliable financial reports. As a result, any failure to maintain effective internal control over financial reporting could result in the loss of investor confidence in the reliability of our financial statements, which in turn could negatively impact the trading price of our ADSs in the U.S. or U.K. Additionally, ineffective internal control over financial reporting could subject us to regulatory investigations, and civil or criminal sanctions.
Past and future grants of share-based awards may have an adverse effect on our financial condition and results of operations and have dilutive impact to your investment.
We adopted three share incentive plans in May 2020, October 2021 and May 2023, which we refer to as the 2020 Plan, 2021 Plan and 2023 Plan, respectively, in this annual report, to grant share-based compensation awards to employees, directors, consultants and senior management to incentivize their performance and align their interests with ours. We adopted a share incentive plan in September 2022, which we refer to as the 2022 Plan in this annual report, to replace the 2021 Plan. Options that were issued under the 2021 Plan were accordingly cancelled and replaced by options issued under the 2022 Plan. The maximum aggregate number of ordinary shares we are authorized to issue pursuant to all awards under the 2020 Plan, 2022 Plan and 2023 Plan are 4,512,276 ordinary shares, 11,775,525 ordinary shares and 4,755,000 ordinary shares, respectively. We have also separately issued share incentive awards to our directors, officers and employees outside of the 2020 Plan, 2022 Plan and 2023 Plan. As of March 31, 2024, we had 20,677,571 Class A ordinary shares underlying outstanding share options, restricted shares and restricted share units. See “Item 6. Directors, Senior Management and Employees—B. Compensation—Share Incentive Awards.”
We believe the granting of share-based awards is of significant importance to our ability to attract and retain key personnel and employees, and we will continue to grant share-based compensation to employees, directors and consultants in the future. As a result, our expenses associated with share- based compensation may increase, which may have an adverse effect on our financial condition and results of operations.
21
Table of Contents
We may be subject to litigation and other claims and legal proceedings, and may not always be successful in defending ourselves against these claims or proceedings.
We may be subject to and involved in lawsuits and other claims in the ordinary course of our business. We may from time to time be subject to lawsuits and other legal proceedings brought by our customers, competitors, employees, business partners, investors, other shareholders of the companies we invest, or other entities against us in the ordinary course of our business. We may also be subject to regulatory proceedings in the ordinary course of our business. We may not be successful in defending ourselves, and the outcomes of these lawsuits and proceedings may be unfavorable to us. Lawsuits and regulatory proceedings against us may also generate negative publicity that significantly harms our reputation, which may adversely affect our customer base, market position and our relationships with our research partners and other business partners. In addition to the related costs, managing and defending litigation and other legal proceedings and related indemnity obligations can significantly divert our management’s attention from operating our business. We may also need to pay damages or settle lawsuits or other claims with a substantial amount of cash, negatively affecting our liquidity. As a result, our business, financial condition and results of operations could be adversely affected.
Risks Relating to Government Regulations
We are subject to extensive legal and regulatory requirements in China for our NGS-based products and services. Any lack of requisite certificates, licenses or permits applicable to our business may have an adverse impact on our business, financial condition and results of operations.
We are engaged in the purchase, manufacturing, sale and usage of certain imported laboratory equipment, NGS-based products and related software. The laws and regulations regulating NGS-based products are still in a preliminary stage of development in China. In accordance with current PRC laws and regulations, certain of these equipment, products and software are regulated as medical devices and are required to obtain and maintain various certificates, licenses and permits, including but not limited to medical device record-filing certificates, medical device manufacturing licenses, medical device registration certificates and medical device operation licenses.
Although we obtained China’s first medical device registration certificate for NGS-based cancer therapy selection, as of the date of this annual report, certain of these equipment, products and software have not obtained the required certificates, licenses or permits. In China, very few NGS-based products have obtained medical device registration certificates issued by the competent Chinese governmental authorities. It is uncertain whether we can obtain all medical device registration certificates for our NGS-based products and how long it will take to obtain such registration certificates.
In addition, we have obtained the NMPA approval for two of our NGS reagent kits and may seek to obtain such approvals for our other NGS reagent kits or any other products currently under development, including our early cancer detection products, from time to time. The NMPA approval is valid for five years and we are in the process of renewing the NMPA approval one of our NMPA-approve product. The NMPA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional pre-clinical, clinical or other studies. The process of obtaining NMPA approvals is inherently uncertain and there is no guarantee that our existing or future products could successfully obtain NMPA approvals in a timely manner, if at all. Delays or failure in obtaining NMPA approvals of our products could result in substantial additional costs, adversely affect our ability to compete with other companies, and negatively affect investors’ confidence in our financial performance and business prospects. Even if the NMPA approval is ultimately granted, we may not successfully maintain or renew the approval and the approval may be withdrawn. Any NMPA approval received may also restrict the intended use and marketing of the product we want to commercialize.
According to the most recently amended Regulations on the Supervision and Administration of Medical Devices which became effective on June 1, 2021, subject to more detailed administrative measures to be enacted by the NMPA and the PRC National Health Commission, or the NHC, qualified medical institutions may, based on clinical demands, conduct research and development on in vitro diagnostic testing reagents if the same type of products are not available at market in China, and may also use such in vitro diagnostic testing reagents internally under the instruction of practicing physicians. According to these provisions, medical institutions may use self-developed in vitro diagnostic testing reagents without medical device registration certificate for specific purposes under specific circumstances. However, since the specific administrative measures are still in the process of being formulated, there is uncertainty as to the specific requirements we must comply with.
As of the date of this annual report, we have not been subject to any penalties from the relevant authorities for the purchase, manufacture, sale and usage of these equipment, products and software. As advised by our PRC counsel, Tian Yuan Law Firm, the risk of penalties imposed by the competent authorities is relatively low. However, we cannot assure you that the competent governmental authorities will not take different views or interpretations from us or our PRC counsel, or enact new detailed or more restrictive rules and regulations. Failure to comply with laws or regulations may subject us to penalties, including fines, confiscation of these equipment, products and software and suspension of business, and our business and results of operations could be adversely affected.
22
Table of Contents
We are subject to ongoing obligations and continued regulatory review. There could be a subsequent discovery of previously unknown problems with our NGS-based products and services. Any government investigation of alleged violations of law could require us to expend significant time and resources and could result in adverse government actions and negative publicity.
Failure to comply with existing or future laws and regulations related to the management of human genetic resources in China could lead to government enforcement actions, which could include civil or criminal fines or penalties, private litigation, other liabilities, and/or adverse publicity. Compliance or the failure to comply with such laws could increase the costs of, limit and cause significant delay in our clinical studies and research and development activities, and could otherwise materially and adversely affect our operating results, business and prospects.
Laws and regulations related to the management of human genetic resources in China are rapidly evolving and the enforcement thereof is likely to remain uncertain for the foreseeable future. On June 10, 1998, the Ministry of Science and Technology, or MOST, and the Ministry of Health jointly established the rules for protecting and utilizing human genetic resources, or HGR, in China. From 2006 to 2016, MOST and other regulatory agencies in China have been focused on HGR legislation, and proactively sought opinions from the public on draft regulations. In 2015, MOST issued a Guideline on HGR and reinforced its legislative efforts in HGR administration. In May 2019, the Regulation on Human Genetic Resources Management, or the HGR Regulation, was put in place. The State Council promulgated the HGR Regulation on May 28, 2019 and it became effective on July 1, 2019.
The HGR Regulation prohibits foreign entities or individuals or such entities established or actually controlled thereby, or “Foreign Persons,” from collecting or preserving China HGR in China, or providing China HGR abroad, whereas activities of collection and preservation of organs, tissues and cells for purposes of clinical diagnosis and treatment, service of blood collection and provision, investigation of illegal activities, doping test and funeral service, are required to be conducted in accordance with other relevant laws and regulations. The HGR Regulation permits Foreign Persons’ limited use of China HGR “to carry out scientific research activities,” which must be conducted through collaboration with Chinese scientific research institutions, higher education institutions, medical institutions, or enterprises, collectively, the “Chinese Entities.” Such activities must be approved by MOST, and the application for approval must be filed jointly by the Foreign Person and the relevant Chinese Entity. The only exception to the approval requirement is “international collaboration in clinical trials” that do not involve the outbound transfer of China HGR materials such as organs, tissues, or cells comprising the human genome, genes, or other genetic substances, collectively, China HGR Materials. Such clinical trial collaboration, however, must still be pre-registered with MOST. There remain significant uncertainties as to how provisions of the HGR Regulation might be interpreted and implemented. A VIE entity actually controlled by a foreign entity through contractual agreements would be deemed as a Foreign Person under the HGR Regulation. Short-term storage of samples of laboratory testing by foreign laboratories or foreign-invested laboratories may also be interpreted as preserving China HGR, thus being subjected to MOST application, approval or pre-registration processes.
On October 17, 2020, the Standing Committee of the NPC promulgated the Biosecurity Law of the PRC which became effective on April 15, 2021. The new law, among other things, restates relevant approval or pre-registration requirements of HGR collection, preservation, utilization and external provision, as provided in the HGR Regulation. Moreover, the promulgation of the new law, which takes the form of national law, further demonstrates the commitments of protecting China HGR and safeguarding state biosecurity by the PRC government.
As a company with a VIE structure since our inception, we are deemed as a Foreign Person under the HGR Regulation. As a result, when conducting or participating in research and clinical studies that involve any of our products, performing clinical studies for any of our pipeline products that are under development (including our early detection products), or providing companion diagnostics services to pharmaceutical companies, we are required to seek approval of or make pre-registration with MOST with respect to our collaborations with Chinese Entities under the HGR Regulation. These procedures could be lengthy and require additional expenses, and there is no assurance that we can complete these pre-registrations, or obtain such approvals, in a timely manner, or at all. As a result, our clinical studies and research and development activities of any of our products or pipeline products that are under development (including our early detection products), and our companion diagnostics development services to pharmaceutical companies may suffer significant delay, experience suspension, rejections, cancellations and other obstacles. As a result, our business, financial conditions, results of operations and prospects could be materially adversely affected.
As of the date of this annual report, we, same as our peer companies in the healthcare industry in China, have received and may continue receiving notices from the relevant governmental authorities requiring us to share HGR-related information with competent government agencies from time to time, and have complied with all such requests. As of the same date, we have not been subject to any penalties from the competent governmental authorities for our business operations or clinical studies involving the use of China HGR. However, regulatory agencies in China may change their enforcement practices. Therefore, prior enforcement activity, or lack of enforcement activity, is not necessarily predictive of future actions. Failure to comply with existing or future HGR laws and regulations, including the HGR Regulation and the Biosecurity Law, may subject us to penalties, including fines, suspension of related activities and confiscation of related HGR and gains generated from conducting these activities.
23
Table of Contents
The evolving government regulations may place additional burdens on our efforts to commercialize our products and services.
The PRC government has introduced various reforms to the Chinese healthcare system in recent years and may continue to do so, with an overall objective of expanding basic medical insurance coverage and improve the quality and reliability of healthcare services. The specific regulatory changes under the reforms still remain uncertain. The implementing measures to be issued may not be sufficiently effective to achieve the stated goals, and as a result, we may not be able to benefit from these reforms to the level we expect, if at all. Moreover, the reforms could give rise to regulatory developments, such as more burdensome administrative procedures, which may have an adverse effect on our business and prospects.
In addition, laws and regulations in China, including those regulating medical devices and supplies, are rapidly evolving. On February 9, 2021, the State Council promulgated the amended Regulations on Supervision and Administration of Medical Devices, which became effective on June 1, 2021. On August 26, 2021, the State Administration for Market Regulation promulgated the new Administrative Measures for the Registration and Record- filing of Medical Devices and the Administrative Measures for the Registration and Record-filing of IVD Reagents, both of which became effective on October 1, 2021. On March 10, 2022, the SAMR promulgated the new Measures for the Supervision and Administration of the Manufacture of Medical Devices and the new Measures for the Supervision and Administration of the Business Operation of Medical Devices which both became effective on May 1, 2022. Changes in these areas could impose more stringent requirements on us and increase our compliance and other operating costs, and we may not be able to achieve or sustain profitability. Changes in government regulations could also prevent, limit or delay regulatory approvals in relation to our NGS-based products and services. Moreover, regulatory authorities may conduct periodic or unannounced inspections on pharmaceutical and medical device companies to check if these companies’ manufacturing, quality control and procurement, among others, are in compliance with relevant laws and regulations. If we are not able to maintain regulatory compliance or pass regulatory inspections, any regulatory approval that has been obtained may be revoked, and we may be required to recall our current or future products, or even to partially suspend or totally shut down our production. In addition, regulatory changes may relax certain requirements that could benefit our competitors or lower market entry barriers and increase competition. Further, regulatory agencies in China may periodically, and sometimes abruptly, change their enforcement practices. Any litigation or governmental investigation or enforcement proceedings against us in China may be protracted and may result in substantial costs and diversion of resources and management attention, negative publicity, damage to our reputation and decline in the price of our ADSs.
Furthermore, China’s regulatory framework governing genetic testing is also in the preliminary stage and rapidly evolving. The evolution of government regulations and their interpretation and enforcement involve significant uncertainties, which may place additional burdens on us or even render it impossible for us to comply with certain regulations. For example, in February 2014, two government agencies jointly published an announcement regarding the clinical application of genetic tests, or Circular 25, which halted the provision of genetic tests unless the clinical laboratory of genetic testing is included in a designated pilot program. Pursuant to Circular 25, in March 2014, the PRC government launched the pilot program that granted permits to NGS laboratories. This pilot program, to our knowledge, has been discontinued. Since no implementing rules for Circular 25 have been promulgated as of the date of this annual report, the provision of genetic testing by biotechnology companies, including us, which were not included in such pilot program, may be deemed by the competent governmental authorities to have violated Circular 25. As advised by our PRC counsel, we believe that the risk of us being found in violation of Circular 25 by providing genetics tests is low given that (i) our central laboratory has obtained the clinical PCR testing laboratory certificate, and we are one of the first biotechnology companies in China that have obtained the NGS laboratory certificate, both issued by the NCCL, according to Administrative Regulations for Clinical Gene Amplification Laboratory of Medical Institutions, and (ii) as of the date of this annual report, the relevant governmental authorities have not imposed any penalties on us, or to our knowledge, on other peer companies conducting genetic testing, for any violation of Circular 25. However, we cannot assure you that the governmental authorities will take the same view with us or our PRC counsel. If the governmental authorities determine that we have violated Circular 25, our business of provision of genetic tests may be halted, which may adversely affect our business and prospects.
We may be exposed to liabilities under various anti-corruption laws and regulations. Any determination that we or our employees have violated these laws and regulations could have an adverse effect on our business or our reputation.
We operate in the healthcare industry in China and are subject to Chinese anti-corruption laws and regulations, which generally prohibit companies and intermediaries from engaging in any bribery, corruption and fraudulent activities, including, among other things, improper payments or other form of bribes to hospitals and physicians in connection with the procurement of products. If we, due to either our own deliberate or inadvertent acts or those of others, fail to comply with applicable anti-corruption laws, our reputation could be harmed and we could incur criminal or civil penalties, other sanctions and/or significant expenses, which could have an adverse effect on our business, including our financial condition, results of operations, cash flows and prospects.
24
Table of Contents
In addition, our procedures and controls to monitor anti-bribery compliance may fail to protect us from reckless or criminal acts committed by our employees. We could be liable for actions taken by our employees, including any violations of applicable law in connection with the marketing or sale of our products and services, including China’s anti-corruption laws and the Foreign Corrupt Practices Act of the U.S., or the FCPA. In particular, if our employees make any payments that are forbidden under the FCPA, we could be subject to civil and criminal penalties imposed by the U.S. government.
Any change in the regulations governing the use of personal data in China, which are still under development, could adversely affect our business and reputation.
Through our products and services, we have access to our tested individuals’ personal data, including their age, gender, disease status and medical records. We use these personal data internally to expand our database and improve the clinical utility of our analytics and reporting system.
Chinese regulations governing the collection and use of personal data are still under development. On June 10, 2021, the Standing Committee of the NPC promulgated the PRC Data Security Law, which became effective on September 1, 2021. The PRC Data Security Law defines “data” referred to therein as any recording of information in electronic or other forms and defines “data processing” as including the collection, storage, use, processing, transmission, provision, disclosure, etc. of data. Any organization or individual that collects data shall do so in a lawful and legitimate manner and shall not obtain data by stealing or other illegal means. Where laws and administrative regulations contain provisions on the purposes and scope of data collection and use, organizations and individuals shall collect and use data within the purposes and scope prescribed by laws and administrative regulations. Data processors shall establish and improve a whole-process data security management system, organize data security education and trainings, and take appropriate technical and other necessary measures to protect data security. Following the PRC Data Security Law, the long anticipate Personal Information Protection Law of the PRC was promulgated on August 20, 2021 and became effective on November 1, 2021, which is considered China’s first comprehensive law in the personal information protection. The Personal Information Protection Law of the PRC emphasizes that the processing of personal information shall have clear and reasonable purposes and be carried out in a way that has minimal impact on personal rights and interests. The collection of personal information shall be limited to the smallest scope necessary for achieving the purpose of processing. No organization or individual may illegally collect, use, process or transmit the personal data of others, or illegally trade, provide or publicly disclose the personal data of others, or engage in personal data processing activities that endanger national security or public interests.
Although we believe that there is currently no PRC legal restriction on our internal use of our tested individuals’ personal data, any change in the regulatory regime in this regard could potentially subject us to more stringent data privacy regulations and affect our ability with regard to the collection and use of these personal data, which in turn could have an adverse effect on our business, financial condition and results of operations. In the future, we plan to expand our business internationally and will be subject to relevant regulatory regimes related to data privacy in those countries, which may be subject us to heightened standards of data protection.
Risks Relating to Our Corporate Structure
If the PRC government finds that the agreements that establish the structure for operating our businesses in China do not comply with applicable PRC laws and regulations, or if these regulations or their interpretations change, we could be subject to severe penalties or be forced to relinquish our interests in those operations.
In accordance with the Negative List (2021 Edition) promulgated on December 27, 2021 and became effective on January 1, 2022, foreign investors are prohibited from investing in businesses related to the research, development, and application of genomic diagnosis and treatment technology.
We are an exempted company limited by shares incorporated under the laws of the Cayman Islands, and Beijing Burning Rock Biotech Limited, our wholly owned subsidiary, or WFOE, is considered a foreign-invested enterprise. To comply with PRC laws and regulations, we conduct substantially all of our business in the PRC through Burning Rock (Beijing) Biotechnology Co., Ltd., the VIE, and its subsidiaries, based on contractual arrangements entered into among WFOE, the VIE and its shareholders.
25
Table of Contents
We believe that, although we do not have equity ownership of the VIE, our corporate structure and contractual arrangements enable us to: (i) be the exclusive provider of business support, technical and consulting services in exchange for a fee; (ii) receive substantially all of the economic benefits and bear the obligation to absorb substantially all of the losses of the VIE; (iii) have an irrevocable and exclusive right to purchase, or to designate one or more persons to purchase, from the registered shareholders all or any part of their equity interests in the VIE at any time and from time to time in our absolute discretion to the extent permitted by PRC laws; (iv) have an irrevocable and exclusive right to purchase, or to designate one or more persons to purchase, from the VIE all or any part of its assets at any time and from time to time in our absolute discretion to the extent permitted by PRC laws; (v) appoint us, any person authorized by us (except the shareholders of the VIE), as exclusive agent and attorney to act on behalf of the shareholders of the VIE on all matters concerning the VIE and to exercise all their rights as a registered shareholder of the VIE in accordance with PRC laws and the articles of the VIE; and (vi) pledge as first-ranking charge all of the equity interests in the VIE to us as collateral security for any and all of the guaranteed debt under the contractual arrangements and to secure performance of the obligations under the contractual arrangements. The contractual arrangements allow the results of operations and assets and liabilities of the VIE and its subsidiaries to be consolidated into our results of operations and assets and liabilities under U.S. GAAP as if they were subsidiaries of our Group.
Our PRC counsel, Tian Yuan Law Firm, is of the opinion that (i) the ownership structure of WFOE and the VIE does not violate applicable PRC laws and regulations currently in effect, and (ii) the contractual arrangements are valid, binding and enforceable in accordance with the applicable PRC laws or regulations currently in effect. However, there can be no assurance that the PRC government authorities will take a view that is not contrary to or otherwise different from the opinion of our PRC counsel stated above. There is also the possibility that the PRC government authorities may adopt new laws, regulations and interpretations that may invalidate the contractual arrangements. If the PRC government determines that we are in violation of PRC laws or regulations or lack the necessary permits or licenses to operate our business, the relevant PRC regulatory authorities, including the NHC, would have broad discretion in dealing with such violations or failures, including, but not limited to:
| • | revoking our business and operating licenses; |
| • | discontinuing or restricting our operations; |
| • | imposing fines or confiscating any of our income that they deem to have been obtained through illegal operations; |
| • | imposing conditions or requirements with which we or WFOE and the VIE may not be able to comply; |
| • | requiring us, WFOE and the VIE to restructure the relevant ownership structure or operations; |
| • | restricting or prohibiting our use of the proceeds from our initial public offering and the concurrent private placement or other of our financing activities to finance the business and operations of the VIE and its subsidiaries; or |
| • | taking other regulatory or enforcement actions that could be harmful to our business. |
Any of these actions could cause significant disruption to our business operations, and may adversely affect our business, financial condition and results of operations. In addition, if the PRC governmental authorities find our legal structure and contractual arrangements to be in violation of PRC laws and regulations, it is unclear what impact these actions would have on us and on our ability to consolidate the financial results of the VIE and its subsidiaries in our consolidated financial statements. If any penalty results in our inability to direct the activities of the VIE and its subsidiaries and/or assert contractual control rights over the assets of the VIE and its subsidiaries that conduct substantially all of our PRC operations, and such a penalty significantly impacts their economic performance and/or our ability to receive economic benefits from the VIE and its subsidiaries, we may not be able to consolidate the VIE and its subsidiaries into our consolidated financial statements in accordance with U.S. GAAP.
Our contractual arrangements with the VIE and its shareholders may not be as effective in providing operational control or enabling us to derive economic benefits as a direct ownership of a controlling equity interest would be.
The VIE and its subsidiaries are consolidated for accounting purposes only, and we do not own any equity interest in any of these entities. We have relied and expect to continue to rely on contractual arrangements with the VIE, its shareholders and subsidiaries to operate our business activities. These contractual arrangements may not be as effective as direct ownership in providing us with control over the VIE and its subsidiaries. For example, the VIE, its subsidiaries or shareholders may fail to fulfill their contractual obligations with us or take other actions that are detrimental to our interests.
26
Table of Contents
If we had direct ownership of the VIE, we would be able to exercise our rights as shareholders to effect changes in their board of directors, which in turn could implement changes, subject to any applicable fiduciary obligations, at the management and operational level. However, under the current contractual arrangements, we rely on the performance by the VIE, its subsidiaries and shareholders of their obligations under the contractual arrangements to exercise control over the VIE and its subsidiaries. As a result, investors in our Company may never directly control equity interests in the VIE and its subsidiaries. The shareholders of the VIE may not act in the best interests of our company or may not perform their obligations under these contracts. These risks exist throughout the period in which we intend to operate our business through the contractual arrangements with the VIE, its subsidiaries and shareholders. If any of these shareholders is uncooperative or any dispute relating to these contracts remains unresolved, we will have to enforce our rights under these contracts through the operations of PRC laws and arbitration, litigation and other legal proceedings, the outcome of which will be subject to uncertainties in the PRC legal system; we may also incur substantial costs to enforce the terms of the arrangements. If we are unable to enforce the contractual arrangements or we experience significant delays or other obstacles in the process of enforcing the contractual arrangements, we may not be able to exert effective control over the VIE and may lose control over its assets. Therefore, our contractual arrangements with the VIE, its subsidiaries and shareholders may not be as effective in ensuring our control over the relevant portion of our business operations as direct ownership would be.
We may lose the ability to use and enjoy assets held by the VIE that are critical to the operation of our business if the VIE declares bankruptcy or becomes subject to a dissolution or liquidation proceeding.
The VIE holds certain assets that are critical to the operation of our business. Under the contractual arrangements entered into by WFOE, the VIE and its shareholders, the VIE may not and its shareholders may not cause it to, sell, transfer, pledge or dispose of in any other manner the legal or beneficial interest in the VIE. They also may not allow any encumbrance of security interest over such equity interest, except for the equity interest pledge agreement in the contractual arrangements, without WFOE’s prior written consent. However, if the shareholders of the VIE or its subsidiaries breach the contractual arrangements and voluntarily liquidate the VIE or its subsidiaries, or if the VIE or its subsidiaries declares bankruptcy and all or part of their assets become subject to liens or rights of third-party creditors or are otherwise disposed of without our consent, we may be unable to continue some or all of our business activities, which could adversely affect our business, financial condition and results of operations. In addition, if the VIE or its subsidiaries undergoes an involuntary liquidation proceeding, third-party creditors may claim rights to some or all of its or their assets, thereby hindering our ability to operate our business, which could adversely affect our business, financial condition and results of operations.
Any failure by the VIE, its subsidiaries or shareholders to perform their obligations under our contractual arrangements with them would have an adverse effect on our business.
Under the contractual arrangements entered into by WFOE, the VIE and its shareholders, these shareholders covenanted that they will not request the VIE to distribute profit or dividends, raise shareholders’ resolution to make such a distribution or vote in favor of any such relevant shareholders’ resolution without WFOE’s prior written consent. If these shareholders receive any income, profit distribution or dividend, except as otherwise determined by us, they must promptly transfer or pay such income, profit distribution or dividend to us or any other person designated by us as service fees to the extent permitted under applicable PRC laws. If the shareholders of the VIE breach the relevant covenants, we may need to resort to legal proceedings to enforce the terms of the contractual arrangements. Any such legal proceedings may be costly and may divert our management’s time and attention away from the operation of our business, and the outcome of such legal proceedings is uncertain. For more information on the cash flow between our Company (and its subsidiaries) and the VIE (and its subsidiaries), see “Item 3. Key Information—Results of Operations and Cash Flows of the VIE And Its Subsidiaries.”
The ultimate beneficial shareholders of the VIE may have conflicts of interest with us, which may adversely affect our business.
The equity interests in the VIE are ultimately beneficially held by certain of our directors, indirect shareholders and employees of these indirect shareholders. However, these ultimate beneficial shareholders may have potential conflicts of interest with us. They may breach, or cause the VIE to breach, the contractual arrangements. We cannot assure you that when conflicts arise, the ultimate beneficial shareholders of the VIE will act in the best interests of our company or that conflicts will be resolved in our favor. If we cannot resolve any conflicts of interest or disputes between us and these shareholders, we would have to rely on legal proceedings, which could result in the disruption of our business and subject us to substantial uncertainty as to the outcome of any such legal proceedings.
27
Table of Contents
We conduct our business operations in the PRC through the VIE and its subsidiaries by way of our contractual arrangements, but certain of the terms of our contractual arrangements may not be enforceable under PRC laws.
All the agreements that constitute our contractual arrangements with the VIE, its subsidiaries and shareholders are governed by PRC laws and provide for the resolution of disputes through arbitration in the PRC. Accordingly, these agreements would be interpreted in accordance with PRC laws, and disputes would be resolved in accordance with PRC legal procedures. The legal environment in the PRC is not as developed as in other jurisdictions and uncertainties in the PRC legal system could limit our ability to enforce the contractual arrangements. If we are unable to enforce the contractual arrangements, or if we suffer significant time delays or other obstacles in the process of enforcing them, it would be very difficult to exert effective control over the VIE and its subsidiaries, and our ability to conduct our business and our financial condition and results of operations may be adversely affected.
The contractual arrangements provide that (i) in the event of a mandatory liquidation required by PRC laws, WFOE may act on behalf of the shareholders of the VIE to exercise all such rights associated with their equity interest; and (ii) in such event, where PRC laws permit, any distribution the shareholders of the VIE are entitled to receive, after deducting their initial capital contribution, will be transferred voluntarily to WFOE. Such provision may not be enforceable under PRC laws in the event of a mandatory liquidation required by PRC laws or bankruptcy liquidation.
Therefore, in the event of a breach of any agreements constituting the contractual arrangements by the VIE, its subsidiaries and/or shareholders, we may not be able to exert effective control over the VIE due to the inability to enforce the contractual arrangements, which could adversely affect our ability to conduct our business.
If we exercise the option to acquire the equity interest and assets of the VIE, this equity interest or asset transfer may subject us to certain limitations and substantial costs.
Pursuant to the contractual arrangements, WFOE or its designated person has the irrevocable and exclusive right to purchase all or any portion of the equity interests in the VIE from the VIE’s shareholders at any time and from time to time in its absolute discretion to the extent permitted by PRC laws. The consideration WFOE pays for such purchases will be an amount equal to then registered capital of the VIE multiplied by the percentage of any equity interest to be purchased in proportion to the total equity interests of the VIE. But if applicable PRC law contains a compulsory requirement regarding transfer of the equity interest, the WFOE or any third party designated is entitled to pay the lowest price permitted by the PRC law as the purchase price. In addition, under the contractual arrangements, WFOE or its designated party has the irrevocable and exclusive right, where permitted by PRC law, to purchase from the VIE all or any portion of its assets, and the purchase price will be the higher of (i) the net book value of the assets to be purchased and (ii) the lowest price permitted by applicable PRC law.
Such transfer of equity or assets may be subject to approvals from, or filings with, competent PRC authorities, such as the Ministry of Commerce, or MOFCOM, the State Administration for Market Regulation, or the SAMR, and/or their local competent branches. In addition, the equity transfer price may be subject to review and tax adjustment by the relevant tax authorities. The assets transfer price to be received by the VIE under the contractual arrangements may also be subject to enterprise income tax, and these amounts could be substantial.
Substantial uncertainties exist with respect to the interpretation and implementation of the Foreign Investment Law and how it may affect the viability of our current corporate structure, corporate governance and business operations.
On March 15, 2019, the Foreign Investment Law was formally passed by the thirteenth National People’s Congress and it became effective on January 1, 2020. The Foreign Investment Law replaced the Law on Sino-Foreign Equity Joint Ventures, the Law on Sino-Foreign Cooperative Joint Ventures and the Law on Foreign-Capital Enterprises and became the legal foundation for foreign investment in the PRC. The Foreign Investment Law stipulates certain forms of foreign investment. However, the Foreign Investment Law does not explicitly stipulate contractual arrangements such as those we rely on as a form of foreign investment.
Notwithstanding the above, the Foreign Investment Law stipulates that foreign investment includes “foreign investors investing through any other methods under laws, administrative regulations or provisions prescribed by the State Council.” Future laws, administrative regulations or provisions prescribed by the State Council may possibly regard contractual arrangements as a form of foreign investment. If this happens, it is uncertain whether our contractual arrangements with the VIE, its subsidiaries and shareholders would be recognized as foreign investment, or whether our contractual arrangements would be deemed to be in violation of the foreign investment access requirements. As well as the uncertainty on how our contractual arrangements will be handled, there is substantial uncertainty regarding the interpretation and the implementation of the Foreign Investment Law. The relevant government authorities have broad discretion in interpreting the law. Therefore, there is no guarantee that our contractual arrangements, the business of the VIE and our financial condition will not be adversely affected.
28
Table of Contents
Depending on future developments under the new Foreign Investment Law, we could be required to unwind the contractual arrangements and/or dispose of the VIE, which would have an adverse effect on our business, financial conditions and result of operations. If our company no longer has a sustainable business after an unwinding or disposal or when such requirements are not complied with, regulators in the U.S. or the U.K., may take enforcement actions against us, which may have an adverse effect on the trading of our Shares or even result in delisting our company.
There may be a potential adverse impact to our company if our contractual arrangements with the VIE, its subsidiaries and shareholders are not treated as domestic investment.
If the operation of our businesses conducted through the VIE is subject to any restrictions pursuant to the Negative List or any successor regulations, and the contractual arrangements are not treated as domestic investment, the contractual arrangements may be regarded as invalid and illegal. If this were to occur, we would not be able to operate the relevant businesses through the contractual arrangements and would lose our rights to receive the economic benefits of the VIE. As a result, we would no longer consolidate the financial results of the VIE into our financial results and we would have to derecognize their assets and liabilities according to the relevant accounting standards. If we do not receive any compensation, we would recognize an investment loss as a result of such derecognition.
Our contractual arrangements may be subject to scrutiny by the PRC tax authorities, and a finding that we owe additional taxes could adversely affect our results of operations and reduce the value of your investment.
Under PRC laws and regulations, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities within ten years after the taxable year during which arrangements and transactions were concluded. The Enterprise Income Tax Law, or the EIT Law, requires every enterprise in China to submit its annual enterprise income tax return, together with a report on transactions with its related parties, to the relevant tax authorities. The tax authorities may impose reasonable adjustments on taxation if they have identified any related party transactions that are inconsistent with arm’s-length principles. We may face adverse tax consequences if the PRC tax authorities determine that the contractual arrangements among our PRC subsidiaries and the VIE do not represent an arm’s-length price and adjust the VIE’s income in the form of a transfer pricing adjustment. A transfer pricing adjustment could, among other things, result in a reduction, for PRC tax purposes, of expense deductions recorded by the VIE, which could in turn increase their tax liabilities. In addition, the PRC tax authorities may impose late payment fees and other penalties to our PRC controlled structured entities for under-paid taxes. Our results of operations may be adversely affected if our tax liabilities increase or if we are found to be subject to late payment fees or other penalties.
If the custodians or authorized users of our controlling non-tangible assets, including chops and seals, fail to fulfill their responsibilities, or misappropriate or misuse these assets, our business and operations may be materially and adversely affected.
Under the PRC law, legal documents for corporate transactions, including agreements and contracts such as the leases and sales contracts that our business relies on, are executed using the chop or seal of the signing entity or with the signature of a legal representative, whose designation is registered and filed with the relevant local branch of the market supervision administration. In order to maintain the physical security of our chops and the chops of our PRC entities, we generally store these items in secured locations accessible only by the authorized personnel of each of our PRC subsidiary and the VIE. Although we monitor such authorized personnel, we cannot assure you that such procedures will prevent all instances of abuse or negligence. Accordingly, if any of our authorized personnel misuses or misappropriates our corporate chops or seals, or our corporate chops or seals are not kept safely, stolen or otherwise used by unauthorized persons or for unauthorized purposes, we could encounter difficulties in maintaining control over the relevant entities and experience significant disruption to our operations. If a designated legal representative obtains control of the chops in an effort to obtain control over any of our PRC subsidiary or the VIE, we, our PRC subsidiaries or the VIE would need to pass a new shareholder or board resolution to designate a new legal representative and we would need to take legal action to seek the return of the chops, apply for new chops with the relevant authorities, or otherwise seek legal redress for the violation of the representative’s fiduciary duties to us, which could involve significant time and resources and divert management attention away from our regular business. In addition, the affected entity may not be able to recover corporate assets that are sold or transferred out of our control in the event of such a misappropriation if a transferee relies on the apparent authority of the representative and acts in good faith.
29
Table of Contents
Risks Relating to Doing Business in the PRC
Recent regulatory developments in China may subject us to additional regulatory review and disclosure requirements, expose us to government interference, or otherwise restrict or completely hinder our ability to offer securities and raise capitals outside China, all of which could materially and adversely affect our business, and cause the value of our securities to significantly decline or become worthless.
As we mainly conduct our business in China, we may be subject to PRC laws relating to, among others, data security and restrictions over foreign investments in scientific research and technical services and other industry sectors set out in the Negative List (2021 Edition). Specifically, we may be subject to PRC laws relating to the collection, use, sharing, retention security, and transfer of confidential and private information, such as personal information and other data. These PRC laws apply not only to third-party transactions, but also to transfers of information between us and our wholly foreign-owned enterprises in China, and other parties with which we have commercial relations. These PRC laws and their interpretations and enforcement continue to develop and are subject to change, and the PRC government may adopt other rules and restrictions in the future.
We are exposed to legal and operational risks associated with our operations in China. The PRC government has significant authority to exert influence on the ability of a company with operations in China, including us, to conduct its business. Changes in China’s economic, political or social conditions or government policies could materially and adversely affect our business and results of operations. We are subject to risks due to the uncertainty of the interpretation and the application of the PRC laws and regulations, including but not limited to the risks of uncertainty about any future actions of the PRC government on U.S. listed companies. We may also be subject to sanctions imposed by PRC regulatory agencies, including CSRC, if we fail to comply with their rules and regulations. Any actions by the PRC government to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in companies having operations in China, including us, could significantly limit or completely hinder our ability to offer or continue to offer securities to investors, and cause the value of our securities to significantly decline or become worthless. These China-related risks could result in a material change in our operations and/or the value of our securities, or could significantly limit or completely hinder our ability to offer securities to investors in the future and cause the value of such securities to significantly decline or become worthless.
The PRC government may exert, at any time, substantial intervention and influence over the manner of our operations. Recently, the PRC government initiated a series of regulatory actions and statements to regulate business operations in China with little advance notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas, adopting new measures to extend the scope of cybersecurity reviews and new laws and regulations related to data security, and expanding the efforts in anti-monopoly enforcement.
On December 28, 2021, the CAC, and 12 other departments jointly promulgated the newly revised Measures for Cybersecurity Review with effect from February 15, 2022 (“Measures”), which provides that (i) a critical information infrastructure operator (“CIIO”) which intends to purchase network products and services shall prejudge the possible risks to national security that may arise after the products and services are put into use and where national security will or may be affected, the operator shall apply with the Cybersecurity Review Office for cybersecurity review, and (ii) a network platform operator (“NPO”) that possesses more than one million users’ personal information must apply for cybersecurity review before listing in a foreign country.
On November 14, 2021, the CAC publicly solicited opinions on the Regulations on the Administration of Cyber Data Security (Draft for Comments) which expanded the scope of application of cybersecurity review, established the data classified and categorized protection system, and defined the relevant rules for cross-border security management of data. It provides that data processors carrying out the following activities shall apply for cybersecurity review: (i) merger, reorganization or division of Internet platform operators that gather and possess a large number of data resources having bearing on the national security, economic development or public interests, which affects or may affect national security; (ii) listing in a foreign country of a data processor that processes the personal information of more than one million persons; (iii) listing in Hong Kong of a data processor, which affects or may affect national security; and (iv) other data processing activities that affect or may affect national security.
According to the above provisions, we will be subject to cybersecurity review if we are identified as a CIIO that procures network products or services which affect or may affect national security after being put into use or NPO that conducts data processing activities which affect or may affect national security, or we have more than one million users’ personal information and plans to be listed abroad.
30
Table of Contents
We and our PRC legal counsel, Tian Yuan Law Firm, are of the view that, as of the date of this annual report, the possibility that we become identified as a CIIO or NPO and accordingly would be subject to the cybersecurity review pursuant to the relevant regulations and policies that have been issued by the CAC is relatively low, due to the following reasons:
| (i). | we have not received any CIIO identification notice as of the date of this annual report, which is required to be issued in a timely manner by competent departments responsible for the security protection work of critical information infrastructures after they have organized the CIIO identification in the industry in accordance with the Regulations on the Security Protection of Critical Information Infrastructures; |
| (ii). | NPO is not defined in the Measures and even if a company has been identified as NPO, whether such a company needs to be subject to cybersecurity review depends on whether its data processing activities will “affect or may affect national security.” As of the date of this annual report, we have not experienced any major information security incident in relation to the theft, leakage, damage, illegal use or illegal export of data or personal information. In addition, all the user data collected by us in business operation are stored in mainland China; |
| (iii). | We process no more than one million users’ personal information; And |
| (iv). | However, according to article 16 of the Measures, the member unit of the cybersecurity review work mechanism (the “Cybersecurity Member Unit”) has the right to initiate review on network products and services and data processing activities that it deems as “affect or may affect national security” at its own discretion. If the Cybersecurity Member Unit decides to take a cybersecurity review on us and we fail such review, it could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline or become worthless. |
Currently, the cybersecurity laws and regulations have not directly affected our business and operations, but in anticipation of the strengthened implementation of cybersecurity laws and regulations and the expansion of our business, we face potential risks if we are deemed as a CIIO under applicable laws. In such case, we must fulfill certain obligations as required under the Cybersecurity Law and other applicable laws, including, among others, storing personal information and important data collected and produced within the PRC territory during our operations in China, which we are already doing in our business, and we may be subject to review when purchasing internet products and services. As the amended Measures of Cybersecurity Review took effect in February 2022, we may be subject to review when conducting data processing activities, and may face challenges in addressing its requirements and make necessary changes to our internal policies and practices in data processing. As of the date of this annual report, we have not been involved in any investigations on cybersecurity review made by the CAC on such basis, and we have not received any inquiry, notice, warning, or sanctions in such respect. Based on the foregoing, we and our PRC legal counsel, Tian Yuan Law Firm, do not expect that, as of the date of this annual report, the current applicable PRC laws on cybersecurity would have a material adverse impact on our business. After consulting with our PRC legal counsel, Tian Yuan Law Firm, we believe that we are in compliance with regulations or policies that have been issued by the CAC as of the date of this annual report in all material aspects, on the following bases: (i) we have set up internal cybersecurity regulations, including data backup and recovery measures and disaster recovery measures; (ii) we have completed the Grade III information security protection filing as required by the relevant regulations and policies issued by relevant authorities; (iii) we inform our users and obtain their consent before collecting their personal information; (iv) we store relevant information in our own servers within the PRC; (v) we have not been investigated or received any request from any CAC authorities as of the date of this annual report; (vi) we have not been subject to any administrative penalties regarding cybersecurity or data security issues as of the date of this annual report; and (vii) we have not been a party to any litigation or arbitration regarding with cybersecurity or data security issues as of the date of this annual report. As advised by Tian Yuan Law Firm, our PRC counsel, as of the date of this annual report, we are not subject to any cybersecurity review under the cybersecurity laws and regulations.
On September 1, 2021, the PRC Data Security Law became effective, which imposes data security and privacy obligations on entities and individuals conducting data-related activities, and introduces a data classification and hierarchical protection system based on the importance of data in economic and social development, as well as the degree of harm it will cause to national security, public interests, or legitimate rights and interests of individuals or organizations when such data is tampered with, destroyed, leaked, or illegally acquired or used. As of the date of this annual report, we have not been involved in any investigations on data security compliance made in connection with the PRC Data Security Law, and we have not received any inquiry, notice, warning, or sanctions in such respect. Based on the foregoing, we do not expect that, as of the date of this annual report, the PRC Data Security Law would have a material adverse impact on our business.
31
Table of Contents
On July 6, 2021, the relevant PRC governmental authorities published the Opinions on Strictly Cracking Down Illegal Securities Activities in Accordance with the Law. These opinions emphasized the need to strengthen the administration over illegal securities activities and the supervision on overseas listings by China-based companies and proposed to take effective measures, such as promoting the construction of relevant regulatory systems to deal with the risks and incidents faced by China-based overseas-listed companies. As these opinions were recently issued, official guidance and related implementation rules have not been issued yet and the interpretation of these opinions remains unclear at this stage. As of the date of this annual report, we have not received any inquiry, notice, warning, or sanctions from the CSRC or any other PRC government authorities. Based on the foregoing and the currently effective PRC laws, we and our PRC legal counsel, Tian Yuan Law Firm, are of the view that, as of the date of this annual report, these opinions do not have a material adverse impact on our business.
On February 17, 2023, the CSRC promulgated the Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies and five related guidelines, collectively, the Overseas Listing Trial Measures, which became effective on March 31, 2023. The Overseas Listing Trial Measures regulate both direct and indirect overseas offering and listing of PRC domestic companies’ securities by adopting a filing-based regulatory regime. Pursuant to the Overseas Listing Trial Measures, initial public offering or listings in overseas markets shall be filed with the CSRC within three working days after the relevant application is submitted overseas. Furthermore, it is stipulated, among others, that an overseas offering and listing shall be prohibited under any of the following circumstances: (i) where such securities offering and listing is explicitly prohibited by provisions in laws, administrative regulations and relevant state rules; (ii) where the intended securities offering and listing may endanger national security as reviewed and determined by competent authorities under the State Council in accordance with law; (iii) where the PRC domestic company intending to make the securities offering and listing, or its controlling shareholders and the actual controller, have committed crimes such as corruption, bribery, embezzlement, misappropriation or property or undermining the order of the socialist market economy during the last three years; (iv) where the PRC domestic company intending to make the securities offering and listing is suspected of committing crimes or major violations of laws and regulations, and is under investigation according to law, and no conclusion has been made thereof; (v) where there are material ownership disputes over equity held by the PRC domestic company’s controlling shareholder or by other shareholders that are controlled by the controlling shareholder and/or actual controller. The Overseas Listing Trial Measures also provide that any overseas offering and listing made by an issuer that meets both the following conditions will be determined as indirect overseas offering and listing of PRC domestic companies: (i) 50% or more of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent accounting year is accounted for by PRC domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in mainland China, or its main places of business are located mainland China, or the senior management personal in charge of its business operation and management are mostly Chinses citizens or domiciled in mainland China. On February 17, 2023, the CSRC issued the Notice on the Administrative Arrangements for the Filing of Overseas Listings by Domestic Enterprises. The CSRC clarified that on March 31, 2023, the effective date of the Overseas Listing Trial Measures, the PRC domestic companies that have been listed overseas by March 31, 2023 are not required to file immediately, and filing should be made as required if they involve refinancing and other filing matters. As the requirements under Overseas Listing Trial Measures are new and evolving, there remains substantial uncertainties as to their interpretation and implementation and how they may impact our ability to raise or utilize fund and business operation. In particular, there are uncertainties as to the form and substance of regulatory requirements and we cannot assure you that, when required, we can complete the requisite filing with such form and content satisfactory to the relevant regulators in a timely manner. Any delays in completing the filing requirements may adversely affect our ability to complete our capital raising activities from the capital markets in the future.
Since these statements and regulatory actions are new, it is highly uncertain how soon legislative or administrative regulation making bodies will respond and what existing or new laws or regulations or detailed implementations and interpretations will be modified or promulgated, if any, and the potential impact such modified or new laws and regulations will have on our daily business operation, our ability to accept foreign investments and conduct follow-on offerings, and listing or continuing listing on a U.S. or other foreign exchanges. In addition, the PRC government has recently published new policies that significantly affected certain industries such as the education and internet industries, and we cannot rule out the possibility that it will in the future release regulations or policies regarding any other industry including the industry in which we operate, which could adversely affect our business, financial condition and results of operations.
We are subject to many of the economic and political risks associated with emerging markets due to our operation in China. Adverse changes in the Chinese or global economic, political and social conditions as well as government policies could adversely affect our business and prospects.
The majority of our operations are in China, one of the world’s largest emerging markets. In light of our operations in an emerging market, we may be subject to risks and uncertainties including fluctuation in GDP, unfavorable or unpredictable treatment in relation to tax matters, exchange controls, restrictions affecting our ability to make cross-border transfer of funds, regulatory proceedings, inflation, currency fluctuations or the absence of, or unexpected changes in, regulations and unforeseeable operational risks. In addition, our business, prospects, financial condition and results of operations may be significantly influenced by political, economic and social conditions in China generally and by continued economic growth in China.
32
Table of Contents
The Chinese economy differs from the economies of most developed countries in many respects, including the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. Although the PRC government has implemented measures that focus on taking into account market forces to effect economic reform and aimed at reducing the state ownership of productive assets and establishing improved corporate governance in business enterprises, a substantial portion of China’s productive assets are still owned by the government.
In addition, the PRC government continues to play a significant role in regulating development through industrial policies. The PRC government exercises significant control over China’s economic growth through its allocation of resources, control of payment of foreign currency-denominated obligations, monetary policy, and preferential treatment for particular industries or companies. The enforcement of laws, the interpretation of rules and regulations and the direction of government policies can change drastically and quickly with little advance notice but with material and adverse effects for the relevant industries and companies. The Chinese government may also intervene or influence over the companies’ operations, including companies that are listed overseas like us, to a significant extent at any time with little advance notice. It may also exert more control over offerings conducted overseas or foreign investments in China-based issuers like us, including disallowing he VIE structure. Any actions by the Chinese government to exert more oversight and control over securities that are listed overseas by China-based companies, or over foreign investment in China- based issuers, could significantly limit or completely hinder our ability to continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless. The occurrence of any of the foregoing can result in a material change in our business operations and a material and adverse impact on the value of our ADSs.
While the Chinese economy has experienced significant growth over the past decades, growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures, which may benefit the overall Chinese economy, may have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations. In addition, the PRC government has from time to time implemented certain measures, including interest rate changes, to control the pace of economic growth. These measures may cause decreased economic activity in China, and, since 2012, the Chinese economy has slowed down. Any prolonged slowdown in the Chinese economy may reduce the demand for our services and adversely affect our business and results of operations.
Geopolitical tensions have led to a worsening relationship between China and the United States and this adverse trend may continue to deteriorate, which could negatively affect our business and results of operations.
Recently there have been heightened tensions in the trade and economic relations between the U.S. and China. The U.S. government has imposed a series of, and proposed to impose additional, new or higher tariffs on products imported from China to penalize China for what it characterizes as unfair trade practices. China has responded by imposing largely commensurate tariffs on products imported from the U.S. Amid these tensions, the U.S. government has imposed and may impose additional measures on entities in China, including sanctions. Although the U.S. and China signed the “Phase One” trade agreement in January 2020, we cannot assure you that a more comprehensive trade deal will be agreed or that tariffs will not be imposed even if such an agreement is reached. If any new tariffs, legislation and/or regulations are implemented, or if existing trade agreements are renegotiated or, in particular, if the U.S. government takes retaliatory trade actions due to the recent U.S.-China trade tension, and China further retaliates in response to new trade policies, treaties and tariffs implemented by the United States, or even if there is news and rumors of any such escalation, it could introduce uncertainties to China’s economy and the global economy, which in turn could affect our business. We currently source some of our reagents and laboratory equipment from vendors based in the U.S. The U.S. government may prohibit these companies from doing business with Chinese companies and the Chinese government may implement countermeasures. If this were to happen, we may be required to seek substitute suppliers, which could adversely affect our operations. Moreover, the potential increase in tariffs may also increase the costs we incur to purchase imported reagents and laboratory equipment. In addition, as a biotechnology company with operations primarily based in China, our international expansion plan to commercialize our products and services in, and export our products and services to, the U.S. could be adversely affected by these or future trade developments. Our current or future operations in the U.S. may be adversely affected by relationship between the two countries. In addition, increased protectionism and the risk of global trade war, which result in weaker global trade and lower levels of economic activity, could reduce the demand for our tests and adversely affect our business.
33
Table of Contents
In addition to trade disputes, political tensions between the United States and China have escalated in recent years due to, among other things, the COVID-19 outbreak, data security and privacy, emerging technologies, “dual-use” commercial technologies, applications that could be deployed for surveillance or military purposes, import/export of technology, sanctions imposed by the U.S. Department of Treasury on certain officials of the Hong Kong Special Administrative Region and the central government of the PRC and the executive orders issued by former U.S. President Donald J. Trump in August 2020 that prohibit certain transactions with certain Chinese companies and their applications. Throughout 2021, 2022 and 2023, the U.S. also imposed sanctions on additional Chinese entities for their alleged involvement in various matters, including human rights violations in Xinjiang Uyghur Autonomous Region, Communist Chinese military companies, and Hong Kong. The policies and measures directed at China and Chinese companies could also discourage U.S. persons and organizations to work for, provide services to or cooperate with Chinese companies, which could hinder our ability to hire or retain qualified personnel and find suitable partners for our business. Furthermore, the adoption by the U.S. government of these policies and measures against Chinese companies could negatively affect certain investors’ sentiment towards our ADSs and their willingness to invest in or hold our ADSs, which may in turn have a negative impact on the trading price of our ADSs in both the U.S. and U.K. We cannot assure you that the current export controls or economic, trade or other sanctions regulations will not have a negative impact on our business operations, or that the related trend will not further deteriorate in the future. In addition, there have been recent media reports on deliberations within the U.S. government regarding potentially limiting or restricting China-based companies from accessing U.S. capital markets. If any such deliberations or policies were to materialize, the resulting legislation may have material and adverse impact on the stock performance of China-based issuers listed in the U.S. or the U.K. Furthermore, policies of the United States tend to be followed by certain other countries, and these countries may adopt similar policies regarding their relationships with China or against Chinese companies and restricting their operations.
Uncertainties in the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and us.
The PRC legal system is a civil law system based on written statutes. Unlike common law systems, it is a system in which prior court decisions have limited value as precedents. Our PRC subsidiaries and the VIE and its subsidiaries are subject to various PRC laws and regulations generally applicable to companies in China. However, since these laws and regulations are relatively new and the PRC legal system continues to rapidly evolve, their interpretation is not always consistent and their enforcement involves uncertainties.
In particular, PRC laws and regulations concerning the cancer genotyping industry are developing and evolving. Although we have taken measures to comply with the laws and regulations applicable to our business operations and to avoid conducting any non-compliant activities under these laws and regulations, the PRC governmental authorities may promulgate new laws and regulations regulating cancer genotyping industries, some of which may have a retroactive effect. We cannot assure you that our business operations would not be deemed to violate any such new PRC laws or regulations. Moreover, developments in the cancer genotyping industry may lead to changes in PRC laws, regulations and policies or in the interpretation and application of existing laws, regulations and policies, which in turn may limit or restrict us, and could adversely affect our business and operations.
From time to time, we may have to rely on administrative and court proceedings to enforce our legal rights. However, since the PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. Furthermore, the PRC legal system is based in part on government policies and internal rules (some of which are not published in a timely manner or at all) that may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until sometime after the violation. These types of uncertainties, including uncertainty over the scope and effect of our contractual, property (including intellectual property) and procedural rights, and any failure to respond to changes in the regulatory environment in China, could adversely affect our business and impede our ability to continue our operations, and may further affect the legal remedies and protections available to investors, which may, in turn, adversely affect the value of your investment.
34
Table of Contents
We may be classified as a “PRC resident enterprise” for PRC enterprise income tax purposes, which could result in unfavorable tax consequences to us and our non-PRC shareholders or ADS holders.
Under the PRC Enterprise Income Tax Law and its implementation rules, an enterprise established outside of the PRC with a “de facto management body” within the PRC is considered a resident enterprise and will be subject to enterprise income tax on its global income at the rate of 25%. The related implementation rules define the term “de facto management body” as the body that exercises full and substantial control over, and overall management of, the business, productions, personnel, accounts and properties of an enterprise. In April 2009, the State Administration of Taxation, or the SAT, issued a circular, known as Circular 82, which provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled enterprise that is incorporated offshore is located in China. Although Circular 82 only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreigners, the criteria set forth in Circular 82 may reflect the SAT’s general position on how the “de facto management body” test should be applied in determining the tax resident status of all offshore enterprises. According to Circular 82, an offshore-incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group will be regarded as a PRC tax resident by virtue of having its “de facto management body” in China. It will be subject to PRC enterprise income tax on its global income only if all of the following conditions are met: (i) the primary location of the day-to-day operational management is in the PRC; (ii) decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in the PRC; (iii) the enterprise’s primary assets, accounting books and records, company seals, and board and shareholder resolutions are located or maintained in the PRC; and (iv) at least 50% of voting board members or senior executives habitually reside in the PRC.
We believe none of our entities outside of China is a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.” As substantially all of our management members are based in China, it remains unclear how the tax residency rule would apply in our case. If the PRC tax authorities determine that we or any of our subsidiaries outside of China is a PRC resident enterprise for PRC enterprise income tax purposes, then we or such subsidiary could be subject to PRC tax at a rate of 25% on its worldwide income, which could reduce our net income. In addition, we would also be subject to PRC enterprise income tax reporting obligations. Furthermore, if the PRC tax authorities determine that we are a PRC resident enterprise for enterprise income tax purposes, dividends paid by us and gains realized on the sale or other disposition of our ordinary shares or ADSs may be subject to PRC tax, at a rate of 10% in the case of non-PRC enterprises or 20% in the case of non-PRC individuals (in each case, subject to the provisions of any applicable tax treaty), if such dividends and gains are deemed to be from PRC sources. It is unclear whether non-PRC shareholders of our company, including the holders of our ADSs, would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that we are treated as a PRC resident enterprise. Any such tax may reduce the returns on your investment in our ADSs.
We may rely on dividends and other distributions from our subsidiaries in China to fund our cash and financing requirements, and any limitation on the ability of our subsidiaries to make payments to us could adversely affect our ability to conduct our business.
As a holding company, we conduct most of our business through our subsidiaries incorporated in China. We may rely on dividends paid by these PRC subsidiaries for our cash needs, including the funds necessary to pay any dividends and other cash distributions to our shareholders, to service any debt we may incur and to pay our operating expenses. The payment of dividends by entities established in China is subject to limitations.
Regulations in China currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. As a result, our PRC subsidiaries are restricted in their ability to transfer a portion of their net assets to us in the form of dividends. In addition, if any of our PRC subsidiaries incurs debt on its own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us. Any limitations on the ability of our PRC subsidiaries to transfer funds to us could adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends and otherwise fund and conduct our business.
Our PRC subsidiaries generate primarily all of their revenue in Renminbi, which is not freely convertible into other currencies. As a result, any restriction on currency exchange may limit the ability of our PRC subsidiaries to use their Renminbi revenues to pay dividends to us.
Historically, in response to the persistent capital outflow and the Renminbi’s depreciation against the U.S. dollar in 2016, the People’s Bank of China, or the PBOC, and the State Administration of Foreign Exchange, or SAFE, have implemented a series of capital control measures, including stricter vetting procedures for China-based companies to remit foreign currency for overseas acquisitions, dividend payments and shareholder loan repayments. The PRC government may continue to strengthen its capital controls and our PRC subsidiary’s dividends and other distributions may be subjected to tighter scrutiny. Any limitation on the ability of our PRC subsidiary to pay dividends or make other distributions to us could adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends, or otherwise fund and conduct our business.
35
Table of Contents
In addition, the EIT Law and its implementation rules provide that a withholding tax rate of up to 10% will be applicable to dividends payable by Chinese companies to non-PRC resident enterprises unless otherwise exempted or reduced according to treaties or arrangements between the PRC central government and governments of other countries or regions where the non-PRC resident enterprises are incorporated.
Fluctuations in exchange rates could have an adverse effect on our results of operations and the value of your investment.
The conversion of RMB into foreign currencies, including U.S. dollars, is based on rates set by the People’s Bank of China. The RMB has fluctuated against the U.S. dollar, at times significantly and unpredictably. The value of RMB against the U.S. dollar and other currencies is affected by changes in China’s political and economic conditions and by China’s foreign exchange policies, among other things. We cannot assure you that RMB will not appreciate or depreciate significantly in value against the U.S. dollar in the future. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between RMB and the U.S. dollar in the future.
