
Tumor Activated Cancer Therapeutics Restoring anti-tumor immune responses to treat cancer patients February 26, 2024 Exhibit 99.2

Forward-looking statements and disclaimers This presentation includes certain forward-looking statements that involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward-looking statements regarding Janux Therapeutics, Inc. (the “Company”). These forward-looking statements include, but are not limited to, those regarding the Company’s ability to bring new treatments to patients in need, the progress and expected timing of the Company’s drug development programs, clinical development plans and timelines, the timing of and plans for regulatory filings, market size and opportunity, the Company’s strategy and intellectual property matters, and estimates regarding the Company’s expenses, capital requirements, and needs for additional financing. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Factors that may cause actual results to differ materially include the risk that compounds that appeared promising in early research do not demonstrate safety and/or efficacy in later preclinical studies or clinical trials, the risk that the Company may not obtain approval to market its product candidates, uncertainties associated with performing clinical trials, regulatory filings and applications, risks associated with reliance on third parties to successfully conduct clinical trials, the risks associated with reliance on outside financing to meet capital requirements, and other risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs. Also, interim results of a clinical trial are not necessarily indicative of final results and one or more of the clinical outcomes may materially change following more comprehensive reviews of the data, as patient enrollment continues, and as more patient data become available. In light of these risks, uncertainties, contingencies and assumptions, the events or circumstances referred to in the forward-looking statements may not occur. For a further list and description of the risks and uncertainties that the Company faces, please refer to the Company’s periodic and other filings with the Securities and Exchange Commission, which are available at www.sec.gov. Such forward-looking statements are current only as of the date they are made, and the Company assumes no obligation to update any forward-looking statements, whether as a result of new information, future events, or otherwise. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. This presentation concerns therapeutic product candidates that are in preclinical and clinical development and which have not yet been approved for marketing by the U.S. Food and Drug Administration. They are currently limited by federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated.

