
Exhibit 99.2 Achieving the Promise of FORCE to Deliver for Patients F O R C E DELIVER CLINICAL UPDATE | SEPTEMBER 3, 2024

Forward-Looking Statements & Disclaimer This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding Dyne’s strategy, future operations, prospects and plans, objectives of management, the potential of the FORCE platform, the anticipated timelines for reporting additional data from the ACHIEVE and DELIVER clinical trials and initiating registrational cohorts, expectations regarding the timing and outcome of interactions with global regulatory authorities and the availability of accelerated approval pathways for DYNE-101 and DYNE-251, and plans to provide future updates on pipeline programs, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Dyne may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; the timing of and Dyne’s ability to enroll patients in clinical trials; whether results from preclinical studies and initial data from early clinical trials will be predictive of the final results of the clinical trials or future trials; uncertainties as to the FDA’s and other regulatory authorities’ interpretation of the data from Dyne's clinical trials and acceptance of Dyne's clinical programs and the regulatory approval process; whether Dyne’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties identified in Dyne’s filings with the Securities and Exchange Commission (SEC), including the Company’s most recent Form 10-Q and in subsequent filings Dyne may make with the SEC. In addition, the forward-looking statements included in this presentation represent Dyne’s views as of the date of this presentation. Dyne anticipates that subsequent events and developments will cause its views to change. However, while Dyne may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Dyne’s views as of any date subsequent to the date of this presentation. This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company’s industry and business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The Company has not independently verified the accuracy and completeness of the information obtained by third parties included in this presentation. In addition, projections, assumptions and estimates of the Company’s future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. 2

Program Opening remarks John Cox, President & CEO DYNE-251 DELIVER Trial in DMD Data Wildon Farwell, M.D., MPH, Chief Medical Officer Closing Remarks John Cox, President & CEO Q&A 3

OUR MISSION Life-transforming therapies for patients with serious muscle diseases 4

Committed to Building the World’s Leading Muscle Disease Company • Potential to transform the treatment paradigm for people living with DMD DELIVER • Best-in-class dystrophin resulting in unprecedented improvements in multiple functional CLINICAL 1 outcomes, including NSAA and SV95C, in multiple cohorts UPDATE 2 • Favorable safety profile to date • Proven team of biopharma executives to deliver on Dyne’s next chapter LEADERSHIP ▪ Doug Kerr (CMO), Johanna Friedl-Naderer (CCO), and Lucia Celona (CHRO) bring decades of global experience across rare disease clinical development, commercial execution, and organizational builds UPDATE • Accelerating commercial preparedness across key functions • Continue to pursue expedited approval pathways globally NEXT • Initiating registrational cohorts in DELIVER trial of DYNE-251 in DMD STEPS • Provide update on the path to registration for DYNE-101 and DYNE-251 by the end of 2024 th 1. NSAA: North Star Ambulatory Assessment; SV95C: Stride Velocity 95 Centile. 2. Data as of August 21, 2024. 5

Program Opening remarks John Cox, President & CEO DYNE-251 DELIVER Trial in DMD Data Wildon Farwell, M.D., MPH, Chief Medical Officer Closing Remarks John Cox, President & CEO Q&A 6

