
Exhibit 99.2 Clinical Update JANUARY 10, 2025

Forward-Looking Statements & Disclaimer This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding Dyne’s strategy, future operations, prospects and plans, objectives of management, the potential of the FORCE platform, the therapeutic potential of DYNE-101 and DYNE-251, the anticipated timelines for reporting additional data from the ACHIEVE and DELIVER clinical trials and initiating and enrolling registrational cohorts, expectations regarding the timing and outcome of interactions with global regulatory authorities and the availability of accelerated approval pathways for DYNE-101 and DYNE-251 and expectations regarding the timing of filing applications for U.S. Accelerated Approval, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Dyne may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; the timing of and Dyne’s ability to enroll patients in clinical trials; whether results from preclinical studies and initial data from early clinical trials will be predictive of the final results of the clinical trials or future trials; uncertainties as to the FDA’s and other regulatory authorities’ interpretation of the data from Dyne's clinical trials and acceptance of Dyne's clinical programs and the regulatory approval process; whether Dyne’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties identified in Dyne’s filings with the Securities and Exchange Commission (SEC), including the Company’s most recent Form 10-Q and in subsequent filings Dyne may make with the SEC. In addition, the forward-looking statements included in this presentation represent Dyne’s views as of the date of this presentation. Dyne anticipates that subsequent events and developments will cause its views to change. However, while Dyne may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Dyne’s views as of any date subsequent to the date of this presentation. This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company’s industry and business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The Company has not independently verified the accuracy and completeness of the information obtained by third parties included in this presentation. In addition, projections, assumptions and estimates of the Company’s future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. 2
Program Opening Remarks John Cox, President & CEO New Data from DYNE-101 ACHIEVE Trial in DM1 Update on DYNE-251 DELIVER Trial in DMD Doug Kerr, M.D., Ph.D., Chief Medical Officer Closing Remarks John Cox, President & CEO 3
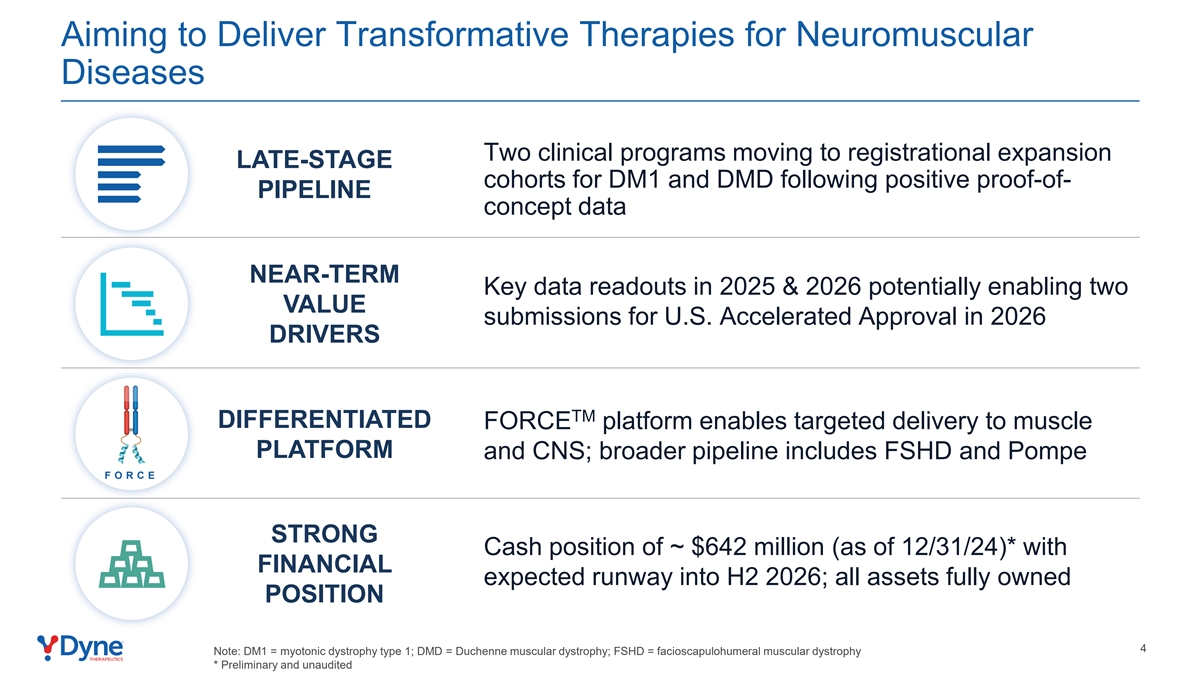
Aiming to Deliver Transformative Therapies for Neuromuscular Diseases Two clinical programs moving to registrational expansion LATE-STAGE cohorts for DM1 and DMD following positive proof-of- PIPELINE concept data NEAR-TERM Key data readouts in 2025 & 2026 potentially enabling two VALUE submissions for U.S. Accelerated Approval in 2026 DRIVERS TM DIFFERENTIATED FORCE platform enables targeted delivery to muscle PLATFORM and CNS; broader pipeline includes FSHD and Pompe F O RC E STRONG Cash position of ~ $642 million (as of 12/31/24)* with FINANCIAL expected runway into H2 2026; all assets fully owned POSITION 4 Note: DM1 = myotonic dystrophy type 1; DMD = Duchenne muscular dystrophy; FSHD = facioscapulohumeral muscular dystrophy * Preliminary and unaudited
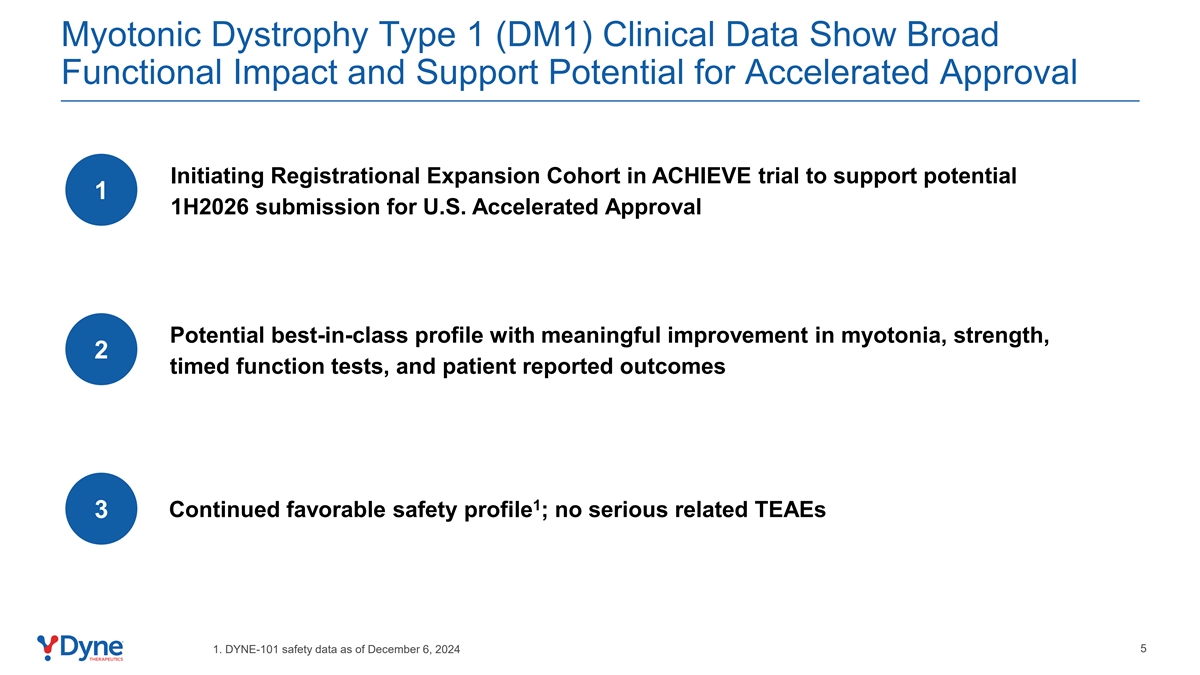
Myotonic Dystrophy Type 1 (DM1) Clinical Data Show Broad Functional Impact and Support Potential for Accelerated Approval Initiating Registrational Expansion Cohort in ACHIEVE trial to support potential 1 1H2026 submission for U.S. Accelerated Approval Potential best-in-class profile with meaningful improvement in myotonia, strength, 2 timed function tests, and patient reported outcomes 1 Continued favorable safety profile ; no serious related TEAEs 3 5 1. DYNE-101 safety data as of December 6, 2024
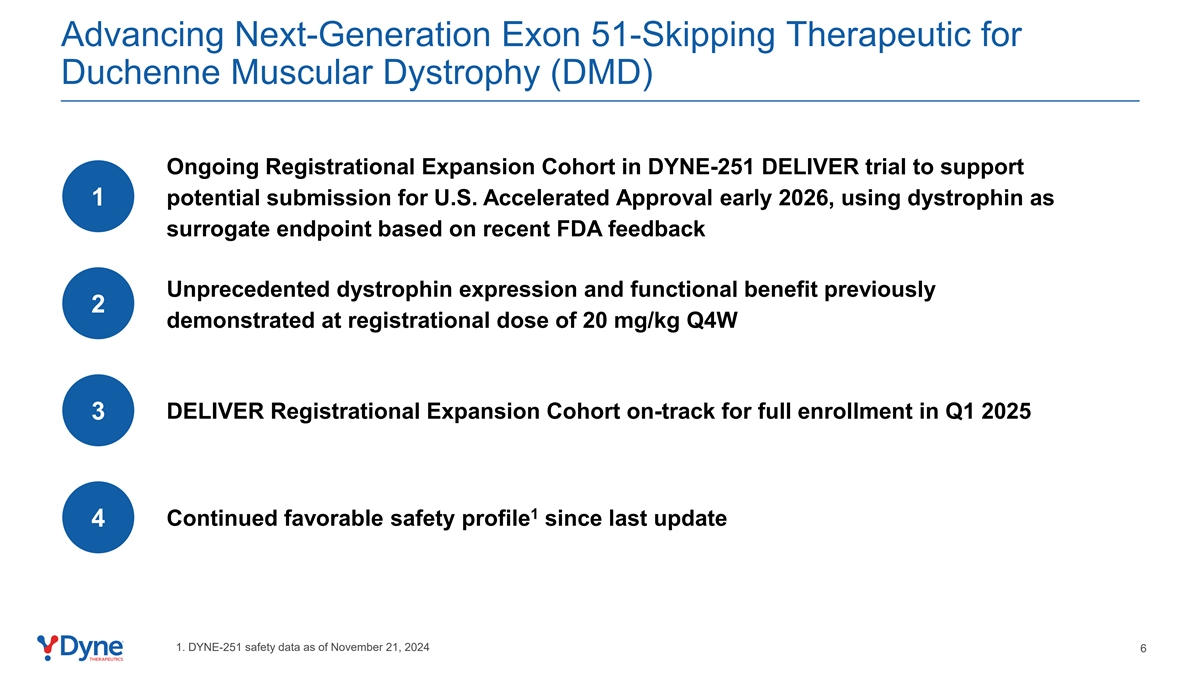
Advancing Next-Generation Exon 51-Skipping Therapeutic for Duchenne Muscular Dystrophy (DMD) Ongoing Registrational Expansion Cohort in DYNE-251 DELIVER trial to support potential submission for U.S. Accelerated Approval early 2026, using dystrophin as 1 surrogate endpoint based on recent FDA feedback Unprecedented dystrophin expression and functional benefit previously 2 demonstrated at registrational dose of 20 mg/kg Q4W DELIVER Registrational Expansion Cohort on-track for full enrollment in Q1 2025 3 1 Continued favorable safety profile since last update 4 1. DYNE-251 safety data as of November 21, 2024 6
Program Opening Remarks John Cox, President & CEO New Data from DYNE-101 ACHIEVE Trial in DM1 Update on DYNE-251 DELIVER Trial in DMD Doug Kerr, M.D., Ph.D., Chief Medical Officer Closing Remarks John Cox, President & CEO 7
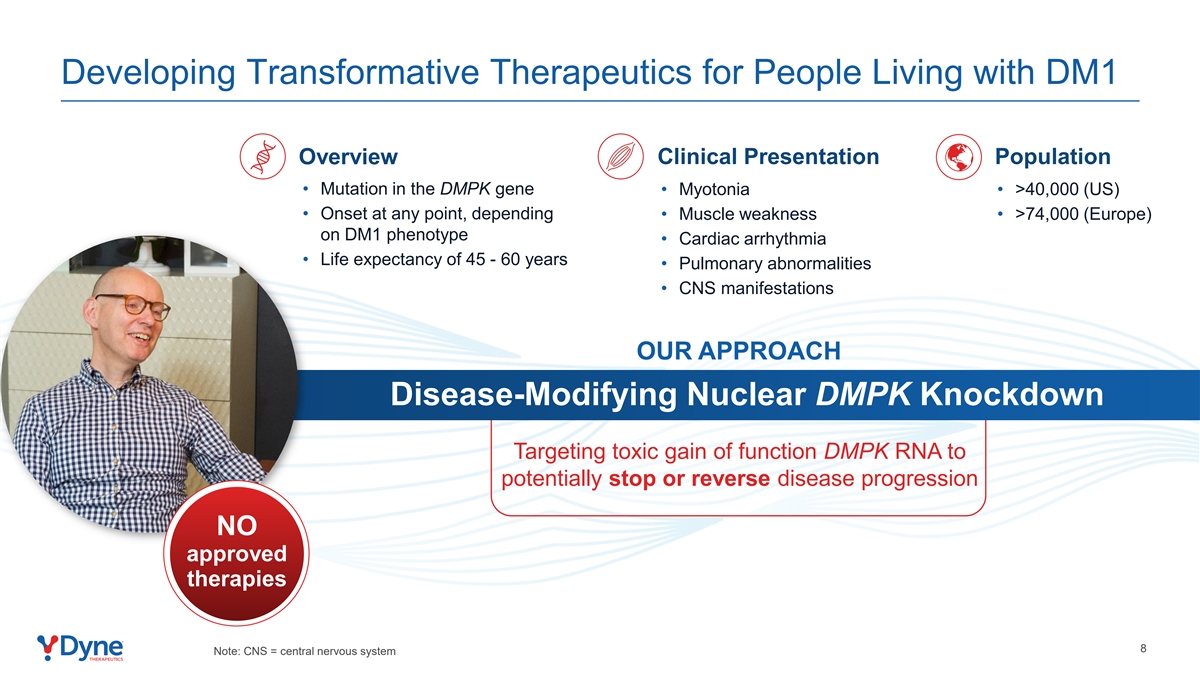
Developing Transformative Therapeutics for People Living with DM1 Clinical Presentation Population Overview • Mutation in the DMPK gene • Myotonia • >40,000 (US) • Onset at any point, depending • Muscle weakness • >74,000 (Europe) on DM1 phenotype • Cardiac arrhythmia • Life expectancy of 45 - 60 years • Pulmonary abnormalities • CNS manifestations OUR APPROACH Disease-Modifying Nuclear DMPK Knockdown Targeting toxic gain of function DMPK RNA to potentially stop or reverse disease progression NO approved therapies 8 Note: CNS = central nervous system
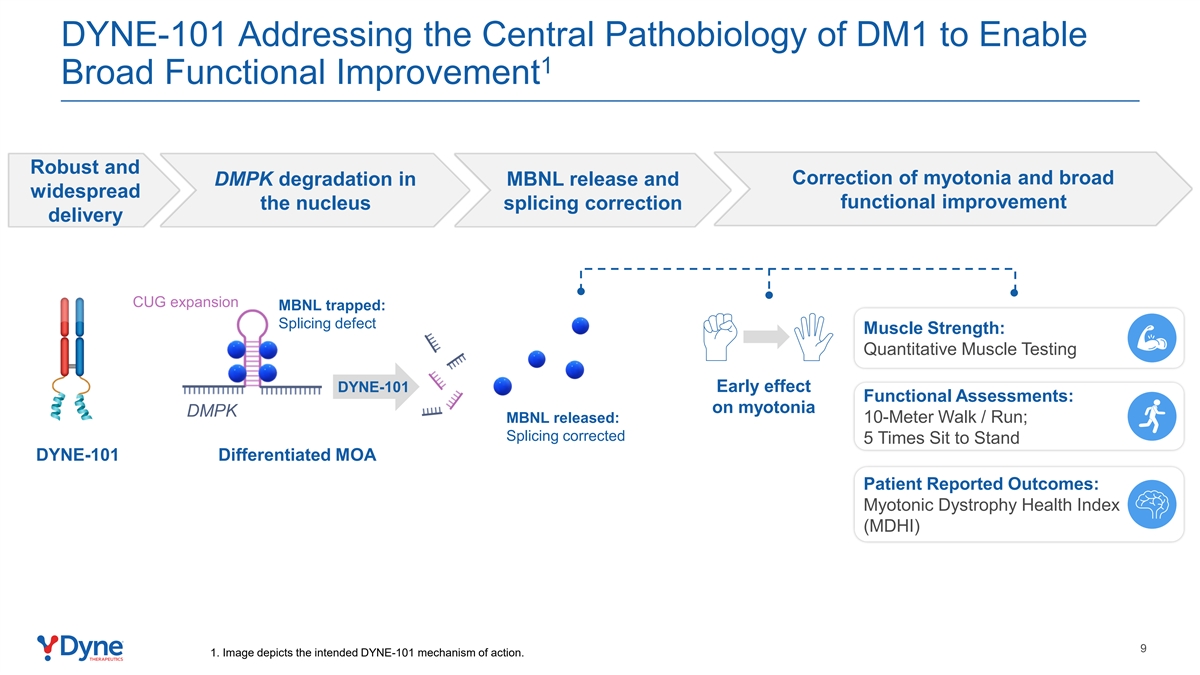
DYNE-101 Addressing the Central Pathobiology of DM1 to Enable 1 Broad Functional Improvement Robust and Correction of myotonia and broad DMPK degradation in MBNL release and widespread functional improvement the nucleus splicing correction delivery CUG expansion MBNL trapped: Splicing defect Muscle Strength: Quantitative Muscle Testing DYNE-101 Early effect Functional Assessments: on myotonia DMPK MBNL released: 10-Meter Walk / Run; Splicing corrected 5 Times Sit to Stand DYNE-101 Differentiated MOA Patient Reported Outcomes: Myotonic Dystrophy Health Index (MDHI) 9 1. Image depicts the intended DYNE-101 mechanism of action.