Janux – multiple near-term high value opportunities in CD3 TCE targets Clinical Pipeline Technology Platform Cash Position *As of September 30, 2023, includes cash and cash equivalents PSMAxCD3-TRACTr to treat mCRPC illustrating a potential best-in-class profile Promising efficacy with favorable safety profile and no CRS > Grade 2 observed Deepening PSA and RECIST responses with increased dose levels EGFRxCD3-TRACTr providing entry point into multiple large indications Deep RECIST response observed in a subject with NSCLC at 0.15mg QW with promising safety profile Early evidence of durable response coupled with no TRAEs or CRS Emerging clinical efficacy and safety data supports applications to additional TCE targets Facilitates development of a highly valuable pipeline and positions Janux to be at the forefront of TCE drug development Robust cash position of $350M* to support program advancement and operating plan through 2027 and Phase 1b trials in both PSMAxCD3 and EGFRxCD3
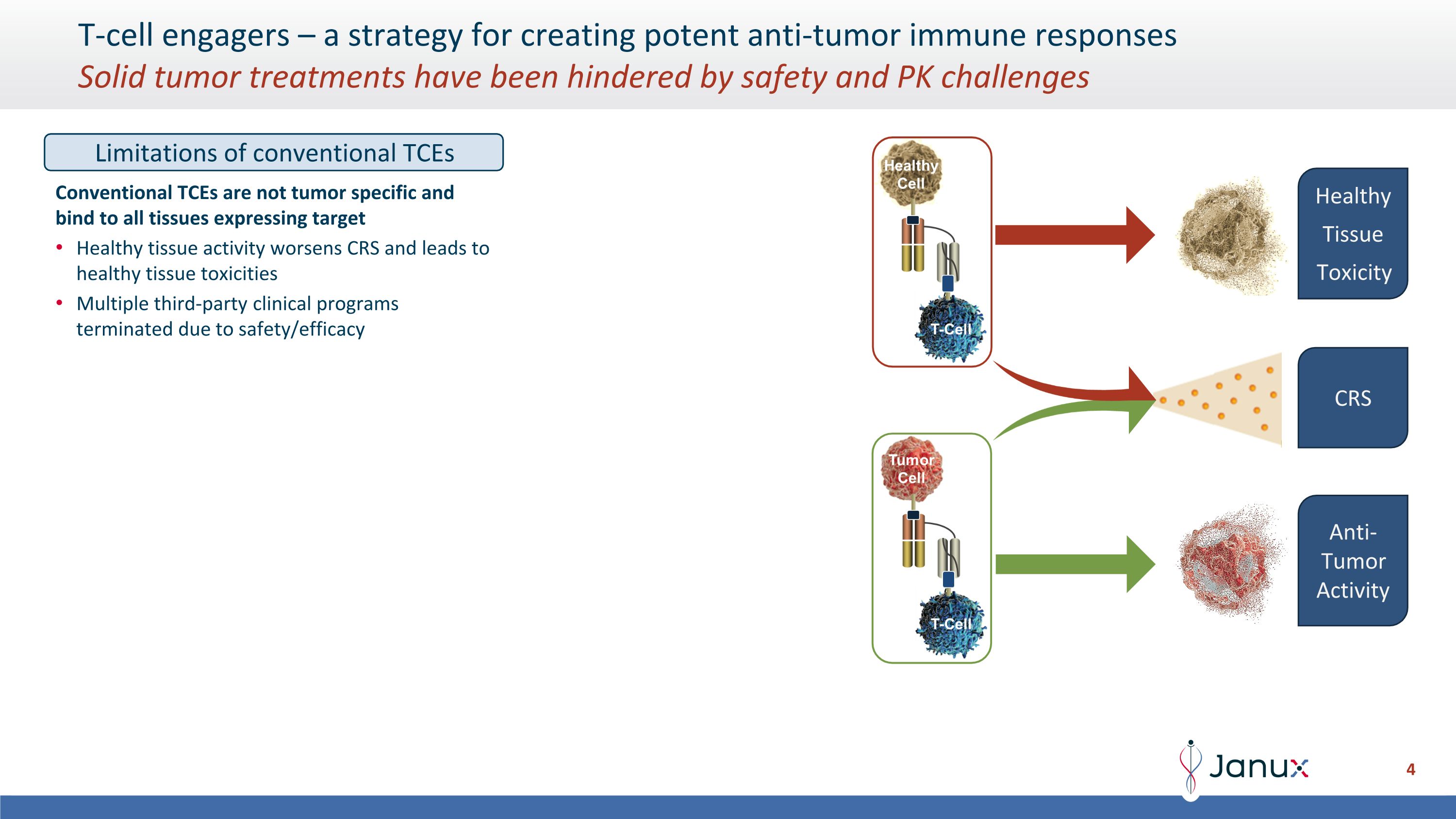
T-cell engagers – a strategy for creating potent anti-tumor immune responses�Solid tumor treatments have been hindered by safety and PK challenges Limitations of conventional TCEs Conventional TCEs are not tumor specific and bind to all tissues expressing target Healthy tissue activity worsens CRS and leads to healthy tissue toxicities Multiple third-party clinical programs terminated due to safety/efficacy Healthy Tissue Toxicity CRS Anti-Tumor Activity
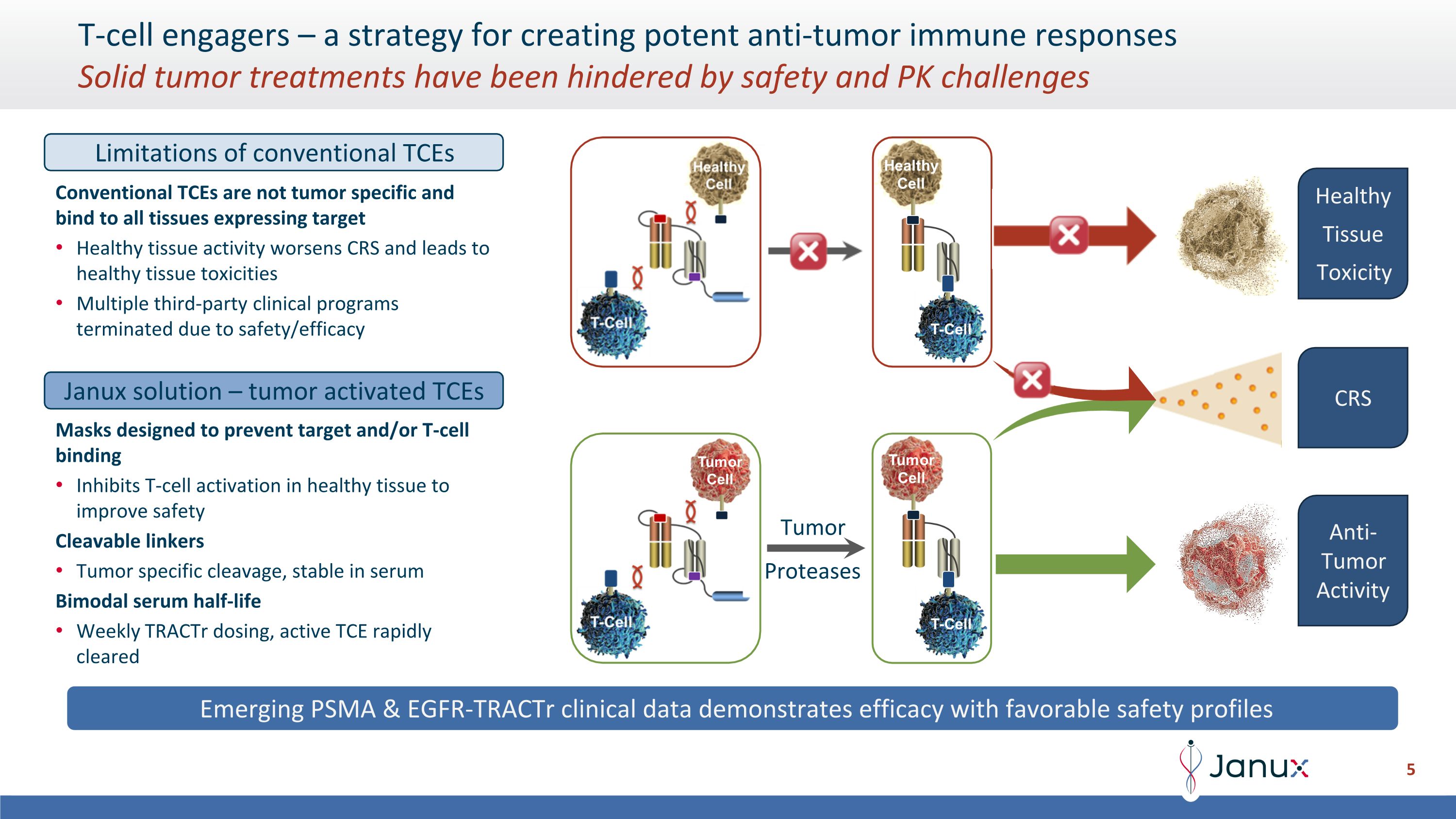
T-cell engagers – a strategy for creating potent anti-tumor immune responses�Solid tumor treatments have been hindered by safety and PK challenges Emerging PSMA & EGFR-TRACTr clinical data demonstrates efficacy with favorable safety profiles Tumor Proteases Limitations of conventional TCEs Conventional TCEs are not tumor specific and bind to all tissues expressing target Healthy tissue activity worsens CRS and leads to healthy tissue toxicities Multiple third-party clinical programs terminated due to safety/efficacy Janux solution – tumor activated TCEs Masks designed to prevent target and/or T-cell binding Inhibits T-cell activation in healthy tissue to improve safety Cleavable linkers Tumor specific cleavage, stable in serum Bimodal serum half-life Weekly TRACTr dosing, active TCE rapidly cleared Healthy Tissue Toxicity CRS Anti-Tumor Activity
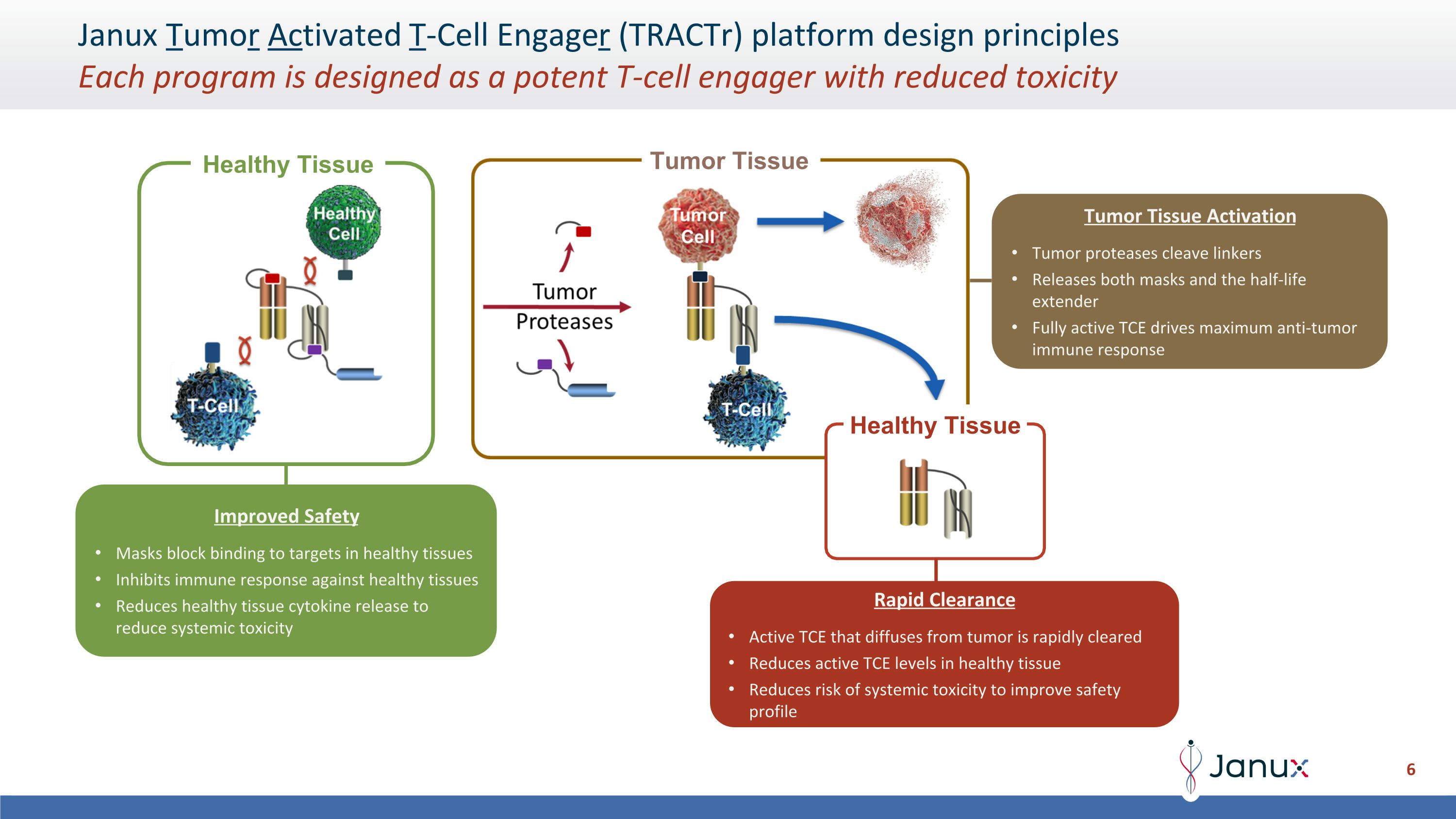
Janux Tumor Activated T-Cell Engager (TRACTr) platform design principles�Each program is designed as a potent T-cell engager with reduced toxicity Healthy Tissue Healthy Tissue Improved Safety Masks block binding to targets in healthy tissues Inhibits immune response against healthy tissues Reduces healthy tissue cytokine release to reduce systemic toxicity Tumor Tissue Activation Tumor proteases cleave linkers Releases both masks and the half-life extender Fully active TCE drives maximum anti-tumor immune response Rapid Clearance Active TCE that diffuses from tumor is rapidly cleared Reduces active TCE levels in healthy tissue Reduces risk of systemic toxicity to improve safety profile Tumor Tissue
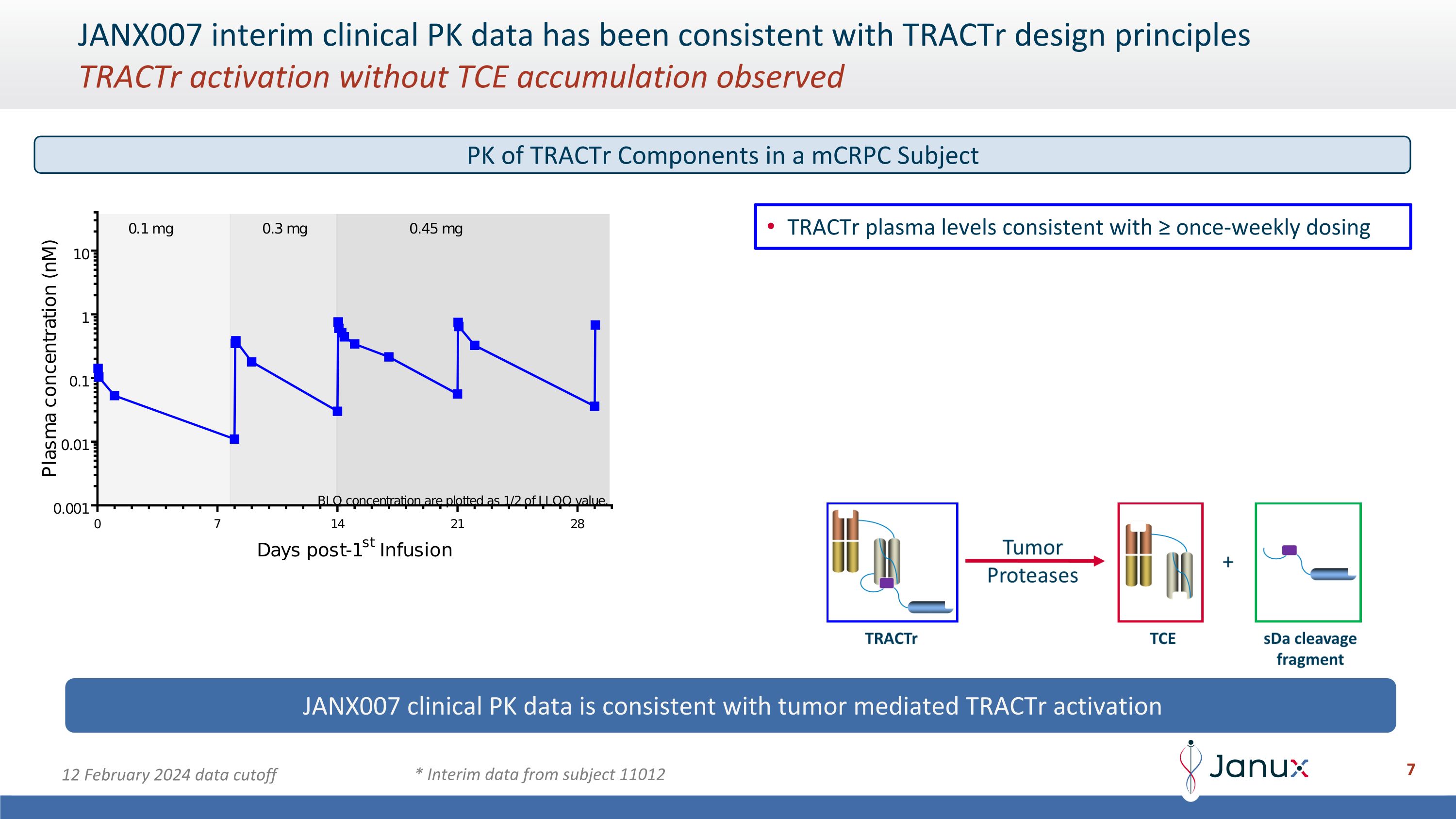