Substantially all of our revenue and costs are denominated in Renminbi. We are a holding company and we rely on dividends paid by our operating subsidiaries in China for our cash needs. Any significant appreciation or depreciation of RMB may materially and adversely affect our revenues, earnings and financial position, and the value of, and any dividends payable on, our ADSs in U.S. dollars. For example, to the extent that we need to convert U.S. dollars we receive into RMB to pay our operating expenses, appreciation of RMB against the U.S. dollar would have an adverse effect on the RMB amount we would receive from the conversion. Conversely, a significant depreciation of RMB against the U.S. dollar may significantly reduce the U.S. dollar equivalent of our earnings, which in turn could adversely affect the price of our ADSs.
Very limited hedging options are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. While we may decide to enter into hedging transactions in the future, the availability and effectiveness of these hedges may be limited and we may not be able to adequately hedge our exposure or at all. In addition, our currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert RMB into foreign currency. As a result, fluctuations in exchange rates may have a material adverse effect on your investment.
The PRC government’s control of foreign currency conversion may limit our foreign exchange transactions, including dividend payments on our ordinary shares.
The PRC government imposes controls on the convertibility of the Renminbi into foreign currencies and, in certain cases, the remittance of currency out of China. We receive substantially all of our revenues in Renminbi. Under our current corporate structure, our company in the Cayman Islands relies on dividend payments indirectly from our PRC subsidiaries to fund any cash and financing requirements we may have. Under existing PRC foreign exchange regulations, payments of current account items, such as profit distributions and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval from SAFE, by complying with certain procedural requirements. Therefore, our PRC subsidiaries are able to pay dividends in foreign currencies to us without prior approval from SAFE, subject to the condition that the remittance of such dividends outside of the PRC complies with certain procedures under PRC foreign exchange regulation. However, approval from or registration with appropriate governmental authorities or commercial banks authorized by such authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses, such as the repayment of loans denominated in foreign currencies.
In light of strong capital outflows from China in 2016, the PRC government has imposed more restrictive foreign exchange policies and stepped up its scrutiny of major outbound capital movements. More restrictions and substantial vetting processes have been put in place by SAFE to regulate cross-border capital account transactions. The PRC government may at its discretion further restrict access to foreign currencies in the future for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currencies to satisfy our foreign currency demands, we may not be able to pay dividends in foreign currencies to our shareholders.
Furthermore, as the interpretation and implementation of these foreign exchange regulations has been constantly evolving, it is unclear how these regulations, and any future regulations concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant government authorities. For example, we may be subject to a more stringent review and approval process with respect to our foreign exchange activities, such as remittance of dividends and foreign-currency-denominated borrowings, which may adversely affect our financial condition and results of operations. In addition, if we decide to acquire a PRC domestic company, we cannot assure you that we or the owners of such company will be able to obtain the necessary approvals or complete the necessary filings and registrations required by the foreign exchange regulations. This may restrict our ability to implement our acquisition strategy and could adversely affect our business and prospects.
36
Table of Contents
Inflation in the PRC could negatively affect our profitability and growth.
The economy of China has experienced significant growth, which has from time to time lead to significant inflation. China’s overall economy is expected to continue to grow. Future increases in China’s inflation may adversely affect our profitability and results of operations.
PRC regulation of loans to and direct investments in PRC entities by offshore holding companies may delay or prevent us from making loans or additional capital contributions to our subsidiaries, which could adversely affect our liquidity and our ability to fund and expand our business.
We are an offshore holding company conducting our operations in China through our PRC subsidiaries. We may make loans to our PRC subsidiaries or we may make additional capital contributions to our wholly foreign-owned subsidiaries in China. Any loans by us to our wholly foreign- owned subsidiaries in China to finance their activities cannot exceed statutory limits and must be registered with the local counterpart of the PRC State Administration of Foreign Exchange, or the SAFE. In addition, a foreign invested enterprise shall use its capital pursuant to the principle of authenticity and self-use within its business scope.
In March 2015, the SAFE promulgated the Circular on Reforming the Administration Measures on Conversion of Foreign Exchange Registered Capital of Foreign-invested Enterprises, or SAFE Circular 19, which took effect and replaced certain previous SAFE regulations from June 1, 2015. The SAFE further promulgated the Circular of the SAFE on Reforming and Regulating Policies on the Control over Foreign Exchange Settlement of Capital Accounts, or SAFE Circular 16, which took effective on June 9, 2016 and, among other things, amended certain provisions of SAFE Circular 19. According to SAFE Circular 19 and SAFE Circular 16, the flow and use of the Renminbi capital converted from foreign currency-denominated registered capital of a foreign-invested company is regulated such that Renminbi capital may not be used for business beyond its business scope, or to provide loans to persons other than affiliates, unless otherwise permitted under its business scope. SAFE Circular 19 and SAFE Circular 16 may limit our ability to transfer the net proceeds from our initial public offering and the concurrent private placement to our PRC subsidiaries and convert the net proceeds into RMB.
In light of the various requirements imposed by PRC regulations on loans to and direct investment in PRC entities by offshore holding companies, we cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals on a timely basis, if at all, with respect to future loans to our PRC subsidiaries or future capital contributions by us to our wholly foreign-owned subsidiaries in China. As a result, uncertainties exist as to our ability to provide prompt financial support to our PRC subsidiaries when needed. If we fail to complete such registrations or obtain such approvals, our ability to use the proceeds we received from our initial public offering and the concurrent private placement and to capitalize or otherwise fund our PRC operations may be negatively affected, which could adversely affect our liquidity and our ability to fund and expand our business.
The M&A Rules and certain other PRC regulations establish complex procedures for some acquisitions of Chinese companies by foreign investors, which could make it more difficult for us to pursue growth through acquisitions in China.
The M&A Rules and some other regulations and rules concerning mergers and acquisitions, have established complex procedures and requirements that restrict merger and acquisition activities by foreign investors. For example, when a foreign investor takes control of a PRC enterprise, it must notify MOFCOM in advance of such change-of-control transaction. Moreover, the Anti-Monopoly Law requires that the anti-trust governmental authority be notified in advance of any concentration of undertaking if certain thresholds are triggered. The security review rules issued by MOFCOM, which became effective in September 2011, specify that certain mergers and acquisitions by foreign investors, for example those that raise “national defense and security” concerns or through which foreign investors may acquire de facto control over domestic enterprises and therefore raise “national security” concerns, are subject to its review. Those rules prohibit any activities attempting to bypass security review, for example by structuring a transaction through a proxy or contractual control arrangements. We may grow our business by acquiring other companies operating in our industry. Complying with the requirements of the regulations described above and other relevant rules to complete these transactions could be time-consuming, and any required approval processes, including obtaining approval from MOFCOM or its local counterparts, may delay or inhibit our ability to complete these transactions, which could affect our ability to expand our business or maintain our market share. Furthermore, according to the M&A Rules, if a PRC entity or individual plans to merger or acquire its related PRC entity through an overseas company legitimately incorporated or controlled by such entity or individual, such a merger and acquisition will be subject to examination and approval by MOFCOM. The application and interpretations of M&A Rules are still uncertain, and there is possibility that the relevant PRC regulators may promulgate new rules or explanations requiring that we obtain approval of MOFCOM for our completed or ongoing mergers and acquisitions. There is no assurance that we can obtain MOFCOM approval for our mergers and acquisitions, and if we fail to obtain those approvals, we may be required to suspend our acquisition and be subject to penalties. Any uncertainties regarding such governmental approval requirements could have an adverse effect on our business, results of operations and corporate structure.
37
Table of Contents
The heightened scrutiny over acquisition transactions by the PRC tax authorities may have a negative impact on our business operations, our acquisition or restructuring strategy or the value of your investment in us.
Pursuant to the Notice on Strengthening Administration of Enterprise Income Tax for Share Transfers by Non-PRC Resident Enterprises, or Circular 698, issued by the SAT, which became effective retroactively as of January 1, 2008, if a non-resident enterprise investor transfers equity interest in a PRC resident enterprise indirectly by way of disposing of equity interest in an overseas holding company, the non-resident enterprise investor, being the transferor, may be subject to PRC enterprise income tax, if the indirect transfer is considered to be an abusive use of company structure without reasonable commercial purposes. As a result, gains derived from such indirect transfers may be subject to PRC withholding tax at a rate of up to 10%. In addition, the relevant PRC resident enterprise may be required to provide necessary assistance to support the enforcement of Circular 698.
On February 3, 2015, the SAT issued a Public Notice Regarding Certain Corporate Income Tax Matters on Indirect Transfer of Properties by Non-Tax Resident Enterprises, or Public Notice 7. Public Notice 7 introduces a new tax regime that is significantly different from Circular 698. Public Notice 7 extends tax jurisdiction to not only indirect transfers set forth under Circular 698 but also to transactions involving the transfer of other taxable assets made through the offshore transfer of a foreign intermediate holding company. In addition, Public Notice 7 provides clearer criteria than Circular 698 on how to assess reasonable commercial purposes and has introduced safe harbors for internal group restructurings and the purchase and sale of equity through a public securities market. Public Notice 7 has new requirements for both foreign transferors and the transferees (or other person who is obligated to pay for the transfer) of the taxable assets. If a non-resident enterprise conducts an “indirect transfer” by transferring the taxable assets indirectly by disposing of the equity interest of an overseas holding company, then the non-resident enterprise, as the transferor, or the transferee or the PRC entity, which directly owned the taxable assets, must report to the relevant tax authority such indirect transfer. As a result, gains derived from such indirect transfer may be subject to PRC enterprise income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes, currently at a rate of up to 10% for the transfer of equity interest in a PRC resident enterprise. Both the transferor and the transferee may be subject to penalties under PRC tax laws if the transferee fails to withhold the taxes and the transferor fails to pay the taxes.
On October 17, 2017, the SAT issued a Public Notice on Issues Concerning the Withholding of Non-resident Enterprise Income Tax at Source, or Public Notice 37, which, among others, repealed the Circular 698 on December 1, 2017. Public Notice 37 further details and clarifies the tax withholding methods in respect of income of non-resident enterprises under Circular 698. And certain rules stipulated in Public Notice 7 are replaced by Public Notice 37. Where the non-resident enterprise fails to declare the tax payable pursuant to Article 39 of the Enterprise Income Tax Law, the tax authority may order it to pay the tax due within required time limits, and the non-resident enterprise is required to declare and pay the tax payable within such time limits specified by the tax authority; however, if the non-resident enterprise voluntarily declares and pays the tax payable before the tax authority orders it to do so within required time limits, it will be deemed that such enterprise has paid the tax in time.
We face uncertainties as to the reporting and other implications of certain past and future transactions where PRC taxable assets are involved, such as offshore restructuring, sale of the shares in our offshore subsidiaries and investments. We may be subject to filing obligations or taxed if we are the transferor in such transactions, and we may be subject to withholding obligations if we are the transferee in such transactions, under Public Notice 7 and Public Notice 37. For transfer of shares in our company by investors who are non-PRC resident enterprises, our PRC subsidiary may be requested to assist in the filing under Public Notice 7 and Public Notice 37. As a result, we may be required to expend valuable resources to comply with Public Notice 7 and Public Notice 37 or to request the relevant transferors from whom we purchase taxable assets to comply with these notices, or to establish that our company should not be taxed under these notices, which may have an adverse effect on our financial condition and results of operations.
You may be subject to PRC income tax on dividends from us or on any gain realized on the transfer of our ADSs.
Under the EIT Law and its implementation rules, PRC withholding tax at the rate of 10% is generally applicable to dividends from PRC sources paid to investors that are resident enterprises outside of China and that do not have an establishment or place of business in China, or that have an establishment or place of business in China but the relevant income is not effectively connected with the establishment or place of business. Any gain realized on the transfer of shares by such investors is subject to 10% PRC income tax if this gain is regarded as income derived from sources within China. Under the PRC Individual Income Tax Law and its implementation rules, dividends from sources within China paid to foreign individual investors who are not PRC residents are generally subject to a PRC withholding tax at a rate of 20% and gains from PRC sources realized by these investors on the transfer of shares are generally subject to 20% PRC income tax. Any such PRC tax liability may be reduced by the provisions of an applicable tax treaty.
38
Table of Contents
Although substantially all of our business operations are in China, it is unclear whether the dividends we pay with respect to our shares or ADSs, or the gains realized from the transfer of our shares or ADSs, would be treated as income derived from sources within China and as a result be subject to PRC income tax if we are considered a PRC resident enterprise. If PRC income tax is imposed on gains realized through the transfer of our ADSs or on dividends paid to our non-resident investors, the value of your investment in our ADSs may be adversely affected. Furthermore, our shareholders whose jurisdictions of residence have tax treaties or arrangements with China may not qualify for benefits under these tax treaties or arrangements.
In addition, pursuant to the Double Tax Avoidance Arrangement between Hong Kong and China, or the Double Tax Avoidance Treaty, and the Notice on Certain Issues with Respect to the Enforcement of Dividend Provisions in Tax Treaties, or the Notice on Tax Treaties, issued on February 20, 2009 by the SAT, if a Hong Kong resident enterprise owns more than 25% of the equity interest of a PRC company at all times during the twelve-month period immediately prior to obtaining a dividend from such company, the 10% withholding tax on such dividend is reduced to 5%, provided that certain other conditions and requirements under the Double Tax Avoidance Treaty and other applicable PRC laws are satisfied at the discretion of the relevant PRC tax authority. However, based on the Notice on Tax Treaties, if the relevant PRC tax authorities determine, in their discretion, that a company benefits from such reduced income tax rate due to a structure or arrangement that is primarily tax-driven, the PRC tax authorities may adjust the preferential tax treatment. Based on the Notice on Issues concerning Beneficial Owner in Tax Treaties, or Circular 9, issued on February 3, 2018 by the SAT and effective on April 1, 2018, when determining the applicant’s status as a “beneficial owner” for purpose of tax treatments in connection with dividends, interests or royalties in the tax treaties, several factors will be taken into account, and it will be analyzed according to the actual circumstances of the specific cases. If our Hong Kong subsidiary is determined by PRC government authorities as receiving benefits from reduced income tax rates due to a structure or arrangement that is primarily tax-driven, the dividends paid by our PRC subsidiaries to our Hong Kong subsidiary will be taxed at a higher rate, which will have an adverse effect on our financial and operational conditions.
We may be subject to penalties, including restrictions on our ability to inject capital into our PRC subsidiaries and on our PRC subsidiaries’ ability to distribute profits to us, if our PRC resident shareholders or beneficial owners fail to comply with relevant PRC foreign exchange regulations.
SAFE has promulgated several regulations that require PRC residents and PRC corporate entities to register with and obtain approval from local branches of SAFE in connection with their direct or indirect offshore investment activities. The Circular on Relevant Issues Relating to Domestic Resident’s Investment and Financing and Roundtrip Investment through Special Purpose Vehicles, or SAFE Circular 37, was promulgated by SAFE in July 2014. SAFE Circular 37 requires PRC residents or entities to register with SAFE or its local branch in connection with their establishment, or control of an offshore entity established, for the purpose of overseas investment or financing. According to the Circular of Further Simplifying and Improving the Policies of Foreign Exchange Administration Applicable to Direct Investment released in February 2015 by SAFE, local banks will examine and handle foreign exchange registration for overseas direct investment, including the initial foreign exchange registration and amendment registration, under SAFE Circular 37 from June 2015. These regulations apply to our shareholders who are PRC residents and may also apply to any offshore acquisitions or investments that we make in the future.
Under these foreign exchange regulations, PRC residents who make, or have previously made, prior to the implementation of these foreign exchange regulations, direct or indirect investments in offshore companies are required to register those investments. In addition, any PRC resident who is a direct or indirect shareholder of an offshore company is required to update its previously filed SAFE registration, to reflect any material change involving its round-trip investment. If any PRC shareholder fails to make the required registration or update the previously filed registration, the PRC subsidiary of that offshore parent company may be restricted from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to their offshore parent company, and the offshore parent company may also be restricted from injecting additional capital into its PRC subsidiary. Moreover, failure to comply with the various foreign exchange registration requirements described above could result in liability under PRC laws for evasion of applicable foreign exchange restrictions, including (i) the requirement by SAFE to return the foreign exchange remitted overseas or into the PRC within a period of time specified by SAFE, with a fine of up to 30% of the total amount of foreign exchange remitted overseas or into PRC and deemed to have been evasive or illegal and (ii) in circumstances involving serious violations, a fine of no less than 30% of and up to the total amount of remitted foreign exchange deemed evasive or illegal.
We are committed to complying with and to ensuring that our shareholders who are subject to these regulations will comply with the relevant SAFE rules and regulations. However, due to the inherent uncertainty in the implementation of the regulatory requirements by the PRC authorities, such registration might not be always practically available in all circumstances as prescribed in those regulations. In addition, we may not always be able to compel them to comply with SAFE Circular 37 or other related regulations. We cannot assure you that SAFE or its local branches will not release explicit requirements or interpret the relevant PRC laws and regulations otherwise. In addition, we may not be fully informed of the identities of all our shareholders or beneficial owners who are PRC residents, and we cannot provide any assurance that all of our shareholders and beneficial owners who are PRC residents will comply with our request to make, obtain or update any applicable registrations or comply with other requirements under SAFE Circular 37 or other related rules in a timely manner.
39
Table of Contents
Because there is uncertainty concerning the reconciliation of these foreign exchange regulations with other approval requirements, it is unclear how these regulations, and any future regulation concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant governmental authorities. We cannot predict how these regulations will affect our business operations or future strategy. For example, we may be subject to a more stringent review and approval process with respect to our foreign exchange activities, such as remittance of dividends and foreign- currency-denominated borrowings, which may adversely affect our results of operations and financial condition. This may restrict our ability to implement our acquisition strategy and could adversely affect our business and prospects.
Any failure to comply with PRC regulations regarding our employee share incentive plans or share option plans may subject plan participants, who are PRC residents, or us to fines and other legal or administrative sanctions.
In February 2012, SAFE promulgated the Notices on Issues Concerning the Foreign Exchange Administration for Domestic Individuals Participating in Stock Incentive Plans of Overseas Publicly-Listed Companies, or SAFE Circular 7. SAFE Circular 7 and other relevant rules and regulations require PRC residents who participate in a stock incentive plan in an overseas publicly tradeable company to register with SAFE or its local branches or the banks and complete certain other procedures. Participants in a stock incentive plan who are PRC residents must retain a qualified PRC agent to conduct the SAFE registration and other procedures with respect to the stock incentive plan on behalf of its participants. Such participants must also retain an overseas entrusted institution to handle matters in connection with their exercise of stock options, the purchase and sale of corresponding stocks or interests and fund transfers. In addition, the PRC agent must amend the SAFE registration with respect to the plan within three months if there is any material change to the stock incentive plan, the PRC agent, or the overseas entrusted institution, or if there are any other material changes in the plan. In addition, SAFE Circular 37 and other relevant rules and regulations stipulate that PRC residents who participate in a share incentive plan of an overseas non-publicly tradeable special purpose company must register with SAFE or its local branches or the banks before they exercise the share options. We and our PRC employees who have been granted share options and restricted shares are subject to these regulations. As of the date of this annual report, we are in the process of applying for such registration under SAFE Circular 7. Failure of our PRC share option holders or restricted shareholders to complete their SAFE registrations may subject them to fines and legal sanctions, and may also limit our ability to contribute additional capital into our PRC subsidiary, limit our PRC subsidiary’s ability to distribute dividends to us, or otherwise adversely affect our business.
The SAT has also issued rules and regulations concerning employee share incentives. Under these rules and regulations, our employees working in the PRC will be subject to PRC individual income tax upon exercise of the share options and/or grant of the restricted shares. Our PRC subsidiaries have obligations to file documents with respect to the granted share options and/or restricted shares with relevant tax authorities and to withhold individual income taxes for their employees upon exercise of the share options and/or grant of the restricted shares. If our employees fail to pay or we fail to withhold their individual income taxes according to relevant rules and regulations, we may face sanctions imposed by the competent governmental authorities.
Our leased property interests may be defective and our right to lease the properties affected by defects may be challenged, which could cause disruption to our business.
As of the date of this annual report, we leased properties for our offices and branch offices in China. Under PRC laws, all lease agreements must be registered with the local housing authorities. As of the date of this annual report, none of the premises we lease have completed the registration of our leases with the local housing authorities. Failure to complete these registrations may expose us to potential monetary fines up to RMB10,000 for each unregistered lease agreement.
We may be subject to penalties under relevant PRC laws and regulations due to failure to be in full compliance with social insurance and housing provident fund regulation.
According to the Social Insurance Law of the PRC promulgated in 2010 and most recently amended in 2018 and the Regulations on Management of Housing Provident Funds promulgated in 1999 and most recently amended in 2019, within a prescribed time limit, we need to register with the relevant social security authority and housing provident fund management center, and to open the relevant accounts and make full contributions for social insurance and housing funds for our employees, and this obligation cannot be delegated to any third party.
40
Table of Contents
Our contributions for some of our employees to the social insurance and housing funds may not have been in compliance with relevant PRC laws and regulations. Some of our subsidiaries or consolidated affiliated entities engaged third-party human resources agencies to pay social insurance and housing funds for some of their employees. As of the date of this annual report, none of these subsidiaries or consolidated affiliated entities had received any notice from the local authorities or any claim or request from these employees in this regard. Under the agreements entered into between the third- party human resources agencies and our relevant subsidiaries or consolidated affiliated entities, the third-party human resources agencies have the obligations to pay social insurance premium and housing provident funds for our relevant employees. However, if the human resource agencies fail to pay the social insurance or housing fund contributions for and on behalf of our employees as required under applicable PRC laws and regulations, we may be subject to penalties imposed by the local social insurance authorities and the local housing fund management centers for failing to discharge our obligations in relation to payment of social insurance and housing funds as an employer.
On July 20, 2018, the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council of the PRC issued the Reform Plan of the State Tax and Local Tax Collection Administration System, or the Tax Reform Plan. Under the Tax Reform Plan, commencing from January 1, 2019, tax authorities are responsible for the collection of social insurance contributions in the PRC. The effect of the Tax Reform Plan is still uncertain. We cannot assure that we will not be required to pay any deemed shortfalls or be subject to penalties or fines regarding social security insurance and housing provident funds contributions, any of which may have an adverse effect on our business and results of operations.
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us, our directors or our management named in this annual report based on foreign laws, and the ability of U.S. authorities to bring actions in China may also be limited.
We are an exempted company limited by shares incorporated under the laws of the Cayman Islands, and we conduct substantially all of our operations in China and substantially all of our assets are located in China. In addition, most of our directors and senior executive officers are nationals or residents in China. As a result, it may be difficult for you to effect service of process upon us or those persons in China. It may also be difficult for you to enforce in the U.S. courts or courts of many other jurisdictions judgments obtained in the U.S. courts or courts of many other jurisdictions based on the civil liability provisions of the U.S. federal securities laws or the comparable laws of many other jurisdictions against us and our directors and officers who reside and whose assets are located in China. There is also uncertainty as to whether the courts of the Cayman Islands or the PRC would recognize or enforce judgments of the U.S. courts or courts of many other jurisdictions against us or such persons predicated upon the civil liability provisions of the securities laws of the U.S. or any state or comparable laws of many other jurisdictions.
The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law and other applicable laws, regulations and interpretations based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions. China does not have any treaties or other forms of reciprocity with the U.S. and many other jurisdictions that provide for the reciprocal recognition and enforcement of judgments from the U.S. and many other jurisdictions. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the U.S and many other jurisdictions. In addition, the SEC, the U.S. Department of Justice and other U.S. authorities and the comparable authorities from many other jurisdictions may also have difficulties in bringing and enforcing actions against us or our directors or officers in the PRC.
Furthermore, shareholder claims that are common in the U.S., including securities law class actions and fraud claims, generally are difficult to pursue as a matter of law or practicality in China. For example, in China, there are significant legal and other obstacles to obtaining information needed for shareholder investigations or litigation outside China or otherwise with respect to foreign entities. Although the local authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such regulatory cooperation with the securities regulatory authorities in the U.S. have not been efficient in the absence of mutual and practical cooperation mechanism. According to Article 177 of the PRC Securities Law which became effective in March 2020, no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within the territory of the PRC. Accordingly, without the consent of the competent PRC securities regulators and relevant authorities, no organization or individual may provide the documents and materials relating to securities business activities to overseas parties. See also “—Risks Relating to The ADSs—You may face difficulties in protecting your interests, and your ability to protect your rights through U.S. courts and English courts may be limited, because we are incorporated under Cayman Islands law” for risks associated with investing in us as a Cayman Islands company.
41
Table of Contents
Recent litigation and negative publicity surrounding China-based companies listed in the U.S. may result in increased regulatory scrutiny of us and negatively impact the trading price of the ADSs and could have an adverse effect upon our business, including our results of operations, financial condition, cash flows and prospects.
We believe that litigation and negative publicity surrounding companies with operations in China that are listed in the U.S. have negatively impacted stock prices for these companies. Various equity-based research organizations have published reports on China-based companies after examining their corporate governance practices, related party transactions, sales practices and financial statements, and these reports have led to special investigations and listing suspensions on U.S. national exchanges. Any similar scrutiny of us, regardless of its lack of merit, could result in a diversion of management resources and energy, potential costs to defend ourselves against rumors, decreases and volatility in the ADS trading price, and increased directors and officers insurance premiums and could have an adverse effect upon our business, including our results of operations, financial condition, cash flows and prospects.
The PCAOB had historically been unable to inspect our auditor in relation to their audit work performed for our financial statements and the inability of the PCAOB to conduct inspections of our auditor in the past has deprived our investors with the benefits of such inspections.
Our auditor, the independent registered public accounting firm that issues the audit report included elsewhere in this annual report, as an auditor of companies that are traded publicly in the United States and a firm registered with the PCAOB, is subject to laws in the U.S. pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards. The auditor is located in mainland China, a jurisdiction where the PCAOB was historically unable to conduct inspections and investigations completely before 2022. As a result, we and investors in the ADSs were deprived of the benefits of such PCAOB inspections. The inability of the PCAOB to conduct inspections of auditors in China in the past has made it more difficult to evaluate the effectiveness of our independent registered public accounting firm’s audit procedures or quality control procedures as compared to auditors outside of China that are subject to the PCAOB inspections. On December 15, 2022, the PCAOB issued a report that vacated its previous determination issued on December 16, 2021 and removed mainland China and Hong Kong from the list of jurisdictions where it is unable to inspect or investigate completely registered public accounting firms. However, if the PCAOB determines in the future that it no longer has full access to inspect and investigate completely accounting firms in mainland China and Hong Kong, and we use an accounting firm headquartered in one of these jurisdictions to issue an audit report on our financial statements filed with the SEC, we and investors in our ADSs would be deprived of the benefits of such PCAOB inspections again, which could cause investors and potential investors in the ADSs to lose confidence in our audit procedures and reported financial information and the quality of our financial statements.
Our ADSs may be prohibited from trading in the United States under the HFCA Act in the future if the PCAOB is unable to inspect or investigate completely our current auditor. The delisting of the ADSs, or the threat of their being delisted, may materially and adversely affect the value of your investment.
The United States adopted Holding Foreign Companies Accountable Act on December 18, 2020, and it was amended by the Consolidated Appropriations Act, 2023 on December 17, 2022, (the amended act, the “HFCA Act”). The HFCA Act states if the SEC determines that we have filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for two consecutive years, the SEC shall prohibit our securities, including our ADSs, from being traded on a U.S. national securities exchange, including the Nasdaq, or in the over-the-counter trading market in the U.S. On December 16, 2021, the PCAOB issued a report to notify the SEC of its determination that the PCAOB was unable to inspect or investigate completely registered public accounting firms headquartered in mainland China and Hong Kong, including our auditor. In May 2022, the SEC conclusively listed us as a Commission-Identified Issuer under the HFCA Act following the filing of our annual report on Form 20-F for the fiscal year ended December 31, 2021. On December 15, 2022, the PCAOB issued a report that vacated its previous determination issued on December 16, 2021 and removed mainland China and Hong Kong from the list of jurisdictions where it is unable to inspect or investigate completely registered public accounting firms. For this reason, we do not expect to be identified as a Commission Identified Issuer under the HFCA Act after we file this annual report on Form 20-F.
42
Table of Contents
Pursuant to amendments made to the HFCA Act, the PCAOB may determine that it is unable to inspect or investigate completely registered public accounting firms in any foreign jurisdictions because of positions taken by any foreign authority, rather than an authority in the location in which the firms are headquartered or in which they have a branch or office, as was the case under the original version of the Act. If the PCAOB determines in the future that it no longer has full access to inspect and investigate completely accounting firms in mainland China and Hong Kong and we use an accounting firm headquartered in one of these jurisdictions to issue an audit report on our financial statements filed with the SEC, we would be identified as a Commission Identified Issuer following the filing of the annual report on Form 20-F for the relevant fiscal year. In accordance with the HFCA Act, our securities would be prohibited from being traded on a national securities exchange or in the over-the-counter trading market in the United States if we are identified as a Commission Identified Issuer for two consecutive years in the future. Although our ADSs have been listed on the Main Market of the London Stock Exchange and are fully fungible with our ADSs listed on the Nasdaq, we cannot assure your that an active trading market for our ADS on the London Stock Exchange will be sustained or that the ADSs can be converted and traded with sufficient market recognition and liquidity, if our ADSs are prohibited from trading in the United States. A prohibition of being able to trade in the United States would substantially impair your ability to sell or purchase our ADSs when you wish to do so, and the risk and uncertainty associated with delisting would have a negative impact on the price of our ADSs. Also, such a prohibition would significantly affect our ability to raise capital on terms acceptable to us, or at all, which would have a material adverse impact on our business, financial condition, and prospects.
Proceedings instituted by the SEC against the Big Four PRC-based accounting firms, including our independent registered public accounting firm, could result in financial statements being determined to not be in compliance with the requirements of the Exchange Act.
In December 2012, the SEC instituted administrative proceedings against the Big Four PRC-based accounting firms in China, including our independent PCAOB-registered public accounting firm, alleging that these firms had violated U.S. securities laws and the SEC’s rules and regulations thereunder by failing to provide to the SEC the firms’ audit work papers with respect to certain other PRC-based companies that are publicly traded in the United States.
On January 22, 2014, the initial administrative law judge presiding over the matter rendered an initial decision that each of the firms had violated the SEC’s rules of practice by failing to produce audit papers and other documents to the SEC. The initial decision censured each of the firms and barred them from practicing before the SEC for a period of six months.
On February 6, 2015, each of the four PRC-based accounting firms agreed to a censure and to pay a fine to the SEC to settle the dispute and avoid suspension of their ability to practice before the SEC and to audit US-listed companies. The settlement required the firms to follow detailed procedures and to seek to provide the SEC with access to Chinese firms’ audit documents via the CSRC. Under the terms of the settlement, the underlying proceeding against the four PRC-based accounting firms was deemed dismissed with prejudice four years after entry of the settlement. The four-year mark occurred on February 6, 2019. While we cannot predict if the SEC will further challenge the four PRC-based accounting firms’ compliance with U.S. law in connection with U.S. regulatory requests for audit work papers or if the results of such a challenge would result in the SEC imposing penalties such as suspensions, if the accounting firms are subject to additional remedial measures, our ability to file our financial statements in compliance with SEC requirements could be affected. A determination that we have not timely filed financial statements in compliance with SEC requirements could ultimately lead to our delisting from the Nasdaq, deregistration from the SEC, or both, which would substantially reduce or effectively terminate the trading of our ADSs in the United States.
In the event that the SEC restarts the administrative proceedings described above, depending upon the final outcome, listed companies in the United States with major China-based operations may find it difficult or impossible to retain auditors in respect of their operations in China, which could result in financial statements being determined not to be in compliance with the requirements of the Exchange Act, including possible delisting. Moreover, any negative news about any such future proceedings against these audit firms may cause investor uncertainty regarding China-based, U.S.- listed companies and the market price of our ADSs may be adversely affected.
If our independent registered public accounting firm was denied, even temporarily, the ability to practice before the SEC and we were unable to timely find another registered public accounting firm to audit and issue an opinion on our financial statements, our financial statements could be determined to be not in compliance with the requirements of the Exchange Act. Such a determination could ultimately lead to the delisting of the ADSs from the Nasdaq or deregistration from the SEC, or both, which would substantially reduce or effectively terminate the trading of the ADSs in the United States.
43
Table of Contents
Risks Relating to Hong Kong
You may have difficulty enforcing judgments in Hong Kong.
BR Hong Kong Limited, one of our subsidiaries incorporate in Hong Kong, wholly owns Beijing Burning Rock Biotech Limited, our WFOE. You may have difficulties in enforcing court judgments obtained in United States courts against our Hong Kong subsidiary, including judgments relating to the federal securities laws of the United States. There is also doubt as to whether courts in Hong Kong will enforce judgments of United States courts based only upon the civil liability provisions of the federal securities laws of the United States, or the securities laws of any state of the United States.
There may be political risks associated with having business connection with Hong Kong.
Hong Kong is a Special Administrative Region of the People’s Republic of China, with its own executive, judicial and legislative branches. Hong Kong enjoys a high degree of autonomy from China under the principle of “one country, two systems.” As a result of this political structure, we enjoy certain benefits, including tax benefits when we hold our WFOE through BR Hong Kong Limited, one of our subsidiaries incorporate in Hong Kong. However, we can give no assurance that Hong Kong will continue to enjoy the same level of autonomy from China. For example, if our Hong Kong subsidiary is deemed as a PRC company when the PRC government no longer treats Hong Kong as “offshore”, we may not be able to enjoy the double tax treaty reached between Hong Kong and mainland China and our Hong Kong company may be subject to the supervision of SAFE, which will lead uncertainty to cross-border capital flows. In particular, China could determine to treat any cash located in Hong Kong as subject to the same distribution rules as mainland China and our cash located in Hong Kong could therefore be subject to the same risks as that located in mainland China. Any intervention by the government of China in the affairs of Hong Kong, in breach of the “one country, two systems” principle, may adversely affect our business and ability to raise capital.
Risks Relating to The ADSs
The trading price of ADSs has been and may continue to be volatile, which could result in substantial losses to investors.
Since our ADSs became listed on Nasdaq on June 12, 2020, the trading price of our ADSs has ranged from US$0.572 to US$39.75 per ADS. Likewise, since our ADSs were admitted to trading on the LSE on November 1, 2022, the trading price of our ADS has ranged from US$0.572 to US$3.80 per ADS. The trading price of our ADSs may continue to be volatile and could fluctuate widely due to factors beyond our control. This may happen because of broad market and industry factors, like the performance and fluctuation of the market prices of other companies with business operations located mainly in China that have listed their securities in the U.S. and/or U.K. A number of Chinese companies have listed or are in the process of listing their securities on U.S. and/or U.K. stock markets. The securities of some of these companies have experienced significant volatility, including price declines in connection with their initial public offerings. The trading performances of these Chinese companies’ securities after their offerings may affect the attitudes of investors toward Chinese companies listed in the U.S. and/or U.K. in general and consequently may impact the trading performance of the ADSs, regardless of our actual operating performance.
In addition to market and industry factors, the price and trading volume for the ADSs may be highly volatile for factors specific to our own operations, including the following:
| • | variations in our revenues, earnings and cash flow; |
| • | announcements of new investments, acquisitions, strategic partnerships or joint ventures by us or our competitors; |
| • | announcements of new services and expansions by us or our competitors; |
| • | failure on our part to realize monetization opportunities as expected; |
| • | changes in financial estimates by securities analysts; |
| • | detrimental adverse publicity about us, our services or our industry; |
| • | additions or departures of key personnel; |
| • | release of lock-up or other transfer restrictions on our outstanding equity securities or sales of additional equity securities; |
| • | regulatory developments affecting us or our industry; and |
| • | potential litigation or regulatory investigations. |
Any of these factors may result in large and sudden changes in the volume and price at which our ADSs will trade in the U.S. or U.K.
44
Table of Contents
Shareholders of public companies have often brought securities class action suits against those companies following periods of instability in the market price of their securities. If we were involved in such a class action suit, it could divert a significant amount of our management’s attention and other resources from our business and operations and require us to incur significant expenses to defend the suit, which could harm our results of operations. Any such class action suit, whether or not successful, could harm our reputation and restrict our ability to raise capital in the future. In addition, if a claim is successfully made against us, we may be required to pay significant damages, which could have an adverse effect on our financial condition and results of operations.
If we fail to meet the applicable listing requirements, Nasdaq or the London Stock Exchange may delist our ADSs from trading on its exchange in which case the liquidity and market price of our ADSs could decline and our ability to raise additional capital would be adversely affected.
Our ADSs are currently listed for trading on the Nasdaq Global Market and the Main Market of the London Stock Exchange. In certain circumstances, our ADSs may be delisted from either of these exchanges. Delisting could have a material and adverse effect on the liquidity of our ADSs and on investors’ ability to sell our ADSs at a satisfactory price.
Nasdaq Global Market
There are a number of requirements that must be met in order for our ADSs to remain listed on the Nasdaq Global Market, including but not limited to the minimum bid price of at least US$1.00 per ADS, and the failure to meet any of these listing standards could result in the delisting of our ADSs from Nasdaq. For examples, we have received written notification from the NASDAQ dated December 29, 2023, indicating that for the last 30 consecutive business days, the closing bid price for our ADSs was below the minimum bid price of US$1.00 per share requirement set forth in NASDAQ Listing Rule. However, we have a compliance period of 180 calendar days, or until June 26, 2024, to regain compliance with NASDAQ’s minimum bid price requirement. We are in the process of changing the ADS-to-Class A ordinary share ratio to regain compliance with the minimum bid price requirement, and we plan to change the ratio of our ADSs to Class A ordinary shares from one ADS representing one Class A ordinary share to one ADS representing ten Class A ordinary shares (the “ADS Ratio Change”). The ADS Ratio Change is expected to become effective on or about May 15, 2024. However, we cannot assure you that we can successfully regain compliance as planned. We also cannot assure you that we will be able to comply with all Nasdaq Listing Rules at all times in the future or regain compliance in a timely manner in case of a default and avoid any subsequent adverse action taken by Nasdaq, including delisting. Any potential delisting of our ADSs from the Nasdaq would make it impossible for our shareholders to sell our ADSs in the public market and will result in decreased liquidity, limited availability of market quotations for our ADSs, limited availability of news and analyst coverage on us and decrease in our ability to issue additional securities. In particular, if the PCAOB cannot inspect our auditors as required by the HFCA Act, the SEC may prohibit the trading of our ADSs. For details, see “Item 3. Key Information—Risk Factors—Risks Relating to Doing Business in the PRC—Our ADSs may be prohibited from trading in the United States under the HFCA Act in the future if the PCAOB is unable to inspect or investigate completely our current auditor. The delisting of the ADSs, or the threat of their being delisted, may materially and adversely affect the value of your investment.”
London Stock Exchange
The United Kingdom Financial Conduct Authority (“FCA”) may cancel the listing of our ADSs on the standard listing segment of the Official List of the FCA if satisfied that there are special circumstances precluding normal and regular dealings in our ADSs. The listing of our ADSs on the Official List may also be cancelled at our request, provided we give at least 20 business days’ notice of the proposed cancellation of the listing. Because the ADSs are listed on the standard listing segment of the Official List, we would not be required to seek shareholder approval before seeking the cancellation of the listing of the ADSs on the Official List.
If securities or industry analysts do not publish research or reports about our business, or if they adversely change their recommendations regarding the ADSs, the market price for the ADSs and trading volume could decline.
The trading market for the ADSs in the U.S. and U.K. will be influenced by research or reports that industry or securities analysts publish about our business. If one or more analysts who cover us downgrade the ADSs, the market price for the ADSs would likely decline. If one or more of these analysts cease to cover us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the market price or trading volume for the ADSs in the U.S. and U.K. to decline.
45
Table of Contents
The sale or availability for sale of substantial amounts of ADSs could adversely affect their market price.
Sales of substantial amounts of ADSs in the public market, or the perception that these sales could occur, could adversely affect the market price of ADSs in the U.S. or U.K. and could impair our ability to raise capital through equity offerings in the future. As of March 31, 2024, we had 102,587,260 ordinary shares issued and outstanding, comprising (i) 85,262,412 Class A ordinary shares (excluding (i) 708,828 Class A ordinary shares issued to our depositary bank for bulk issuance of ADSs reserved for future issuances upon the exercise or vesting of awards granted under our share incentive plans, and (ii) 3,023,138 Class A Ordinary Shares as treasury stock), and (ii) 17,324,848 Class B ordinary shares. Among these shares, 59,779,446 Class A ordinary shares are in the form ADSs (including the 3,023,138 Class A Ordinary Shares treated as treasury stock), which are freely transferable without restriction or additional registration under Securities Act. The remaining outstanding ordinary shares may also be sold in public market, subject to volume and other restrictions as applicable under Rules 144 and 701 under the Securities Act and the applicable lock-up agreements, if any. To the extent shares are released before the expiration of the applicable lock-up period and sold into the market, the market price of the ADSs in the U.S. or U.K. could decline.
If a large number of our ordinary shares or securities convertible into our ordinary shares are sold in the public market after they become eligible for sale, the sales could adversely affect the trading price of the ADSs in the U.S. or U.K. and impede our ability to raise future capital. In addition, any ordinary shares that we issue under our share incentive plan or pursuant to any award agreements would dilute the percentage ownership held by ADS holders.
Because we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of the ADSs for return on your investment.
We currently intend to retain most, if not all, of our available funds and any future earnings to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in the ADSs as a source for any future dividend income.
Our board of directors has complete discretion as to whether to distribute dividends, subject to our memorandum and articles of association and certain requirements of Cayman Islands law. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiary, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return on your investment in the ADSs will likely depend entirely upon any future price appreciation of the ADSs. You may not realize a return on your investment in our ADSs and you may even lose your entire investment in the ADSs.
Our directors, officers and principal shareholders have substantial influence over our company and their interests may not be aligned with the interests of our other shareholders.
As of March 31, 2024, our directors and officers collectively own an aggregate of 61.6% of the total voting power of our outstanding ordinary shares. As a result, they have substantial influence over our business, including significant corporate actions such as change of directors, mergers, change of control transactions and other significant corporate actions.
Our directors, offices, and principal shareholders may take actions that are not in the best interest of us or our other shareholders. The concentration of ownership may discourage, delay or prevent a change in control of our company, which could deprive our shareholders of an opportunity to receive a premium for their shares as part of a sale of our company and may reduce the price of the ADSs in the U.S. or U.K. These actions may be taken even if they are opposed by shareholders, including ADS holders. In addition, the significant concentration of share ownership may adversely affect the trading price of the ADSs in the U.S. or U.K. due to investors’ perception that conflicts of interest may exist or arise.
We believe we were a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for our prior taxable year and there is significant risk that we will be a PFIC for our current taxable year and in future taxable years, which could result in significant adverse U.S. federal income tax consequences to U.S. Holders of ADSs or Class A ordinary shares.
A non-U.S. corporation will be a passive foreign investment company, or PFIC, for any taxable year if either (i) at least 75% of its gross income for such year consists of certain types of “passive” income; or (ii) at least 50% of the value of its assets (generally based on a quarterly average) during such year is attributable to assets that produce passive income or are held for the production of passive income.
Based on our financial statements, the manner in which we conduct our business, the trading price of our Class A ordinary shares and ADSs, the value and nature of our assets, and the sources and nature of our income we believe we were a PFIC for our prior taxable year. Additionally, there is a significant risk that we will be a PFIC for our current taxable year and in future taxable years. The determination of whether we are a PFIC must be made annually based on the facts and circumstances at that time.
46
Table of Contents
If we are a PFIC for any taxable year during which a U.S. Holder (as defined in “Item 10. Additional Information—E. Taxation—United States Federal Income Tax Considerations”) holds the ADSs or Class A ordinary shares, such U.S. Holder may incur significantly increased U.S. federal income tax on (i) gain recognized on the sale or other disposition of the ADSs or Class A ordinary shares and (ii) distributions on the ADSs or Class A ordinary shares and such U.S. Holder may be subject to burdensome reporting requirements.
Further, if we are a PFIC for any year during which a U.S. Holder holds ADSs or Class A ordinary shares, we generally will continue to be treated as a PFIC for all subsequent years during which such U.S. Holder holds our ADSs or Class A ordinary shares, even if we no longer satisfy the tests described above, unless the U.S. Holder makes a special “purging” election on U.S. Internal Revenue Service (“IRS”) Form 8621. See “Item 10. Additional Information—E. Taxation—United States Federal Income Tax Considerations—Passive Foreign Investment Company Rules” for more details. U.S. Holders are urged to consult their own tax advisors regarding the U.S. federal income tax consequences of holding stock or ADSs in a PFIC.
Our memorandum and articles of association contain anti-takeover provisions that could have an adverse effect on the rights of holders of our ordinary shares and the ADSs.
Our memorandum and articles of association contain provisions to limit the ability of others to acquire control of our company or cause us to engage in change-of-control transactions. These provisions could have the effect of depriving our shareholders of an opportunity to sell their shares at a premium over prevailing market prices by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transaction. Our board of directors has the authority, without further action by our shareholders, to issue preferred shares in one or more series and to fix their designations, powers, preferences, privileges, and relative participating, optional or special rights and the qualifications, limitations or restrictions, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights associated with our ordinary shares. Preferred shares could be issued quickly with terms calculated to delay or prevent a change in control of our company or make removal of management more difficult. If our board of directors decides to issue preferred shares, the price of the ADSs in the U.S. or U.K. may fall and the voting and other rights of the holders of our ordinary shares and the ADSs may be adversely affected.
You may face difficulties in protecting your interests, and your ability to protect your rights through U.S. courts and English courts may be limited, because we are incorporated under Cayman Islands law.
We are an exempted company limited by shares incorporated under the laws of the Cayman Islands. Our corporate affairs are governed by our memorandum and articles of association, as amended, the Companies Act (As Revised) of the Cayman Islands and the common law of the Cayman Islands. The rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from the common law of England, the decisions of whose courts are of persuasive authority, but are not binding, on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedent in the U.K or in some jurisdictions in the U.S. In particular, the Cayman Islands has a less developed body of securities laws than the U.S. or the U.K. The U.K and some U.S. states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate law than the Cayman Islands. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action in a federal court of the U.S. or the courts of England and Wales.
Shareholders of Cayman Islands exempted companies like us have no general rights under Cayman Islands law to inspect corporate records or to obtain copies of lists of shareholders of these companies (other than copies of our memorandum and articles of association and register of mortgages and charges, and any special resolutions passed by our shareholders). Under Cayman Islands law, the names of our current directors can be obtained from a search conducted at the Registrar of Companies. Our directors have discretion under our memorandum and articles of association to determine whether or not, and under what conditions, our corporate records may be inspected by our shareholders, but are not obliged to make them available to our shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
47
Table of Contents
As a company incorporated in the Cayman Islands, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq corporate governance requirements and the UK Corporate Governance Code published by the Financial Reporting Council in July 2018; these practices may afford less protection to shareholders than they otherwise would under rules and regulations applicable to U.S. or U.K. domestic issuers.
As a result of all of the above, our public shareholders may have more difficulties in protecting their interests in the face of actions taken by management, members of the board of directors or controlling shareholders than they would as public shareholders of a company incorporated in the U.S or U.K.
We are a foreign private issuer within the meaning of the rules under the Exchange Act, and as such we are exempt from certain provisions applicable to U.S. domestic public companies.
Because we are a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the securities rules and regulations in the U.S. that are applicable to U.S. domestic issuers, including:
| • | the rules under the Exchange Act requiring the filing of quarterly reports on Form 10-Q or current reports on Form 8-K with the SEC; |
| • | the sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations in respect of a security registered under the Exchange Act; |
| • | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and |
| • | the selective disclosure rules by issuers of material nonpublic information under Regulation FD. |
We are required to file an annual report on Form 20-F within four months of the end of each fiscal year. In addition, we publish our results on a quarterly basis through press releases, distributed pursuant to the rules and regulations of the Nasdaq Global Market. Press releases relating to financial results and material events will also be furnished to the SEC on Form 6-K. However, the information we are required to file with or furnish to the SEC will be less extensive and less timely than that required to be filed with the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections or information that would be made available to you were you investing in a U.S. domestic issuer, and it may be more difficult for overseas regulators to conduct investigation or collect evidence within China.
48
Table of Contents
The voting rights of holders of ADSs are limited by the terms of the deposit agreement, and you may not be able to exercise your right to direct the voting of the underlying Class A ordinary shares which are represented by your ADSs.
As a holder of ADSs, you will not have any direct right to attend general meetings of our shareholders or to cast any votes at such meetings. You will only be able to exercise the voting rights which attach to the underlying Class A ordinary shares which are represented by your ADSs indirectly by giving voting instructions to the depositary in accordance with the provisions of the deposit agreement. Under the deposit agreement, you may vote only by giving voting instructions to the depositary, as the holder of the underlying Class A ordinary shares which are represented by your ADSs. Upon receipt of your voting instructions, if voting is by poll, the depositary will try, as far as is practicable, to vote the Class A ordinary shares underlying your ADSs in accordance with your instructions, if such instructions are timely received by the depositary. If voting is by show of hands, the depositary will vote all ordinary shares held on deposit at that time in accordance with the voting instructions received from a majority of holders of ADSs who provide timely instructions. You will not be able to directly exercise any right to vote with respect to the underlying Class A ordinary shares unless you cancel your ADSs, withdraw the shares and become the registered holder of such shares prior to the record date for the general meeting. Under our memorandum and articles of association, the minimum notice period required to be given by our company to our registered shareholders for convening a general meeting is seven (7) calendar days. When a general meeting is convened, you may not receive sufficient advance notice to enable you to cancel our ADSs and withdraw the underlying Class A ordinary shares which are represented by your ADSs and become the registered holder of such shares prior to the record date for the general meeting to allow you to attend the general meeting or to vote directly with respect to any specific matter or resolution which is to be considered and voted upon at the general meeting. In addition, under our memorandum and articles of association for the purposes of determining those shareholders who are entitled to attend and vote at any general meeting, our directors may close our register of members and/or fix in advance a record date for such meeting, and such closure of our register of members or the setting of such a record date may prevent you from withdrawing the underlying Class A ordinary shares which are represented by your ADSs and becoming the registered holder of such shares prior to the record date, so that you would not be able to attend the general meeting or to vote directly. Where any matter is to be put to a vote at a general meeting, the depositary will, if we request, and subject to the terms of the deposit agreement, endeavor to notify you of the upcoming vote and to deliver our voting materials to you. We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote the underlying Class A ordinary shares which are represented by your ADSs. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for their manner of carrying out your voting instructions. This means that you may not be able to exercise your right to direct the voting of the underlying Class A ordinary shares which are represented by your ADSs, and you may have no legal remedy if the underlying Class A ordinary shares are not voted as you requested.
You may not receive dividends or other distributions on our shares and you may not receive any value for them, if it is illegal or impractical to make them available to you.
The depositary has agreed to pay you any cash dividends or other distributions it or the custodian receives on shares or other deposited securities underlying your ADSs, after deducting its fees and expenses. You will receive these distributions in proportion to the number of shares your ADSs represent. However, the depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make a distribution to a holder of ADSs if it consists of securities that require registration under the Securities Act but that are not properly registered or distributed under an applicable exemption from registration. The depositary may also determine that it is not practicable to distribute certain property through the mail. Additionally, the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may determine not to distribute such property. We have no obligation to register under U.S. securities laws any ADSs, ordinary shares, rights or other securities received through such distributions. We also have no obligation to take any other action to permit the distribution of ADSs, ordinary shares, rights or anything else to holders of ADSs. This means that you may not receive distributions we make on our ordinary shares or any value for them if it is illegal or impractical for us to make them available to you. These restrictions may cause a material decline in the value of the ADSs in the U.S. or U.K.
49
Table of Contents
Your right to participate in any future rights offerings may be limited, which may cause dilution to your holdings.
Shareholders do not have pre-emptive rights and we may, without shareholder consent, issue additional ordinary shares, ADSs, warrants, rights, units and debt securities for general corporate purposes, including, but not limited to, working capital, capital expenditures, investments, acquisitions and repayment or refinancing of borrowings. We also issue ordinary shares to our executive officers, employees and independent directors as part of their compensation. This may have the effect of diluting the interests of your holding. Additionally, to the extent that pre-emptive rights are granted, shareholders in certain jurisdictions may experience difficulties or may be unable to exercise their pre-emptive rights. For example, we cannot make such rights available to holders in the U.S. unless we register both the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. Under the deposit agreement, the depositary will not make rights available unless both the rights and the underlying securities to be distributed to ADS holders are either registered under the Securities Act or exempt from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective and we may not be able to establish a necessary exemption from registration under the Securities Act. Accordingly, you may be unable to participate in our rights offerings in the future and may experience dilution in your holdings.
The different characteristics of the capital markets in the U.K and the U.S. may negatively affect the trading prices of our ordinary shares and ADSs.
We are subject to the London Stock Exchange and Nasdaq listing and regulatory requirements concurrently. The London Stock Exchange and the Nasdaq Global Market have different trading hours, trading characteristics (including trading volume and liquidity), trading and listing rules, and investor bases (including different levels of retail and institutional participation). As a result of these differences, the trading prices of our ADSs may not be the same, even allowing for currency differences. Fluctuations in the price of our ADSs due to circumstances peculiar to the U.S. capital markets could materially and adversely affect the price of the ADSs trade on the London Stock Exchange, or vice versa. Certain events having significant negative impact specifically on the U.S. capital markets may result in a decline in the trading price of our ADSs traded on the London Stock Exchange notwithstanding that such event may not impact the trading prices of securities listed in London generally or to the same extent, or vice versa. Because of the different characteristics of the U.S. and U.K. capital markets, the historical market prices of our ADSs in the Nasdaq Global Market may not be indicative of the trading performance of the ADSs in the London Stock Exchange, and vice versa.
Having our ADSs trade on more than one market may result in increased volatility and price variations between such markets.
Our ADSs are currently traded on the Nasdaq Global Market and the Main Market of the London Stock Exchange. The ADSs listed on the London Stock Exchange are fully fungible with the ADSs listed on the Nasdaq Global Market and vice versa. Custodial and depositary links have been established between Euroclear, Clearstream and DTC to facilitate the cross-market transfers of the ADSs associated with secondary market trading. Subject to compliance with U.S. securities law, U.K. securities law and the terms of the deposit agreement, holder of ADSs may settle their trades through Euroclear, Clearstream or DTC.
Trading in the ADSs on these markets could be made at different times due to different time zones, trading days and public holidays in the U.K. and the U.S. The trading prices of the ADSs on these two markets may differ due to this and other factors. Trading of a small number of ADSs on one market could adversely impact the price of the ADSs on that market significantly and could, in turn, impact the price on the other market. Any decrease in the trading price of the ADSs on one of these markets could cause a decrease in the trading price of the ADSs on the other market. Additionally, as there is no direct trading or settlement between the two stock markets, the time required to move the ADSs from one market to another may vary, and there is no certainty of when ADSs that are moved will be available for trading or settlement.
The time required for cross-market transfers and settlement may be longer than expected and investors may not be able to settle or effect any sale of their securities during this period.
The time differences between London and New York, differences as to public holiday, and unforeseen market circumstances or other factors may delay the cross-market transfers and settlement of ADSs. Additionally, as there is no direct trading or settlement between the two stock markets, the time required to move the ADSs from one market to another may vary. Investors will be prevented from settling or effecting the sale of their securities during such periods of delay. In addition, there is no assurance that any such cross-market transfers and settlement will be completed in accordance with the timelines investors may anticipate.
50
Table of Contents
You may be subject to limitations on transfer of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
Our dual-class share structure with different voting rights will limit your ability to influence corporate matters and could discourage others from pursuing any change of control transactions that holders of our Class A ordinary shares and the ADSs may view as beneficial.
We have a dual-class share structure such that our ordinary shares consist of Class A ordinary shares and Class B ordinary shares. In respect of matters requiring the votes of shareholders, holders of Class A ordinary shares are entitled to one vote per share, while holders of Class B ordinary shares are entitled to six votes per share based on our dual-class share structure. Each Class B ordinary share is convertible into one Class A ordinary share at any time by the holder thereof, while Class A ordinary shares are not convertible into Class B ordinary shares under any circumstances. Upon any transfer of Class B ordinary shares by a holder thereof to any person or entity which is not an affiliate of such holder and under certain other circumstances, such Class B ordinary shares shall be automatically and immediately converted into the equal number of Class A ordinary shares. If any of such Class B ordinary shares are converted into Class A ordinary shares or cancelled for any reasons, our board of directors will have the authority without further action by our shareholders to issue additional Class B ordinary shares, which will be dilutive to our Class A ordinary shareholders and ADS holders.
As of March 31, 2024, our founder, chairman of the board of directors and chief executive officer, Mr. Yusheng Han, beneficially owns all of our issued Class B ordinary shares. The Class B ordinary shares constitute 16.9% of our total issued and outstanding share capital and 54.0% of the aggregate voting power of our issued and outstanding share capital due to the disparate voting powers associated with our dual-class share structure. See “Item 6. Directors, Senior Management and Employees—E. Share Ownership.” As a result of the dual-class share structure and the concentration of ownership, our founder and chief executive officer, Mr. Yusheng Han, has considerable influence over matters such as decisions regarding change of directors, mergers, change of control transactions and other significant corporate actions. He may take actions that are not in the best interest of us or our other shareholders. This concentration of ownership may discourage, delay or prevent a change in control of our company, which could have the effect of depriving our other shareholders of the opportunity to receive a premium for their shares as part of a sale of our company and may reduce the price of the ADSs in the U.S. or the U.K. This concentrated control will limit your ability to influence corporate matters and could discourage others from pursuing any potential merger, takeover or other change of control transactions that holders of Class A ordinary shares and ADSs may view as beneficial.
The dual-class structure of our ordinary shares may adversely affect the trading market for and the trading price of the ADSs in the U.S. or the U.K.
Certain shareholder advisory firms have announced changes to their eligibility criteria for inclusion of shares of public companies on certain indices, including the S&P 500, to exclude companies with multiple classes of shares and companies whose public shareholders hold no more than 5% of total voting power from being added to such indices. In addition, several shareholder advisory firms have announced their opposition to the use of multiple class structures. As a result, the dual class structure of our ordinary shares may prevent the inclusion of the ADSs representing Class A ordinary shares in such indices and may cause shareholder advisory firms to publish negative commentary about our corporate governance practices or otherwise seek to cause us to change our capital structure. Any such exclusion from indices could result in a less active trading market for the ADSs in either the U.S. or the U.K. Any actions or publications by shareholder advisory firms critical of our corporate governance practices or capital structure could also adversely affect the value of the ADSs.
