
The Wnt Company – Powering Regeneration 2021 © 2021 Surrozen, Inc. Exhibit 99.2

Legal Disclaimers © 2021 Surrozen, Inc. This presentation (“Presentation”) is for informational purposes only to assist interested parties in making their own evaluation with respect to the proposed business combination (the “Business Combination’”) between Consonance-HFW Acquisition Corp. (“CHFW”) and Surrozen, Inc. (“Surrozen” or the “Company”). The information contained herein does not purport to be all-inclusive and none of CHFW, Surrozen, J.P. Morgan Securities LLC (“JPM”) or BofA Securities, Inc. (“BofA”) nor any of their respective affiliates nor any of its or their control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this presentation, you confirm that you are not relying upon the information contained herein to make any decision. Forward–looking statements. Certain statements in this Presentation may be considered forward-looking statements. Forward-looking statements generally relate to future events or CHFW’s or Surrozen’s future financial or operating performance. For example, statements concerning the following include forward-looking statements: Surrozen’s ability to identify, develop and commercialize drug candidates; the initiation, cost, timing, progress and results of research and development activities, preclinical or and clinical trials with respect to SZN-1326, SZN-043, and potential future drug candidates; estimates of Surrozen’s total addressable market, future revenue, expenses, capital requirements and its needs for additional financing; Surrozen’s ability to advance SZN-1326, SZN-043, or other future product candidates into, and successfully complete, preclinical studies and clinical studies; and the potential effects of the Business Combination on CHFW and Surrozen and related capital raising activities. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by CHFW and its management, and Surrozen and its management, as the case may be, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in CHFW’s final prospectus relating to its initial public offering, dated November 18, 2020, and other filings with the SEC, including its Annual Report on Form 10-K filed on March 31, 2021 (the “Annual Report”), as well as factors associated with companies, such as Surrozen, that are engaged in preclinical studies and other research and development activities in the biopharma industry, including uncertainty in the timing or results of preclinical studies and clinical trials, product acceptance and/or receipt of regulatory approvals for product candidates, including any delays and other impacts from the COVID-19 pandemic. Nothing in this Presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements in this Presentation, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Neither CHFW nor Surrozen undertakes any duty to update these forward-looking statements. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and Surrozen’s own internal estimates and research. In addition, all of the market data included in this Presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while Surrozen believes its internal research is reliable, such research has not been verified by any independent source. This Presentation contains certain financial, including pro forma, information of the Company. Neither the Company’s independent auditors, nor the independent registered public accounting firms of CHFW, audited, reviewed, compiled, or performed any procedures with respect to the projections for the purpose of their inclusion in this Presentation, and accordingly, neither of them expressed an opinion or provided any other form of assurance with respect thereto for the purpose of this Presentation.

Legal Disclaimers © 2021 Surrozen, Inc. Additional Information. In connection with the proposed Business Combination, CHFW intends to file with the SEC a registration statement on Form S-4 , which will include a prospectus with respect to the securities of CHFW to be issued in connection with the business combination to Surrozen stockholders and as well as a proxy statement with respect to the shareholder meeting of CHFW to vote on the business combination and related matters. After the registration statement is declared effective, CHFW will mail a definitive proxy statement/prospectus relating to the proposed Business Combination to its shareholders. This Presentation does not contain all the information that should be considered concerning the proposed Business Combination and is not intended to form the basis of any investment decision or any other decision in respect of the Business Combination. CHFW ‘s shareholders, Surrozen stockholders and other interested persons are advised to read, when available, the preliminary proxy statement/prospectus and the amendments thereto and the definitive proxy statement/prospectus and other documents filed in connection with the proposed Business Combination, as these materials will contain important information about Surrozen, CHFW and the Business Combination. When available, the definitive proxy statement/prospectus and other relevant materials for the proposed Business Combination will be mailed to shareholders of CHFW as of a record date to be established for voting on the proposed Business Combination. Shareholders will also be able to obtain copies of the preliminary proxy statement/prospectus, the definitive proxy statement/prospectus and other documents filed with the SEC, without charge, once available, at the SEC’s website at www.sec.gov, or by directing a request to: Consonance-HFW Acquisition Corp., 1 Palmer Square, Suite 305, Princeton, NJ 08540. Participants in the Solicitation. CHFW and its directors and executive officers may be deemed participants in the solicitation of proxies from CHFW ‘s shareholders with respect to the proposed Business Combination. A list of the names of those directors and executive officers and a description of their interests in CHFW is contained in CHFW’s Annual Report, which was filed with the SEC and is available free of charge at the SEC’s web site at www.sec.gov, or by directing a request to Consonance-HFW Acquisition Corp., 1 Palmer Square, Suite 305, Princeton, NJ 08540. Additional information regarding the interests of such participants will be contained in the proxy statement/prospectus for the proposed Business Combination when available. No offer or Solicitation. This communication is for informational purposes only and does not constitute a proxy statement or a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the potential transaction, or form a part of, an offer to sell or the solicitation of an offer to sell or an offer to buy or the solicitation of an offer to buy any securities, and there shall be no sale of securities, in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended, and otherwise in accordance with applicable law.