JANX007 interim clinical PK data has been consistent with TRACTr design principles�TRACTr activation without TCE accumulation observed TRACTr plasma levels consistent with ≥ once-weekly dosing JANX007 clinical PK data is consistent with tumor mediated TRACTr activation * Interim data from subject 11012 12 February 2024 data cutoff PK of TRACTr Components in a mCRPC Subject
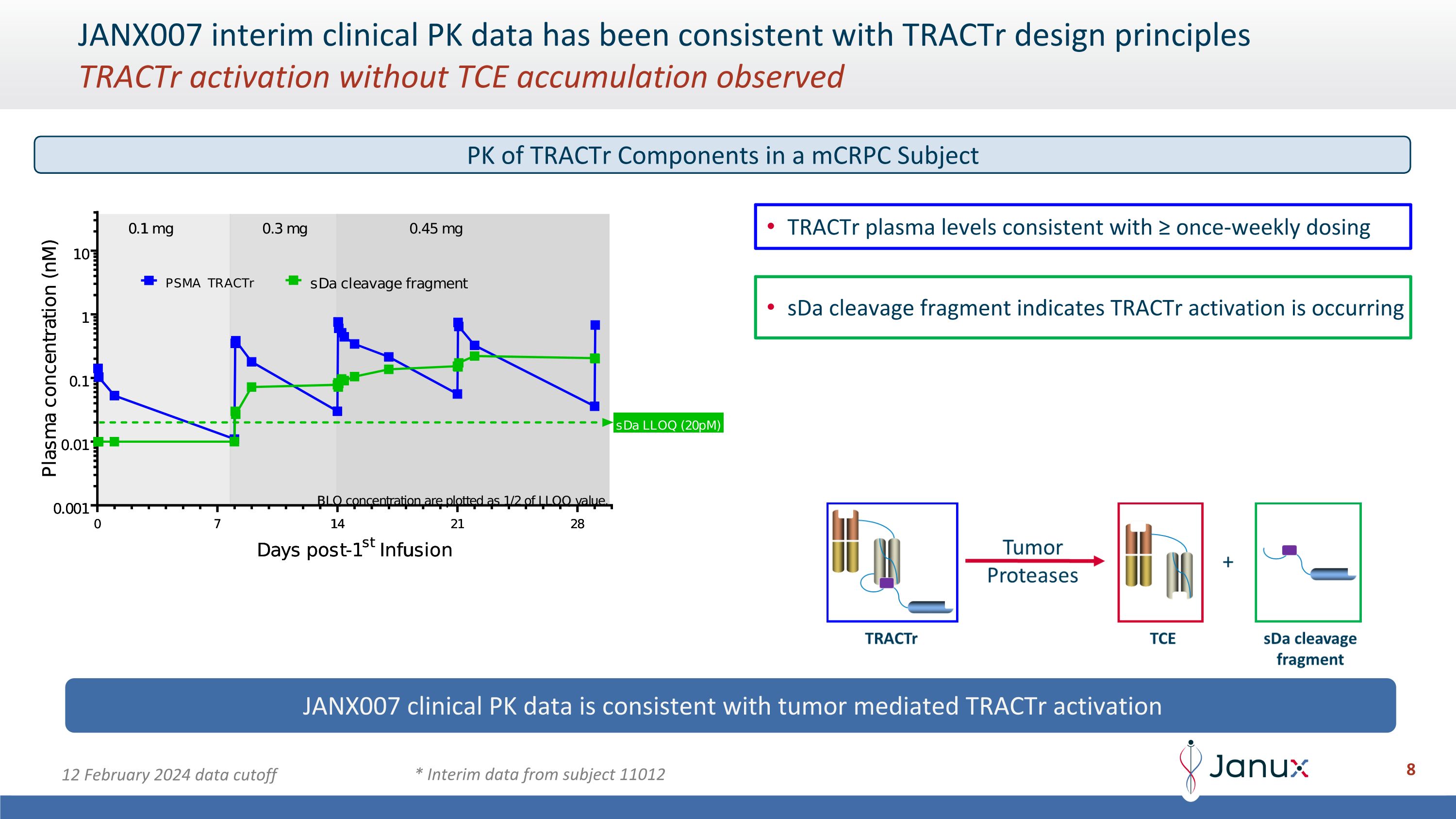
JANX007 interim clinical PK data has been consistent with TRACTr design principles�TRACTr activation without TCE accumulation observed TRACTr plasma levels consistent with ≥ once-weekly dosing sDa cleavage fragment indicates TRACTr activation is occurring JANX007 clinical PK data is consistent with tumor mediated TRACTr activation * Interim data from subject 11012 12 February 2024 data cutoff PK of TRACTr Components in a mCRPC Subject
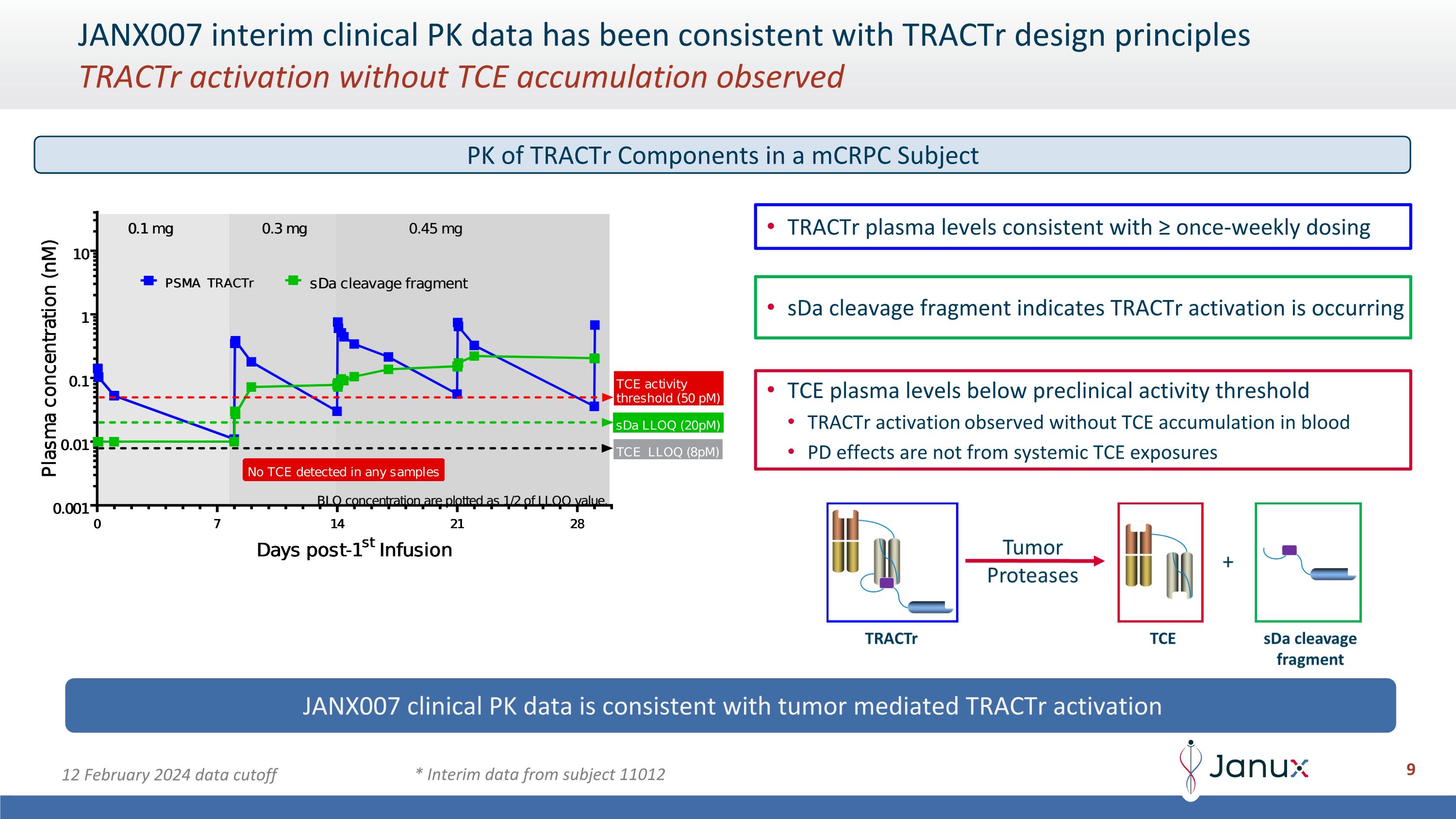
JANX007 interim clinical PK data has been consistent with TRACTr design principles�TRACTr activation without TCE accumulation observed TRACTr plasma levels consistent with ≥ once-weekly dosing sDa cleavage fragment indicates TRACTr activation is occurring TCE plasma levels below preclinical activity threshold TRACTr activation observed without TCE accumulation in blood PD effects are not from systemic TCE exposures JANX007 clinical PK data is consistent with tumor mediated TRACTr activation * Interim data from subject 11012 12 February 2024 data cutoff PK of TRACTr Components in a mCRPC Subject

PSMA-TRACTr Program JANX007
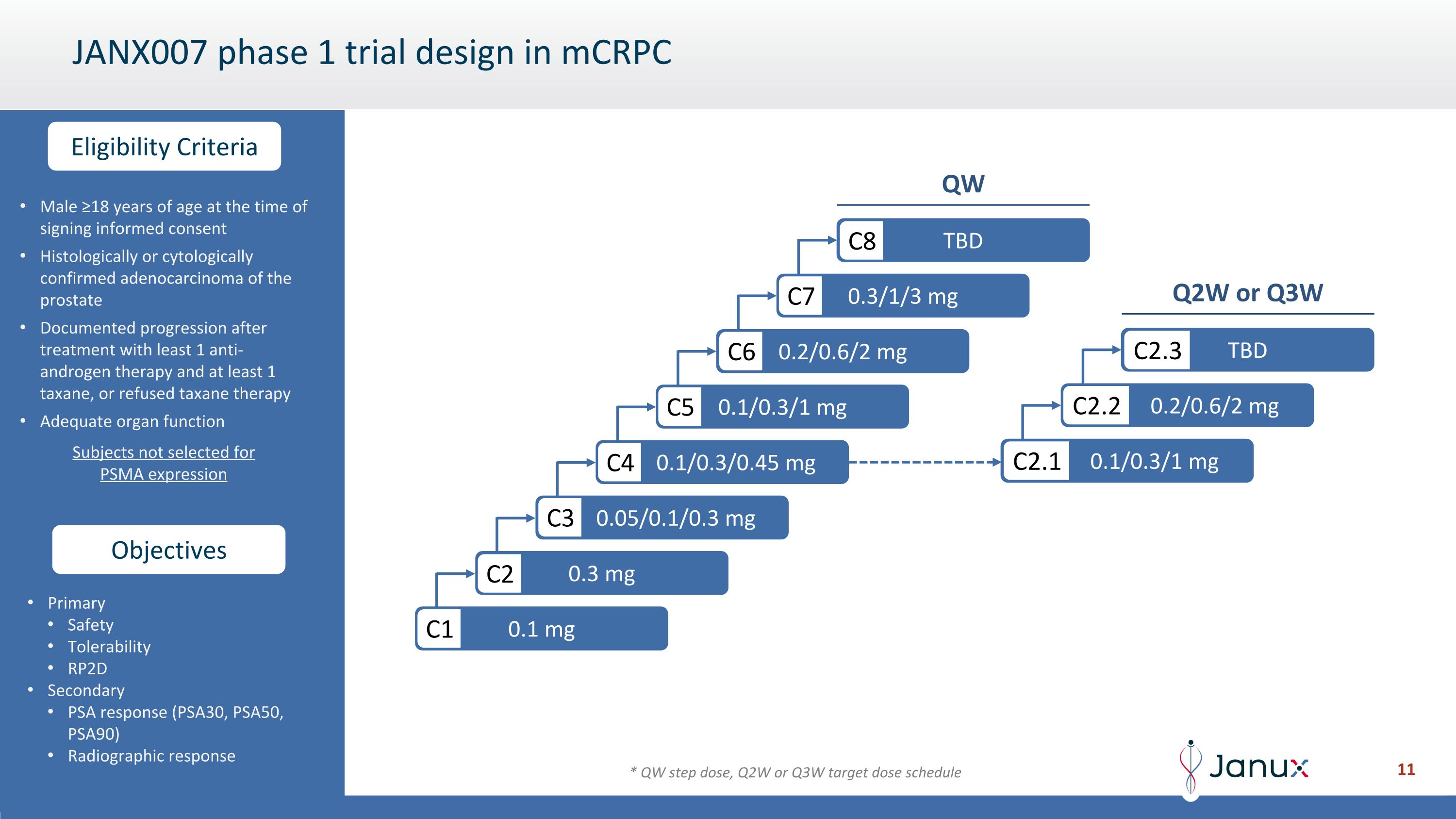
Male ≥18 years of age at the time of signing informed consent Histologically or cytologically confirmed adenocarcinoma of the prostate Documented progression after treatment with least 1 anti-androgen therapy and at least 1 taxane, or refused taxane therapy Adequate organ function Subjects not selected for PSMA expression Primary Safety Tolerability RP2D Secondary PSA response (PSA30, PSA50, PSA90) Radiographic response JANX007 phase 1 trial design in mCRPC 0.1 mg C1 0.3 mg C2 0.05/0.1/0.3 mg C3 0.1/0.3/0.45 mg C4 0.1/0.3/1 mg C5 0.2/0.6/2 mg C6 0.3/1/3 mg C7 TBD C8 Objectives Eligibility Criteria 0.1/0.3/1 mg C2.1 0.2/0.6/2 mg C2.2 TBD C2.3 QW Q2W or Q3W * QW step dose, Q2W or Q3W target dose schedule
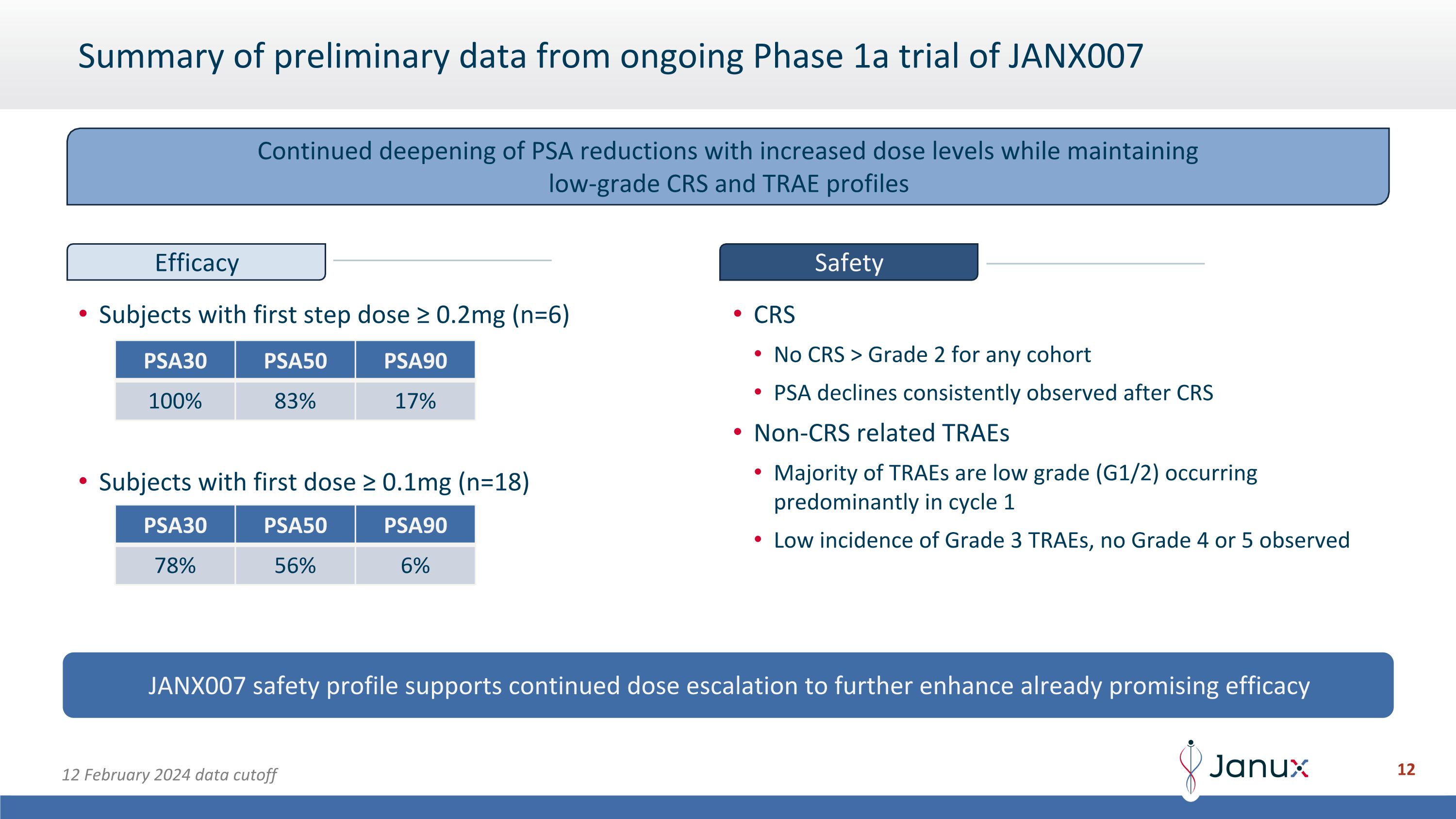
Summary of preliminary data from ongoing Phase 1a trial of JANX007 Subjects with first step dose ≥ 0.2mg (n=6) Subjects with first dose ≥ 0.1mg (n=18) CRS No CRS > Grade 2 for any cohort PSA declines consistently observed after CRS Non-CRS related TRAEs Majority of TRAEs are low grade (G1/2) occurring predominantly in cycle 1 Low incidence of Grade 3 TRAEs, no Grade 4 or 5 observed JANX007 safety profile supports continued dose escalation to further enhance already promising efficacy 12 February 2024 data cutoff Continued deepening of PSA reductions with increased dose levels while maintaining low-grade CRS and TRAE profiles Efficacy Safety PSA30 PSA50 PSA90 100% 83% 17% PSA30 PSA50 PSA90 78% 56% 6%
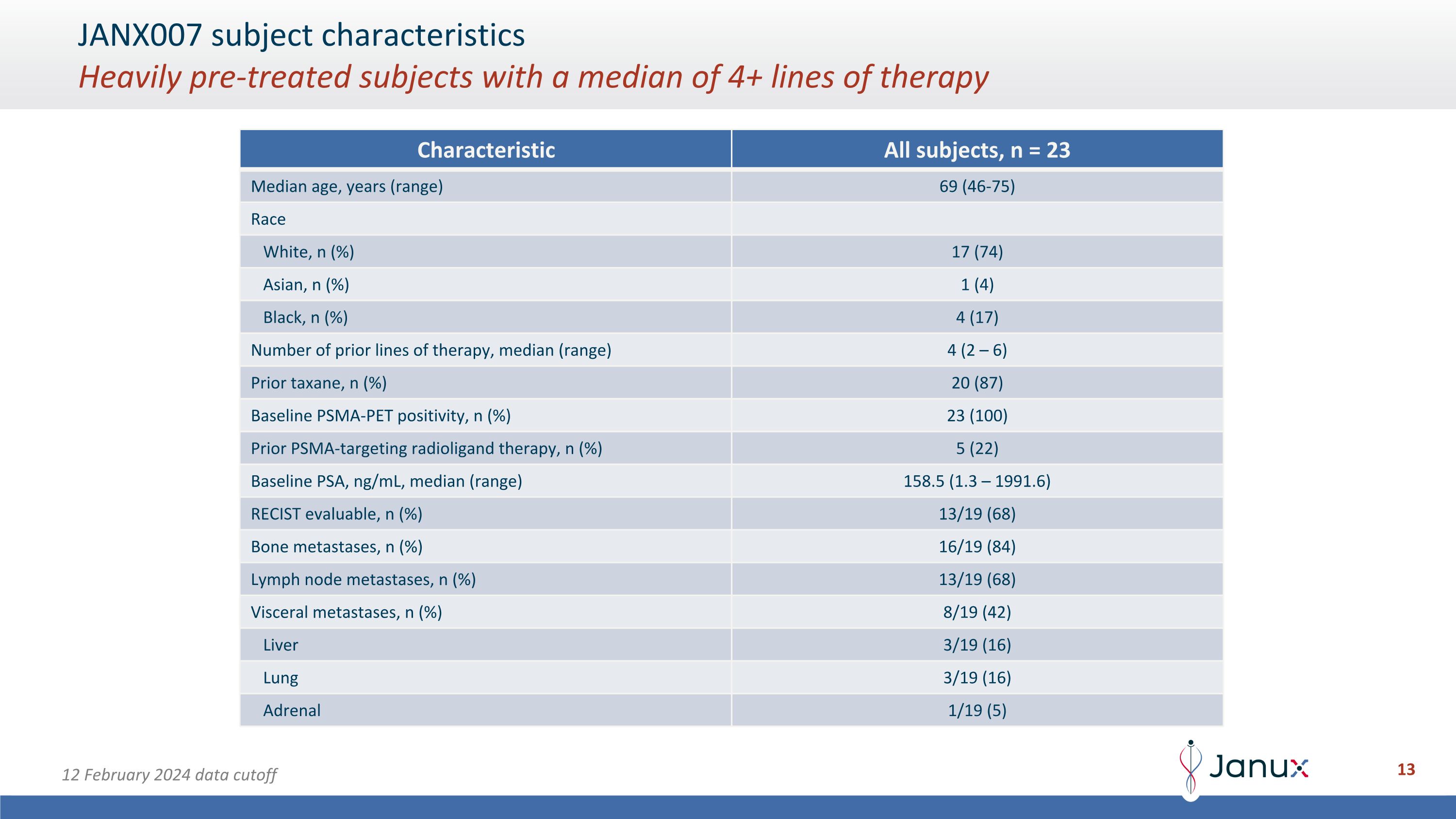