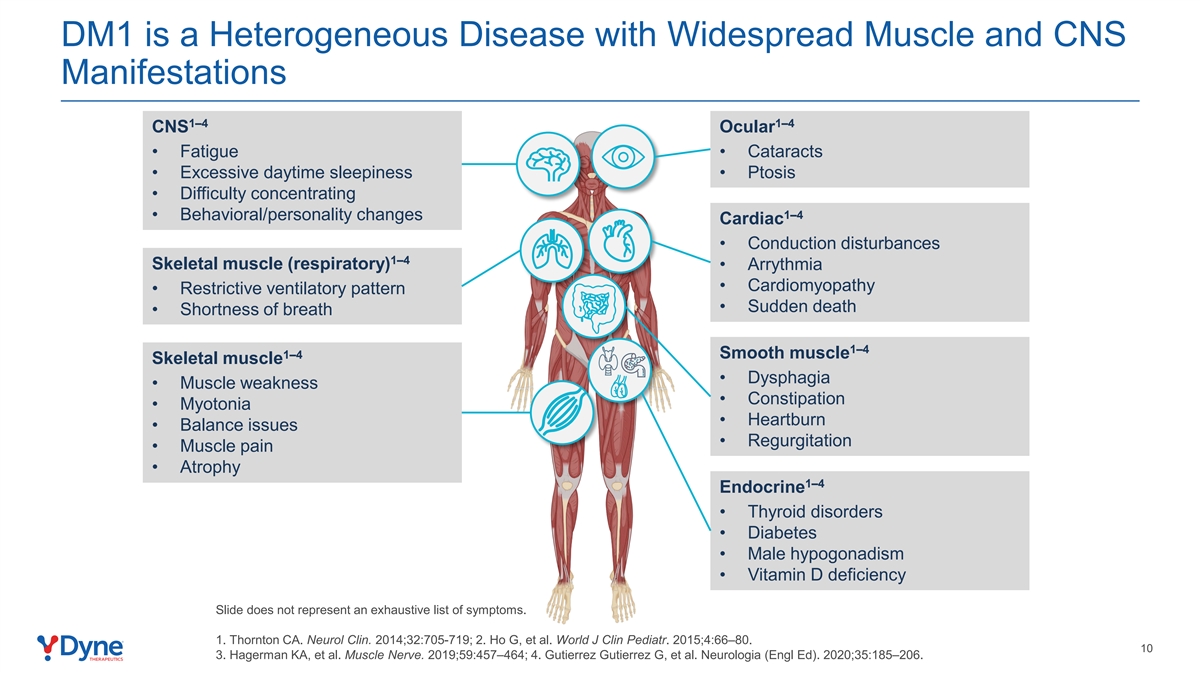
DM1 is a Heterogeneous Disease with Widespread Muscle and CNS Manifestations 1–4 1–4 CNS Ocular • Fatigue • Cataracts • Excessive daytime sleepiness • Ptosis • Difficulty concentrating 1–4 • Behavioral/personality changes Cardiac • Conduction disturbances 1–4 Skeletal muscle (respiratory) • Arrythmia • Cardiomyopathy • Restrictive ventilatory pattern • Sudden death • Shortness of breath 1–4 1–4 Smooth muscle Skeletal muscle • Dysphagia • Muscle weakness • Constipation • Myotonia • Heartburn • Balance issues • Regurgitation • Muscle pain • Atrophy 1–4 Endocrine • Thyroid disorders • Diabetes • Male hypogonadism • Vitamin D deficiency Slide does not represent an exhaustive list of symptoms. 1. Thornton CA. Neurol Clin. 2014;32:705-719; 2. Ho G, et al. World J Clin Pediatr. 2015;4:66–80. 10 3. Hagerman KA, et al. Muscle Nerve. 2019;59:457–464; 4. Gutierrez Gutierrez G, et al. Neurologia (Engl Ed). 2020;35:185–206.
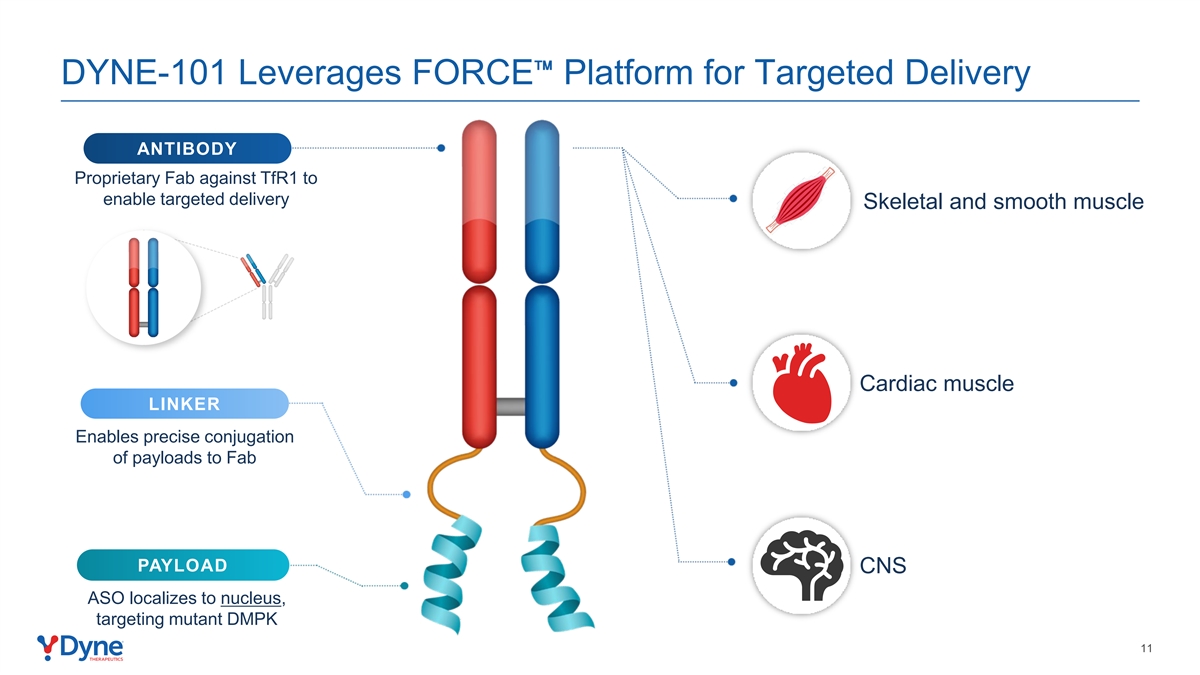
DYNE-101 Leverages FORCE Platform for Targeted Delivery ANTIBODY Proprietary Fab against TfR1 to enable targeted delivery Skeletal and smooth muscle Cardiac muscle LINKER Enables precise conjugation of payloads to Fab PAYLOAD CNS ASO localizes to nucleus, targeting mutant DMPK 11
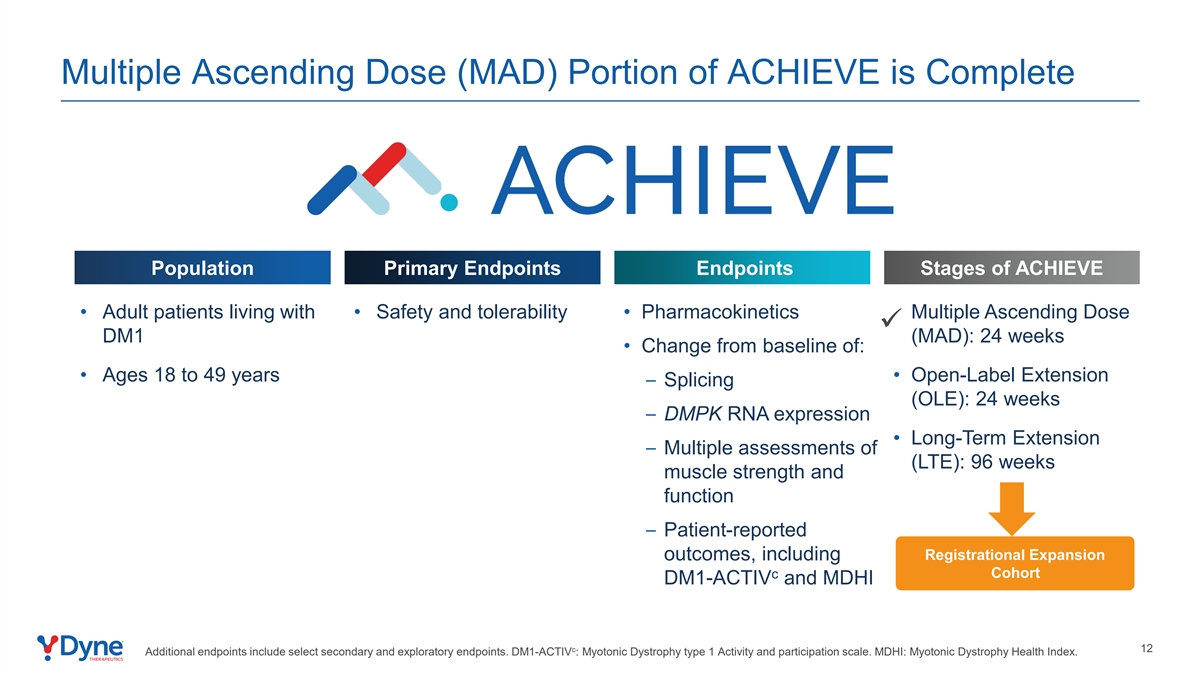
Multiple Ascending Dose (MAD) Portion of ACHIEVE is Complete Population Primary Endpoints Endpoints Stages of ACHIEVE • Adult patients living with • Safety and tolerability • Pharmacokinetics • Multiple Ascending Dose ü DM1 (MAD): 24 weeks • Change from baseline of: • Ages 18 to 49 years • Open-Label Extension – Splicing (OLE): 24 weeks – DMPK RNA expression • Long-Term Extension – Multiple assessments of (LTE): 96 weeks muscle strength and function – Patient-reported Registrational Expansion outcomes, including c Cohort DM1-ACTIV and MDHI c 12 Additional endpoints include select secondary and exploratory endpoints. DM1-ACTIV : Myotonic Dystrophy type 1 Activity and participation scale. MDHI: Myotonic Dystrophy Health Index.