Building a Global DMD Franchise of Transformative Therapies Clinical Presentation Population Overview • Mutation in the DMD gene that • Muscle weakness • ~12,000 - 15,000 (US) encodes for dystrophin • Progressive loss of function • ~ 25,000 (Europe) • Onset in first few years of life • Loss of ambulation • Life expectancy ~30 years • Respiratory/cardiac failure OUR APPROACH Potential Best-in-class Targeted Exon Skipping Increase dystrophin expression and enable less frequent dosing to potentially Current Approved stop or reverse disease progression Exon 51 Therapies Only Increased Dystrophin Production <1% 7
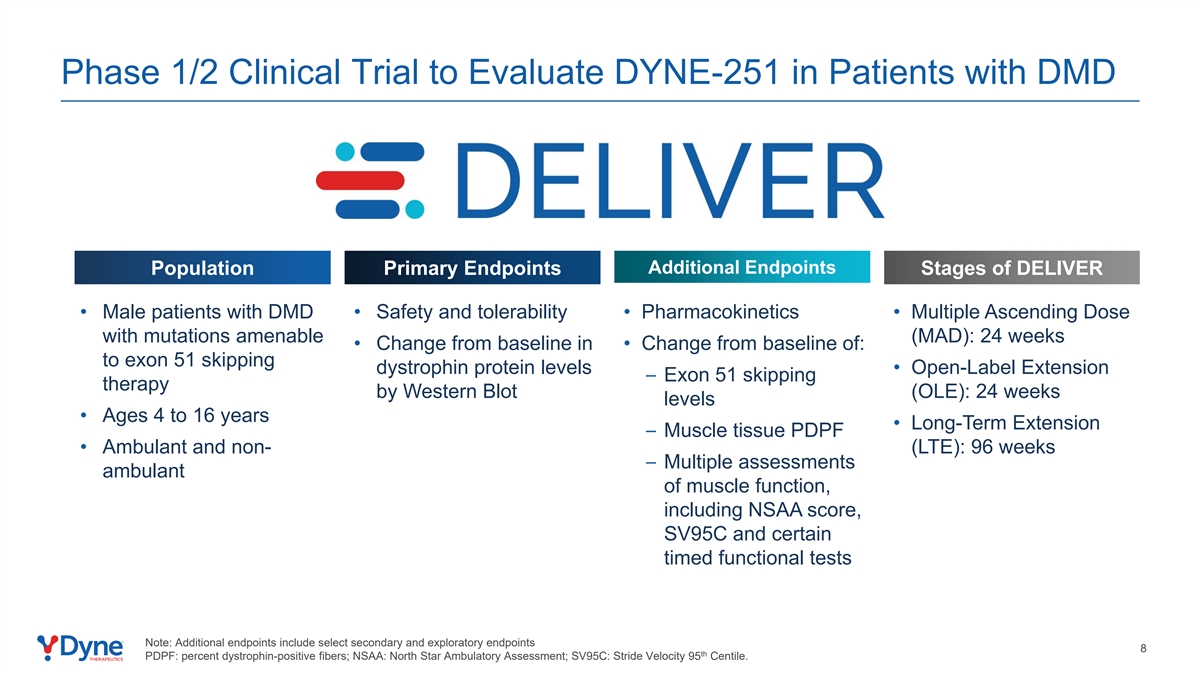
Phase 1/2 Clinical Trial to Evaluate DYNE-251 in Patients with DMD Additional Endpoints Population Primary Endpoints Stages of DELIVER • Male patients with DMD • Safety and tolerability • Pharmacokinetics • Multiple Ascending Dose with mutations amenable (MAD): 24 weeks • Change from baseline in • Change from baseline of: to exon 51 skipping dystrophin protein levels • Open-Label Extension – Exon 51 skipping therapy by Western Blot (OLE): 24 weeks levels • Ages 4 to 16 years • Long-Term Extension – Muscle tissue PDPF • Ambulant and non- (LTE): 96 weeks – Multiple assessments ambulant of muscle function, including NSAA score, SV95C and certain timed functional tests Note: Additional endpoints include select secondary and exploratory endpoints 8 th PDPF: percent dystrophin-positive fibers; NSAA: North Star Ambulatory Assessment; SV95C: Stride Velocity 95 Centile.

DELIVER Trial Design Global, Randomized, Placebo-Controlled Stage Evaluating Administration of DYNE-251 in Ambulant and Non-Ambulant Male DMD Patients with Mutations Amenable to Exon 51 Skipping Therapy MAD Study Details Registrational Registrational Cohorts Initiating Dose: TBD (≥20 mg/kg, Q4W or Q8W) Cohorts • IV administration of DYNE- 251 or placebo every 4 weeks 20 mg/kg Q4W Fully enrolled or every 8 weeks 1 Dose Optimization N=8 (3:1) Cohorts 10 mg/kg Q4W • Muscle biopsies: Baseline Fully enrolled N=8 (3:1) 2 and 24 weeks 5 mg/kg Q4W Fully enrolled • Patients in MAD study N=6 (2:1) Starting Dose escalated to highest tolerable Cohorts 2.8 mg/kg Q4W Fully enrolled dose in OLE and LTE N=6 (2:1) 1.4 mg/kg Q4W* Fully enrolled N=6 (2:1) 0.7 mg/kg Q4W* Fully enrolled N=6 (2:1) Global Trial Designed to be Registrational and to Enable Rapid Achievement of Predicted Pharmacologically Active Dose Levels Doses provided refer to PMO component of DYNE-251. Cohorts randomized to active arm or placebo. 1. All participants in DELIVER starting dose and dose optimization cohorts are currently receiving 20 mg/kg dose, including 32 participants dose escalated following the placebo-controlled period 9 from starting doses lower than 20 mg/kg and 14 participants initiated at 40 mg/kg who are now being dosed at 20 mg/kg following evaluation of the safety profile at 40 mg/kg. 2. Muscle biopsies taken at baseline and 24 weeks in 2.8 mg/kg Q4W cohort to 20 mg/kg Q4W cohort; biopsies not taken in 0.7 mg/kg and 1.4 mg/kg cohorts. Rando Randomiza mization tion