JANX007 subject characteristics�Heavily pre-treated subjects with a median of 4+ lines of therapy 12 February 2024 data cutoff Characteristic All subjects, n = 23 Median age, years (range) 69 (46-75) Race White, n (%) 17 (74) Asian, n (%) 1 (4) Black, n (%) 4 (17) Number of prior lines of therapy, median (range) 4 (2 – 6) Prior taxane, n (%) 20 (87) Baseline PSMA-PET positivity, n (%) 23 (100) Prior PSMA-targeting radioligand therapy, n (%) 5 (22) Baseline PSA, ng/mL, median (range) 158.5 (1.3 – 1991.6) RECIST evaluable, n (%) 13/19 (68) Bone metastases, n (%) 16/19 (84) Lymph node metastases, n (%) 13/19 (68) Visceral metastases, n (%) 8/19 (42) Liver 3/19 (16) Lung 3/19 (16) Adrenal 1/19 (5)
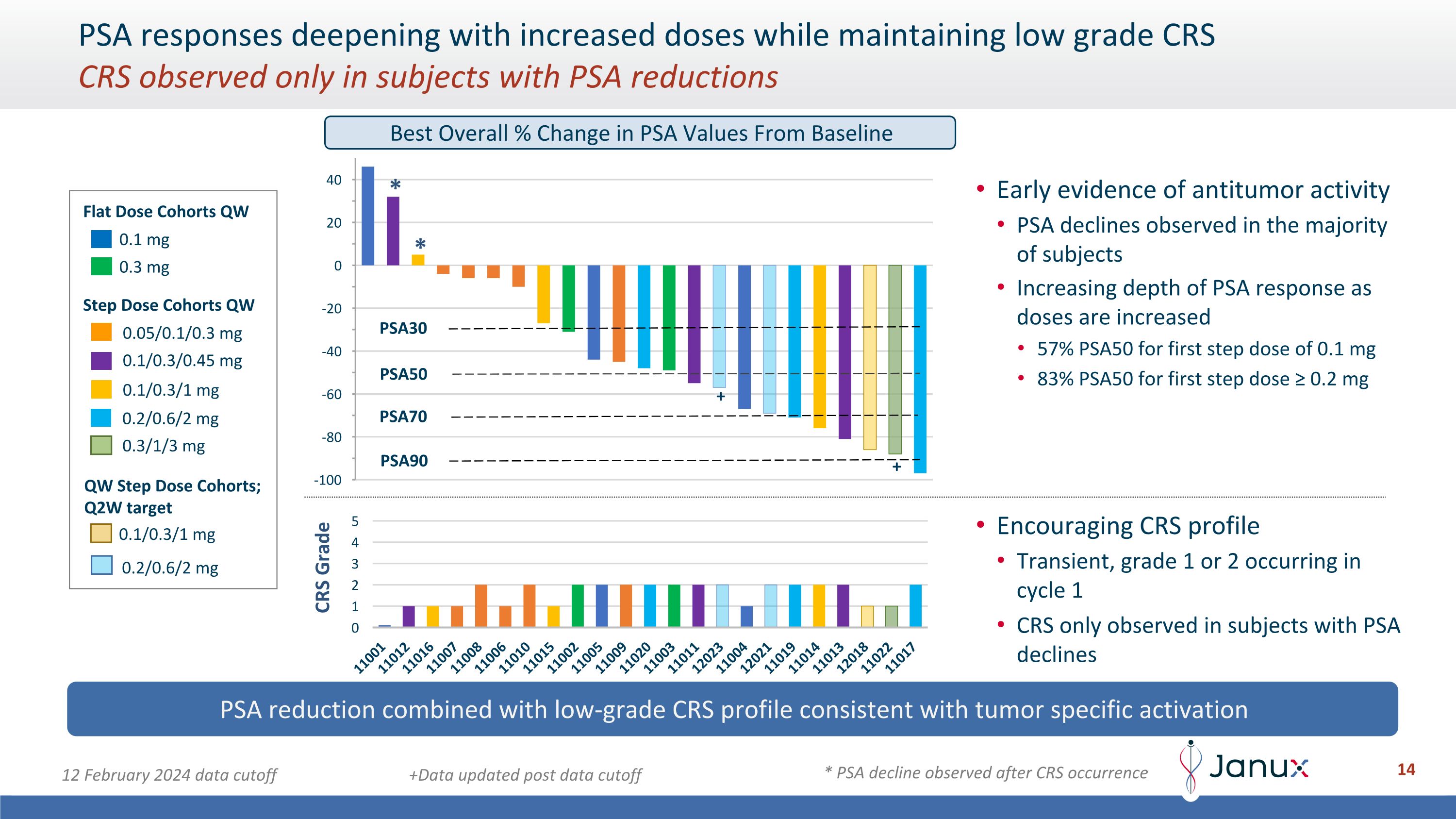
PSA responses deepening with increased doses while maintaining low grade CRS�CRS observed only in subjects with PSA reductions PSA reduction combined with low-grade CRS profile consistent with tumor specific activation CRS Grade * PSA decline observed after CRS occurrence Encouraging CRS profile Transient, grade 1 or 2 occurring in cycle 1 CRS only observed in subjects with PSA declines Early evidence of antitumor activity PSA declines observed in the majority of subjects Increasing depth of PSA response as doses are increased 57% PSA50 for first step dose of 0.1 mg 83% PSA50 for first step dose ≥ 0.2 mg * PSA30 PSA50 PSA90 PSA70 * 0.1 mg 0.3 mg 0.05/0.1/0.3 mg Flat Dose Cohorts QW Step Dose Cohorts QW 0.1/0.3/1 mg 0.1/0.3/0.45 mg 0.1/0.3/1 mg QW Step Dose Cohorts; Q2W target 0.2/0.6/2 mg 0.2/0.6/2 mg 0.3/1/3 mg +Data updated post data cutoff + + 12 February 2024 data cutoff Best Overall % Change in PSA Values From Baseline
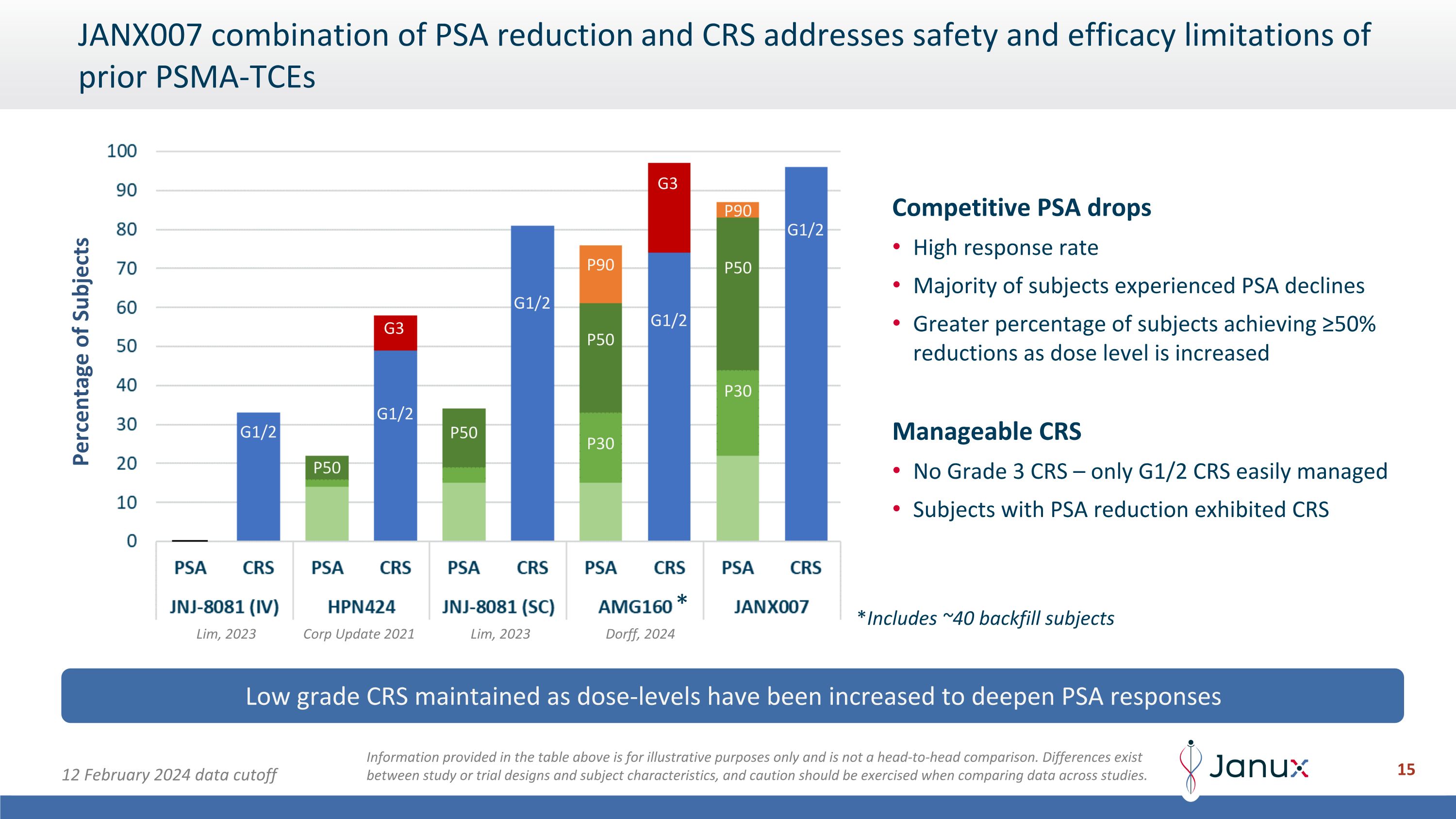
JANX007 combination of PSA reduction and CRS addresses safety and efficacy limitations of prior PSMA-TCEs Low grade CRS maintained as dose-levels have been increased to deepen PSA responses Information provided in the table above is for illustrative purposes only and is not a head-to-head comparison. Differences exist between study or trial designs and subject characteristics, and caution should be exercised when comparing data across studies. Percentage of Subjects Competitive PSA drops High response rate Majority of subjects experienced PSA declines Greater percentage of subjects achieving ≥50% reductions as dose level is increased Manageable CRS No Grade 3 CRS – only G1/2 CRS easily managed Subjects with PSA reduction exhibited CRS P50 P50 P50 P50 P30 P30 G3 G3 G1/2 P90 P90 G1/2 G1/2 G1/2 G1/2 * *Includes ~40 backfill subjects 12 February 2024 data cutoff Lim, 2023 Lim, 2023 Corp Update 2021 Dorff, 2024
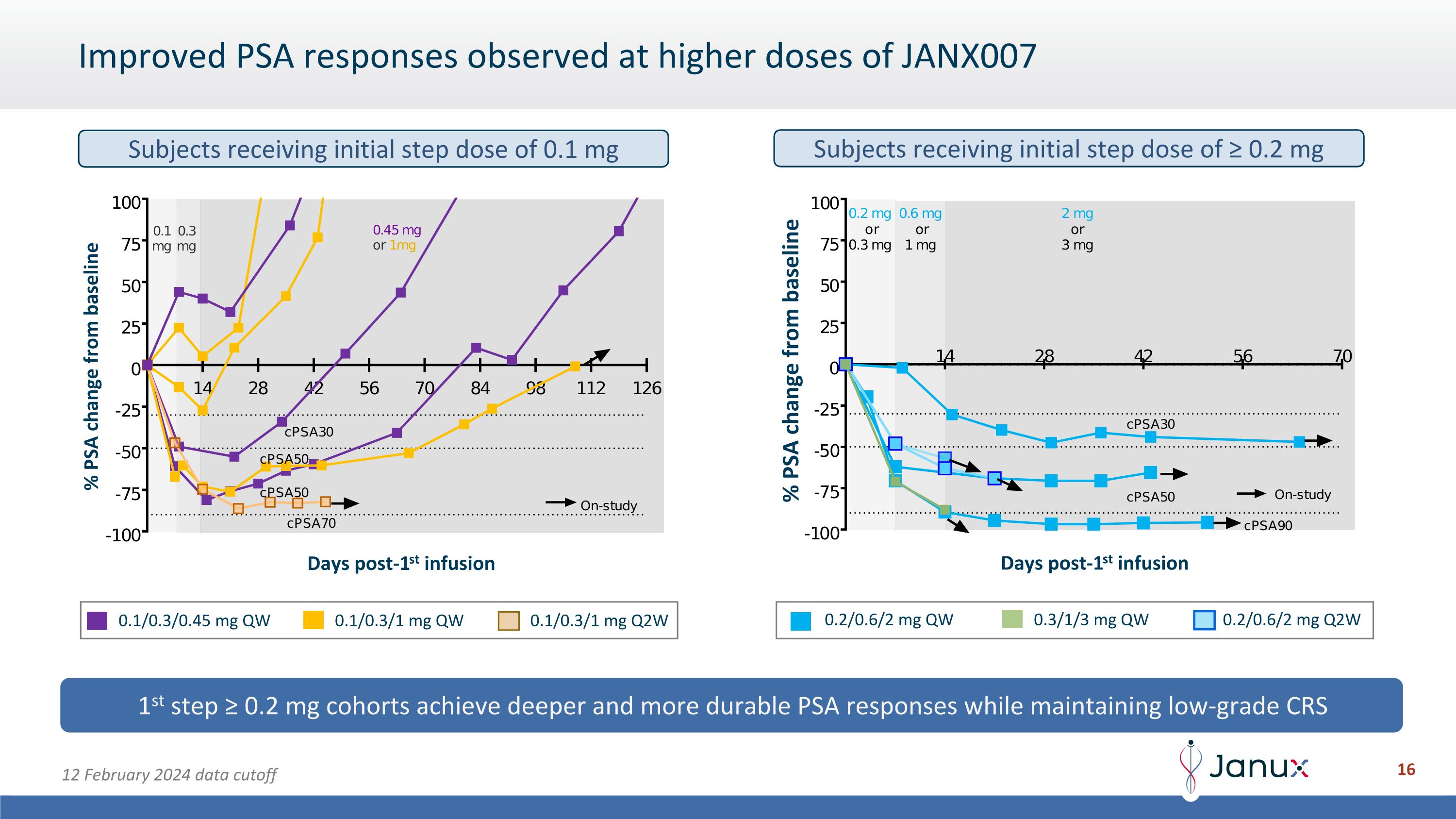
Improved PSA responses observed at higher doses of JANX007 Days post-1st infusion 1st step ≥ 0.2 mg cohorts achieve deeper and more durable PSA responses while maintaining low-grade CRS 12 February 2024 data cutoff % PSA change from baseline % PSA change from baseline Days post-1st infusion 0.1/0.3/1 mg QW 0.1/0.3/0.45 mg QW 0.1/0.3/1 mg Q2W 0.2/0.6/2 mg QW 0.2/0.6/2 mg Q2W 0.3/1/3 mg QW Subjects receiving initial step dose of 0.1 mg Subjects receiving initial step dose of ≥ 0.2 mg