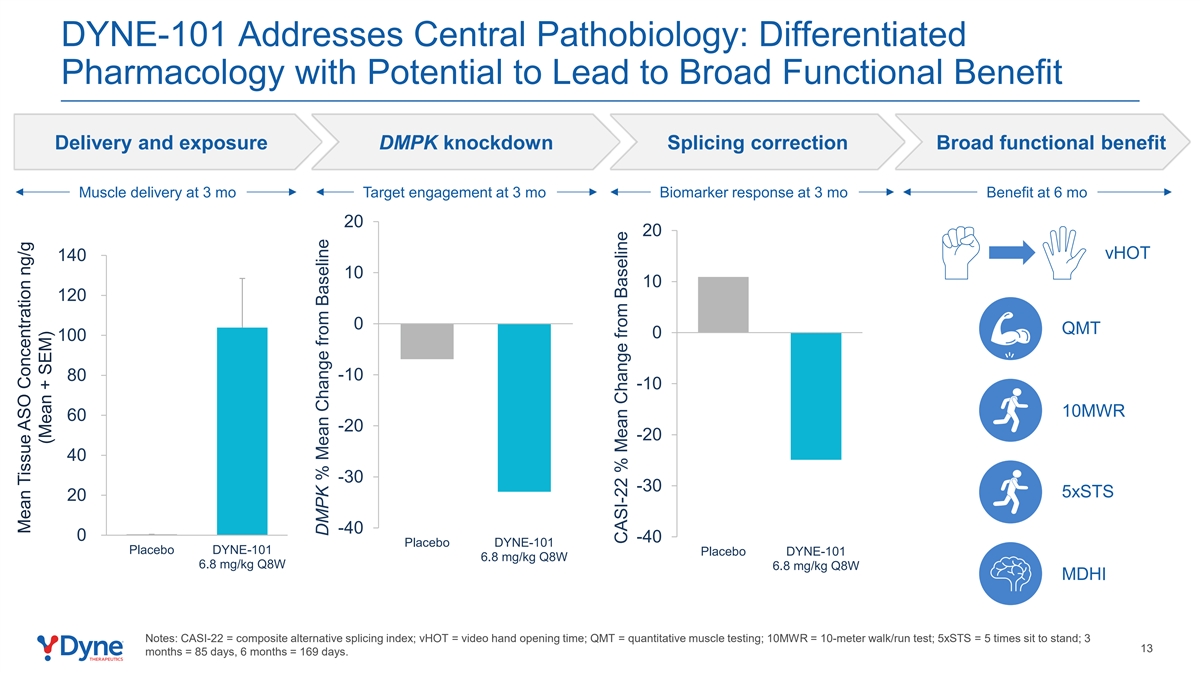
DYNE-101 Addresses Central Pathobiology: Differentiated Pharmacology with Potential to Lead to Broad Functional Benefit Delivery and exposure DMPK knockdown Splicing correction Broad functional benefit Muscle delivery at 3 mo Target engagement at 3 mo Biomarker response at 3 mo Benefit at 6 mo 20 20 vHOT 140 10 10 120 0 QMT 0 100 80 -10 -10 10MWR 60 -20 -20 40 -30 -30 5xSTS 20 -40 0 -40 Placebo DYNE-101 Placebo DYNE-101 Placebo DYNE-101 6.8 mg/kg Q8W 6.8 mg/kg Q8W 6.8 mg/kg Q8W MDHI Notes: CASI-22 = composite alternative splicing index; vHOT = video hand opening time; QMT = quantitative muscle testing; 10MWR = 10-meter walk/run test; 5xSTS = 5 times sit to stand; 3 13 months = 85 days, 6 months = 169 days. Mean Tissue ASO Concentration ng/g (Mean + SEM) DMPK % Mean Change from Baseline CASI-22 % Mean Change from Baseline
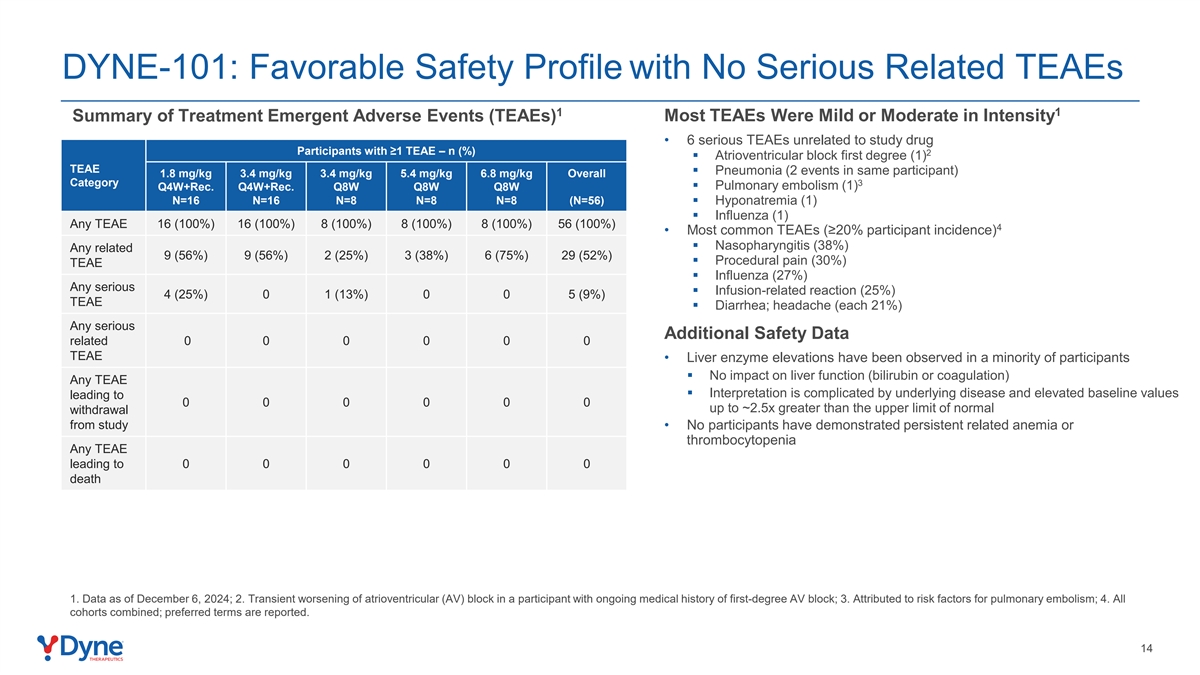
DYNE-101: Favorable Safety Profile with No Serious Related TEAEs 1 1 Summary of Treatment Emergent Adverse Events (TEAEs) Most TEAEs Were Mild or Moderate in Intensity • 6 serious TEAEs unrelated to study drug Participants with ≥1 TEAE – n (%) 2 § Atrioventricular block first degree (1) TEAE § Pneumonia (2 events in same participant) 1.8 mg/kg 3.4 mg/kg 3.4 mg/kg 5.4 mg/kg 6.8 mg/kg Overall Category 3 § Pulmonary embolism (1) Q4W+Rec. Q4W+Rec. Q8W Q8W Q8W N=16 N=16 N=8 N=8 N=8 (N=56)§ Hyponatremia (1) § Influenza (1) Any TEAE 16 (100%) 16 (100%) 8 (100%) 8 (100%) 8 (100%) 56 (100%) 4 • Most common TEAEs (≥20% participant incidence) § Nasopharyngitis (38%) Any related 9 (56%) 9 (56%) 2 (25%) 3 (38%) 6 (75%) 29 (52%) § Procedural pain (30%) TEAE § Influenza (27%) Any serious § Infusion-related reaction (25%) 4 (25%) 0 1 (13%) 0 0 5 (9%) TEAE § Diarrhea; headache (each 21%) Any serious Additional Safety Data related 0 0 0 0 0 0 TEAE • Liver enzyme elevations have been observed in a minority of participants § No impact on liver function (bilirubin or coagulation) Any TEAE § Interpretation is complicated by underlying disease and elevated baseline values leading to 0 0 0 0 0 0 up to ~2.5x greater than the upper limit of normal withdrawal from study • No participants have demonstrated persistent related anemia or thrombocytopenia Any TEAE leading to 0 0 0 0 0 0 death 1. Data as of December 6, 2024; 2. Transient worsening of atrioventricular (AV) block in a participant with ongoing medical history of first-degree AV block; 3. Attributed to risk factors for pulmonary embolism; 4. All cohorts combined; preferred terms are reported. 14
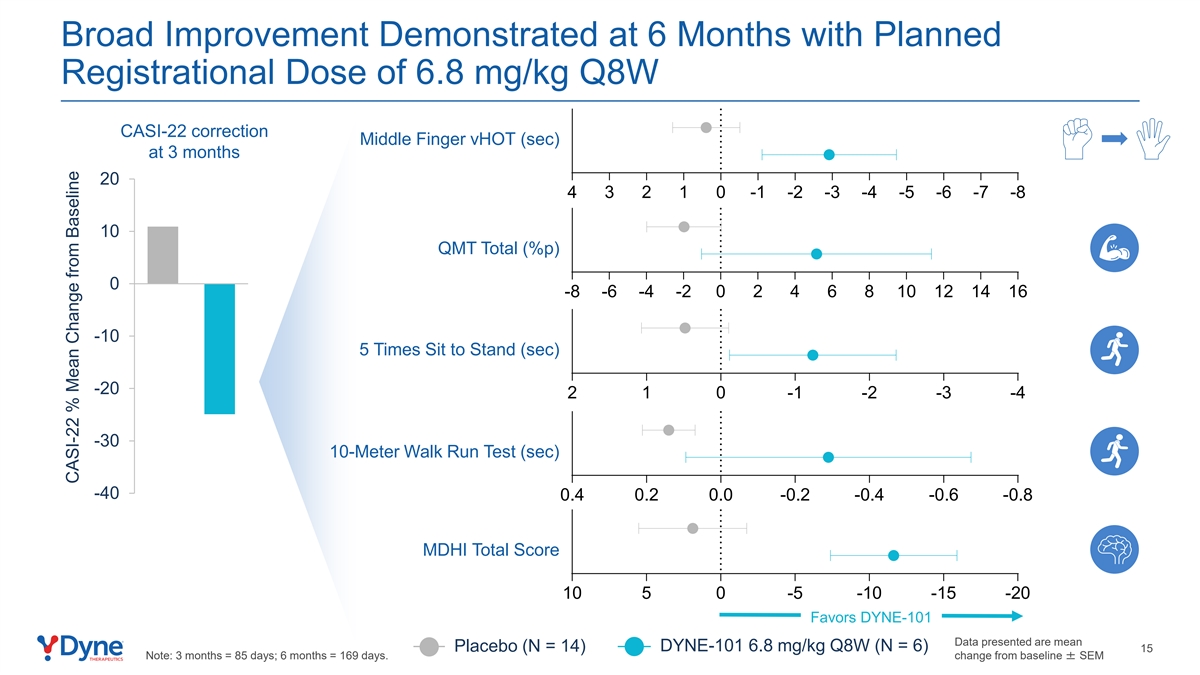
Broad Improvement Demonstrated at 6 Months with Planned Registrational Dose of 6.8 mg/kg Q8W CASI-22 correction Middle Finger vHOT (sec) at 3 months 20 4 3 2 1 0 -1 -2 -3 -4 -5 -6 -7 -8 10 QMT Total (%p) 0 -8 -6 -4 -2 0 2 4 6 8 10 12 14 16 -10 5 Times Sit to Stand (sec) -20 2 1 0 -1 -2 -3 -4 -30 10-Meter Walk Run Test (sec) -40 0.4 0.2 0.0 -0.2 -0.4 -0.6 -0.8 MDHI Total Score 10 5 0 -5 -10 -15 -20 Favors DYNE-101 Data presented are mean DYNE-101 6.8 mg/kg Q8W (N = 6) Placebo (N = 14) 15 Note: 3 months = 85 days; 6 months = 169 days. change from baseline ± SEM CASI-22 % Mean Change from Baseline

Registrational Expansion Cohort Timeline Enables Potential Submission for U.S. Accelerated Approval in H1 2026 Accelerated Approval Path Enables Potential Profile Speed to Filing with Functional Benefit Planned Primary Endpoint (3 months) • Change from baseline in CASI N ~ 32, 3:1 ACHIEVE 6.8 mg/kg Registrational Placebo Controlled Period Extension Planned Secondary Endpoints Q8W Expansion (6 months) Cohort • Change from baseline in • vHOT (middle finger) Full Enrollment Splicing Data Functional • 10MWR (mid-2025) (3 months) Endpoints (6 months) • QMT • 5xSTS • MDHI Total Score Potential Submission for U.S. Accelerated Approval (H1 2026) 16
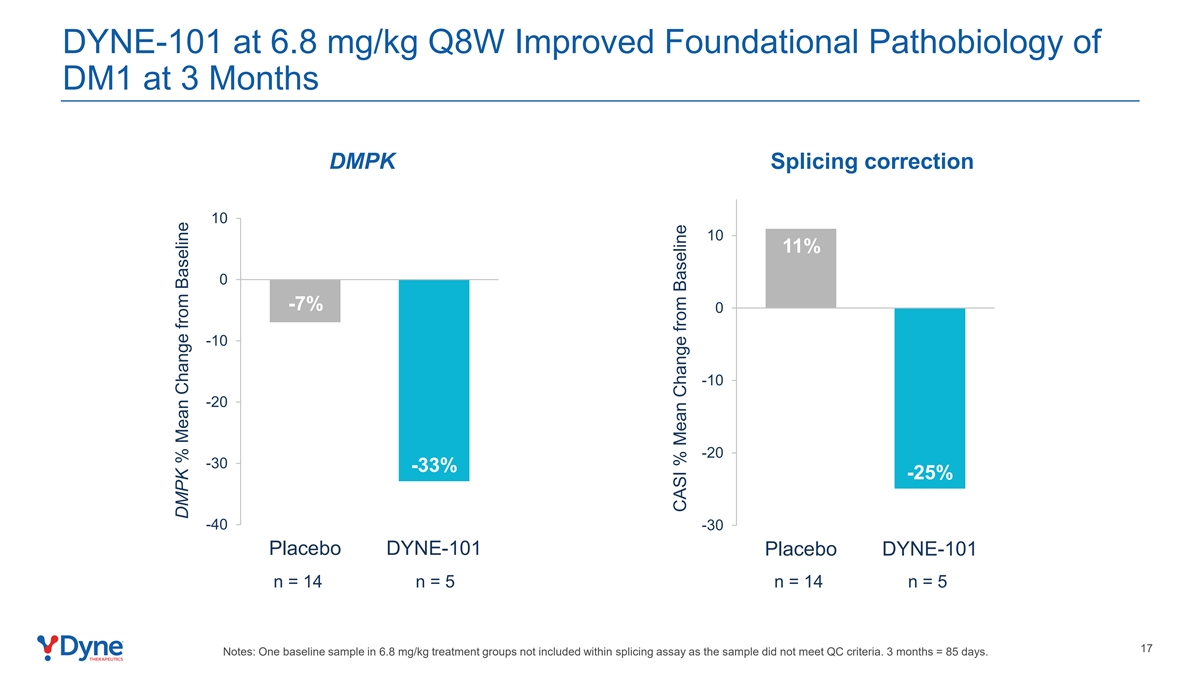
DYNE-101 at 6.8 mg/kg Q8W Improved Foundational Pathobiology of DM1 at 3 Months DMPK Splicing correction 10 10 11% 0 -7% 0 -10 -10 -20 -20 -30 -33% -25% -40 -30 Placebo DYNE-101 Placebo DYNE-101 n = 14 n = 5 n = 14 n = 5 17 Notes: One baseline sample in 6.8 mg/kg treatment groups not included within splicing assay as the sample did not meet QC criteria. 3 months = 85 days. DMPK % Mean Change from Baseline CASI % Mean Change from Baseline