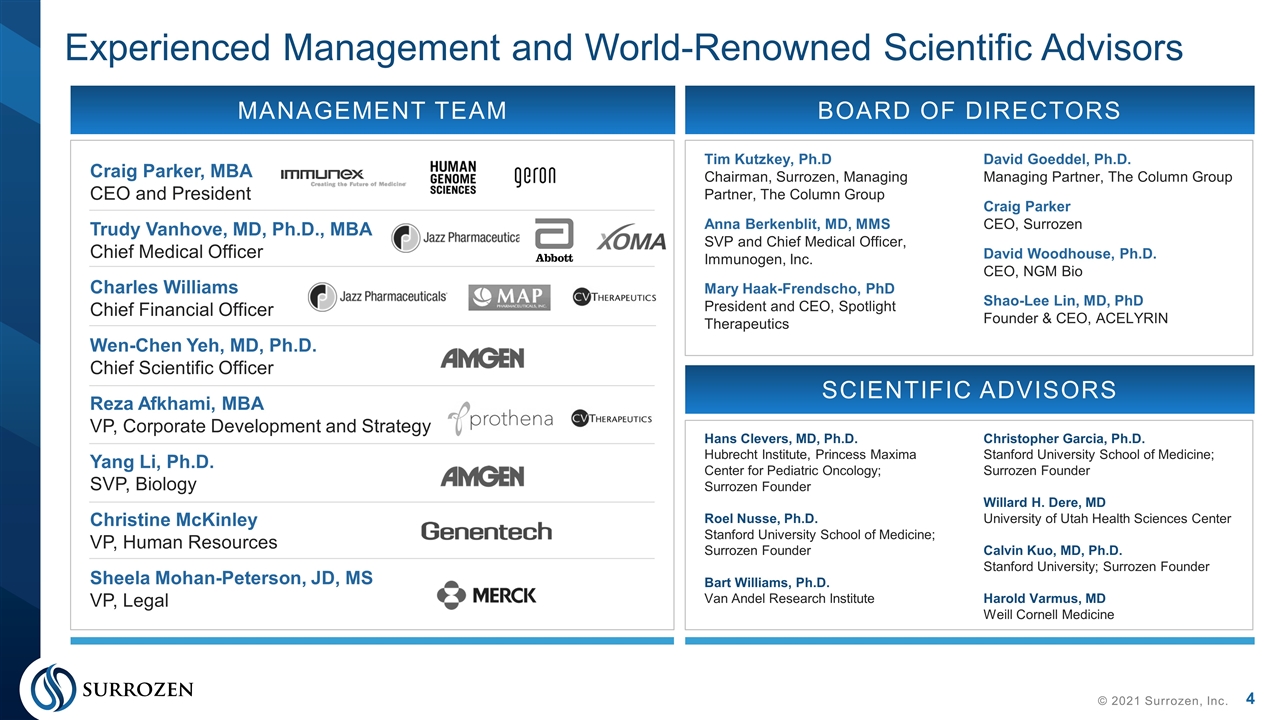
Craig Parker, MBA CEO and President Trudy Vanhove, MD, Ph.D., MBA Chief Medical Officer Charles Williams Chief Financial Officer Wen-Chen Yeh, MD, Ph.D. Chief Scientific Officer Reza Afkhami, MBA VP, Corporate Development and Strategy Yang Li, Ph.D. SVP, Biology Christine McKinley VP, Human Resources Sheela Mohan-Peterson, JD, MS VP, Legal Experienced Management and World-Renowned Scientific Advisors © 2021 Surrozen, Inc. MANAGEMENT TEAM BOARD OF DIRECTORS Tim Kutzkey, Ph.D Chairman, Surrozen, Managing Partner, The Column Group Anna Berkenblit, MD, MMS SVP and Chief Medical Officer, Immunogen, Inc. Mary Haak-Frendscho, PhD President and CEO, Spotlight Therapeutics David Goeddel, Ph.D. Managing Partner, The Column Group Craig Parker CEO, Surrozen David Woodhouse, Ph.D. CEO, NGM Bio Shao-Lee Lin, MD, PhD Founder & CEO, ACELYRIN SCIENTIFIC ADVISORS Hans Clevers, MD, Ph.D. Hubrecht Institute, Princess Maxima Center for Pediatric Oncology; Surrozen Founder Roel Nusse, Ph.D. Stanford University School of Medicine; Surrozen Founder Bart Williams, Ph.D. Van Andel Research Institute Christopher Garcia, Ph.D. Stanford University School of Medicine; Surrozen Founder Willard H. Dere, MD University of Utah Health Sciences Center Calvin Kuo, MD, Ph.D. Stanford University; Surrozen Founder Harold Varmus, MD Weill Cornell Medicine