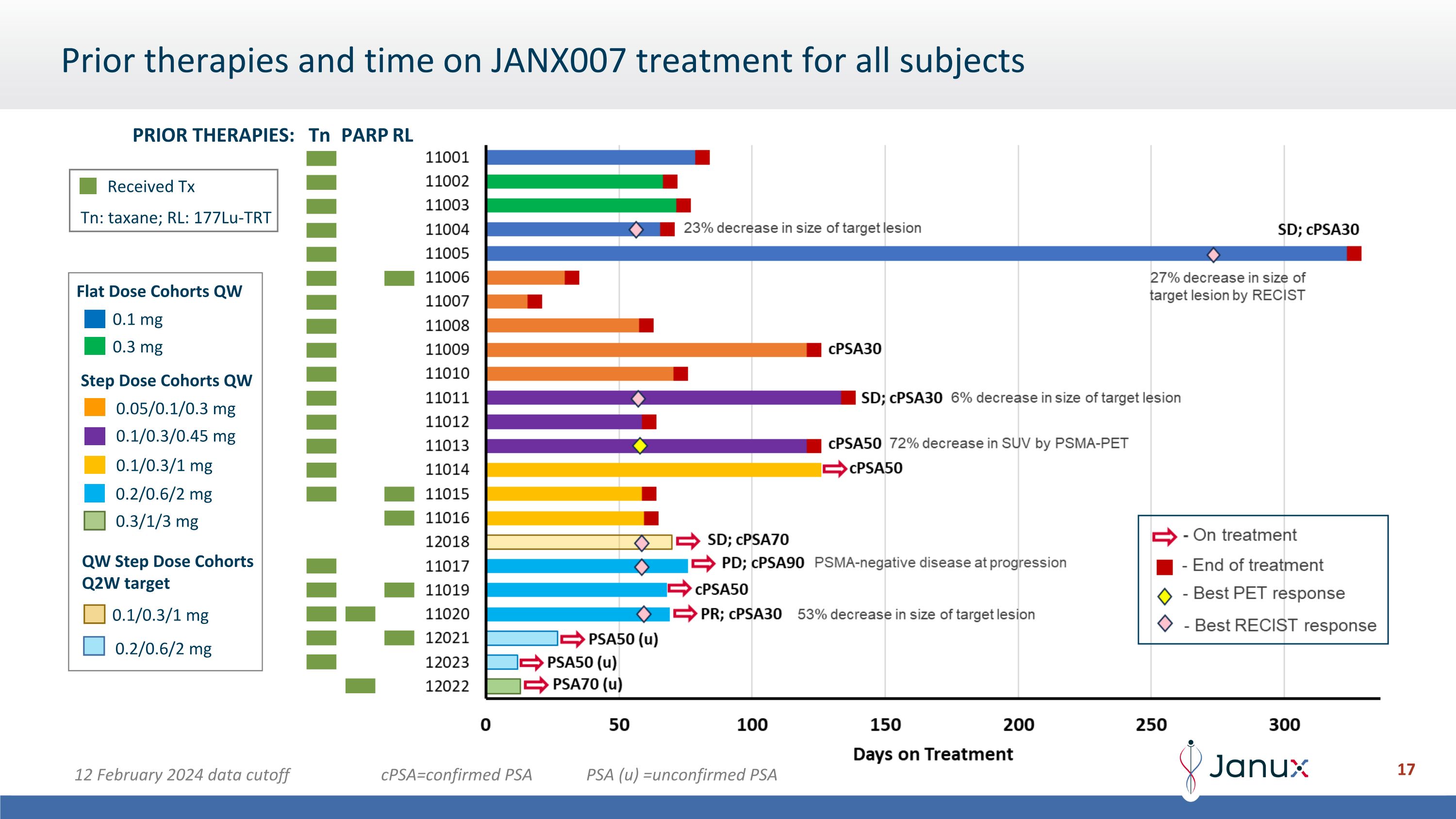
Step Dose Cohorts QW QW Step Dose Cohorts Q2W target 0.1 mg 0.3 mg 0.05/0.1/0.3 mg Flat Dose Cohorts QW 0.1/0.3/1 mg 0.1/0.3/0.45 mg 0.1/0.3/1 mg 0.2/0.6/2 mg 0.2/0.6/2 mg 0.3/1/3 mg Prior therapies and time on JANX007 treatment for all subjects Tn PARP RL PRIOR THERAPIES: Tn: taxane; RL: 177Lu-TRT Received Tx 12 February 2024 data cutoff cPSA=confirmed PSA PSA (u) =unconfirmed PSA
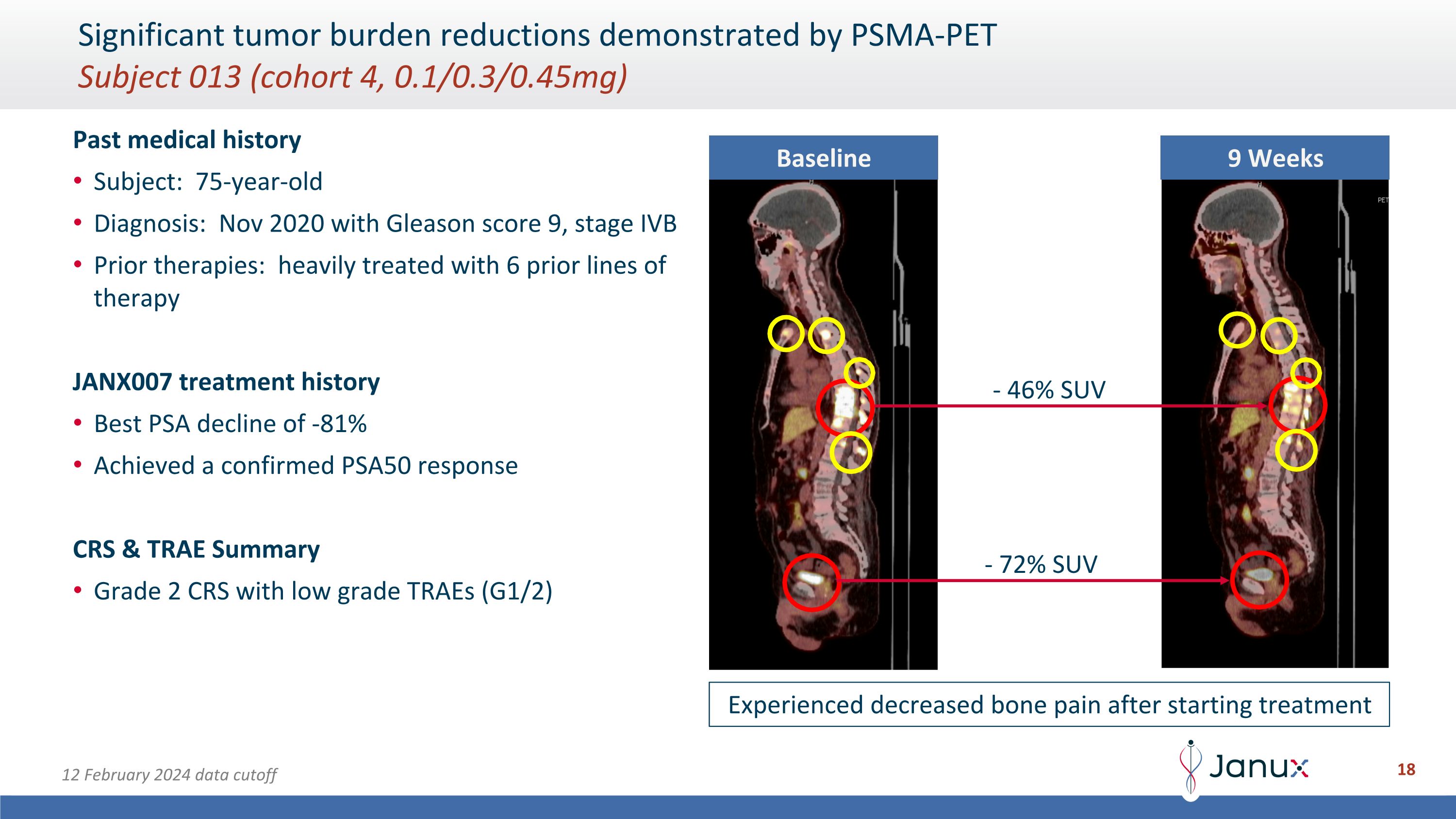
Significant tumor burden reductions demonstrated by PSMA-PET�Subject 013 (cohort 4, 0.1/0.3/0.45mg) Past medical history Subject: 75-year-old Diagnosis: Nov 2020 with Gleason score 9, stage IVB Prior therapies: heavily treated with 6 prior lines of therapy JANX007 treatment history Best PSA decline of -81% Achieved a confirmed PSA50 response CRS & TRAE Summary Grade 2 CRS with low grade TRAEs (G1/2) Baseline 9 Weeks - 46% SUV - 72% SUV Experienced decreased bone pain after starting treatment 12 February 2024 data cutoff
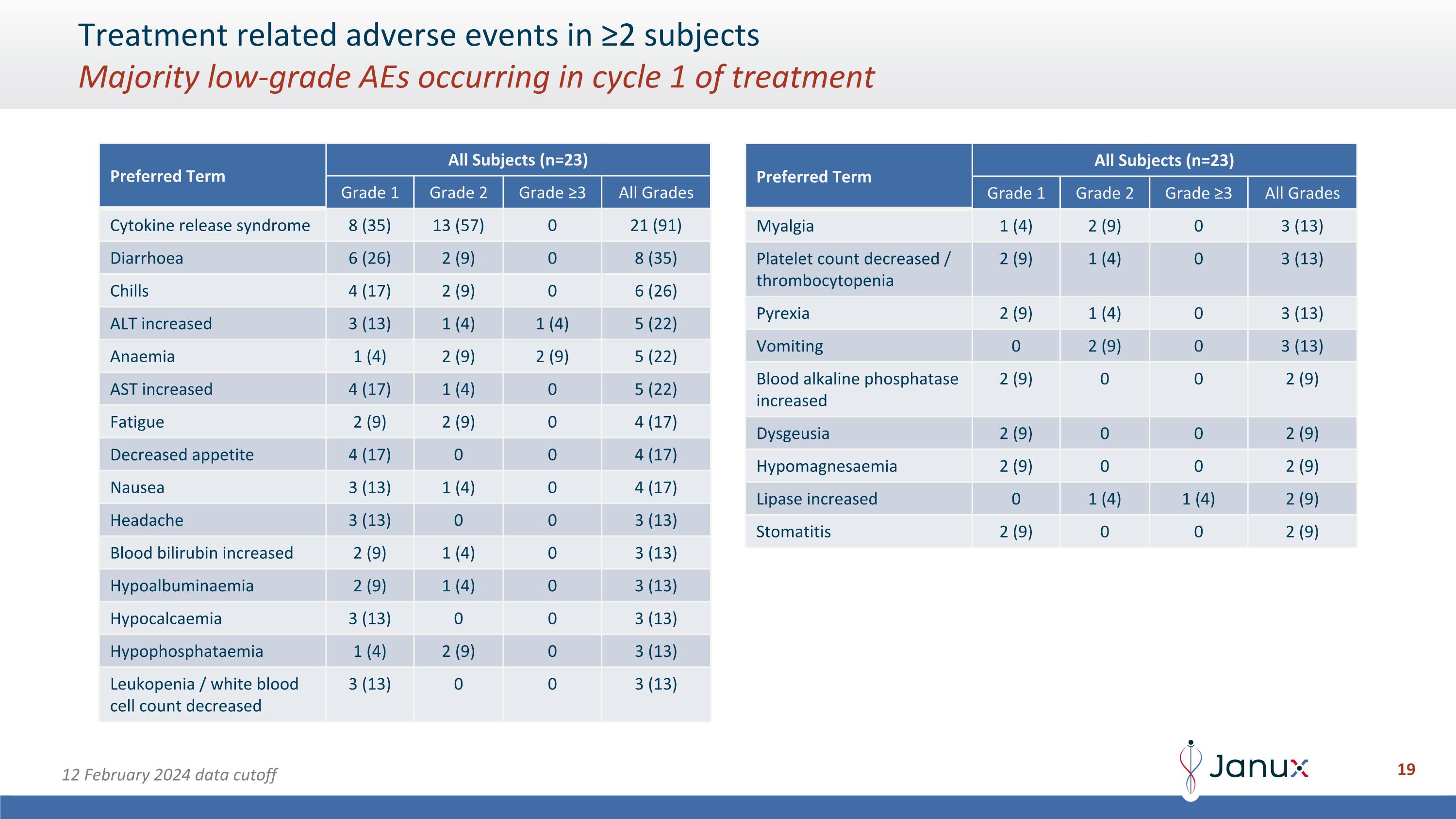
Treatment related adverse events in ≥2 subjects�Majority low-grade AEs occurring in cycle 1 of treatment Preferred Term All Subjects (n=23) Grade 1 Grade 2 Grade ≥3 All Grades Cytokine release syndrome 8 (35) 13 (57) 0 21 (91) Diarrhoea 6 (26) 2 (9) 0 8 (35) Chills 4 (17) 2 (9) 0 6 (26) ALT increased 3 (13) 1 (4) 1 (4) 5 (22) Anaemia 1 (4) 2 (9) 2 (9) 5 (22) AST increased 4 (17) 1 (4) 0 5 (22) Fatigue 2 (9) 2 (9) 0 4 (17) Decreased appetite 4 (17) 0 0 4 (17) Nausea 3 (13) 1 (4) 0 4 (17) Headache 3 (13) 0 0 3 (13) Blood bilirubin increased 2 (9) 1 (4) 0 3 (13) Hypoalbuminaemia 2 (9) 1 (4) 0 3 (13) Hypocalcaemia 3 (13) 0 0 3 (13) Hypophosphataemia 1 (4) 2 (9) 0 3 (13) Leukopenia / white blood cell count decreased 3 (13) 0 0 3 (13) Preferred Term All Subjects (n=23) Grade 1 Grade 2 Grade ≥3 All Grades Myalgia 1 (4) 2 (9) 0 3 (13) Platelet count decreased / thrombocytopenia 2 (9) 1 (4) 0 3 (13) Pyrexia 2 (9) 1 (4) 0 3 (13) Vomiting 0 2 (9) 0 3 (13) Blood alkaline phosphatase increased 2 (9) 0 0 2 (9) Dysgeusia 2 (9) 0 0 2 (9) Hypomagnesaemia 2 (9) 0 0 2 (9) Lipase increased 0 1 (4) 1 (4) 2 (9) Stomatitis 2 (9) 0 0 2 (9) 12 February 2024 data cutoff
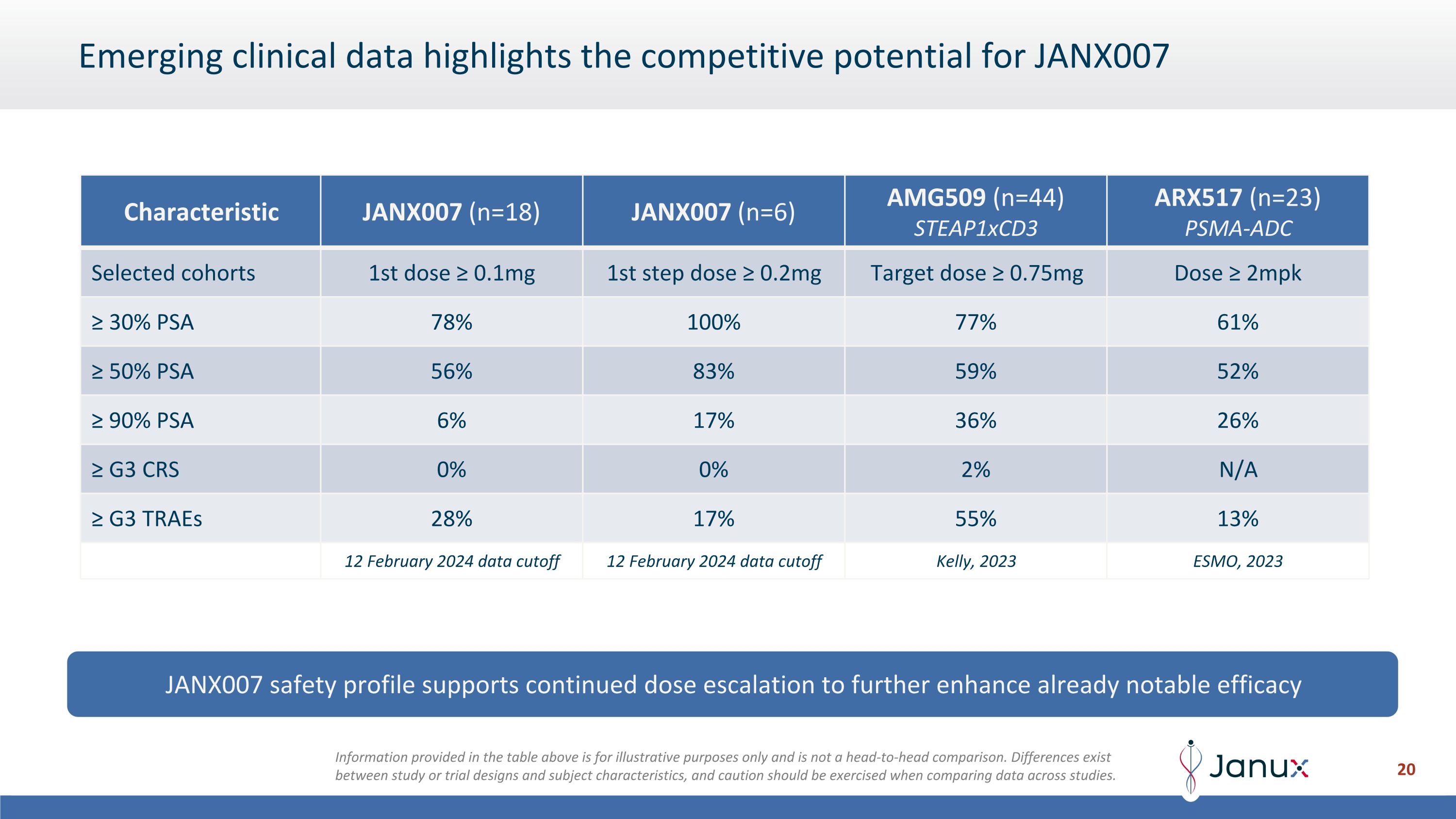
Emerging clinical data highlights the competitive potential for JANX007 JANX007 safety profile supports continued dose escalation to further enhance already notable efficacy Characteristic JANX007 (n=18) JANX007 (n=6) AMG509 (n=44) STEAP1xCD3 ARX517 (n=23) PSMA-ADC Selected cohorts 1st dose ≥ 0.1mg 1st step dose ≥ 0.2mg Target dose ≥ 0.75mg Dose ≥ 2mpk ≥ 30% PSA 78% 100% 77% 61% ≥ 50% PSA 56% 83% 59% 52% ≥ 90% PSA 6% 17% 36% 26% ≥ G3 CRS 0% 0% 2% N/A ≥ G3 TRAEs 28% 17% 55% 13% 12 February 2024 data cutoff 12 February 2024 data cutoff Kelly, 2023 ESMO, 2023 Information provided in the table above is for illustrative purposes only and is not a head-to-head comparison. Differences exist between study or trial designs and subject characteristics, and caution should be exercised when comparing data across studies.