ADSs holders may not be entitled to a jury trial with respect to claims arising under the deposit agreement, which could result in less favorable outcomes to the plaintiffs in any such action.
The deposit agreement governing the ADSs representing our shares provides that, to the fullest extent permitted by law, ADS holders waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our shares, the ADSs or the deposit agreement, including any claim under the U.S. federal securities laws.
If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable based on the facts and circumstances of that case in accordance with the applicable state and federal law. To our knowledge, the enforceability of a contractual pre-dispute jury trial waiver in connection with claims arising under the federal securities laws has not been finally adjudicated by the United States Supreme Court. However, we believe that a contractual pre-dispute jury trial waiver provision is generally enforceable, including under the laws of the State of New York, which govern the deposit agreement, by a federal or state court in the City of New York, which has nonexclusive jurisdiction over matters arising under the deposit agreement. In determining whether to enforce a contractual pre-dispute jury trial waiver provision, courts will generally consider whether a party knowingly, intelligently and voluntarily waived the right to a jury trial. We believe that this is the case with respect to the deposit agreement and the ADSs. It is advisable that you consult legal counsel regarding the jury waiver provision before entering into the deposit agreement.
51
Table of Contents
If you or any other holders or beneficial owners of ADSs bring a claim against us or the depositary in connection with matters arising under the deposit agreement or the ADSs, including claims under federal securities laws, you or such other holder or beneficial owner may not be entitled to a jury trial with respect to such claims, which may have the effect of limiting and discouraging lawsuits against us and the depositary. If a lawsuit is brought against either or both of us and the depositary under the deposit agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different outcomes than a trial by jury would have, including results that could be less favorable to the plaintiffs in any such action.
Nevertheless, if this jury trial waiver provision is not permitted by applicable law, an action could proceed under the terms of the deposit agreement with a jury trial. No condition, stipulation or provision of the deposit agreement or ADSs serves as a waiver by any holder or beneficial owner of ADSs or by us or the depositary of compliance with any substantive provision of the U.S. federal securities laws and the rules and regulations promulgated thereunder.
Certain judgments obtained against us by our shareholders may not be enforceable.
We are an exempted company limited by shares incorporated under the laws of the Cayman Islands. We conduct substantially all of our operations in China and substantially all of our assets are located in China. In addition, a majority of our directors and executive officers reside within China, and most of the assets of these persons are located within China. As a result, it may be difficult or impossible for you to effect service of process within the U.S. or the U.K. or elsewhere outside of China upon these individuals, or to bring an action against us or against these individuals in the U.S. or the U.K. or elsewhere outside of China in the event that you believe your rights have been infringed under the U.S. federal securities laws or any securities laws in the U.K. or otherwise. Even if you are successful in bringing an action of this kind, the laws of the Cayman Islands and of the PRC may render you unable to enforce a judgment against our assets or the assets of our directors and officers.
There is no statutory enforcement in the Cayman Islands of judgments obtained in the federal or state courts of the United States (and the Cayman Islands are not a party to any treaties for the reciprocal enforcement or recognition of such judgments), the courts of the Cayman Islands will, at common law, recognize and enforce a foreign monetary judgment of a foreign court of competent jurisdiction without any re-examination of the merits of the underlying dispute based on the principle that a judgment of a competent foreign court imposes upon the judgment debtor an obligation to pay the liquidated sum for which such judgment has been given, provided such judgment (a) is given by a foreign court of competent jurisdiction, (b) imposes on the judgment debtor a liability to pay a liquidated sum for which the judgment has been given, (c) is final, (d) is not in respect of taxes, a fine or a penalty, and € was not obtained in a manner and is not of a kind the enforcement of which is contrary to natural justice or the public policy of the Cayman Islands. However, the Cayman Islands courts are unlikely to enforce a judgment obtained from the U.S. courts under civil liability provisions of the U.S. federal securities law if such judgment is determined by the courts of the Cayman Islands to give rise to obligations to make payments that are penal or punitive in nature. A Cayman Islands court may stay enforcement proceedings if concurrent proceedings are being brought elsewhere.
The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions, as well as public policy considerations and conditions set forth in applicable provisions of other PRC laws relating to the enforcement of civil liability. China does not have treaties providing for the reciprocal recognition and enforcement of court judgments with many countries, including the U.S., U.K. and most other Western countries. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment against us or our director and officers if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the U.S., the U.K. or the Cayman Islands. These limitations may deprive investors of effective legal recourse for claims related to investment in the ADSs.
In terms of actions brought by securities regulatory authorities of the U.K. or other countries, there are also significant legal obstacles to obtaining requisite information. In particular, pursuant to Article 177 of the PRC Securities Law which became effective in March 2020, no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within China. Accordingly, without the consent of the competent PRC securities regulators and relevant authorities, no organization or individual may provide the documents and materials relating to securities business activities to overseas parties.
See also “—You may face difficulties in protecting your interests, and your ability to protect your rights through U.S. courts and English courts may be limited, because we are incorporated under Cayman Islands law” for risks associated with investing in us as a Cayman Islands company.
52
Table of Contents
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
We commenced our operation in January 2014 through Burning Rock (Beijing) Biotechnology Co., Ltd., a PRC company. In March 2014, we incorporated Burning Rock Biotech Limited in the Cayman Islands as our offshore holding company in order to facilitate foreign investment in our company. Subsequently, we established BR Hong Kong Limited as our intermediate holding company in April 2014, which in turn established a wholly-owned PRC subsidiary, Beijing Burning Rock Biotech Limited, our WFOE, in June 2014. In the same month, our WFOE entered into a series of contractual arrangements with Burning Rock (Beijing) Biotech Limited and its then shareholders, and Burning Rock (Beijing) Biotechnology Co., Ltd. became our variable interest entity, or VIE. These contractual arrangements were amended and restated in October 2019. See “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements.”
We conduct our NGS-based products and services business primarily through the wholly-owned subsidiaries of the VIE, Guangzhou Burning Rock Dx Co., Ltd. and Guangzhou Burning Rock Medical Equipment Co., Ltd., which were established in March 2014 and January 2015, respectively.
On June 12, 2020, our ADSs commenced trading on NASDAQ Global Market under the symbol “BNR.” We raised from our initial public offering US$234.9 million net proceeds, after the underwriters exercised in full their option to purchase additional ADSs. Concurrently with our initial public offering, we raised US$25 million from Lake Bleu Prime Healthcare Master, in a private placement.
On December 8, 2020, we completed a registered follow-on public offering by certain selling shareholders of 2,243,000 ADSs at a public offering price of US$25.75 per ADS. We did not receive any proceeds from the follow-on public offering.
On June 21, 2022, we announced a share repurchase plan, under which we may repurchase our Class A ordinary shares in the form of ADSs with an aggregate value of up to US$10 million during a 12-month period. As of the date of this annual report, the share repurchase program was completed in full and we repurchased a total of 3,023,138 Class A ordinary shares in the form of ADSs. The repurchased shares have been retained as treasury shares.
On November 1, 2022, our ADSs were admitted to the standard listing segment of the Official List of the Financial Conduct Authority and to trading on the Main Market of the London Stock Exchange plc under the ticker “BNR.”
On November 7, 2022, we filed a prospectus supplement to sell up to an aggregate of US$100,000,000 of our ADSs through an at-the-market equity offering program. The ADSs will be offered through or to Cowen and Company, LLC as the sales agent pursuant to a sales agreement dated November 7, 2022 between us and Cowen and Company, LLC.
Our principal executive offices are located at No. 5, Xingdao Ring Road North, International Bio Island, Guangzhou, the People’s Republic of China. Our telephone number at this address is +86 020-3403 7871. Our registered office in the Cayman Islands is located at the offices of Maples Corporate Services Limited at PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands.
Investors should submit any inquiries to the address and telephone number of our principal executive offices. Our main website is http://www.brbiotech.com. The information contained on our website is not a part of this annual report.
SEC maintains an internet site (http://www.sec.gov), which contains reports, proxy and information statements, and other information regarding us that file electronically with the SEC.
B. Business Overview
We aim to transform precision oncology and early cancer detection. We are China’s leading NGS-based cancer therapy selection company. Our cancer therapy selection platform is built upon our advanced proprietary technologies, comprehensive portfolio of products and a two-pronged market-driven commercial infrastructure addressing both larger hospitals through our in-hospital model and smaller hospitals through our central laboratory model.
Our advanced technology platform integrates cutting-edge proprietary cancer therapy selection technologies using both tissue and liquid biopsies, including assay biochemistry, bioinformatics and a patented laboratory information management system. Our proprietary HS library preparation technology allows us to work with poor quality and limited volume samples and enables enhanced sensitivity—capabilities that are critical to effectively deploying NGS-based cancer therapy selection, especially in China. Our in-depth cancer genomics insights, accumulated from the large number of tests we have performed since our inception, enable us to process and accurately analyze genomic information. We have achieved a turnaround time of approximately six days for regular oncology products, approximately 25 days for MRD products and approximately nine days for early detection products.
53
Table of Contents
We offer a broad spectrum of NGS-based products. Our NGS-based cancer therapy selection test products are used to assist physicians in selecting the most effective therapy for cancer patients. We primarily offer 15 NGS-based cancer therapy selection and prognosis prediction tests applicable to a broad range of cancer types, including lung cancer, gastrointestinal cancer, prostate cancer, breast cancer, lymphomas, thyroid cancer, colorectal cancer, ovarian cancer, pancreatic cancer, and bladder cancer, using both tissue and liquid biopsy samples. Our core products, including OncoCompass™ IO, OncoScreen™ IO and OncoCompass™ Target, perform on par with those of our global peers. We launched our MRD product, brPROPHET™, in March 2022, which has demonstrated superior sensitivity and specificity to fixed panel in pre-operative ctDNA detection and post-operative MRD calling among relapsed patients. In January 2023 and October 2023, our early detection product, OverC™ Multi-Cancer Detection Blood Test, or OverC™, was granted Breakthrough Device Designation by the FDA and the NMPA, respectively, making this product the only test globally that has received such designation from both the FDA and NMPA. This product is intended for early detection of multiple cancer types, including esophageal, liver, lung, ovarian, and pancreatic cancer, in adults of either sex, aged 50 to 75 years old, at average risk for cancers. We are the clear leader in the lung cancer segment of China’s NGS-based cancer therapy selection market. We believe we offer the best NGS-based products and services in China, and we have won the trust of pharmaceutical companies, physicians, hospitals and patients with our high quality standards, superior product performance and strong service support. Our products are recognized by the medical, pharmaceutical and scientific communities, as evidenced by (i) the use of our products by oncology key opinion leaders in clinical trials and research studies they initiate, and (ii) our collaborations on clinical trials and research studies with leading pharmaceutical companies including AstraZeneca (NYSE: AZN), Bayer (ETR: BAYN), Johnson & Johnson (NYSE: JNJ), CStone (HKEX: 2616), BeiGene (HKEX: 6160), Abbisko Therapeutics (HKEX: 2256), IMPACT Therapeutics, Boehringer Ingelheim and Merck KGaA (ETR: MRK), primarily by providing central laboratory services and companion diagnostics development services to these pharmaceutical companies. The results of these clinical trials and research studies have been published in over 200 peer-reviewed articles, and the results of research studies using our products have been published in numerous peer-reviewed articles.
We pioneered a two-pronged commercial infrastructure, consisting of both central and in-hospital laboratories, to maximize market penetration and create higher barriers to entry.
| • | Central laboratory model: Our central laboratory processes cancer patients’ tissue and liquid biopsy samples delivered to us from hospitals across China and issues test reports. This model has enabled us to become China’s largest provider of NGS-based cancer therapy selection tests while building relationships with over 6,700 physicians from 829 hospitals across China. Our central laboratory also supports our collaborations with pharmaceutical companies. As our central laboratory model has become a relatively mature business segment, we have shifted our strategic focus to the in-hospital model, which we believe has higher growth potential. Accordingly, while revenue from our central laboratory model has accounted for, and is expected to continue to account for, a substantial part of our total revenue, we expect revenue from our central laboratory model to be more stable in the future. |
| • | In-hospital model: Chinese hospitals generally prefer to conduct laboratory tests in-house. However, despite the large and growing demand for NGS-based cancer therapy selection tests, hospitals face multiple challenges in adopting these tests, which have technically sophisticated workflows. In 2016, we became China’s first NGS-based cancer therapy selection company to offer an in-hospital model, providing turn-key solutions to address Chinese hospitals’ challenges in adopting NGS-based cancer therapy selection. We help our partner hospitals establish their in-hospital laboratories, install laboratory equipment and systems, and provide ongoing training and support. With these laboratories, equipment and systems in place, we sell them our reagent kits on a recurring basis, which allow them to perform testing on their own in a standardized manner. As of December 31, 2023, we have partnered with 87 hospitals in 40 cities across China. We have invested and expect to continue investing substantially in our in-hospital model, as we expect it to become an increasingly important segment of China’s NGS-based cancer therapy selection market. Revenue from our in-hospital model has grown substantially since we entered into this model, and revenue from our in-hospital model constituted a substantial part of our revenue in 2023, reaffirming our strategic judgment about the development of China’s NGS-based cancer therapy selection market. |
In addition to our NGS-based cancer therapy selection tests, we are also investing in our development of early cancer detection tests. Early cancer detection can substantially increase the chances of successful treatment and therefore presents enormous market opportunities. However, it is extremely difficult to develop liquid biopsy-based early cancer detection tests with the sensitivity and specificity needed for the tests to be clinically useful. Our targeted DNA methylation-based library preparation technologies and bioinformatics effectively address these challenges by enhancing the signal-to-noise ratio on the most informative cancer-associated methylation loci and blocks, enabling us to detect extremely low circulating levels of cancer biomarkers to facilitate accurate early detection of multiple cancers. Our early cancer detection technologies have demonstrated an overall sensitivity of 80.6% across six cancer types (including lung cancer, colorectal cancer, liver cancer, ovarian cancer, pancreatic cancer and esophageal cancer) at various stages, with 98.3% specificity (meaning 98.3% of asymptomatic participants test negative for any cancer). We will continue our research and development efforts in early cancer detection, with the aim of developing pan-cancer early detection products.
Our revenue increased by 10.9% from RMB507.9 million in 2021 to RMB563.2 million in 2022 and decreased by 4.6% to RMB537.4 million (US$75.7 million) in 2023. Our gross profit increased by 4.4% from RMB364.1million in 2021 to RMB380.0 million in 2022 and decreased by 4.4% to RMB363.2 million (US$51.2 million) in 2023. Our gross profit margin was 71.7%, 67.5% and 67.6% in 2021, 2022, and 2023 respectively.
54
Table of Contents
Our Technologies
NGS-Based Cancer Therapy Selection Technologies
The adoption of NGS-based cancer therapy selection in China presents a number of challenges, including (i) library preparation and probe hybridization using the low-quality FFPE samples containing degraded or low quantities of DNA that are common in China, and (ii) Chinese hospitals typically prefer to perform tests in-house rather than outsourcing to third parties, but lack the required expertise, knowledge and skills to perform NGS-based cancer therapy selection tests. We have developed the proprietary assay biochemistry and bioinformatics described below that underlie our current product portfolio and effectively address those challenges.
HS Library Preparation Technology—Enhancing Capture Efficiency for Low-Quality FFPE Samples
The low quality FFPE samples available in China often fail to meet the minimum quality and quantity thresholds required for standard NGS-based cancer therapy selection. Our proprietary High Sensitivity, or HS, library preparation technology improves the capture efficiency of low-quality FFPE samples and enables us to maximize the capture of unique DNA molecules, which are used to make up the sequencing library. This technology improves by approximately 80% the library conversion and library complexity—a measure of the number of unique DNA molecules present in a DNA library—of DNA libraries derived from FFPE samples, enabling us to work with low-quality FFPE samples. When applied to liquid biopsy ctDNA samples, our HS library preparation technology shows similar improvements in library complexity, enabling us to work with liquid biopsy ctDNA samples as small as 10-nanograms. Building upon our HS library preparation technology, we have further developed our capture-based personalized MRD assay, brPROPHET™.
The diagram below illustrates the significant improvements in complexity and overall quality of DNA libraries derived from clinical FFPE samples of different quality levels (from the highest level “A+” to the lowest level “C”) using our HS library preparation technology, each as compared with conventional library preparation methods:
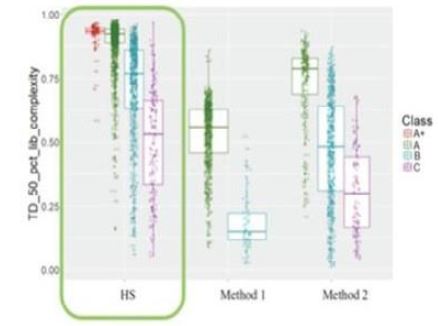
Comparison of FFPE DNA library complexity and quality at 500X raw depth
55
Table of Contents
The diagram below illustrates the improvements (denoted as “after”) in library complexity of liquid biopsy ctDNA samples achieved using our HS library preparation technology:
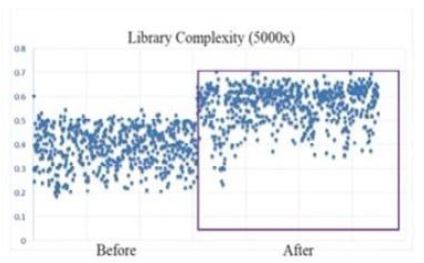
Liquid Biopsy Technologies—Enabling Super-High Sensitivity in ctDNA Samples Through Signal-Noise Ratio Enhancement
Compared to tissue biopsies, NGS-based ctDNA liquid biopsies require higher technological capabilities and expertise because of the low concentrations of ctDNA in liquid biopsy samples. In addition to our HS technology, we have also developed our UMI technology and corresponding bioinformatics, which improve the signal detection and noise control capabilities of our liquid biopsy-based tests and accurately distinguish true origin of DNA fragments from those that are duplicated, contaminated, erroneous or otherwise irrelevant. These technologies increase test sensitivity and lower our ctDNA detection limit by five to ten times to 0.1% or lower, which significantly enhances the accuracy of our liquid biopsy-based tests.
The diagram below illustrates the noise reduction achieved by applying our UMI technology in ctDNA sample library preparation:
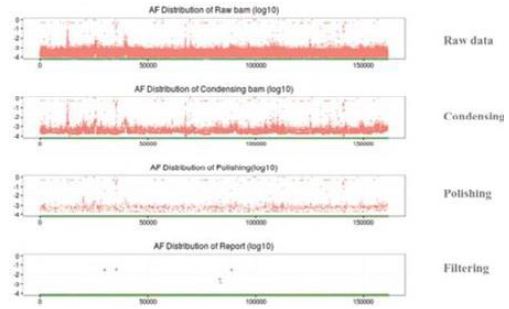
MSI Calling Algorithms—World-Class NGS-Based Algorithms Detecting MSI in Tissue and Liquid Biopsies
Polymerase chain reaction-, or PCR-, based methods have been the conventional method for detecting microsatellite instability, or MSI, an important biomarker for immune-oncology treatment selection. We have developed proprietary NGS-based MSI calling algorithms, prettyMSI and bMSISEA, which enable our tests to accurately detect the presence of MSI in tissue and ctDNA samples, respectively. By incorporating these algorithms, our tissue and liquid biopsy-based tests provide patients a one-stop, cost-effective solution for the detection of genomic alterations of targeted genes and MSI in a single test. According to CIC, our MSI calling algorithms have higher sensitivity than substantially all other published MSI algorithms.
56
Table of Contents
In 2018, our prettyMSI algorithm was clinically validated in an MSI detection study with the results published in a 2018 March Journal of Molecular Diagnostics article “A novel and reliable method to detect microsatellite instability in colorectal cancer by next-generation sequencing.” In 2020, our bMSISEA algorithm was clinically validated in an MSI detection study, the result of which will be published in an article titled “Detection of microsatellite instability from circulating tumor DNA by targeted deep sequencing”, that has been submitted to and accepted by the same journal. In 2019, one of our products using the prettyMSI algorithm was endorsed and recommended in Chinese Experts Consensus on MSI testing.
Since 2021, a series of publication utilizing therapy selection technology described above had been published in top-tier journals to demonstrate analytical validity, clinical validity, and clinical utilities. In 2021, the SEQC2 study initiated by MAQC/FDA was published in multiple journals. Among them, the ctDNA panel sequencing study was published in a Nature Biotechnology article titled “Evaluating the analytical validity of circulating tumor DNA sequencing assays for precision oncology.” Our ctDNA panel OncoCompassTM Target demonstrated the best sensitivity, specificity and reproducibility. The graphs below demonstrated the performance of our OncoCompassTM Target (indicated as “BRP) in comparison with other products.
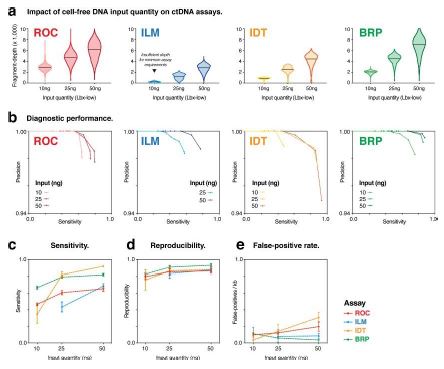
Regarding the clinical utilities, Our OncoScreenTM IO/OncoCompassTM IO panel was used in the China cohort Clinical Testing Assay (CTA) of Capivasertib Phase 3 trial (CAPItello-291) sponsored by AstroZeneca. The results had been published in an article titled “Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer” on The New England Journal of Medicine. The trial results ultimately led to the FDA approval of Capivasertib in the U.S. with CDx requirement of AKT1-pathway alteration. We are currently closely working with the NMPA and AstroZeneca on our collaboration to develop CDx product for Capivasertib in China.
Automated NGS Library Preparation System—Enabling Automation and Standardization of In-Hospital Laboratories
Hospitals in China generally lack the expertise necessary to conduct NGS-based cancer therapy selection. In addition, the conventional process flows that most Chinese hospitals use not only make the testing process time consuming, but also introduce contamination risk in the library preparation stage, which reduces testing accuracy. We have been a pioneer in helping Chinese hospitals address these challenges, and in September 2019, we launched Magnis BR, China’s first and only capture-based fully automated NGS library preparation system, and associated library preparation reagents, which we co-developed with Agilent. Magnis BR and its associated reagents are particularly suitable for Chinese hospitals because they fully automate the NGS library preparation process, converting DNA samples into sequencing-ready libraries in around nine hours. Magnis BR can process 112 samples per week. We presented the analytical validation data of Magnis BR at the Association for Molecular Pathology (AMP) 2020 annual meeting in a platform presentation. In 2022, our Magnis BR received clearance as a Class II medical device for IVD use in China.
57
Table of Contents
Molecular Residual Disease (MRD)
The disease monitoring and surveillance utility of ctDNA test had been drawing extensive academic and clinical interest in recent years. Using ctDNA test to identify ctDNA positivity after definitive therapy such as post-surgery can bring prognostic and potentially predictive value. In 2020, we started developing our MRD technology—brPROPHET. It utilizes tumor-informed approach to identify up to 50 loci through whole exome sequencing, or WES, of tumor and paired normal samples. A personalized hybridization capture probe set is created for each patient. Ultra-deep sequencing leveraging on HS-UMI technology described above is also used in the process to enable high sensitivity of tumor-informed ctDNA detection. The limit of detection of the brPROPHET can be as low as 40ppm. At the AACR Annual Meeting in 2023, we presented the results of the study in relation to MEthylation Based Dynamic Analysis for Lung Cancer) (MEDAL) (the “MEDAL study”), which demonstrated that brPROPHET™ detected more MRD events with ultra-low ctDNA abundance but meaningful prognostic significance and proved important insights for guiding the standardization of future surveillance applications by evaluating the dynamic change of longitudinal MRD. In September 2023, the MEDAL study was published in a Cancer Cell article titled “Individualized tumor-informed circulating tumor DNA analysis for postoperative monitoring of non-small cell lung cancer.”
The diagrams below illustrate the analytical validity and clinical validity of the MEDAL study:
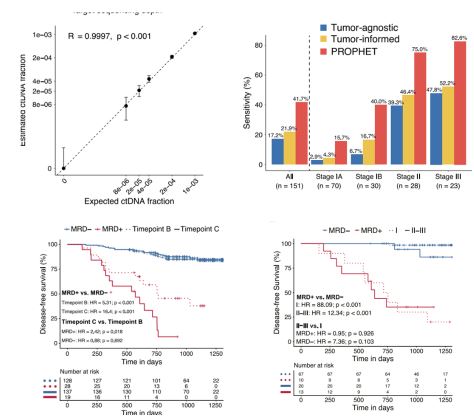
Early Cancer Detection Technologies
In 2016, we started our research and development on the use of targeted DNA methylation in early cancer detection. Early cancer detection can substantially increase the chances of successful treatment, and accordingly presents enormous market opportunities. However, it is extremely difficult to develop liquid biopsy-based early cancer detection tests with the sensitivity and specificity needed for the tests to be clinically useful. To effectively address the technical challenges of early cancer detection, we have developed targeted DNA methylation-based library preparation technologies and bioinformatics that sensitively detect extremely low circulating levels of cancer biomarkers by enhancing the signal-to-noise ratio on the most informative cancer-associated methylation loci and blocks, facilitating the accurate early detection of multiple cancers.
We have built on our technology platform to develop proprietary technologies for early cancer detection using analysis of change in DNA methylation, a promising biomarker associated with the initiation of certain cancers. BrELSA™ is our proprietary targeted DNA-methylation-based library preparation technology for early cancer detection. It significantly increases the conversion rate, and maximizes the preservation, of sequenceable DNA fragments; it also ensures that the methylation sites of pathogenic significance are captured. These capabilities allow us to prepare sequenceable libraries using liquid biopsy samples as small as 5 to 10 milligrams. We also use targeted DNA methylation reinforced malignancy non-invasive detection, or brMERMAID™, our proprietary bioinformatics and statistical algorithm for the early detection of multiple types of cancers. We train brMERMAID™ with real world clinical samples and its machine learning capability enables continuous performance improvements as it incorporates data from additional clinical samples. The combination of brELSA™ and brMERMAID™ enables highly sensitive, accurate and robust early cancer detection results that are on par with global leaders.
58
Table of Contents
At the American Association of Cancer Research (AACR) Annual Meeting 2019, we presented a poster that demonstrated the data of early detection of lung cancer using our methylation profiling method combining brELSA™ and brMERMAID™. In the Special Conference on Advances in Liquid Biopsies hosted by AACR in 2020, we presented our data regarding early detection of lung, colorectal and liver cancers with brELSA™ and brMERMAID™ in a poster titled “Multiplatform analysis of early-stage cancer signatures in blood.” At the AACR Virtual Annual Meeting II, we presented our new data regarding early detection of ovarian cancer in a poster titled “Methylation profiling of circulating tumor DNA for the detection of ovarian cancer.” At the European Society for Medical Oncology (ESMO) Asia Virtual Congress 2020, we presented our new data regarding early detection of lung, colorectal, liver, ovarian, pancreatic, and esophageal cancers in a presentation titled “Early detection and localization of multiple cancers using a blood-based methylation assay (ELSA-seq).” At the AACR Annual Meeting 2022, we presented a poster titled “Analytical performance of ELSA-seq, a blood-based test for early detection of multiple cancers,” in which we evaluated the analytical performance of a refined test version of ELSA-seq and demonstrated the high sensitivity of the detection data.
The diagram below illustrates our early cancer detection workflow incorporating brELSA™ and brMERMAID™:
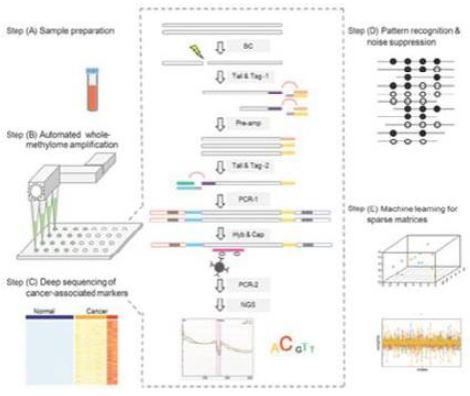
Step (A) Sample preparation: 8-10 ml of venous blood is collected and processed to isolate circulating cell-free DNA, or cfDNA, which is a cancer biomarker.
Step (B) Automated whole-methylome amplification: DNA Libraries are prepared using a method called whole methylome bisulfite sequencing, or WGBS, in an automated way. WGBS is a widely used method to profile the methylation landscape of the whole genome. The detailed sub-steps are shown in the center of the above diagram.
Step (C) Deep sequencing of cancer-associated markers: Probes are used to capture the specific genomic regions associated with common types of cancer, and the captured regions are then sequenced at high depth. The detailed sub-steps are shown in the center of the above diagram.
Step (D) Pattern recognition & noise suppression: After the methylation changes are detected, statistical algorithms are used to differentiate signals from noise in the sequencing data and the signals are then categorized into specific patterns.
Step (E) Machine learning for sparse matrices: An algorithm is built to differentiate tumor samples from normal samples. This algorithm combines numerous random and scarce methylation patterns to address challenges arising from low circulating levels of tumor DNA in early-stage cancer patients.
59
Table of Contents
The graphs below show that our brELSA™ technology enables higher recovery of circulating DNA in library preparation and sequencing as compared to two commercially available kits. The high recovery rate and deep sequencing of targeted methylated region facilitates the ultra-high detection sensitivity, with the limit of detection as low as 0.001%:
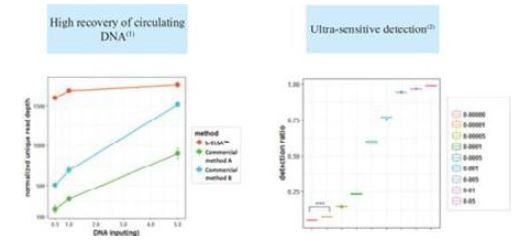
| (1) | The graph shows the unique read depth (Y-axis) observed with different quantities of DNA input (X-axis) of E.coli (DH5a)—a type of bacteria used in labs worldwide as a host for DNA sequences, using brELSA™ and two commercially available kits when sequenced to ~ 2,000X median depth. It shows that brELSA™’s unique read depth is consistently higher than the other two kits, which in turn enables higher recovery of circulating DNA in library preparation and sequencing. |
| (2) | The x-axis denotes cell lines with various known proportions of methylation sites, with the exact proportion numbers (from 0.00000, or 0.000% to 0.05, or 5%) as indicated in the box on the right; the y-axis denotes the percentage of methylation sites being recognized as positive using brELSA™. This graph demonstrates that even for the most signal-scarce sample—0.00001 (0.001%) tumor cell DNA shown as the yellow bar in the graph—the overall sample can still be recognized as positive, as indicated by the three asterisks in the graph. This result shows that brELSA™ has ultra-high detection sensitivity, with a limit of detection as low as 0.001%. |
We have upgraded our 3-cancer early cancer detection test that detects lung, intestinal and liver cancers to a 6-cancer test, which is our OverC™, that detects lung, colorectal, liver, ovarian, pancreatic and esophageal cancers. We plan to ultimately upgrade this test to a pan-cancer test, with improved accuracy in determining the origin of tissue compared to the 3-cancer test and OverC™. We have successfully signed contracts with a small number of pilot hospitals in China for the commercialization of our OverC™, and expect to focus on customer education and contracting more hospitals. We are developing and validating cancer early detection products for coverage of more cancer types. In 2023, the 6-cancer THUNDER study was published in an Annals of Oncology article titled “Unintrusive multi-cancer detection by circulating cell-free DNA methylation sequencing (THUNDER): development and independent validation studies.” In 2023, the OverC™ was also granted Breakthrough Device Designation by both FDA and NMPA. The table below sets forth the sensitivity of our 6-cancer test for the detection of stage I-IV lung, colorectal, liver, ovarian, pancreatic and esophageal cancers at 98.3% specificity.
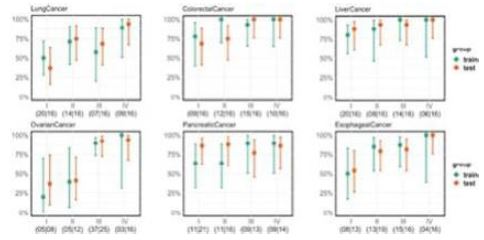
60
Table of Contents
We have started the development and analytical validation for our pan-cancer test, including to initiate two prospective, multi-center studies, the PREDICT study and the PRESCIENT study to further develop and validate our pan-cancer early detection test.
The PRESCIENT study, which was launched in May 2021, was the first blood-based, pan-cancer early-detection study in China using a multi- omics approach.
In June 2022, we launched PREVENT (a Prospective multi-canceR Early-detection and interVENTional) study, China’s first prospective interventional study to evaluate the performance of OverC™ in the asymptomatic population. The study is expected to enroll 12,500 asymptomatic individuals, to evaluate the performance of OverC™ in detecting six types of cancers, including lung, liver, colorectal, esophageal, pancreatic and ovarian cancers.
Our Products
We primarily offer various NGS-based tissue and liquid biopsy cancer therapy selection and prognosis prediction tests, catering to different clinical and affordability needs of the different cancer patient segments.
Key Therapy Selection Tests
The table below sets forth the 13 key therapy selection tests we currently offer:
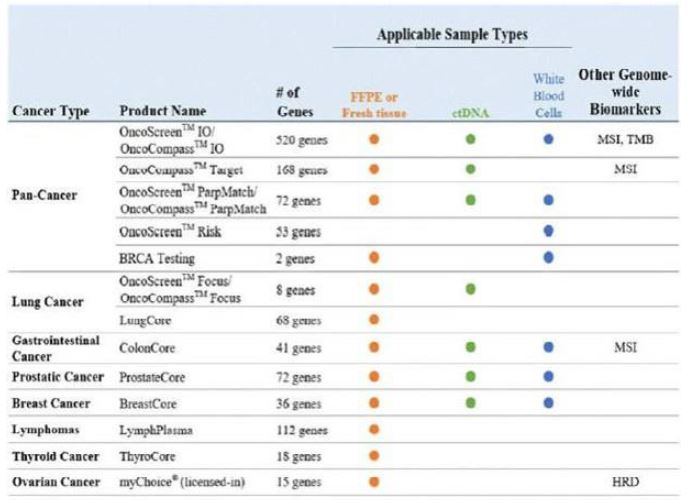
OncoCompassTM IO/OncoScreenTM IO
In 2015, we launched our pan-cancer test OncoScreen, which we upgraded to OncoScreen™ IO, our pan-cancer test for tissue samples, and OncoCompass™ IO, the corresponding test for liquid biopsy samples, in 2017. OncoScreen™ IO and OncoCompass™ IO reflect the latest developments in targeted therapy and immunotherapy. These tests profile 520 genes associated with most solid tumors, such as lung cancer, colorectal cancer, breast cancer, ovarian cancer, bladder cancer and prostate cancer, for which a targeted therapy has been approved by the FDA or NMPA or is in current clinical development. In addition to detecting the genomic alternations of the targeted genes, OncoScreen™ IO and OncoCompass™ IO also detect important immune-oncology biomarkers including TMB and MSI, as well as rare but clinically actionable biomarkers, such as NTRK fusions, which provide important insights for therapy selection.
61
Table of Contents
The table below sets forth the key specifications of OncoScreen™ IO and OncoCompass™ IO:
Product and Operational Specifications | OncoScreen™ IO/ OncoCompass™ IO | |||
Number of genes | 520 | |||
Immunotherapy biomarkers | TMB, MSI | |||
Limit of detection (on hot-spot mutations) | 1.7-2 | % | ||
Maximum turnaround time(1) | 10 days | |||
| (1) | For the year ended December 31, 2023. |
The design and performance of OncoScreen™ IO and OncoCompass™ IO has been endorsed by their adoption in over 20 clinical trials and studies. For example, OncoScreen™ IO was selected by CStone in its Phase III clinical trial of CS1001—one of CStone’s core product candidates that targets PD-L1—to detect TMB, which can potentially identify the patients who may benefit from treatment of CS1001. Janssen, a subsidiary of Johnson & Johnson, selected our OncoScreen™ IO and OncoCompass™ IO in a clinical study to conduct analysis of blood samples collected from patients with various kinds of advanced solid tumors. OncoScreen™ IO also participated in the FDA-initiated SEQC2 study for global tissue-based NGS assay comparison. OncoScreen, OncoScreen™ IO and OncoCompass™ IO were also used in research studies that resulted in publications in high-impact journals, including Clinical Cancer Research and Cancer Cell. Our OncoScreenTM IO was also featured in a phase 3 multi-regional clinical trial sponsored by AstraZeneca, one of our pharma clients This clinical trial assessed the efficacy and safety of Capivasertib–fulvestrant therapy in advanced breast cancer patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) cancer whose disease had progressed during or after aromatase inhibitor therapy. This clinical study further resulted in the 2023 June The New England Journal of Medicine article titled “Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer.”
OncoCompassTM Target
In 2015, we launched OncoCompass™ Target, our ctDNA liquid biopsy-based test for NSCLC, which we upgraded to a pan-cancer test for all solid tumors. This test analyzes 168 genes that are related to the development of NSCLC and solid tumors, including all genes that have a targeted therapy that is FDA- or NMPA-approved or NCCN-recommended. It provides information with optimal clinical value for cancer patients, especially advanced-stage cancer patients who do not have accessible tissue, for various solid tumors across treatment stages, from baseline profiling, dynamic monitoring to MRD detection. The table below sets forth the key specifications of OncoCompass™ Target:
Product and Operational Specifications | OncoCompass™ Targe | |||
Number of genes | 168 | |||
Immunotherapy biomarkers | MSI | |||
Limit of detection (defined at 80% sensitivity) | 0.2 | % | ||
Percentage of samples processed within 7 days(1) | 95 | % | ||
| (1) | For the year ended December 31, 2023. |
Our OncoCompass™ Target demonstrates consistently high sensitivity in liquid biopsies for biomarkers that are difficult to detect using conventional methods. For example, our OncoCompass™ Target can detect actionable mutations among treatment-naive stage IV NSCLC patients with sensitivity of 96% and specificity greater than 99%. In a separate study, OncoCompass™ Target detected ALK fusion with a sensitivity of 79%. From a real-world cohort of 1016 patients with paired tissue and plasma samples tested simultaneously, OncoCompass™ Target could detect at least one actionable mutation among 74% patients from tissues samples, 61% from plasma samples, or 76% from either.
The performance of OncoCompass™ Target has been validated in clinical trials and research studies led by international and domestic pharmaceutical companies and leading oncology key opinion leaders, including:
| • | A 2017 study that was published in the Journal of Thoracic Oncology titled “Capture-based targeted ultradeep sequencing in paired tissue and plasma samples demonstrates differential subclonal ctDNA-releasing capability in advanced lung cancer,” in which OncoCompass™ Target presented high concordance between the paired tissue and plasma samples, illustrating its high clinical feasibility and utility. In this study, the specificity of OncoCompass™ Target for all targeted genomic alterations was higher than 99%, and the sensitivity of OncoCompass™ Target was 87.2% for all targeted genomic alterations and 96.2% for the known actionable driver mutations among the 7 NCCN-recommended genes. |
62
Table of Contents
| • | Our OncoCompass™ Target was applied in the exploratory biomarker sub-study within the BENEFIT study, which was an innovatively designed prospective study where patients were tested for EGFR mutations based solely on liquid biopsy and recruited to test the efficacy of Gefitinib among EGFR-mutant patients. The BENEFIT study was published in the Lancet Respiratory Medicine titled “Detection of EGFR mutations in plasma circulating tumor DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicenter clinical trial”. In this study, concurrent mutations identified by OncoCompass™ Target were able to further stratify EGFR-mutant patients into groups with differential response to Gefetinib. |
| • | Our OncoCompass™ Target was selected by AstraZeneca as the only NGS-based product for its Tagrisso (Osimertinib) Phase III diagnostic methods comparison study. |
| • | Our OncoCompass™ Target was selected in our companion diagnostics (“CDx”) collaboration with Merck for the MET inhibitor tepotinib for the China market. |
OncoCompass™ Target has also been used in a number of high impact research studies, with results published in over 50 peer-reviewed articles in academic journals, including Journal of Thoracic Cancer, Annals of Oncology and Lancet Respiratory Medicine. For example, our OncoCompass™ Target was used in the following research studies: (1) a research study that resulted in the 2018 January Annals of Oncology article titled “Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy,” which we jointly published with Professor Yi-Long Wu; (2) a research study that resulted in the 2018 July Journal of Thoracic Onology article titled “First-in-human Phase I study of AC0010, a mutant-selective EGFR inhibitor in non-small cell lung cancer: safety, efficacy and potential mechanism of resistance,” which we jointly published with Professor Li Zhang; (3) a research study that resulted in the 2020 February Journal of Thoracic Cancer article titled “Detection of non-reciprocal/reciprocal ALK translocation as poor predictive marker in first-line crizotinib-treated ALK-rearranged non-small cell lung cancer patients,” which we jointly published with Professor Nong Yang; (4) a research study that resulted in the 2019 December Translational Lung Cancer Research article titled “Parallel serial assessment of somatic mutation and methylation profile from circulating tumor DNA predicts treatment response and impending disease progression in osimertinib-treated lung adenocarcinoma patients,” which we jointly published with Professor Yuan Chen; and (5) a research study that resulted in the 2020 April Translational Lung Cancer Research article titled “Circulating tumor DNA clearance predicts prognosis across treatment regimen in a large real-world longitudinally monitored advanced non-small cell lung cancer cohort”, which we jointly published with Professor Shun Lu.
These published studies provide further evidence of OncoCompass™ Target’s accurate and consistent test performance.
ColonCore
ColonCore, which we launched in 2016, is capable of simultaneously assessing 22 microsatellite loci related to MSI status and detecting mutations in 41 genes associated with gastrointestinal cancers. It has been validated in multiple studies in China on NGS-based detection of MSI from both tissue and plasma samples. According to a 2018 March Journal of Molecular Diagnostics article titled “A novel and reliable method to detect microsatellite instability in colorectal cancer by next-generation sequencing,” the specificity and sensitivity of ColonCore were 100% and 97.9%, respectively. Our ColonCore was also endorsed and recommended in Chinese Experts Consensus on MSI Testing.
Key Prognosis Prediction Tests
brPROPHET™
brPROPHET™, which we have launched in March 2022, is our self-developed MRD product based on personalized approach. It has demonstrated superior sensitivity and specificity to fixed panel in pre-operative ctDNA detection and post-operative MRD calling among relapsed patients. A recent study published in September 2023 in Cancer Cell highlighted the advancements enabled by brPROPHET™ in the treatment of early-stage non-small cell lung cancer (NSCLC). In MEDAL-PROPHET study, brPROPHETTM demonstrated superior performance in head-to-head comparisons with tumor-agnostic fixed-panel and tumor-informed fixed-panel MRD assays.
Other Products
We also offer a number of Magnis BR-customized version of our key products. In addition, in November 2020, we entered into a development and commercialization agreement with Myriad Genetics, Inc. (NASDAQ: MYGN, “Myriad”) to in-license Myriad myChoice® tumor testing in China. This test enables physicians to identify patients with tumors that have lost the ability to repair double-stranded DNA breaks, resulting in potentially increased susceptibility to DNA-damaging drugs such as platinum drugs or PARP inhibitors.
63
Table of Contents
In December 2021, we launched OncoMaster™, an automatic NGS data analysis and report interpretation machine, which can complement our in-hospital model through providing simple, efficient and accurate data analysis solutions.
Certifications and Regulatory Approvals
We are committed to developing and maintaining high quality standards for our laboratory and products. As part of this effort, we voluntarily sought and obtained certifications from the relevant U.S. certifying authorities. We have also obtained Conformite Europeenne (“CE”) marking for certain of our products, including OncoScreen™ IO, OncoCompass™ Target and OncoScreen™ Focus, enabling us to sell these products within the European Union and certain other jurisdictions recognizing CE marking. We have also obtained the NCCL certification for our central laboratory and the NMPA approval for two NGS-based reagent kits. We are the first company in China that has an NGS laboratory that has been certified by the CLIA and the NCCL and accredited by the CAP. We are also the first company in China with NMPA-approved NGS-based reagent kits. We have two NMPA- approved NGS-based reagent kits as of the date of this annual report. In particular, in March 2022, the NMPA approved our human nine-gene mutation joint detection kit (reversible termination sequencing) (LungCure™ CDx) for non-small cell lung cancer as a class III medical device. This approval demonstrates our industry-leading capability of working with the NMPA on bringing an innovative NGS-based diagnostic product to the China market. In addition, we have one early cancer detection product, OverC™, that has been granted Breakthrough Device Designation by both the FDA and the NMPA. We believe these certifications and regulatory approvals demonstrate the efficiency, accuracy and consistency of our testing services.
The U.S.
We aspire to become a world-class cancer diagnostics company, and we believe an integral step to achieving this goal is for our laboratory to comply with world-class certification requirements. Accordingly, we voluntarily applied for and obtained the following certifications and accreditations:
FDA application. In January 2023, our OverC™ was granted Breakthrough Device Designation by the FDA, which is the third of its kind globally. Under the FDA’s Breakthrough Devices Program, the Breakthrough Device Designation is granted to certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions such as cancer. This program is designed to provide patients and healthcare providers with timely access to medical devices granted the designation by speeding up their development, assessment, and review. This designation demonstrated our industry-leading R&D capabilities in developing cancer early detection products.
CLIA certification. The Clinical Laboratory Improvement Amendments, or the CLIA, mandate specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality control, quality assurance and inspections. These standards are intended to ensure that CLIA-certified laboratories’ testing services are accurate, reliable and timely. In the U.S., clinical laboratories must be CLIA-certified by the Centers for Medicare & Medicaid Services, or the CMS, before they can accept human samples for diagnostic testing. In January 2017, our central laboratory became the first NGS laboratory in China to be CLIA-certified—one and a half years ahead of our competitors. In October 2020, we successfully renewed our CLIA certification.
CAP accreditation. The CAP accredits laboratories performing testing on specimens from human beings or animals, using methodologies and clinical application within the expertise of the program. In the U.S., the CMS has deemed CAP standards to be equal to or more stringent than CLIA regulations. Our central laboratory was accredited by the CAP and we successfully renewed this accreditation in April 2022.
China
Cancer genotyping is a nascent and rapidly evolving industry. Given the nature of the industry, relevant regulatory authorities in China, similar to their counterparts in the U.S., are constantly drafting and refining the regulatory requirements to implement quality management systems in the industry. We are one of the pioneers in China’s cancer genotyping industry, and have worked with regulators to share our insights on the nature of the NGS technology while seeking comprehensive approvals, setting high industry standards. We have obtained the following certifications in China:
NCCL certification. The NCCL is the supervising authority of NGS laboratories in China. Our central laboratory in Guangzhou was the second and one of the only three NGS laboratories in China to have passed comprehensive review by the provincial centers for clinical laboratories led by the NCCL. In May 2018, we were certified by, and received NGS laboratory certification from, the Guangdong branch of the NCCL, and the certificate is valid for five years. Our central laboratory also renewed our clinical PCR testing laboratory certificate issued by Guangdong Branch of the NCCL in May 2021, and the certificate is valid for five years.
64
Table of Contents
NMPA approval. We are a pioneer in our industry in seeking and obtaining the NMPA approval. In September 2016, our OncoScreen™ Focus was the first innovative medical device in the oncology application field that was approved to enter the “Innovative Device Pathway,” a fast-track review for innovative medical device, similar to the FDA’s “Breakthrough Device Program.” In July 2018, our OncoScreen™ Focus was approved by the NMPA and became the NMPA’s first approved NGS-based reagent kit. In March 2022, the NMPA approved our human nine-gene mutation joint detection kit (reversible termination sequencing) (LungCure™ CDx) for non-small cell lung cancer as a class III medical device. This approval demonstrates our industry-leading capability of working with the NMPA on bringing an innovative NGS-based diagnostic product to the China market. We plan to seek approval for more reagent kits with the NMPA. In October 2023, our OverC™ also received the Breakthrough Device Designation by the NMPA in October 2023, making it the only test globally that has received Breakthrough Device Designation from both the FDA and the NMPA.
Other Jurisdictions
We have also obtained CE marking for four of our products, including OncoScreen™ Focus CDx Tissue Kit, OncoCompass™ Target Cancer Mutation Profiling Liquid Kit, OncoScreen™ Plus Cancer Mutation Profiling Tissue Kit and OverC™ Multi-Cancer Detection Blood Test. CE marking is mandatory for certain products intended for sale within the European Union and certain other jurisdictions recognizing CE marking. It demonstrates these products’ conformity with European health, safety, and environmental protection standards.
Academic Collaborations
We seek to raise the profile of our technologies and products in China’s medical community and encourage their adoption through two principal channels: collaborations with oncology key opinion leaders—where we either collaborate with them and co-author papers or through studies conducted by oncology key opinion leaders using our products, both of which are published in leading academic journals; and collaboration with pharmaceutical companies—where we collaborate with them on targeted therapies and immunotherapies under clinical investigation.
Physicians look to peer experts and key opinion leaders in the medical community for guidance in research, diagnosis and treatment. We believe our relationships with oncology key opinion leaders, as well as the resulting peer-to-peer interaction they have generated, have been instrumental in raising the awareness of our technology platform and driving adoption of our products.
We form academic collaborations with oncology key opinion leaders where our products are used in clinical trials and research studies on cancer targeted therapies and immunotherapies, the results of which have been published in over 200 peer-reviewed articles in the Journal of Clinical Oncology, Lancet Respiratory Medicine, Clinical Cancer Research, Journal of Thoracic Oncology, Annals of Oncology and other academic journals.
65
Table of Contents
The table below highlights some of our publication collaborations with influential oncology key opinion leaders based on these clinical trials and research studies:
Collaborating Key Opinion Leaders | Journal Title | Article Title | Our Products | |||
| Qiang Gao and Jia Fan, oncologists residing in Zhongshan Hospital, Fudan University | Annals of Oncology | Unintrusive multi-cancer detection by circulating cell-free DNA methylation sequencing (THUNDER): development and independent validation studies | Our OverC™ was validated in the THUNDER study, a large- scale prospective six-cancer case-control study | |||
| Juan Zhou and Minwei Bao, oncologists residing in the Shanghai Pulmonary Hospital & Thoracic Cancer Institute, Tongji University School of Medicine | BMC Medicine | Increased blood-based intratumor heterogeneity (bITH) is associated with unfavorable outcomes of immune checkpoint inhibitors plus chemotherapy in non-small cell lung cancer | Our products: Our OncoCompass™ IO was applied to discover a novel liquid-based predictive biomarker for immunotherapy | |||
| Jie Wang, head of department of medicine in the Cancer Hospital of Chinese Academy of Medical Sciences, vice president of CSCO | Lancet Respiratory Medicine | Detection of EGFR mutations in plasma circulating tumor DNA as a selection criterion for first-line Gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicenter clinical trial | Our OncoCompass™ Target was used for the NGS-based cancer therapy selection of plasma ctDNA in the study | |||
| Qing Zhou, deputy head of the Lung Research Institute of Guangdong Provincial People’s Hospital, secretary of CTONG | EBioMedicine | Analysis of resistance mechanisms to Abivertinib, a third-generation EGFR tyrosine kinase inhibitor, in patients with EGFR T790M- positive non-small cell lung cancer from a phase I trial | Our OncoScreen was selected in the biomarker study | |||
| Ying Yuan, deputy head of department of medicine of the Second Affiliated Hospital of Zhejiang University School of Medicine, member and secretary of the Committee of Colorectal Cancer of China Anti-Cancer Association | Journal of Molecular Diagnostics | A novel and reliable method to detect microsatellite instability in colorectal cancer by next- generation sequencing | Our ColonCore and the corresponding MSI calling algorithm were used in the validation study | |||
| Naixin Liang, oncologist residing in Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences | Nature Biomedical Engineering | Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning | Our ELSA-seq was used for study of circulating tumor DNA methylation markers for the early detection of lung cancer | |||
Collaborations with Pharmaceutical Companies
We collaborate with leading international and domestic pharmaceutical companies on clinical trials and research studies, primarily by providing central laboratory services and companion diagnostics development services. Our NGS-based cancer therapy selection tests are also regularly used by leading pharmaceutical companies, such as AstraZeneca, Bayer, Johnson & Johnson, CStone, Abbisko Therapeutics, Boehringer Ingelheim, and Merck, in their clinical studies and clinical trials or as CDx development for their respective drug candidates. These services enable pharmaceutical companies to identify molecularly defined patient populations enrolled in specific clinical trials or to better understand how targeted oncology therapy and immunotherapy drug candidates are working on patients, which in turn guides their drug development process. In order to form collaborations with pharmaceutical companies, we must go through their rigorous quality assurance audits and technical validations to demonstrate that the design, specification and performance of our tests as well as our testing workflow meet their quality and technical requirements.
66
Table of Contents
Distribution
We pioneered a two-pronged commercial infrastructure, consisting of both central and in-hospital laboratories, to maximize market penetration and create higher barriers to entry:
| • | Central laboratory model. Since 2014, we have offered our cancer therapy selection tests under a central laboratory model. Under this model, cancer patients’ tissue and liquid biopsy samples are delivered to our central laboratory in Guangzhou for processing, and we issue test reports generally within six days from our receipt of the tissue and liquid biopsy samples, 25 days for samples for our MRD products and nine days for samples for our early detection products. Our central laboratory also supports our collaborations with pharmaceutical companies; and |
| • | In-hospital model. In China, cancer patients typically go to top oncology hospitals for cancer treatment. These hospitals generally prefer to conduct laboratory tests in-house. Although the complexities of NGS-based cancer therapy selection have so far limited the number of hospitals to have their own laboratory facilities for these tests, we believe that the in-hospital segment presents enormous market opportunities and will become an increasingly important segment of China’s cancer genotyping market. Given this opportunity, in 2016, we began offering turn-key solutions under our in-hospital model, enabling our partner hospitals that use our reagent kits to perform testing on their own in a standardized manner with our ongoing training and support. |
Central Laboratory Model
We began offering NGS-based cancer therapy selection services under a central laboratory model in 2014, and we have become the market leader in the central laboratory segment of China’s NGS-based cancer therapy selection market. Under our central laboratory model, cancer patients’ treating physicians order our cancer therapy selection tests for their patients during the diagnostic process, have the patients’ liquid biopsy or tissue samples shipped to our central laboratory in Guangzhou for testing, and design treatment plans based on our test results. Our test reports communicate the actionable genomic alterations in a patient’s cancer and match those alterations with potentially relevant treatment options, including targeted therapies and immunotherapies, according to predicted efficacy or resistance. Patients pay us for these tests with out-of-pocket payments.
We have established a dedicated sales and marketing team that focuses on expanding our brand awareness and growing our coverage of hospitals and physicians across China. Our marketing efforts for our central laboratory model include educating hospitals and physicians on the benefits of our tests and the clinical data supporting our test results. We also work with medical professional societies to promote the awareness of the clinical benefits of our tests and NGS-based cancer therapy selection in general, and we sponsor or present at medical, scientific or industry exhibitions and conferences and pursue or support scientific studies of our tests and the publication of results in academic journals.
Since our inception, over 6,700 physicians from 829 hospitals across China have ordered our cancer therapy selection tests under our central laboratory model. The table below sets forth the key operating data for our central laboratory model for the periods presented:
| Year ended December 31, | ||||||||||||
| 2021 | 2022 | 2023 | ||||||||||
Number of patients tested(1) | 28,199 | 27,353 | 20,129 | |||||||||
Number of ordering physicians(2) | 1,105 | 949 | 745 | |||||||||
Number of ordering hospitals(3) | 339 | 272 | 228 | |||||||||
| (1) | A patient who took multiple tests in different quarters of a given period is counted only once. |
| (2) | Represents physicians who on average order at least one test from us every month during a relevant period under the central laboratory model. |
| (3) | Represents hospitals whose residing physicians who on average order at least one test from us every month during a relevant period under the central laboratory model. |
| Three months ended | ||||||||||||||||||||||||||||||||
| March 31, 2022 | June 30, 2022 | September 30, 2022 | December 31, 2022 | March 31, 2023 | June 30, 2023 | September 30, 2023 | December 31, 2023 | |||||||||||||||||||||||||
Number of patients tested | 7,743 | 8,060 | 7,989 | 6,419 | 6,139 | 6,585 | 5,502 | 5,156 | ||||||||||||||||||||||||
Number of ordering physicians(1) | 994 | 767 | 897 | 797 | 792 | 725 | 643 | 620 | ||||||||||||||||||||||||
Number of ordering hospitals(2) | 318 | 264 | 257 | 238 | 241 | 225 | 213 | 202 | ||||||||||||||||||||||||
| (1) | Represents physicians who on average order at least one test from us every month during a relevant period under the central laboratory model. |
| (2) | Represents hospitals whose residing physicians who on average order at least one test from us every month during a relevant period under the central laboratory model. |
67
Table of Contents
In-hospital Model
Despite the large and growing demand, Chinese hospitals face multiple challenges in adopting NGS-based cancer therapy selection testing in house, which has technically sophisticated workflows such as library preparation and complex data analysis and interpretation. As a result, these hospitals are in urgent need of high-performing and greatly standardized technologies and products that adhere to their rigorous quality requirements and operating protocols. Strategically focusing on the in-hospital segment of China’s cancer genotyping industry since our inception, in 2016 we became the first company in China to offer Chinese hospitals a turn-key solution and ongoing support that effectively addresses their challenges in adopting NGS-based cancer therapy selection.
The flow chart below sets forth the key steps of our in-hospital model:
| (1) | Typically include tests conducted by the hospitals to compare our tests against conventional cancer therapy selection methods, as well as against those offered by other NGS-based cancer therapy selection companies. |
To form collaborations with partner hospitals, we must complete each partner hospitals’ rigorous onboarding process, including (i) benchmarking tests conducted by the hospitals, including comparisons of our tests against conventional cancer therapy selection methods such as PCR and FISH, as well as against those offered by other NGS-based cancer therapy selection companies, and (ii) other comprehensive assessments to evaluate our technical and service capabilities. Throughout this process, our dedicated in-hospital model sales and technical support teams, working closely with our research and development, medical support and other teams, collaborate with our partner hospitals to redesign their in-hospital laboratories, complete tender processes, source laboratory equipment and supplies, install laboratory systems and customize the hospitals’ testing workflow, data analysis and report generation—all while ensuring compliance with the hospitals’ rigorous quality and operating protocols.