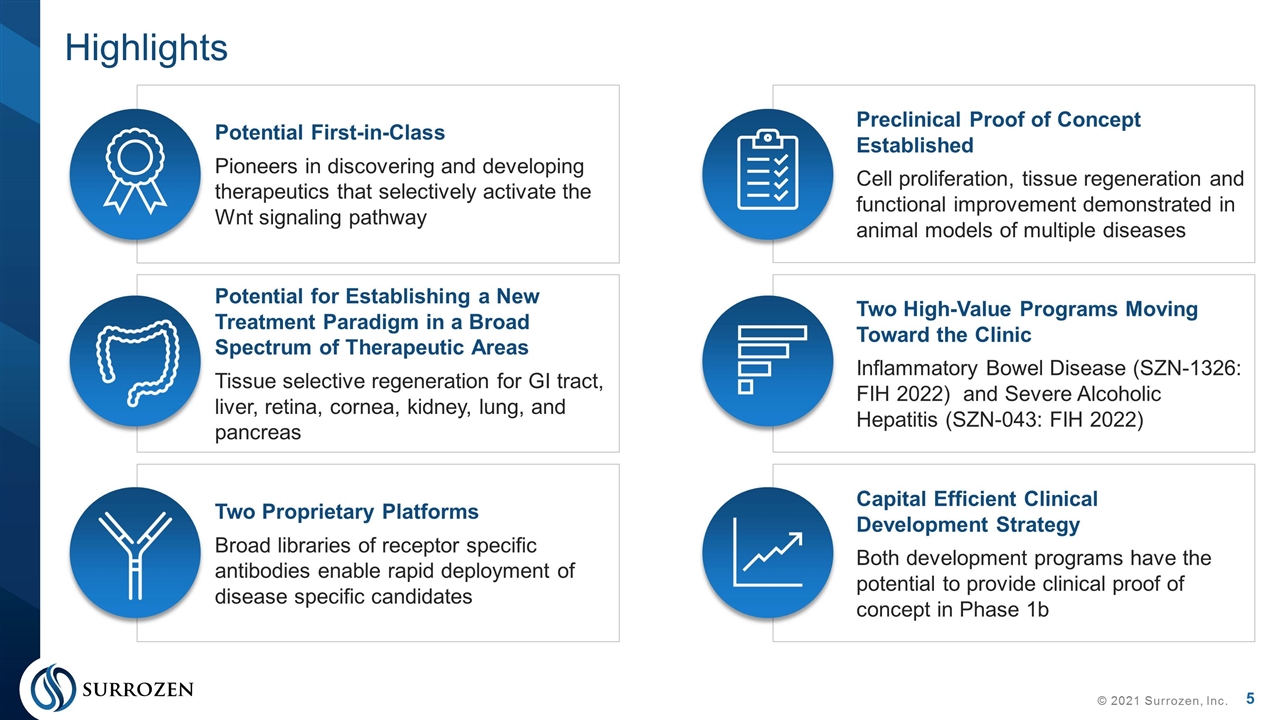
Potential First-in-Class Pioneers in discovering and developing therapeutics that selectively activate the Wnt signaling pathway Highlights © 2021 Surrozen, Inc. Potential for Establishing a New Treatment Paradigm in a Broad Spectrum of Therapeutic Areas Tissue selective regeneration for GI tract, liver, retina, cornea, kidney, lung, and pancreas Two Proprietary Platforms Broad libraries of receptor specific antibodies enable rapid deployment of disease specific candidates Preclinical Proof of Concept Established Cell proliferation, tissue regeneration and functional improvement demonstrated in animal models of multiple diseases Two High-Value Programs Moving Toward the Clinic Inflammatory Bowel Disease (SZN-1326: FIH 2022) and Severe Alcoholic Hepatitis (SZN-043: FIH 2022) Capital Efficient Clinical Development Strategy Both development programs have the potential to provide clinical proof of concept in Phase 1b
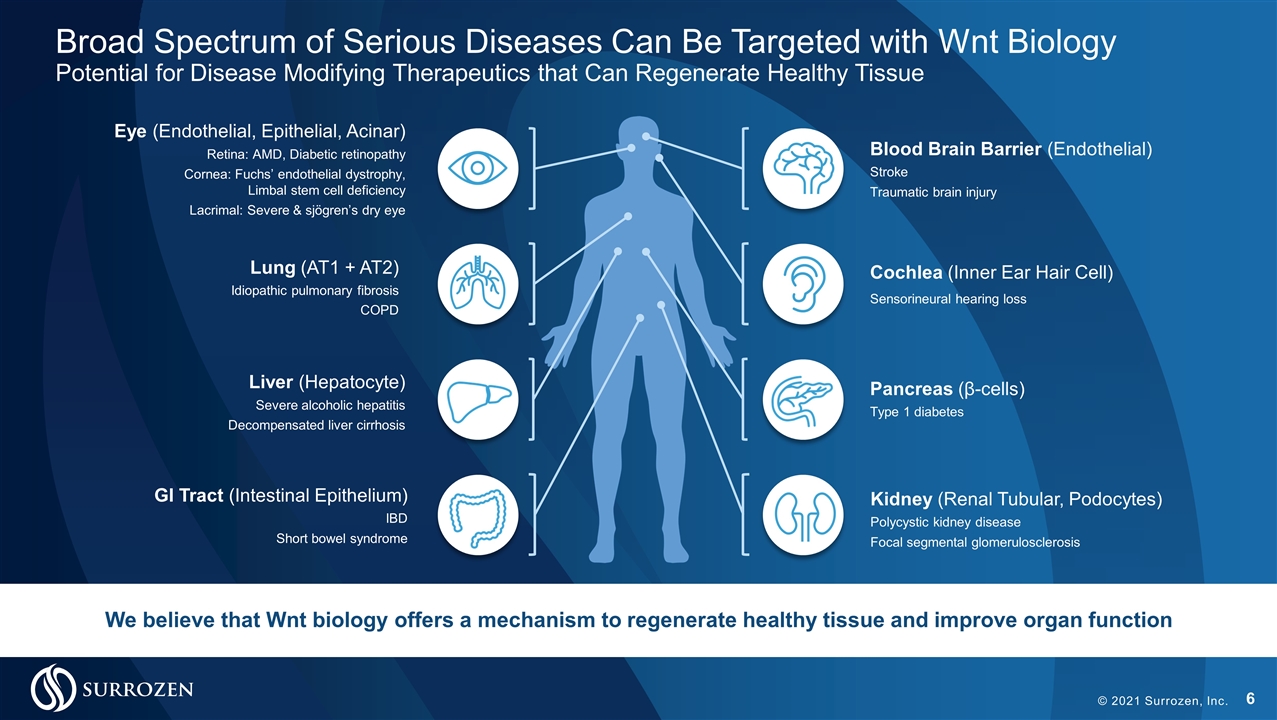
Broad Spectrum of Serious Diseases Can Be Targeted with Wnt Biology Potential for Disease Modifying Therapeutics that Can Regenerate Healthy Tissue © 2021 Surrozen, Inc. We believe that Wnt biology offers a mechanism to regenerate healthy tissue and improve organ function