JANX007 positioning in mCRPC Target PSMA is a clinically validated (Pluvicto®) and highly expressed target that is expressed in >80%† of mCRPC patients JANX007 Single-agent efficacy and safety data in heavily pre-treated, late-stage mCRPC patients Supports single-agent development in 3L+ and 2L+ settings Non-overlapping toxicity provides opportunity to treat Pluvicto® responders and non-responders Switch maintenance therapy to JANX007 following Pluvicto® provides opportunity to deepen and prolong responses Additional opportunity for PSMA directed therapy for Pluvicto® non-responders Opportunity in 2L and 3L settings Complementary combination opportunity with enzalutamide Enzalutamide upregulates PSMA, the target for JANX007 treatment Combination with enzalutamide may provide synergy to overcome enzalutamide resistance mechanisms Opportunity in 1L and 2L settings † Bostwick DG, 1998
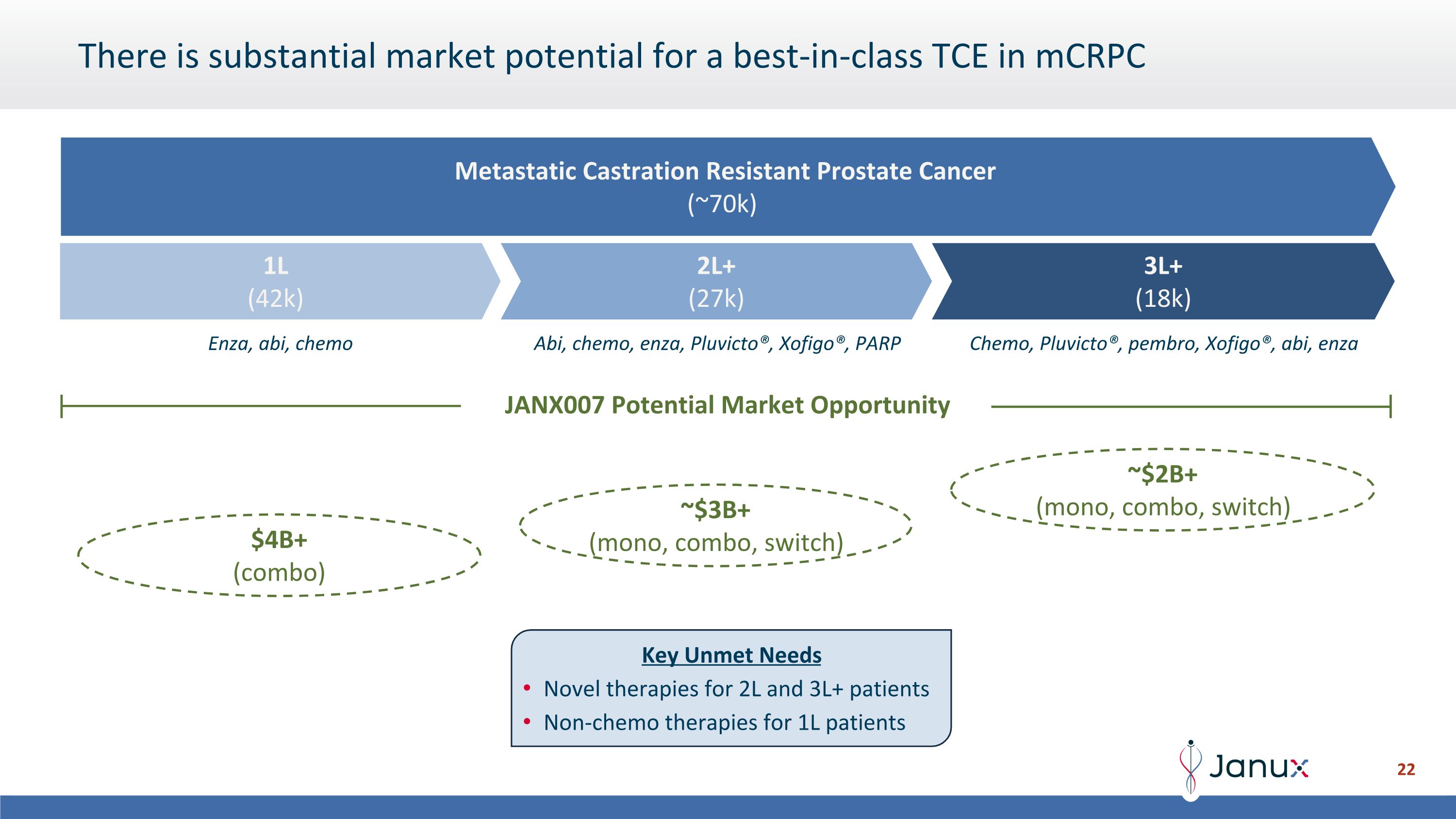
There is substantial market potential for a best-in-class TCE in mCRPC JANX007 Potential Market Opportunity 2L+ (27k) 3L+ (18k) 1L (42k) Metastatic Castration Resistant Prostate Cancer (~70k) Enza, abi, chemo Abi, chemo, enza, Pluvicto®, Xofigo®, PARP Chemo, Pluvicto®, pembro, Xofigo®, abi, enza ~$2B+ (mono, combo, switch) $4B+ (combo) ~$3B+ (mono, combo, switch) Key Unmet Needs Novel therapies for 2L and 3L+ patients Non-chemo therapies for 1L patients

EGFR-TRACTr Program JANX008
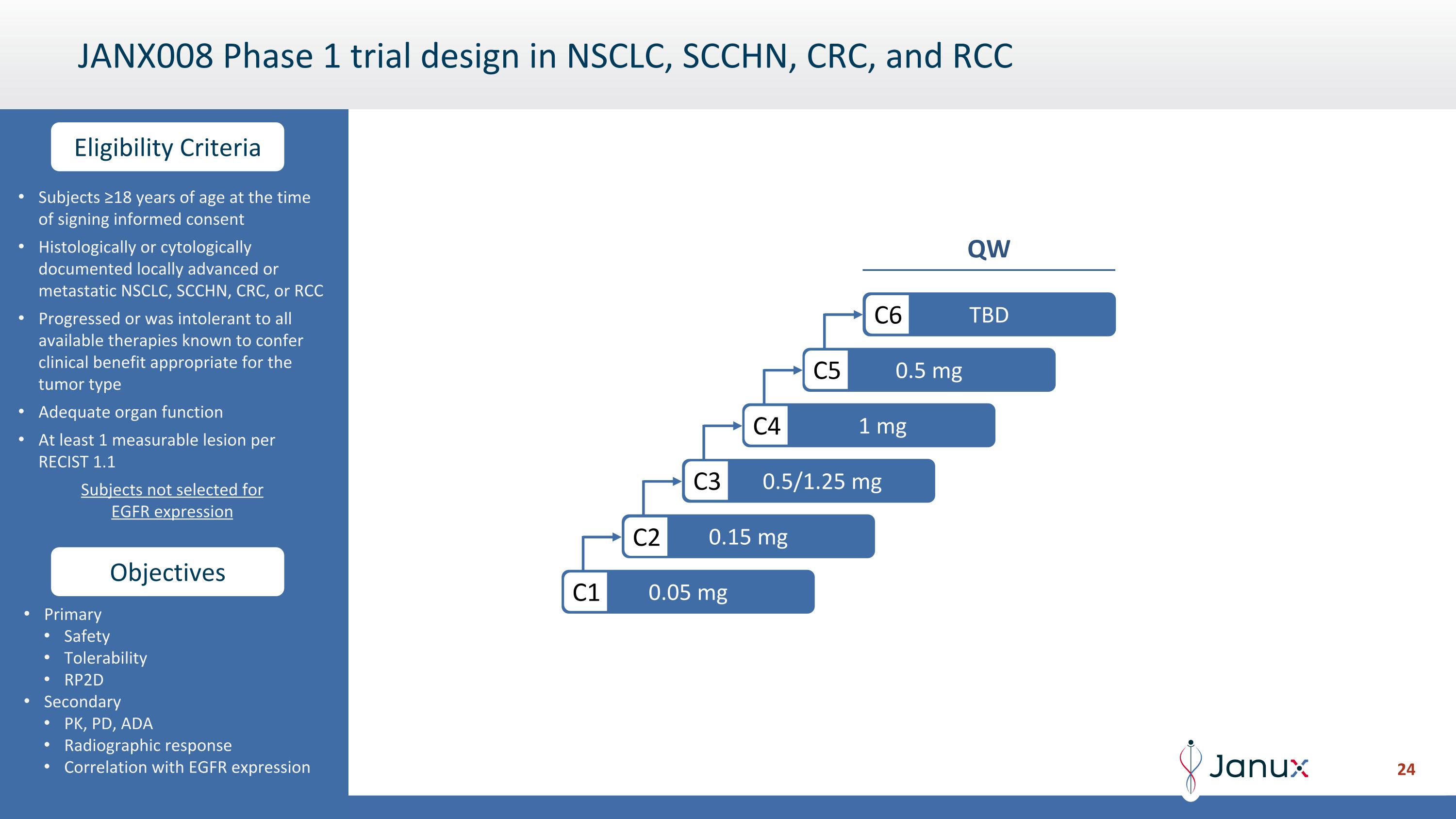
Objectives Eligibility Criteria JANX008 Phase 1 trial design in NSCLC, SCCHN, CRC, and RCC Subjects ≥18 years of age at the time of signing informed consent Histologically or cytologically documented locally advanced or metastatic NSCLC, SCCHN, CRC, or RCC Progressed or was intolerant to all available therapies known to confer clinical benefit appropriate for the tumor type Adequate organ function At least 1 measurable lesion per RECIST 1.1 Subjects not selected for EGFR expression Primary Safety Tolerability RP2D Secondary PK, PD, ADA Radiographic response Correlation with EGFR expression 0.05 mg C1 0.15 mg C2 0.5/1.25 mg C3 1 mg C4 0.5 mg C5 TBD C6 QW
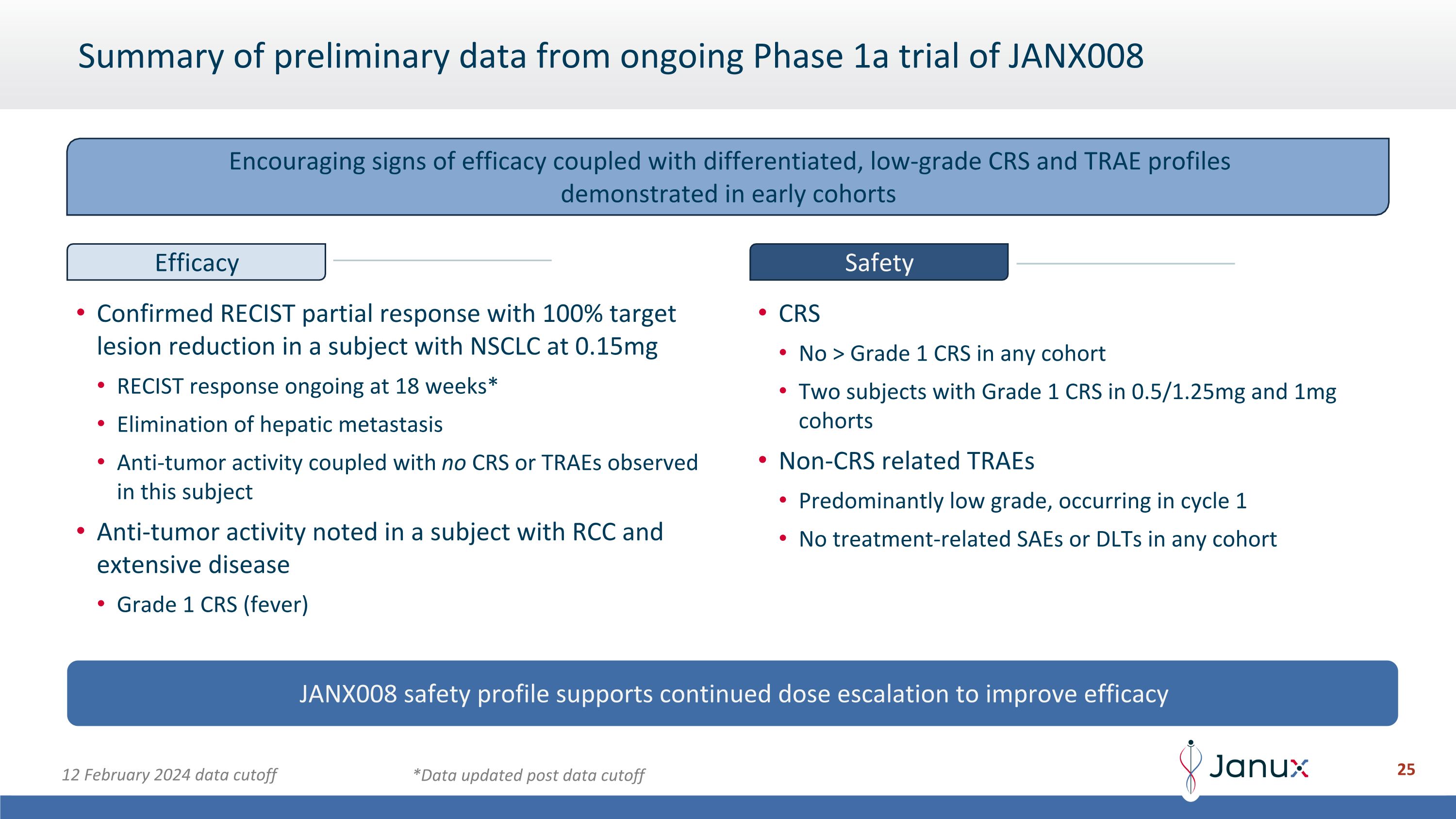
Summary of preliminary data from ongoing Phase 1a trial of JANX008 Confirmed RECIST partial response with 100% target lesion reduction in a subject with NSCLC at 0.15mg RECIST response ongoing at 18 weeks* Elimination of hepatic metastasis Anti-tumor activity coupled with no CRS or TRAEs observed in this subject Anti-tumor activity noted in a subject with RCC and extensive disease Grade 1 CRS (fever) CRS No > Grade 1 CRS in any cohort Two subjects with Grade 1 CRS in 0.5/1.25mg and 1mg cohorts Non-CRS related TRAEs Predominantly low grade, occurring in cycle 1 No treatment-related SAEs or DLTs in any cohort JANX008 safety profile supports continued dose escalation to improve efficacy 12 February 2024 data cutoff *Data updated post data cutoff Encouraging signs of efficacy coupled with differentiated, low-grade CRS and TRAE profiles demonstrated in early cohorts Efficacy Safety