Once an in-hospital laboratory is in operation, the partner hospital purchases our products to perform NGS-based cancer therapy selection on a recurring basis. We are dedicated to continuously optimizing the operations of these in-hospital laboratories and maintaining our relationships with our partner hospitals. We frequently conduct onsite visits and provide remote technical support, such as data analytics support, to ensure optimal laboratory performance. In September 2019, we launched our fully automated NGS library preparation system, Magnis BR, and associated library preparation reagents, which we co-developed with Agilent. Magnis BR and its associated reagents are particularly suitable for Chinese hospitals because they fully automate the NGS library preparation process and convert DNA samples into sequencing-ready libraries in around nine hours, which help partner hospitals streamline their testing workflow, reduce manual labor and minimize risks.
Through our strategic focus—supported by our high-quality products and industry-leading technological capabilities—we have become the market leader in the in-hospital segment of China’s NGS-based cancer therapy selection market. Our in-hospital model represents a stable and growing revenue stream that consists of fees from initial facilitation of the hospitals’ laboratory equipment purchases followed by recurring sales of our products.
As of December 31, 2023, we have partnered with 87 hospitals in 40 cities across China, to establish in-hospital laboratories. The table below sets forth the cumulative numbers of our partner hospitals as of the dates indicated:
| As of December 31, | ||||||||||||
| 2021 | 2022 | 2023 | ||||||||||
Pipeline partner hospitals(1) | 23 | 28 | 28 | |||||||||
Contracted partner hospitals(2) | 41 | 49 | 59 | |||||||||
Total number of partner hospitals | 64 | 77 | 87 | |||||||||
| (1) | Refers to hospitals that have established in-hospital laboratories, completed laboratory equipment installation and commenced pilot testing using our products. It generally takes 12 to 30 months for hospitals to progress from pipeline partner hospitals to contracted partner hospitals, which generate recurring revenue from the sale of reagent kits. |
68
Table of Contents
Operations
We primarily perform cancer therapy selection using both tissue and liquid biopsy tests under the central laboratory model in our NCCL- and CLIA-certified, CAP-accredited central laboratory in Guangzhou. Our central laboratory currently has an annual capacity of over 100,000 tests, which is expected to increase to 200,000 tests by the end of 2024 through the adoption of automation systems and laboratory expansions. We have achieved a turnaround time of approximately six days for regular oncology products, approximately 25 days for MRD products and approximately nine days for early detection products. Our test reports contain comprehensive information about the detected actionable genomic alterations and recommend targeted therapies and immunotherapies for each genomic alteration, according to predicted efficacy and resistance.
We have applied good clinical practices, or GCP, to the operations of our central laboratory. Our GCP system consists of a quality control, or QC, system, a quality assurance, or QA, system and a corrective and preventive action, or CAPA, management system. We have incorporated these comprehensive quality control measures in all stages of our testing process to ensure the high-quality, consistency, and timeliness of our testing results. We have also participated in various proficiency tests and external quality assessments for the testing services we offer, including, among others, ctDNA testing, NGS solid tumor testing, and BRCA testing and interpretation. Our industry-leading technological capabilities and QC system have resulted in our operational excellence. For example, the testing success rate of our OncoCompass™ Target is more than 99% (represents the proportion of clinical samples tested by OncoCompass™ Target that passed our quality control standards—including cfDNA extraction amount, pre-library quality, library quality and sequencing data quality—and therefore test reports were successfully generated), which we believe is on par with world-class genomic testing companies.
We have GMP-standard manufacturing facilities in Guangzhou for the manufacturing of our reagent kits, with an aggregate annual production capacity of 400,000 kits. We plan to substantially increase our production capacity to meet rising market demand by installing automated workstations in our manufacturing facilities. We have adopted various QC measures to ensure that we comply with all applicable regulations, standards and internal policies during the manufacturing process. Our manufacturing facilities successfully obtained ISO13485 certification, which was last renewed in October 2021. This certification is valid for three years. This ISO standard demonstrates that we have a comprehensive quality management system for the design and manufacture of medical devices.
We typically source sequencers, reagents and certain other laboratory supplies used in our laboratory operations from trading companies that procure laboratory supplies from a variety of manufacturers. We generally enter into short-term supply agreements with our suppliers on an as-needed basis, each specifying the quantity, quality, warranty, delivery and payment terms and other customary terms for the respective batch of laboratory equipment and supply we purchase. Our suppliers generally grant us a credit term of 30 to 90 days, and are responsible for the repair and maintenance of the laboratory equipment and supplies they supply.
Research and Development
Our research and development efforts are primarily focused on the following areas:
Development of, and improvement on, NGS-based cancer therapy selection products. Based on clinical market demand and scientific progress, we design a series of different panels to meet different clinical needs. In particular, we are continuously working on designing products that require lower sample input and have higher library conversion rate and shorter hands-on time. We are also working to increase the automation of NGS-based cancer therapy selection products to alleviate manual workload and improve therapy selection precision. Our bioinformatics team will continue improving our data analysis algorithms and developing our analysis pipeline. Our validation team is working on thoroughly evaluating the sensitivity, specificity, reproducibility and accuracy of each product before launch.
Development of more reagent kits for NMPA approval. We are developing a number of products targeting different cancers for the NMPA approval. For each product, we will implement strict design control process, perform analytical validation, and conform the manufacturing to GMP and ISO13485 standards. We are also developing the corresponding software solutions for these products.
Development and validation of MRD detection products. Building upon brPROPHET™, we are continuously conducting analytical and clinical validation studies on our UMI-based liquid biopsy products for their sensitivity and utility for MRD detection, which could demonstrate clinical benefits for early-stage patients by predicting their risk of recurrence after treatment.
Development of early cancer detection technologies and products. Building upon brELSA™, our targeted DNA methylation-based library preparation method, and brMERMAID™, our machine learning algorithm, we will keep improving the biochemistry behind our technologies to enhance background noise suppression, allowing for more accurate qualification and enabling our tests to be compatible with more sequencers, as well as improving our early detection prediction models for cancer detection sensitivity, specificity and tissue origin determination accuracy.
69
Table of Contents
Development of automation solutions for current and future products. To alleviate complicated workflow for NGS-based cancer therapy selection products, we are developing multiple automation solutions to streamline the workflow and reduce human intervention and turnaround time. Solutions we are now developing include robotic liquid handling system and corresponding laboratory information management system integration to work with high, medium, and low throughput laboratory requirement.
Research and technology development on additional clinically actionable biomarkers. We are also conducting research and development on additional clinically actionable biomarkers. For example, we are developing a technology to sequence RNA samples to detect clinically significant RNA alterations, which is expected to be a useful supplement to DNA sequencing. Leveraging our industry-leading NGS-based cancer therapy selection products, we are further collaborating with pharmaceutical companies to accommodate their exploratory research for clinically actionable biomarkers and development needs for CDx.
In 2021, 2022 and 2023, our research and development expenses was RMB367.9 million, RMB421.9 million and RMB347.0 million (US$48.9 million), respectively.
Intellectual Property
We protect our intellectual property rights through a combination of patents, trademarks, copyrights, trade secrets, including know-how, license agreements, confidentiality agreements and procedures, non-disclosure agreements with third parties, employee disclosure and invention assignment agreements and other contractual rights.
Our patent strategy is focused on seeking coverage for our core technologies and specific follow-on applications, implementations for detecting and monitoring cancer by determining genomic alterations, and evaluating the status of specific biomarkers in liquid or tissue samples. In addition, we file for patent protection on our on-going research and development, particularly into early-stage cancer screening.
Our patents and patents applications are primarily related to our proprietary library preparation technologies, algorithms and laboratory equipment and processes. As of December 31, 2023, we held 29 patents in China, which will expire between 2025 and 2042. We held five patents in Hong Kong, which will expire between 2038 and 2042. We held one patent in Japan, which will expire in 2038. We held one patent in Brazil, which will expire in 2039. As of the same date, we had 30 pending patent applications in China, twelve pending patent applications in Hong Kong, five pending patent applications in the United States, three pending patent applications in European Patent Office, two pending patent applications in Japan, two pending patent application in Canada, one pending patent applications in Brazil, one pending patent application in Singapore, two pending patent applications in Australia, and 17 international applications strategically filed under the Patent Cooperation Treaty, or PCT, of which two are the basis of pending registration for our MSI (MicroSatellite Instability) calling algorithms in the U.S., European Patent Office and Japan, two are the basis of pending registration for brELSA™, our targeted DNA-methylation based library preparation method for early cancer detection, in the U.S., Canada, Brazil, Singapore, Australia, China, Hong Kong, Japan and the European Patent Office, six are for OverC™, our multi-cancer detection blood test for early cancer testing, and another one is for brPROPHET™, our MRD (Minimal Resideal Disease) algorithms.
The table below sets forth details of our key patents:
Description of patent | Use and application | Jurisdiction | Expiration date | |||
| A library preparation method and associated reagents (HS library preparation technology) | Our cancer therapy selection tests | China | 2036 | |||
| A composition of matter that detects the presence of MSI in liquid biopsy samples (related to bMSISEA) | Tests such as ColonCore and pan-cancer tests | China, Hong Kong | 2038 | |||
| A automation method of the management and reporting of quality control of laboratory processes | Our laboratory information management system | China | 2035 | |||
| A NGS-based method to simultaneously detect MSI and genomic mutations in liquid biopsy samples (bMSISEA) | Our cancer therapy selection tests that detect MSI in liquid biopsy samples, such as ColonCore | China, Hong Kong, Brazil | 2038, 2039 | |||
| A NGS-based method to simultaneously detect MSI and genomic mutations in tissue samples (prettyMSI) | Our cancer therapy selection tests that detect MSI in tissue samples, such as OncoScreen™ IO | China, Japan | 2037, 2038 | |||
| A NGS-based method to multi-cancer detection for early cancer testing in A NGS-based in blood | Our multi-cancer detection blood test for early cancer testing, such as OverC™ | China, Hong Kong | 2042 | |||
70
Table of Contents
The table below sets forth details of our key pending patent applications:
Description of patent application | Use and application | Jurisdiction | Expected | |||
| A NGS-based method to simultaneously detect MSI and genomic mutations in liquid biopsy samples (bMSISEA) | Our cancer therapy selection tests that detect MSI in liquid biopsy samples, such as ColonCore | PCT (currently under review by patent offices in Japan, the U.S., Canada, Brazil, Australia and the European Patent Office) | 2039 | |||
| A NGS-based method to simultaneously detect MSI and genomic mutations in tissue samples (prettyMSI) | Our cancer therapy selection tests that detect MSI in tissue samples, such as OncoScreen™ IO | Hong Kong, PCT (currently under review by patent offices in Japan, the U.S. and the European Patent Office) | 2038 | |||
| Compositions and methods for preparing nucleic acid libraries (brELSA™) | Our targeted DNA-methylation based library preparation method for early cancer detection | PCT (currently under review by the patent office in China, Hong Kong, the U.S., Japan, Canada, Brazil, Australia, Singapore and the European Patent Office) | 2039 | |||
| A detection method for variant nucleic acid (brPROPHET™) | Our MRD detection assay and bioinformatics algorithms | China, PCT (to be entered into national phases by 2024) | 2041 | |||
| Methylation sequencing method and device (OverC™) | Our multi-cancer detection blood test for early cancer testing | China, PCT (to be entered into national phases by 2023) | 2042 | |||
| Aa high-precision gene hybridization capture probe for hybridizing and capturing methylation variation regions related to various cancers | Our multi-cancer detection blood test for early cancer testing, such as OverC™ | China, Hong Kong, PCT (to be entered into national phases by 2024) | 2042 | |||
As of December 31, 2023, we have also registered 11 software copyrights related to our laboratory process quality control management, report automation, and sequencing result analysis.
As of December 31, 2023, we had registered 579 trademarks in China, including “  ”, “BURNING ROCK DX”, “
”, “BURNING ROCK DX”, “ 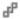 ” and product and service names, and 37 trademark applications pending in China. We had registered 20 trademarks, including “BURNING ROCK DX”, “ONCOSCREEN” , “CanCatch” , “OverC” AND “ONCOCOMPASS” in European Union, the U.K, United States, Japan. and Australia, and twelve trademark applications pending in the US, Canada, Japan,and Brazil. We also own four registered domain names, including our official website.
” and product and service names, and 37 trademark applications pending in China. We had registered 20 trademarks, including “BURNING ROCK DX”, “ONCOSCREEN” , “CanCatch” , “OverC” AND “ONCOCOMPASS” in European Union, the U.K, United States, Japan. and Australia, and twelve trademark applications pending in the US, Canada, Japan,and Brazil. We also own four registered domain names, including our official website.
Environmental, Social and Governance
We believe sustainability and social values are fundamental to our continued growth. We aspire to create a lasting positive environmental, social, and governance (“ESG”) impacts on our business partners and the broader community whom our products and services and business operation may impact. Having a central laboratory conducting tests for our collaborating physicians and hospitals and manufacturing facilities for the production of reagent kits, we acknowledge our potential environmental impacts and social responsibilities on environmental protection.
We target to implement a number of company-wide measures to ensure compliance with the stringent regulatory requirements and standard operating procedures relating to emissions of air, water and other materials, bio-waste generation and treatment, handling, use, storage, treatment and disposal of hazardous substances, worker health and safety requirements, and emergency planning and response. Specifically, we are considering implementing climate-related monitoring and disclosures based on the recommendations of the Task Force on Climate-related Financial Disclosures (“TCFD”) under the Financial Stability Board (“FSB”) to identify risks and opportunities related to climate change, and improve management to reduce greenhouse gas emissions in our operations and mitigate the impact on climate change. We acknowledge that the current climate-related disclosures contained in this document are not fully consistent with TCFD recommendations and that further work is required to enhance the identification, impact and reporting for climate-related risks and opportunities.
As of the date of this annual report, we are not subject to significant environmental or climate-related risks. However, growing concerns about climate change and greenhouse gas emissions have led to the adoption of various regulations and policies in jurisdictions where we operate. The estimated magnitude of resulting impacts will be evaluated over short-, medium and long-term horizons. In recent years, changing weather patterns due to climate change have increased the frequency of extreme weather conditions. Disasters created by extreme conditions could cause significant interruption to our operations, including the indirect interruption as a result of the adverse impacts on our suppliers, customers and business partners. In the medium to long-term, governmental legislation and regulations in response to potential impacts of climate change, as well as the compliance requirements from our suppliers, customers and business partners in relation to our ESG practice, may potentially impact our business operation and subject us to additional compliance costs and restrictions. We support the TCFD and its recommendations and we are committed to assessing the impacts of climate risks in the coming financial years.
71
Table of Contents
Competition
We are China’s number one NGS-based cancer therapy selection company. China’s cancer genotyping industry is highly competitive. Our major competitors include domestic NGS-based cancer therapy selection and MRD companies, such as AmoyDx, BGI and Geneseeq. Our competitors may have more expertise, experience and financial resources, stronger business relationships in developing and commercializing their products and services, more mature technologies, greater market adoption among physicians, patients and others in the medical community, broader test menus, or greater brand recognition than we do. We also cannot assure you that our technologies will not become obsolete if we cannot keep pace with the constantly changing technologies in the industry.
Regulation
We are subject to a variety of PRC laws, rules and regulations affecting many aspects of our business. This section summarizes the principal PRC laws, rules and regulations that we believe are relevant to our business and operations.
Regulations on Foreign Investment
Investment in China by foreign investors are regulated by the Catalog of Industries for Encouraging Foreign Investment, as promulgated by the MOFCOM and the NDRC on December 27, 2020 and effective on January 27, 2021, and the Special Administrative Measures for Access of Foreign Investment (2021 Edition), or the Negative List, as promulgated on December 27, 2021 and effective on January 1, 2022. Industries not listed in the Negative List are generally permitted and open to foreign investment, unless specifically prohibited or restricted by the PRC laws and regulations. According to the Negative List, foreign investors are permitted to access to the medical device industry, whereas foreign investors are prohibited from investing in businesses involving the development and application of genomic diagnosis and treatment technology.
In addition, a foreign-invested enterprise in the PRC is required to comply with other regulations on its incorporation, operation and changes. On March 15, 2019, the National People’s Congress adopted the Foreign Investment Law of the PRC, which became effective on January 1, 2020. Pursuant to the Foreign Investment Law of the PRC, China will grant national treatment to foreign invested entities, except for those foreign invested entities that operate in industries that fall within “restricted” or “prohibited” categories as prescribed in the Negative List to be released or approved by the State Council.
On December 26, 2019, the State Council promulgated the Implementation Rules to the Foreign Investment Law, which became effective on January 1, 2020. The implementation rules further clarify that the state encourages and promotes foreign investment, protects the lawful rights and interests of foreign investors, regulates foreign investment administration, continues to optimize foreign investment environment, and advances a higher- level opening. On December 30, 2019, the MOFCOM and SAMR jointly promulgated the Measures for Information Reporting on Foreign Investment, which became effective on January 1, 2020. Pursuant to the Measures for Information Reporting on Foreign Investment, where a foreign investor carries out investment activities in China directly or indirectly, the foreign investor or the foreign-invested enterprise shall submit the investment information to the competent commerce department.
Regulations on Human Genetic Resources
Regulation on the Management of Human Genetic Resources
The Regulation on the Management of Human Genetic Resources, as promulgated by the State Council on May 28, 2019 and effective on July 1, 2019, regulates the collection, preservation, usage and external provision of China’s human genetic resources. According to this regulation, “human genetic resource” includes human genetic resource materials and information. Human genetic resource materials refer to organs, tissues, cells and other genetic materials containing human genome, genes and other genetic materials. Human genetic resource information refers to information, such as data, generated by human genetic resources materials. The Administrative Department of Science and Technology under the State Council is responsible for the management of human genetic resources at the national level, and the administrative departments of science and technology under the provincial governments are responsible for the management of human genetic resources at local level and are vertically directed by the central government. Foreign entities, individuals and such entities established or actually controlled thereby are not allowed to collect or preserve China’s human genetic resources (including organs, tissues, cells and other genetic materials of human genome and gene) or provide human genetic resources abroad, while they are prohibited from using China’s human genetic resources unless they have obtained an approval from relevant PRC government authority or have filed with relevant government authority for international cooperation with a Chinese entity.
72
Table of Contents
Biosecurity Law
On October 17, 2020, the Standing Committee of the National People’s Congress adopted the Biosecurity Law of the People’s Republic of China, or the Biosecurity Law, which became effective on April 15, 2021. The Biosecurity Law establishes an integrated system to regulate biosecurity related activities in China, including the security regulation of HGR and biological resources. The Biosecurity Law for the first time expressly declares that China has sovereignty over its HGR, and further endorsed the Regulation on the Management of Human Genetic Resources, as promulgated by the State Council on May 28, 2019 by recognizing the fundamental regulatory principles and systems established by it over the utilization of Chinese HGR by foreign entities in China. Although the Biosecurity Law does not provide any specific new regulatory requirements for HGR, because it is a law adopted by China’s highest legislative authority, it gives China’s major regulatory authority of HGR, the Ministry of Science and Technology, significantly more power and discretion to regulate HGR, and it is expected that the overall regulatory landscape of Chinese HGR will evolve and become even more rigorous and sophisticated. Failure to comply with the requirement under the Biosecurity Law will result in the penalties, including fines, suspension of related activities and confiscation of related HGR and gains generated from conducting these activities.
Regulation on Medical Institutions and Medical Devices
Regulatory Authorities
The newly formed NMPA under the State Administration for Market Regulation is the government authority that monitors and supervises the administration of pharmaceutical products, medical devices and cosmetics. The NMPA’s predecessor, the CFDA, was established in March 2013 and separated from the Ministry of Health of the PRC, or the MOH, as part of an institutional reform of the State Council. Predecessors of the NMPA also include the former State Food and Drug Administration, or the SFDA, which was established in March 2003 and the State Drug Administration, or the SDA, that was established in August 1998. The primary responsibilities of the NMPA include:
| • | monitoring and supervising the administration of pharmaceutical products, medical devices and cosmetics in the PRC; |
| • | formulating administrative rules and policies concerning the supervision and administration of the pharmaceutical, medical device and cosmetics industry; |
| • | evaluating, registering and approving of new drugs, generic drugs, imported drugs and traditional Chinese medicine; |
| • | approving and issuing permits for the manufacture and export/import of pharmaceutical products and medical devices, and approving the establishment of enterprises to be engaged in the manufacture and distribution of pharmaceutical products; and |
| • | examining and evaluating the safety of pharmaceutical products, medical devices and cosmetics and handling significant accidents involving these products. |
The National Health and Family Planning Commission, or the NHFPC, has been renamed as the National Health Commission, or the NHC. The NHC is an authority at the ministerial level under the State Council and is primarily responsible for national public health. The NHC combines the responsibilities of the former NHFPC, the Leading Group Overseeing Medical and Healthcare Reform under the State Council, the China National Working Commission on Aging, partial responsibilities of the Ministry of Industry and Information Technology in relation to tobacco control, and partial responsibilities from the State Administration of Work Safety in relation to occupational safety. The predecessor of NHFPC is the MOH. Following the establishment of the SFDA in 2003, the MOH was put in charge of the overall administration of national health in the PRC excluding the pharmaceutical industry.
Medical Institutions Laws and Regulations
The Regulation on the Administration of Medical Institutions as promulgated by the State Council in 1994 and revised in 2016 and 2022 provides the requirements for the establishment and administration of medical institutions. The establishment of medical institutions must comply with local governments’ plans for the establishment of medical institutions and the basic standards for medical institutions. To establish a medical institution, an entity or individual will be subject to the examination and approval of the health administrative department of the local government at or above the county level if required by the regulations of the State Council. A medical institution other than a clinic providing medical services must register and obtain a Medical Institution Practice License. An entity or individual that has not obtained a Medical Institution Practice License or filed for record may not carry out diagnosis or treatment activities. The revised Rules for Implementation of the Administrative Regulation on Medical Institutions, as promulgated by the NHFPC in February 2017, further regulates the approval on the establishment, registration, validation and practice of medical institutions.
Guangzhou Burning Rock Dx Co., Ltd., a subsidiary of the VIE, obtained a Medical Institution Practice License in September 2017, with a five- year validity from March 2015 to March 2020. This license was renewed in February 2020, and the renewed license has a five-year validity until February 2025.
73
Table of Contents
The Measures for the Administration of Clinical Testing Laboratories in Medical Institutions, which was promulgated by the MOH in February 2006 and became effective in June 2006 and was revised in July 2020, provides regulations on the examination, establishment, quality management and safety practice of clinical testing laboratories in medical institutions.
The Measures for the Administration of Clinical Gene Amplification Testing Laboratories in Medical Institutions, as promulgated by the MOH in December 2010, provides the requirements for medical institutions to carry out clinical gene amplification test techniques. A clinical gene amplification testing laboratory refers to a laboratory that detects specific DNA or RNA by amplification to perform disease diagnosis, treatment monitoring and prognosis determination. The MOH is responsible for supervising and administering clinical gene amplification testing laboratories in medical institutions nationwide. The health administrative authorities at the provincial level are responsible for supervising and administering clinical gene amplification testing laboratories in medical institutions within their respective administrative regions. This regulation also provides the examination and establishment of clinical gene amplification testing laboratories, laboratory quality management and laboratory supervision and management.
The Notice for the Basic Standards for Clinical Testing Laboratories (for Trial Implementation), as promulgated by the NHFPC in July 2016, further provides the standards and requirements for clinical testing laboratories.
The Notice for the Further Administration of Clinical Gene Amplification Testing Laboratories in Medical Institutions as promulgated by the Guangdong Health Department in September 2012 provides that medical institutions carrying out clinical gene amplification test techniques must apply for technical access from the Guangdong Health Department, and the Guangdong Clinical Laboratory Center is authorized as the technical auditing institution of clinical gene amplification testing technology.
The Notice for the Further Administration of Department Office and Medical Technology in Clinical Institutions, as promulgated by the Guangdong Health Department in May 2016, further provides for the management of medical technology. Clinical gene amplification testing technology, as a limited medical technology, is subject to the examination and approval of the Guangdong Health Department.
Guangzhou Burning Rock Dx Co., Ltd., a subsidiary of the VIE, obtained its Certificate of Clinical Gene Amplification Testing Laboratory in August 2015, with a five-year validity from August 2015 to August 2020, and the certificate was successfully renewed in May 2021. Guangzhou Burning Rock Dx Co., Ltd. obtained its Certificate of High Throughput Sequencing Testing Laboratory in May 2018, with a five-year validity from May 2018 to May 2023.
Medical Devices Administration Laws and Regulations
According to the Notice on Strengthening the Management of Products and Technologies Related to Clinical Use of Gene Sequencing, as promulgated by the CFDA and NHFPC in February 2014, gene sequencing diagnostic products (including gene sequencer and related diagnostic reagents and software) are regulated as medical devices and must be registered pursuant to relevant regulations.
The Regulations on the Supervision and Administration of Medical Devices, as amended by the State Council in May 2017 and February 2021, regulates entities that engage in the research and development, production, operation, use, supervision and administration of medical devices in the PRC. Medical devices are classified according to their risk levels. Class I medical devices are medical devices with low risks, and the safety and effectiveness of which can be ensured through routine administration. Class II medical devices are medical devices with moderate risks, which are strictly controlled and administered to ensure their safety and effectiveness. Class III medical devices are medical devices with relatively high risks, which are strictly controlled and administered through special measures to ensure their safety and effectiveness. The evaluation of the risk levels of medical devices take into consideration the medical devices’ objectives, structural features, methods of use and other factors. Registration certificates are required for Class II and Class III medical devices. The classification of specific medical devices is stipulated in the Medical Device Classification Catalog, which was issued by the CFDA on August 31, 2017 and became executive on August 1, 2018. According to the most recently amended Regulations on the Supervision and Administration of Medical Devices which became effective on June 1, 2021, qualified medical institutions may, based on clinical demands, conduct research and development on in vitro diagnostic testing reagents if the same type of products are not available at market in China, and may also use such in vitro diagnostic testing reagents internally under the instruction of practicing physicians. Specific administrative measures shall be formulated by the medical products administration of the State Council in conjunction with the competent department of health of the State Council.
74
Table of Contents
The Administrative Measures for the Registration of Medical Devices, or the Medical Devices Registration Measures, as promulgated by the CFDA in October 2014, provide that Class I medical devices are subject to record-filing, while Class II and Class III medical devices are subject to registration. According to the Medical Devices Registration Measures, the registration and record-filing of IVD reagents that are regulated as medical devices are governed by the Administrative Measures for the Registration of IVD Reagents, which was first promulgated by the CFDA and took effect on July 30, 2014, and amended on January 25, 2017. Pursuant to the Administrative Measures for the Registration of IVD Reagents, Class I IVD reagents are subject to filing, and Class II and Class III IVD reagents are subject to inspection, approval and registration. On August 26, 2021, the SAMR promulgated the Administrative Measures for the Registration and Record-filing of Medical Devices and the Administrative Measures for the Registration and Record-filing of IVD Reagents, both of which became effective on October 1, 2021, and replaced the Medical Devices Registration Measures and the Administrative Measures for the Registration of IVD Reagents respectively.
According to the Opinions on Deepening the Reform of the Evaluation and Approval System and Inspiring Innovation of Drugs and Medical Devices, the evaluation and approval for the application of innovative medical devices will be prioritized. In November 2018, the NMPA released the Special Review Procedures for Innovative Medical Devices, which provides that the NMPA will prioritize applications for qualified innovative medical devices. These rules specify requirements for the application of innovative medical devices, including certificates, intellectual property, process and results of product research and development and other technical documents.
The Measures for the Supervision and Administration of the Manufacture of Medical Devices, as promulgated by the CFDA in November 2017, regulates entities that engage in the manufacturing of medical devices in the PRC. The food and drug administration authorities at or above the county level regulate medical device manufacturing within their administrative regions, including manufacturing-related licensing and filing, contract manufacturing and manufacturing quality controls. Production permits are required for the manufacture of Class II and Class III medical devices. A medical device production license is valid for five years, which may be extended upon expiration in accordance with relevant administrative provisions. Medical device manufacturers are not required to obtain a medical device operation license to sell their self-manufactured products. On March 10, 2022, the SAMR promulgated the new Measures for the Supervision and Administration of the Manufacture of Medical Devices which became effective on May 1, 2022.
The Good Manufacturing Practice Rules for Medical Devices, as promulgated by the CFDA on December 29, 2014 and effective on March 1, 2015, provide basic principles for quality control systems for medical devices manufacturing, and these rules are applicable to the entire process of design and development, production, sales and post-sale services of medical devices.
The Measures for the Supervision and Administration of the Business Operation of Medical Devices, as promulgated by the CFDA in November 2017, regulates entities conducting the business operation of medical devices in the PRC. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and effectiveness of the device. Business activities involving medical devices are regulated in accordance with the classification of each of the medical devices. No filing or license is required for business activities involving Class I medical devices. Filing is required for business activities involving Class II medical devices, and licenses are required for business activities involving Class III medical devices. A medical device operation license is valid for five years, which may be extended upon expiration in accordance with relevant administrative provisions. Medical devices manufacturing enterprises engaging in the sale of self-produced products are not required to obtain a medical device operation license. On March 10, 2022, the SAMR promulgated the new Measures for the Supervision and Administration of the Business Operation of Medical Devices which became effective on May 1, 2022.
According to the Supervision and Administration of Medical Devices, entities are prohibited from using or operating unregistered or unfiled, expired, invalid or obsolete medical devices or those without a certificate of conformity.
Pursuant to the Notice on Strengthening the Administration of Import and Use of Pharmaceutical and Medical Devices, as promulgated by the CFDA in October 2010, medical institutions may only purchase qualified medical devices from enterprises with a medical device manufacture license or a medical device operation license.
Guangzhou Burning Rock Dx Co., Ltd., a subsidiary of the VIE, obtained Class I medical devices record-filing certificates for our general kit for sequencing reaction, nucleic acid extraction or purification reagent, sequencing kit for gene sequencing and library kit for gene sequencing (DNA interruption linking) in May 2016, January 2017, April 2017 and December 2017, respectively. Guangzhou Burning Rock Dx Co., Ltd. also obtained a Class III medical device registration certificate for our human EGFR/ALK/BRAF/KRAS fusion gene mutation detection kit (reversible termination sequencing) and mutation gene analysis software for non-small cell lung cancer in July 2018 and August 2019, respectively. In March 2022, Guangzhou Burning Rock Dx Co., Ltd. obtained from the NMPA a Class III medical device registration certificate for our human nine-gene mutation joint detection kit (reversible termination sequencing) (LungCure™ CDx) for non-small cell lung cancer.
Guangzhou Burning Rock Dx Co., Ltd. obtained a Class III medical device manufacture license for our human EGFR/ALK/BRAF/KRAS fusion gene mutation detection kit (reversible termination sequencing) in August 2018, with a term of five years.
Guangzhou Burning Rock Medical Devices Co., Ltd. obtained a medical device operation license for Class III medical devices in December 2020, with a term of five years.
75
Table of Contents
Medical Devices Subject to Cold Chain Management
According to the Guidelines for Cold Chain (Transport & Storage) Management of Medical Devices, as promulgated by the CFDA in September 2016, medical devices subject to cold chain management, such as our reagent kits, are medical devices requiring refrigeration and frozen management in the process of transportation and storage in accordance with relevant instructions and labels. Medical device manufacturers and wholesalers must equip with cold storage, refrigerated vehicles and containers, and other facilities and equipment, which fit the variety and scale of the medical devices they produce or operate. To ensure proper temperature control during transportation, operators must choose a reasonable means of transportation, and take adequate temperature control measures based on transportation conditions, which, among others, include the quantity of medical devices subject to cold- chain management, the distance and time requirements, and the temperature requirements. Operators who engage third-party carriers must examine the carrier’s qualifications and capabilities, and enter into relevant agency agreements for transportation.
Tendering Processes for Medical Devices
The Chinese government has implemented measures to encourage pooled procurement of expensive medical consumables through tendering processes. In June 2007, MOH issued the Notice on Further Strengthening the Administration of Centralized Procurement of Medical Devices, which requires that all non-profit medical institutions established by local governments, associations or state-owned enterprises participate in the centralized procurement. Public tendering will be the principal method for centralized procurement.
Policies on NGS-based Cancer Therapy Selection
In recent years, China has introduced a series of policies that support the development of NGS-based cancer therapy selection. The table below presents a selection of these policies introduced by relevant governmental authorities in China from 2014 to 2021:
Date | Authority | Key messages | ||
| February 2014 | NMPA | The NMPA (former CFDA) issued a Notice on Special Approval Procedures for Innovative Medical Devices (Trial), which significantly accelerated the approval process for NGS products. | ||
| March 2014 | State Council | The State Council published Regulation on the Supervision and Administration of Medical Devices, which provides that reagents related to human gene testing are Class III medical devices. NGS products are managed as medical devices. | ||
| February 2015 | NHC | The NHC published Guidelines for Personalized Medical Testing Applications of Sequencing Technology, which provides guidance on sample collection, transportation, receiving, processing, testing and inspection of project development, verification, and validation, basic principles of quality control, result reporting, and the possible problems and countermeasures, to provide standardized guidance on precision medicine based on sequencing technology application. | ||
| July 2015 | NHC | The NHC published Guidelines for Individualized Treatment and Detection of Tumors, which provides for the standardization of testing technology, laboratory access and quality assurance. It includes specific requirements for clinical and medical laboratories to ensure the accuracy of genotyping test results. | ||
| February 2016 | NHC | The NHC published Notice of the General Office of the National Health and Family Planning Commission on Issues Related to the Management of Clinical Testing Projects, which covers strengthening the management of clinical inspection projects, standardizing the clinical inspection work of medical institutions, meeting the needs of clinical medical treatment, and ensuring the quality and safety of medical treatment. | ||
| May 2017 | State Council | The State Council published Amendments to the Regulations on the Supervision and Administration of Medical Devices, which regulates Class III medical devices, including NGS products, under product registration management. It also provided detailed requirements for Class III medical device registration. | ||
| September 2018 | NHC | The NHC published Guidelines for Clinical Application of New Cancer Drugs to guide the clinical application of cancer drugs. The guidelines cover 7 types of tumors including respiratory system, digestive system, blood tumor, urinary system, breast cancer and 42 types of cancer drugs, providing clear guidance for precision medicine. | ||
| September 2021 | NMPA | The NMPA published Guidelines for Clinical Trials of In Vitro Diagnostic Reagents, which provides basic principles for IVD reagent clinical trials, provides recommendations in principle for clinical trial design, identifies key factors to consider during clinical trials, and provides reference for technical review departments in reviewing clinical trial data. | ||
76
Table of Contents
Other Significant PRC Regulations Affecting Our Business Activities
Commercial Bribery Regulations
The Standing Committee of the National People’s Congress adopted the Anti-Unfair Competition Law, which became effective on December 1, 1993 and was amended on November 4, 2017 and April 23, 2019, respectively, with the most recent amendment coming into force on April 23, 2019. The Anti-Unfair Competition Law provides that a business operator commits a crime if it offers money or any other bribes in the course of selling or purchasing products.
Medical device companies involved in criminal investigations or administrative proceedings related to bribery are listed in the Adverse Records of Commercial Briberies by their respective provincial health and family planning administrative departments. Pursuant to the Provisions on the Establishment of Adverse Records of Commercial Briberies in the Medicine Purchase and Sales Industry, which became effective on March 1, 2014, provincial health and family planning administrative departments are responsible for formulating the implementing measures for the establishment of Adverse Records of Commercial Briberies. If a company is listed in the Adverse Records of Commercial Briberies for the first time, its products may not be purchased by public medical institutions. A company will not be penalized by the relevant PRC government authorities merely by virtue of having contractual relationships with sales agents or third-party promoters who are engaged in bribery activities, so long as such company and its employees are not utilizing the sales agents or third-party promoters for the implementation of, or acting in conjunction with them in, the prohibited bribery activities. In addition, a company is under no legal obligation to monitor the operating activities of its sales agents and third-party promoters, and will not be subject to penalties or sanctions by relevant PRC government authorities as a result of failure to monitor their operating activities.
Product Liability Regulations
In addition to a strict new medical products approval process, certain PRC laws have been promulgated to protect the rights of consumers and to strengthen the control of medical products in China. Under current PRC law, manufacturers and vendors of defective products in China may incur liability for loss and injury caused by such products. Pursuant to the General Principles of the Civil Law of the PRC promulgated on April 12, 1986 and amended on August 27, 2009, a defective product that causes property damage or physical injury to any person may subject the manufacturer or vendor of that product to civil liability for such damage or injury. On May 28, 2020, the Third Session of the 13th National People’s Congress passed the Civil Code of the PRC which took effect on January 1, 2021, and replaced the General Principles of the Civil Law of the PRC. The Civil Code of the PRC provides that the defective product that causes any property damage or physical injury to any person may subject the manufacturer or vendor of that product to civil liability for such damage or injury.
On February 22, 1993, the Product Quality Law of the PRC, or the Product Quality Law, was promulgated to supplement the General Principles of the Civil Law of the PRC aiming to protect the legitimate rights and interests of the end-users and consumers and to strengthen the supervision and control of the quality of products. The Product Quality Law was revised by the National People’s Congress on July 8, 2000, August 27, 2009 and December 29, 2018. Pursuant to the revised Product Quality Law, manufacturers who produce defective products may be subject to civil or criminal liability and have their business licenses revoked.
The Law of the PRC on the Protection of the Rights and Interests of Consumers was promulgated on October 31, 1993 and was amended on August 27, 2009 and October 25, 2013 to protect consumers’ rights when they purchase or use goods and accept services. All business operators must comply with this law when they manufacture or sell goods and/or provide services to customers. Under the amendments made on October 25, 2013, all business operators must pay high attention to protecting customers’ privacy and must strictly keep confidential any consumer information they obtain during their business operations. In addition, in extreme situations, pharmaceutical product manufacturers and operators may be subject to criminal liability if their goods or services lead to the death or injuries of customers or other third parties.
We are not aware of any material product liability related litigation or other legal proceedings against us arising from the gene testing products or services that we provide to our customers.
Law of PRC Tort Liabilities
On May 28, 2020, the Third Session of the 13th National People’s Congress passed the Civil Code of the PRC which took effect on January 1, 2021, and replaced the Tort Law of the PRC. Under the Civil Code of the PRC, if damages to persons are caused by defective products due to the fault of a third party, such as the parties providing transportation or warehousing services, the producers and the sellers of the products have a right to recover their respective losses from such third parties. If defective products are identified after they have been distributed, the producers or the sellers must take remedial measures, such as issuance of a warning or recall of products, in a timely manner. The producers or the sellers will be liable under tort if they fail to take remedial measures in a timely manner or have not made efforts to take remedial measures, thus causing damages. If the products are produced or sold with known defects and cause deaths or severe adverse health issues, the infringed party has a right to claim punitive damages in addition to compensatory damages.
77
Table of Contents
Intellectual Property Laws and Regulations
China has made substantial efforts to promulgate comprehensive legislation governing intellectual property rights, including laws and regulations on patents, trademarks, copyrights and domain names.
Patents
Pursuant to the PRC Patent Law, most recently amended in October 2020, and its implementation rules, most recently amended in January 2010, patents in China fall into three categories: invention, utility model and design. An invention patent is granted to a new technical solution proposed in respect of a product or method or an improvement of a product or method. A utility model is granted to a new technical solution that is practicable for application and proposed in respect of the shape, structure (or a combination of both) of a product. A design patent is granted to a new design of a certain product in shape (overall or partial), pattern (or a combination of both) and in color, shape and pattern combinations aesthetically suitable for industrial application. Under the PRC Patent Law, the term of patent protection starts from the date of application. Patents relating to invention are effective for twenty years, utility model patents are effective for ten years and design patents are effective for fifteen years from the date of application. The PRC Patent Law adopts the principle of “first-to-file” system, which provides that where more than one person files a patent application for the same invention, a patent will be granted to the person who first files the application.
Existing patents can be narrowed, invalidated or unenforceable due to a variety of grounds, including lack of novelty, creativity, and deficiencies in patent application. In China, a patent must have novelty, creativity and practical applicability. Under the PRC Patent Law, novelty means that before a patent application is filed, no identical invention or utility model has been publicly disclosed in any publication in China or overseas or has been publicly used or made known to the public by any other means, whether in or outside of China, nor has any other person filed with the patent authority an application that describes an identical invention or utility model and is recorded in patent application documents or patent documents published after the filing date. Creativity means that, compared with existing technology, an invention has prominent substantial features and represents notable progress, and a utility model has substantial features and represents any progress. Practical applicability means an invention or utility model can be manufactured or used and may produce positive results. Patents in China are filed with the China National Intellectual Property Administration, or CNIPA. Normally, the CNIPA publishes an application for an invention patent within 18 months after the filing date, which may be shortened at the request of applicant. The applicant must apply to the CNIPA for a substantive examination within three years from the date of application.
The PRC Patent Law provides that, for an invention or utility model completed in China, any applicant (not limited to Chinese companies and individuals), before filing a patent application outside of China, must first submit it to the CNIPA for a confidential examination. Failure to comply with this requirement will result in the denial of any Chinese patent for the relevant invention. This added requirement of confidential examination by the CNIPA has raised concerns by foreign companies that conduct research and development activities in China or outsource research and development activities to service providers in China.
Patent Enforcement
Unauthorized use of patents without consent from owners of patents, forgery of patents belonging to other persons, or engaging in other patent infringement acts, will subject the infringers to infringement liability. Serious offenses such as forgery of patents may be subject to criminal penalties.
When a dispute arises out of the infringement of a patent owner’s patent rights, PRC law requires that the parties first attempt to settle the dispute through mutual consultation. However, if the dispute cannot be settled through mutual consultation, the patent owner, or an interested party who believes the patent is being infringed, may either file a civil legal suit or file an administrative complaint with the relevant patent administration authority. A Chinese court may issue a preliminary injunction upon the request of the patent owner or an interested party before instituting any legal proceedings or during the proceedings. Damages for infringement are calculated as the loss suffered by the patent holder arising from the infringement, and if the loss suffered by the patent holder arising from the infringement cannot be determined, the damages for infringement are calculated as the benefit gained by the infringer from the infringement. If it is difficult to ascertain damages in this manner, damages may be determined using a reasonable multiple of the license fee under a contractual license. Statutory damages may be awarded in circumstances where damages cannot be determined by the calculation standards described above. The damage calculation methods will be applied in the order described above. Generally, a patent owner has the burden of proving that the patent is being infringed. However, if the owner of an invention patent for manufacturing process of a new product alleges infringement of its patent, the alleged infringer has the burden of proof.
As of December 31, 2023, we held 29 patents in China, which will expire between 2025 and 2042. We held five patents in Hong Kong, which will expire between 2038 and 2042. We held one patent in Brazil, which will expire in 2039. As of the same date, we had 30 pending patent applications in China, 12 pending patent applications in Hong Kong, five pending patent applications in the United States, three pending patent applications in European Patent Office, two pending patent applications in Japan, two pending patent applications in Canada, one pending patent applications in Brazil, one pending patent application in Singapore, two pending patent applications in Australia, and 17 international applications strategically filed under the Patent Cooperation Treaty, or PCT, of which one is the basis of pending registration for our MSI calling algorithms in the U.S., European Patent Office and Japan two are the basis of pending registration for brELSA™, our targeted DNA-methylation based library preparation method for early cancer detection, in the U.S., Canada, Brazil, Singapore, Australia, China, Hong Kong, Japan and the European Patent Office, six are for OverC™, our multi-cancer detection blood test for early cancer testing, and another one is for brPROPHET™, our MRD (Minimal Resideal Disease) algorithms.
78
Table of Contents
Trade Secrets
According to the PRC Anti-Unfair Competition Law, the term “trade secrets” refers to technical and business information that is unknown to the public, has utility and may create business interests or profits for its legal owners or holders, and is maintained as a secret by its legal owners or holders.
Under the PRC Anti-Unfair Competition Law, which was promulgated on September 2, 1993 and was amended on November 4, 2017 and April 23, 2019, respectively, business persons are prohibited from infringing others’ trade secrets by: (1) obtaining the trade secrets from the legal owners or holders by any unfair methods such as theft, bribery, intimidation, solicitation or coercion; (2) disclosing, using or permitting others to use the trade secrets obtained illegally under item (1) above; (3) disclosing, using or permitting others to use the trade secrets in violation of any contractual agreements or any confidentiality obligation or the requirements of the legal owners or holders to keep such trade secrets in confidence; or (4) abetting a person, or tempting, or aiding a person into or in acquiring, disclosing, using, or allowing another person to use the trade secret of the right holder in violation of his or her non-disclosure obligation or the requirements of the right holder for keeping the trade secret confidential. If a third party knows or should have known that an employee or former employee of the right owner of trade secrets or any other entity or individual conducts any of the illegal acts listed above, but still accepts, publishes, uses or allows any other to use such secrets, this practice will be deemed as an infringement of trade secrets. A party whose trade secrets are being misappropriated may petition for administrative corrections, and regulatory authorities may stop any illegal activities and fine infringing parties in the amount of RMB100,000 to RMB1,000,000, and where the circumstance is serious, the fine will be RMB500,000 to RMB5,000,000. Alternatively, persons whose trade secrets are being misappropriated may file lawsuits in a Chinese court for loss and damages incurred due to the misappropriation.
The measures to protect trade secrets include oral or written non-disclosure agreements or other reasonable measures to require the employees of, or persons in business contact with, legal owners or holders to keep trade secrets confidential. Once the legal owners or holders have asked others to keep trade secrets confidential and have adopted reasonable protection measures, the requested persons bear the responsibility for keeping the trade secrets confidential.
Trademarks
The PRC Trademark Law and its implementation rules protect registered trademarks. The Trademark Office of CNIPA is responsible for the registration and administration of trademarks throughout the PRC. The Trademark Law has adopted a “first-to-file” principle with respect to trademark registration. As of December 31, 2023, we had 579 registered trademarks and 37 pending trademark applications in the PRC.
Copyright
Pursuant to the Copyright Law of the PRC, as amended, copyrights include personal rights such as the right of publication and that of attribution as well as property rights such as the rights of production and distribution. Reproducing, distributing, performing, projecting, broadcasting or compiling a work or communicating the same to the public via an information network without permission from the owner of the copyright therein, unless otherwise provided in the Copyright Law of the PRC, constitutes infringements of copyrights. The infringer must, according to the circumstances of the case, undertake to cease the infringement, take remedial action, and offer an apology or pay damages.
Pursuant to the Computer Software Copyright Protection Regulations promulgated on December 20, 2001 and amended in January 8, 2011 and January 30, 2013, a software copyright owner may complete registration formalities with a software registration authority recognized by the State Council’s copyright administrative department. A software copyright owner may authorize others to exercise that copyright, and is entitled to receive remuneration. As of December 31, 2023, we had eleven software copyrights.
Domain Names
Domain names are protected under the Administrative Measures on the Internet Domain Names promulgated by the Ministry of Industry and Information Technology. The Ministry of Industry and Information Technology is the main regulatory body responsible for the administration of PRC internet domain names As of December 31, 2023, we had four registered domain names, including our official website.
PRC Regulation on Data Protection
The Basic Standards for Medical Laboratories (for Trial Implementation), as promulgated by the NHFPC in 2016, provides that medical laboratories must establish information management and patient privacy protection policies. The Measures for the Administration of General Population Health Information (for Trial Implementation) as promulgated by the NHFPC in 2014 sets forth the operational measures for patient privacy protection in medical institutions. The measures regulate the collection, use, management, safety and privacy protection of general population health information by medical institutions. Medical institutions must establish information management departments responsible for general population health information and establish quality control procedures and relevant information systems to manage this information. Medical institutions must adopt stringent procedures to verify the general population health data collected, timely update and maintain the data, establish policies on the authorized use of this information, and establish safety protection systems, policies, practice and technical guidance to avoid divulging confidential or private information.
79
Table of Contents
According to the Cybersecurity Law of the PRC promulgated by the Standing Committee of the NPC on November 7, 2016 and taking effect on June 1, 2017, network operators shall comply with laws and administrative regulations and fulfill their obligations to safeguard security of the network when conducting business and providing services. Those who provide services through networks shall take technical measures and other necessary measures in accordance with laws, administrative regulations and the compulsory requirements of national standards to safeguard the safe and stable operation of the networks, respond to network security incidents effectively, prevent illegal and criminal activities, and maintain the integrity, confidentiality and usability of network data. Network operators collecting and using personal information shall abide by the principles of legality, justification and necessity, publish the rules for collection and use, explicitly state the purposes, means and scope of the information collection and use, and obtain the consent of the persons whose data is gathered. The network operator shall not collect the personal information irrelevant to the services it provides or collect or use the personal information in violation of the provisions of laws, administrative regulations or the agreements between both parties.
On June 10, 2021, the Standing Committee of the NPC promulgated the Data Security Law of the PRC, or the Data Security Law, which became effective on September 1, 2021. The Data Security Law defines “data” as any recording of information in electronic or other forms, and defines “data processing” as including the collection, storage, use, processing, transmission, provision, disclosure, etc. of data. The Data Security Law requires that data collection shall be conducted in a legitimate and proper manner, and theft or illegal collection of data is not permitted. The PRC government shall establish a data classified and categorized protection system. Data concerning national security, lifelines of the national economy, important aspects of people’s livelihood, and major public interests are core data, and shall be subject to a stricter management system. Data processors shall establish and improve the whole-process data security management rules, organize and implement data security education and trainings, and take appropriate technical measures and other necessary measures to protect data security. In case of data security incidents, responding measures shall be taken immediately, and disclosure to users and report to the competent authorities shall be made in a timely manner.
On August 20, 2021, the Standing Committee of the NPC promulgated the Personal Information Protection Law of the PRC, or the Personal Information Protection Law, which became effective on November 1, 2021. The Personal Information Protection Law sets forth that the personal information of natural persons shall be protected by law, and no organization or individual may infringe upon the personal information rights and interests of natural persons. The processing of personal information shall have clear and reasonable purposes, be directly related to the purposes of processing, and be carried out in a way that has minimal impact on personal rights and interests. The collection of personal information shall be limited to the smallest scope necessary for achieving the purpose of processing, and personal information shall not be collected excessively. Personal information processors shall bear responsibility for their personal information processing activities, and adopt necessary measures to safeguard the security of the personal information they process. Otherwise, the personal information processors may be ordered to make correction or suspend or terminate the provision of services, or be imposed confiscation of illegal income, fines or other penalties.
On December 28, 2021, the Cyberspace Administration of China, or the CAC, and 12 other departments jointly promulgated the newly revised Measures for Cybersecurity Review with effect from February 15, 2022, which provides that (i) a critical information infrastructure operator which intends to purchase network products and services shall prejudge the possible risks to national security that may arise after the products and services are put into use and where national security will or may be affected, the operator shall apply with the Cybersecurity Review Office for cybersecurity review, and (ii) a network platform operator that possesses more than one million users’ personal information must apply for cybersecurity review before listing in a foreign country.
On November 14, 2021, the CAC publicly solicited opinions on the Regulations on the Administration of Cyber Data Security (Draft for Comments) which expanded the scope of application of cybersecurity review, established the data classified and categorized protection system, and defined the relevant rules for cross-border security management of data. It provides that data processors carrying out the following activities shall apply for cybersecurity review: (i) merger, reorganization or division of Internet platform operators that gather and possess a large number of data resources having bearing on the national security, economic development or public interests, which affects or may affect national security; (ii) listing in a foreign country of a data processor that processes the personal information of more than one million persons; (iii) listing in Hong Kong of a data processor, which affects or may affect national security; and (iv) other data processing activities that affect or may affect national security.
To comply with these laws and regulations, we have required our customers and research partners to consent to, or obtain consent from the tested individuals to, our collection and use of their personal information for our genetic tests. We have also established information security systems to protect tested individuals’ privacy, including data access restrictions and monitoring, data storage, database encryption and backup procedures.
PRC Regulation on Labor Protection
Under the Labor Law of the PRC, effective on January 1, 1995 and subsequently amended on August 27, 2009 and December 29, 2018, the PRC Employment Contract Law, effective on January 1, 2008 and subsequently amended on December 28, 2012 and the Implementing Regulations of the Employment Contract Law, effective on September 18, 2008, employers must establish a comprehensive management system to protect the rights of their employees, including a system governing occupational health and safety to provide employees with occupational training to prevent occupational injury. Employers are also required to truthfully inform prospective employees of the job description, working conditions, location, occupational hazards and status of safe production as well as remuneration and other conditions as requested by the PRC Employment Contract Law.
80
Table of Contents
Pursuant to the Law of Manufacturing Safety of the PRC, effective on November 1, 2002 and amended on August 27, 2009, August 31, 2014 and June 10, 2021, manufacturers must establish a comprehensive management system to ensure manufacturing safety in accordance with applicable laws, regulations, national standards, and industrial standards. Manufacturers not meeting relevant legal requirements are not permitted to commence manufacturing activities.
Pursuant to the Administrative Measures Governing the Production Quality of Pharmaceutical Products effective on March 1, 2011, manufacturers of pharmaceutical products must establish production safety and labor protection measures in connection with the operation of their manufacturing equipment and manufacturing process.
Pursuant to applicable PRC laws, rules and regulations, including the Social Insurance Law, which became effective on July 1, 2011 and amended on December 29, 2018, the Interim Regulations on the Collection and Payment of Social Security Funds, which became effective on January 22, 1999 and amended on March 24, 2019, the Interim Measures concerning the Maternity Insurance of Employees, which become effective on January 1, 1995, and the Regulations on Work-related Injury Insurance, which became effective on January 1, 2004 and was subsequently amended on December 20, 2010, employers must contribute, on behalf of their employees, to a number of social security funds, including funds for basic pension insurance, unemployment insurance, basic medical insurance, work-related injury insurance and maternity insurance. If an employer fails to make social insurance contributions timely and in full, the social insurance collecting authority will order the employer to make up outstanding contributions within the prescribed time period and impose a late payment fee at the rate of 0.05% per day from the date on which the contribution becomes due. If such an employer fails to make the overdue contributions within the time limit, the relevant administrative department may impose a fine equivalent to one to three times the overdue amount.
Regulations Relating to Foreign Exchange Registration of Offshore Investment by PRC Residents
In July 2014, SAFE issued SAFE Circular 37 and its implementation guidelines. Pursuant to SAFE Circular 37 and its implementation guidelines, PRC residents (including PRC institutions and individuals) must register with local branches of SAFE in connection with their direct or indirect offshore investment in an overseas special purpose vehicle, or SPV, directly established or indirectly controlled by PRC residents for the purposes of offshore investment and financing with their legally owned assets or interests in domestic enterprises, or their legally owned offshore assets or interests. PRC residents required to make these registrations are also required to amend their registrations with SAFE when there is a change to the basic information of the SPV, such as changes of a PRC resident individual shareholder, the name or operating period of the SPV, or when there is a significant change to the SPV, such as changes of the PRC individual resident’s increase or decrease of its capital contribution in the SPV, or any share transfer or exchange, merger, division of the SPV. In February 2015, SAFE further promulgated the Circular of the State Administration of Foreign Exchange on Further Simplifying and Improving Policies for the Foreign Exchange Administration of Direct Investment, or the SAFE Circular 13, effective June 2015. SAFE Circular 13 amends SAFE Circular 37 by requiring PRC residents or entities to register with qualified banks rather than the SAFE or its local branch in connection with their establishment or control of an offshore entity established for the purpose of overseas investment or financing. Failure to comply with the registration procedures set forth in these regulations may result in restrictions being imposed on the foreign exchange activities of the relevant onshore company, including the payment of dividends and other distributions to its offshore parent or affiliate, the capital inflow from the offshore entities and settlement of foreign exchange capital, and may also subject relevant onshore company or PRC residents to penalties under PRC foreign exchange administration regulations.
Regulations Relating to Employee Stock Incentive Plan
In February 2012, SAFE promulgated the Notices on Issues Concerning the Foreign Exchange Administration for Domestic Individuals Participating in Stock Incentive Plans of Overseas Publicly Listed Companies, or the Stock Option Rules. In accordance with the Stock Option Rules and relevant rules and regulations, PRC citizens or non-PRC citizens residing in China for a continuous period of not less than one year, who participate in any stock incentive plan of an overseas publicly listed company, subject to a few exceptions, must register with SAFE through a domestic qualified agent, which could be a PRC subsidiary of such overseas listed company, and complete certain procedures. We and our employees who are PRC citizens or who reside in China for a continuous period of not less than one year and who participate in our stock incentive plan will be subject to these regulations. In addition, the SAT has issued circulars concerning employee share options or restricted shares. Under these circulars, employees working in the PRC who exercise share options, or whose restricted shares vest, will be subject to PRC individual income tax, or the IIT. The PRC subsidiaries of an overseas listed company have obligations to file documents related to employee share options or restricted shares with relevant tax authorities and to withhold IIT of these employees related to their share options or restricted shares. If the employees fail to pay, or the PRC subsidiaries fail to withhold, their IIT in accordance with relevant laws, rules and regulations, the PRC subsidiaries may face sanctions imposed by the tax authorities or other PRC government authorities.
81
Table of Contents
Regulations Relating to Dividend Distributions
The principal regulations governing distributions of dividends paid by wholly foreign-owned enterprises include:
| • | Company Law of the PRC (1993), as amended in 1999, 2004, 2005, 2013, and 2018; |
| • | Foreign Investment Law of the PRC; and |
| • | Implementation Rules to the Foreign Investment Law. |
Under these laws and regulations, foreign-invested enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a wholly foreign-owned enterprise in China is required to set aside at least 10% of its after-tax profit (based on PRC accounting standards) each year to its statutory reserve funds until the accumulative amount of such reserves reach 50% of its registered capital. These reserves are not distributable as cash dividends. A foreign-invested enterprise has the discretion to allocate a portion of its after-tax profits to discretionary reserve funds. A PRC company may not distribute any profits until any losses from prior fiscal years have been offset. Profits retained from prior fiscal years may be distributed together with distributable profits from the current fiscal year.
Regulations Relating to Foreign Exchange
The principal regulations governing foreign currency exchange in China are the Foreign Exchange Administration Regulations, most recently amended in August 2008. Under the Foreign Exchange Administration Regulations, payments of current account items, such as profit distributions and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval from SAFE by complying with certain procedural requirements. However, approval from or registration with appropriate government authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses, such as the repayment of foreign currency-denominated loans.