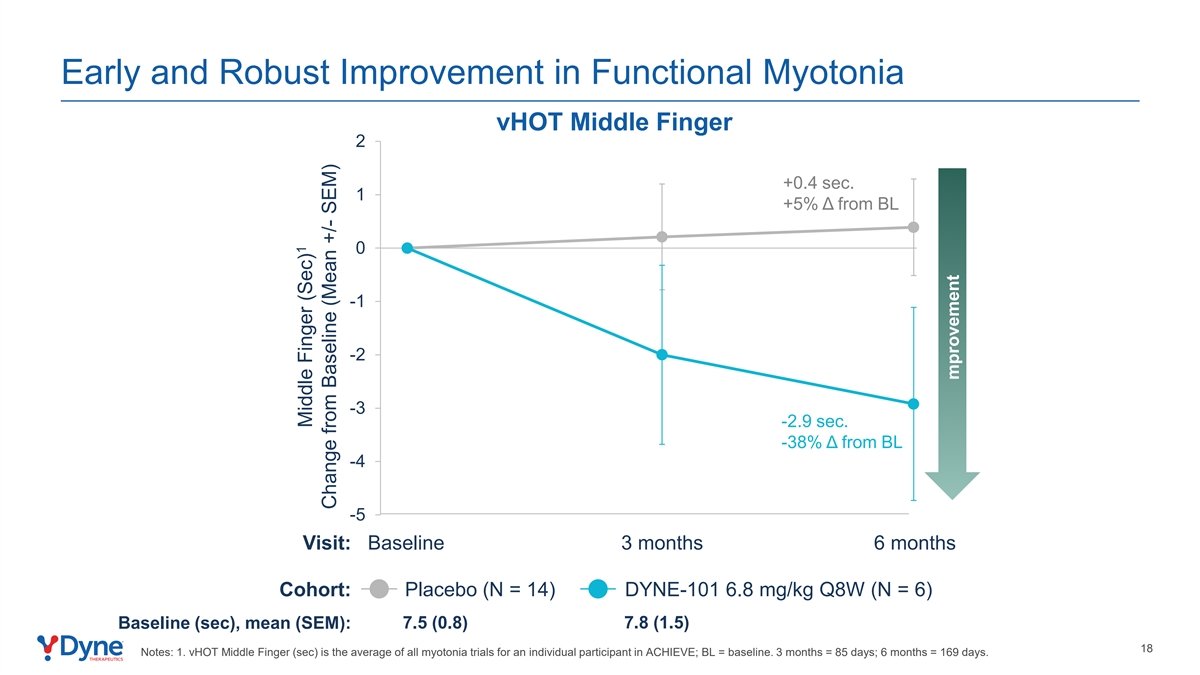
Early and Robust Improvement in Functional Myotonia vHOT Middle Finger 2 +0.4 sec. 1 +5% Δ from BL 0 -1 -2 -3 -2.9 sec. -38% Δ from BL -4 -5 Visit: Baseline 3 months 6 months Cohort: Placebo (N = 14) DYNE-101 6.8 mg/kg Q8W (N = 6) Baseline (sec), mean (SEM): 7.5 (0.8) 7.8 (1.5) 18 Notes: 1. vHOT Middle Finger (sec) is the average of all myotonia trials for an individual participant in ACHIEVE; BL = baseline. 3 months = 85 days; 6 months = 169 days. 1 Middle Finger (Sec) Change from Baseline (Mean +/- SEM) mprovement
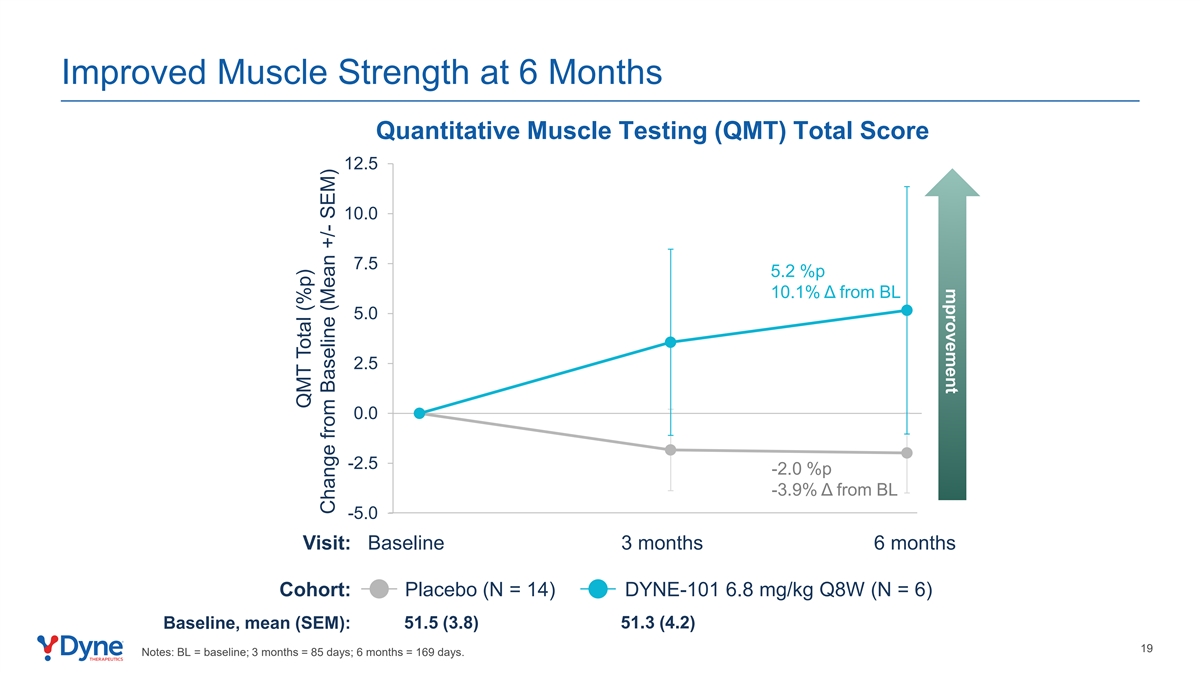
mprovement Improved Muscle Strength at 6 Months Quantitative Muscle Testing (QMT) Total Score 12.5 10.0 7.5 5.2 %p 10.1% Δ from BL 5.0 2.5 0.0 -2.5 -2.0 %p -3.9% Δ from BL -5.0 Visit: Baseline 3 months 6 months Cohort: Placebo (N = 14) DYNE-101 6.8 mg/kg Q8W (N = 6) Baseline, mean (SEM): 51.5 (3.8) 51.3 (4.2) 19 Notes: BL = baseline; 3 months = 85 days; 6 months = 169 days. QMT Total (%p) Change from Baseline (Mean +/- SEM)
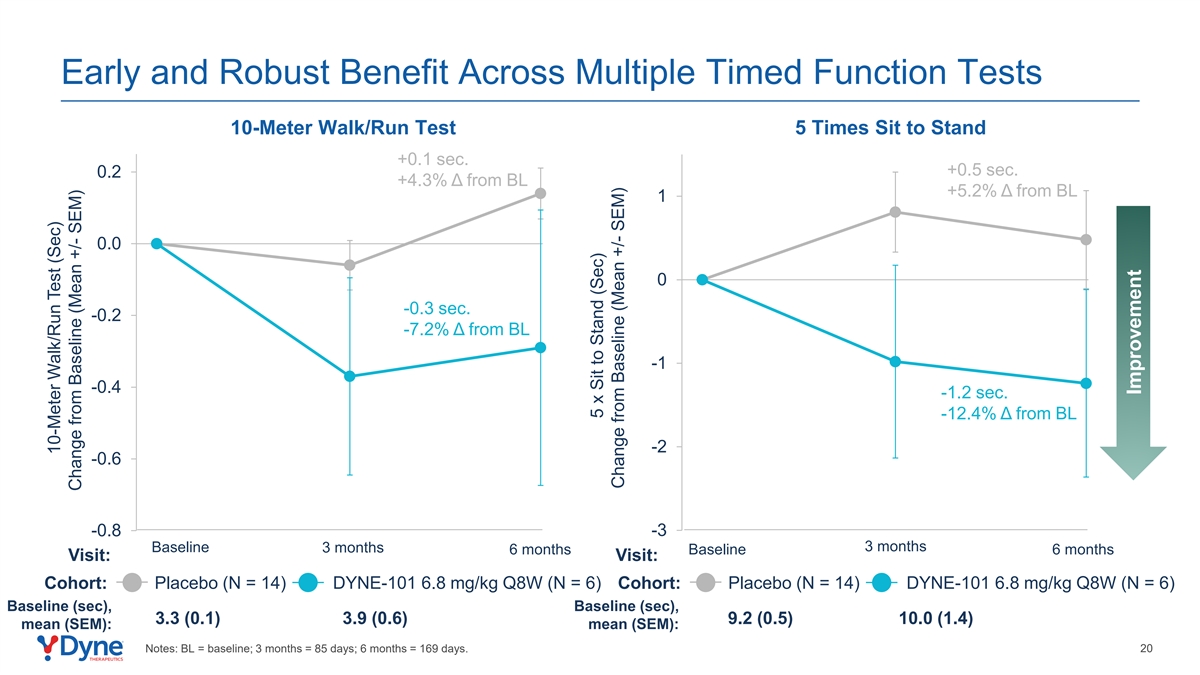
Early and Robust Benefit Across Multiple Timed Function Tests 10-Meter Walk/Run Test 5 Times Sit to Stand +0.1 sec. +0.5 sec. 0.2 +4.3% Δ from BL +5.2% Δ from BL 1 0.0 0 -0.3 sec. -0.2 -7.2% Δ from BL -1 -0.4 -1.2 sec. -12.4% Δ from BL -2 -0.6 -0.8 -3 3 months Baseline 3 months 6 months Baseline 6 months Visit: Visit: Cohort: Placebo (N = 14) DYNE-101 6.8 mg/kg Q8W (N = 6) Cohort: Placebo (N = 14) DYNE-101 6.8 mg/kg Q8W (N = 6) Baseline (sec), Baseline (sec), 3.3 (0.1) 3.9 (0.6) 9.2 (0.5) 10.0 (1.4) mean (SEM): mean (SEM): Notes: BL = baseline; 3 months = 85 days; 6 months = 169 days. 20 10-Meter Walk/Run Test (Sec) Change from Baseline (Mean +/- SEM) 5 x Sit to Stand (Sec) Change from Baseline (Mean +/- SEM) Improvement
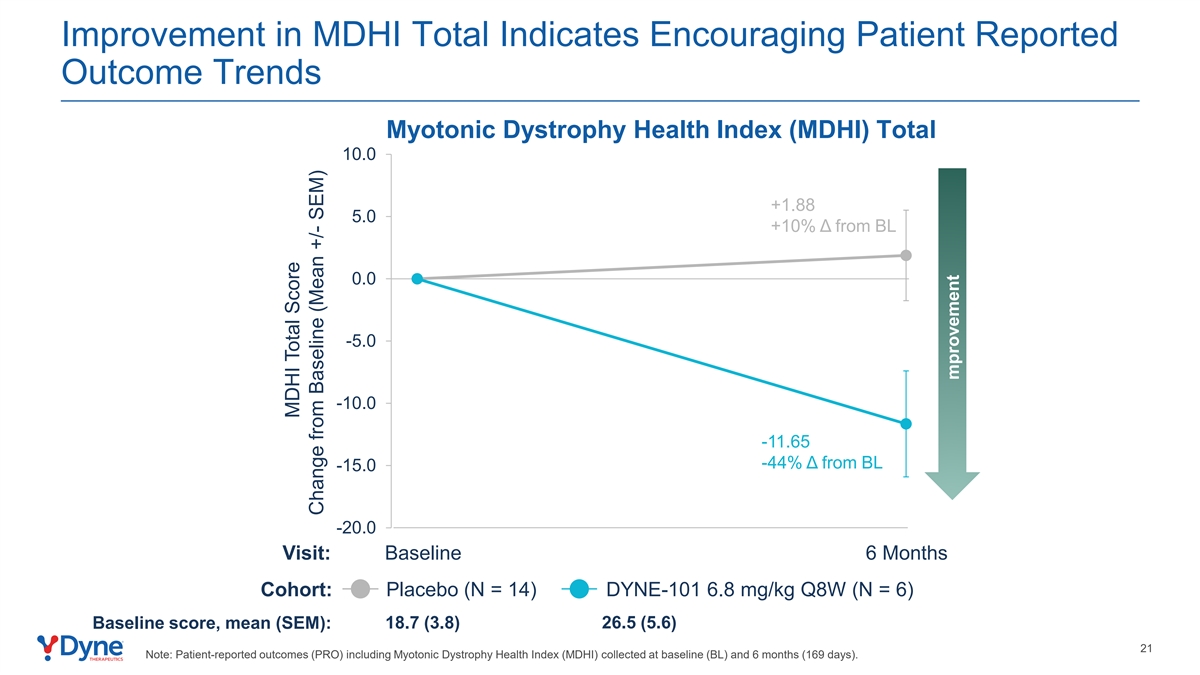
Improvement in MDHI Total Indicates Encouraging Patient Reported Outcome Trends Myotonic Dystrophy Health Index (MDHI) Total 10.0 +1.88 5.0 +10% Δ from BL 0.0 -5.0 -10.0 -11.65 -44% Δ from BL -15.0 -20.0 Visit: Baseline 6 Months Cohort: Placebo (N = 14) DYNE-101 6.8 mg/kg Q8W (N = 6) Baseline score, mean (SEM): 18.7 (3.8) 26.5 (5.6) 21 Note: Patient-reported outcomes (PRO) including Myotonic Dystrophy Health Index (MDHI) collected at baseline (BL) and 6 months (169 days). MDHI Total Score Change from Baseline (Mean +/- SEM) mprovement
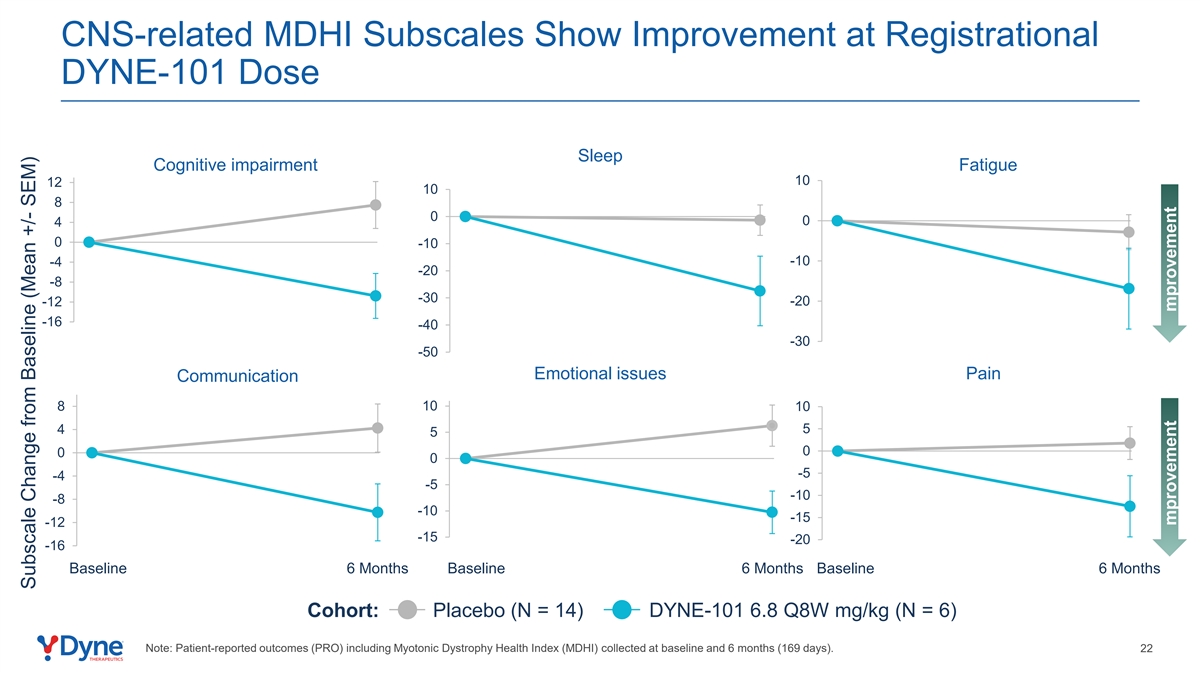
CNS-related MDHI Subscales Show Improvement at Registrational DYNE-101 Dose Sleep Cognitive impairment Fatigue 10 12 10 8 0 0 4 0 -10 -10 -4 -20 -8 -30 -12 -20 -16 -40 -30 -50 Emotional issues Pain Communication 8 10 10 4 5 5 0 0 0 -5 -4 -5 -10 -8 -10 -15 -12 -15 -20 -16 Baseline 6 Months Baseline 6 Months Baseline 6 Months Cohort: Placebo (N = 14) DYNE-101 6.8 Q8W mg/kg (N = 6) Note: Patient-reported outcomes (PRO) including Myotonic Dystrophy Health Index (MDHI) collected at baseline and 6 months (169 days). 22 Subscale Change from Baseline (Mean +/- SEM) mprovement mprovement
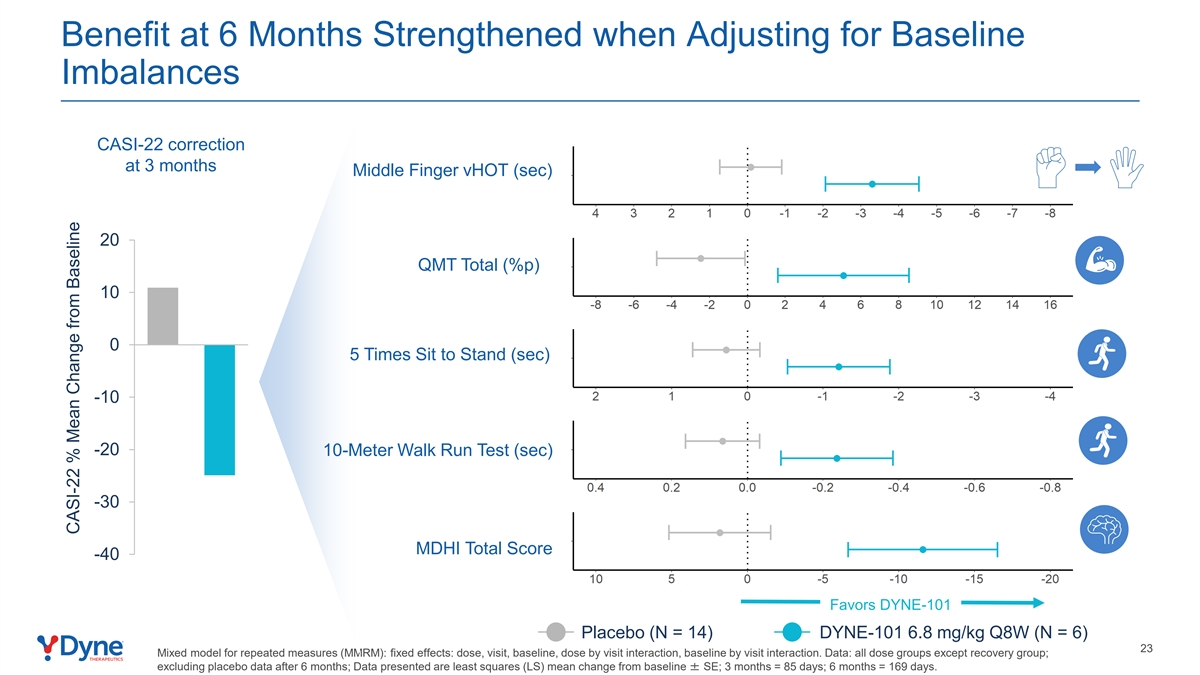
Benefit at 6 Months Strengthened when Adjusting for Baseline Imbalances CASI-22 correction at 3 months Middle Finger vHOT (sec) 20 QMT Total (%p) 10 0 5 Times Sit to Stand (sec) -10 -20 10-Meter Walk Run Test (sec) -30 MDHI Total Score -40 Favors DYNE-101 Placebo (N = 14) DYNE-101 6.8 mg/kg Q8W (N = 6) 23 Mixed model for repeated measures (MMRM): fixed effects: dose, visit, baseline, dose by visit interaction, baseline by visit interaction. Data: all dose groups except recovery group; excluding placebo data after 6 months; Data presented are least squares (LS) mean change from baseline ± SE; 3 months = 85 days; 6 months = 169 days. CASI-22 % Mean Change from Baseline
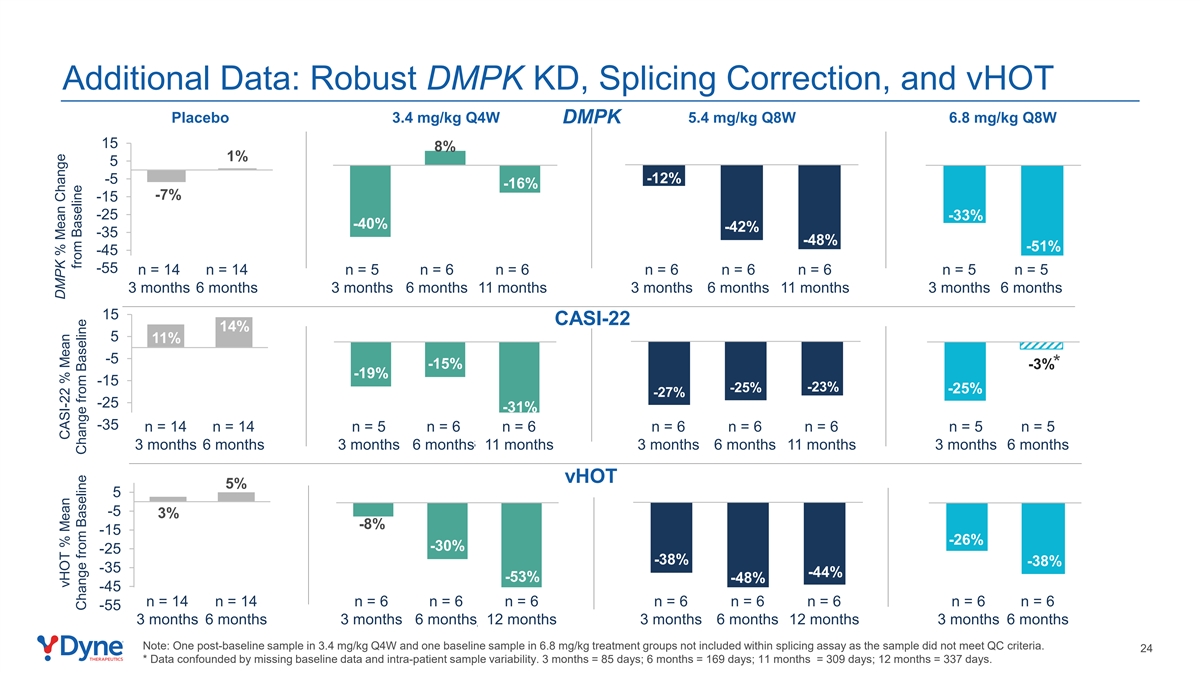