Cochlea (Inner Ear Hair Cell) Sensorineural hearing loss Lung (AT1 + AT2) Idiopathic pulmonary fibrosis COPD Kidney (Renal Tubular, Podocytes) Polycystic kidney disease Focal segmental glomerulosclerosis Pancreas (β-cells) Type 1 diabetes Blood Brain Barrier (Endothelial) Stroke Traumatic brain injury Eye (Endothelial, Epithelial, Acinar) Retina: AMD, Diabetic retinopathy Cornea: Fuchs’ endothelial dystrophy, Limbal stem cell deficiency Lacrimal: Severe & sjögren’s dry eye Liver (Hepatocyte) Severe alcoholic hepatitis Decompensated liver cirrhosis GI Tract (Intestinal Epithelium) IBD Short bowel syndrome

© 2021 Surrozen, Inc. Our Novel Approach Overcomes Previous Challenges Technologies, Expertise and Strategy Help Establish a New Paradigm Our antibodies have desirable drug-like properties: Technologies confer desirable PK, stability and manufacturability properties Our mechanisms mimic normal physiologic responses: Antibodies copy natural regeneration and repair process including negative feedback pathways and self-limiting components Identification of diseased tissue sensitivity: Discovered diseased tissue responds to Wnt signaling while we see little or no activity in healthy and non-targeted tissue; no evidence of hyperplasia or dysplasia Wnt biology expertise: Understand, and continue to profile, expression