In August 2008, SAFE issued the Circular on the Relevant Operating Issues Concerning the Improvement of the Administration of the Payment and Settlement of Foreign Currency Capital of Foreign-Invested Enterprises, or SAFE Circular No. 142, regulating the conversion by a foreign-invested enterprise of foreign currency-registered capital into RMB by restricting how the converted RMB may be used. SAFE Circular No. 142 provides that RMB capital converted from foreign currency registered capital of a foreign-invested enterprise may only be used for purposes within the enterprise’s business scope approved by the applicable government authority and may not be used for equity investments within China. SAFE also strengthened its oversight of the flow and use of Renminbi capital converted from foreign currency registered capital of foreign-invested enterprises. The use of such Renminbi capital may not be changed without SAFE’s approval, and such Renminbi capital may not in any case be used to repay Renminbi loans if the proceeds of such loans have not been used. In March 2015, SAFE issued SAFE Circular No. 19, which took effective and replaced SAFE Circular No. 142 on June 1, 2015. Although SAFE Circular No. 19 allows for the use of Renminbi converted from the foreign currency-denominated capital for equity investments in China, the restrictions continue to apply as to foreign-invested enterprises’ use of the converted Renminbi for purposes beyond the business scope, for entrusted loans or for inter-company Renminbi loans. SAFE promulgated the Notice of the SAFE on Reforming and Standardizing the Foreign Exchange Settlement Management Policy of Capital Account, or Circular 16, effective on June 9, 2016, which reiterates some of the rules set forth in Circular 19, but changes the prohibition against using Renminbi capital converted from foreign currency-denominated registered capital of a foreign-invested company to issue Renminbi entrusted loans to a prohibition against using such capital to issue loans to non-associated enterprises. Violations of SAFE Circular 19 or Circular 16 could result in administrative penalties.
In November 2012, SAFE promulgated the Circular of Further Improving and Adjusting Foreign Exchange Administration Policies on Foreign Direct Investment, which substantially amended and simplified foreign exchange procedures. Pursuant to this circular, the opening of various special purpose foreign exchange accounts (e.g., pre-establishment expenses accounts, foreign exchange capital accounts and guarantee accounts), the reinvestment of lawful incomes derived by foreign investors in China (e.g. profit, proceeds of equity transfer, capital reduction, liquidation and early repatriation of investment), and purchase and remittance of foreign exchange as a result of capital reduction, liquidation, early repatriation or share transfer in a foreign-invested enterprise no longer require SAFE approval, and multiple capital accounts for the same entity may be opened in different provinces, which was not previously permitted. In addition, SAFE promulgated the Circular on Printing and Distributing the Provisions on Foreign Exchange Administration over Domestic Direct Investment by Foreign Investors and the Supporting Documents in May 2013 and amended in October 2018, which specifies that the administration by SAFE or its local branches over direct investment by foreign investors in the PRC shall be conducted by way of registration and banks shall process foreign exchange business relating to the direct investment in China based on the registration information provided by SAFE and its branches.
Furthermore, SAFE Circular No. 13 delegates the authority to enforce the foreign exchange registration in connection with the inbound and outbound direct investment under relevant SAFE rules to certain banks and therefore further simplifies the foreign exchange registration procedures for inbound and outbound direct investment.
82
Table of Contents
Regulations on Enterprise Income Tax
Pursuant to the EIT Law effective as of January 2008 and as last amended in December 2018, the income tax rate for both domestic and foreign- invested enterprises is 25% with certain exceptions. To clarify certain provisions in the EIT Law, the State Council promulgated the Implementation Rules of the EIT Law in December 2007, which became effective in January 2008 and as amended in April 2019. Under the EIT Law and the Implementation Rules of the EIT Law, enterprises are classified as either “resident enterprises” or “non-resident enterprises.” Besides enterprises established within the PRC, enterprises established outside of China whose “de facto management bodies” are located in China are considered “resident enterprises” and are subject to the uniform 25% enterprise income tax rate for their global income. In addition, the EIT Law provides that a non-resident enterprise refers to an entity established under foreign law whose “de facto management body” is not within the PRC, but has an establishment or place of business in the PRC, or does not have an establishment or place of business in the PRC but has income sourced within the PRC.
The Implementation Rules of the EIT Law provide that since January 2008, an income tax rate of 10% will normally be applicable to dividends declared to non-PRC resident enterprise investors that do not have an establishment or place of business in the PRC, or have such establishment or place of business but the relevant income is not effectively connected with the establishment or place of business, to the extent such dividends are derived from sources within the PRC. The income tax on dividends may be reduced pursuant to a tax treaty between China and the jurisdictions in which the non-PRC shareholders reside.
Other PRC National- and Provincial-Level Laws and Regulations
We are subject to changing regulations under many other laws and regulations administered by governmental authorities at the national, provincial and municipal levels, some of which are or may become applicable to our business. These laws and regulations governing both the disclosure and the use of confidential patient medical information may become more restrictive.
We also comply with numerous additional national and provincial laws relating to matters such as safe working conditions, manufacturing practices, environmental protection and fire hazard control in all material aspects. We believe that we are currently in compliance with these laws and regulations; however, we may be required to incur significant costs to comply with these laws and regulations in the future. Unanticipated changes in existing regulatory requirements or adoption of new requirements could therefore have a material adverse effect on our business, results of operations and financial condition.
C. Organizational Structure
The chart below sets forth our corporate structure and identifies our principal subsidiaries as of the date of this annual report:
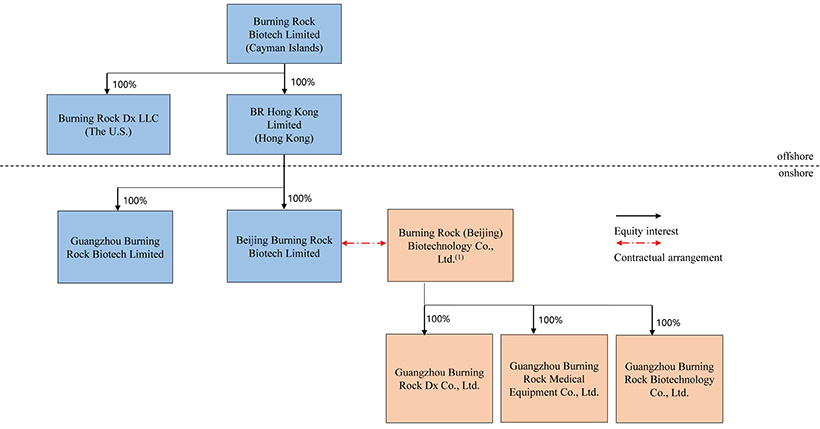
83
Table of Contents
| (1) | Shareholders of Burning Rock (Beijing) Biotechnology Co., Ltd., the VIE, include (i) Mr. Yusheng Han, our founder, chairman of the board of directors and chief executive officer, who holds 45.9% of the equity interests in the VIE, (ii) Mr. Xia Nan, an affiliate of Northern Light Venture Capital III, Ltd., who holds 18.1% of the equity interests in the VIE, (iii) Mr. Gang Lu, our director, and Mr. Jin Zhao, our former director, who hold 7.1% and 8.8% of the equity interests in the VIE, respectively, (iv) Growth No. 12 Investment (Shenzhen) Partnership (Limited Partnership), an affiliate of a principal shareholder, which holds 6.0% of the equity interests in the VIE, and (v) seven minority shareholders, who in aggregate hold 14.1% of the equity interests in the VIE, including Dr. Shaokun (Shannon) Chuai, our former chief scientific officer. |
Contractual Arrangements
Investment in China by foreign investors is subject to certain restriction under PRC laws and regulations, in particular, the Catalog of Industries for Encouraging Foreign Investment, and the Special Administrative Measures for Access of Foreign Investment (2021 Edition), or the Negative List. Industries not listed in the Negative List are generally permitted and open to foreign investment, unless specifically prohibited or restricted by the PRC laws and regulations. While foreign investors are given access to the medical device industry according to Negative list, foreign ownership is prohibited in businesses involving the development and application of genomic diagnosis and treatment technology. We are a company incorporated in the Cayman Islands, and, as a result, our subsidiaries in China are considered foreign-owned enterprises. To comply with the PRC laws and regulations described above, we primarily conduct our business in China through the VIE and its subsidiaries in China, based on a series of contractual arrangements among the VIE, its shareholders and our WFOE.
Agreement that Allows Us to Receive Economic Benefits from the VIE
Exclusive Business Cooperation Agreement
Pursuant to the exclusive business cooperation agreement, as amended and restated on October 21, 2019, which was entered into between the WFOE and the VIE, WFOE or its designated party has the exclusive right to provide the VIE with business support, technology service, consulting service and other services. In exchange for these services, the VIE will pay a service fee, equal to the VIE’s profit before tax, after recovering any accumulated losses of the VIE and its subsidiaries from the preceding fiscal year, and deducting working capital, expenses, tax and a reasonable amount of operating profit according to applicable tax law principles and tax practice. Without the prior written consent of the WFOE, the VIE may not accept any services covered by this agreement from any third party, and may not cooperate with any third party in respect of the same. The WFOE will exclusively own the proprietary rights, ownership, interests and intellectual property rights produced or created in connection with the performance of this agreement. Unless terminated by the WFOE, this agreement will remain effective for ten years. The WFOE may at its sole discretion unilaterally extend the term of this agreement prior to its expiration upon notice to the VIE.
Agreement that Provides Us with Options to Purchase the Equity Interests in and Assets of the
VIE Exclusive Option Agreement
Pursuant to the exclusive option agreement, as amended and restated on October 21, 2019, which was entered into among the WFOE, the VIE and its shareholders, the shareholders of the VIE have irrevocably and unconditionally granted the WFOE or its designated party an exclusive option, where permitted by the PRC law, to purchase all or any portion of their respective equity interests in the VIE. The purchase price for any equity interest upon exercise of this option will be calculated as then registered capital of the VIE multiplied by the percentage of such equity interest in proportion to the total equity of the VIE. However, if applicable PRC law contains compulsory requirement regarding transfer of equity interest, the WFOE or any third party designated by the WFOE is entitled to pay the lowest price permitted by the PRC law as purchase price. In addition, pursuant to this agreement, the VIE has irrevocably and unconditionally granted the WFOE or its designated party an exclusive option, where permitted by applicable PRC law, to purchase all or any portion of its assets. The purchase price upon exercise of this option will be the higher of (i) the net book value of the assets to be purchased or (ii) the lowest price permitted by applicable PRC law.
Without the prior written consent of the WFOE, the shareholders of the VIE may not, in any manner, supplement, modify or amend the articles of associations and by-laws of the VIE; increase or reduce its registered capital or change the structure of registered capital in other manners; sell, transfer, pledge or dispose of its assets, legal or beneficial interests in business or revenue or allow any encumbrance on the same; assume, inherit, guarantee any debt, or allow the existence of any debt, except for debts incurred in the ordinary course of business and debts known and agreed in writing by the WFOE; cause the VIE to enter into any material contract outside the ordinary course of business; cause the VIE to provide loans, credits or guarantees in any form to any other persons; cause or permit the VIE to merge, consolidate with, acquire or invest in any other persons, or acquired or invested by any other persons; cause the VIE to liquidate, dissolve or de-registrate; request the VIE to distribute dividends to its shareholders, or propose or vote in favor of any shareholders’ resolution for such distribution of dividends. This agreement will remain effective until all equity interests in the VIE held by its shareholders has been transferred to the WFOE or its designated party in accordance with provisions of this agreement. The WFOE may at its sole discretion unilaterally terminate this agreement prior to its expiration upon notice to the VIE.
84
Table of Contents
Agreements that Provide Us with Effective Control over the VIE
Equity Interest Pledge Agreement
Pursuant to the equity interest pledge agreement, as amended and restated on October 21, 2019, which was entered into among WFOE, the VIE and its shareholders, each shareholder of the VIE has pledged all of its respective equity interests in the VIE to the WFOE to guarantee the performance of the VIE and its shareholders of their respective obligations under the exclusive business cooperation agreement, the exclusive option agreement, the agreement for power of attorney as well as their respective liabilities arising from any breach of any obligation thereunder. If the VIE or any of its shareholders breaches any obligation under these agreements, the WFOE, as pledgee, may dispose of the pledged equity interest and have priority to be compensated by the proceeds from the disposal of such equity. Each of the shareholders of the VIE agrees that before its obligations under these agreements are discharged and the amounts payable under these agreements are fully paid, it will not dispose of the pledged equity interest, create or allow any encumbrance on the pledged equity interest without the prior written consent of the WFOE. The equity interest pledge agreement will remain effective until the VIE and its shareholders have discharged all their obligations and fully paid all the amounts payable under these agreements. We completed the registration of the pledge of equity interest with the relevant office of the State Administration for Market Regulation on November 25, 2019 in accordance with applicable PRC law and regulations.
Agreement for Power of Attorney
Pursuant to the agreement for power of attorney, as amended and restated on October 21, 2019, which was entered into among the WFOE, the VIE and its shareholders, each shareholder of the VIE irrevocably authorizes the WFOE or its designated person to act as the attorney-in-fact to exercise all such shareholder’s voting and other rights associated with the shareholder’s equity interests in the VIE, such as the right to appoint or remove directors, supervisors and officers, as well as the right to sell, transfer, pledge or dispose of all or any portion of the equity interests held by such shareholder, or of the assets held by the VIE. The parties have agreed that the WFOE is entitled to unilaterally amend, modify or supplement this agreement for power of attorney and the other parties will cooperate where there is a request in respect of the same by the WFOE. This agreement for power of attorney will remain effective until it is terminated by the WFOE.
Spousal Consent Letters
The spouses of Yusheng Han, Gang Lu, Zhigang Wu, Dan Zhou, Peijing Si, Dong Yin and Jin Zhao each signed a spousal consent letter on October 21, 2019. Under these letters, each signing spouse has agreed that he or she is aware of the equity interests beneficially owned by his or her spouse in the VIE and the relevant contractual arrangements in connection with such equity interests. Each signing spouse has unconditionally and irrevocably confirmed that he or she does not have any equity interest in the VIE and will not take any action that may interfere with the contractual arrangement including any claims in respect of the equity interests held by his or her spouse. Each signing spouse has further confirmed that in any event he or she is conferred with any equity interest, he or she is willing to be bound by the relevant contractual arrangements unconditionally as if being a party thereof, and undertakes to take all necessary measures for the performance of those arrangements.
Financial Support Undertaking Letter
Pursuant to the financial support undertaking letter addressed to the VIE, dated October 21, 2019, we undertake to provide unlimited financial support to the VIE to the extent permissible under the applicable PRC laws and regulations, regardless of whether the VIE has incurred an operational loss. The form of financial support includes but is not limited to cash, entrusted loans and borrowings. We will not request repayment of any outstanding loans or borrowings from the VIE if it or its shareholders do not have sufficient funds or are unable to repay such loans or borrowings. The letter is effective until the earlier of (i) the date on which all of the equity interests of the VIE have been acquired by us or our designee, and (ii) the date on which we, in our sole and absolute discretion, unilaterally terminates the applicable financial support undertaking letter.
Voting Proxy Agreement
Pursuant to the voting proxy agreement entered into between our company and our WFOE, dated October 21, 2019, our WFOE irrevocably and unconditionally undertakes to exercise its rights under the agreement for power of attorney, as amended and restated on October 21, 2019, by and among our WFOE, the VIE and its shareholders, in accordance with our company’s instruction.
In the opinion of Tian Yuan Law Firm, our PRC counsel:
| • | the ownership structure of the VIE and our WFOE in China currently does not violate any applicable PRC laws or regulations currently in effect; and |
| • | the contractual arrangements among our WFOE, VIE and the shareholders of the VIE governed by PRC law are valid, binding and enforceable in accordance with their terms and applicable PRC laws or regulations currently in effect and currently do not and will not violate any applicable PRC laws or regulations currently in effect. |
85
Table of Contents
However, there are substantial uncertainties regarding the interpretation and application of current and future PRC laws, regulations and rules. Accordingly, the PRC regulatory authorities may in the future take a view that is contrary to or otherwise different from the above opinion of our PRC legal counsel. See “Item 3. Key Information—D. Risk Factors—Risks Relating to Our Corporate Structure—If the PRC government finds that the agreements that establish the structure for operating our businesses in China do not comply with applicable PRC laws and regulations, or if these regulations or their interpretations change, we could be subject to severe penalties or be forced to relinquish our interests in those operations” and “Item 3. Key Information—D. Risk Factors—Risks Relating to Doing Business in the PRC—Uncertainties in the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and us.” for more details.
D. Property, Plants and Equipment
Our corporate headquarters, central laboratory and manufacturing facilities are primarily located in Guangzhou, China. We also have a research and development center in Shanghai and offices in Beijing. These facilitates have an aggregate of over 30,000 square meters. We currently lease all of our facilities. We believe that we will be able to obtain adequate facilities, principally through leasing, to accommodate our future expansion.
ITEM 4A. UNSOLVED STAFF COMMENTS
None.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
A. Operating Results
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our consolidated financial statements and the related notes included elsewhere in this annual report on Form 20-F. This discussion may contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Item 3. Key Information—D. Risk Factors” or in other parts of this annual report on Form 20-F.
Overview
We aim to transform precision oncology and early cancer detection. We are China’s leading NGS-based cancer therapy selection company. Our cancer therapy selection platform is built upon our advanced proprietary technologies, comprehensive portfolio of products and a two-pronged market- driven commercial infrastructure addressing both larger hospitals through our in-hospital model and smaller hospitals through our central laboratory model.
We primarily offer cancer therapy selection tests under our central laboratory model, where our central laboratory processes cancer patients’ tissue and liquid biopsy samples delivered to us from hospitals across China and issues test reports. In 2021, 2022 and 2023, 28,199, 27,353 and 20,129 patients took our tests, respectively. In 2021, 2022 and 2023, revenue from sale of cancer therapy selection tests under our central laboratory model contributed 62.9%, 55.9% and 43.3% of our total revenues, respectively.
In 2016, we became China’s first NGS-based cancer therapy selection company to offer an in-hospital model, providing turn-key solutions to address Chinese hospitals’ challenges in adopting NGS-based cancer therapy selection. Under this model, we have partnered with 87 hospitals to establish in-hospital laboratories, enabling our partner hospitals to perform NGS-based cancer therapy selection on their own using our reagent kits. In 2021, 2022 and 2023, revenue from fees we received for facilitating the hospitals’ purchases of laboratory equipment and sales of reagent kits and laboratory equipment under the in-hospital model contributed 32.5%, 31.1% and 35.1% of our total revenues, respectively.
We also generate a small portion of revenue from pharma research and development services we provide to pharmaceutical companies and hospitals, which contributed 4.6%, 13.0% and 21.6% of our total revenues in 2021, 2022 and 2023, respectively.
Our revenue increased by 10.9% from RMB507.9 million in 2021 to RMB563.2 million in 2022. Our revenue underwent a 4.6% decline from RMB563.2 million to RMB537.4 million (US$75.7 million) in 2023. Similarly, our gross profit increased by 4.4% from RMB364.1 million in 2021 to RMB380.0 million in 2022, followed by a decrease by 4.4% from RMB380.0 million to RMB363.2 million (US$ 51.2 million) in 2023. Our gross profit margin was 71.7%, 67.5% and 67.6% in 2021, 2022 and 2023, respectively. We incurred net loss of RMB796.7 million, RMB971.2 million and RMB653.7 million (US$92.1 million) in 2021, 2022 and 2023, respectively.
86
Table of Contents
Key Factors Affecting Our Results of Operations
We believe there are several important factors that have impacted and that we expect will continue to impact our operating performance and results of operations, including:
| • | market adoption of our cancer therapy selection products and services; |
| • | testing volume and hospital coverage under our central laboratory model; |
| • | success of our in-hospital model; and |
| • | our ability to successfully develop early cancer detection products. |
Market Adoption of Our Cancer Therapy Selection Products and Services
We currently derive substantially all of our revenues from the sale of our therapy selection tests. We expect our continued growth and business prospects to depend significantly on our ability to increase market adoption of our cancer therapy selection tests, as well as our ability to increase physician and patient awareness of cancer therapy selection in China in general. Although China’s cancer genotyping industry is expected to continue to grow rapidly, cancer therapy selection companies like us face challenges in raising awareness and adoption of their products and services by physicians, patients, hospitals and others in China’s medical community. Among these challenges are that cancer therapy selection tests can be prohibitively expensive and the interpretation of testing results can be time consuming and require knowledge and skills that are not yet widely available in China. We have approached these challenges by building and continually advancing a robust technology platform that we believe will allow us to address many of these challenges.
To increase the market awareness and adoption of our cancer therapy selection tests, we conduct marketing activities to educate hospitals, physicians and pharmaceutical companies on the benefits of our cancer therapy selection products and services. We also participate in research studies and clinical trials in cooperation with oncology key opinion leaders and pharmaceutical companies that validate our cancer therapy selection tests and technologies.
Testing Volume and Hospital Coverage under Our Central Laboratory Model
Our revenue and results of operations are primarily dependent on testing volume and hospital coverage under our central laboratory model. In 2021, 2022 and 2023, revenue from sale of cancer therapy selection tests under our central laboratory model contributed 62.9%, 55.9% and 43.3% of our total revenues, respectively. We expect the central laboratory model to continue to contribute a significant portion of our revenue going forward. As such, our results of operations are affected, and will continue to be affected, by the volume of testing and hospital coverage under our central laboratory model. In 2021, 2022 and 2023, 28,199, 27,353 and 20,129 patients took our tests, respectively. To generate sufficient volumes of demand for our central laboratory business, we will need to maintain and continue to develop relationships with hospitals and physicians. We may need to hire additional sales and marketing staff to support our growth.
Success of Our In-hospital Model
Since 2016, we have been actively expanding our cancer therapy selection business under the in-hospital model, where we offer Chinese hospitals a turn-key solution that allows them to perform cancer therapy selection tests using our products in in-hospital laboratories that we help them establish.
The in-hospital segment is expected to become an increasingly important segment of China’s NGS-based cancer therapy selection market. Although there are substantial challenges in getting hospitals to adopt the in-hospital model, once the in-hospital laboratories, equipment and systems are in place, we sell them our reagent kits on a recurring basis, creating high barrier to entry and high customer loyalty.
Despite the large and rapidly growing demand and higher customer loyalty, establishing in-hospital laboratories usually involves long ramp-up periods—from laboratory design, tender, laboratory equipment sourcing and system installation to ongoing training and support. Accordingly, our in-hospital model requires significant upfront investment, which in turn may affect our short-term results of operations. In addition, revenue from this model depends on our partner hospitals’ clinical needs and budgets for cancer therapy selection products and services, which are beyond our control.
Our Ability to Successfully Develop Early Cancer Detection Products
Investing in the research and development of new products is critical to our long-term competitiveness. In 2016, we started our research and development on the use of targeted DNA methylation in early cancer detection. Developing early cancer detection product candidates requires a significant investment of resources over a prolonged period of time, and we expect to continue to make sustained investment in this area.
87
Table of Contents
Key Components of Results of Operations
Revenues
Our revenues consist of revenues from services and revenues from sales of products, and are derived from three sources: (i) central laboratory business; (ii) in-hospital business; and (iii) pharma research and development services. The table below sets forth a breakdown of our revenues in absolute amount and as a percentage of our total revenues for the periods indicated:
| Year ended December 31, 2021 | ||||||||||||||||||||||||||||||||
| Central laboratory business | In-hospital business | Pharma research and development services | Total revenues | |||||||||||||||||||||||||||||
| RMB | % of total revenues | RMB | % of total revenues | RMB | % of total revenues | RMB | % of total revenues | |||||||||||||||||||||||||
| (in thousands, except for%) | ||||||||||||||||||||||||||||||||
Revenues from services | 319,353 | 69.2 | (281 | ) | (0.1 | ) | 23,393 | 4.6 | 342,465 | 67.4 | ||||||||||||||||||||||
Revenues from sales of products | — | — | 165,397 | 32.6 | — | — | 165,397 | 32.6 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| 319,353 | 69.2 | 165,116 | 32.5 | 23,393 | 4.6 | 507,862 | 100.0 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| Year ended December 31, 2022 | ||||||||||||||||||||||||||||||||
| Central laboratory business | In-hospital business | Pharma research and development services | Total revenues | |||||||||||||||||||||||||||||
| RMB | % of total revenues | RMB | % of total revenues | RMB | % of total revenues | RMB | % of total revenues | |||||||||||||||||||||||||
| (in thousands, except for%) | ||||||||||||||||||||||||||||||||
Revenues from services | 314,770 | 55.9 | 3,606 | 0.6 | 73,172 | 13.0 | 391,548 | 69.5 | ||||||||||||||||||||||||
Revenues from sales of products | — | — | 171,690 | 30.5 | — | — | 171,690 | 30.5 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| 314,770 | 55.9 | 175,296 | 31.1 | 73,172 | 13.0 | 563,238 | 100.0 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| Year ended December 31, 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Central laboratory business | In-hospital business | Pharma research and development services | Total revenues | |||||||||||||||||||||||||||||||||||||||||||||
| RMB | US$ | % of total revenues | RMB | US$ | % of total revenues | RMB | US$ | % of total revenues | RMB | US$ | % of total revenues | |||||||||||||||||||||||||||||||||||||
| (in thousands, except for%) | ||||||||||||||||||||||||||||||||||||||||||||||||
Revenues from services | 232,812 | 32,791 | 43.3 | (3,704 | ) | (522 | ) | (0.7 | ) | 115,922 | 16,327 | 21.6 | 345,030 | 48,596 | 64.2 | |||||||||||||||||||||||||||||||||
Revenues from sales of products | — | — | — | 192,405 | 27,100 | 35.8 | — | — | — | 192,405 | 27,100 | 35.8 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
| 232,812 | 32,791 | 43.3 | 188,701 | 26,578 | 35.1 | 115,922 | 16,327 | 21.6 | 537,435 | 75,696 | 100.0 | |||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Central laboratory business
Central laboratory business revenue is generated from sales of our cancer therapy selection tests to individual patients. Patients pay us for these tests with out-of-pocket payments after their physicians have ordered our tests. We recognize revenue upon the delivery of test reports to the individual patients.
In-hospital business
Under our in-hospital business, we (i) in some instances facilitate the hospitals’ procurement of laboratory equipment required to set up their in-hospital laboratories, for which we charge a fee, and (ii) sell our reagent kits to hospitals for them to perform cancer therapy selection testing in the in-hospital laboratories we helped them establish. Revenues from fees we receive for facilitating laboratory equipment purchases are recorded on a net basis when we have completed our facilitation services. Revenues from reagent kit sales are recorded on a gross basis when the reagent kits are delivered to hospitals.
Pharma research and development services
We provide pharmaceutical research and development services to international and domestic pharmaceutical companies primarily in relation to the development of targeted therapies and immunotherapies for various types of cancer, and to hospitals for their studies on cancer diagnosis and treatment. We also provide companion diagnostics development service to pharmaceutical companies.
88
Table of Contents
Cost of Revenues
Our cost of revenues consists of cost of services and cost of goods sold and are incurred from three sources: (i) the cost of revenues for our central laboratory business, which primarily includes cost of laboratory consumables used in cancer therapy selection testing, the manufacturing cost of our reagent kits, personnel cost and depreciation and amortization, (ii) the cost of revenues for our in-hospital business, which primarily includes the cost of materials, manufacturing costs of our reagent kits and personnel cost, and (iii) the cost of revenues for pharma research and development services, which primarily includes costs of laboratory consumables used in pharma research and development services. The following table sets forth a breakdown of our cost of revenues for the periods indicated.
| Year ended December 31, | ||||||||||||||||
| 2021 | 2022 | 2023 | ||||||||||||||
| RMB | RMB | RMB | US$ | |||||||||||||
| (in thousands) | ||||||||||||||||
Cost of revenues: | ||||||||||||||||
Central laboratory business | 81,088 | 82,123 | 49,473 | 6,968 | ||||||||||||
In-hospital business | 50,315 | 63,296 | 72,570 | 10,221 | ||||||||||||
Pharma research and development services | 12,313 | 37,780 | 52,165 | 7,348 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total cost of revenues | 143,716 | 183,199 | 174,208 | 24,537 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating Expenses
Our operating expenses include research and development expenses, selling and marketing expenses and general and administrative expenses. The following table sets forth a breakdown of these expenses for the periods indicated.
| Year ended December 31, | ||||||||||||||||
| 2021 | 2022 | 2023 | ||||||||||||||
| RMB | RMB | RMB | US$ | |||||||||||||
| (in thousands) | ||||||||||||||||
Operating expenses: | ||||||||||||||||
Research and development expenses | 367,858 | 421,868 | 347,016 | 48,876 | ||||||||||||
Selling and marketing expenses | 303,096 | 370,294 | 247,711 | 34,889 | ||||||||||||
General and administrative expenses | 490,256 | 568,284 | 437,821 | 61,666 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating expenses | 1,161,210 | 1,360,446 | 1,032,548 | 145,431 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Research and Development Expenses
Our research and development expenses primarily consist of (i) expenses incurred for clinical and non-clinical activities performed by third-party contract research organizations, and (ii) salaries and benefits for research and development personnel and the cost of materials for our research and development projects and products. We expect that our research and development expenses will increase as we continue to invest in the research and development of our early cancer detection and cancer therapy selection products and technologies.
Selling and Marketing Expenses
Our selling and marketing expenses primarily consist of staff costs for personnel engaged in sales and marketing functions, travel and entertainment expenses and conference expenses. Base salary of our sales and marketing personnel represents a very significant portion of staff costs, with the remainder being performance-based bonuses for these personnel. We expect that our selling and marketing expenses will increase as we continue to expand our sales and marketing teams and engage in sales and marketing activities to increase the adoption and market awareness of our products.
General and Administrative Expenses
Our general and administrative expenses primarily consist of staff costs for personnel engaged in general and administrative functions, professional service fees, depreciation and amortization and travel and office expenses. We expect our general and administrative expenses to continue increasing to support our business growth, but we expect that they will eventually decrease as a percentage of our revenues as we achieve increased economies of scale.
89
Table of Contents
Taxation
Cayman Islands
We are an exempted company incorporated in the Cayman Islands. The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation, and there is currently no estate duty, inheritance tax or gift tax. There are no other taxes likely to be material to us levied by the government of the Cayman Islands except for stamp duties that may be applicable on instruments executed in, or after execution brought within, the jurisdiction of the Cayman Islands. In addition, the Cayman Islands does not impose withholding tax on dividend payments.
Hong Kong
Before April 1, 2018, our subsidiary incorporated in Hong Kong was subject to Hong Kong profit tax at a rate of 16.5%. Since April 1, 2018, our subsidiary incorporated in Hong Kong has been subject to Hong Kong profit tax at a rate of 8.25% on assessable profits up to HK$2,000,000 and 16.5% on any part of assessable profits over HK$2,000,000. Hong Kong has an anti-fragmentation measure under which a corporate group must nominate only one company in the group to benefit from the progressive rates. No Hong Kong profit tax has been levied on us as we did not have assessable profit that was earned in or derived from our Hong Kong subsidiary in 2021, 2022 or 2023. Hong Kong does not impose a withholding tax on dividends.
China
For our operations in the PRC, we are subject to a general PRC enterprise income tax rate of 25%. Guangzhou Burning Rock Dx Co., Ltd., a subsidiary of the VIE, has been qualified as a high and new technology enterprise, or HNTE, since November 2016, and accordingly is entitled to a reduced income tax rate of 15%.
Dividends paid by our wholly foreign-owned subsidiaries in China to our intermediary holding company in Hong Kong will be subject to a withholding tax rate of 10%, unless they qualify for an exemption. If our intermediary holding company in Hong Kong satisfies all the requirements under the Arrangement between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income and receives approval from the relevant tax authority, then dividends paid to it by our wholly foreign-owned subsidiaries in China will be subject to a withholding tax rate of 5% instead. Effective from November 1, 2015, the above- mentioned approval requirement has been abolished, but a Hong Kong entity is still required to file an application package with the relevant tax authority, and settle the overdue taxes if the preferential 5% tax rate is denied based on the subsequent review of the application package by the relevant tax authority.
If our holding company in the Cayman Islands or any of our subsidiaries outside of China is deemed to be a “resident enterprise” under the PRC Enterprise Income Tax Law, it will be subject to enterprise income tax on its worldwide income at a rate of 25%.
Pursuant to applicable PRC laws and regulations, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities. We may be subject to adverse tax consequences and our consolidated results of operations may be adversely affected if the PRC tax authorities determine that the contractual arrangements among our PRC subsidiaries and their shareholders are not on an arm’s length basis and constitute favorable transfer pricing.
Critical Accounting Polices, Judgments and Estimates
An accounting policy is considered critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time such estimate is made, and if different accounting estimates that reasonably could have been used, or changes in the accounting estimates that are reasonably likely to occur periodically, could materially impact the consolidated financial statements.
We prepare our consolidated financial statements in accordance with U.S. GAAP. The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the balance sheet dates and the reported amounts of revenues and expenses during the reporting periods. Our accounting policies are more fully described in Note 2 of the consolidated financial statements.
We base our estimates on historical experience and various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results could materially differ from those estimates. We believe the following discussion addresses our most critical accounting policies, which are those that are most important to the portrayal of our financial condition and results of operations and require management’s most difficult, subjective and complex judgments.
90
Table of Contents
The following descriptions of critical accounting policies, judgments and estimates should be read in conjunction with our consolidated financial statements and accompanying notes and other disclosures included in this annual report. When reviewing our financial statements, you should consider (i) our selection of critical accounting policies, (ii) the judgments and other uncertainties affecting the application of these policies and (iii) the sensitivity of reported results to changes in conditions and assumptions.
Revenue Recognition
We derive revenue from our central laboratory business, in-hospital business and pharma research and development services. We recognize revenue to depict the transfer of promised products or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those products or services. For businesses that enter primarily short-term contracts, we apply the practical expedient which allows costs to obtain a contract to be expensed when incurred if the amortization period of the assets that would otherwise have been recognized is one year or less.
Revenue from central laboratory business
Revenue from central laboratory business is primarily generated through the sales of our cancer therapy selection test to individual patient customers. Individual patients prepay the consideration in full, and the transaction price for each contract is fixed at contract inception.
Patients can choose to purchase a single cancer therapy selection test, as a package which consists of multiple cancer therapy selection tests of the same type or a combination of different types of cancer therapy selection tests. Each cancer therapy selection test represents a single performance obligation. Revenue is allocated to each performance obligation based on the relative standalone selling price method. We record revenue at a point in time when each cancer therapy selection testing report is delivered to the patient.
We launched cancer therapy selection testing packages (“Monitoring Packages”) in 2017. Each monitoring package contains a fixed number of the same type cancer therapy selection tests which can be used up to two years from purchase date. The portion of the cancer therapy selection tests within the Monitoring Packages, which are not expected to be used by the patient prior to expiration based on historical usage rates, are referred to as a “breakage.” We recognize the expected breakage amount as revenue in proportion to the total number of tests expected to be performed for patients prior to the expiration date. If we are not expected to be entitled to a breakage amount due to the lack of historical experience, the expected breakage amount is recognized as revenue at the end of the two-year period when the monitoring package expires. We evaluate our breakage estimates periodically based upon our historical experience with each type of Monitoring Packages recent usage pattern prior to the expiration period. The historical usage rates may not be reflective of the actual usage rates due to changes in patient behavior and medical advancements. The determination of whether we have accumulated sufficient historical experience to determine breakage amount and changes in the actual patients’ usage rates may significantly impact the amount of breakage revenue recognized for the period. We recognized breakage income of RMB14.2 million, RMB26.4 million and RMB10.9 million (US$1.5 million) for the years ended December 31, 2021, 2022 and 2023, respectively.
Revenue from in-hospital business
Revenue from in-hospital business is primarily generated through reagent kit sales and providing facilitation services for laboratory equipment sold to hospitals. We manufacture the reagent kits and sell to the hospitals when the hospitals submit a purchase order. Each reagent kit represents a single performance obligation. We do not provide rights of return for the reagent kits sold other than returns of defective products. Returns for defective products were not material for the periods presented. The contracts with hospital customers from reagent kit sales of in-hospital business often contain bundles of reagent kits to customers. Each kit represents a single performance obligation. We allocate the transaction price to each kit on a relative standalone selling price basis using the expected cost plus a margin method. We estimate the standalone selling price based on its pricing strategies considering market conditions, entity-specific factors, product mix sold to customers over time and our expected gross margin for different customers. We recognize revenue on the sales of reagent kits at a point in time when the reagent kits are delivered to hospitals customers. For the facilitation services, we purchase the laboratory equipment from third-party suppliers when a hospital submits purchase request and resells the laboratory equipment to the hospital. We act as an agent in facilitating laboratory equipment sales as we do not control the laboratory equipment prior to its delivery to hospitals and do not have inventory risks. The facilitation services for each piece of laboratory equipment represent a single performance obligation. We record revenue on a net basis at the point in time when we have completed our facilitation services.
91
Table of Contents
Revenue from pharma research and development services
We provide pharma research and development services to pharmaceutical companies for developing new targeted therapies and immunotherapies on various types of cancers and to hospitals for their studies on cancer diagnosis and treatment. The pharma research and development services include a range of cancer therapy selection test services, analytical validation services and project management services. We deliver an analysis report upon completion of services. The testing services, analytical validation services and project management services are not distinct within the context of the contract because we are using these services as inputs to produce the analysis report. We recognize services revenue over the period in which these services are provided because we do not create an asset with alternative use to us and we have an enforceable right to payment for the performance completed to date. We recognize revenue using an output method to measure progress, utilizing cancer therapy selection testing performed to-date as our measure of progress.
We also provide companion diagnostics development service to pharmaceutical companies. We recognize revenue using an input method to measure progress for these arrangements. We determined the pattern of revenue recognition over time would most faithfully represent the economic value of the companion diagnostics services being provided based on the external costs incurred over the term of the development and clinical validation phases of the project.
Pharmaceutical companies may also separately engage us to perform multiple cancer therapy selection tests without an analysis of the test results. Each therapy selection test is capable of being distinct and separately identifiable from other promises in the contracts and therefore, represent distinct performance obligations. Revenue is allocated to each cancer therapy selection test using a relative standalone selling price basis. We record revenue at a point in time, when each cancer therapy selection test result is delivered to the pharmaceutical companies and hospitals.
Accounts Receivable, Contract Assets and Allowance for Credit Losses
We recognize contract assets when we satisfy our performance obligations before the customer pays consideration or before payment is due. We transfer contract assets to “accounts receivable” when its right to payment becomes unconditional.
We record the allowance for credit losses as an offset to accounts receivable and contract assets, with estimated credit losses charged to “General and administrative expenses” in the consolidated statements of comprehensive loss. We assess credit loss by reviewing accounts receivable and contract assets on a collective basis where similar characteristics exist, primarily based on similar business segments, service or product offerings and on an individual basis when we identify specific customers with known disputes or collectability issues. We apply a migration roll rate method that considers historical collectability based on past due status, the age of the accounts receivable and contract asset balances, credit quality of our customers based on ongoing credit evaluations, current economic conditions, and reasonable and supportable forecasts of future economic conditions and other factors that may affect our ability to collect from customers. We write off accounts receivable and contract assets are deemed uncollectible when after all collection efforts have ceased.
Fair Value of Share Options
We determined the fair value of share-based payment awards granted without market conditions using the Black-Scholes model and determined the fair value of share-based payment awards granted with market conditions using the Monte Carlo Simulation model. The Black-Scholes models and Monte Carlo Simulation model all require subjective assumptions, including the grant date fair value of the ordinary shares, expected volatility, the exercise multiple, the risk-free rate and the dividend yield. We used the grant date closing ADS price quoted on NASDAQ exchange to determine the fair value of our ordinary shares. For expected volatility, we have referenced historical volatility of several comparable companies in the same industry. The exercise multiple was estimated as the average ratio of the stock price to the exercise price of when employees would decide to voluntarily exercise their vested options. The risk-free rate for periods within the contractual life of the options is based on the market yield of U.S. Treasury Bonds in effect at the time of grant. The dividend yield is based on our expected dividend policy over the contractual life of the options.
The assumptions used to estimate the fair value of the share options granted are as follows:
| For the year ended December 31, | ||||||
| 2021 | 2022 | 2023 | ||||
Risk-free interest rate | 0.97%–2.07% | 1.52%–3.94% | 3.48%–4.61% | |||
Dividend yield | 0% | 0% | 0% | |||
Expected volatility range | 47.67%–72.98% | 48.20%–56.00% | 47.90%–50.69% | |||
Exercise multiple | N/A | 2.80 | N/A | |||
Contractual life | 10 years | 10 years | 10 years | |||
Fair market value per ordinary share as at valuation dates | US$9.53– US$30.50 | US$0.013– US$9.29 | US$0.93– US$3.11 | |||
92
Table of Contents
These assumptions represented our best estimates, but the estimates involve inherent uncertainties and the application of our judgment. As a result, if we use significantly different assumptions or estimates when valuing our options, our share-based compensation expense could be materially different.
Recent accounting pronouncements
A list of recent relevant accounting pronouncements is included in Note 2 “Summary of Significant Accounting Policies” to our consolidated financial statements included elsewhere in this annual report.
Results of Operations
The following table sets forth our results of operations for the periods indicated:
| Year ended December 31, | ||||||||||||||||||||||||||||
| 2021 | 2022 | 2023 | ||||||||||||||||||||||||||
| RMB | % of total revenues | RMB | % of total revenues | RMB | US$ | % of total revenues | ||||||||||||||||||||||
| (in thousands, except for%) | ||||||||||||||||||||||||||||
Revenues: | ||||||||||||||||||||||||||||
Revenues from services | 342,465 | 67.4 | 391,548 | 69.5 | 345,030 | 48,596 | 64.2 | |||||||||||||||||||||
Revenues from sales of products | 165,397 | 32.6 | 171,690 | 30.5 | 192,405 | 27,100 | 35.8 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total revenues | 507,862 | 100.0 | 563,238 | 100.0 | 537,435 | 75,696 | 100.0 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Cost of revenues(1): | ||||||||||||||||||||||||||||
Cost of services | (93,401 | ) | (18.4 | ) | (119,903 | ) | (21.3 | ) | (101,638 | ) | (14,315 | ) | (18.9 | ) | ||||||||||||||
Cost of goods sold | (50,315 | ) | (9.9 | ) | (63,296 | ) | (11.2 | ) | (72,570 | ) | (10,222 | ) | (13.5 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total cost of revenues | (143,716 | ) | (28.3 | ) | (183,199 | ) | (32.5 | ) | (174,208 | ) | (24,537 | ) | (32.4 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Gross profit | 364,146 | 71.7 | 380,039 | 67.5 | 363,227 | 51,159 | 67.6 | |||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||
Research and development expenses(1) | (367,858 | ) | (72.4 | ) | (421,868 | ) | (74.9 | ) | (347,016 | ) | (48,876 | ) | (64.6 | ) | ||||||||||||||
Selling and marketing expenses(1) | (303,096 | ) | (59.7 | ) | (370,294 | ) | (65.7 | ) | (247,711 | ) | (34,889 | ) | (46.1 | ) | ||||||||||||||
General and administrative expenses(1) | (490,256 | ) | (96.5 | ) | (568,284 | ) | (100.9 | ) | (437,821 | ) | (61,666 | ) | (81.5 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total operating expenses | (1,161,210 | ) | (228.6 | ) | (1,360,446 | ) | (241.5 | ) | (1,032,548 | ) | (145,431 | ) | (192.1 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Loss from operations | (797,064 | ) | (156.9 | ) | (980,407 | ) | (174.0 | ) | (669,321 | ) | (94,272 | ) | (124.5 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Interest income, net | 1,921 | 0.4 | 9,458 | 1.7 | 17,956 | 2,529 | 3.3 | |||||||||||||||||||||
Other income, net | 199 | — | 152 | — | 484 | 68 | 0.1 | |||||||||||||||||||||
Foreign exchange (loss) gain, net | (854 | ) | (0.2 | ) | 1,549 | 0.3 | (420 | ) | (59 | ) | (0.1 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Loss before income tax | (795,798 | ) | (156.7 | ) | (969,248 | ) | (172.0 | ) | (651,301 | ) | (91,734 | ) | (121.2 | ) | ||||||||||||||
Income tax expenses | (899 | ) | (0.2 | ) | (1,985 | ) | (0.4 | ) | (2,388 | ) | (336 | ) | (0.4 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Net loss | (796,697 | ) | (156.9 | ) | (971,233 | ) | (172.4 | ) | (653,689 | ) | (92,070 | ) | (121.6 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
| (1) | Share-based compensation expenses were allocated as follows: |
| Year ended December 31, | ||||||||||||||||
| 2021 | 2022 | 2023 | ||||||||||||||
| RMB | RMB | RMB | US$ | |||||||||||||
| (in thousands) | ||||||||||||||||
Cost of revenues | 1,504 | 1,783 | 2,313 | 326 | ||||||||||||
Research and development expenses | 29,637 | 52,873 | 53,474 | 7,532 | ||||||||||||
Selling and marketing expenses | 9,612 | 8,525 | 9,658 | 1,360 | ||||||||||||
General and administrative expenses | 241,680 | 263,603 | 195,274 | 27,504 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | 282,433 | 326,784 | 260,719 | 36,722 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Year Ended December 31, 2023 Compared to Year Ended December 31, 2022
Revenues
Our revenues decreased by 4.6% to RMB537.4 million (US$75.7 million) for 2023 from RMB563.2 million for 2022, primarily due to a decrease in revenues generated from services to RMB345.0 million (US$48.6 million) for 2023 from RMB391.5 million for 2022, offset by an increase in revenues from sales of products to RMB192.4 million (US$27.1 million) for 2023 from RMB171.7 million for 2022. We derived our revenues from three sources:
| • | Central laboratory business. Our revenue generated from central laboratory business decreased by 26.0% to RMB232.8 million (US$32.8 million) for 2023 from RMB314.8 million for 2022. In 2023, 20,129 patients took our tests, compared to 27,353 patients in 2022. |
93
Table of Contents
| • | In-hospital business. Our revenue generated from in-hospital business increased by 7.6% to RMB188.7 million (US$26.6 million) for 2023 from RMB175.3 million for 2022, primarily due to the demand of our services from ten new contracted partner hospitals added to our in-hospital channel in 2023. The number of our contracted partner hospitals increased from 49 as of December 31, 2022 to 59 as of December 31, 2023. |
| • | Pharma research and development services. Our revenue generated from pharma research and development services increased by 58.4% to RMB115.9 million (US$16.3 million) for 2023 from RMB73.2 million for 2022, primarily attributable to increased development and testing services performed for our pharma customers as well as an increasing number of collaborative projects with pharmaceutical companies, where we provided a range of services including experimental design, performance evaluation and related services. |
Cost of Revenues
Our cost of revenues decreased by 4.9% to RMB174.2 million (US$24.5 million) for 2023 from RMB183.2 million for 2022. This decrease was primarily attributable to a decrease in cost of services to RMB101.6 million (US$14.3 million) for 2023 from RMB119.9 million for 2022, and offset by an increase in cost of goods sold to RMB72.6 million (US$10.2 million) for 2023 from RMB63.3 million for 2022.
The decrease in cost of revenues from 2022 to 2023 was primarily due to a decrease in the cost of revenues for our central laboratory business, as we shifted our focus to the in-hospital business.
| • | Central laboratory business. Cost of revenue for central laboratory business was RMB49.5 million (US$7.0 million) for 2023, representing a 39.8% decrease from RMB82.1 million for 2022, primarily due to the decreases in our inventory write-downs and royalty and license fee. |
| • | In-hospital business. Cost of revenue for in-hospital business was RMB72.6 million (US$10.2 million) for 2023, representing a 14.7% increase from RMB63.3 million for 2022, primarily due to the increased selling volume as well as renovation depreciation in relation to our new laboratory. |
| • | Pharma research and development services. Cost of revenue for pharma research and development services was RMB52.2 million (US$7.3 million) for 2023, representing a 38.1% increase from RMB37.8 million for 2022, in line with the revenue growth of this segment. |
Gross Profit and Gross Margin
Our gross profit decreased by 4.4% to RMB363.2 million (US$51.2 million) for 2023 from RMB380.0 million for 2022, primarily due to a decrease of sales volume in the central laboratory business. Our gross margin increased to 67.6% for 2023 from 67.5% for 2022.
The table below sets forth a breakdown of our gross profit and gross profit margin for the periods indicated:
| Year ended December 31, | ||||||||||||||||||||
| 2022 | 2023 | |||||||||||||||||||
| RMB | Gross profit margin (%) | RMB | US$ | Gross profit margin (%) | ||||||||||||||||
| (in thousands, except %) | ||||||||||||||||||||
Central laboratory business | 232,647 | 73.9 | 183,339 | 25,823 | 78.7 | |||||||||||||||
In-hospital business | 112,000 | 63.9 | 116,131 | 16,357 | 61.5 | |||||||||||||||
Pharma research and development services | 35,392 | 48.4 | 63,757 | 8,979 | 55.0 | |||||||||||||||
Total | 380,039 | 67.5 | 363,227 | 51,159 | 67.6 | |||||||||||||||
| • | Central laboratory business. Gross profit for central laboratory business was RMB183.3 million (US$25.8 million) for 2023, representing a 21.2% decrease from RMB232.6 million for 2022, primarily attributable to a decrease of revenue from our monitoring packages. Our gross margin for central laboratory business increased to 78.7% for 2023 from 73.9% for 2022, primarily due to the decreases in inventory write-downs and royalty and license fee. |
| • | In-hospital business. Gross profit for in-hospital business was RMB116.1 million (US$16.4 million) for 2023, representing a 3.7% increase from RMB112.0 million for 2022, primarily due to the volume growth, partially offset by the increased depreciation in relation to our laboratories which was put into use in March and May 2022, respectively. Our gross margin for in-hospital business decreased to 61.5% for 2023 from 63.9% for 2022, primarily due to the increased depreciation in relation to our laboratories. |
| • | Pharma research and development services. Gross profit for pharma research and development services was RMB63.8 million (US$9.0 million) for 2023, representing a 80.1% increase from RMB35.4 million for 2022, primarily due to the increased testing services performed for pharma customers and the increasing number of collaborative projects with pharmaceutical companies. Our gross margin for pharma research and development services increased to 55.0% for 2023 from 48.4% for 2022, primarily due to a higher proportion of projects with higher gross profit margin undertaken in 2023. |
94
Table of Contents
Operating Expenses
Research and development expenses
Our research and development expenses decreased by 17.7% to RMB347.0 million (US$48.9 million) for 2023 from RMB421.9 million for 2022, primarily due to (i) a decrease in the expenditure for detection research, (ii) a decrease in royalty and license fee, and (iii) a decrease in staff cost resulted from the reorganization of our research and development department to improve operating efficiency.
Selling and marketing expenses
Our selling and marketing expenses decreased by 33.1% to RMB247.7 million (US$34.9 million) for 2023 from RMB370.3 million for 2022, primarily due to (i) a decrease in staff cost resulted from the reorganization of our sales department to improve operating efficiency, (ii) a decrease in conference fee and marketing fees, and (iii) a decrease in entertainment fees.
General and administrative expenses
Our general and administrative expenses decreased by 23.0% to RMB437.8 million (US$61.7 million) for 2023 from RMB568.3 million for 2022, primarily due to (i) a decrease in professional service fee, and (ii) a decrease in allowance for doubtful accounts resulted from accelerated settlement with customers with long accounts receivable, and (iii) a decrease in staff cost resulted from the reorganization of our general and administrative department to improve operating efficiency.
Interest Income
Our interest income increased by 89.8% to RMB18.0 million (US$2.5 million) for 2023 from RMB9.5 million for 2022, primarily due to the increase in interest income as a result of the U.S. interest rate hike.
Net Loss
Our net loss decreased by 32.7% to RMB653.7 million (US$92.1 million) for 2023 from RMB971.2 million for 2022, primarily due to (i) a decrease in headcount related expenses, (ii) a decrease in professional service fees, and (iii) a decrease in operating expenses as mentioned above.
Year Ended December 31, 2022 Compared to Year Ended December 31, 2021
Revenues
Our revenues increased by 10.9% to RMB563.2 million for 2022 from RMB507.9 million for 2021, primarily attributable to an increase in revenues generated from services to RMB391.5 million for 2022 from RMB342.5 million for 2021 and to a lesser extent, revenues from sales of products to RMB171.7 million for 2022 from RMB165.4 million for 2021. We derived our revenues from three sources:
| • | Central laboratory business. Our revenue generated from central laboratory business decreased by 1.4% to RMB314.8 million for 2022 from RMB319.4 million for 2021. In 2022, 27,353 patients took our tests, compared to 28,199 patients in 2021. |
| • | In-hospital business. Our revenue generated from in-hospital business increased by 6.2% to RMB175.3 million for 2022 from RMB165.1 million for 2021, primarily attributable to (i) increased demand from existing hospitals and (ii) demand from eight new contracted partner hospitals added to our in-hospital channel in 2022. The number of our contracted partner hospitals increased from 41 as of December 31, 2021 to 49 as of December 31, 2022. |
| • | Pharma research and development services. Our revenue generated from pharma research and development services increased by 212.8% to RMB73.2 million for 2022 from RMB23.4 million for 2021, primarily attributable to increased development and testing services performed for our pharma customers. |
Cost of Revenues
Our cost of revenues increased by 27.5% to RMB183.2 million for 2022 from RMB143.7 million for 2021. This increase was primarily attributable to an increase in cost of services to RMB119.9 million for 2022 from RMB93.4 million for 2021, and to a lesser extent, an increase in cost of goods sold to RMB63.3 million for 2022 from RMB50.3 million for 2021.
95
Table of Contents
The increase in cost of revenues from 2021 to 2022 was primarily due to an increase in the cost of revenues for our in-hospital business and pharma research and development services.
| • | Central laboratory business. Cost of revenue for central laboratory business was RMB82.1 million for 2022, representing a 1.2% increase from RMB81.1 million for 2021, primarily due to the increased depreciation in relation to our new laboratory and inventory write-downs. |
| • | In-hospital business. Cost of revenue for in-hospital business was RMB63.3 million for 2022, representing a 25.8% increase from RMB50.3 million for 2021, primarily due to the increased selling volume as well as rental expense and renovation depreciation in relation to our new laboratory. |
| • | Pharma research and development services. Cost of revenue for pharma research and development services was RMB37.8 million for 2022, representing a 207.3% increase from RMB12.3 million for 2021, which was in line with the revenue growth of this segment. |
Gross Profit and Gross Margin
Our gross profit increased by 4.4% to RMB380.0 million for 2022 from RMB364.1 million for 2021, primarily due to increased testing under our pharma research and development services performed for our pharma customers and growth in companion diagnostics development services. Our gross margin decreased to 67.5% for 2022 from 71.7% for 2021.
The table below sets forth a breakdown of our gross profit and gross profit margin for the periods indicated:
| Year ended December 31, | ||||||||||||||||
| 2021 | 2022 | |||||||||||||||
| RMB | Gross profit margin (%) | RMB | Gross profit margin (%) | |||||||||||||
| (in thousands, except %) | ||||||||||||||||
Central laboratory business | 238,265 | 74.6 | 232,647 | 73.9 | ||||||||||||
In-hospital business | 114,801 | 69.5 | 112,000 | 63.9 | ||||||||||||
Pharma research and development services | 11,080 | 47.4 | 35,392 | 48.4 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | 364,146 | 71.7 | 380,039 | 67.5 | ||||||||||||
| • | Central laboratory business. Gross profit for central laboratory business was RMB232.6 million for 2022, representing a 2.4% decrease from RMB238.3 million for 2021, primarily attributable to the increased depreciation in relation to our new laboratory and inventory write-downs, partially offset by the increased revenue from our monitoring packages. Our gross margin for central laboratory business decreased to 73.9% for 2022 from 74.6% for 2021, primarily due to the same reason. |
| • | In-hospital business. Gross profit for in-hospital business was RMB112.0 million for 2022, representing a 2.4% decrease from RMB114.8 million for 2021, primarily due to the volume growth in Shanghai and Beijing as COVID-19 impact lessened in 2022, partially offset by the increased depreciation in relation to our new laboratory. Our gross margin for in-hospital business decreased to 63.9% for 2022 from 69.5% for 2021, primarily due to increased depreciation in relation to our new laboratory. |
| • | Pharma research and development services. Gross profit for pharma research and development services was RMB35.4 million for 2022, representing a 218.9% increase from RMB11.1 million for 2021, primarily due to the increased testing services performed for pharma customers. Our gross margin for pharma research and development services remained relatively stable at 48.4 % and 47.4% for 2022 and 2021, respectively. |
Operating Expenses
Research and development expenses
Our research and development expenses increased by 14.7% to RMB421.9 million for 2022 from RMB367.9 million for 2021, primarily due to (i) an increase in expenditure for early cancer detection research, (ii) an increase in depreciation expenses for new buildings; and (iii) an increase in amortized expense on share-based compensation.
Selling and marketing expenses
Our selling and marketing expenses increased by 22.2% to RMB370.3 million for 2022 from RMB303.1 million for 2021, primarily due to (i) an increase in staff cost of sales and marketing personnel in anticipation of the commercialization of our early detection products and pharma research and development services; (ii) an increase in conference fee and marketing fees; and (iii) an increase in amortized expense on share- based compensation.
96
Table of Contents
General and administrative expenses
Our general and administrative expenses increased by 15.9% to RMB568.3 million for 2022 from RMB490.3 million for 2021, primarily due to (i) an increase in depreciation expenses for new buildings, and (ii) an increase in allowance for credit loss in relation to accounts receivables; and (iii) an increase in amortized expense on share-based compensation.
Interest Income, Net
Our interest income, net increased by 392.3% to RMB9.5 million for 2022 from RMB1.9 million for 2021, primarily due to the increase in interest income as a result of the U.S. interest rate hike.
Net Loss
Our net loss increased by 21.9% to RMB971.2 million for 2022 from RMB796.7 million for 2021, primarily due to an increase in operating expenses as mentioned above, which was in line with the continued growth of our business. The increase in net loss was partially offset by our increased total revenues.
B. Liquidity and Capital Resources
Our principal sources of liquidity have been proceeds from our initial public offering and concurrent private placement, equity contributions from our shareholders and bank borrowings. In June 2020, we completed our initial public offering in which we issued and sold an aggregate of 15,525,000 ADSs, representing 15,525,000 Class A ordinary shares, resulting in net proceeds to us of US$234.9 million. Concurrently with our initial public offering, we also raised US$25 million from Lake Bleu Prime Healthcare Master Fund Limited, by selling 1,515,151 Class A ordinary shares to it in a private placement.
As of December 31, 2023, we had cash and cash equivalents and restricted cash of RMB615.2 million (US$86.6 million), primarily consisting of bank deposits.
We believe that our cash and cash equivalents and restricted cash, together with our cash generated from financing activities, our initial public offering and private placement, will be sufficient to meet our current and anticipated needs for general corporate purposes for at least the next 12 months. We may, however, decide to expand our business through additional equity and debt financing. The issuance and sale of additional equity would result in further dilution to our shareholders. The incurrence of indebtedness would result in increased fixed obligations and could result in operating covenants that would restrict our operations.