Additional Data: Robust DMPK KD, Splicing Correction, and vHOT Placebo 3.4 mg/kg Q4W 5.4 mg/kg Q8W 6.8 mg/kg Q8W DMPK 15 8% 1% 5 -5 -12% -16% -7% -15 -25 -33% -40% -42% -35 -48% -51% -45 -55 n = 14 n = 14 n = 5 n = 6 n = 6 n = 6 n = 6 n = 6 n = 5 n = 5 3 months 6 months 3 months 6 months 11 months 3 months 6 months 11 months 3 months 6 months 15 CASI-22 14% 5 11% -5 -15% -3%* -19% -15 -23% -25% -25% -27% -25 -31% -35 n = 14 n = 14 n = 5 n = 6 n = 6 n = 6 n = 6 n = 6 n = 5 n = 5 3 months 6 months 3 months 6 months 11 months 3 months 6 months 11 months 3 months 6 months 3 months 6 months 3 months 6 months 11 months 3 months 6 months 11 months 3 months 6 months vHOT 5% 5 -5 3% -8% -15 -26% -30% -25 -38% -38% -35 -44% -53% -48% -45 n = 14 n = 14 n = 6 n = 6 n = 6 n = 6 n = 6 n = 6 n = 6 n = 6 -55 3 months 6 months 3 months 6 months 12 months 3 months 6 months 12 months 3 months 6 months 3 months 6 months 3 months 6 months 12 months 3 months 6 months 12 months 3 months 6 months Note: One post-baseline sample in 3.4 mg/kg Q4W and one baseline sample in 6.8 mg/kg treatment groups not included within splicing assay as the sample did not meet QC criteria. 24 * Data confounded by missing baseline data and intra-patient sample variability. 3 months = 85 days; 6 months = 169 days; 11 months = 309 days; 12 months = 337 days. DMPK % Mean Change vHOT % Mean CASI-22 % Mean from Baseline Change from Baseline Change from Baseline
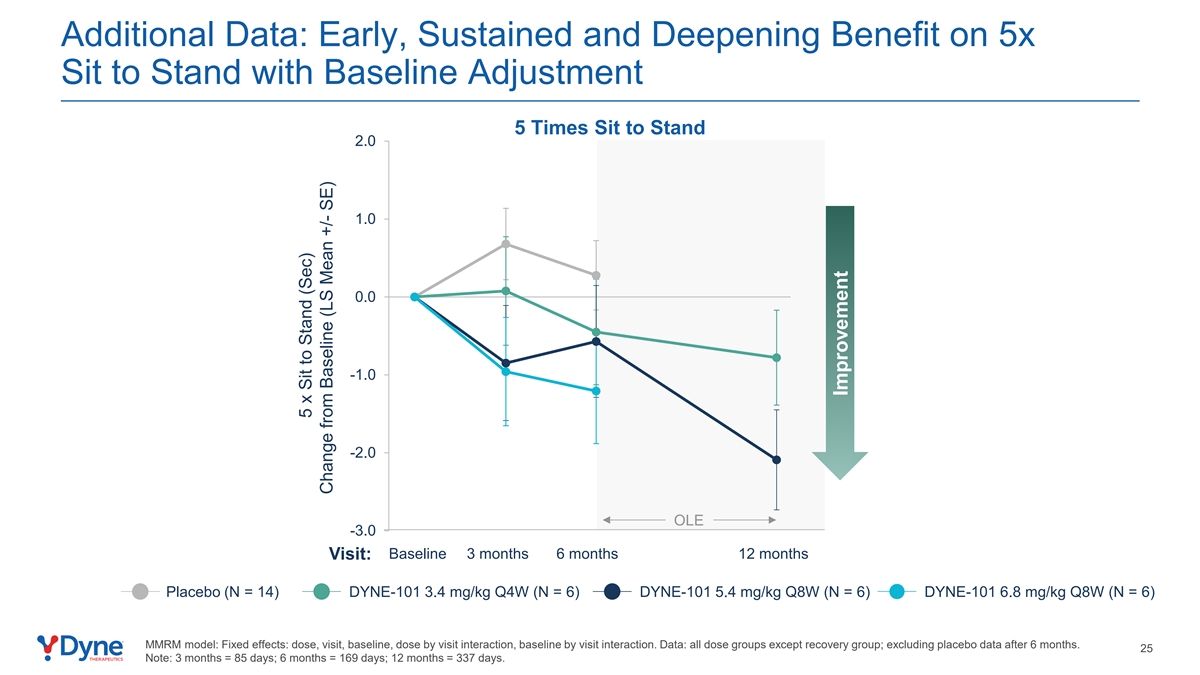
Additional Data: Early, Sustained and Deepening Benefit on 5x Sit to Stand with Baseline Adjustment 5 Times Sit to Stand 2.0 1.0 0.0 -1.0 -2.0 OLE -3.0 Baseline 3 months 6 months 12 months Visit: Placebo (N = 14) DYNE-101 3.4 mg/kg Q4W (N = 6) DYNE-101 5.4 mg/kg Q8W (N = 6) DYNE-101 6.8 mg/kg Q8W (N = 6) MMRM model: Fixed effects: dose, visit, baseline, dose by visit interaction, baseline by visit interaction. Data: all dose groups except recovery group; excluding placebo data after 6 months. 25 Note: 3 months = 85 days; 6 months = 169 days; 12 months = 337 days. 5 x Sit to Stand (Sec) Change from Baseline (LS Mean +/- SE) Improvement
Myotonic Dystrophy Type 1 (DM1) Clinical Data Show Broad Functional Impact and Support Potential for Accelerated Approval Initiating Registrational Expansion Cohort: primary endpoint of splicing correction at 3 ü months, supported by functional endpoints and PROs; full enrollment expected mid-2025 6.8 mg/kg Q8W dose showed robust splicing correction at 3 months and broad ü functional improvement, starting at 3 months and continuing at 6 months 1 Continued favorable safety profile ; no serious related TEAEs ü 26 1. DYNE-101 safety data as of December 6, 2024
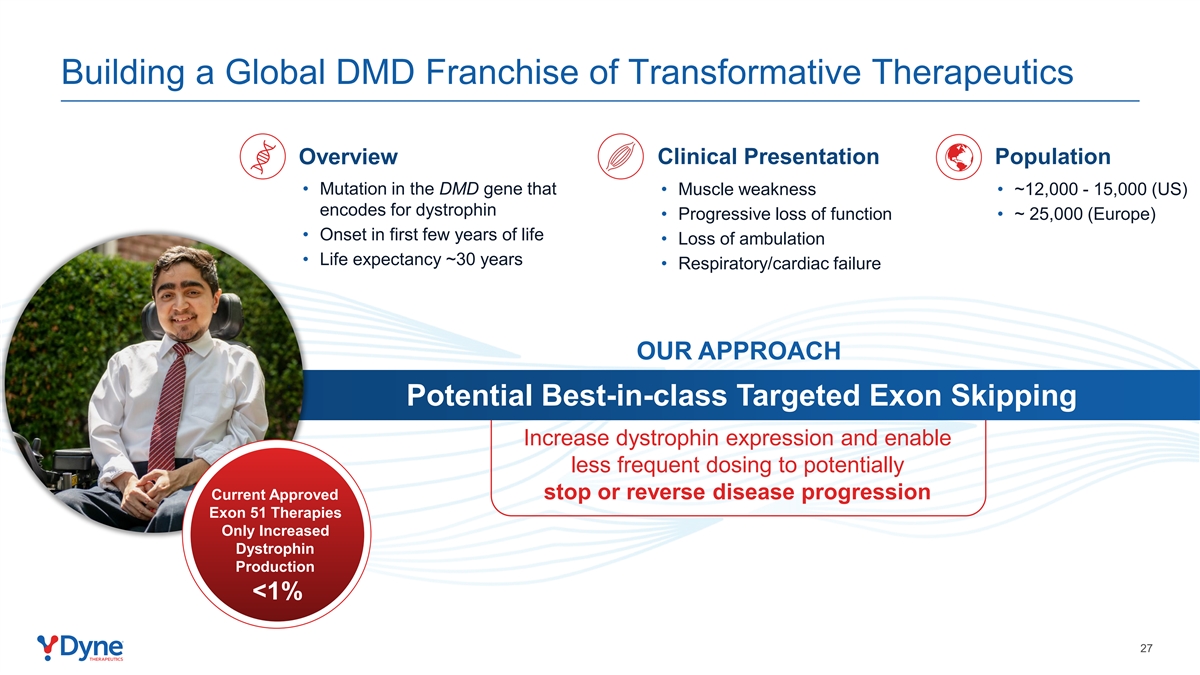
Building a Global DMD Franchise of Transformative Therapeutics Clinical Presentation Population Overview • Mutation in the DMD gene that • Muscle weakness • ~12,000 - 15,000 (US) encodes for dystrophin • Progressive loss of function • ~ 25,000 (Europe) • Onset in first few years of life • Loss of ambulation • Life expectancy ~30 years • Respiratory/cardiac failure OUR APPROACH Potential Best-in-class Targeted Exon Skipping Increase dystrophin expression and enable less frequent dosing to potentially Current Approved stop or reverse disease progression Exon 51 Therapies Only Increased Dystrophin Production <1% 27
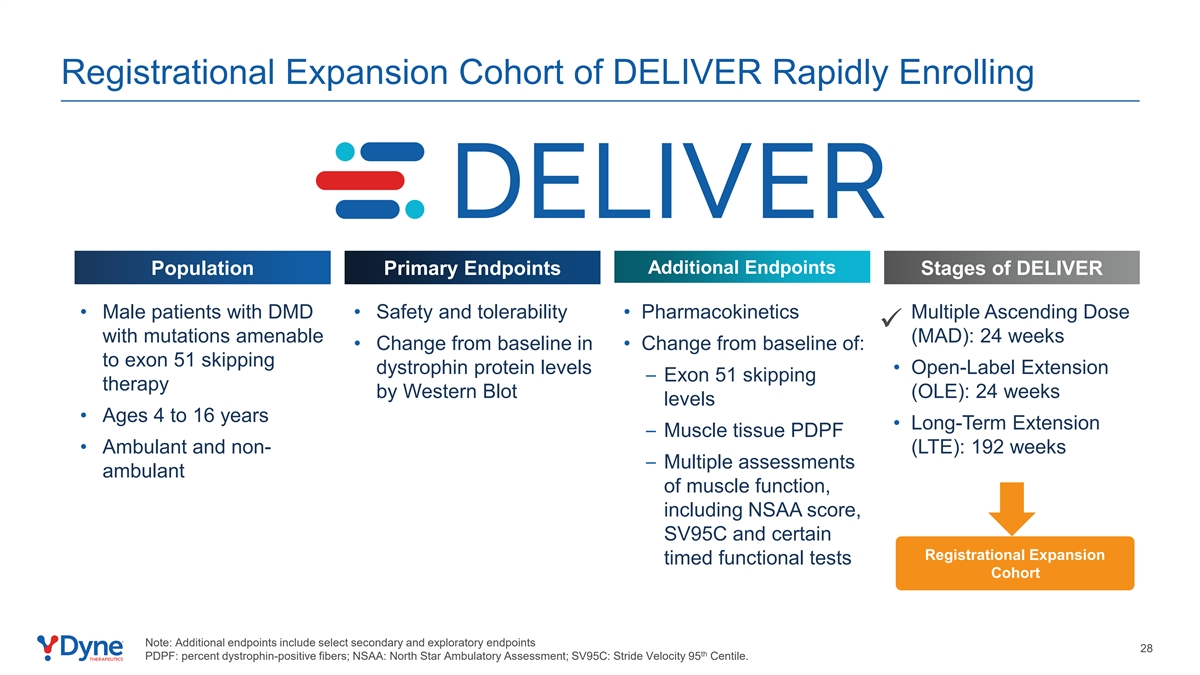
Registrational Expansion Cohort of DELIVER Rapidly Enrolling Additional Endpoints Population Primary Endpoints Stages of DELIVER • Male patients with DMD • Safety and tolerability • Pharmacokinetics • Multiple Ascending Dose ü with mutations amenable (MAD): 24 weeks • Change from baseline in • Change from baseline of: to exon 51 skipping dystrophin protein levels • Open-Label Extension – Exon 51 skipping therapy by Western Blot (OLE): 24 weeks levels • Ages 4 to 16 years • Long-Term Extension – Muscle tissue PDPF • Ambulant and non- (LTE): 192 weeks – Multiple assessments ambulant of muscle function, including NSAA score, SV95C and certain Registrational Expansion timed functional tests Cohort Note: Additional endpoints include select secondary and exploratory endpoints 28 th PDPF: percent dystrophin-positive fibers; NSAA: North Star Ambulatory Assessment; SV95C: Stride Velocity 95 Centile.