DELIVER Baseline Participant Characteristics: By Cohort mean (SD) or n(%) 0.7 mg/kg 1.4 mg/kg 2.8 mg/kg 5 mg/kg 10 mg/kg 20 mg/kg (N=6) (N=6) (N=6) (N=6) (N=8) (N=8) Age (years) 10.8 (2.2) 7.8 (3.3) 10.7 (2.9) 8.3 (2.8) 6.6 (2.2) 8.1 (2.4) 2 BMI (kg/m ) 19.5 (3.4) 18.6 (2.3) 22.6 (6.3) 20.9 (1.6) 18.3 (3.2) 18.6 (5.1) Age of Symptom Onset (years) 3.7 (1.8) 4.5 (2.1) 2.8 (1.8) 3.7 (3.1) 2.8 (1.6) 2.9 (2.0) 1 Corticosteroid dosing regimen (n (%)) Daily 4 (66.7%) 4 (66.7%) 5 (83.3%) 6 (100.0%) 8 (100.0%) 8 (100.0%) Other 2 (33.3%) 3 (50.0%) 2 (33.3%) 0 0 2 (25.0%) Prior DMD Therapy (n (%)) Eteplirsen 4 (66.7%) 2 (33.3%) 5 (83.3%) 1 (16.7%) 1 (12.5%) 0 Other 2 (33.3%) 1 (16.7%) 0 0 1 (12.5%) 2 (25.0%) NSAA Total Score 22.2 (7.2) 22.8 (10.5) 20.3 (9.0) 21.0 (7.0) 25.3 (6.4) 15.6 (5.1) 10 Meter Run/Walk (sec) 6.1 (1.5) 6.3 (5.2) 6.9 (3.6) 5.1 (1.5) 4.6 (1.9) 7.7 (3.8) Time Rise From Floor (sec) 8.5 (4.0) 3.1 (0.3) 6.9 (4.9) 5.0 (2.6) 6.3 (5.6) 5.1 (2.3) th Stride Velocity 95 Percentile (m/sec) N/A N/A N/A N/A 1.9 (0.5) 1.4 (0.5) 10 Note: Q4W and placebo arms are reported together for baseline characteristics. N/A: not applicable as data not collected. 1. Historical corticosteroid regimen based on medical history; a participant can be counted in multiple categories.
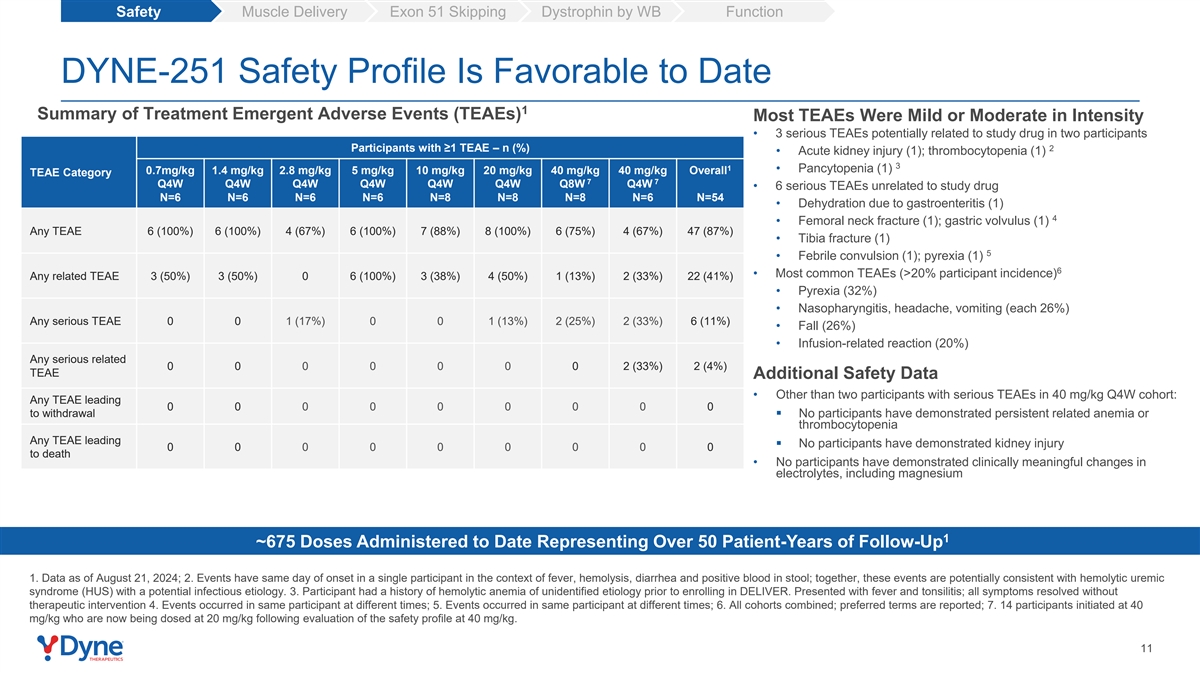
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Safety Profile Is Favorable to Date 1 Summary of Treatment Emergent Adverse Events (TEAEs) Most TEAEs Were Mild or Moderate in Intensity • 3 serious TEAEs potentially related to study drug in two participants Participants with ≥1 TEAE – n (%) 2 • Acute kidney injury (1); thrombocytopenia (1) 3 1 • Pancytopenia (1) 0.7mg/kg 1.4 mg/kg 2.8 mg/kg 5 mg/kg 10 mg/kg 20 mg/kg 40 mg/kg 40 mg/kg Overall TEAE Category 7 7 Q4W Q4W Q4W Q4W Q4W Q4W Q8W Q4W • 6 serious TEAEs unrelated to study drug N=6 N=6 N=6 N=6 N=8 N=8 N=8 N=6 N=54 • Dehydration due to gastroenteritis (1) 4 • Femoral neck fracture (1); gastric volvulus (1) Any TEAE 6 (100%) 6 (100%) 4 (67%) 6 (100%) 7 (88%) 8 (100%) 6 (75%) 4 (67%) 47 (87%) • Tibia fracture (1) 5 • Febrile convulsion (1); pyrexia (1) 6 • Most common TEAEs (>20% participant incidence) Any related TEAE 3 (50%) 3 (50%) 0 6 (100%) 3 (38%) 4 (50%) 1 (13%) 2 (33%) 22 (41%) • Pyrexia (32%) • Nasopharyngitis, headache, vomiting (each 26%) Any serious TEAE 0 0 1 (17%) 0 0 1 (13%) 2 (25%) 2 (33%) 6 (11%) • Fall (26%) • Infusion-related reaction (20%) Any serious related 0 0 0 0 0 0 0 2 (33%) 2 (4%) TEAE Additional Safety Data • Other than two participants with serious TEAEs in 40 mg/kg Q4W cohort: Any TEAE leading 0 0 0 0 0 0 0 0 0 to withdrawal▪ No participants have demonstrated persistent related anemia or thrombocytopenia Any TEAE leading ▪ No participants have demonstrated kidney injury 0 0 0 0 0 0 0 0 0 to death • No participants have demonstrated clinically meaningful changes in electrolytes, including magnesium 1 ~675 Doses Administered to Date Representing Over 50 Patient-Years of Follow-Up 1. Data as of August 21, 2024; 2. Events have same day of onset in a single participant in the context of fever, hemolysis, diarrhea and positive blood in stool; together, these events are potentially consistent with hemolytic uremic syndrome (HUS) with a potential infectious etiology. 3. Participant had a history of hemolytic anemia of unidentified etiology prior to enrolling in DELIVER. Presented with fever and tonsilitis; all symptoms resolved without therapeutic intervention 4. Events occurred in same participant at different times; 5. Events occurred in same participant at different times; 6. All cohorts combined; preferred terms are reported; 7. 14 participants initiated at 40 mg/kg who are now being dosed at 20 mg/kg following evaluation of the safety profile at 40 mg/kg. 11

Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Drove Dose Dependent Delivery of PMO to Muscle PMO Muscle Concentration 4,500 3,527 3,000 2,158 1,500 657 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months Baseline 6 Months n=7 n=8 n=4 n=4 n=4 n=5 n=6 n=6 Placebo DYNE-251 5 mg/kg Q4W DYNE-251 10 mg/kg Q4W DYNE-251 20 mg/kg Q4W 12 1. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER. 1 Mean Tissue PMO Concentration (ng/g) + SEM
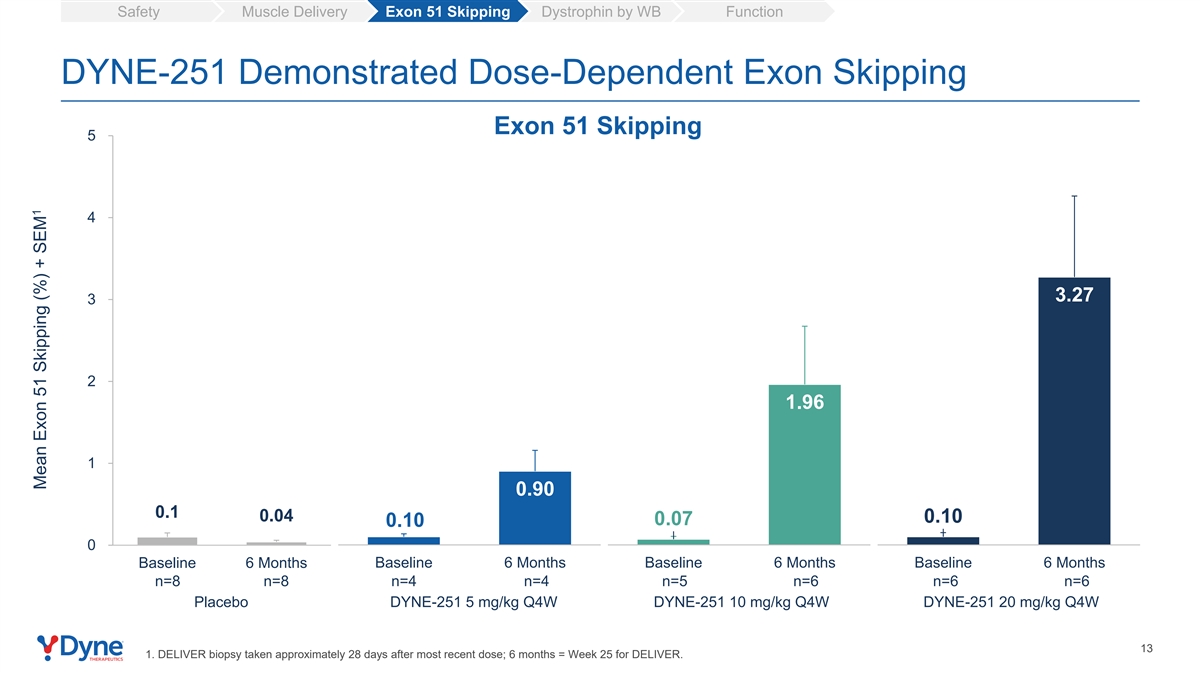
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Demonstrated Dose-Dependent Exon Skipping Exon 51 Skipping 5 4 3.27 3 2 1.96 1 0.90 0.1 0.04 0.10 0.07 0.10 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months Baseline 6 Months n=8 n=8 n=4 n=4 n=5 n=6 n=6 n=6 Placebo DYNE-251 5 mg/kg Q4W DYNE-251 10 mg/kg Q4W DYNE-251 20 mg/kg Q4W 13 1. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER. 1 Mean Exon 51 Skipping (%) + SEM
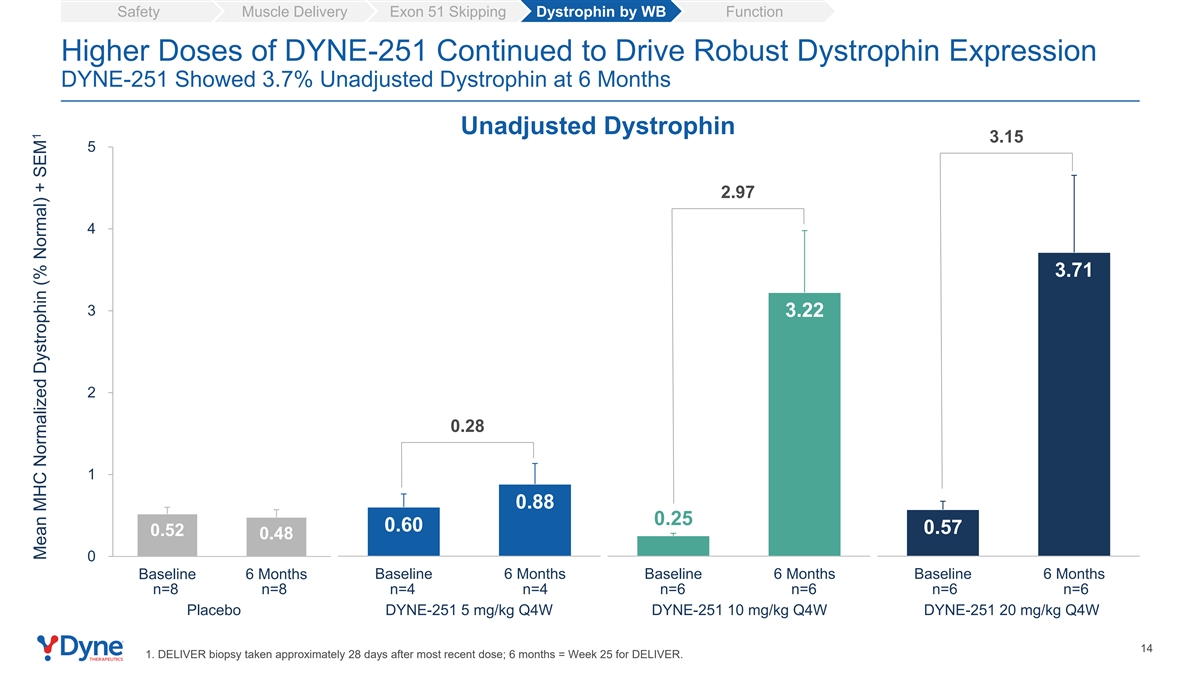
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function Higher Doses of DYNE-251 Continued to Drive Robust Dystrophin Expression DYNE-251 Showed 3.7% Unadjusted Dystrophin at 6 Months Unadjusted Dystrophin 3.15 5 2.97 4 3.71 3 3.22 2 0.28 1 0.88 0.25 0.60 0.57 0.52 0.48 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months Baseline 6 Months n=8 n=8 n=4 n=4 n=6 n=6 n=6 n=6 Placebo DYNE-251 5 mg/kg Q4W DYNE-251 10 mg/kg Q4W DYNE-251 20 mg/kg Q4W 14 1. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER. 1 Mean MHC Normalized Dystrophin (% Normal) + SEM

Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Positioned as a Potentially Best-in-Class Next Generation Exon Skipper, Achieving 8.7% Muscle Content Adjusted Dystrophin at 6 Months Muscle Content Adjusted Dystrophin 12 7.37 6.94 Muscle-content Dystrophin (MHC normalized) adjusted = 1 % Muscle Content dystrophin 8.72 8 7.64 4 0.44 1.84 1.63 1.58 1.35 1.19 0.70 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months Baseline 6 Months n=7 n=8 n=4 n=4 n=5 n=6 n=6 n=5 Placebo DYNE-251 5 mg/kg Q4W DYNE-251 10 mg/kg Q4W DYNE-251 20 mg/kg Q4W 15 1. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER. 1 Mean MCA MHC Normalized Dystrophin (% Normal) + SEM
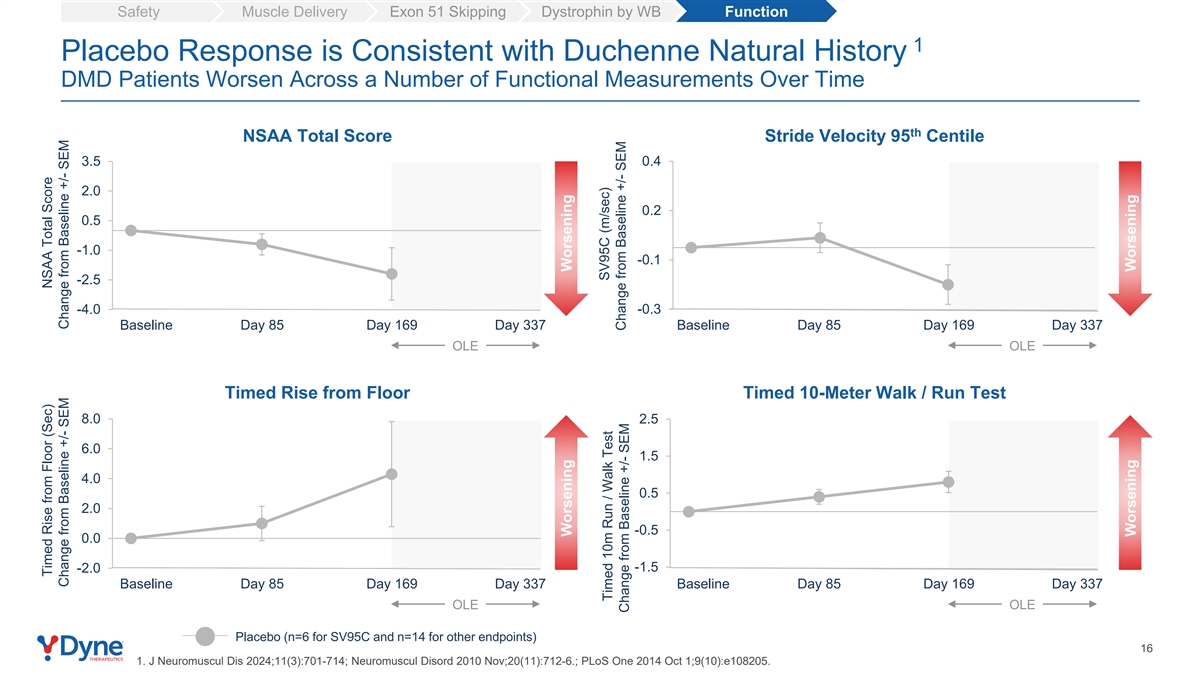
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function 1 Placebo Response is Consistent with Duchenne Natural History DMD Patients Worsen Across a Number of Functional Measurements Over Time th NSAA Total Score Stride Velocity 95 Centile 3.5 0.4 2.0 0.2 0.5 -1.0 -0.1 -2.5 -4.0 -0.3 Baseline Day 85 Day 169 Day 337 Baseline Day 85 Day 169 Day 337 OLE OLE Timed Rise from Floor Timed 10-Meter Walk / Run Test 8.0 2.5 6.0 1.5 4.0 0.5 2.0 -0.5 0.0 -1.5 -2.0 Baseline Day 85 Day 169 Day 337 Baseline Day 85 Day 169 Day 337 OLE OLE Placebo (n=6 for SV95C and n=14 for other endpoints) 16 1. J Neuromuscul Dis 2024;11(3):701-714; Neuromuscul Disord 2010 Nov;20(11):712-6.; PLoS One 2014 Oct 1;9(10):e108205. Timed Rise from Floor (Sec) NSAA Total Score Change from Baseline +/- SEM Change from Baseline +/- SEM Worsening Worsening SV95C (m/sec) Timed 10m Run / Walk Test Change from Baseline +/- SEM Change from Baseline +/- SEM Worsening Worsening
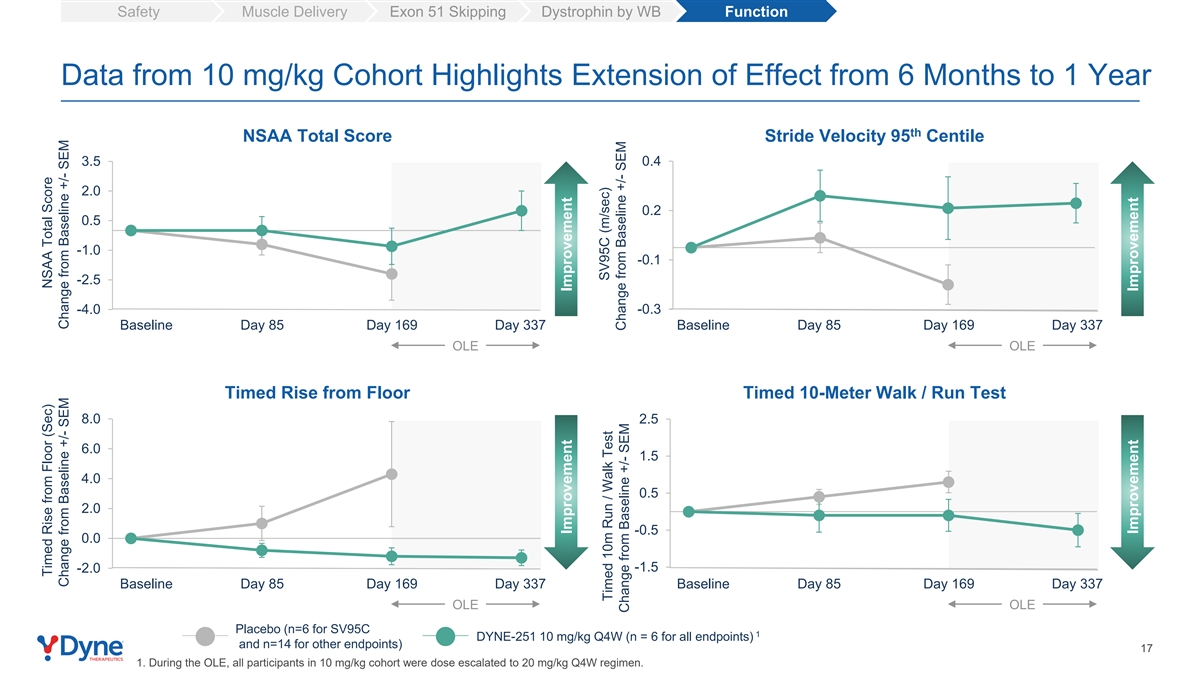