Substantially all of our revenues in the foreseeable future are likely to continue to be denominated in Renminbi. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior SAFE approval as long as certain routine procedural requirements are fulfilled. Therefore, our PRC subsidiaries are allowed to pay dividends in U.S. dollars to us without prior SAFE approval by following these routine procedural requirements. However, approval from or registration with competent government authorities is required where the Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses, such as the repayment of loans denominated in foreign currencies. The PRC government may at its discretion restrict access to foreign currencies for current account transactions in the future.
The following table sets forth selected cash flow statement information for the periods indicated:
| Year ended December 31, | ||||||||||||||||
| 2021 | 2022 | 2023 | ||||||||||||||
| RMB | RMB | RMB | US$ | |||||||||||||
| (in thousands) | ||||||||||||||||
Net cash used in operating activities | (477,886 | ) | (456,808 | ) | (255,783 | ) | (36,026 | ) | ||||||||
Net cash generated from (used in) investing activities | 81,697 | (7,463 | ) | (9,300 | ) | (1,309 | ) | |||||||||
Net cash used in financing activities | (52,899 | ) | (86,239 | ) | (48,832 | ) | (6,878 | ) | ||||||||
Effect of exchange rate changes on cash and cash equivalents and restricted cash | (37,006 | ) | 36,666 | 3,863 | 543 | |||||||||||
Net decrease in cash, cash equivalents and restricted cash | (486,094 | ) | (513,844 | ) | (310,052 | ) | (43,670 | ) | ||||||||
Cash, cash equivalents and restricted cash at the beginning of year | 1,925,206 | 1,439,112 | 925,268 | 130,321 | ||||||||||||
Cash, cash equivalents and restricted cash at the end of year | 1,439,112 | 925,268 | 615,216 | 86,651 | ||||||||||||
97
Table of Contents
Operating Activities
Net cash used in operating activities for 2023 was RMB255.8 million (US$36.0 million), while our net loss for the same period was RMB653.7 million (US$92.1 million). The difference was primarily due to adjustment for non-cash and non-operating items of RMB450.1 million (US$63.4 million), primarily including share-based compensation of RMB260.7 million (US$36.7 million), depreciation and amortization of RMB133.4 million (US$18.8 million), non-cash operating lease expenses of RMB35.2 million (US$5.0 million), and changes in working capital. The changes in working capital primarily reflected (i) a decrease in inventories of RMB57.3 million, primarily attributable to our adjustment in response to changes in order demand and improvement in inventory management level, and (ii) a decrease in prepayments and other current and non-current assets of RMB12.4 million, primarily attributable to the decrease of prepaid research and development expenses, and partially offset by (i) a decrease in operating lease liabilities of RMB37.7 million, primarily attributable to the termination of office leases, (ii) a decrease in accounts payable of RMB31.1 million, primarily attributable to the timely settlement of payment and reduction of inventories, and (iii) a decrease in accrued liabilities and other current liabilities of RMB19.2 million, primarily attributed to the settlement of guaranteed return for certain eligible employees under our ESOP plans.
Net cash used in operating activities for 2022 was RMB456.8 million, while our net loss for the same period was RMB971.2 million. The difference was primarily due to adjustment for non-cash and non-operating items of RMB525.6 million, primarily including share-based compensation of RMB326.8 million, depreciation and amortization of RMB124.1 million, non-cash lease expense of RMB37.4 million, and changes in working capital. The changes in working capital primarily reflected (i) an increase in accrued liabilities and other current liabilities of RMB33.7 million primarily attributable to the payment of guaranteed return we will be liable to certain eligible employees under our ESOP plans if such employees request us to repurchase their eligible shares, and (ii) a decrease in other non-current assets of RMB24.2 million primarily attributable to the decrease of non-current deductible input VAT, and partially offset by (i) a decrease in operating lease liabilities of RMB37.8 million, primarily attributable to some termination of leasing offices (ii) an increase in inventories of RMB21.7 million, primarily attributable to our overall business growth, and (iii) an increase in accounts receivable and contract asset of RMB42.2 million, primarily attributed to the continued growth of our in-hospital business.
Net cash used in operating activities for 2021 was RMB477.9 million, while our net loss for the same period was RMB796.7 million. The difference was primarily due to adjustment for non-cash and non-operating items of RMB381.2 million, primarily including share-based compensation of RMB282.4 million, depreciation and amortization of RMB47.8 million, non-cash lease expense of RMB 35.3 million, and changes in working capital. The changes in working capital primarily reflected (i) an increase in deferred revenue of RMB68.5 million primarily as a result of our overall business growth, (ii) an increase in accrued liabilities and other current liabilities of RMB44.2 million primarily attributable to our increased payroll payables and increased accounts payable for leasehold improvement, and (iii) a decrease in operating lease liabilities of RMB29.7 million primarily as a result of adoption of ASC842, the new lease accounting principle, and partially offset by (i) an increase in inventories of RMB67.0 million, primarily attributable to our overall business growth and (ii) an increase in contract asset of RMB30.8 million, primarily attributed to the continued growth of our in-hospital business.
Investing Activities
Net cash generated from investing activities for 2023 was RMB9.3 million (US$1.3million), primarily due to purchase of property and equipment of RMB8.1 million.
Net cash used in investing activities for 2022 was RMB7.5 million, primarily due to proceeds from maturity of short-term investment of RMB65.6 million, partially offset by purchase of property and equipment of RMB70.3 million.
Net cash generated from investing activities for 2021 was RMB81.7million, primarily due to proceeds from maturity of short-term investment of RMB358.5 million, partially offset by purchase of property and equipment of RMB204.3 million and purchase of short-term investment of RMB63.9 million.
Financing Activities
Net cash used in financing activities for 2023 was RMB48.8 million (US$6.9 million), primarily due to refund of consideration for Employee Share Incentive Program of RMB41.8 million and purchase of treasury shares of RMB7.0 million.
Net cash used in financing activities for 2022 was RMB86.2 million, primarily due to our purchase of ADSs from the open market of RMB71.8 million.
Net cash used in financing activities for 2021 was RMB52.9 million, primarily due to the cash outflow of repayment of long-term borrowings of RMB34.7 million.
98
Table of Contents
Long-term borrowings
As of December 31, 2023, we did not have any long-term borrowings.
Capital Expenditures
Our capital expenditures were RMB206.9 million, RMB70.5 million and RMB9.4 million (US$1.3 million) for 2021, 2022 and 2023, respectively. These capital expenditures included the purchase of property, equipment and computer software. We will continue to make capital expenditures to meet the needs of our business’ expected growth. We intend to fund our future capital expenditure with our existing cash balance and proceeds from our initial public offering and the concurrent private placement.
Material Cash Requirements
Our material cash requirements as of December 31, 2023 and any subsequent interim period primarily included our operating lease obligations. Our operating lease obligations primarily represent our obligations for leasing office premises, which include all future cash outflows under ASC Topic 842, Leases. For further information, see Note 10 to our audited consolidated financial statements included elsewhere in this annual report.
The following table sets forth our contractual obligations by specified categories as of December 31, 2023:
| Payments due by period | ||||||||||||||||||||
| Total | less than 1 year | 1-3 years | 3-5 years | more than 5 years | ||||||||||||||||
| (RMB in thousands) | ||||||||||||||||||||
Operating lease obligations | 12,900 | 9,091 | 3,809 | — | — | |||||||||||||||
Other than those shown above, we did not have any significant capital and other commitments, long-term obligations or guarantees as of December 31, 2023.
Holding Company Structure
We are a holding company with no material operations of its own. We conduct our NGS-based cancer diagnostic business primarily through the VIE’s subsidiaries in China. As a result, our ability to pay dividends depends upon dividends paid by our WFOE. If our WFOE or any newly formed PRC subsidiaries incur debt on their own behalf in the future, the instruments governing their debt may restrict their ability to pay dividends to us. In addition, our WFOE is permitted to pay dividends to us only out of its retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. Under PRC law, each of our WFOE, VIE and their respective subsidiaries is required to set aside at least 10% of its after-tax profits each year, if any, to fund certain statutory reserve funds until such reserve funds reach 50% of its registered capital. In addition, our WFOE and the VIE may allocate a portion of its after-tax profits based on PRC accounting standards to a discretionary surplus fund at its discretion. The statutory reserve funds and the discretionary funds are not distributable as cash dividends. Remittance of dividends by a wholly foreign-owned company out of China is subject to examination by the banks designated by SAFE. Our WFOE has not paid any dividends and will not be able to pay dividends until it generates accumulated profits and meets the requirements for statutory reserve funds.
C. Research and Development, Patents and Licenses, etc.
See “Item 4. Information on the Company—B. Business Overview—Research and Development” and “—Intellectual Property”.
99
Table of Contents
D. Trend Information
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the current fiscal year that are reasonably likely to have a material adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E. Critical Accounting Estimate
We believe the following areas involve critical estimates: the current expected credit loss model rate in relation to ASC 326, Financial Instruments – Credit Losses, and standalone selling price of performance obligations for in-hospital business.
For information on our critical accounting estimates, see “Item 5. Operating and Financial Review and Prospects—A. Operating Results—Critical Accounting Policies, Judgments and Estimates” and Note 2 to our consolidated financial statements included elsewhere in this annual report.
Credit loss allowance for accounts receivables and contract assets
Upon adoption of ASC 326, we record the allowance for credit losses as an offset to accounts receivable and contract assets, with estimated credit losses charged to “general and administrative expenses” in the consolidated statements of comprehensive loss. We assess credit loss by reviewing accounts receivable and contract assets on a collective basis where similar characteristics exist, primarily based on similar business segments, service or product offerings and on an individual basis when we identify specific customers with known disputes or collectability issues. We apply a migration roll rate method that considers historical collectability based on past due status, the age of the accounts receivable and contract asset balances, credit quality of our customers based on ongoing credit evaluations, current economic conditions, reasonable and supportable forecasts of future economic conditions and other factors that may affect our ability to collect from customers. Our estimates of these inputs require subjective management judgment and are inherently uncertain. Changes in our estimates of these inputs may affect our financial condition and results of operations.
Determining the standalone selling price of the performance obligation for in-hospital business
The contracts with customers from reagent kit sales of in-hospital business often contain bundles of reagent kits to customers. Each kit represents a single performance obligation. We allocate the total transaction consideration to each performance obligation based on a relative standalone selling price basis using the expected cost plus a margin method. We estimate standalone selling price by using market conditions, entity-specific factors, including that product mix sold to customers remains over time and our expected gross margin for different customers. Changes in these assumptions and inputs could directly affects the amount and timing of revenue recognized under these arrangements.
F. Safe Harbor
See “Forward-Looking Statements” in this annual report.
100
Table of Contents
ITEM 6. DIRECTORS, SENIOR MANANGEMENT AND EMPLOYEES
A. Directors and Senior Management
The following table sets forth information regarding our directors and executive officers as of the date of this annual report.
Directors and Executive Officers | Age | Position/Title | ||
| Yusheng Han | 45 | Founder, chairman of the board of directors and chief executive officer | ||
| Zhihong (Joe) Zhang | 48 | Director and chief technology officer | ||
| Leo Li | 39 | Director and chief financial officer | ||
| Gang Lu | 52 | Director | ||
| Feng Deng | 60 | Director | ||
| Wendy Hayes | 54 | Independent director | ||
| Min-Jui Richard Shen | 59 | Independent director | ||
| Licen Lisa Xu | 58 | Independent director |
Mr. Yusheng Han is our founder, chairman of the board of directors and chief executive officer. Mr. Han has 19 years of experience in life science. From June 2011 to November 2013, he was an associate in Northern Light Venture Capital where he focused on investment in the healthcare industry and helped the firm invest in successful companies. From July 2005 to May 2009, Mr. Han worked at BioTek Instruments, Inc. as its general manager in China. During his term with BioTek Instruments China, he built and led teams across marketing, sales and post-sale. From September 2003 to May 2005, he served as the product specialist of Gene Company Limited. Mr. Han received a bachelor’s degree in biochemistry from Jilin University in July 2000, and a master’s degree in cell biology in Peking Union Medical College in June 2003. He obtained a Master of Business Administration degree from Columbia Business School in May 2011.
Dr. Zhihong (Joe) Zhang has served as our chief technology officer in March 2016 and our director since June 2023. Prior to joining us, Dr. Zhang was a staff scientist of Illumina, Inc., and a senior fellow of Howard Hughes Medical Institute and University of Washington. He obtained a bachelor’s and master’s degree in biochemistry and molecular biology from Fudan University in 1997 and 2000, and a Ph.D. degree in molecular genetics and microbiology from Duke University in 2005.
Mr. Leo Li has served as our chief financial officer since the third quarter of 2019 and our director since the first quarter of 2020. Prior to joining us, Mr. Li served as the chief financial officer of Weidai Ltd., a NYSE-listed leading auto-backed financing solution provider in China. Prior to Weidai Ltd., Mr. Li served as an investment director and later an executive director of Vision Knight Capital, or VKC, a private equity fund focusing on China’s internet-driven sectors. Prior to VKC, Mr. Li worked at Morgan Stanley Asia Ltd. Mr. Li attended University of Oxford from 2004 to 2008 and received a four-year Master of Physics degree. Mr. Li is a Chartered Financial Analyst.
Mr. Gang Lu has served as our director since June 2014. In 2009, Mr. Lu joined Legend Star, a venture capital headquartered in Beijing, and he is now a partner of Legend Star and leads investment in healthcare, specialized in the fields of innovative medicine, biological and genetic technology, and innovative medical service. Mr. Lu holds a bachelor’s degree in electromagnetic engineering from Xidian University and a Master of Business Administration degree from Tsinghua University.
Mr. Feng Deng has served as our director since August 2016. Mr. Deng has over 20 years of experience in venture capital, computer science and telecommunication industry. He founded Northern Light Venture Capital in January 2006 and served as its founding managing partner, focusing on investment in technology, media and telecom, or TMT, clean technology, healthcare and consumer sectors. From February 2004 to February 2005, he served as the vice president in strategy in Juniper Networks. From October 1997 to February 2004, Mr. Deng served as the vice president in engineering, chief strategy officer and a director of NetScreen Technologies Inc. Prior to NetScreen, he worked at Intel Corporation as a systems architect from July 1993 to October 1997. He holds a bachelor’s and a master’s degree in electronic engineering from Tsinghua University, a master’s degree in computer engineering from the University of Southern California, and a Master of Business Administration degree from the Wharton Business School of the University of Pennsylvania.
Ms. Wendy Hayes has served as our independent director since June 2020. Ms. Hayes has served as an independent director of Tuanche Limited (NASDAQ: TC) since November 2018, iHuman Inc. (NYSE: IH) since October 2020, SciClone Pharmaceuticals (Holdings) Limited (HKEX: 6600) since March 2021, Apollomics Inc. (NASDAQ: APLM) since March 2023 and SharkNinja, Inc. (NYSE: SN) since July 2023. Between May 2013 and September 2018, Ms. Hayes served as the inspections leader at the Public Company Accounting Oversight Board in the United States. Prior to that, Ms. Hayes was an audit partner at Deloitte (China). Ms. Hayes received her bachelor’s degree in international finance from University of International Business and Economics in 1991, and her executive MBA from Cheung Kong Graduate School of Business in 2012. Ms. Hayes is a certified public accountant in the United States (California) and China.
101
Table of Contents
Dr. Min-Jui Richard Shen has served as our independent director since June 2020. Dr. Shen is the managing director of RS Technology Ventures, LLC, a strategic advisory and investment company that he founded in 2016. From 2021 to 2022, Dr. Shen served as the senior vice president of research and development at Pacific Biosciences of California, Inc., a leading provider of high-quality and accurate sequencing platforms. From 2020 to 2021, Dr. Shen was Chief Product Officer and then the President of Omniome, a privately held start-up that was acquired by Pacific Biosciences of California, Inc. in 2021. Dr. Shen worked at Illumina, Inc., a life sciences tools provider, from 2000 to 2016. He served in many roles at Illumina, the last of which was vice president for oncology research and development. Before joining Illumina, Dr. Shen worked at Myriad Genetics, Inc., a molecular diagnostics company, from 1998 to 2000. Dr. Shen earned his bachelor’s degree in biochemistry from University of California, Los Angeles, and his Ph.D. degree in biochemistry and molecular biology from Louisiana State University Medical Center. Additionally, Dr. Shen is a member of the External Advisory Board of the Parker H. Petit Institute for Bioengineering and Bioscience at the Georgia Institute of Technology.
Dr. Licen Lisa Xu has served as our independent director since June 2022. Dr. Xu has 17 years of experience in driving the productization and lifecycle for life science and biomedical products, from product concept and research to development and commercialization. She is the chief commercial officer of FlashDx Inc., a molecular diagnostics sample-to-answer solution company, and an independent board member of Ribbon Biolab, a company specialized in the production of synthetic DNAs. Dr. Xu served as a vice president on strategic product management and lifecycle management at Roche Diagnostics, a subsidiary of Roche Holding AG for the development of diagnostic tests, instruments and digital solutions, and a vice president on strategic marketing and product management at Illumina Inc. Dr. Xu has a Ph.D. degree in molecular biology from University of Zurich and a master of science degree in biophysics from Tsinghua University, and completed the business executive education program from Wharton Business School, University of Pennsylvania.
Board Diversity Disclosure
The following information was provided by our directors on a voluntary basis.
Nasdaq Board Diversity Matrix (As of date of this annual report)
| Country of Principal Executive Offices | China | |||||||
| Foreign Private Issuer | Yes | |||||||
| Disclosure Prohibited under Home Country Law | No | |||||||
| Total Number of Directors | 8 | |||||||
| Female | Male | Non-Binary | Did No Disclose Gender | |||||
| Part I: Gender Identity | ||||||||
| Directors | 2 | 6 | 0 | 0 | ||||
| Part II: Demographic Background | ||||||||
| Underrepresented Individual in Home Country Jurisdiction | 0 | |||||||
| LGBTQ+ | 0 | |||||||
| Did Not Disclose Demographic Background | 0 | |||||||
UK FCA Diversity Disclosures (As at December 31, 2023)
As at December 31, 2023, 100% of the members of the board of directors identified as having a minority ethnic background. Nonetheless, we acknowledge that the Company does not, at this time, meet all of the diversity targets specified in FCA Policy Statement PS22/3 on diversity and inclusion on company boards and executive management. Specifically:
| • | None of the following senior positions are held by women: chairman of the board of directors, chief executive officer, senior independent director or chief financial officer; and |
| • | Less than 40% of the individuals on the board of directors are women. |
While the Company has not adopted a formal diversity policy, the Nominating and Corporate Governance Committee review annually the current composition of the board of directors with regards to characteristics such as independence, knowledge, skills, experience and diversity. In considering succession plans for senior positions, due attention will be given to the FCA board diversity targets set out in PS22/3, and we are confident that future appointments will, as a whole, continue to support the Company’s diversity aims.
102
Table of Contents
The information presented in the following tables was collected on a self-reporting basis. The board of directors were provided with the tables below, and asked to complete them based on how they identify. The Company’s approach to data collection is consistent for the purposes of all diversity-related reporting requirements under the UK Listing Rules and across all individuals in relation to whom data is being reported.
Reporting on sex/gender representation as at December 31, 2023
| Number of board of director members | Percentage of the board of directors | Number of senior positions on the board of directors | Number in executive management(1) | Percentage of executive management(1) | ||||||||||||||||
Men | 6 | 75 | % | 3 | 3 | 100 | % | |||||||||||||
Women | 2 | 25 | % | 0 | 0 | 0 | ||||||||||||||
Not specified/ prefer not to say | — | — | — | — | — | |||||||||||||||
| (1) | The Company’s executive management consists of the Chief Executive Officer, the Chief Financial Officer and the Chief Technology Officer, each of whom are also on the board of directors. |
Reporting on ethnicity representation as at December 31, 2023
| Number of board of director members | Percentage of the board of directors | Number of senior positions on the board of directors | Number in executive management(1) | Percentage of executive management(1) | ||||||||||||||||
White British or other White (including minority-white groups) | — | — | — | — | — | |||||||||||||||
Mixed/Multiple Ethnic Groups | — | — | — | — | — | |||||||||||||||
Asian/ Asian British | 8 | 100 | % | 3 | 3 | 100 | % | |||||||||||||
Black/African/Caribbean/Black British | — | — | — | — | — | |||||||||||||||
Other ethnic group, including Arab | — | — | — | — | — | |||||||||||||||
Not specified/ prefer not to say | — | — | — | — | — | |||||||||||||||
| (1) | The Company’s executive management consists of the Chief Executive Officer, the Chief Financial Officer and the Chief Technology Officer, each of whom are also on the board of directors. |
B. Compensation
In 2023, we paid an aggregate of approximately RMB6.1 million (US$0.9 million) in cash to our directors and executive officers. Our PRC subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, unemployment insurance, employment injury insurance, maternity insurance and other statutory benefits and a housing provident fund.
Employment Agreements and Indemnification Agreements
We have entered into employment agreements with each of our executive officers. Under these agreements, each of our executive officers is employed for a specified time period. We may terminate employment for cause, at any time, without advance notice or remuneration, for certain acts of the executive officer, such as conviction or plea of guilty to a felony or any crime involving moral turpitude, negligent or dishonest acts to our detriment, or misconduct. If the executive officer otherwise fails to perform agreed duties, we may terminate employment upon one-week to 30-day advance written notice. We may also terminate an executive officer’s employment upon mutual agreement or 30-day advance written notice. In such case of termination by us, we will provide severance payments to the executive officer as expressly required by applicable law of the jurisdiction where the executive officer is based. Our executive officer may resign at any time upon mutual agreement or 30-day advance written notice.
Each executive officer has agreed to hold, both during and after the termination or expiration of his or her employment agreement, in strict confidence and not to use, except as required in the performance of his or her duties in connection with the employment or pursuant to applicable law, any of our confidential information or trade secrets, any confidential information or trade secrets of our clients or prospective clients, or the confidential or proprietary information of any third party received by us and for which we have confidential obligations. The executive officers have also agreed to disclose in confidence to us all information with economic value, including but not limited to inventions, works and software, which they conceive, develop or reduce to practice during the executive officer’s employment with us and one year following the last date of employment, and to assign all right, title and interest in them to us, and assist us in obtaining and enforcing patents, copyrights and other legal rights for information with economic value.
103
Table of Contents
We have entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we may agree to indemnify our directors and executive officers against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being a director or officer of our company.
Share Incentive Awards
2023 Equity Incentive Plan
In June 2023, our board of directors approved our 2023 Equity Incentive Plan, or the 2023 Plan, to provide incentives to employees, directors and consultants and promote the success of our business. The maximum number of shares that may be issued pursuant all awards under our 2023 Plan is 4,755,000 Class A ordinary shares (the “Awards”).
The following paragraphs describe the principal terms of the 2023 Plan:
Type of awards. The 2023 Plan permits the awards of options, restricted shares, restricted share units that the plan administrator decides.
Plan administration. Our compensation committee will administer the 2023 Plan. The compensation committee will determine the participants to receive awards, the time, type and number of awards to be granted to each participant, and the terms and conditions of each award grant.
Award agreement. Awards granted under the 2023 Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each award, which may include the term of the award, the provisions applicable in the event of the grantee’s employment or service terminates, and our authority to unilaterally or bilaterally amend, modify, suspend, cancel or rescind the award.
Eligibility. We may grant awards to employees, directors and consultants of our company or any of our affiliates.
Vesting schedule. In general, the plan administrator determines the vesting schedule, which is specified in the relevant award agreement.
Exercise of options. The plan administrator determines the exercise price per share for each award, which is stated in the award agreement and shall be no less than the par value of any such shares. The vested portion of option will expire if not exercised prior to the time as the plan administrator determines at the time of its grant. However, the maximum exercisable term is ten years from the date of a grant.
Transfer Restrictions. Awards may not be transferred in any manner by the participant other than in accordance with the exceptions provided in the 2023 Plan or the relevant award agreement or otherwise determined by the plan administrator, such as transfers by will or the laws of descent and distribution.
Termination and Amendment. Unless terminated earlier, the 2023 Plan has a term of ten years. The plan administrator has the authority to amend or terminate the 2023 Plan. Except with respect to amendments made by the plan administrator, no termination, amendment or modification may diminish any of the rights of the participant under any award pursuant to the 2023 Plan unless agreed by the participant.
As of March 31, 2024, there were 1,243,667 ordinary shares underlying outstanding options granted to our executive officers and employees under our 2023 Plan.
2022 Long-term Equity Incentive Plan
In August 2022, our board of directors approved our 2022 Long-term Equity Incentive Plan, or the 2022 Plan, which was subsequently approved by our shareholders in the annual general meeting in September 2022. The 2022 Plan and the grant of the awards thereunder replaced our 2021 Long-term Equity Incentive Plan, or the 2021 Plan, and the share incentive awards granted thereunder, which were approved by our board of directors in November 2021 and shareholders in December 2021. Following the approval of the 2022 Plan, the 2021 Plan and all awards granted thereunder were automatically and immediately cancelled. The 2022 Plan will grant options to senior management and employees. The maximum number of shares that may be issued pursuant all awards under our 2022 Plan is 11,775,525 Class A ordinary shares (the “Awards”).
104
Table of Contents
The following paragraphs describe the principal terms of the 2022 Plan:
Type of awards. The 2022 Plan permits the awards of options.
Allocation and Plan administration. As approved by our board of directors and shareholders, the Awards consist of three tranches of options (the “Pool I Awards”, “Pool II Awards” and “Pool III Awards”) and will be allocated as follows:
| • | Pool I Awards: 0 to Mr. Yusheng Han, our chairman and CEO; 60% to our other officers and employees; and the remaining 40% will be reserved for future grants to our officers and employees (none of the reserved portion will be granted to Mr. Han), details of which will be determined by the compensation committee. |
| • | Pool II Awards: 62.07% (or options to purchase 3,532,658 Class A ordinary shares) to Mr. Han; 22.76% to our other officers and employees; and the remaining 15.17% will be reserved for future grants to our officers and employees (none of the reserved portion will be granted to Mr. Han), details of which will be determined by the compensation committee. |
| • | Pool III Awards: 45% (or options to purchase 1,766,329 Class A ordinary shares) to Mr. Han; 33% to our other officers and employees; and the remaining 22% will be reserved for future grants to our officers and employees (none of the reserved portion will be granted to Mr. Han), details of which will be determined by the compensation committee. |
Award agreement. Awards granted under the 2022 Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each award, which may include the term of the award, the provisions applicable in the event of the grantee’s employment or service terminates, and our authority to unilaterally or bilaterally amend, modify, suspend, cancel or rescind the award.
Eligibility. We may grant awards to senior management and employees of our company under the 2022 Plan.
Vesting schedule. The options will vest in three batches, consisting of (1) 2,158,846 Class A ordinary shares for the Pool I Awards, (2) 5,691,504 Class A ordinary shares for the Pool II Awards, and (3) 3,925,175 Class A ordinary shares for the Pool III Awards. The Pool I Awards will vest as follows:
| 1) | 40% of the Pool I Awards will vest once (a) our valuation (based on the 60-day average closing share price of our publicly traded shares) reaches US$2 billion by the fifth anniversary of the grant date (the “Pool I valuation target”), and (b) the grantee remains employed by us at the time when the Pool I valuation target is achieved; and |
| 2) | the remaining 60% of the Pool I Awards will vest once (a) the Pool I valuation target has been achieved, (b) the grantee remains employed by us at the time when the Pool I valuation target is achieved, and (c) the grantee has been employed by us for five (5) years after the date of grant. |
The Pool II Awards will vest as follows:
| 1) | 40% of the Pool II Awards will vest once (a) our valuation (based on the 60-day average closing share price of our publicly traded shares) reaches US$4 billion by the seventh anniversary of the grant date (the “Pool II valuation target”), and (b) the grantee remains employed by us at the time when the Pool II valuation target is achieved, and |
| 2) | the remaining 60% of the Pool II Awards will vest once (a) the Pool II valuation target has been achieved, (b) the grantee remains employed by us at the time when the Pool II valuation target is achieved, and (c) the grantee has been employed by us for five (5) years after the date of grant. |
The Pool III Awards will vest as follows:
| 1) | 40% of the Pool III Awards will vest once (a) our valuation (based on the 60-day average closing share price of our publicly traded shares) reaches US$10 billion by the seventh anniversary of the grant date (the “Pool III valuation target”), and (b) the grantee remains employed by us at the time when the Pool III valuation target is achieved, and; |
| 2) | the remaining 60% of the Pool III Awards will vest once (a) the Pool III valuation target has been achieved, (b) the grantee remains employed by us at the time when the Pool III valuation target is achieved, and (c) the grantee has been employed by us for five (5) years after the date of grant. |
Exercise of options. The grantee can exercise the vested portion of options granted under our 2022 Plan at any time but no more than ten years after the grant date. The exercise price of the Awards shall be US$3.33 per share, which is equivalent to our 60-day average closing share price immediately prior to September 8, 2022, the date of shareholder approval.
Transfer Restrictions. Awards may not be transferred in any manner by the participant other than in accordance with the exceptions provided in the 2022 Plan or the relevant award agreement or otherwise determined by the Compensation Committee, such as transfers by will or the laws of descent and distribution.
105
Table of Contents
Termination and Amendment. Our board of directors has the authority to amend or terminate the 2022 Plan, but no amendments, alternation or discontinuation made by the board of director, (a) without the approval (but only to extent such approval is required by the principal national securities exchange on which the shares are listed or admitted to trading, and subject to certain other exceptions) of the shareholders of our company, if such action would increase the total number of shares reserved for the purposes of our 2022 Plan or change the maximum number of shares for which awards may be granted to any participant under the 2022 Plan, (b) such action may diminish any of the rights of the participant under any award pursuant to the 2022 Plan unless agreed by the participant. In addition, the Allocated Awards can only be amended at a shareholders’ general meeting, at which meeting our C-level officers and/or entities they beneficially own shall abstain from voting.
As of March 31, 2024, there were 9,893,478 ordinary shares underlying outstanding options granted to our executive officers and employees under our 2022 Plan.
2020 Share Incentive Plan
In May 2020, our board of directors and shareholders approved our 2020 Share Incentive Plan, or the 2020 Plan, to provide incentives to employees, directors and consultants and promote the success of our business. The maximum number of ordinary shares that may be issued pursuant to all awards under our 2020 Plan is 4,512,276 ordinary shares.
The following paragraphs describe the principal terms of the 2020 Plan:
Type of awards. The 2020 Plan permits the awards of options, restricted shares, restricted share units that the plan administrator decides.
Plan administration. Our compensation committee will administer the 2020 Plan. The compensation committee will determine the participants to receive awards, the time, type and number of awards to be granted to each participant, and the terms and conditions of each award grant.
Award agreement. Awards granted under the 2020 Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each award, which may include the term of the award, the provisions applicable in the event of the grantee’s employment or service terminates, and our authority to unilaterally or bilaterally amend, modify, suspend, cancel or rescind the award.
Eligibility. We may grant awards to employees, directors and consultants of our company or any of our affiliates.
Vesting schedule. In general, the plan administrator determines the vesting schedule, which is specified in the relevant award agreement.
Exercise of options. The plan administrator determines the exercise price per share for each award, which is stated in the award agreement and shall be no less than the par value of any such shares. The vested portion of option will expire if not exercised prior to the time as the plan administrator determines at the time of its grant. However, the maximum exercisable term is ten years from the date of a grant.
Transfer Restrictions. Awards may not be transferred in any manner by the participant other than in accordance with the exceptions provided in the 2020 Plan or the relevant award agreement or otherwise determined by the plan administrator, such as transfers by will or the laws of descent and distribution.
Termination and Amendment. Unless terminated earlier, the 2020 Plan has a term of ten years. The plan administrator has the authority to amend or terminate the 2020 Plan. Except with respect to amendments made by the plan administrator, no termination, amendment or modification may diminish any of the rights of the participant under any award pursuant to the 2020 Plan unless agreed by the participant.
As of March 31, 2024, we granted certain number of restricted share units under our 2020 Plan to three independent directors, which represent approximately 1% of our total outstanding ordinary shares as of the same date.
Other Share Incentive Awards
We also granted options to our directors, officers and employees other than under our 2020 Plan and 2022 Plan. As of March 31, 2024, there were 6,227,135 ordinary shares underlying outstanding options granted to our directors, officers and employees outside our 2020 Plan and 2022 Plan. These options bear a per share exercise price of US$0.0002 or US$13.6184, and will expire between September 30, 2025 and January 31, 2032.
106
Table of Contents
In December 2020, we issued an aggregate of 743,955 restricted shares at purchase prices between US$13.2000 and US$20.0229 per share to certain employees. These restricted shares are subject to a three-year lock-up period. By August 2023, we had repurchased all these shares from these employees.
C. Board Practice
Board of Directors
Our board of directors consists of nine directors. A director is not required to hold any shares in our company by way of qualification. A director may vote with respect to any contract, proposed contract or arrangement in which he is materially interested, provided that (a) such director, if his or her interest in such contract or arrangement is material, has declared the nature of his or her interest at the earliest meeting of the board at which it is practicable for him or her to do so, either specifically or by way of a general notice and (b) if such contract or arrangement is a transaction with a related party, such transaction has been approved by the audit committee. The directors may exercise all the powers of the company to borrow money, mortgage its undertaking, property and uncalled capital, and issue debentures or other securities whenever money is borrowed or as security for any obligation of the company or of any third party.
Committees of the Board of Directors
We have established an audit committee, a compensation committee and a nominating and corporate governance committee. We have adopted a charter for each of these committees. Each committee’s members and functions are described below.
Audit Committee. Our audit committee consists of Ms. Wendy Hayes and Dr. Min-Jui Richard Shen. Ms. Wendy Hayes is the chairman of our audit committee. We have determined that Ms. Wendy Hayes and Dr. Min-Jui Richard Shen each satisfies the “independence” requirements of Rule 5605(c)(2) of the Listing Rules of the Nasdaq Stock Market and meets the independence standards under Rule 10A-3 under the Exchange Act, as amended. We have determined that Ms. Wendy Hayes qualifies as an “audit committee financial expert.” The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
| • | appointing the independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by the independent auditors; |
| • | reviewing with the independent auditors any audit problems or difficulties and management’s response; |
| • | discussing the annual audited financial statements with management and the independent auditors; |
| • | reviewing the adequacy and effectiveness of our accounting and internal control policies and procedures and any steps taken to monitor and control major financial risk exposures; |
| • | reviewing and approving all proposed related party transactions; |
| • | meeting separately and periodically with management and the independent auditors; and |
| • | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
Compensation Committee. Our compensation committee consists of Mr. Yusheng Han, Mr. Feng Deng and Dr. Licen Lisa Xu. Mr. Yusheng Han is the chairman of our compensation committee. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
| • | reviewing and approving, or recommending to the board for its approval, the compensation for our chief executive officer and other executive officers; |
| • | reviewing and recommending to the board for determination with respect to the compensation of our non-employee directors; |
| • | reviewing periodically and approving any incentive compensation or equity plans, programs or similar arrangements; and |
| • | selecting compensation consultant, legal counsel or other adviser only after taking into consideration all factors relevant to that person’s independence from management. |
107
Table of Contents
Nominating and Corporate Governance Committee. Our nominating and corporate governance committee consists of Mr. Gang Lu, Ms. Wendy Hayes and Mr. Yusheng Han. Mr. Gang Lu is the chairman of our nomination committee. We have determined that Ms. Wendy Hayes satisfies the “independence” requirements of the Listing Rules of the Nasdaq Stock Market. The nominating and corporate governance committee assists the board in selecting individuals qualified to become our directors, and determining the composition of the board and its committees. The nominating and corporate governance committee is responsible for, among other things:
| • | selecting and recommending to the board nominees for election by the shareholders or appointment by the board; |
| • | reviewing annually with the board the current composition of the board with regards to characteristics such as independence, knowledge, skills, experience and diversity; |
| • | making recommendations on the frequency and structure of board meetings and monitoring the functioning of the committees of the board; and |
| • | advising the board periodically with regards to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to the board on all matters of corporate governance and on any remedial action to be taken. |
Duties of Directors
Under Cayman Islands law, our directors owe fiduciary duties to our company, including a duty of loyalty, a duty to act honestly, and a duty to act in what they consider in good faith to be in our best interests. Our directors must also exercise their powers only for a proper purpose. Our directors also have a duty to exercise skills they actually possess and such care and diligence that a reasonably prudent person would exercise in comparable circumstances. It was previously considered that a director need not exhibit in the performance of his duties a greater degree of skill than what may reasonably be expected from a person of his knowledge and experience. However, English and Commonwealth courts have moved towards an objective standard with regard to the required skill and care, and these authorities are likely to be followed in the Cayman Islands. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association, as amended and restated from time to time, and the class rights vested thereunder in the holders of the shares. Our company has the right to seek damages if a duty owed by our directors is breached. A shareholder may in certain circumstances have rights to damages if a duty owed by the directors is breached.
Our board of directors has all the powers necessary for managing, and for directing and supervising, our business affairs. The functions and powers of our board of directors include, among others:
| • | convening shareholders’ annual general meetings and reporting its work to shareholders at such meetings; |
| • | declaring dividends and distributions; |
| • | appointing officers and determining the term of office of the officers; |
| • | exercising the borrowing powers of our company and mortgaging the property of our company; and |
| • | approving the transfer of shares in our company, including the registration of such shares in our share register. |
Terms of Directors and Officers
Our directors may be elected by an ordinary resolution of our shareholders. Alternatively, our board of directors may, by the affirmative vote of a simple majority of the directors present and voting at a board meeting appoint any person as a director to fill a casual vacancy on our board or as an addition to the existing board. One-third of our directors (or, if the number of our directors is not a multiple of three, the number nearest to but not greater than one-third) will retire from office by rotation at each annual general meeting. In addition, a director will cease to be a director if he (i) becomes bankrupt or makes any arrangement or composition with his creditors; (ii) dies or is found to be or becomes of unsound mind; (iii) resigns his office by notice in writing; (iv) without special leave of absence from our board, is absent from meetings of our board for three consecutive meetings and our board resolves that his office be vacated; or (v) is removed from office pursuant to any other provision of our articles of association.
Our officers are appointed by and serve at the discretion of the board of directors, and may be removed by our board of directors.
108
Table of Contents
D. Employees
As of December 31, 2021, 2022 and 2023, we had 1,394, 1,138 and 786 employees, respectively. Most of our employees are located in China, with a small number located in the United States. The following table sets forth the number of our employees by function as of December 31, 2023.
| As of December 31, 2023 | ||||||||
| Number | % of Total Employees | |||||||
Functions: | ||||||||
Technology, Research and Development | 174 | 22.1 | % | |||||
Medical Affairs | 84 | 10.7 | % | |||||
Operations and Quality Assurance | 177 | 22.5 | % | |||||
Sales and Marketing | 271 | 34.5 | % | |||||
General and Administration | 80 | 10.2 | % | |||||
|
|
|
| |||||
Total number of employees | 786 | 100.0 | % | |||||
|
|
|
| |||||
As required by PRC laws and regulations, we participate in various employee social security plans that are organized by municipal and provincial governments, including pension, medical insurance and unemployment insurance and housing fund. We are required under PRC laws to make contributions to employee benefit plans at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by the local government from time to time.
E. Share Ownership
Except as specifically noted, the following table sets forth information with respect to the beneficial ownership of our ordinary shares as of March 31, 2024 by:
| • | each of our directors and executive officers; |
| • | each person known to us to own beneficially more than 5% of our ordinary shares. |
The calculations in the table below are based on 102,587,260 ordinary shares issued and outstanding as of March 31, 2024, comprising (i) 85,262,412 Class A ordinary shares, excluding (a) 708,828 Class A ordinary shares issued to our depositary bank for bulk issuance of ADSs reserved for future issuances upon the exercise or vesting of awards granted under share incentive plans, and (b) 3,023,138 Class A Ordinary Shares as treasury stock, and (ii) 17,324,848 Class B ordinary shares.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person.
| Ordinary Shares Beneficially Owned | ||||||||||||||||||||
| Class A Ordinary Shares | Class B Ordinary Shares | Total Ordinary Shares | % of Beneficial Ownership† | % of Aggregate Voting Power†† | ||||||||||||||||
Directors and Executive Officers**: | ||||||||||||||||||||
Yusheng Han(1) | 85,123 | 17,324,848 | 17,409,971 | 17.0 | % | 55.0 | % | |||||||||||||
Zhihong (Joe) Zhang | * | — | * | * | * | |||||||||||||||
Leo Li | * | — | * | * | * | |||||||||||||||
Gang Lu | — | — | — | — | — | |||||||||||||||
Feng Deng(2) | 11,880,245 | — | 11,880,245 | 11.6 | % | 6.3 | % | |||||||||||||
Wendy Hayes | — | — | — | — | — | |||||||||||||||
Min-Jui Richard Shen | * | — | * | * | * | |||||||||||||||
Licen Lisa Xu | * | — | * | * | * | |||||||||||||||
All Directors and Executive Officers as a Group | 12,888,799 | 17,324,848 | 30,213,647 | 29.3 | % | 61.6 | % | |||||||||||||
Principal Shareholders: | ||||||||||||||||||||
Quantum Boundary Holdings Limited(1) | 85,123 | 17,324,848 | 17,409,971 | 17.0 | % | 55.0 | % | |||||||||||||
Northern Light Venture Capital III, Ltd.(3) | 11,880,245 | — | 11,880,245 | 11.6 | % | 6.3 | % | |||||||||||||
Entities affiliated with LYFE Capital(4) | 8,338,381 | — | 8,338,381 | 8.1 | % | 4.4 | % | |||||||||||||
Sequoia Capital China(5) | 6,846,567 | — | 6,846,567 | 6.7 | % | 3.6 | % | |||||||||||||
Investment funds affiliated with CMB(6) | 7,017,385 | — | 7,017,385 | 6.8 | % | 3.7 | % | |||||||||||||
Kynam Capital Management, LP(7) | 8,694,426 | — | 8,694,426 | 8.5 | % | 4.6 | % | |||||||||||||
109
Table of Contents
| * | Less than 1% of our total ordinary shares outstanding as of March 31, 2024. |
| ** | Except as otherwise indicated below, the business address of our directors and executive officers is No. 5, Xingdao Ring Road North, International Bio Island, Guangzhou, China. |
| † | For each person and group included in this column, percentage ownership is calculated by dividing the number of shares beneficially owned by such person or group by the sum of the total number of shares outstanding, and the number of shares such person or group has the right to acquire upon exercise of an option, warrant or other right within 60 days after March 31, 2024. The total number of ordinary shares issued and outstanding as of March 31, 2024 is 102,587,260, including 85,262,412 Class A ordinary shares and 17,324,848 Class B ordinary shares. |
| †† | For each person and group included in this column, percentage of voting power is calculated by dividing the voting power beneficially owned by such person or group by the voting power of all of our Class A and Class B ordinary shares as a single class. Each holder of Class A ordinary shares is entitled to one vote per share and each holder of our Class B ordinary shares is entitled to six votes per share on all matters submitted to them for a vote. Our Class A ordinary shares and Class B ordinary shares vote together as a single class on all matters submitted to a vote of our shareholders, except as may otherwise be required by law. Our Class B ordinary shares are convertible at any time by the holder thereof into Class A ordinary shares on a one-for-one basis. |
| (1) | Represents (i) 32,958 Class A Ordinary Shares directly held by Quantum Boundary Holdings Limited, a British Virgin Island company, (ii) 52,165 Class A ordinary shares issuable to Mr. Yusheng Han upon exercise of options within 60 days of March 31, 2024, and (iii) 17,324,848 Class B ordinary shares directly held by Quantum Boundary Holdings Limited. Quantum Boundary Holdings Limited is indirectly wholly owned and ultimately controlled by a family trust, a trust established under the laws of the Republic of Singapore and managed by J.P. Morgan Trust Company (Singapore) Pte. Ltd as the trustee. |
Mr. Han is the settlor of the trust. Mr. Han and his family members are the beneficiaries of the trust. The register address of Quantum Boundary Holdings Limited is at Vistra Corporate Services Centre, Wickhams Cay II, Road Town, Tortola, VG1110, British Virgin Islands.
| (2) | Consists of the shares listed in footnote (3) below. For purpose of this section, Mr. Feng Deng, one of our directors, beneficially owns the shares held by Northern Light Venture Capital III, Ltd. |
| (3) | Represents (i) 10,542,529 Class A ordinary shares held by Northern Light Venture Fund III, L.P., or NLVF III, a Cayman Islands exempted limited liability partnership, (ii) 1,188,025 Class A ordinary shares held by Northern Light Strategic Fund III, L.P., or NLSF III, a Cayman Islands exempted limited liability partnership, and (iii) 149,691 Class A ordinary shares held by Northern Light Partners Fund III, L.P., or NLPF III, a Cayman Islands exempted limited liability partnership, as reported in the Schedule 13G filed on February 13, 2023. Northern Light Venture Capital III, Ltd., or NLVC, is the general partner of Northern Light Partners III, L.P., which in turn is the general partner of NLVF III, NLSF III and NLPF III. NLVC and Feng Deng, a director of NLVC, may be deemed to beneficially own the shares owned by NLVF III, NLSF III and NLPF III. The registered addresses of NLVC, NLVF III, NLSF III and NLPF III are Floor 4, Willow House, Cricket Square, Grand Cayman, KY1-9010, Cayman Islands. |
| (4) | Represents (i) 6,013,684 Class A ordinary shares (in the form of ADSs) held by LYFE Capital Stone (Hong Kong) Limited, a Hong Kong private company limited by shares, (ii) 1,597,425 Class A ordinary shares (in the form of ADSs) held by LYFE Mount Whitney Limited, a Hong Kong private company limited by shares, and (iii) 727,272 Class A ordinary shares (in the form of ADSs) represented by 727,272 ADSs held by LYFE Capital Fund II, L.P., a Cayman Islands partnership, as reported in the Schedule 13G filed on February 7, 2022. LYFE Capital Stone (Hong Kong) Limited is owned by LYFE Capital Fund, L.P., and LYFE Capital Fund—A, L.P. LYFE Mount Whitney Limited is owned by LYFE Capital Fund II, L.P., Pantheon Access Co-investment Program, L.P.—Series 81 and Pantheon International PLC. LYFE Capital GP, L.P. is the general partner of LYFE Capital Fund, L.P. LYFE Capital GP II, L.P. is the general partner of LYFE Capital Fund II, L.P. LYFE Capital Management Limited is, in turn, the general partner of LYFE Capital GP, L.P. and LYFE Capital GP II, L.P. Mr. Jin Zhao and Mr. Zhengkun Yu, through their control over LYFE Capital Management Limited, share the voting and investment power with respect to all of our shares held by LYFE Capital Stone (Hong Kong) Limited and LYFE Mount Whitney Limited. LYFE Capital Fund II, L.P is an affiliate of LYFE Mount Whitney Limited. The registered address of LYFE Capital Stone (Hong Kong) Limited is Suite 1113A, 11/F, Ocean Centre, Harbour City, 5 Canton Road, Tsim Sha Tsui, Kowloon, Hong Kong. The registered address of LYFE Mount Whitney Limited is Suite 1113A, 11/F, Ocean Centre, Harbour City, 5 Canton Road, Tsim Sha Tsui, Kowloon, Hong Kong. |
| (5) | Represents (i) 3,004,874 Class A ordinary shares held by HSG Venture V Holdco I, Ltd., an exempted company with limited liability incorporated under the law of the Cayman Islands, (ii) 3,840,808 Class A ordinary shares (including 212,121 Class A ordinary shares in the form of ADSs) held by HSG Venture VI Holdco, Ltd., an exempted company with limited liability incorporated under the law of the Cayman Islands, and (iii) 885 Class A ordinary shares held by Mr. Neil Nanpeng Shen, as reported in the Schedule 13G filed on February 14, 2024. HSG Venture V Holdco I, Ltd. is wholly-owned by HongShan Capital Venture Fund V, L.P. The general partner of HongShan Capital Venture Fund V, L.P. is HSG Venture V Management, L.P., whose general partner is HSG Holding Limited. HSG Venture VI Holdco, Ltd. is wholly-owned by HongShan Capital Venture Fund VI, L.P. The general partner of HongShan Capital Venture Fund VI, L.P. is HSG Venture VI Management, L.P., whose general partner is HSG Holding Limited. HSG Holding Limited is wholly-owned by SNP China Enterprises Limited, which in turn is wholly-owned by Mr. Neil Nanpeng Shen. The registered address of SCC Venture V Holdco I, Ltd. and SCC Venture VI Holdco, Ltd. is Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. The address for Mr. Shen is Suite 3613, 36/F Two Pacific Place, 88 Queensway, Hong Kong. |
110
Table of Contents
| (6) | Represents (i) 5,964,435 Class A ordinary shares (including 3,829,927 Class A ordinary shares in the form of ADSs) held by EverGreen SeriesC Limited Partnership, a Cayman Islands exempted limited partnership, and (ii) 1,052,950 Class A ordinary shares(including 800,000 Class A ordinary shares in the form of ADSs) held by CMBI Private Equity Series SPC on behalf of and for the account of Biotechnology Fund IV SP, a segregated portfolio company incorporated under the law of Cayman Islands, whose management shares wholly owned by CMB International Private Investment Limited, an exempted company with limited liability incorporated under the law of Cayman Islands, as reported in the Schedule 13G filed on February 13, 2023. CMB International Private Investment Limited is also the general partner of EverGreen SeriesC Limited Partnership, and holds voting and dispositive power of the shares held by EverGreen SeriesC Limited Partnership. CMB International Private Investment Limited is ultimately controlled by China Merchants Bank Co., Limited (HKEX: 3968). The registered address of CMBI Private Equity Series SPC and EverGreen SeriesC Limited Partnership is the offices of Harneys Fiduciary (Cayman) Limited of 4th Floor, Harbour Place, 103 South Church Street, P.O. Box 10240, Grand Cayman KY1-1002, Cayman Islands. |
| (7) | Represents 8,694,426 Class A ordinary shares (in the form of ADSs) held Kynam Capital Management, LP, as reported in the Schedule 13G filed on February 14, 2024. Kynam Capital Management, LP is a Delaware company and its address is 221 Elm Road. Princeton, NJ 08540, U.S. |
To our knowledge, as of March 31, 2024, 56,075,045 issued and outstanding Class A ordinary shares, representing approximately 54.7% of our total issued and outstanding ordinary shares, were held by three record shareholders with registered addresses in the United States. None of our outstanding Class B ordinary shares are held by record holders in the United States. The number of beneficial owners of our ADSs in the United States is likely to be much larger than the number of record holders of our ordinary shares in the United States.
We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
F. Disclosure of Registrant’s Action to Recover Erroneously Awarded Compensation
We have adopted a policy relating to recovery of erroneously awarded compensation (the “Clawback Policy”), a form of which is included as Exhibit 97.1 attached herein.
During or after the year ended December 31, 2023, we were not required to prepare an accounting restatement that required recovery of erroneously awarded compensation pursuant to the Clawback Policy. As of December 31, 2023, there was no outstanding balance of erroneously awarded compensation to be recovered from the application of the Clawback Policy to a prior restatement.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
See “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
B. Related Party Transactions
Contractual Arrangements with Our Variable Interest Entities and Their Shareholders
See “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements.”
Registration Rights
Pursuant to the fifth amended and restated shareholders agreement dated January 10, 2020, we have granted certain registration rights to holders of our then preferred shares. Set forth below is a description of the registration rights granted under the agreement.
Demand Registration Right. At any time after the earlier of (i) the five (5) year after the closing of Series A+ financing (i.e. August 27, 2015) or (ii) the date that is six (6) months following the taking effect of a registration statement of an IPO, holder(s) together holding ten percent (10%) or more of the outstanding registrable securities may request in writing that we file a registration statement under Securities Act covering at least fifteen percent (15%) of the registrable securities. Within twenty (20) days after receipt of such a request, we shall use our best efforts to effect a registration of the registrable securities specified in the request, together with any registrable securities of any holder who requests in writing to join such registration. We are not be obligated to effect more than three (3) such registrations pursuant to the demand registration right, and we are not obligated to register registrable securities if we have, within the six-month period preceding the date of such request, effected a registration under the Securities Act pursuant to the exercise of the holders’ demand registration rights or Form F-3 registration right, or in which the holders had an opportunity to participate in a piggyback registration, unless the registrable securities of the holders were excluded from such registration. In addition, we have the right to defer filing of a registration statement for a period up to ninety (90) days after receipt of such request if, in the good faith judgment of our board of directors, the filing of a registration statement would be materially detrimental to us and our shareholders, but we cannot exercise this right more than once in any twelve-month period. Neither can we register any other of our shares during such twelve-month period.
111
Table of Contents
Piggyback Registration Right. If we propose to file a registration statement under the Securities Act for purposes of effecting a public offering of our securities (including registration statements relating to secondary offerings of our securities, but excluding registration statements relating to a demand registration or a piggyback registration, or to any employee benefit plan or a corporate reorganization), we must afford holders of registrable securities an opportunity to include in that registration all or any part of their registrable securities then held.
Registration on Form F-3. Any holder of registrable securities may request us in writing to effect a registration on the Form F-3 (or an equivalent registration in a jurisdiction outside of the U.S.) and any related qualification or compliance with respect to the registrable securities owned by such holder. Upon such request, we shall cause the registrable securities specified in the request, together with any registrable securities of any holder who requests in writing to join such registration, to be registered and effect any related qualification or compliance, provided that (i) Form F-3 is available for such offering by the holder, (ii) the registrable securities proposed to be sold to the public has an aggregate price in an amount of not less than US$500,000, and (iii) in no jurisdiction in which we would be required to qualify to do business or to execute a general consent to service of process in effecting such registration, qualification or compliance. We are not obligated to register registrable securities if we have, within the six-month period preceding the date of such request, effected a registration under the Securities Act, unless the registrable securities of the holders were excluded from such registration. In addition, we have the right to defer filing of the Form F-3 registration statement no more than once during any twelve-month period and for a period up to sixty (60) days after receipt of such request if, in the good faith judgment of our board of directors, the filing of a registration statement would be materially detrimental to us and our shareholders, provided that we will not register any other of our shares during such sixty-day period.
Expenses of Registration. We will pay all expenses relating to registration, fillings or qualifications, with certain limited exception, and each holder participating in a registration will bear its proportionate share of all selling expenses or other amounts payable to underwriters or brokers, if any, in connection with the offering by such holder.
Termination of Registration Rights. Such registration rights would terminate upon the earlier of (i) the date that is five (5) years after the closing of our initial public offering in June 2020, or (ii) such time at which all registrable securities held by the holders of our then preferred shares may be sold without restriction under Rule 144(k) of the Securities Act within a ninety-day period.
Employment Agreements and Indemnification Agreements
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment Agreements and Indemnification Agreements.”
Share Incentive Awards
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Share Incentive Awards.”
Other Related Party Transactions
Transactions with EaSuMed
We invested in and are currently a minority shareholder of EaSuMed Holding Ltd., or EaSuMed, a medical service provider. In 2023, we paid service fees in the amount of RMB18.0 thousand (US$3.0 thousand) to EaSuMed, which was mainly related to consulting services EaSuMed provided to us.
C. Interest of Experts and Counsel
Not applicable.
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report.
Legal Proceedings
From time to time, we may become a party to various legal or administrative proceedings arising in the ordinary course of our business, including actions with respect to intellectual property infringement, violation of third-party licenses or other rights, breach of contract and labor and employment claims. We are currently not a party to, and we are not aware of any threat of, any legal or administrative proceedings that, in the opinion of our management, are likely to have any material and adverse effect on our business, financial condition, cash-flow or results of operations.
112
Table of Contents
Dividend Policy
Our board of directors has discretion on whether to distribute dividends, subject to certain restrictions under Cayman Islands law, namely that our company may only pay dividends out of profits or share premium, and provided always that in no circumstances may a dividend be paid if this would result in our company being unable to pay its debts as they fall due in the ordinary course of business. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant. We do not have any present plan to pay any cash dividends on our ordinary shares in the foreseeable future. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand our business.
We are a holding company incorporated in the Cayman Islands. We may rely on dividends from our subsidiaries in China for our cash requirements, including any payment of dividends to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us.
If we pay any dividends on our ordinary shares, we will pay those dividends which are payable in respect of the underlying Class A ordinary shares represented by our ADSs to the depositary, as the registered holder of such Class A ordinary shares, and the depositary then will pay such amounts to our ADS holders in proportion to the underlying Class A ordinary shares represented by the ADSs held by such ADS holders, subject to the terms of the deposit agreement, including the fees and expenses payable thereunder. Cash dividends on our ordinary shares, if any, will be paid in U.S. dollars.
B. Significant Changes
Except as disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
ITEM 9. THE OFFER AND LISTING
A. Offering and Listing Details
Our ADSs, each representing one Class A ordinary share, have been listed on the NASDAQ Global Market since June 12, 2020 and the London Stock Exchange since November 1, 2022. Our ADSs trade on both exchanges under the symbol “BNR.” In 2022, no significant trading suspensions occurred.
B. Plan of Distribution
Not applicable.
C. Markets
Our ADSs have been traded on the NASDAQ Global Market since June 12, 2020 and the London Stock Exchange since November 1, 2022, on both exchanges under the symbol “BNR”.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expense of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
113
Table of Contents
B. Memorandum and Articles of Association
The following are summaries of material provisions of our tenth amended and restated memorandum and articles of association, as currently in effect, insofar as they relate to the material terms of our ordinary shares.
Ordinary Shares
General. Holders of Class A ordinary shares and Class B ordinary shares will have the same rights except for voting and conversion rights. All of our outstanding ordinary shares are fully paid and non-assessable. Certificates representing the ordinary shares are issued in registered form. Our shareholders who are non-residents of the Cayman Islands may freely hold and transfer their ordinary shares.
Conversion. Each Class B ordinary share is convertible into one (1) Class A ordinary share at any time by the holder thereof. Class A ordinary shares are not convertible into Class B ordinary shares under any circumstances. Upon any sale, transfer, assignment or disposition of any Class B ordinary share by a holder thereof to any person who is not an affiliate of such holder, or upon a change of control of any Class B ordinary share to any person who is not an affiliate of the registered shareholder of such Class B ordinary share, such Class B ordinary share shall be automatically and immediately converted into one Class A ordinary share. Furthermore, each Class B ordinary share will be automatically converted into one Class A ordinary share, if (i) at any time the holder thereof and the affiliates of such holder collectively hold less than 5% of the total number of our issued and outstanding shares, or (ii) at any time the holder thereof and the affiliates of such holder collectively hold less than 8.5% of the total number of our issued and outstanding shares and the holder thereof is no longer providing services to us in a position equivalent to or above vice president.
Dividends. The holders of our ordinary shares are entitled to such dividends as may be declared by our board of directors. Our current articles of association provide that dividends may be declared and paid out of our profits, realized or unrealized, or from any reserve set aside from profits which our board of directors determine is no longer needed. Dividends may also be declared and paid out of share premium account or any other fund or account which can be authorized for this purpose in accordance with the Companies Act. Holders of ordinary shares and Class B ordinary shares will be entitled to the same amount of dividends, if declared.