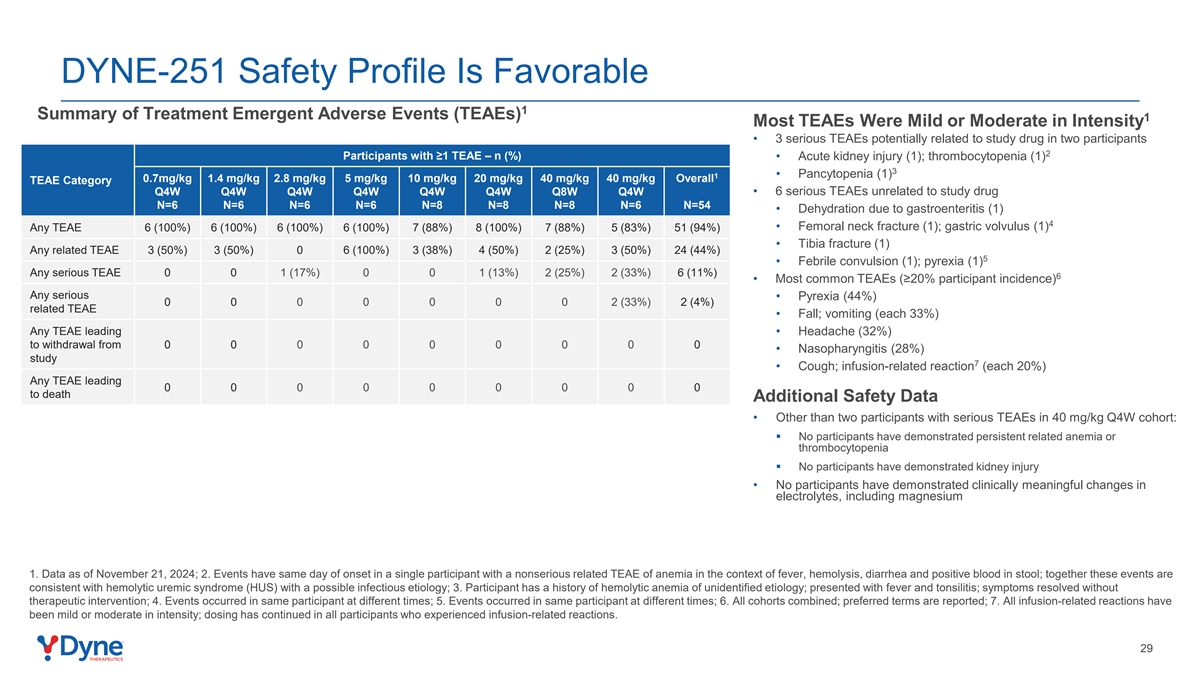
DYNE-251 Safety Profile Is Favorable 1 Summary of Treatment Emergent Adverse Events (TEAEs) 1 Most TEAEs Were Mild or Moderate in Intensity • 3 serious TEAEs potentially related to study drug in two participants 2 Participants with ≥1 TEAE – n (%) • Acute kidney injury (1); thrombocytopenia (1) 3 • Pancytopenia (1) 1 0.7mg/kg 1.4 mg/kg 2.8 mg/kg 5 mg/kg 10 mg/kg 20 mg/kg 40 mg/kg 40 mg/kg Overall TEAE Category • 6 serious TEAEs unrelated to study drug Q4W Q4W Q4W Q4W Q4W Q4W Q8W Q4W N=6 N=6 N=6 N=6 N=8 N=8 N=8 N=6 N=54 • Dehydration due to gastroenteritis (1) 4 • Femoral neck fracture (1); gastric volvulus (1) Any TEAE 6 (100%) 6 (100%) 6 (100%) 6 (100%) 7 (88%) 8 (100%) 7 (88%) 5 (83%) 51 (94%) • Tibia fracture (1) Any related TEAE 3 (50%) 3 (50%) 0 6 (100%) 3 (38%) 4 (50%) 2 (25%) 3 (50%) 24 (44%) 5 • Febrile convulsion (1); pyrexia (1) Any serious TEAE 0 0 1 (17%) 0 0 1 (13%) 2 (25%) 2 (33%) 6 (11%) 6 • Most common TEAEs (≥20% participant incidence) Any serious • Pyrexia (44%) 0 0 0 0 0 0 0 2 (33%) 2 (4%) related TEAE • Fall; vomiting (each 33%) Any TEAE leading • Headache (32%) to withdrawal from 0 0 0 0 0 0 0 0 0 • Nasopharyngitis (28%) study 7 • Cough; infusion-related reaction (each 20%) Any TEAE leading 0 0 0 0 0 0 0 0 0 to death Additional Safety Data • Other than two participants with serious TEAEs in 40 mg/kg Q4W cohort: § No participants have demonstrated persistent related anemia or thrombocytopenia § No participants have demonstrated kidney injury • No participants have demonstrated clinically meaningful changes in electrolytes, including magnesium 1. Data as of November 21, 2024; 2. Events have same day of onset in a single participant with a nonserious related TEAE of anemia in the context of fever, hemolysis, diarrhea and positive blood in stool; together these events are consistent with hemolytic uremic syndrome (HUS) with a possible infectious etiology; 3. Participant has a history of hemolytic anemia of unidentified etiology; presented with fever and tonsilitis; symptoms resolved without therapeutic intervention; 4. Events occurred in same participant at different times; 5. Events occurred in same participant at different times; 6. All cohorts combined; preferred terms are reported; 7. All infusion-related reactions have been mild or moderate in intensity; dosing has continued in all participants who experienced infusion-related reactions. 29
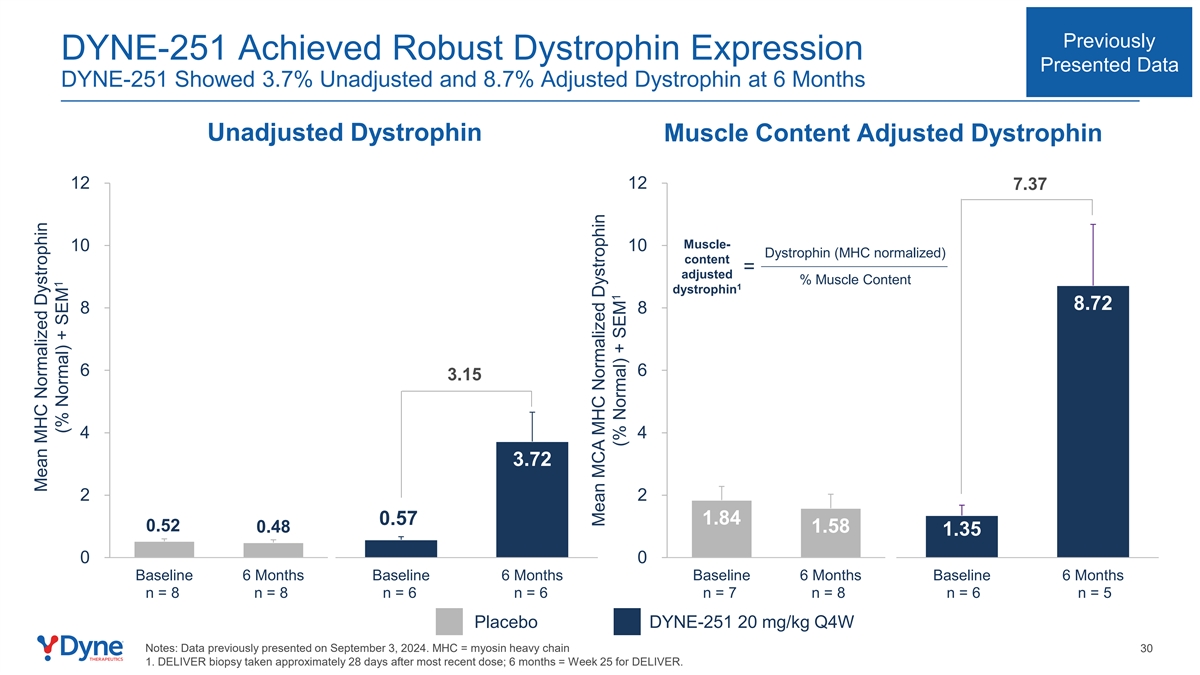
Previously DYNE-251 Achieved Robust Dystrophin Expression Presented Data DYNE-251 Showed 3.7% Unadjusted and 8.7% Adjusted Dystrophin at 6 Months Unadjusted Dystrophin Muscle Content Adjusted Dystrophin 12 12 7.37 Muscle- 10 10 Dystrophin (MHC normalized) content = adjusted % Muscle Content 1 dystrophin 8.72 8 8 6 6 3.15 4 4 3.72 2 2 0.57 1.84 0.52 0.48 1.58 1.35 0 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months Baseline 6 Months n = 8 n = 8 n = 6 n = 6 n = 7 n = 8 n = 6 n = 5 Placebo DYNE-251 20 mg/kg Q4W Notes: Data previously presented on September 3, 2024. MHC = myosin heavy chain 30 1. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER. Mean MHC Normalized Dystrophin 1 (% Normal) + SEM Mean MCA MHC Normalized Dystrophin 1 (% Normal) + SEM
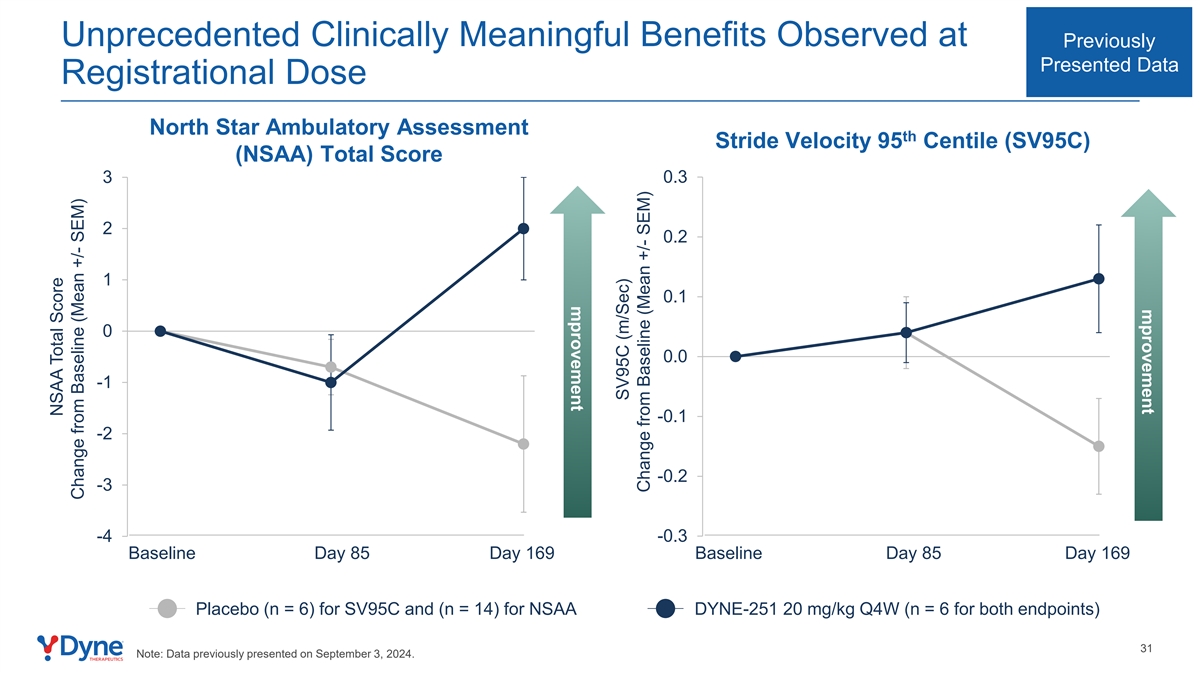