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function Data from 10 mg/kg Cohort Highlights Extension of Effect from 6 Months to 1 Year th NSAA Total Score Stride Velocity 95 Centile 3.5 0.4 2.0 0.2 0.5 -1.0 -0.1 -2.5 -4.0 -0.3 Baseline Day 85 Day 169 Day 337 Baseline Day 85 Day 169 Day 337 OLE OLE Timed Rise from Floor Timed 10-Meter Walk / Run Test 8.0 2.5 6.0 1.5 4.0 0.5 2.0 -0.5 0.0 -1.5 -2.0 Baseline Day 85 Day 169 Day 337 Baseline Day 85 Day 169 Day 337 OLE OLE Placebo (n=6 for SV95C 1 DYNE-251 10 mg/kg Q4W (n = 6 for all endpoints) and n=14 for other endpoints) 17 1. During the OLE, all participants in 10 mg/kg cohort were dose escalated to 20 mg/kg Q4W regimen. Timed Rise from Floor (Sec) NSAA Total Score Change from Baseline +/- SEM Change from Baseline +/- SEM Improvement Improvement SV95C (m/sec) Timed 10m Run / Walk Test Change from Baseline +/- SEM Change from Baseline +/- SEM Improvement Improvement
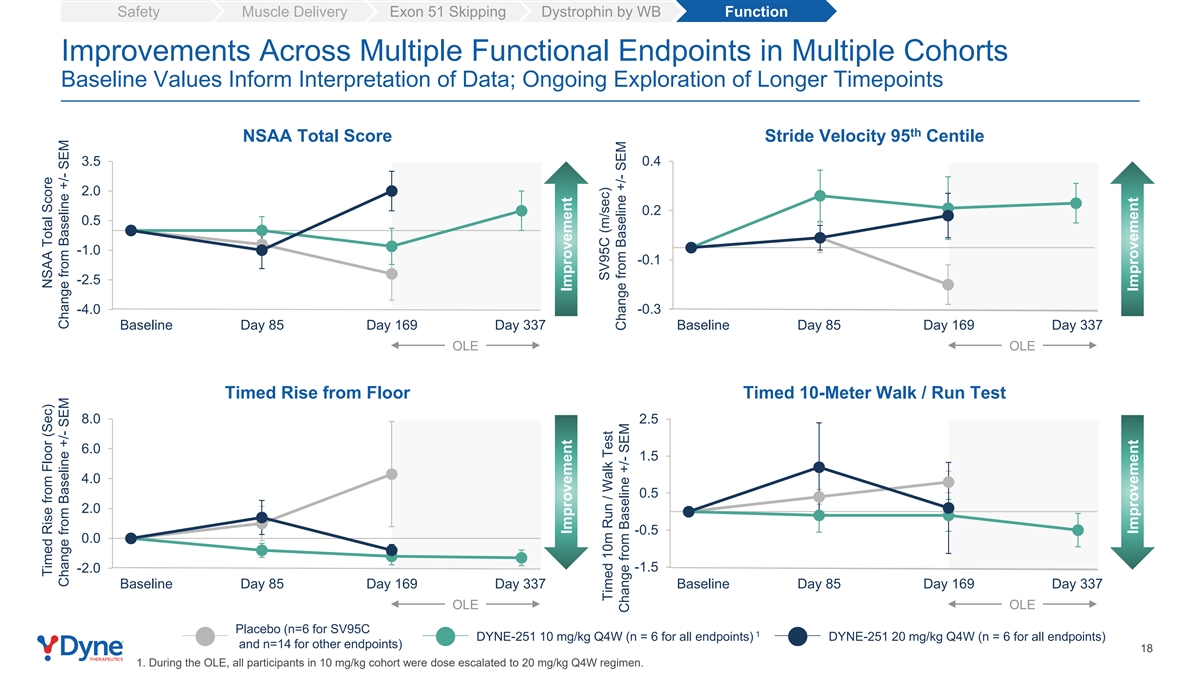
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function Improvements Across Multiple Functional Endpoints in Multiple Cohorts Baseline Values Inform Interpretation of Data; Ongoing Exploration of Longer Timepoints th NSAA Total Score Stride Velocity 95 Centile 3.5 0.4 2.0 0.2 0.5 -1.0 -0.1 -2.5 -4.0 -0.3 Baseline Day 85 Day 169 Day 337 Baseline Day 85 Day 169 Day 337 OLE OLE Timed Rise from Floor Timed 10-Meter Walk / Run Test 8.0 2.5 6.0 1.5 4.0 0.5 2.0 -0.5 0.0 -1.5 -2.0 Baseline Day 85 Day 169 Day 337 Baseline Day 85 Day 169 Day 337 OLE OLE Placebo (n=6 for SV95C 1 DYNE-251 10 mg/kg Q4W (n = 6 for all endpoints) DYNE-251 20 mg/kg Q4W (n = 6 for all endpoints) and n=14 for other endpoints) 18 1. During the OLE, all participants in 10 mg/kg cohort were dose escalated to 20 mg/kg Q4W regimen. Timed Rise from Floor (Sec) NSAA Total Score Change from Baseline +/- SEM Change from Baseline +/- SEM Improvement Improvement SV95C (m/sec) Timed 10m Run / Walk Test Change from Baseline +/- SEM Change from Baseline +/- SEM Improvement Improvement

Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function th DYNE-251 Drove Clinically Meaningful Improvements in Stride Velocity 95 Centile SV95C is a Qualified Primary Endpoint for Duchenne Trials in Europe and Leveraged Across Global Trials th Stride Velocity 95 Centile (SV95C) 0.4 • SV95C is a digital objective endpoint of ambulatory 0.2 performance in patients’ normal daily environment • Patients in DELIVER wore the device on each ankle for 3 weeks prior to the clinic visits -0.1 • The change from baseline met 1 the published MCID by the EMA -0.3 OLE Baseline Day 85 Day 169 Day 337 Visit: 2 Placebo (n = 6) 10 mg/kg Q4W (n = 6) 20 mg/kg Q4W (n = 6) 1. Minimal clinically important difference (MCID) as defined by EMA in its qualification opinion for SV95C as primary endpoint in studies in ambulatory DMD studies. 2. During the OLE, all 19 participants in 10 mg/kg cohort were dose escalated to 20 mg/kg Q4W regimen. SV95C (m/sec) Change from Baseline +/- SEM Improvement

Opportunity to Build a Global DMD Franchise: Leading with DYNE-251, Payloads Identified for Exons 53, 45, 44 Exon 51 – 13% Exon 53 – 8% Approximately Exon 45 – 8% Exon 44 – 6% Exon 50 – 4% 80% of patients Exon 52 – 4% Exon 43 – 4% have genotypes amenable Exon 55 – 2% to exon skipping Exon 8 – 2% Other exon skips – ~30% May not be amenable to exon skipping – ~20% 20