Voting Rights. Holders of Class A ordinary shares and Class B ordinary shares shall, at all times, vote together as one class on all matters submitted to a vote by the members. Each Class A ordinary share shall be entitled to one vote on all matters subject to vote at general and special meetings of our company and each Class B ordinary share shall be entitled to six (6) votes on all matters subject to vote at general and special meetings of our company.
Voting at any meeting of shareholders is by show of hands unless a poll is demanded. A poll may be demanded by the chairman of such meeting or any one or more shareholders who together hold not less than 10% of the nominal value of the total issued voting shares of our company present in person or by proxy. An ordinary resolution to be passed at a meeting by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the ordinary shares cast at a meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes cast attaching to the outstanding ordinary shares at a meeting. A special resolution will be required for important matters such as making changes to our current memorandum and articles of association.
Transfer of Ordinary Shares. Subject to the restrictions contained in our current articles of association, any of our shareholders may transfer all or any of his or her ordinary shares by an instrument of transfer in the usual or common form or any other form approved by our board of directors.
Our board of directors may, in its absolute discretion, decline to register any transfer of any ordinary share which is not fully paid up or on which we have a lien. Our board of directors may also decline to register any transfer of any ordinary share unless:
| • | the instrument of transfer is lodged with us, accompanied by the certificate for the ordinary shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; |
| • | the instrument of transfer is in respect of only one class of ordinary shares; |
| • | the instrument of transfer is properly stamped, if required; |
| • | in the case of a transfer to joint holders, the number of joint holders to whom the ordinary share is to be transferred does not exceed four; and |
| • | a fee of such maximum sum as the NASDAQ Global Market may determine to be payable or such lesser sum as our directors may from time to time require is paid to us in respect thereof. |
If our directors refuse to register a transfer, they shall, within three months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal.
114
Table of Contents
The registration of transfers may, after compliance with any notice required of the NASDAQ Global Market, be suspended and the register of members closed at such times and for such periods as our board of directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register of members closed for more than 30 days in any year as our board may determine.
Liquidation. On a return of capital on winding up or otherwise (other than on conversion, redemption or purchase of ordinary shares), assets available for distribution among the holders of ordinary shares shall be distributed among the holders of the ordinary shares on a pro rata basis. If our assets available for distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by our shareholders proportionately.
Calls on Ordinary Shares and Forfeiture of Ordinary Shares. Our board of directors may from time to time make calls upon shareholders for any amounts unpaid on their ordinary shares in a notice served to such shareholders at least 14 clear days prior to the specified time of payment. The ordinary shares that have been called upon and remain unpaid are subject to forfeiture.
Redemption of Ordinary Shares. The Companies Act and our current articles of association permit us to purchase our own shares. In accordance with our current articles of association and provided the necessary shareholders or board approval have been obtained, we may issue shares on terms that are subject to redemption, at our option or at the option of the holders of these shares, on such terms and in such manner, including out of capital, as may be determined by our board of directors.
Variations of Rights of Shares. All or any of the special rights attached to any class of shares may, subject to the provisions of the Companies Act, be materially adversely varied with the written consent of the holders of all of the issued shares of that class or with the sanction of an ordinary resolution passed at a general meeting of the holders of the shares of that class. The rights conferred upon the holders of the shares of any class issued shall not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such existing class of shares. The rights of the holders of any shares shall not be deemed to be materially adversely varied by the creation or issue of shares with preferred or other rights including, without limitation, the creation of shares with enhanced or weighted voting rights.
General Meetings of Shareholders
Shareholders’ meetings may be convened by a majority of our board of directors or our chairman. Advance notice of at least seven (7) calendar days is required for the convening of our annual general shareholders’ meeting and any other general meeting of our shareholders. A quorum required for and throughout a meeting of shareholders consists of at least one shareholder entitled to vote and present in person or by proxy or (in the case of a shareholder being a corporation) by its duly authorized representative representing not less than one-third of all voting power of our share capital in issue.
Inspection of Books and Records
Holders of our ordinary shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. (other than copies of our memorandum and articles of association and register of mortgages and charges, and any special resolutions passed by our shareholders). Under Cayman Islands law, the names of our current directors can be obtained from a search conducted at the Registrar of Companies. However, we will in our articles provide our shareholders with the right to inspect our list of shareholders and to receive annual audited financial statements.
Changes in Capital
We may from time to time by ordinary resolution:
| • | increase the share capital by such sum, to be divided into shares of such classes and amount, as the resolution shall prescribe; |
| • | consolidate and divide all or any of our share capital into shares of a larger amount than our existing shares; |
| • | sub-divide our existing shares, or any of them into shares of a smaller amount; or |
| • | cancel any shares which, at the date of the passing of the resolution, have not been taken or agreed to be taken by any person and diminish the amount of our share capital by the amount of the shares so canceled. |
We may by special resolution, subject to any confirmation or consent required by the Companies Act, reduce our share capital or any capital redemption reserve in any manner permitted by law.
115
Table of Contents
Registered Office and Objects
Our registered office in the Cayman Islands is located at the offices of Maples Corporate Services Limited, P.O. Box 309, Ugland House, Grand Cayman KY1-1104, Cayman Islands, or at such other location within the Cayman Islands as our directors may from time to time decide. The objects for which our company is established are unrestricted and we have full power and authority to carry out any object not prohibited by the Companies Act or any other law of the Cayman Islands.
Board of Directors
See “Item 6. Directors, Senior Management and Employees—C. Board Practice.”
Exempted Company
We are an exempted company with limited liability incorporated under the Companies Act. The Companies Act in the Cayman Islands distinguishes between ordinary resident companies and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands may apply to be registered as an exempted company. The requirements for an exempted company are essentially the same as for an ordinary company except for the exemptions and privileges listed below:
| • | an exempted company does not have to file an annual return of its shareholders with the Registrar of Companies; |
| • | an exempted company’s register of members is not open to inspection; |
| • | an exempted company does not have to hold an annual general meeting; |
| • | an exempted company may issue no par value shares; |
| • | an exempted company may obtain an undertaking against the imposition of any future taxation (such undertakings are usually given for 20 years in the first instance); |
| • | an exempted company may register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands; |
| • | an exempted company may register as a limited duration company; and |
“Limited liability” means that the liability of each shareholder is limited to the amount unpaid by the shareholder on the shares of the company (except in exceptional circumstances, such as involving fraud, the establishment of an agency relationship or an illegal or improper purpose or other circumstances in which a court may be prepared to pierce or lift the corporate veil). We are subject to reporting and other informational requirements of the Exchange Act, as applicable to foreign private issuers. The NASDAQ Global Market rules require that every company listed on the NASDAQ Global Market hold an annual general meeting of shareholders. In addition, our current articles of association allow directors to call special meeting of shareholders pursuant to the procedures set forth in our articles.
Differences in Corporate Law
The Companies Act is modeled after that of England and Wales but does not follow recent statutory enactments in England. In addition, the Companies Act differs from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of the significant differences between the provisions of the Companies Act applicable to us and the laws applicable to companies incorporated in the State of Delaware.
Mergers and Similar Arrangements
A merger of two or more constituent companies under Cayman Islands law requires a plan of merger or consolidation to be approved by the directors of each constituent company and authorization by a special resolution of the members of each constituent company.
A merger between a Cayman parent company and its Cayman subsidiary or subsidiaries does not require authorization by a resolution of shareholders of that Cayman subsidiary if a copy of the plan of merger is given to every member of that Cayman subsidiary to be merged unless that member agrees otherwise. For this purpose a company is a “parent” of a subsidiary if it holds issued shares that together represent at least ninety percent (90%) of the votes at a general meeting of the subsidiary.
The consent of each holder of a fixed or floating security interest over a constituent company is required unless this requirement is waived by a court in the Cayman Islands.
116
Table of Contents
Dissenting shareholders have the right to be paid the fair value of their shares (which, if not agreed between the parties, will be determined by the Cayman Islands court) if they follow the required procedures, subject to certain exceptions. The exercise of dissenter rights will preclude the exercise by the dissenting shareholder of any other rights to which he or she might otherwise be entitled by virtue of holding shares, save for the right to seek relief on the grounds that the merger or consolidation is void or unlawful. Court approval is not required for a merger or consolidation which is effected in compliance with these statutory procedures.
In addition, there are statutory provisions that facilitate the reconstruction and amalgamation of companies by way of schemes of arrangement, provided that the arrangement is approved by a majority in number of each class of shareholders and creditors with whom the arrangement is to be made, and who must, in addition, represent three-fourths in value of each such class of shareholders or creditors, as the case may be, that are present and voting either in person or by proxy at a meeting, or meetings, convened for that purpose. The convening of the meetings and subsequently the arrangement must be sanctioned by the Grand Court of the Cayman Islands. While a dissenting shareholder has the right to express to the court the view that the transaction ought not to be approved, the court can be expected to approve the arrangement if it determines that:
| • | the statutory provisions as to the required majority vote have been met; |
| • | the shareholders have been fairly represented at the meeting in question and the statutory majority are acting bona fide without coercion of the minority to promote interests adverse to those of the class; |
| • | the arrangement is such that may be reasonably approved by an intelligent and honest man of that class acting in respect of his interest; and |
| • | the arrangement is not one that would more properly be sanctioned under some other provision of the Companies Act. |
The Companies Act also contains a statutory power of compulsory acquisition which may facilitate the “squeeze out” of dissentient minority shareholder upon a takeover offer. When a takeover offer is made and accepted by holders of 90% of the shares within four months, the offeror may, within a two-month period commencing on the expiration of such four month period, require the holders of the remaining shares to transfer such shares on the terms of the offer. An objection can be made to the Grand Court of the Cayman Islands but this is unlikely to succeed in the case of an offer which has been so approved unless there is evidence of fraud, bad faith or collusion.
If an arrangement and reconstruction by way of scheme of arrangement is thus approved, or if a takeover offer is made and accepted, in accordance with the foregoing statutory procedures, the dissenting shareholder would have no rights comparable to appraisal rights, save that objectors to a takeover offer may apply to the Grand Court of the Cayman Islands for various orders that the Grand Court of the Cayman Islands has a broad discretion to make, which would otherwise ordinarily be available to dissenting shareholders of Delaware corporations, providing rights to receive payment in cash for the judicially determined value of the shares.
Shareholders’ Suits
In principle, we will normally be the proper plaintiff and as a general rule a derivative action may not be brought by a minority shareholder. However, based on English authorities, which would in all likelihood be of persuasive authority in the Cayman Islands, there are exceptions to the foregoing principle, including when:
| • | a company acts or proposes to act illegally or ultra vires; |
| • | the act complained of, although not ultra vires, could only be effected duly if authorized by more than a simple majority vote that has not been obtained; and |
| • | those who control the company are perpetrating a “fraud on the minority.” |
Indemnification of Directors and Executive Officers and Limitation of Liability
Cayman Islands law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Our current memorandum and articles of association permit indemnification of officers and directors for losses, damages, costs and expenses incurred in their capacities as such unless such losses or damages arise from dishonesty or fraud which may attach to such directors or officers. This standard of conduct is generally the same as permitted under the Delaware General Corporation Law for a Delaware corporation. In addition, we intend to enter into indemnification agreements with our directors and senior executive officers that will provide such persons with additional indemnification beyond that provided in our current memorandum and articles of association.
117
Table of Contents
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Anti-Takeover Provisions in the Memorandum and Articles of Association
Some provisions of our current memorandum and articles of association may discourage, delay or prevent a change in control of our company or management that shareholders may consider favorable, including provisions that authorize our board of directors to issue preferred shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preferred shares without any further vote or action by our shareholders.
However, under Cayman Islands law, our directors may only exercise the rights and powers granted to them under our current memorandum and articles of association, as amended and restated from time to time, for what they believe in good faith to be in the best interests of our company.
Directors’ Fiduciary Duties
Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he or she reasonably believes to be in the best interests of the corporation. He or she must not use his or her corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, a director must prove the procedural fairness of the transaction, and that the transaction was of fair value to the corporation.
As a matter of Cayman Islands law, a director of a Cayman Islands company is in the position of a fiduciary with respect to the company and therefore it is considered that he owes the following duties to the company—a duty to act bona fide in the best interests of the company, a duty not to make a profit based on his or her position as director (unless the company permits him to do so) and a duty not to put himself in a position where the interests of the company conflict with his or her personal interest or his or her duty to a third party. A director of a Cayman Islands company owes to the company a duty to act with skill and care. It was previously considered that a director need not exhibit in the performance of his or her duties a greater degree of skill than may reasonably be expected from a person of his or her knowledge and experience. However, English and Commonwealth courts have moved towards an objective standard with regard to the required skill and care and these authorities are likely to be followed in the Cayman Islands.
Shareholder Action by Written Consent
Under the Delaware General Corporation Law, a corporation may eliminate the right of shareholders to act by written consent by amendment to its certificate of incorporation. Our current memorandum and articles of association provide that shareholders may approve corporate matters by way of a unanimous written resolution signed by or on behalf of each shareholder who would have been entitled to vote on such matter at a general meeting without a meeting being held.
Shareholder Proposals
Under the Delaware General Corporation Law, a shareholder has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings.
As an exempted Cayman Islands company, we are not obliged by law to call shareholders’ annual general meetings.
118
Table of Contents
Cumulative Voting
Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director. As permitted under Cayman Islands law, our current memorandum and articles of association do not provide for cumulative voting. As a result, our shareholders are not afforded any less protections or rights on this issue than shareholders of a Delaware corporation.
Removal of Directors
Under the Delaware General Corporation Law, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. Under our current memorandum and articles of association, directors may be removed by an ordinary resolution of shareholders.
Transactions with Interested Shareholders
The Delaware General Corporation Law contains a business combination statute applicable to Delaware corporations whereby, unless the corporation has specifically elected not to be governed by such statute by amendment to its certificate of incorporation, it is prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or a group who or which owns or owned 15% or more of the target’s outstanding voting stock within the past three years. This has the effect of limiting the ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The statute does not apply if, among other things, prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware corporation to negotiate the terms of any acquisition transaction with the target’s board of directors.
Cayman Islands law has no comparable statute. As a result, we cannot avail ourselves of the types of protections afforded by the Delaware business combination statute. However, although Cayman Islands law does not regulate transactions between a company and its significant shareholders, it does provide that such transactions must be entered into bona fide in the best interests of the company and for a proper corporate purpose and not with the effect of constituting a fraud on the minority shareholders.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board. Under Cayman Islands law, a company may be wound up by either an order of the courts of the Cayman Islands or by a special resolution of its members or, if the company is unable to pay its debts as they fall due, by an ordinary resolution of its members. The court has authority to order winding up in a number of specified circumstances including where it is, in the opinion of the court, just and equitable to do so.
Under the Companies Act and our current memorandum and articles of association, our company may be dissolved, liquidated or wound up with the sanction of a special resolution at a meeting.
Variation of Rights of Shares
Under the Delaware General Corporation Law, a corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise. Under our current memorandum and articles of association, if our share capital is divided into more than one class of shares, we may materially adversely vary the rights attached to any class only with the sanction of an ordinary resolution passed at a general meeting of the holders of the shares of that class or the written consent the holders of all of the issued shares of that class.
Amendment of Governing Documents
Under the Delaware General Corporation Law, a corporation’s governing documents may be amended with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. As permitted by Cayman Islands law, our current articles of association may only be amended by a special resolution of shareholders.
119
Table of Contents
Rights of Non-Resident or Foreign Shareholders
There are no limitations imposed by our current memorandum and articles of association on the rights of non-resident or foreign shareholders to hold or exercise voting rights on our shares. In addition, there are no provisions in our current memorandum and articles of association that require our company to disclose shareholder ownership above any particular ownership threshold.
Directors’ Power to Issue Shares
Subject to applicable law, our board of directors is empowered to issue or allot shares or grant options and warrants with or without preferred, deferred, qualified or other special rights or restrictions.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company,” “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions,” or elsewhere in this annual report on Form 20-F.
D. Exchange Controls
See “Item 4. Information on the Company—B. Business Overview—Regulation—Regulations Relating to Foreign Exchange.”
E. Taxation
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to us levied by the government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or brought within the jurisdiction of, the Cayman Islands. The Cayman Islands is not party to any double tax treaties that are applicable to any payments made to or by our company. There are no exchange control regulations or currency restrictions in the Cayman Islands.
Payments of dividends and capital in respect of our ADSs or ordinary shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of a dividend or capital to any holder of ADSs or ordinary shares, nor will gains derived from the disposal of ADSs or ordinary shares be subject to Cayman Islands income or corporation tax.
No stamp duty is payable in respect of the issue of ADSs or ordinary shares or on an instrument of transfer in respect of ADSs or ordinary shares.
PRC Taxation
Under the PRC Enterprise Income Tax Law and its implementation rules, an enterprise established outside China with a “de facto management body” within China is considered as a resident enterprise. The implementation rules define the term “de facto management body” as the body that exercises full and substantial control and overall management over the business, productions, personnel, accounts and properties of an enterprise. In April 2009, the SAT issued the Circular Regarding the Determination of Chinese-Controlled Overseas Incorporated Enterprises as PRC Tax Resident Enterprises on the Basis of De Facto Management Bodies, known as Circular 82, which provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled enterprise that is incorporated offshore is located in China. Although this circular only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreigners, the criteria set forth in the circular may reflect the SAT’s general position on how the “de facto management body” test should be applied in determining the tax resident status of all offshore enterprises. According to Circular 82, an offshore incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group will be regarded as a PRC tax resident by virtue of having its “de facto management body” in China only if all of the following conditions are met: (i) the primary location of the day-to-day operational management is in China; (ii) decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in China; (iii) the enterprise’s primary assets, accounting books and records, company seals, and board and shareholder resolutions, are located or maintained in China; and (iv) at least 50% of voting board members or senior executives habitually reside in China. In 2011, the SAT issued the Administrative Measures for Enterprise Income Tax of Chinese-Controlled Overseas Incorporated Resident Enterprises (Trial Version), or Bulletin No. 45, which further clarifies certain issues related to the determination of tax resident status and competent tax authorities. It also specifies that when provided with a copy of Recognition of Residential Status from a resident Chinese- controlled offshore-incorporated enterprise, a payer does not need to withhold income tax when paying certain PRC-sourced income such as dividends, interest and royalties to such Chinese-controlled offshore-incorporated enterprise.
120
Table of Contents
We believe that we are not a PRC resident enterprise for PRC tax purposes. We are not controlled by a PRC enterprise or PRC enterprise group and we do not believe that we meet all of the conditions above. We are a company incorporated outside China and our records (including the minutes and resolutions of our board of directors and the resolutions of our shareholders) are maintained outside China. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.”
If the PRC tax authorities determine that we are a PRC resident enterprise for enterprise income tax purposes, we may be required to withhold a 10% withholding tax from dividends we pay to our shareholders that are non-resident enterprises, including the holders of our ADSs. In addition, non-resident enterprise shareholders (including our ADS holders) may be subject to a 10% PRC tax on gains realized on the sale or other disposition of ADSs or Class A ordinary shares, if such income is treated as sourced from within China. It is unclear whether our non-PRC individual shareholders (including our ADS holders) would be subject to any PRC tax on dividends or gains obtained by such non-PRC individual shareholders in the event we are determined to be a PRC resident enterprise. If any PRC tax were to apply to such dividends or gains, it would generally apply at a rate of 20% unless a reduced rate is available under an applicable tax treaty. However, it is also unclear whether our non-PRC shareholders would be able to claim the benefits of any tax treaties between their country of tax residence and China in the event that we are treated as a PRC resident enterprise. See “Item 3. Key Information—D. Risk Factors—Risks Relating to Doing Business in the PRC—You may be subject to PRC income tax on dividends from us or on any gain realized on the transfer of our ADSs.”
United States Federal Income Tax Considerations
The following is a summary of material U.S. federal income tax considerations that are likely to be relevant to the purchase, ownership and disposition of our Class A ordinary shares or ADSs by a U.S. Holder (as defined below).
This summary is based on provisions of the Internal Revenue Code of 1986, as amended (the “Code”), and regulations, rulings and judicial interpretations thereof, in force as of the date hereof. Those authorities may be changed at any time, perhaps retroactively, so as to result in U.S. federal income tax consequences different from those summarized below.
This summary is not a comprehensive discussion of all of the tax considerations that may be relevant to a particular investor’s decision to purchase, hold, or dispose of Class A ordinary shares or ADSs. In particular, this summary is directed only to U.S. Holders that hold Class A ordinary shares or ADSs as capital assets and does not address particular tax consequences that may be applicable to U.S. Holders who may be subject to special tax rules, such as banks, brokers or dealers in securities or currencies, traders in securities electing to mark to market, financial institutions, insurance companies, tax-exempt entities, regulated investment companies, entities or arrangements that are treated as partnerships for U.S. federal income tax purposes (or the partners therein), holders that own or are treated as owning 10% or more of our stock by vote or value, persons holding Class A ordinary shares or ADSs as part of a hedging or conversion transaction or a straddle, or persons whose functional currency is not the U.S. dollar. Moreover, this summary does not address state, local or foreign taxes, the U.S. federal estate and gift taxes, or the Medicare contribution tax applicable to net investment income of certain non-corporate U.S. Holders, or alternative minimum tax consequences of acquiring, holding or disposing of Class A ordinary shares or ADSs.
For purposes of this summary, a “U.S. Holder” is a beneficial owner of Class A ordinary shares or ADSs that is a citizen or resident of the U.S. or a U.S. domestic corporation or that otherwise is subject to U.S. federal income taxation on a net income basis in respect of such Class A ordinary shares or ADSs.
You should consult your own tax advisors about the consequences of the acquisition, ownership and disposition of the Class A ordinary shares or ADSs, including the relevance to your particular situation of the considerations discussed below and any consequences arising under foreign, state, local or other tax laws.
ADSs
In general, if you are a U.S. Holder of ADSs, you will be treated, for U.S. federal income tax purposes, as the beneficial owner of the underlying Class A ordinary shares that are represented by those ADSs. References to “shares” below apply to both Class A ordinary shares and ADSs, unless the context indicates otherwise.
Deposits and withdrawals of Class A ordinary shares by U.S. Holders in exchange for ADSs will not result in the realization of gain or loss for U.S. federal income tax purposes.
121
Table of Contents
Taxation of Dividends
As discussed in “Dividend Policy,” we do not have any present plan to pay any cash dividends on our shares in the foreseeable future. Subject to the discussion below under “Passive Foreign Investment Company Rules,” the gross amount of any distribution of cash or property with respect to our shares (including amounts, if any, withheld in respect of PRC taxes) that is paid out of our current or accumulated earnings and profits (as determined for U.S. federal income tax purposes) will generally be includible in your taxable income as ordinary dividend income on the day on which you receive the dividend, in the case of Class A ordinary shares, or the date the depositary receives the dividends, in the case of ADSs, and will not be eligible for the dividends-received deduction allowed to U.S. corporations under the Code.
We do not expect to maintain calculations of our earnings and profits in accordance with U.S. federal income tax principles. U.S. Holders therefore should expect that distributions generally will be treated as dividends for U.S. federal income tax purposes.
In the event that we are deemed to be a PRC resident enterprise under the PRC Enterprise Income Tax Law (see “E. Taxation—PRC Taxation”), a U.S. Holder may be subject to PRC withholding taxes on dividends paid on our shares. In that case, we may, however, be eligible for the benefits of the Agreement Between the Government of the United States of America and the Government of the People’s Republic of China for the Avoidance of Double Taxation and the Prevention of Tax Evasion with Respect to Taxes on Income (the “Treaty”).
Dividend distributions will constitute income from sources outside the U.S. and, for U.S. Holders that elect to claim foreign tax credits, generally will constitute “passive category income” for foreign tax credit purposes. Subject to generally applicable limitations and conditions PRC dividend withholding tax paid at the appropriate rate applicable to the U.S. Holder may be eligible for a credit against such U.S. Holder’s U.S. federal income tax liability. These generally applicable limitations and conditions include new requirements adopted by the IRS in regulations promulgated in December 2021 and any PRC tax will need to satisfy these requirements in order to be eligible to be a creditable tax for a U.S. Holder. In the case of a U.S. Holder that either (i) is eligible for, and properly elects, the benefits of the Treaty, or (ii) consistently elects to apply a modified version of these rules under recently issued temporary guidance and complies with specific requirements set forth in such guidance, the PRC tax on dividends will be treated as meeting the new requirements and therefore as a creditable tax. In the case of all other U.S. Holders, the application of these requirements to the PRC tax on dividends is uncertain and we have not determined whether these requirements have been met. If the PRC dividend tax is not a creditable tax for a U.S. Holder or the U.S. Holder does not elect to claim a foreign tax credit for any foreign income taxes paid or accrued in the same taxable year, the U.S. Holder may be able to deduct the PRC tax in computing such U.S. Holder’s taxable income for U.S. federal income tax purposes. The availability and calculation of foreign tax credits and deductions for foreign taxes depend on a U.S. Holder’s particular circumstances and involve the application of complex rules to those circumstances. The temporary guidance discussed above also indicates that the Treasury and the IRS are considering proposing amendments to the December 2021 regulations and that the temporary guidance can be relied upon until additional guidance is issued that withdraws or modifies the temporary guidance. U.S. Holders should consult their own tax advisors regarding the application of these rules to their particular situations. U.S. Holders should consult their own tax advisors regarding the application of these rules to their particular situations.
U.S. Holders that receive distributions of additional shares or rights to subscribe for shares as part of a pro rata distribution to all our shareholders generally will not be subject to U.S. federal income tax in respect of the distributions, unless the U.S. Holder has the right to receive cash or property, in which case the U.S. Holder will be treated as if it received cash equal to the fair market value of the distribution.
Taxation of Dispositions of Shares
Subject to the discussion below under “Passive Foreign Investment Company Rules,” upon a sale, exchange or other taxable disposition of the shares, U.S. Holders will realize gain or loss for U.S. federal income tax purposes in an amount equal to the difference between the amount realized on the disposition and the U.S. Holder’s adjusted tax basis in the shares, both as determined in U.S. dollars. Such gain or loss will be capital gain or loss, and will generally be long-term capital gain or loss if the shares have been held for more than one year. Long-term capital gain realized by a non-corporate U.S. Holder is subject to taxation at a preferential rate. The deductibility of capital losses is subject to limitations.
122
Table of Contents
A U.S. Holder generally will not be entitled to credit any PRC tax imposed on the sale or other disposition of the shares against such U.S. Holder’s U.S. federal income tax liability, except in the case of either (i) a U.S. Holder that is eligible for, and properly elects to claim, the benefits of the Treaty, or (ii) a U.S. Holder that consistently elects to apply a modified version of the U.S. foreign tax credit rules that is permitted under recently issued temporary guidance and complies with the specific requirements set forth in such guidance. Capital gain or loss recognized by a U.S. Holder on the sale or other disposition of the shares generally will be U.S. source gain or loss for U.S. foreign tax credit purposes (except to the extent that the U.S. Holder establishes the right to treat gain as foreign source income under the Treaty). Consequently, even if the withholding tax qualifies as a creditable tax, a U.S. Holder may not be able to credit the tax against its U.S. federal income tax liability unless such credit can be applied (subject to generally applicable conditions and limitations) against tax due on other income treated as derived from foreign sources. If the PRC tax is not a creditable tax or is not claimed as a credit by the U.S. holder pursuant to the Treaty, the tax would reduce the amount realized on the sale or other disposition of the shares even if the U.S. Holder has elected to claim a foreign tax credit for other taxes in the same year. The temporary guidance discussed above also indicates that the Treasury and the IRS are considering proposing amendments to the December 2021 regulations and that the temporary guidance can be relied upon until additional guidance is issued that withdraws or modifies the temporary guidance. U.S. Holders should consult their own tax advisors regarding the application of the foreign tax credit rules to a sale or other disposition of the shares and any PRC tax imposed on such sale or disposition.
Passive Foreign Investment Company Rules
Special U.S. tax rules apply to companies that are considered to be PFICs. We will be classified as a PFIC in a particular taxable year if, either:
| • | 75% or more of our gross income for the taxable year is passive income; or |
| • | the average percentage of the value of our assets (generally based on a quarterly average) that is attributable to assets that produce or are held for the production of passive income is at least 50%. |
For this purpose, passive income generally includes dividends, interest, gains from certain commodities transactions, rents, royalties and the excess of gains over losses from the disposition of assets that produce passive income. Goodwill is treated as an active asset under the PFIC rules to the extent attributable to activities that produce active income. Cash generally is a passive asset for these purposes. If we own at least 25% (by value) of the stock of another corporation, for purposes of determining whether we are a PFIC, we will be treated as owning our proportionate share of the other corporation’s assets and receiving our proportionate share of the other corporation’s income. Although the law in this regard is not entirely clear, we treat our VIE as being owned by us for U.S. federal income tax purposes because we control its management decisions and are entitled to substantially all of the economic benefits associated with it.
Based on our financial statements, the manner in which we conduct our business, the trading price of our Class A ordinary shares or ADSs, the value and nature of our assets, and the sources and nature of our income, we believe that we were a PFIC for our prior taxable year. Additionally, there is a significant risk that we will be a PFIC for our current taxable year and in future taxable years. The determination of whether we are a PFIC must be made annually based on the facts and circumstances at that time.
If we are classified as a PFIC for any taxable year in which a U.S. Holder holds our stock, we will generally continue to be treated as a PFIC with respect to such U.S. Holder for all succeeding years during which such holder owns our shares, even if we cease to meet the threshold requirements for PFIC status (unless the U.S. Holder makes a special “purging” election on IRS Form 8621 with respect to our shares once we are no longer a PFIC). U.S. Holders are urged to consult their own tax advisors about the application of the PFIC rules to us.
If we are classified as a PFIC for any taxable year during which a U.S. Holder holds shares and such U.S. Holder does not make the election described below, such U.S. Holder will be subject to a special tax at ordinary income tax rates on “excess distributions” (generally, any distributions that a U.S. Holder receives in a taxable year that are greater than 125 percent of the average annual distributions that such U.S. Holder has received in the preceding three taxable years, or its holding period, if shorter), as well as any gain that such U.S. Holder recognizes on the sale or other disposition of its shares. Under these rules (a) the excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period for the shares, (b) the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which we are a PFIC will be taxed as ordinary income, and (c) the amount allocated to each of the other taxable years will be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit will be imposed with respect to the resulting tax attributable to each such other taxable year.
Classification as a PFIC may also have other adverse tax consequences, including, in the case of individuals, the denial of a step-up in the basis of shares at death.
123
Table of Contents
If we are a PFIC for any taxable year during which a U.S. Holder holds our shares and we have any direct, and in certain circumstances, indirect subsidiaries that are PFICs (each a “Subsidiary PFIC”), the U.S. Holder will be treated as owning its pro rata share of the stock of each such Subsidiary PFIC for purposes of the application of these rules, and the U.S. Holder generally would be subject to similar rules with respect to distributions to us by, and dispositions by us of the stock of, any of our direct or indirect subsidiaries that are PFICs. U.S. Holders are urged to consult their own tax advisors about the application of the PFIC rules to any of our subsidiaries.
A U.S. Holder may be able to avoid the interest charge described above by electing to mark its shares to market, provided the shares are considered “marketable.” The shares will be marketable if they are regularly traded on certain U.S. stock exchanges, including the NASDAQ Global Market, or on a non-U.S. stock exchange if (i) the non-U.S. stock exchange is regulated or supervised by a governmental authority in the country in which the exchange is located, (ii) the non-U.S. stock exchange has trading volume, listing, financial disclosure, surveillance and other requirements designed to prevent fraudulent and manipulative acts and practices, remove impediments to, and perfect the mechanism of, a free and open, fair and orderly, market and to protect investors, (iii) the laws of the country in which the exchange is located and the rules of the exchange ensure that these requirements are actually enforced, and (iv) the rules of the exchange ensure active trading during any calendar year during which they are traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. It should be noted that only the ADSs, and not the Class A ordinary shares, are listed on the NASDAQ Global Market. Consequently, a mark-to-market election is not expected to be available for a U.S. Holder that holds Class A ordinary shares that are not represented by ADSs.
If the U.S. Holder makes a mark-to-market election with respect to its ADSs, the holder will be required in any year in which we are a PFIC to include as ordinary income the excess of the fair market value of its ADSs at year-end over the holder’s basis in those ADSs. If at the end of the U.S. Holder’s taxable year for a year in which we were a PFIC, the holder’s basis in the ADSs exceeds their fair market value, the holder will be entitled to deduct the excess as an ordinary loss, but only to the extent of the holder’s net mark-to-market gains from previous years. The holder’s adjusted tax basis in the ADSs will be adjusted to reflect any income or loss recognized under these rules. In addition, any gain the U.S. Holder recognizes upon the sale or other disposition of its ADSs in a year in which we were a PFIC will be taxed as ordinary income in the year of sale and any loss will be treated as an ordinary loss to the extent of the U.S. Holder’s net mark-to-market gains from previous years. Once made, the election cannot be revoked without the consent of the IRS unless the ADSs cease to be marketable.
However, a U.S. Holder will not be able to make a mark-to-market election with respect to the stock of any Subsidiary PFIC. Therefore, the U.S. Holder would continue to be subject to the excess distribution rules with respect to any of our subsidiaries that are PFICs, any distributions received by us from a subsidiary that is a PFIC and any gain recognized by us upon a sale of equity interests in a subsidiary that is a PFIC, even if the U.S. Holder has made a mark-to-market election with respect to our ADSs. The interaction of the mark-to-market rules and the rules governing lower-tier PFICs is complex and uncertain, and investors should therefore consult their own tax advisors regarding the availability of the mark-to-market election as well as the application of the PFIC rules to their ownership of our shares.
In some cases, a shareholder of a PFIC may be subject to alternative treatment by making a valid qualified electing fund election, or QEF election. If a QEF election is made, such U.S. Holder generally will be required to include in income on a current basis its pro rata share of the PFIC’s ordinary income and net capital gains, regardless of whether or not such earnings and gains are actually distributed to such U.S. Holder. To make a QEF election, the PFIC must provide shareholders with certain information compiled according to U.S. federal income tax principles. We do not intend, however, to prepare or provide the information that would enable U.S. Holders to make QEF elections.
A U.S. Holder that owns an equity interest in a PFIC generally must annually file IRS Form 8621, and may be required to file other IRS forms. A failure to file one or more of these forms as required may toll the running of the statute of limitations in respect of each of the holder’s taxable years for which such form is required to be filed. As a result, the taxable years with respect to which the U.S. Holder fails to file the form may remain open to assessment by the IRS indefinitely, until the form is filed.
You should consult your own tax advisor regarding the U.S. federal income tax considerations discussed above and the desirability of making a mark-to-market election.
124
Table of Contents
Foreign Financial Asset Reporting
Certain U.S. Holders that own specified foreign financial assets with an aggregate value in excess of U.S.$50,000 on the last day of the taxable year or U.S. $75,000 (and in some circumstances, higher thresholds) at any time during the taxable year are generally required to file an information statement along with their tax returns, currently on IRS Form 8938, with respect to such assets. Specified foreign financial assets include any financial accounts held at a non-U.S. financial institution, as well as securities issued by a non-U.S. issuer that are not held in accounts maintained by financial institutions. The understatement of income attributable to “specified foreign financial assets” in excess of U.S.$5,000 extends the statute of limitations with respect to the tax return to six years after the return was filed. U.S. Holders who fail to report the required information could be subject to substantial penalties. Prospective investors are encouraged to consult with their own tax advisors regarding the possible application of these rules, including the application of the rules to their particular circumstances.
Backup Withholding and Information Reporting
Dividends paid on shares to a U.S. Holder and proceeds from the sale or other disposition of the shares by a U.S. Holder generally may be subject to the information reporting requirements of the Code and may be subject to backup withholding unless the U.S. Holder provides an accurate taxpayer identification number and makes any other required certification or otherwise establishes an exemption. Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a refund or credit against the U.S. Holder’s U.S. federal income tax liability, provided the required information is furnished to the IRS in a timely manner.
A holder that is not a U.S. Holder may be required to comply with certification and identification procedures in order to establish its exemption from information reporting and backup withholding.
UK Taxation
The following is a summary of certain aspects of the UK tax treatment of an investment in the ADSs. It is for general information only and is not an exhaustive analysis of all UK tax consequences of acquiring, holding or disposing of the ADSs. It does not constitute legal or tax advice.
The comments set out below are based on current UK tax law as applied in England and Wales and HM Revenue & Customs published practice (which may not be binding on HM Revenue & Customs) as at the date of this Prospectus, both of which are subject to change, possibly with retrospective effect.
The following comments are intended as a general guide and, except where expressly stated otherwise, apply only to holders of the ADSs who: (i) are: (x) resident and, in the case of an individual, domiciled, for tax purposes in the UK and to whom “split year” treatment does not apply; or (y) hold ADSs through a permanent establishment or branch within the UK; (ii) do not have a permanent establishment or fixed base outside the UK with which the holding of the ADSs (and dividends paid in respect of the ADSs) is connected; (iii) hold ADSs as an investment (other than under an Individual Savings Account (including Lifetime ISAs) or a Self-investment Personal Pension); and (iv) are, or are treated as, the absolute beneficial owners thereof. For the avoidance of doubt, the following comments do not apply to holders of the ADSs who are temporarily non-resident in the UK.
Certain categories of holders of ADSs, including those carrying on certain financial activities, those subject to specific tax regimes or benefiting from certain reliefs or exemptions, those connected with the Company or Group and those for whom the ADSs are employment related securities, may be subject to special rules and this summary does not apply to such holders. This summary also does not apply to any holder of ADSs who, alone or with certain associated persons, is (or has been) interested or treated as interested in more than 5% of the ordinary share capital of the Company.
Each investor’s specific circumstances will impact on their UK taxation position, and all investors are recommended to obtain their own bespoke taxation advice. Holders of ADSs and prospective holders of ADSs who are in any doubt about their tax position, or who are resident or otherwise subject to taxation in a jurisdiction outside the UK, should consult their own professional advisers immediately.
This summary assumes that: (a) the ADSs and the underlying Shares are not registered in a register kept in the UK by or on behalf of the Company; (b) the ADSs and the underlying Shares are not paired with shares issued by a company incorporated in the UK; (c) the underlying Shares will not be held by, and the ADSs will not be issued by, a depositary (or nominee or agent for a depositary) incorporated in the UK or acting from an office within the UK; (d) dividends paid in respect of the underlying Shares will be treated as income distributions for UK tax purposes; and (e) the holders of ADSs are the absolute beneficial owners of the underlying Shares and of any dividends paid in respect thereof for UK tax purposes.
125
Table of Contents
Taxation of the Company
The board of directors intend to conduct the affairs of the Company so that the Company does not become resident in the UK for taxation purposes or otherwise subject to UK corporation tax. The summary below is written on the basis that the Company is and remains resident for tax purposes solely in the Cayman Islands.
Taxation of Dividends
The Company will not be required to withhold amounts on account of UK tax at source when paying a dividend. Except where expressly stated otherwise, the following paragraphs proceed on the basis that no withholding tax is levied in the Cayman Islands or any other jurisdiction on dividend payments in respect of the ADSs.
Holders of ADSs subject to UK Income Tax
Dividends received by a holder of ADSs subject to UK income tax will generally be subject to tax as dividend income.
For UK resident individual holders, the first £500 (the “Dividend Allowance”) of the gross amount of any UK and non-UK source dividend income and certain other distributions in respect of shares (including any dividends received in respect of the underlying Shares) (“Dividend Income”) received by such an individual in a tax year will be taxed at a nil rate (and so no income tax will be payable in respect of such amounts).
Where the total Dividend Income of such an individual exceeds the Dividend Allowance (such excess being referred to as the “Taxable Excess”), then the Taxable Excess will be subject to tax depending on the tax rate band or bands it falls within. The relevant tax rate band is determined by reference to the total income of a UK resident individual holder of ADSs charged to income tax (including the Dividend Income charged at a nil rate by virtue of the Dividend Allowance) less relevant reliefs and allowances (including the personal allowance of such individual). The Taxable Excess is treated as the top slice of that total income. So, on that basis:
| • | To the extent that the Taxable Excess falls below the basic rate limit, a UK resident individual holder of ADSs will be subject to tax on it at the dividend basic rate of 8.75%. |
| • | To the extent that the Taxable Excess falls above the basic rate limit but below the higher rate limit, a UK resident individual holder of ADSs will be subject to tax on it at the dividend higher rate of 33.75%. |
| • | To the extent that the Taxable Excess falls above the higher rate limit, a UK resident individual holder of ADSs will be subject to tax on it at the dividend additional rate of 39.35%. |
The taxation of dividends received by trusts depends on the type of trust. Trustees should seek their own bespoke taxation advice.
Corporate Holders of ADSs
Holders of ADSs who are within the charge to corporation tax in respect of ADSs will be subject to corporation tax on any dividends paid by the Company unless (subject to special rules for such holders of ADSs that are small companies) the dividends fall within an exempt class and certain other conditions are met. The position of each holder of ADSs will depend on its own individual circumstances, although it would normally be expected that the dividends paid by the Company would fall within an exempt class.
If the conditions to fall within an exempt class are not met (or cease to be satisfied), or a holder of ADSs elects for an otherwise exempt dividend to be taxable, the holder of ADSs will be subject to UK corporation tax on dividends received from the Company at the rate of corporation tax applicable to that holder (the main rate is currently 25%).
Credit for PRC Tax
If PRC tax is withheld from dividends paid by the Company, credit may be given against UK tax on the same income subject to general rules regarding the calculation and availability of such credit, including a requirement to take all reasonable steps to minimize the amounts of PRC tax on such dividends, including claiming any available allowances and reliefs. Where a dividend paid by the Company is treated as exempt from UK corporation tax, a UK resident company will not be entitled to claim relief by way of credit in the UK in respect of any PRC tax paid by such holder, either directly or by deduction, in respect of that dividend.
Taxation of Capital Gains
Holders of ADSs who are resident in the UK may, depending on their circumstances (including the availability of exemptions or reliefs), be liable to UK taxation on chargeable gains in respect of gains arising from a sale or other disposal (or deemed disposal) of ADSs.
126
Table of Contents
Holders of ADSs subject to UK Capital Gains Tax
For a holder of ADSs subject to UK capital gains tax, a disposal (or deemed disposal) of the ADSs may give rise to a chargeable gain or an allowable loss for the purposes of UK capital gains tax.
Upon the disposal or deemed disposal of the ADSs, and after all allowable deductions, a UK resident individual holder’s taxable chargeable gains would, for the 2024/2025 tax year, generally be taxed at (i) 10% if the aggregate of the taxable chargeable gains and other taxable income for the year of that UK resident individual holder does not exceed the basic rate income tax limit; or (ii) 20%, where the aggregate of the taxable chargeable gains and other taxable income for the year of the relevant holder does exceed the basic rate income tax limit. A UK resident individual holder is entitled to realize an annual exempt amount of gains (£3,000 for the 2024/2025 tax year) without being liable to UK capital gains tax.
Corporate Holders of ADSs
For a holder of ADSs that is within the charge to UK corporation tax, a disposal (or deemed disposal) of the ADSs may give rise to a chargeable gain subject to UK corporation tax (currently at a main rate of 25%) or an allowable loss for the purposes of UK corporation tax.
Stamp Duty and Stamp Duty Reserve Tax (“SDRT”)
The following comments are intended as a general guide to the current UK stamp duty and SDRT position, and apply regardless of whether or not an investor is resident in the UK. Certain categories of person (including specified market intermediaries) are entitled to exemption from stamp duty and SDRT on purchases of securities in specified circumstances.
No UK stamp duty or SDRT will be payable on (i) the issue of the ADSs, (ii) the delivery of the ADSs into DTC, Euroclear or Clearstream or (iii) any dealings in the ADSs once they are delivered into such clearance systems, where such dealings are effected in book-entry form in accordance with the procedures of DTC, Euroclear or Clearstream (as applicable) and not by written instrument of transfer.
No SDRT will be payable in respect of any agreement to transfer the ADSs or underlying shares, on the basis of the assumptions set out above in this summary.
Assuming that any document effecting a transfer of the ADSs or the underlying Shares, or containing an agreement to transfer an equitable interest in the ADSs or the underlying Shares is neither (i) executed in the UK, nor (ii) relates to any property situate, or to any matter or thing done or to be done, in the UK (the term “matter or thing done or to be done” is very wide and can cover the involvement of UK bank accounts in payment mechanics), then no UK stamp duty should be required to be paid on such document.
Even if a document effecting a transfer of the ADSs or the underlying Shares, or containing an agreement to transfer an equitable interest in the ADSs or the underlying Shares is (i) executed in the UK and/or (ii) relates to any property situate, or to any matter or thing done or to be done, in the UK, in practice it should not be necessary to pay any UK stamp duty on such document unless the document is required for any purpose in the UK. If it is necessary to pay UK stamp duty, it may also be necessary to pay interest and penalties.
Inheritance Tax
The ADSs should be assets situated outside the UK for the purposes of UK inheritance tax provided the Shares (and any other property represented by the ADSs) are situated outside the UK, the ADSs are not registered in any register kept in the UK and the Depositary (or any nominee or agent for the Depositary) remains a non-UK incorporated company acting from an office outside the UK. The Shares are assets situated outside the United Kingdom for these purposes on the basis that they are not registered in any register kept in the UK.
A gift of such assets by, or the death of, an individual holder of such assets who is domiciled or is deemed to be domiciled in the UK may (subject to certain exemptions and reliefs) give rise to a liability to UK inheritance tax. Generally, UK inheritance tax is not chargeable on gifts to individuals if the transfer is made more than seven complete years prior to the death of the donor. For inheritance tax purposes, a transfer of assets at less than full market value may be treated as a gift and particular rules apply to gifts where the donor reserves or retains some benefit. Special rules apply to close companies and to trustees of certain settlements who hold ADSs that may bring them within the charge to inheritance tax.
127
Table of Contents
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We have filed a registration statement, including relevant exhibits, with the SEC on Form F-1 (Registration No. 333-238596) under the Securities Act to register the issuance and sale of our Class A ordinary shares represented by ADSs in relation to our initial public offering. We have also filed a related registration statement on Form F-6 (Registration No. 333-238921) with the SEC to register the ADSs representing our Class A ordinary shares.
We are subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Accordingly, we are required to file reports, including annual reports on Form 20-F, and other information with the SEC. All information filed with the SEC can be obtained over the internet at the SEC’s website at www.sec.gov or inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of documents, upon payment of a duplicating fee, by writing to the SEC.
As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we intend to furnish the depositary with our annual reports, which will include a review of operations and annual audited consolidated combined financial statements prepared in conformity with IFRS, and all notices of shareholders’ meetings and other reports and communications that are made generally available to our shareholders. The depositary will make such notices, reports and communications available to holders of ADSs and, if we so request, will mail to all record holders of ADSs the information contained in any notice of a shareholders’ meeting received by the depositary from us.
I. Subsidiary Information
Not applicable.
ITEM 11. QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
Credit risk
Our credit risk is mainly associated with cash and cash equivalents, restricted cash, short-term investment, long-term investment, and accounts receivable. We place our cash and cash equivalents, restricted cash, short-term investment and long-term investment with reputable financial institutions of high credit quality. As of December 31, 2023, approximately 99.99% of our cash and cash equivalents and restricted cash were held at major financial institutions located in the PRC, and approximately 0.01% were deposited with major financial institutions located outside the PRC. There has been no recent history of default related to these financial institutions. We continue to monitor the credit worthiness of these financial institutions.
Accounts receivables, typically unsecured and denominated in Renminbi, are derived from revenues earned from reputable customers. As of December 31, 2023, we had no single customer with a receivable balance exceeding 10% of the total accounts receivable balance. We manage credit risk of accounts receivable through ongoing monitoring of the outstanding balances.
Foreign currency exchange risk
From July 21, 2005, the RMB is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. For U.S. dollar against RMB, there was depreciation of approximately 2.3%, appreciation of approximately 8.2% and depreciation of approximately 2.9% in the years ended December 31, 2021, 2022 and 2023, respectively. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the RMB and the U.S. dollar in the future.
128
Table of Contents
Our functional currency and reporting currency are the US$ and the RMB, respectively. Most of our revenues and costs are denominated in RMB, while a portion of cash and cash equivalents are denominated in US$. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the Renminbi and the US$ in the future. Any significant fluctuation of the valuation of RMB may materially affect the Group’s cash flows, revenues, earnings and financial position, and the value of any dividends payable on the ADS in US$.
Substantially all of our business is transacted in Renminbi, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the PBOC or other authorized financial institution at exchange rates quoted by the PBOC. Approval of foreign currency payments by the PBOC or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices and signed contracts.
Interest rate risk
Fluctuations in market interest rates may negatively affect our financial condition and results of operations. We did not have any borrowings as of December 31, 2023. As a result, we are not exposed to fair value interest rate risk due to our borrowings with fixed interest rates. We have not been exposed, nor do we anticipate to be exposed, to material risks due to changes in interest rates, and we have not used any derivative financial instruments to manage our interest risk exposure. We may incur additional borrowings or other facilities in the future. In addition, our future financial condition and results of operations may be affected due to changes in market interest rates.
Inflation
Since our inception, inflation has not materially affected our results of operations. According to the National Bureau of Statistics of China, the year-over-year percent change in the consumer price index for 2021, 2022 and 2023 were increases of 0.9%, 2.0% and 0.2%, respectively. Although we have not been materially affected by inflation, we may be affected if China experiences higher rates of inflation in the future.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
129
Table of Contents
D. American Depositary Shares
Fees and Charges
As an ADS holder, you will be required to pay the following fees under the terms of the deposit agreement:
Service | Fees | |
• Issuance of ADSs (e.g., an issuance of ADS upon a deposit of Class A ordinary shares, upon a change in the ADS(s)-to-Shares ratio, or for any other reason), excluding ADS issuances as a result of distributions of Class A ordinary shares | Up to U.S. 5¢ per ADS issued | |
• Cancellation of ADSs (e.g., a cancellation of ADSs for delivery of deposited property, upon a change in the ADS(s)-to-Shares ratio, or for any other reason) | Up to U.S. 5¢ per ADS cancelled | |
• Distribution of cash dividends or other cash distributions (e.g., upon a sale of rights and other entitlements) | Up to U.S. 5¢ per ADS cancelled | |
• Distribution of ADSs pursuant to (i) stock dividends or other free stock distributions, or (ii) exercise of rights to purchase additional ADSs | Up to U.S. 5¢ per ADS held Up | |
• Distribution of securities other than ADSs or rights to purchase additional ADSs (e.g., upon a spin-off) | Up to U.S. 5¢ per ADS held | |
• ADS Services | Up to U.S. 5¢ per ADS held on the applicable record date(s) established by the depositary | |
• Registration of ADS transfers (e.g., upon a registration of the transfer of registered ownership of ADSs, upon a transfer of ADSs into DTC and vice versa, or for any other reason) | Up to U.S. 5¢ per ADS (or fraction thereof) transferred | |
• Conversion of ADSs of one series for ADSs of another series (e.g., upon conversion of Partial Entitlement ADSs for Full Entitlement ADSs, or upon conversion of Restricted ADSs (each as defined in the Deposit Agreement) into freely transferable ADSs, and vice versa). | Up to U.S. 5¢ per ADS (or fraction thereof) converted | |
As an ADS holder you will also be responsible to pay certain charges such as:
| • | taxes (including applicable interest and penalties) and other governmental charges; |
| • | the registration fees as may from time to time be in effect for the registration of Class A ordinary shares on the share register and applicable to transfers of Class A ordinary shares to or from the name of the custodian, the depositary or any nominees upon the making of deposits and withdrawals, respectively; |
| • | certain cable, telex and facsimile transmission and delivery expenses; |
| • | the fees, expenses, spreads, taxes and other charges of the depositary and/or service providers (which may be a division, branch or affiliate of the depositary) in the conversion of foreign currency; |
| • | the reasonable and customary out-of-pocket expenses incurred by the depositary in connection with compliance with exchange control regulations and other regulatory requirements applicable to Class A ordinary shares, ADSs and ADRs; and |
| • | the fees, charges, costs and expenses incurred by the depositary, the custodian, or any nominee in connection with the ADR program. |
130
Table of Contents
ADS fees and charges for (i) the issuance of ADSs, and (ii) the cancellation of ADSs are charged to the person for whom the ADSs are issued (in the case of ADS issuances) and to the person for whom ADSs are cancelled (in the case of ADS cancellations). In the case of ADSs issued by the depositary into DTC, the ADS issuance and cancellation fees and charges may be deducted from distributions made through DTC, and may be charged to the DTC participant(s) receiving the ADSs being issued or the DTC participant(s) holding the ADSs being cancelled, as the case may be, on behalf of the beneficial owner(s) and will be charged by the DTC participant(s) to the account of the applicable beneficial owner(s) in accordance with the procedures and practices of the DTC participants as in effect at the time. ADS fees and charges in respect of distributions and the ADS service fee are charged to the holders as of the applicable ADS record date. In the case of distributions of cash, the amount of the applicable ADS fees and charges is deducted from the funds being distributed. In the case of (i) distributions other than cash and (ii) the ADS service fee, holders as of the ADS record date will be invoiced for the amount of the ADS fees and charges and such ADS fees and charges may be deducted from distributions made to holders of ADSs. For ADSs held through DTC, the ADS fees and charges for distributions other than cash and the ADS service fee may be deducted from distributions made through DTC, and may be charged to the DTC participants in accordance with the procedures and practices prescribed by DTC and the DTC participants in turn charge the amount of such ADS fees and charges to the beneficial owners for whom they hold ADSs. In the case of (i) registration of ADS transfers, the ADS transfer fee will be payable by the ADS Holder whose ADSs are being transferred or by the person to whom the ADSs are transferred, and (ii) conversion of ADSs of one series for ADSs of another series, the ADS conversion fee will be payable by the Holder whose ADSs are converted or by the person to whom the converted ADSs are delivered.
In connection with the listing of the ADSs on the London Stock Exchange (LSE) in 2022, we and the depositary have agreed to supplement the deposit agreement, as of October 31, 2022, to provide, inter alia, that the depositary is to take into consideration the rules and regulations of the LSE in managing the ADR program so long as the ADSs are listed on the LSE (e.g. in the context of corporate actions affecting the ADSs and the shares represented by ADSs). In addition, we and the depositary have agreed to use commercially reasonable efforts to establish certain elective and non-elective procedures to remove the ADSs from settlement in the U.S. into non-U.S. clearing systems in the event that the ADSs are delisted from applicable U.S. securities exchanges and the trading of ADSs on U.S. securities exchanges and on the over-the-counter market are suspended by regulatory action of the SEC.
In the event of refusal to pay the depositary fees, the depositary may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder. Certain depositary fees and charges (such as the ADS services fee) may become payable shortly after the closing of the ADS offering. Note that the fees and charges you may be required to pay may vary over time and may be changed by us and by the depositary. You will receive prior notice of such changes. The depositary may reimburse us for certain expenses incurred by us in respect of the ADR program, by making available a portion of the ADS fees charged in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary agree from time to time.
Fees and Other Payments Made by the Depositary to Us
The depositary may make payments to us or reimburse us for certain costs and expenses, by making available a portion of the ADS fees collected in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary bank agree from time to time. In 2023, we received US$18,662, net of withholding taxes, from the depository for expenses incurred in connection with the establishment and maintenance of the ADS program.
131
Table of Contents
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
Material Modifications to the Rights of Security Holders
See “Item 10. Additional Information—B. Memorandum and Articles of Association” for a description of the rights of securities holders, which remain unchanged.
Use of Proceeds
The following “Use of Proceeds” information relates to the registration statement on Form F-1, as amended (File No. 333-238596) in relation to our initial public offering, which was declared effective by the SEC on June 11, 2020. In June 2020, we completed our initial public offering in which we issued and sold an aggregate of 13,500,000 ADSs (excluding ADSs offered in the exercise of the over-allotment options), representing 13,500,000 Class A ordinary shares. In the same month, the underwriters for our initial public offering exercised their over-allotment options in full to purchase an addition of 2,025,000 ADSs. The net proceeds we received from the initial public offering and the exercise of over-allotment options totaled approximately US$234.9 million. Morgan Stanley & Co. LLC, BofA Securities, Inc. and Cowen and Company, LLC were the representatives of the underwriters for our initial public offering.
For the period from June 11, 2020, the date that the registration statement on Form F-1 was declared effective by the SEC, to December 31, 2023, the total expenses incurred for our company’s account in connection with our initial public offering was approximately US$2.5 million, which included US$2.3 million in underwriting discounts and commissions for the initial public offering and approximately US$0.2 million in other costs and expenses for our initial public offering. None of the transaction expenses included payments to directors or officers of our company or their associates, persons owning more than 10% or more of our equity securities or our affiliates.
For the period from June 11, 2020, the date that the registration statement on Form F-1 was declared effective by the SEC, to December 31, 2023, we used (i) US$25.8 million of the net proceeds from our initial public offering to renovate our laboratories and offices, (ii) US$20.6 million to purchase and installation of machinery and equipment, (iii) US$1.5 million to supplement our working capital, (iv) US$35.1 million for early detection related research and development, and (v) US$115.7 million of for other operating and corporate administration expenses. None of the net proceeds from the initial public offering were paid, directly or indirectly, to any of our directors or officers or their associates, persons owning 10% or more of our equity securities or our affiliates.
We still intend to use the remainder of the proceeds from our initial public offering as disclosed in our registration statements on Form F-1.
ITEM 15. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, as required by Rule 13a-15(b) under the Exchange Act.
Based upon that evaluation, our management has concluded that, as of December 31, 2023, our disclosure controls and procedures were effective in ensuring that the information required to be disclosed by us in the reports that we file and furnish under the Exchange Act was recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. Management, with the participation of the chief executive officer, the chief financial officer, has assessed the effectiveness of internal control over financial reporting as of December 31, 2023. Management’s assessment was based on the framework in “Internal Control – Integrated Framework (2013)”, issued by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO.
132
Table of Contents
Based on that assessment, our management concluded that, as of December 31, 2023, the Company’s internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of its financial reporting and the preparation of its financial statements for external purposes, in accordance with generally accepted accounting principles.