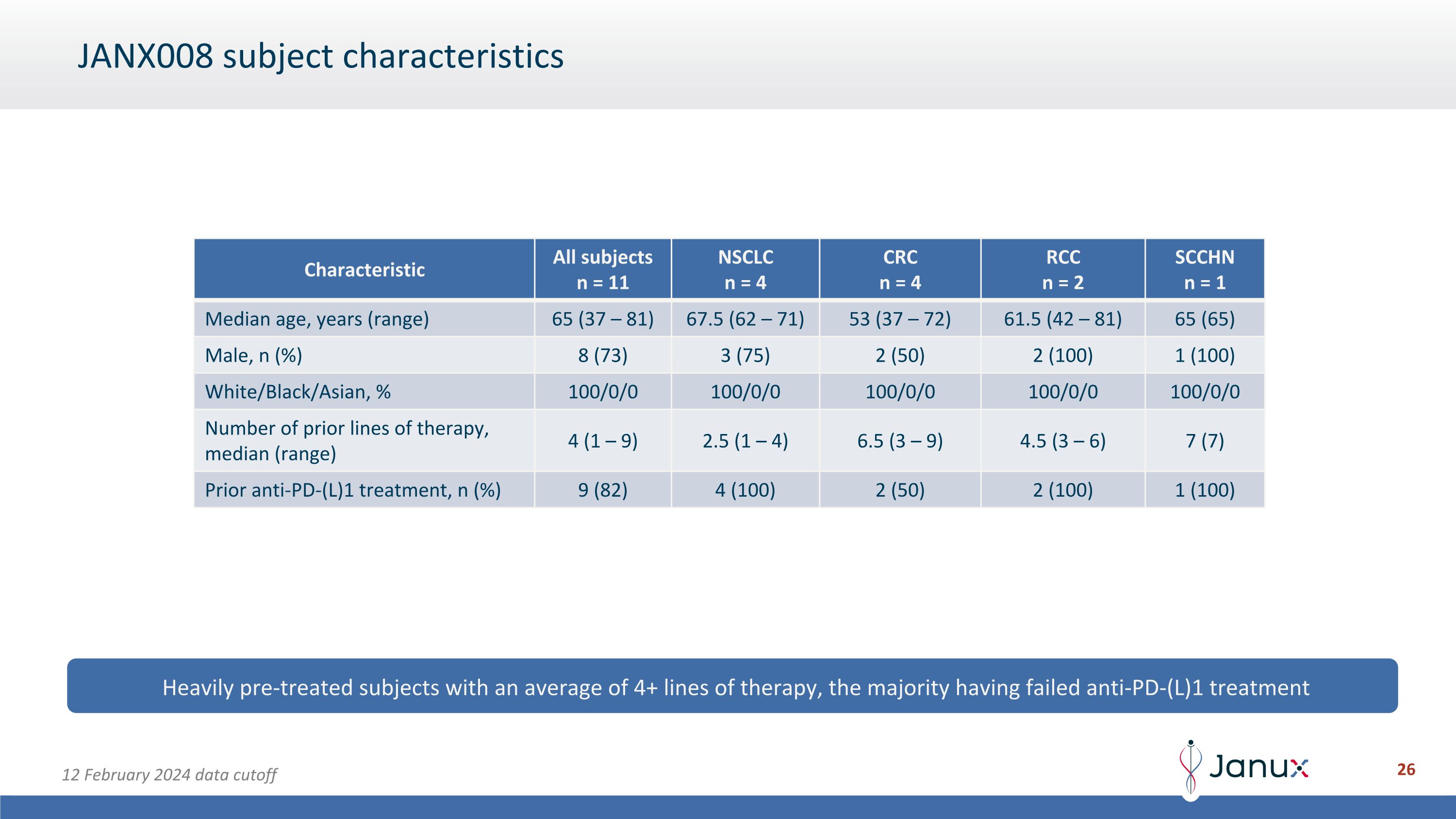
JANX008 subject characteristics Heavily pre-treated subjects with an average of 4+ lines of therapy, the majority having failed anti-PD-(L)1 treatment Characteristic All subjects n = 11 NSCLC n = 4 CRC n = 4 RCC n = 2 SCCHN n = 1 Median age, years (range) 65 (37 – 81) 67.5 (62 – 71) 53 (37 – 72) 61.5 (42 – 81) 65 (65) Male, n (%) 8 (73) 3 (75) 2 (50) 2 (100) 1 (100) White/Black/Asian, % 100/0/0 100/0/0 100/0/0 100/0/0 100/0/0 Number of prior lines of therapy, median (range) 4 (1 – 9) 2.5 (1 – 4) 6.5 (3 – 9) 4.5 (3 – 6) 7 (7) Prior anti-PD-(L)1 treatment, n (%) 9 (82) 4 (100) 2 (50) 2 (100) 1 (100) 12 February 2024 data cutoff
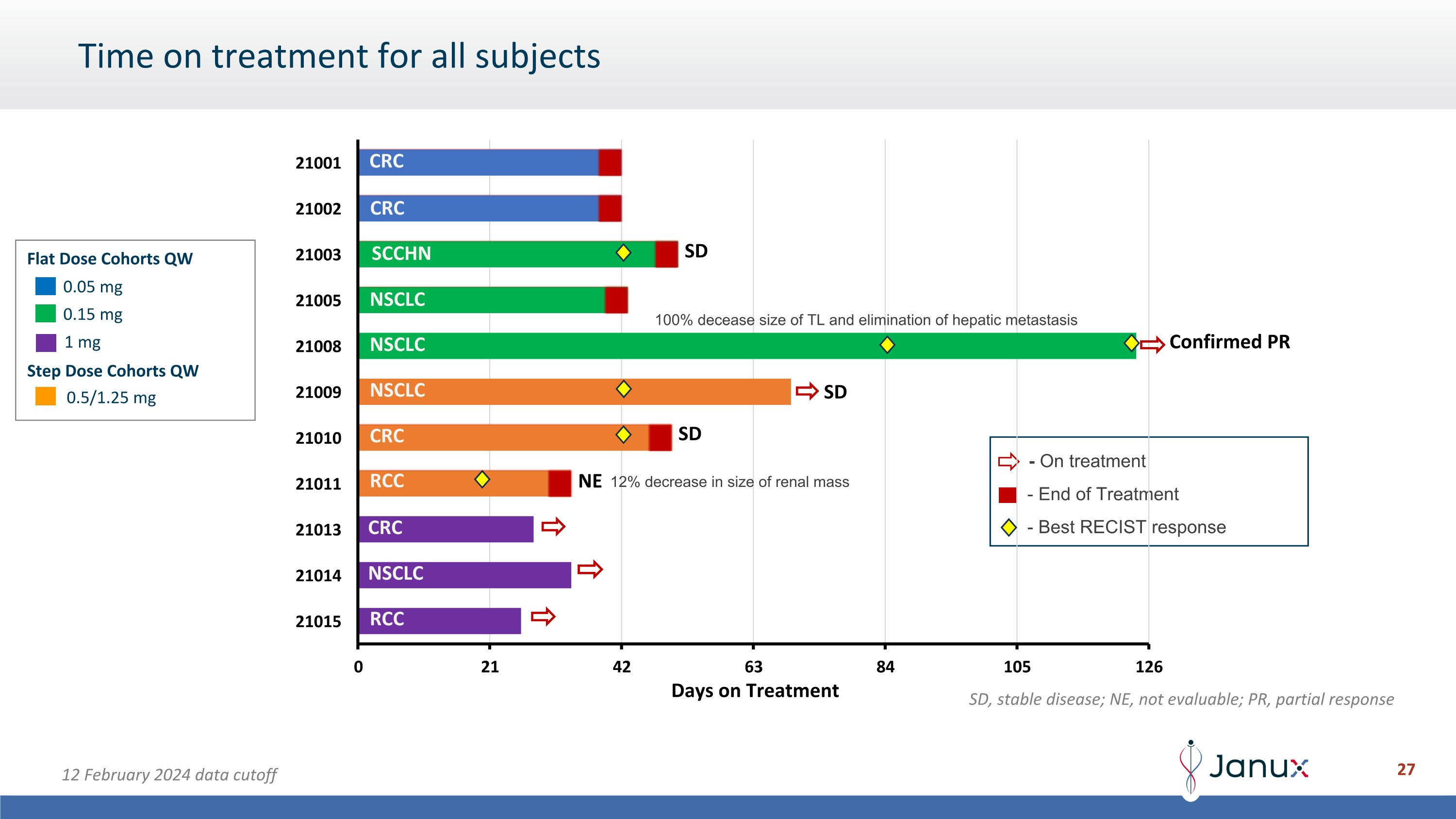
Time on treatment for all subjects - End of Treatment - On treatment 0.05 mg 0.15 mg 0.5/1.25 mg Flat Dose Cohorts QW Step Dose Cohorts QW 1 mg SD Confirmed PR SD SD CRC CRC CRC RCC RCC NSCLC NSCLC NSCLC NSCLC SCCHN - Best RECIST response 100% decease size of TL and elimination of hepatic metastasis 12% decrease in size of renal mass NE SD, stable disease; NE, not evaluable; PR, partial response 12 February 2024 data cutoff CRC
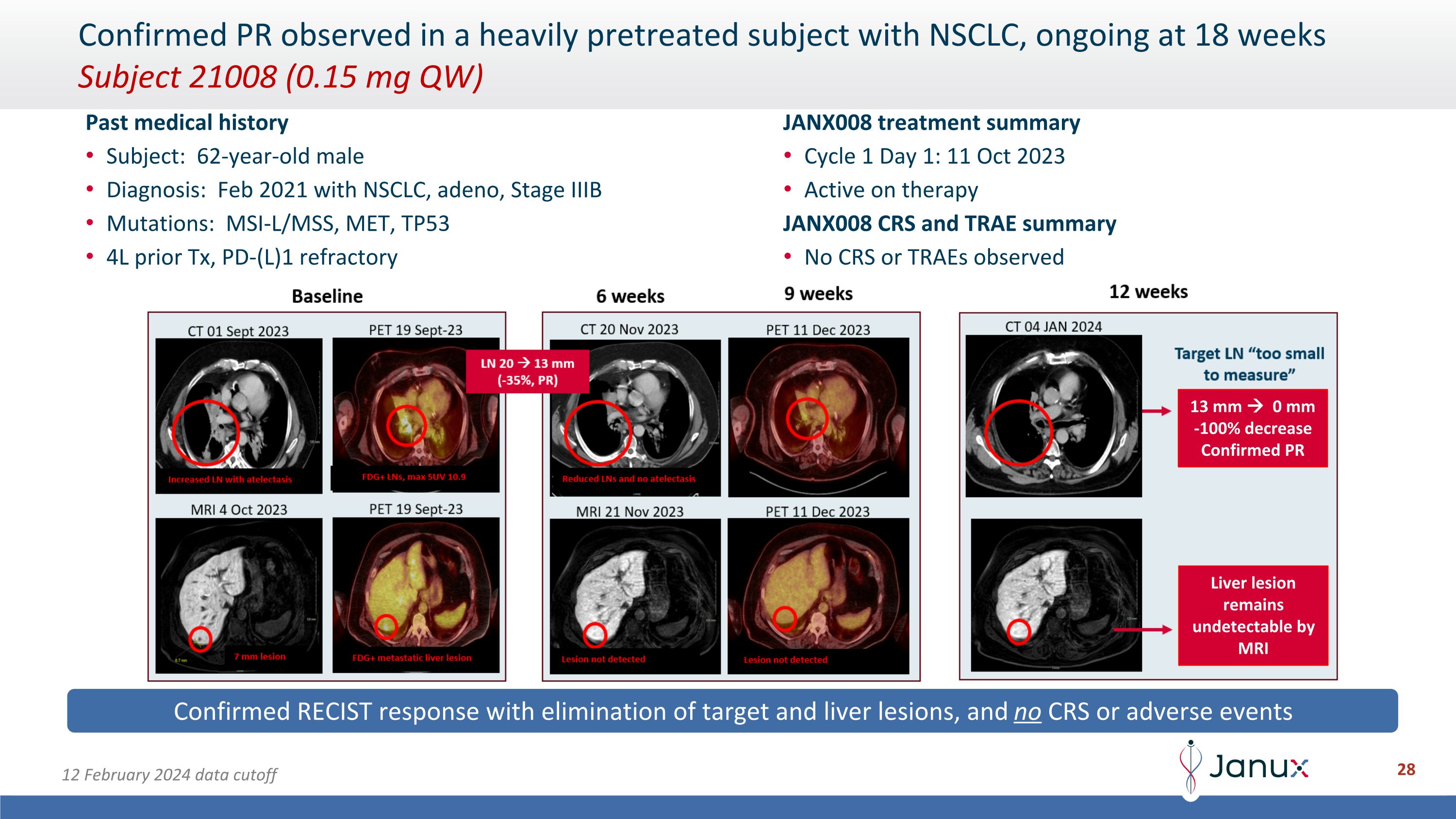
Past medical history Subject: 62-year-old male Diagnosis: Feb 2021 with NSCLC, adeno, Stage IIIB Mutations: MSI-L/MSS, MET, TP53 4L prior Tx, PD-(L)1 refractory JANX008 treatment summary Cycle 1 Day 1: 11 Oct 2023 Active on therapy JANX008 CRS and TRAE summary No CRS or TRAEs observed Confirmed RECIST response with elimination of target and liver lesions, and no CRS or adverse events Confirmed PR observed in a heavily pretreated subject with NSCLC, ongoing at 18 weeks�Subject 21008 (0.15 mg QW) Liver lesion remains undetectable by MRI 13 mm 0 mm -100% decrease Confirmed PR 12 February 2024 data cutoff

Early anti-tumor activity observed in a subject with RCC�Subject 21011 (0.5/1.25 mg QW) Past medical history Subject: 42-year-old male Diagnosis: Jan 2023 with RCC, clear cell, stage IV Mutations: MSI-L/MSS, pMMR Prior therapies: three prior lines of therapy JANX008 treatment summary Cycle 1 Day 1: 20 Nov 2023 Discontinued treatment due to adverse event (G3 aspiration pneumonia, unrelated to drug)* JANX008 CRS summary Grade 1 CRS Radiographic activity* Baseline: extensive L renal mass (11 cm) Complete resolution of cancer-related back pain (managed with narcotics) after 2 doses of JANX008 Off-schedule day 17 CT scan demonstrated a 12% decrease in diameter of renal mass *Data updated post data cutoff 12 February 2024 data cutoff
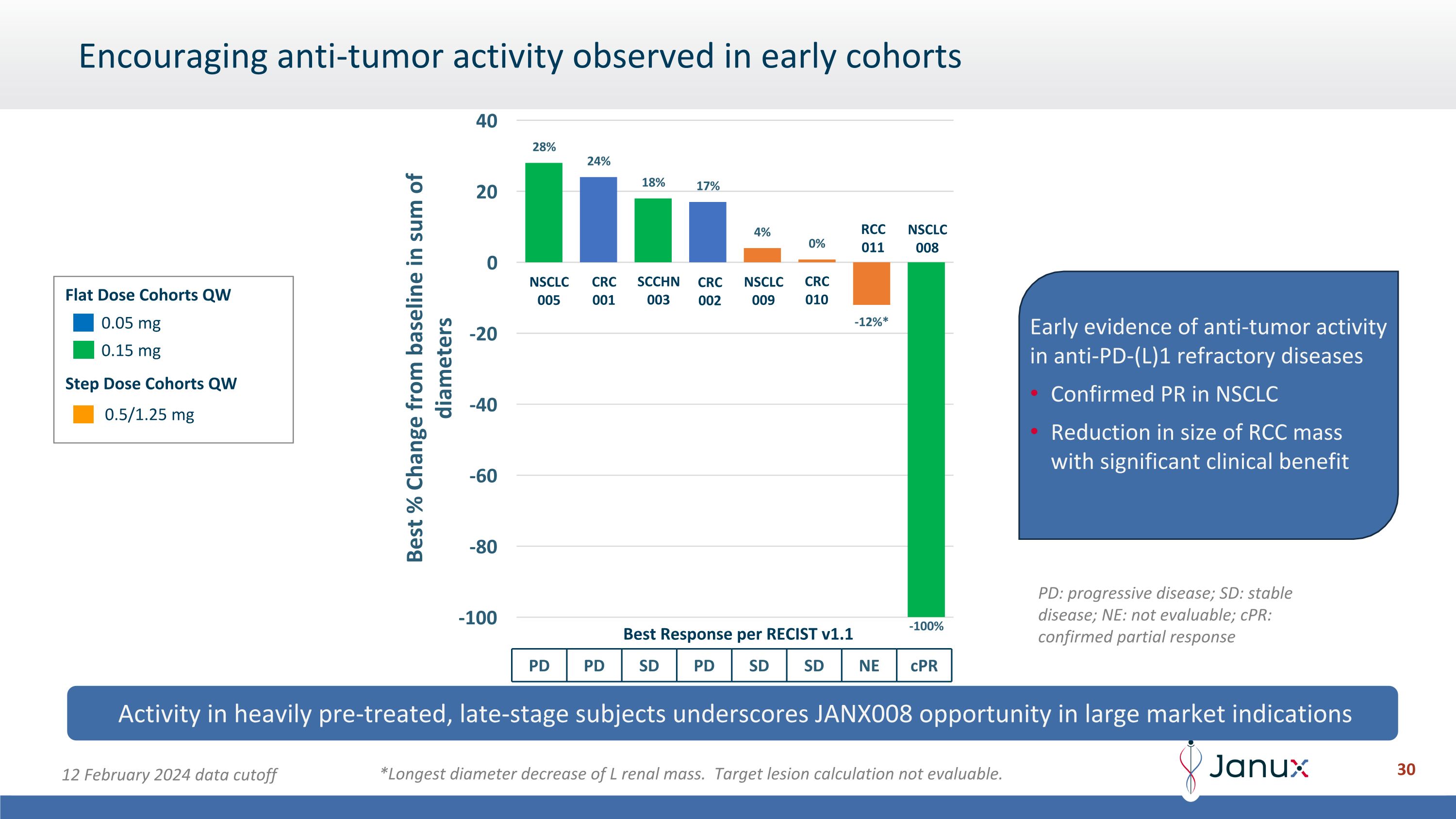
Encouraging anti-tumor activity observed in early cohorts Activity in heavily pre-treated, late-stage subjects underscores JANX008 opportunity in large market indications 0.05 mg 0.15 mg 0.5/1.25 mg Flat Dose Cohorts QW Step Dose Cohorts QW Best Response per RECIST v1.1 PD PD SD PD SD SD NE cPR NSCLC 005 CRC 001 CRC 002 SCCHN 003 NSCLC 008 CRC 010 RCC 011 NSCLC 009 Early evidence of anti-tumor activity in anti-PD-(L)1 refractory diseases Confirmed PR in NSCLC Reduction in size of RCC mass with significant clinical benefit *Longest diameter decrease of L renal mass. Target lesion calculation not evaluable. 12 February 2024 data cutoff PD: progressive disease; SD: stable disease; NE: not evaluable; cPR: confirmed partial response