Advancing DYNE-251 Towards Potentially Registrational Data Set Unprecedented level of dystrophin generated, with 3.7% ✓ unadjusted and 8.7% muscle content adjusted dystrophin Improvements in multiple functional outcomes, including SV95C, an approvable endpoint in Europe, in multiple ✓ cohorts Favorable safety profile with ~675 doses administered ✓ 1 representing over 50 patient-years of follow up to date Supports further development of DMD global franchise ✓ Initiating registrational cohorts based on regulatory interactions and strength of data Update on path to registration for DYNE-251 expected by YE 2024 1. Data as of August 21, 2024. 21

Program Opening remarks John Cox, President & CEO DYNE-251 DELIVER Trial in DMD Data Wildon Farwell, M.D., MPH, Chief Medical Officer Closing Remarks John Cox, President & CEO Q&A 22

Driving Towards Potentially Transformative DM1 and DMD Therapies Delivered on the Promise of FORCE: Enhanced Delivery of Therapeutics to Muscle Compelling Impact on Key Disease Biomarkers and Improvements in Multiple Functional Endpoints in Both DM1 and DMD Favorable Safety & Tolerability Profile Fully Enrolled Through 6.8 mg/kg Initiating Registrational Cohorts Pursuing Expedited Approvals for Both Programs with Update on Registrational Pathway by YE 2024 23

Strengthening the Team to Deliver on Dyne’s Next Chapter Doug Kerr Johanna Friedl-Naderer Lucia Celona Chief Medical Officer Chief Commercial Officer Chief Human Resources Officer Proven Team of Biopharma Executives Prepared to Advance Multiple Programs to Market 24

Building the World’s Leading Muscle Disease Company Dynamo Culture Own Muscle Delivery & Win in DM1, DMD, FSHD Leverage FORCE 25

Achieving the Promise of FORCE to Deliver for Patients F O R C E DELIVER CLINICAL UPDATE | SEPTEMBER 3, 2024

