Due to its inherent limitations, internal control over financial reporting may not prevent or detect misstatements, and can only provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Attestation Report of the Registered Public Accounting Firm
Our independent registered public accounting firm, Ernst & Young Hua Ming LLP, has audited the effectiveness of our company’s internal control over financial reporting as of December 31, 2023, as stated in its report, which appears on pages F-4 and F-5 of this annual report on Form 20-F.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financing reporting (as defined in Rule 13a-15(f) of the Exchange Act) during the period covered by this annual report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 15A. AUDIT COMMITTEE FINANCIAL EXPERT
Our board of directors has determined that Ms. Wendy Hayes qualifies as an “audit committee financial expert,” and that Ms. Wendy Hayes and Dr. Min-Jui Richard Shen each satisfies the “independence” requirements of Rule 5605(c)(2) of the Listing Rules of the Nasdaq Stock Market and meets the independence standards under Rule 10A-3 under the Exchange Act, as amended.
ITEM 15B. CODE OF ETHICS
Our board of directors adopted a code of business conduct and ethics that applies to our directors, officers and employees in January 2020. We have posted a copy of our code of business conduct and ethics on our website at: https://ir.brbiotech.com/, where you can obtain a copy without charge.
ITEM 15C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by Ernst & Young Hua Ming LLP, our principal external auditors, for the periods indicated.
| Year ended December 31. | ||||||||||||
| 2022 | 2023 | |||||||||||
| RMB | RMB | US$ | ||||||||||
| (in thousands) | ||||||||||||
Audit fees(1) | 4,497 | 5,212 | 734 | |||||||||
Tax fees(2) | 86 | 521 | 73 | |||||||||
|
|
|
|
|
| |||||||
Total | 4,583 | 5,733 | 807 | |||||||||
|
|
|
|
|
| |||||||
| (1) | Audit fees include the aggregate fees billed in each of the fiscal period listed for professional services rendered by our independent public accountant in relation to the audit of our annual financial statements, our internal controls over financial reporting, agreed upon procedures of our quarterly financial statements, and statutory audits for certain of our subsidiaries. |
| (2) | Tax fees include the aggregate fees billed in each of the fiscal period listed for professional services rendered by our independent public accountant for tax compliance, tax advice, and tax planning. |
The policy of our audit committee is to pre-approve all audit and non-audit services provided by Ernst & Young Hua Ming LLP, including audit services, audit-related services, tax services and other services as described above, other than those for de minimis services which are approved by the audit committee prior to the completion of the audit.
ITEM 15D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
133
Table of Contents
ITEM 15E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
We announced a Share Repurchase Program approved by our board of directors on June 21, 2022. Under the terms of the approved program, we may repurchase our Class A ordinary shares in the form of ADSs with an aggregate value of up to US$10 million during a 12-month period. The Share Repurchase Program was completed in full. We repurchased a total of 3,023,138 Class A ordinary shares in the form of ADSs. The following table sets forth information about our purchases of ADSs in 2023.
| Period | (a) Total Number of ADSs Purchased | (b) Average Price Paid per ADSs (US$) | (c) Total Number of ADSs Purchased as Part of Publicly Announced Plans or Programs | (d) Approximately Dollar Value of ADSs that May Yet be Purchased Under the Plans or Programs (in US$ million | ||||||||||||
August 23, 2022 | 3,023,138 | 2.83 | 3,023,138 | — | ||||||||||||
ITEM 15F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 15G. CORPORATE GOVERNANCE
As a Cayman Islands company listed on the NASDAQ Global Market, we are subject to the NASDAQ Global Market corporate governance listing standards. However, NASDAQ Global Market rules permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in the Cayman Islands, which is our home country, may differ significantly from the NASDAQ Global Market corporate governance listing standards. We opt to follow our home country practices and rely on certain exemptions provided by the NASDAQ Global Market corporate governance listing standards to a foreign private issuer, including exemptions from the requirements to have:
| • | majority of independent directors on our board of directors; |
| • | a minimum of three members in our audit committee; |
| • | only independent directors being involved in the selection of director nominees and determination of executive officer compensation; |
| • | regularly scheduled executive sessions of independent directors; and |
| • | a quorum of annual general meeting which is no less than 33 1/3% of our outstanding shares. |
As a result of our reliance on the corporate governance exemptions available to foreign private issuers, holders of our ADSs will not have the same protection afforded to shareholders of companies that are subject to all of NASDAQ Global Market corporate governance requirements.
ITEM 15H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 15I. DISCLOSURE REGARDING FOREIGN JURISDICTION THAT PREVENT INSPECTIONS
Not applicable.
ITEM 15J. INSIDER TRADING POLICIES
Our board of directors adopted an Insider Trading Policy on November 21, 2023, and a copy of the Insider Trading Policy is filed as an exhibit to this annual report.
134
Table of Contents
ITEM 15K. CYBERSECURITY
We have developed and maintained a cybersecurity risk management program, consisting of cybersecurity policies, procedures, compliance and awareness programs to mitigate risk and to ensure compliance with security, availability and confidentiality. The cybersecurity process has been integrated into our overall risk management system and process, and is solely internally managed. Pursuant to our cybersecurity policy, the Board is responsible for the oversight of risks from cybersecurity threats, including full authority in monitoring, resolving, disclosing and otherwise handling matters related to or arising out any cybersecurity incidents. The Board has also delegated authorities to certain Board committee and management. Our Audit Committee is responsible for identifying risks that threaten achievement of the control activities and reporting such risks and any material cybersecurity incidents to the Board on a quarterly basis. We also established a dedicated management team comprising cybersecurity officers from each department. This management team is responsible for developing cybersecurity management strategies and harmonizing and publishing cybersecurity management rules and specifications; regularly studying relevant legal and regulatory compliance matters, and identifying any compliance risks that we face; developing the operating procedures for cybersecurity management and providing clarifications on such procedures, including the emergency plans, in relation to cybersecurity incidents; developing data classification standards, assisting business departments in effective data asset identification and classification management, and implementing the cybersecurity matters in the business operation context; and developing and organizing cybersecurity education and training sessions, and reporting major cybersecurity matters to the Audit Committee. The risk assessment for our cybersecurity occurs as business needs change, and covers identification of risks that could act against our objectives as well as specific risks related to a compromise to the security of data.
The oversight of cybersecurity threats is undertaken by our head of IT department. The level of each identified risk is evaluated based on its potential impact, prioritizing high-scoring risks for immediate action and mitigation. A thorough analysis is conducted to assess if the risk aligns with our risk acceptance criteria, determining whether it should be accepted or mitigated. Mitigation plans are developed, specifying the responsible individual or department, and may take into account budgetary considerations.
As of the date of this annual report, we are not aware of any material risks from cybersecurity threats that have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations or financial condition.
PART III
ITEM 16. FINANCIAL STATEMENTS
We have elected to provide financial statements pursuant to Item 18.
ITEM 17. FINANCIAL STATEMENTS
Our consolidated financial statements are included at the end of this annual report.
135
Table of Contents
ITEM 18. EXHIBITS
136
Table of Contents
| * | Filed herewith |
| ** | Furnished herewith |
137
Table of Contents
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing its annual report on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| Burning Rock Biotech Limited | ||
| By: | /s/ Yusheng Han | |
| Name: | Yusheng Han | |
| Title: | Chairman of the Board of Directors and Chief Executive Officer | |
Date: April 29, 2024
138
Table of Contents
Page | ||||
| F-2 | ||||
| F-6 | ||||
F- 7 | ||||
F- 9 | ||||
F-1 2 | ||||
F-1 4 | ||||
Revenue recognition – determining the standalone selling price of the Company’s performance obligation for in-hospital business | ||
| Description of the Matter | As discussed in Note 2 to the consolidated financial statements, the Company’s contracts with customers from in-hospital business often contain bundles of reagent kits to hospital customers. Each kit represents a single performance obligation. The Company allocates the transaction price to each kit on a relative standalone selling price basis using the expected cost plus a margin method and recognizes revenue when the reagent kits are delivered to the hospital customers. | |
| Auditing the Company’s estimated standalone selling prices for the in-hospital business was complex and required subjective auditor judgment due to the significant management judgement to develop these estimates. The standalone selling price is based on the product mix sold to customers over time and the Company’s expected gross margin for different customers, which directly affects the amount and timing of revenue recognized under these arrangements. | ||
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of the Company’s internal controls over the stand-alone selling price, including the Company’s controls over the review of product offerings, contracts, pricing information used to estimate the standalone selling prices and historical margin analysis. To test the estimated standalone selling price of each performance obligation, our audit procedures included, among others, testing the underlying data used in management’s calculations for completeness and accuracy as well as evaluating the significant assumptions including the product mix sold to customers over time and expected gross margin for different customers used in the estimate by analyzing historical experience and other factors, such as changes in customer demand for product mix, changes in internal cost structure and the Company’s pricing strategies. We interviewed management to understand the details of the Company’s pricing strategies and hospital customers to confirm the product mix on a sample basis and compared such information to the Company’s annual sales register. We further assessed the reasonableness of management’s expected margin by comparing to management’s historical margin analysis. | |
As of December 31, | ||||||||||||||
Notes | 2022 | 2023 | ||||||||||||
RMB | RMB | US$ | ||||||||||||
ASSETS | ||||||||||||||
Current assets: | ||||||||||||||
Cash and cash equivalents | 905,451 | 615,096 | 86,634 | |||||||||||
Restricted cash | 19,817 | 120 | 17 | |||||||||||
Accounts receivable (net of allowance of RMB42,914 and RMB38,396 (US$5,408) as of December 31, 2022 and 2023, respectively) | 4 | 109,954 | 126,858 | 17,868 | ||||||||||
Contract assets (net of allowance of RMB35,770 and RMB58,790 (US$8,280) as of December 31, 2022 and 2023, respectively) | 5 | 41,757 | 22,748 | 3,204 | ||||||||||
Inventories, net | 6 | 130,321 | 69,020 | 9,721 | ||||||||||
Prepayments and other current assets | 7 | 51,462 | 50,254 | 7,079 | ||||||||||
Total current assets | 1,258,762 | 884,096 | 124,523 | |||||||||||
Non-current assets: | ||||||||||||||
Equity method investment | 690 | 337 | 47 | |||||||||||
Convertible note receivable | 5,105 | 5,320 | 749 | |||||||||||
Property and equipment, net | 8 | 251,829 | 131,912 | 18,579 | ||||||||||
Intangible assets, net | 9 | 1,986 | 964 | 136 | ||||||||||
Operating right-of-use | 10 | 48,205 | 12,284 | 1,730 | ||||||||||
Other non-current assets | 20,890 | 5,088 | 718 | |||||||||||
Total non-current assets | 328,705 | 155,905 | 21,959 | |||||||||||
TOTAL ASSETS | 1,587,467 | 1,040,001 | 146,482 | |||||||||||
LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||||||
Current liabilities | ||||||||||||||
Accounts payable | 50,947 | 18,061 | 2,544 | |||||||||||
Deferred revenue | 147,633 | 130,537 | 18,386 | |||||||||||
Accrued liabilities and other current liabilities | 11 | 173,832 | 104,935 | 14,780 | ||||||||||
Customer deposits | 1,803 | 1,197 | 169 | |||||||||||
Current portion of operating lease liabilities | 10 | 37,236 | 8,634 | 1,216 | ||||||||||
Total current liabilities | 411,451 | 263,364 | 37,095 | |||||||||||
Non-current liabilities | ||||||||||||||
Other non-current liabilities | 4,124 | 4,537 | 638 | |||||||||||
Non-current portion of operating lease liabilities | 10 | 13,551 | 3,690 | 520 | ||||||||||
Total non-current liabilities | 17,675 | 8,227 | 1,158 | |||||||||||
TOTAL LIABILITIES | 429,126 | 271,591 | 38,253 | |||||||||||
Commitments and contingencies | 17 | |||||||||||||
Shareholders’ equity: | ||||||||||||||
Class A ordinary shares (par value of US$0.0002 per share; 230,000,000 shares authorized; 85,318,596 and 85,115,600 shares issued and outstanding as of December 31, 2022 and 2023, respectively) | 117 | 116 | 16 | |||||||||||
Class B ordinary shares (par value of US$0.0002 per share; 20,000,000 shares authorized; 17,324,848 shares issued and outstanding as of December 31, 2022 and 2023) | 21 | 21 | 3 | |||||||||||
Treasury stock | 13 | (58,919 | ) | (65,896 | ) | (9,281 | ) | |||||||
Additional paid-in capital | 4,582,790 | 4,849,337 | 683,015 | |||||||||||
Accumulated deficits | (3,199,946 | ) | (3,853,635 | ) | (542,773 | ) | ||||||||
Accumulated other comprehensive loss | (165,722 | ) | (161,533 | ) | (22,751 | ) | ||||||||
Total shareholders’ equity | 1,158,341 | 768,410 | 108,229 | |||||||||||
TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | 1,587,467 | 1,040,001 | 146,482 | |||||||||||
For the years ended December 31, | ||||||||||||||||||
Notes | 2021 | 2022 | 2023 | |||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||||
Revenues: | ||||||||||||||||||
| Revenues from services | 342,465 | 391,548 | 345,030 | 48,596 | ||||||||||||||
| Revenues from sales of products | 165,397 | 171,690 | 192,405 | 27,100 | ||||||||||||||
Total revenues | 3 | 507,862 | 563,238 | 537,435 | 75,696 | |||||||||||||
Cost of revenues: | ||||||||||||||||||
| Cost of services (including related party amounts of RMB208, nil and RMB18 (US$3) for the years ended December 31, 2021, 2022 and 2023, respectively) | (93,401 | ) | (119,903 | ) | (101,638 | ) | (14,315 | ) | ||||||||||
| Cost of goods sold | (50,315 | ) | (63,296 | ) | (72,570 | ) | (10,222 | ) | ||||||||||
Total cost of revenues | (143,716 | ) | (183,199 | ) | (174,208 | ) | (24,537 | ) | ||||||||||
Gross profit | 364,146 | 380,039 | 363,227 | 51,159 | ||||||||||||||
Operating expenses: | ||||||||||||||||||
| Research and development expenses | (367,858 | ) | (421,868 | ) | (347,016 | ) | (48,876 | ) | ||||||||||
| Selling and marketing expenses (including related party amounts of RMB235, RMB201 and nil for the years ended December 31, 2021, 2022 and 2023, respectively) | 16 | (303,096 | ) | (370,294 | ) | (247,711 | ) | (34,889 | ) | |||||||||
| General and administrative expenses (including related party amounts of RMB1,752, nil and nil for the years ended December 31, 2021, 2022 and 2023, respectively) | 16 | (490,256 | ) | (568,284 | ) | (437,821 | ) | (61,666 | ) | |||||||||
Total operating expenses | (1,161,210 | ) | (1,360,446 | ) | (1,032,548 | ) | (145,431 | ) | ||||||||||
Loss from operations | (797,064 | ) | (980,407 | ) | (669,321 | ) | (94,272 | ) | ||||||||||
| Interest income | 3,457 | 9,356 | 17,956 | 2,529 | ||||||||||||||
| Interest expenses | (1,536 | ) | 102 | — | — | |||||||||||||
| Other income, net (including related party income of RMB628, nil and nil for the years ended December 31, 2021, 2022 and 2023, respectively) | 199 | 152 | 484 | 68 | ||||||||||||||
| Foreign exchange (loss) gain, net | (854 | ) | 1,549 | (420 | ) | (59 | ) | |||||||||||
Loss before income tax | (795,798 | ) | (969,248 | ) | (651,301 | ) | (91,734 | ) | ||||||||||
| Income tax expenses | 14 | (899 | ) | (1,985 | ) | (2,388 | ) | (336 | ) | |||||||||
Net loss | (796,697 | ) | (971,233 | ) | (653,689 | ) | (92,070 | ) | ||||||||||
For the years ended December 31, | ||||||||||||||||||
Notes | 2021 | 2022 | 2023 | |||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||||
Net loss attributable to Burning Rock Biotech Limited | (796,697 | ) | (971,233 | ) | (653,689 | ) | (92,070 | ) | ||||||||||
Loss per share for class A and class B ordinary shares: | 15 | |||||||||||||||||
| Class A ordinary shares - basic and diluted | (7.65 | ) | (9.35 | ) | (6.38 | ) | (0.90 | ) | ||||||||||
| Class B ordinary shares - basic and diluted | (7.65 | ) | (9.35 | ) | (6.38 | ) | (0.90 | ) | ||||||||||
Weighted average shares outstanding used in loss per share computation: | 15 | |||||||||||||||||
| Class A ordinary shares - basic and diluted | 86,883,011 | 86,584,100 | 85,071,691 | 85,071,691 | ||||||||||||||
| Class B ordinary shares - basic and diluted | 17,324,848 | 17,324,848 | 17,324,848 | 17,324,848 | ||||||||||||||
Other comprehensive (loss) income, net of tax of nil: | ||||||||||||||||||
| Foreign currency translation adjustments | (39,480 | ) | 41,347 | 4,189 | 590 | |||||||||||||
Total comprehensive loss | (836,177 | ) | (929,886 | ) | (649,500 | ) | (91,480 | ) | ||||||||||
Total comprehensive loss attributable to Burning Rock Biotech Limited | (836,177 | ) | (929,886 | ) | (649,500 | ) | (91,480 | ) | ||||||||||
Ordinary shares | Treasury stock | Additional paid-in capital | Accumulated deficits | Accumulated other comprehensive loss | Total shareholders’ equity | |||||||||||||||||||||||
Number of shares | Amount | |||||||||||||||||||||||||||
RMB | RMB | RMB | RMB | RMB | RMB | |||||||||||||||||||||||
Balance as of January 1, 2021 | 104,781,929 | 137 | — | 4,006,616 | (1,418,160 | ) | (167,589 | ) | 2,421,004 | |||||||||||||||||||
| Net loss | — | — | — | — | (796,697 | ) | — | (796,697 | ) | |||||||||||||||||||
| Adoption of ASC 326 | — | — | — | — | (13,856 | ) | — | (13,856 | ) | |||||||||||||||||||
| Other comprehensive loss | — | — | — | — | — | (39,480 | ) | (39,480 | ) | |||||||||||||||||||
| Share-based compensation (note 12) | — | — | — | 282,433 | — | — | 282,433 | |||||||||||||||||||||
| Purchase of treasury stock (note 13) | (61,026 | ) | — | (4,270 | ) | — | — | — | (4,270 | ) | ||||||||||||||||||
| Exercise of options (note 12) | 425,407 | — | 4,270 | (4,270 | ) | — | — | — | ||||||||||||||||||||
| Issuance of restricted shares (note 12) | 2,424 | — | — | — | — | — | — | |||||||||||||||||||||
| Refund for prepaid subscription for forfeited restricted shares | (39,885 | ) | — | — | (3,823 | ) | — | — | (3,823 | ) | ||||||||||||||||||
Balance as of December 31, 2021 | 105,108,849 | 137 | — | 4,280,956 | (2,228,713 | ) | (207,069 | ) | 1,845,311 | |||||||||||||||||||
Ordinary shares | Treasury stock | Additional paid-in capital | Accumulated deficits | Accumulated other comprehensive loss | Total shareholders’ equity | |||||||||||||||||||||||
Number of shares | Amount | |||||||||||||||||||||||||||
RMB | RMB | RMB | RMB | RMB | RMB | |||||||||||||||||||||||
Balance as of January 1, 2022 | 105,108,849 | 137 | — | 4,280,956 | (2,228,713 | ) | (207,069 | ) | 1,845,311 | |||||||||||||||||||
| Net loss | — | — | — | (971,233 | ) | — | (971,233 | ) | ||||||||||||||||||||
| Other comprehensive income | — | — | — | — | 41,347 | 41,347 | ||||||||||||||||||||||
| Share-based compensation (note 12) | — | — | 326,784 | — | — | 326,784 | ||||||||||||||||||||||
| Purchase of treasury stock (note 13) | (3,187,025 | ) | — | (62,177 | ) | (9,657 | ) | — | — | (71,834 | ) | |||||||||||||||||
| Exercise of options (note 12) | 746,042 | 1 | 3,258 | (3,258 | ) | — | — | 1 | ||||||||||||||||||||
| Issuance of restricted shares (note 12) | 2,425 | — | — | — | — | — | — | |||||||||||||||||||||
| Refund for prepaid subscription for forfeited restricted shares | (26,847 | ) | — | — | (12,035 | ) | — | — | (12,035 | ) | ||||||||||||||||||
Balance as of December 31, 2022 | 102,643,444 | 138 | (58,919 | ) | 4,582,790 | (3,199,946 | ) | (165,722 | ) | 1,158,341 | ||||||||||||||||||
| Ordinary shares | Treasury stock | Additional paid-in capital | Accumulated deficits | Accumulated other comprehensive loss | Total shareholders’ equity | |||||||||||||||||||||||
| Number of shares | Amount | |||||||||||||||||||||||||||
| RMB | RMB | RMB | RMB | RMB | RMB | |||||||||||||||||||||||
| Balance as of January 1, 2023 | 102,643,444 | 138 | (58,919 | ) | 4,582,790 | (3,199,946 | ) | (165,722 | ) | 1,158,341 | ||||||||||||||||||
| Net loss | — | — | — | (653,689 | ) | — | (653,689 | ) | ||||||||||||||||||||
| Other comprehensive income | — | — | — | — | 4,189 | 4,189 | ||||||||||||||||||||||
| Share-based compensation (note 12) | — | — | 260,719 | — | — | 260,719 | ||||||||||||||||||||||
| Purchase of treasury stock (note 13) | (449,783 | ) | (1 | ) | (6,977 | ) | 5,907 | — | — | (1,071 | ) | |||||||||||||||||
| Exercise of options (note 12) | 258,646 | — | — | — | — | — | — | |||||||||||||||||||||
| Refund for prepaid subscription for forfeited restricted shares | (11,859 | ) | — | — | (79 | ) | — | — | (79 | ) | ||||||||||||||||||
| Balance as of December 31, 2023 | 102,440,448 | 137 | (65,896 | ) | 4,849,337 | (3,853,635 | ) | (161,533 | ) | 768,410 | ||||||||||||||||||
| Balance as of December 31, 2023 (US$) | 102,440,448 | 19 | (9,281 | ) | 683,015 | (542,773 | ) | (22,751 | ) | 108,229 | ||||||||||||||||||
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
Cash flows from operating activities: | ||||||||||||||||
| Net loss | (796,697 | ) | (971,233 | ) | (653,689 | ) | (92,070 | ) | ||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||
| Depreciation and amortization | 47,766 | 124,096 | 133,400 | 18,789 | ||||||||||||
| Allowance for credit losses | 12,115 | 24,921 | 18,502 | 2,606 | ||||||||||||
| Inventory reserve | 2,619 | 11,202 | 1,105 | 156 | ||||||||||||
| Loss on disposal of equipment | 178 | 1,418 | 1,008 | 142 | ||||||||||||
| Share of loss from an equity method investee | 483 | 294 | 361 | 51 | ||||||||||||
| Share-based compensation | 282,433 | 326,784 | 260,719 | 36,722 | ||||||||||||
| Accrued interest | 295 | — | — | — | ||||||||||||
| Change in fair value of convertible note receivable | — | (105 | ) | (215 | ) | (30 | ) | |||||||||
Non-cash operating lease expenses | 35,317 | 37,447 | 35,196 | 4,957 | ||||||||||||
Derecognition of right-of-use | — | (473 | ) | (13 | ) | (2 | ) | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||||||
| Accounts receivable | (18,930 | ) | (21,505 | ) | (12,386 | ) | (1,745 | ) | ||||||||
| Contract assets | (30,838 | ) | (20,658 | ) | (4,011 | ) | (565 | ) | ||||||||
| Amounts due from related parties | 212 | — | — | — | ||||||||||||
| Inventories | (66,999 | ) | (21,654 | ) | 57,277 | 8,067 | ||||||||||
Prepayments and other current and non-current assets | (34,859 | ) | 33,919 | 12,353 | 1,740 | |||||||||||
| Accounts payable | (5,102 | ) | 16,632 | (31,124 | ) | (4,384 | ) | |||||||||
| Deferred revenue | 68,469 | 4,762 | (17,096 | ) | (2,408 | ) | ||||||||||
| Accrued liabilities and other current liabilities | 44,244 | 33,662 | (19,247 | ) | (2,711 | ) | ||||||||||
| Customer deposits | (148 | ) | 831 | (606 | ) | (85 | ) | |||||||||
| Deferred government grants | (263 | ) | 663 | 427 | 60 | |||||||||||
| Operating lease liabilities | (29,735 | ) | (37,811 | ) | (37,744 | ) | (5,316 | ) | ||||||||
Other non-current liabilities | 11,554 | — | — | — | ||||||||||||
Net cash used in operating activities | (477,886 | ) | (456,808 | ) | (255,783 | ) | (36,026 | ) | ||||||||
Cash flows from investing activities: | ||||||||||||||||
| Proceeds from maturity of short-term investments | 358,504 | 65,598 | — | — | ||||||||||||
| Proceeds from disposal of equipment | 565 | 2,414 | 84 | 12 | ||||||||||||
| Prepayment for property and equipment | (6,590 | ) | (8,259 | ) | — | — | ||||||||||
| Purchase of property and equipment | (204,329 | ) | (62,031 | ) | (8,104 | ) | (1,141 | ) | ||||||||
| Purchase of intangible assets | (2,529 | ) | (185 | ) | (1,280 | ) | (180 | ) | ||||||||
Purchase of convertible n ote | — | (5,000 | ) | — | — | |||||||||||
| Purchase of short-term investments | (63,924 | ) | — | — | — | |||||||||||
Net cash generated from (used in) investing activities | 81,697 | (7,463 | ) | (9,300 | ) | (1,309 | ) | |||||||||
For the years ended December 31, | ||||||||||||||||||||
Notes | 2021 | 2022 | 2023 | |||||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||||||
Cash flows from financing activities: | — | — | — | |||||||||||||||||
| Financing lease obligation payments | (5,111 | ) | — | — | — | |||||||||||||||
| Refund of consideration for Employee Share Incentive Program | (3,823 | ) | (12,035 | ) | (41,855 | ) | (5,895 | ) | ||||||||||||
| Repayment of short-term borrowings | (5,000 | ) | (2,370 | ) | — | — | ||||||||||||||
| Repayment of long-term borrowings | (34,695 | ) | — | — | — | |||||||||||||||
| Purchase of treasury stock | (4,270 | ) | (71,834 | ) | (6,977 | ) | (983 | ) | ||||||||||||
Net cash used in financing activities | (52,899 | ) | (86,239 | ) | (48,832 | ) | (6,878 | ) | ||||||||||||
| Effect of exchange rate on cash, cash equivalents and restricted cash | (37,006 | ) | 36,666 | 3,863 | 543 | |||||||||||||||
Net decreases in cash, cash equivalents and restricted cash | (486,094 | ) | (513,844 | ) | (310,052 | ) | (43,670 | ) | ||||||||||||
| Cash, cash equivalents and restricted cash at the beginning of year | 1,925,206 | 1,439,112 | 925,268 | 130,321 | ||||||||||||||||
Cash, cash equivalents and restricted cash at the end of year | 1,439,112 | 925,268 | 615,216 | 86,651 | ||||||||||||||||
For the years ended December 31, | ||||||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||||||
Supplemental disclosures of cash flow information: | ||||||||||||||||||||
| Interest expense paid | 1,517 | 877 | — | — | ||||||||||||||||
| Income tax expense paid | 1,105 | 98 | — | — | ||||||||||||||||
Supplemental disclosures of non-cash information: | ||||||||||||||||||||
Purchase of property and equipment included in prepayments and other non-current assets | 14,017 | 7,794 | 4,656 | 656 | ||||||||||||||||
| Purchase of property and equipment included in accounts payable | (32,700 | ) | (6,645 | ) | 1,762 | 248 | ||||||||||||||
Reconciliation of cash, cash equivalents and restricted cash: | ||||||||||||||||||||
| Cash and cash equivalents | 1,431,317 | 905,451 | 615,096 | 86,634 | ||||||||||||||||
| Restricted cash | 7,795 | 19,817 | 120 | 17 | ||||||||||||||||
| Total cash, cash equivalents and restricted cash shown in the statements of cash flows | 1,439,112 | 925,268 | 615,216 | 86,651 | ||||||||||||||||
1. | ORGANIZATION AND BASIS OF PRESENTATION |
Entity | Date of incorporation | Place of incorporation | Percentage of legal ownership by the Company | Principal activities | ||||||||||
Subsidiaries | ||||||||||||||
| BR Hong Kong Limited | April 1, 2014 | Hong Kong | 100 | % | Investment holding | |||||||||
| Beijing Burning Rock Biotech Co., Ltd. (the “WFOE”) | June 13, 2014 | PRC | 100 | % | Trading | |||||||||
| Burning Rock Biotechnology (Shanghai) Co., Ltd. | July 4, 2016 | PRC | 100 | % | Research and development | |||||||||
| Burning Rock Dx LLC | August 28, 2019 | United States | 100 | % | Cancer therapy selection test | |||||||||
VIE | ||||||||||||||
| Burning Rock (Beijing) Biotechnology Co., Ltd. | January 7, 2014 | PRC | Nil | Holding | ||||||||||
VIE’s subsidiaries | ||||||||||||||
| Guangzhou Burning Rock Dx Co., Ltd. | March 18, 2014 | PRC | Nil | Cancer therapy selection test and sales of reagent kits | ||||||||||
| Guangzhou Burning Rock Medical Equipment Co., Ltd. | January 6, 2015 | PRC | Nil | Facilitation of laboratory equipment sales and sales of reagent kits | ||||||||||
1. | ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED) |
1. | ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED) |
(1) | Exclusive Option Agreement |
| • | The VIE irrevocably grants the WFOE an exclusive asset purchase option whereby the WFOE has the right to purchase or designate another party to purchase part or all of the assets of the VIE as permitted under the PRC laws. The purchase price of the VIE’s assets is equal to the book value of the assets or the minimum price as permitted by applicable PRC law, whichever is higher; and |
| • | The WFOE has the right to unilaterally amendment, supplement and termination of this agreement. |
(2) | Exclusive Business Cooperation Agreement |
| • | In exchange for these services, the VIE will pay a service fee, equal to the VIE’s profit before tax, after recovering any accumulated losses of the VIE and its subsidiaries from the preceding fiscal year, and deducting working capital, expenses, tax and a reasonable amount of operating profit according to applicable tax law principles and tax practice; and |
| • | The agreement will be in effect for 10 years unless the WFOE unilaterally terminates the agreement by giving written notification at least thirty days prior to the expiration of the agreement. The WFOE may at its sole discretion unilaterally extend the term of this agreement prior to its expiration upon notice to the VIE. |
(3) | Equity Interest Pledge Agreement |
| • | The Nominee Shareholders pledged all of their respective equity interests in the VIE to the WFOE as continuing first priority security interest to guarantee the performance of these Nominee Shareholders and the VIE’s obligations under the power of attorney, the exclusive option agreement and the exclusive business cooperation agreement; and |
1. | ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED) |
(3) | Equity Interest Pledge Agreement (continued) |
| • | This agreement will remain effective until all the contractual obligations have been satisfied in full under all the agreements mentioned above. |
(4) | Financial Support Undertaking Letter |
| • | Pursuant to the financial support undertaking letter, the Company is obligated and hereby undertakes to provide unlimited financial support to the VIE, to the extent permissible under the applicable PRC laws and regulations, whether or not any such operational loss is actually incurred. The Company will not request repayment of the loans or borrowings if the VIE or its Nominee Shareholders do not have sufficient funds or are unable to repay. |
(5) | Voting Proxy Agreement |
| • | Pursuant to the voting proxy agreement, the WFOE irrevocably and unconditionally commits to execute its rights under the power of attorney in accordance with the instructions from the Company. |
1 | ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED) |
1 | ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED) |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| Cash and cash equivalents | 376,735 | 314,724 | 44,328 | |||||||||
| Restricted cash | 10,277 | 20 | 3 | |||||||||
| Accounts receivable (net of allowance of RMB45,647 and RMB38,123 (US$5,370) as of December 31, 2022 and 2023, respectively) | 109,948 | 126,851 | 17,867 | |||||||||
| Contract assets (net of allowance of RMB35,770 and RMB58,790 (US$8,280) as of December 31, 2022 and 2023, respectively) | 41,757 | 22,748 | 3,204 | |||||||||
| Inter-company receivables* | 245,391 | 343,342 | 48,359 | |||||||||
| Inventories | 114,340 | 61,169 | 8,615 | |||||||||
| Prepayments and other current assets | 19,215 | 16,855 | 2,374 | |||||||||
Total current assets | 917,663 | 885,709 | 124,750 | |||||||||
| Property and equipment, net | 59,465 | 45,533 | 6,413 | |||||||||
| Intangible assets, net | 161 | 38 | 5 | |||||||||
Other non-current assets | 10,165 | 2,398 | 338 | |||||||||
Operating right-of-use | 28,805 | 4,524 | 637 | |||||||||
Total non-current assets | 98,596 | 52,493 | 7,393 | |||||||||
TOTAL ASSETS | 1,016,259 | 938,202 | 132,143 | |||||||||
| Accounts payable | 10,139 | 11,209 | 1,579 | |||||||||
| Deferred revenue | 145,117 | 127,631 | 17,976 | |||||||||
| Inter-company payables* | 1,601,116 | 1,693,577 | 238,535 | |||||||||
| Accrued liabilities and other current liabilities | 107,240 | 71,144 | 10,021 | |||||||||
| Customer deposits | 1,803 | 1,197 | 169 | |||||||||
| Current portion of operating lease liabilities | 25,953 | 3,639 | 513 | |||||||||
Total current liabilities | 1,891,368 | 1,908,397 | 268,793 | |||||||||
Other non-current liabilities | 1,901 | 4,288 | 604 | |||||||||
Non-current portion of operating lease liabilities | 4,640 | — | — | |||||||||
Total non-current liabilities | 6,541 | 4,288 | 604 | |||||||||
TOTAL LIABILITIES | 1,897,909 | 1,912,685 | 269,397 | |||||||||
| * | Inter-company receivables/payables represent balances of VIE and subsidiaries of the VIE due from/to the Company and the Group’s consolidated subsidiaries. |
1 | ORGANIZATION AND BASIS OF PRESENTATION (CONTINUED) |
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| Revenues | 526,071 | 557,667 | 537,502 | 75,706 | ||||||||||||
| Net loss | (508,803 | ) | (605,934 | ) | (340,575 | ) | (47,969 | ) | ||||||||
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| Net cash used in operating activities | (257,506 | ) | (139,381 | ) | (2,768 | ) | (391 | ) | ||||||||
| Net cash used in investing activities | (11,265 | ) | (37,088 | ) | (606 | ) | (86 | ) | ||||||||
| Net cash generated from (used in) financing activities | 304,623 | 377,630 | (68,894 | ) | (9,704 | ) | ||||||||||
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
Category | Estimated Useful Life | |
| Machinery and laboratory equipment | 5 years | |
| Vehicles | 6 years | |
| Furniture and tools | 5 years | |
| Electronic equipment | 3 years | |
| Leasehold improvements | Lesser of lease terms or estimated useful lives of the assets |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
Category | Estimated Useful Life | |
| Computer software | 3 years |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) |
3 | SEGMENT REPORTING |
For the year ended December 31, 2021 | ||||||||||||||||
| Central laboratory business | In-hospital business | Pharma research and development services | Total | |||||||||||||
RMB | RMB | RMB | RMB | |||||||||||||
Revenues: | ||||||||||||||||
| Revenues from services | 319,353 | (281 | ) | 23,393 | 342,465 | |||||||||||
| Revenues from sales of products | — | 165,397 | — | 165,397 | ||||||||||||
Total revenues | 319,353 | 165,116 | 23,393 | 507,862 | ||||||||||||
Cost of revenues: | (81,088 | ) | (50,315 | ) | (12,313 | ) | (143,716 | ) | ||||||||
Gross profit | 238,265 | 114,801 | 11,080 | 364,146 | ||||||||||||
3 | SEGMENT REPORTING (CONTINUED) |
For the year ended December 31, 2022 | ||||||||||||||||
| Central laboratory business | In-hospital business | Pharma research and development services | Total | |||||||||||||
RMB | RMB | RMB | RMB | |||||||||||||
Revenues: | ||||||||||||||||
| Revenues from services | 314,770 | 3,606 | 73,172 | 391,548 | ||||||||||||
| Revenues from sales of products | — | 171,690 | — | 171,690 | ||||||||||||
Total revenues | 314,770 | 175,296 | 73,172 | 563,238 | ||||||||||||
Cost of revenues: | (82,123 | ) | (63,296 | ) | (37,780 | ) | (183,199 | ) | ||||||||
Gross profit | 232,647 | 112,000 | 35,392 | 380,039 | ||||||||||||
For the year ended December 31, 2023 | ||||||||||||||||||||
| Central laboratory business | In-hospital business | Pharma research and development services | Total | |||||||||||||||||
RMB | RMB | RMB | RMB | US$ | ||||||||||||||||
Revenues: | ||||||||||||||||||||
| Revenues from services | 232,812 | (3,704 | ) | 115,922 | 345,030 | 48,596 | ||||||||||||||
| Revenues from sales of products | — | 192,405 | — | 192,405 | 27,100 | |||||||||||||||
Total revenues | 232,812 | 188,701 | 115,922 | 537,435 | 75,696 | |||||||||||||||
Cost of revenues: | (49,473 | ) | (72,570 | ) | (52,165 | ) | (174,208 | ) | (24,537 | ) | ||||||||||
Gross profit | 183,339 | 116,131 | 63,757 | 363,227 | 51,159 | |||||||||||||||
3 | SEGMENT REPORTING (CONTINUED) |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| PRC | 286,797 | 127,866 | 18,010 | |||||||||
| United States | 34,127 | 21,418 | 3,017 | |||||||||
320,924 | 149,284 | 21,027 | ||||||||||
For the year ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| PRC | 504,052 | 553,304 | 534,928 | 75,343 | ||||||||||||
| United States | 3,810 | 9,934 | 2,507 | 353 | ||||||||||||
507,862 | 563,238 | 537,435 | 75,696 | |||||||||||||
4 | ACCOUNTS RECEIVABLE, NET |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| Accounts receivable | 152,868 | 165,254 | 23,276 | |||||||||
| Allowance for credit losses | (42,914 | ) | (38,396 | ) | (5,408 | ) | ||||||
109,954 | 126,858 | 17,868 | ||||||||||
As of December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| Balance at the beginning of the year | 24,215 | 39,166 | 42,914 | 6,044 | ||||||||||||
| Adoption of ASC 326 | 11,358 | — | — | — | ||||||||||||
| Provisions/(Reversals) | 3,593 | 3,748 | (4,518 | ) | (636 | ) | ||||||||||
| Balance at the end of the year | 39,166 | 42,914 | 38,396 | 5,408 | ||||||||||||
5 | CONTRACT ASSETS, NET |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| Contract assets | 77,527 | 81,538 | 11,484 | |||||||||
| Allowance for credit losses | (35,770 | ) | (58,790 | ) | (8,280 | ) | ||||||
41,757 | 22,748 | 3,204 | ||||||||||
As of December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| Balance at the beginning of the year | 3,497 | 14,478 | 35,770 | 5,038 | ||||||||||||
| Adoption of ASC 326 | 2,383 | — | — | — | ||||||||||||
| Provisions | 8,598 | 21,292 | 23,020 | 3,242 | ||||||||||||
| Balance at the end of the year | 14,478 | 35,770 | 58,790 | 8,280 | ||||||||||||
6 | INVENTORIES, NET |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| Raw materials | 87,428 | 42,703 | 6,014 | |||||||||
| Work in progress | 7,586 | 5,258 | 741 | |||||||||
| Finished goods | 35,307 | 21,059 | 2,966 | |||||||||
130,321 | 69,020 | 9,721 | ||||||||||
7 | PREPAYMENTS AND OTHER CURRENT ASSETS |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| Deductible input VAT | 18,416 | 17,027 | 2,398 | |||||||||
| Prepayments | 20,523 | 15,845 | 2,232 | |||||||||
| Deposits | 2,094 | 13,405 | 1,888 | |||||||||
| Interest receivable | 103 | 1,179 | 166 | |||||||||
| Others | 10,326 | 2,798 | 395 | |||||||||
51,462 | 50,254 | 7,079 | ||||||||||
8 | PROPERTY AND EQUIPMENT, NET |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| Machinery and laboratory equipment | 265,476 | 275,209 | 38,763 | |||||||||
| Vehicles | 2,808 | 2,229 | 314 | |||||||||
| Furniture and tools | 19,031 | 18,192 | 2,562 | |||||||||
| Electronic equipment | 51,774 | 52,442 | 7,386 | |||||||||
| Leasehold improvements | 187,379 | 187,390 | 26,393 | |||||||||
| Construction in progress | 2,659 | 548 | 77 | |||||||||
529,127 | 536,010 | 75,495 | ||||||||||
| Accumulated depreciation | (277,298 | ) | (404,098 | ) | (56,916 | ) | ||||||
251,829 | 131,912 | 18,579 | ||||||||||
9 | INTANGIBLE ASSETS, NET |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| Computer software | 10,654 | 11,590 | 1,633 | |||||||||
| Accumulated amortization | (8,668 | ) | (10,626 | ) | (1,497 | ) | ||||||
1,986 | 964 | 136 | ||||||||||
10 | LEASES |
For the year ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| Operating lease cost | 34,785 | 37,447 | 35,197 | 4,957 | ||||||||||||
| Short-term lease cost | 1,740 | 2,267 | 1,971 | 278 | ||||||||||||
| Finance lease cost: | ||||||||||||||||
| Amortization of ROU assets | 3,846 | — | — | — | ||||||||||||
| Interest expense on lease liabilities | 295 | — | — | — | ||||||||||||
| Sublease income | (187 | ) | — | — | — | |||||||||||
Total lease cost | 40,479 | 39,714 | 37,168 | 5,235 | ||||||||||||
For the year ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
Other information | ||||||||||||||||
Cash paid for amounts included in the measurement of lease liabilities: | ||||||||||||||||
Operating cash flows used for operating leases | 30,388 | 37,811 | 37,700 | 5,310 | ||||||||||||
Operating cash flows used for finance leases | 295 | — | — | — | ||||||||||||
Financing cash flows used for finance leases | 5,111 | — | — | — | ||||||||||||
ROU assets obtained in exchange for operating lease liabilities | 90,272 | 4,311 | — | — | ||||||||||||
10 | LEASE (CONTINUED) |
Future lease payments under operating leases as of December 31, 2023 were as follows: Years ending December 31, | Operating leases | |||||||
RMB | US$ | |||||||
| 2024 | 9,091 | 1,281 | ||||||
| 2025 | 3,809 | 536 | ||||||
| Total future lease payments | 12,900 | 1,817 | ||||||
| Less: imputed interest | (576 | ) | (81 | ) | ||||
Total lease liability balance | 12,324 | 1,736 | ||||||
11 | ACCRUED LIABILITIES AND OTHER CURRENT LIABILITIES |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
| Accrued payroll and welfare | 70,989 | 50,862 | 7,164 | |||||||||
| Other taxes and surcharge | 11,025 | 20,619 | 2,904 | |||||||||
| Accrued reimbursement expenses | 23,197 | 12,881 | 1,814 | |||||||||
| Professional service fees | 10,042 | 1,628 | 229 | |||||||||
| Guaranteed return to eligible employees* | 38,972 | — | — | |||||||||
| Others | 19,607 | 18,945 | 2,669 | |||||||||
173,832 | 104,935 | 14,780 | ||||||||||
| * | Balance represented guaranteed return to eligible employees resulting from modification of Employee Share Incentive Plan No. 1 (the “ESIP No. 1”), and Employee Share Incentive Plan No. 2 (the “ESIP No. 2”) as described in Note 12. |
12 | SHARE-BASED COMPENSATION |
12 | SHARE-BASED COMPENSATION (CONTINUED) |
12 | SHARE-BASED COMPENSATION (CONTINUED) |
For the years ended December 31, | ||||||
2021 | 2022 | 2023 | ||||
| Risk-free interest rate | 0.97%-2.07% | 1.52%-3.94% | 3.48%-4.61% | |||
| Dividend yield | 0% | 0% | 0% | |||
| Expected volatility range | 47.67%-72.98% | 48.20%-56.00% | 47.90%-50.69% | |||
| Exercise multiple | N/A | 2.8 | N/A | |||
| Contractual life | 10 years | 10 years | 10 years | |||
| Fair market value per ordinary share as at valuation dates | US$9.53 - US$30.50 | US$0.013- US$ | US$0.93- US$ 3.11 | |||
12 | SHARE-BASED COMPENSATION (CONTINUED) |
Number of options | Weighted- average exercise price | Weighted- average grant date fair value | Weighted average remaining contractual term | Aggregate intrinsic value | ||||||||||||||||
US$ per option | US$ per option | Years | US$ | |||||||||||||||||
Outstanding, January 1, 2023 | 20,060,112 | 2.0527 | 8.43 | 8.93 | 19,280 | |||||||||||||||
| Granted | 3,824,912 | 0.0002 | 3.07 | |||||||||||||||||
| Exercised | (235,905 | ) | 0.0002 | 7.50 | ||||||||||||||||
| Forfeited | (2,643,578 | ) | 1.6232 | 5.37 | ||||||||||||||||
Outstanding, December 31, 2023 | 21,005,541 | 1.7560 | 7.84 | 5.61 | 10,441 | |||||||||||||||
Vested and expected to vest at December 31, 2023 | 21,005,541 | 1.7560 | 7.84 | 5.61 | 10,441 | |||||||||||||||
Exercisable at December 31, 2023 | 894,333 | 4.1228 | 7.73 | 5.61 | 722 | |||||||||||||||
12 | SHARE-BASED COMPENSATION (CONTINUED) |
Number of shares | Weighted- average grant date fair value | Weighted average remaining contractual term | Aggregate intrinsic value | |||||||||||||
US$ per unit | Years | US$ | ||||||||||||||
| Outstanding, December 31, 2022 | 46,710 | 2.37 | 9.48 | 106 | ||||||||||||
| Granted | 8,612 | 2.58 | ||||||||||||||
| Exercised | (23,992 | ) | 2.36 | |||||||||||||
| Outstanding, December 31, 2023 | 31,330 | 2.43 | ||||||||||||||
| Vested and expected to vest at December 31, 2023 | 33,879 | 2.40 | 8.72 | 33 | ||||||||||||
12 | SHARE-BASED COMPEN SAT ION (CONTINUED) |
Number of shares | Weighted average grant date fair value | |||||||
US$ per share | ||||||||
| Outstanding as of December 31, 2022 | 251,008 | 8.71 | ||||||
| Vested | (251,008 | ) | 8.71 | |||||
| Outstanding as of December 31, 2023 | — | — | ||||||
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| Cost of revenues | 1,504 | 1,783 | 2,313 | 326 | ||||||||||||
| Research and development expenses | 29,637 | 52,873 | 53,474 | 7,532 | ||||||||||||
| Selling and marketing expenses | 9,612 | 8,525 | 9,658 | 1,360 | ||||||||||||
| General and administrative expenses | 241,680 | 263,603 | 195,274 | 27,504 | ||||||||||||
282,433 | 326,784 | 260,719 | 36,722 | |||||||||||||
13 | TREASURY STOCK |
14 | INCOME TAXES |
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| PRC | (704,003 | ) | (892,612 | ) | (604,997 | ) | (85,211 | ) | ||||||||
Non-PRC | (91,795 | ) | (76,636 | ) | (46,304 | ) | (6,522 | ) | ||||||||
| Total loss before income tax | (795,798 | ) | (969,248 | ) | (651,301 | ) | (91,734 | ) | ||||||||
14 | INCOME TAX (CONTINUED) |
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
Current income tax expenses | 899 | 1,985 | 2,388 | 336 | ||||||||||||
Deferred income tax expenses | — | — | — | — | ||||||||||||
Total income tax expenses | 899 | 1,985 | 2,388 | 336 | ||||||||||||
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
Loss before income tax | (795,798 | ) | (969,248 | ) | (651,301 | ) | (91,734 | ) | ||||||||
PRC statutory income tax rate | 25 | % | 25 | % | 25 | % | 25 | % | ||||||||
Income tax at statutory tax rate | (198,950 | ) | (242,312 | ) | (162,825 | ) | (22,933 | ) | ||||||||
Effect of different tax rates | (10,702 | ) | 3,391 | (439 | ) | (62 | ) | |||||||||
Effect of PRC preferential tax rates | 24,598 | 1,102 | 10,258 | 1,445 | ||||||||||||
Research and development super-deduction | (9,897 | ) | (13,376 | ) | (21,812 | ) | (3,072 | ) | ||||||||
Non-deductible expenses | 84,029 | 66,254 | 69,646 | 9,809 | ||||||||||||
Non-taxable income | (872 | ) | (575 | ) | (1,807 | ) | (255 | ) | ||||||||
Expiration of tax attributes | — | 6,540 | 12,421 | 1,749 | ||||||||||||
Deferred only adjustment | — | — | (3,083 | ) | (434 | ) | ||||||||||
TP adjustment | — | 7,342 | 12,011 | 1,692 | ||||||||||||
Interest and penalty | — | — | 219 | 31 | ||||||||||||
Tax rate change | — | 1,102 | (13,453 | ) | (1,895 | ) | ||||||||||
Provision to return | — | (120 | ) | 2,722 | 383 | |||||||||||
Changes in valuation allowance | 112,693 | 172,637 | 98,530 | 13,878 | ||||||||||||
Income tax expenses | 899 | 1,985 | 2,388 | 336 | ||||||||||||
14 | INCOME TAX (CONTINUED) |
A December 31,s of | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
Deferred tax assets: | ||||||||||||
| Accruals and reserves | 28,725 | 34,972 | 4,926 | |||||||||
| Net operating loss carried forward | 182,191 | 217,850 | 30,684 | |||||||||
| Government grants | 99 | — | — | |||||||||
| Depreciation and amortization | 4,160 | 476 | 67 | |||||||||
| Excessive education fee | 593 | 556 | 78 | |||||||||
| Capitalized research and development expense | 5,581 | 20,513 | 2,889 | |||||||||
Research and development expense recognition | 215,026 | 258,008 | 36,340 | |||||||||
Deferred revenue recognition | 1,161 | 775 | 109 | |||||||||
| Excessive donation expense carried forward | 2,533 | 1,881 | 265 | |||||||||
| Operating lease liabilities | 10,405 | 3,113 | 438 | |||||||||
Gross deferred tax assets | 450,474 | 538,144 | 75,796 | |||||||||
| Less: Valuation allowance | (436,833 | ) | (535,363 | ) | (75,404 | ) | ||||||
| Total deferred tax assets | 13,641 | 2,781 | 392 | |||||||||
Deferred tax liabilities: | ||||||||||||
Operating right - of- use assets | (13,075 | ) | (2,781 | ) | (392 | ) | ||||||
| Depreciation and amortization | (566 | ) | — | — | ||||||||
| Total deferred tax liabilities | (13,641 | ) | (2,781 | ) | (392 | ) | ||||||
Net deferred tax assets | — | — | — | |||||||||
14 | INCOME TAX (CONTINUED) |
For the years ended December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
Balance at beginning of the year | — | 7,342 | 1,034 | |||||||||
| Additions | 7,342 | 12,011 | 1,692 | |||||||||
Balance at end of the year | 7,342 | 19,353 | 2,726 | |||||||||
15 | LOSS PER SHARE |
For the years ended December 31, | ||||||||||||||||||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||||||||||||||||||
Class A | Class B | Class A | Class B | Class A | Class B | |||||||||||||||||||||||||||
RMB | RMB | RMB | RMB | RMB | US$ | RMB | US$ | |||||||||||||||||||||||||
Numerator: | ||||||||||||||||||||||||||||||||
Net loss attributable to ordinary shareholders | (664,244 | ) | (132,453 | ) | (809,298 | ) | (161,935 | ) | (543,089 | ) | (76,492 | ) | (110,600 | ) | (15,578 | ) | ||||||||||||||||
Denominator: | ||||||||||||||||||||||||||||||||
Weighted-average number of ordinary shares outstanding | 87,556,581 | 17,324,848 | 87,005,610 | 17,324,848 | 85,188,137 | 85,188,137 | 17,324,848 | 17,324,848 | ||||||||||||||||||||||||
| Effect of unvested restricted shares | (673,570 | ) | — | (421,510 | ) | — | (116,446 | ) | (116,446 | ) | — | — | ||||||||||||||||||||
Weighted-average number of ordinary shares outstanding – basic and diluted | 86,883,011 | 17,324,848 | 86,584,100 | 17,324,848 | 85,071,691 | 85,071,691 | 17,324,848 | 17,324,848 | ||||||||||||||||||||||||
Loss per share - basic and diluted | (7.65 | ) | (7.65 | ) | (9.35 | ) | (9.35 | ) | (6.38 | ) | (0.90 | ) | (6.38 | ) | (0.90 | ) | ||||||||||||||||
16 | RELATED PARTY TRANSACTIONS |
a) | Related parties |
Name of related parties | Relationship | |
| EaSuMed Holding Ltd. | Equity method investee | |
| Guangzhou Burning Rock Biological Engineering Co., Ltd. | Company controlled by the Chief Executive Officer |
b) | The Group had the following related party transactions: |
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
Service received from: | ||||||||||||||||
| EaSuMed Holding Ltd. | 2,195 | 201 | 18 | 3 | ||||||||||||
Rental income from: | ||||||||||||||||
| Guangzhou Burning Rock Biological Engineering Co., Ltd. | 187 | — | — | — | ||||||||||||
Equipment usage service income from: | ||||||||||||||||
| Guangzhou Burning Rock Biological Engineering Co., Ltd. | 441 | — | — | — | ||||||||||||
17 | COMMITMENTS AND CONTINGENCIES |
18 | RESTRICTED NET ASSETS |
19 | CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY |
19 | CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY (CONTINUED) |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
ASSETS | ||||||||||||
Current assets: | ||||||||||||
| Cash and cash equivalents | 71,858 | 14,171 | 1,996 | |||||||||
| Restricted cash | 2,120 | 62 | 9 | |||||||||
| Inter-company receivables | 3,057,703 | 3,152,747 | 444,055 | |||||||||
| Prepayments and other current assets | 121 | 315 | 44 | |||||||||
Total current assets | 3,131,802 | 3,167,295 | 446,104 | |||||||||
Non-current assets: | ||||||||||||
| Equity method investment | 690 | 337 | 47 | |||||||||
| Property and equipment, net | 1,278 | 627 | 89 | |||||||||
Total non-current assets | 1,968 | 964 | 136 | |||||||||
Total assets | 3,133,770 | 3,168,259 | 446,240 | |||||||||
LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||||
Current liabilities: | ||||||||||||
| Inter-company payables | 1,964,454 | 2,399,333 | 337,939 | |||||||||
| Accrued liabilities and other current liabilities | 10,975 | 516 | 73 | |||||||||
Total current liabilities | 1,975,429 | 2,399,849 | 338,012 | |||||||||
Total liabilities | 1,975,429 | 2,399,849 | 338,012 | |||||||||
19 | CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY (CONTINUED) |
As of December 31, | ||||||||||||
2022 | 2023 | |||||||||||
RMB | RMB | US$ | ||||||||||
LIABILITIES AND SHAREHOLDERS’ EQUITY (CONTINUED) | ||||||||||||
Shareholders’ equity: | ||||||||||||
| Class A ordinary shares (par value of US$0.0002 per share; 230,000,000 shares authorized; 85,318,596 and 85,115,600 shares issued and outstanding as of December 31, 2022 and 2023, respectively) | 117 | 116 | 16 | |||||||||
| Class B ordinary shares (par value of US$0.0002 per share; 20,000,000 shares authorized; 17,324,848 shares issued and outstanding as of December 31, 2022 and 2023) | 21 | 21 | 3 | |||||||||
| Treasury stock | (58,919 | ) | (65,896 | ) | (9,281 | ) | ||||||
Additional paid-in capital | 4,582,790 | 4,849,337 | 683,015 | |||||||||
| Accumulated deficits | (3,199,946 | ) | (3,853,635 | ) | (542,774 | ) | ||||||
| Accumulated other comprehensive loss | (165,722 | ) | (161,533 | ) | (22,751 | ) | ||||||
Total shareholders’ equity | 1,158,341 | 768,410 | 108,228 | |||||||||
TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | 3,133,770 | 3,168,259 | 446,240 | |||||||||
19 | CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY (CONTINUED) |
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
Revenues | — | — | — | — | ||||||||||||
Cost of revenues | — | — | — | — | ||||||||||||
Gross profit | — | — | — | — | ||||||||||||
Operating expenses: | ||||||||||||||||
| General and administrative expenses | (48,566 | ) | (31,041 | ) | (11,616 | ) | (1,636 | ) | ||||||||
| Share of losses in subsidiaries, the VIE and the VIE’s subsidiaries | (750,015 | ) | (943,534 | ) | (645,522 | ) | (90,920 | ) | ||||||||
Total operating expenses | (798,581 | ) | (974,575 | ) | (657,138 | ) | (92,556 | ) | ||||||||
Loss from operations | (798,581 | ) | (974,575 | ) | (657,138 | ) | (92,556 | ) | ||||||||
| Interest income | 2,365 | 3,636 | 3,811 | 537 | ||||||||||||
| Other expense, net | (481 | ) | (294 | ) | (362 | ) | (51 | ) | ||||||||
Loss before income taxes | (796,697 | ) | (971,233 | ) | (653,689 | ) | (92,070 | ) | ||||||||
| Income tax expenses | — | — | — | — | ||||||||||||
Net loss | (796,697 | ) | (971,233 | ) | (653,689 | ) | (92,070 | ) | ||||||||
Other comprehensive (loss) income, net of tax of nil: | — | — | — | — | ||||||||||||
| Foreign currency translation adjustments | (39,480 | ) | 41,347 | 4,189 | 590 | |||||||||||
Total comprehensive loss | (836,177 | ) | (929,886 | ) | (649,500 | ) | (91,480 | ) | ||||||||
19 | CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY (CONTINUED) |
For the years ended December 31, | ||||||||||||||||
2021 | 2022 | 2023 | ||||||||||||||
RMB | RMB | RMB | US$ | |||||||||||||
| Net cash used in operating activities | (51,411 | ) | (19,357 | ) | (19,253 | ) | (2,712 | ) | ||||||||
| Net cash (used in) generated from investing activities | (1,179,603 | ) | 125,576 | (41,305 | ) | (5,818 | ) | |||||||||
| Net cash used in financing activities | — | (66,850 | ) | — | — | |||||||||||
| Effect of exchange rate changes | (484 | ) | 1,508 | 813 | 115 | |||||||||||
Net (decrease) increase in cash, cash equivalents and restricted cash | (1,231,498 | ) | 40,877 | (59,745 | ) | (8,415 | ) | |||||||||
| Cash, cash equivalents and restricted cash at the beginning of year | 1,264,599 | 33,101 | 73,978 | 10,420 | ||||||||||||
Cash, cash equivalents and restricted cash at the end of year | 33,101 | 73,978 | 14,233 | 2,005 | ||||||||||||