mprovement mprovement Unprecedented Clinically Meaningful Benefits Observed at Previously Presented Data Registrational Dose North Star Ambulatory Assessment th Stride Velocity 95 Centile (SV95C) (NSAA) Total Score 0.3 3 2 0.2 1 0.1 0 0.0 -1 -0.1 -2 -0.2 -3 -4 -0.3 Baseline Day 85 Day 169 Baseline Day 85 Day 169 Placebo (n = 6) for SV95C and (n = 14) for NSAA DYNE-251 20 mg/kg Q4W (n = 6 for both endpoints) 31 Note: Data previously presented on September 3, 2024. NSAA Total Score Change from Baseline (Mean +/- SEM) SV95C (m/Sec) Change from Baseline (Mean +/- SEM)
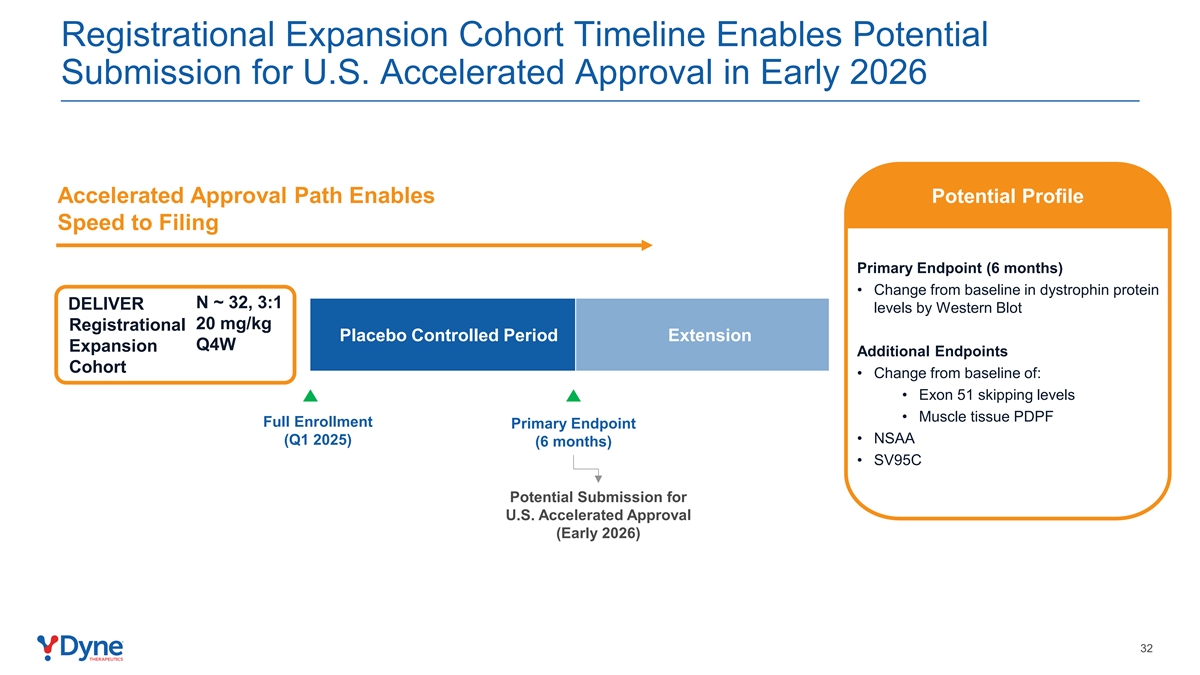
Registrational Expansion Cohort Timeline Enables Potential Submission for U.S. Accelerated Approval in Early 2026 Accelerated Approval Path Enables Potential Profile Speed to Filing Primary Endpoint (6 months) • Change from baseline in dystrophin protein N ~ 32, 3:1 DELIVER levels by Western Blot 20 mg/kg Registrational Placebo Controlled Period Extension Q4W Expansion Additional Endpoints Cohort • Change from baseline of: • Exon 51 skipping levels • Muscle tissue PDPF Full Enrollment Primary Endpoint • NSAA (Q1 2025) (6 months) • SV95C Potential Submission for U.S. Accelerated Approval (Early 2026) 32
Program Opening Remarks John Cox, President & CEO New Data from DYNE-101 ACHIEVE Trial in DM1 Update on DYNE-251 DELIVER Trial in DMD Doug Kerr, M.D., Ph.D., Chief Medical Officer Closing Remarks John Cox, President & CEO 33
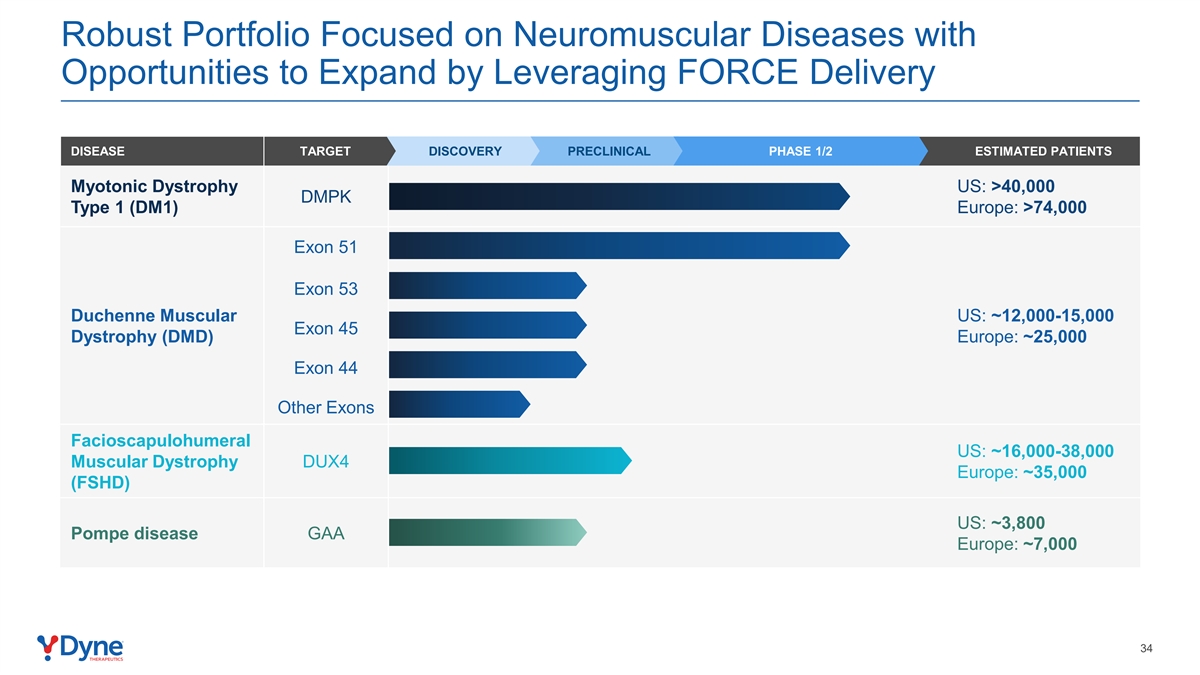
Robust Portfolio Focused on Neuromuscular Diseases with Opportunities to Expand by Leveraging FORCE Delivery DISEASE TARGET DISCOVERY PRECLINICAL PHASE 1/2 ESTIMATED PATIENTS Myotonic Dystrophy US: >40,000 DMPK Type 1 (DM1) Europe: >74,000 Exon 51 Exon 53 Duchenne Muscular US: ~12,000-15,000 Exon 45 Dystrophy (DMD) Europe: ~25,000 Exon 44 Other Exons Facioscapulohumeral US: ~16,000-38,000 Muscular Dystrophy DUX4 Europe: ~35,000 (FSHD) US: ~3,800 Pompe disease GAA Europe: ~7,000 34
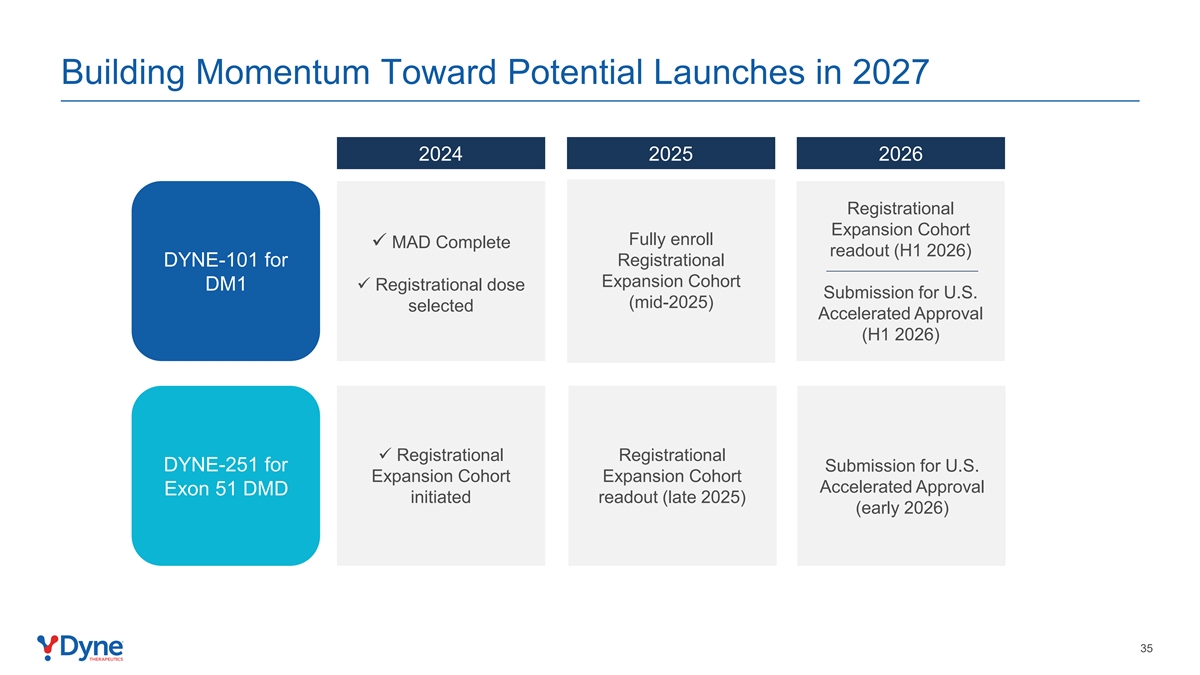
Building Momentum Toward Potential Launches in 2027 2024 2025 2026 Registrational Expansion Cohort Fully enroll ü MAD Complete readout (H1 2026) DYNE-101 for Registrational Expansion Cohort DM1ü Registrational dose Submission for U.S. (mid-2025) selected Accelerated Approval (H1 2026) ü Registrational Registrational DYNE-251 for Submission for U.S. Expansion Cohort Expansion Cohort Accelerated Approval Exon 51 DMD initiated readout (late 2025) (early 2026) 35
Q&A 36

Appendix: Additional Study Results 37
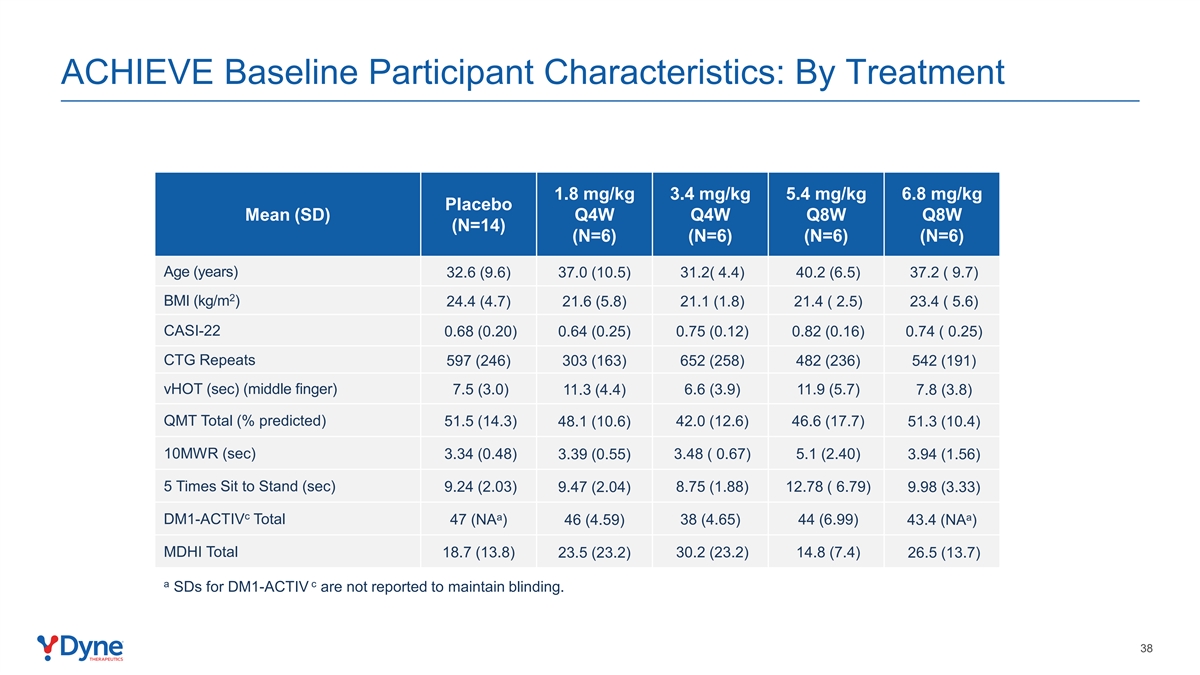
ACHIEVE Baseline Participant Characteristics: By Treatment 1.8 mg/kg 3.4 mg/kg 5.4 mg/kg 6.8 mg/kg Placebo Mean (SD) Q4W Q4W Q8W Q8W (N=14) (N=6) (N=6) (N=6) (N=6) Age (years) 32.6 (9.6) 37.0 (10.5) 31.2( 4.4) 40.2 (6.5) 37.2 ( 9.7) 2 BMI (kg/m ) 24.4 (4.7) 21.6 (5.8) 21.1 (1.8) 21.4 ( 2.5) 23.4 ( 5.6) CASI-22 0.68 (0.20) 0.64 (0.25) 0.75 (0.12) 0.82 (0.16) 0.74 ( 0.25) CTG Repeats 597 (246) 303 (163) 652 (258) 482 (236) 542 (191) vHOT (sec) (middle finger) 7.5 (3.0) 11.3 (4.4) 6.6 (3.9) 11.9 (5.7) 7.8 (3.8) QMT Total (% predicted) 51.5 (14.3) 48.1 (10.6) 42.0 (12.6) 46.6 (17.7) 51.3 (10.4) 10MWR (sec) 3.34 (0.48) 3.48 ( 0.67) 5.1 (2.40) 3.39 (0.55) 3.94 (1.56) 5 Times Sit to Stand (sec) 9.24 (2.03) 9.47 (2.04) 8.75 (1.88) 12.78 ( 6.79) 9.98 (3.33) c a a DM1-ACTIV Total 47 (NA ) 46 (4.59) 38 (4.65) 44 (6.99) 43.4 (NA ) MDHI Total 18.7 (13.8) 23.5 (23.2) 30.2 (23.2) 14.8 (7.4) 26.5 (13.7) a c SDs for DM1-ACTIV are not reported to maintain blinding. 38
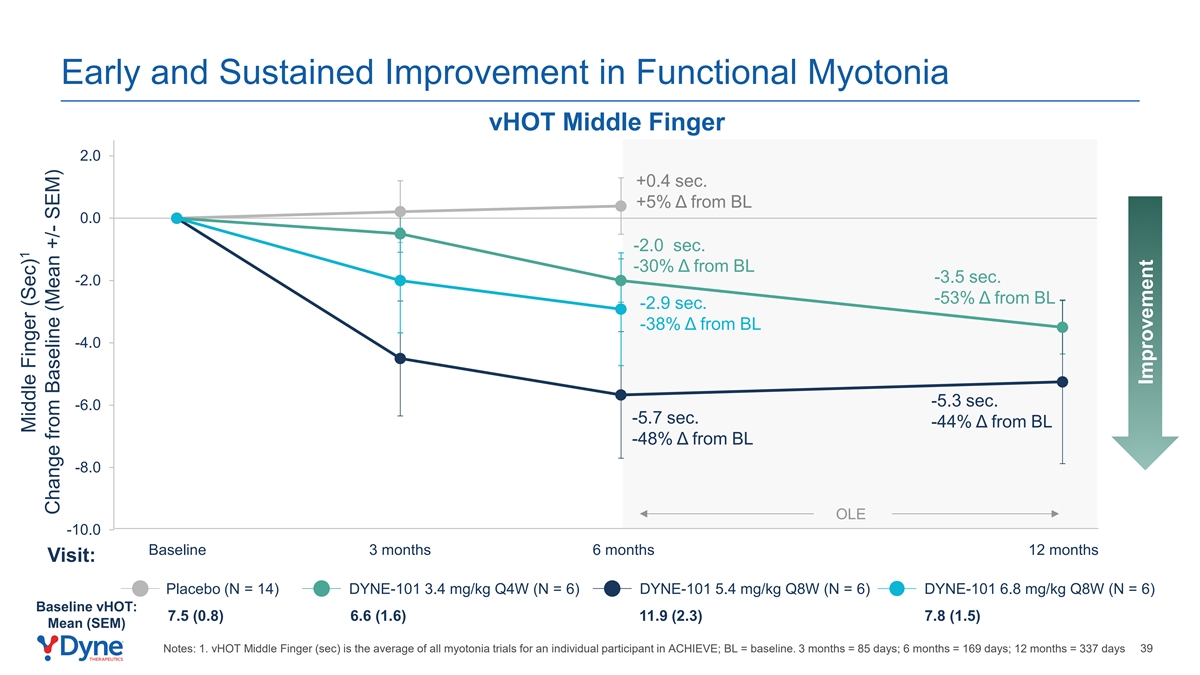