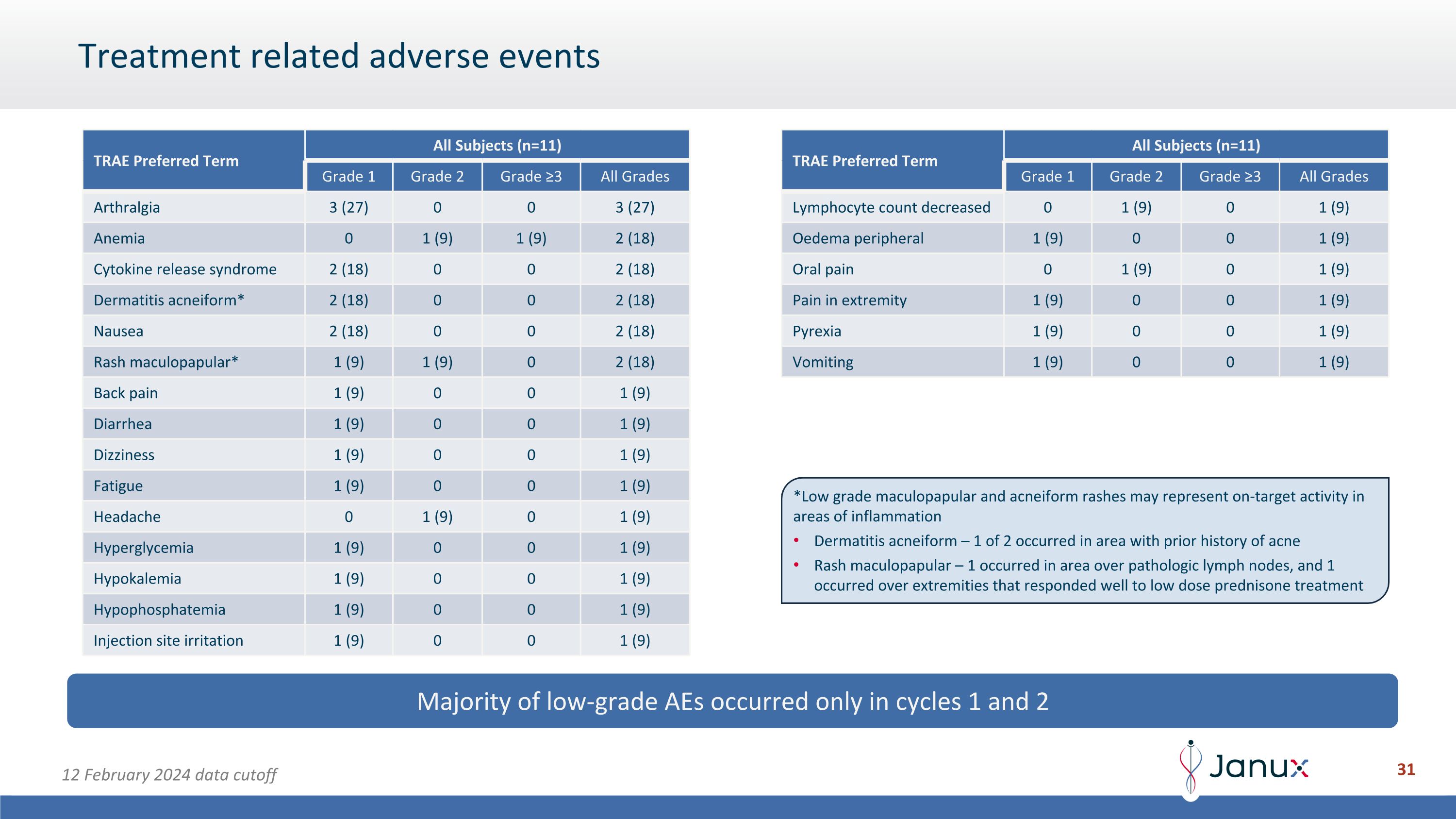
Treatment related adverse events Majority of low-grade AEs occurred only in cycles 1 and 2 TRAE Preferred Term All Subjects (n=11) Grade 1 Grade 2 Grade ≥3 All Grades Arthralgia 3 (27) 0 0 3 (27) Anemia 0 1 (9) 1 (9) 2 (18) Cytokine release syndrome 2 (18) 0 0 2 (18) Dermatitis acneiform* 2 (18) 0 0 2 (18) Nausea 2 (18) 0 0 2 (18) Rash maculopapular* 1 (9) 1 (9) 0 2 (18) Back pain 1 (9) 0 0 1 (9) Diarrhea 1 (9) 0 0 1 (9) Dizziness 1 (9) 0 0 1 (9) Fatigue 1 (9) 0 0 1 (9) Headache 0 1 (9) 0 1 (9) Hyperglycemia 1 (9) 0 0 1 (9) Hypokalemia 1 (9) 0 0 1 (9) Hypophosphatemia 1 (9) 0 0 1 (9) Injection site irritation 1 (9) 0 0 1 (9) TRAE Preferred Term All Subjects (n=11) Grade 1 Grade 2 Grade ≥3 All Grades Lymphocyte count decreased 0 1 (9) 0 1 (9) Oedema peripheral 1 (9) 0 0 1 (9) Oral pain 0 1 (9) 0 1 (9) Pain in extremity 1 (9) 0 0 1 (9) Pyrexia 1 (9) 0 0 1 (9) Vomiting 1 (9) 0 0 1 (9) 12 February 2024 data cutoff *Low grade maculopapular and acneiform rashes may represent on-target activity in areas of inflammation Dermatitis acneiform – 1 of 2 occurred in area with prior history of acne Rash maculopapular – 1 occurred in area over pathologic lymph nodes, and 1 occurred over extremities that responded well to low dose prednisone treatment
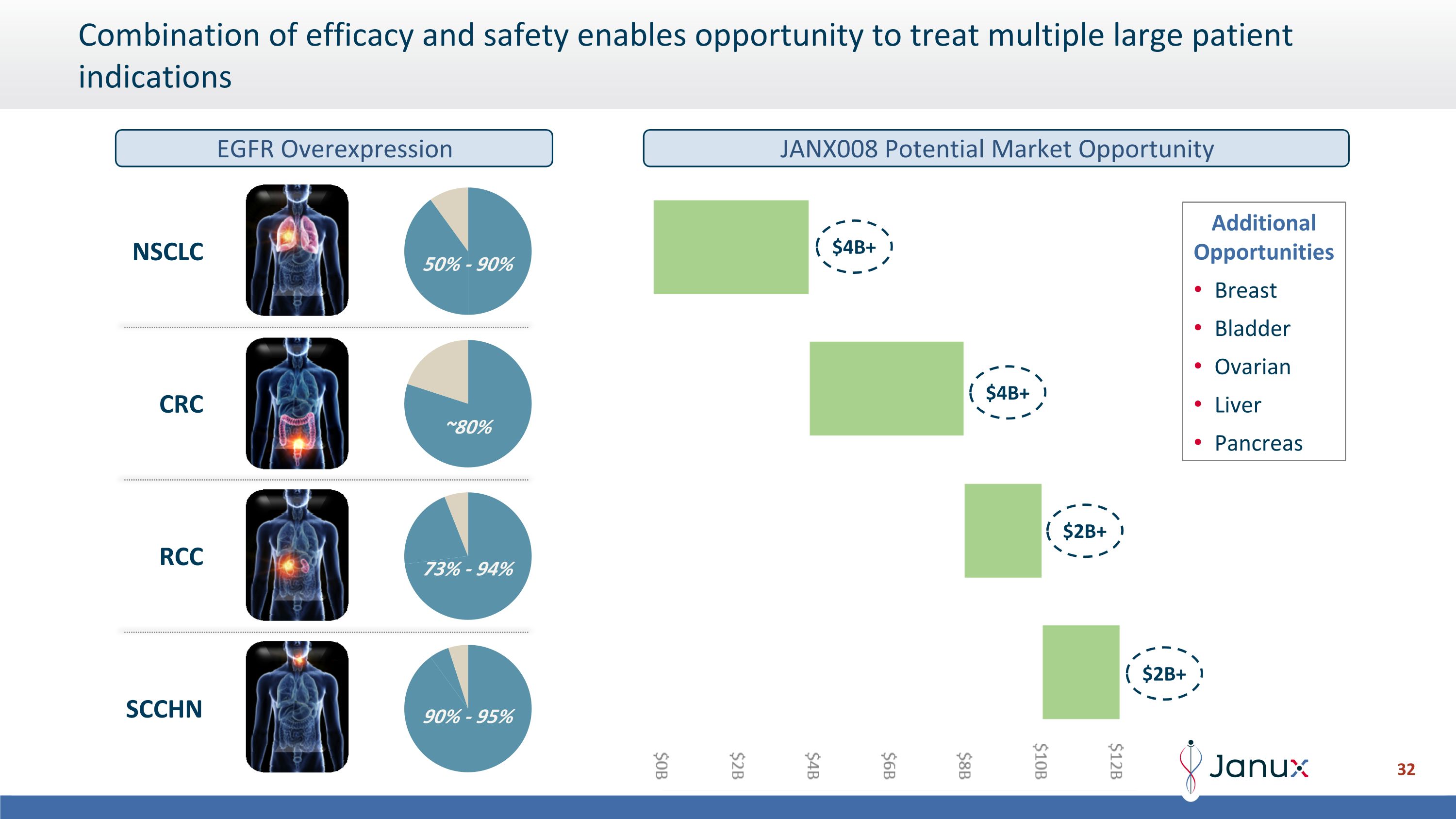
Combination of efficacy and safety enables opportunity to treat multiple large patient indications Additional Opportunities Breast Bladder Ovarian Liver Pancreas 50% - 90% ~80% 73% - 94% 90% - 95% NSCLC CRC RCC SCCHN $2B+ $4B+ $2B+ $4B+ EGFR Overexpression JANX008 Potential Market Opportunity

Janux clinical update summary Proof of Principle Large Opportunity Forward Momentum PSMA and EGFR-TRACTr clinical programs showcase an early display of efficacy combined with a differentiated favorable safety profile in heavily pre-treated, late state cancer patients Clinical data provides compelling proof-of principle for the TRACTr platform in a setting where many other approaches have failed due to material safety issues or lack of efficacy PSMA and EGFR-TRACTr clinical programs underscore the potential to not only be first-in-class but best-in-class in large market segments TRACTr platform provides entry point to solid tumor targets that are intractable with conventional TCE approaches Constitutes the vast majority of attractive anti-tumor targets PSMA-TRACTr - we plan to continue dose escalation to identify expansion doses in the second half of 2024 EGFR-TRACTr - we plan to continue dose escalation optimization for the remainder of 2024

David Campbell, Ph.D.�President and CEO 10955 Vista Sorrento Parkway San Diego, CA 92130 dcampbell@januxrx.com

