Early and Sustained Improvement in Functional Myotonia vHOT Middle Finger 2.0 +0.4 sec. +5% Δ from BL 0.0 -2.0 sec. -30% Δ from BL -3.5 sec. -2.0 -53% Δ from BL -2.9 sec. -38% Δ from BL -4.0 -5.3 sec. -6.0 -5.7 sec. -44% Δ from BL -48% Δ from BL -8.0 OLE -10.0 Baseline 3 months 6 months 12 months Visit: Placebo (N = 14) DYNE-101 3.4 mg/kg Q4W (N = 6) DYNE-101 5.4 mg/kg Q8W (N = 6) DYNE-101 6.8 mg/kg Q8W (N = 6) Baseline vHOT: 7.5 (0.8) 6.6 (1.6) 11.9 (2.3) 7.8 (1.5) Mean (SEM) Notes: 1. vHOT Middle Finger (sec) is the average of all myotonia trials for an individual participant in ACHIEVE; BL = baseline. 3 months = 85 days; 6 months = 169 days; 12 months = 337 days 39 1 Middle Finger (Sec) Change from Baseline (Mean +/- SEM) Improvement
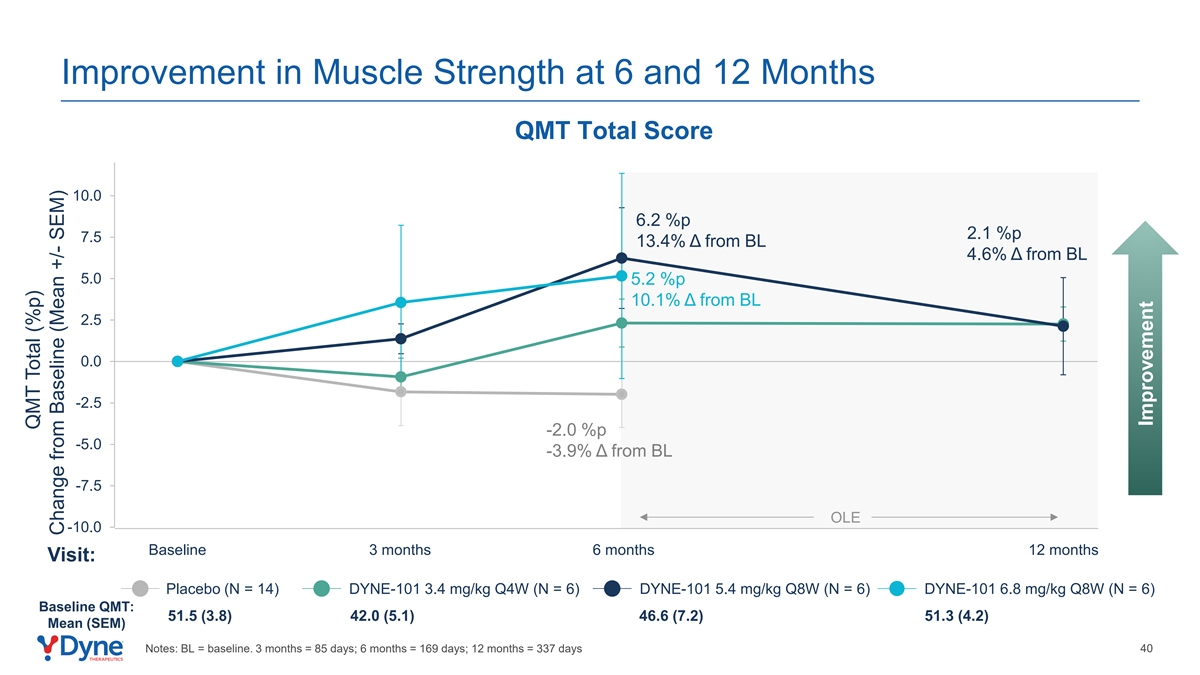
Improvement in Muscle Strength at 6 and 12 Months QMT Total Score 10.0 6.2 %p 2.1 %p 7.5 13.4% Δ from BL 4.6% Δ from BL 5.0 5.2 %p 10.1% Δ from BL 2.5 0.0 -2.5 -2.0 %p -5.0 -3.9% Δ from BL -7.5 OLE -10.0 Baseline 3 months 6 months 12 months Visit: Placebo (N = 14) DYNE-101 3.4 mg/kg Q4W (N = 6) DYNE-101 5.4 mg/kg Q8W (N = 6) DYNE-101 6.8 mg/kg Q8W (N = 6) Baseline QMT: 51.5 (3.8) 42.0 (5.1) 46.6 (7.2) 51.3 (4.2) Mean (SEM) Notes: BL = baseline. 3 months = 85 days; 6 months = 169 days; 12 months = 337 days 40 QMT Total (%p) Change from Baseline (Mean +/- SEM) Improvement
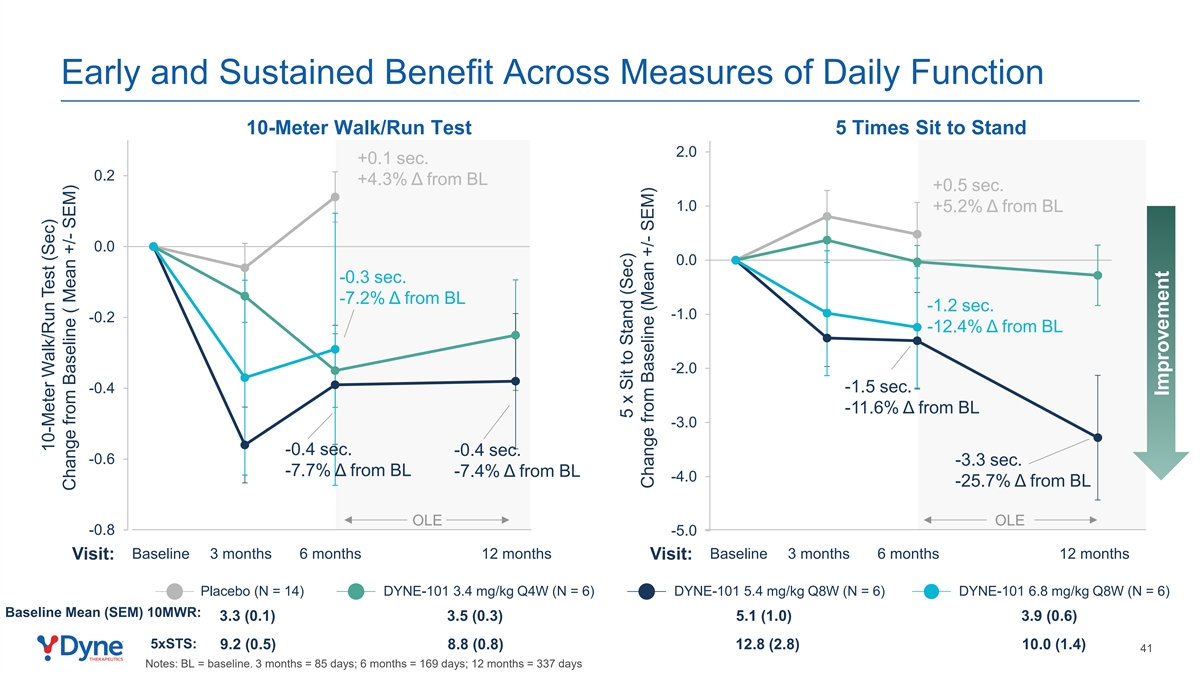
Early and Sustained Benefit Across Measures of Daily Function 10-Meter Walk/Run Test 5 Times Sit to Stand 2.0 +0.1 sec. 0.2 +4.3% Δ from BL +0.5 sec. 1.0 +5.2% Δ from BL 0.0 0.0 -0.3 sec. -7.2% Δ from BL -1.2 sec. -1.0 -0.2 -12.4% Δ from BL -2.0 -1.5 sec. -0.4 -11.6% Δ from BL -3.0 -0.4 sec. -0.4 sec. -0.6 -3.3 sec. -7.7% Δ from BL -7.4% Δ from BL -4.0 -25.7% Δ from BL OLE OLE -0.8 -5.0 Baseline 3 months 6 months 12 months Baseline 3 months 6 months 12 months Visit: Visit: Placebo (N = 14) DYNE-101 3.4 mg/kg Q4W (N = 6) DYNE-101 5.4 mg/kg Q8W (N = 6) DYNE-101 6.8 mg/kg Q8W (N = 6) Baseline Mean (SEM) 10MWR: 3.3 (0.1) 3.5 (0.3) 5.1 (1.0) 3.9 (0.6) 5xSTS: 9.2 (0.5) 8.8 (0.8) 12.8 (2.8) 10.0 (1.4) 41 Notes: BL = baseline. 3 months = 85 days; 6 months = 169 days; 12 months = 337 days 10-Meter Walk/Run Test (Sec) Change from Baseline ( Mean +/- SEM) 5 x Sit to Stand (Sec) Change from Baseline (Mean +/- SEM) Improvement
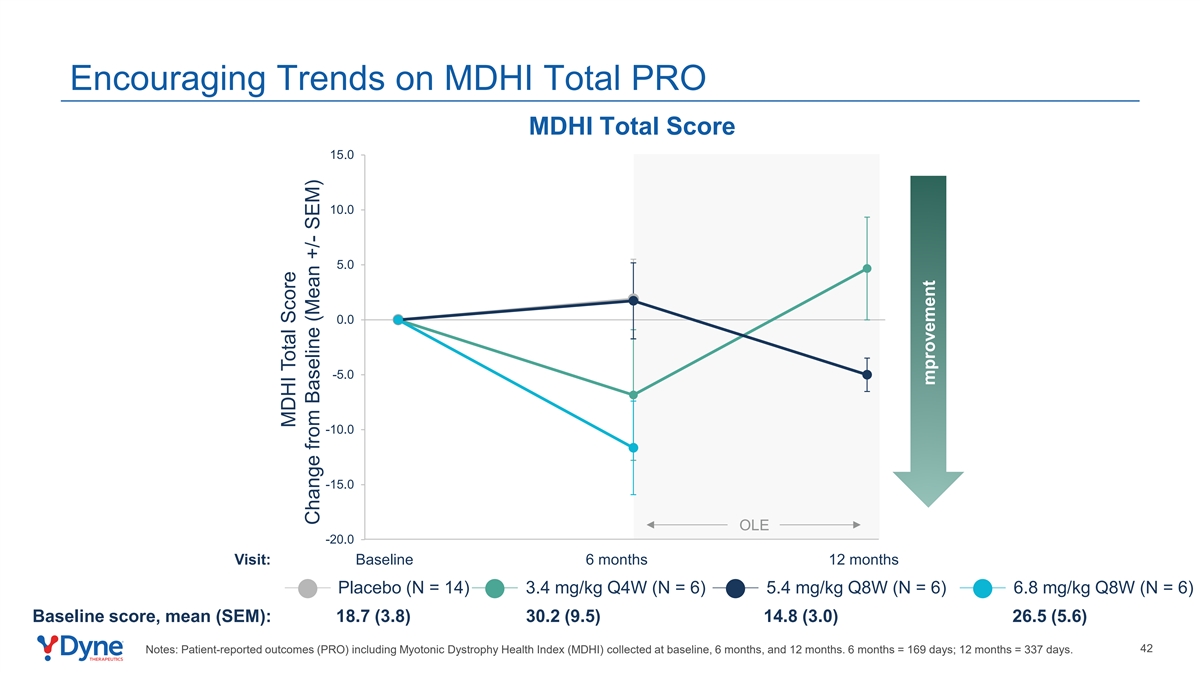
Encouraging Trends on MDHI Total PRO MDHI Total Score 15.0 10.0 5.0 0.0 -5.0 -10.0 -15.0 OLE -20.0 Visit: Baseline 6 months 12 months Placebo (N = 14) 3.4 mg/kg Q4W (N = 6) 5.4 mg/kg Q8W (N = 6) 6.8 mg/kg Q8W (N = 6) Baseline score, mean (SEM): 18.7 (3.8) 30.2 (9.5) 14.8 (3.0) 26.5 (5.6) 42 Notes: Patient-reported outcomes (PRO) including Myotonic Dystrophy Health Index (MDHI) collected at baseline, 6 months, and 12 months. 6 months = 169 days; 12 months = 337 days. MDHI Total Score Change from Baseline (Mean +/- SEM) mprovement
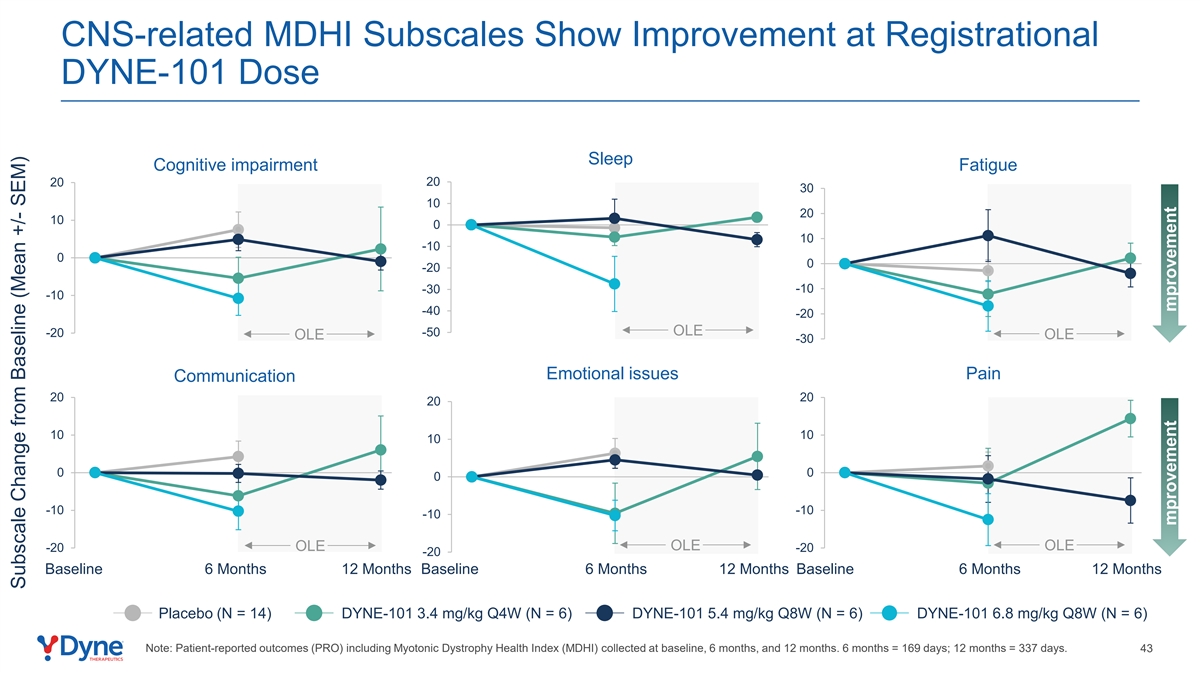
CNS-related MDHI Subscales Show Improvement at Registrational DYNE-101 Dose Sleep Cognitive impairment Fatigue 20 20 30 10 20 10 0 10 -10 0 0 -20 -10 -30 -10 -40 -20 -50 OLE -20 OLE OLE -30 Emotional issues Pain Communication 20 20 20 10 10 10 0 0 0 -10 -10 -10 OLE OLE OLE -20 -20 -20 Baseline 6 Months 12 Months Baseline 6 Months 12 Months Baseline 6 Months 12 Months Placebo (N = 14) DYNE-101 3.4 mg/kg Q4W (N = 6) DYNE-101 5.4 mg/kg Q8W (N = 6) DYNE-101 6.8 mg/kg Q8W (N = 6) Note: Patient-reported outcomes (PRO) including Myotonic Dystrophy Health Index (MDHI) collected at baseline, 6 months, and 12 months. 6 months = 169 days; 12 months = 337 days. 43 Subscale Change from Baseline (Mean +/- SEM) mprovement mprovement