patterns of FZDs, LRPs and R-Spondins across disease states Selective targeting with potency: Achieved individual FZD receptor selectivity and tissue specificity while preserving potency Our strategy limits risk: Focus on severe disease, short term-dosing, and potential local administration There is an approved drug precedent: Romosozumab, an anti-sclerostin antibody, enhances Wnt signaling in bone. Proven safety with one year of dosing in thousands of osteoporosis patients
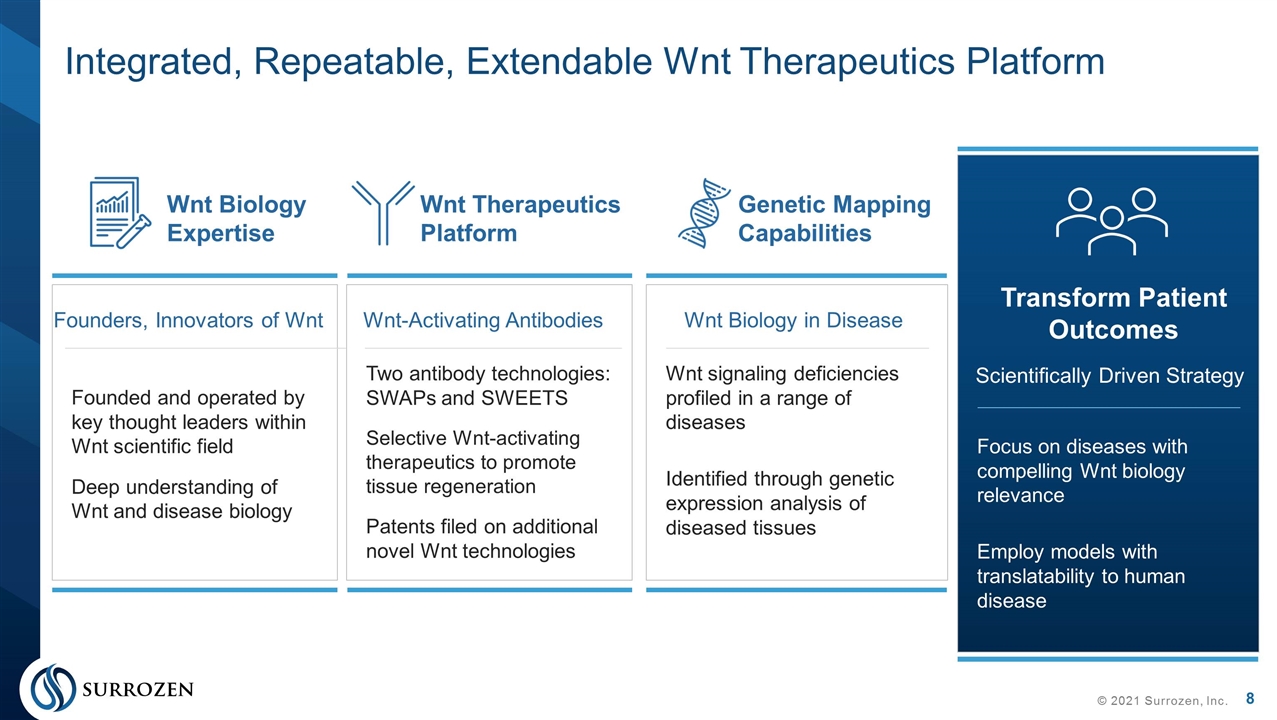
Founded and operated by key thought leaders within Wnt scientific field Deep understanding of Wnt and disease biology Two antibody technologies: SWAPs and SWEETS Selective Wnt-activating therapeutics to promote tissue regeneration Patents filed on additional novel Wnt technologies Wnt signaling deficiencies profiled in a range of diseases Identified through genetic expression analysis of diseased tissues Integrated, Repeatable, Extendable Wnt Therapeutics Platform © 2021 Surrozen, Inc. Founders, Innovators of Wnt Wnt-Activating Antibodies Wnt Biology in Disease Wnt Biology Expertise Wnt Therapeutics Platform Genetic Mapping Capabilities Focus on diseases with compelling Wnt biology relevance Employ models with translatability to human disease Scientifically Driven Strategy Transform Patient Outcomes
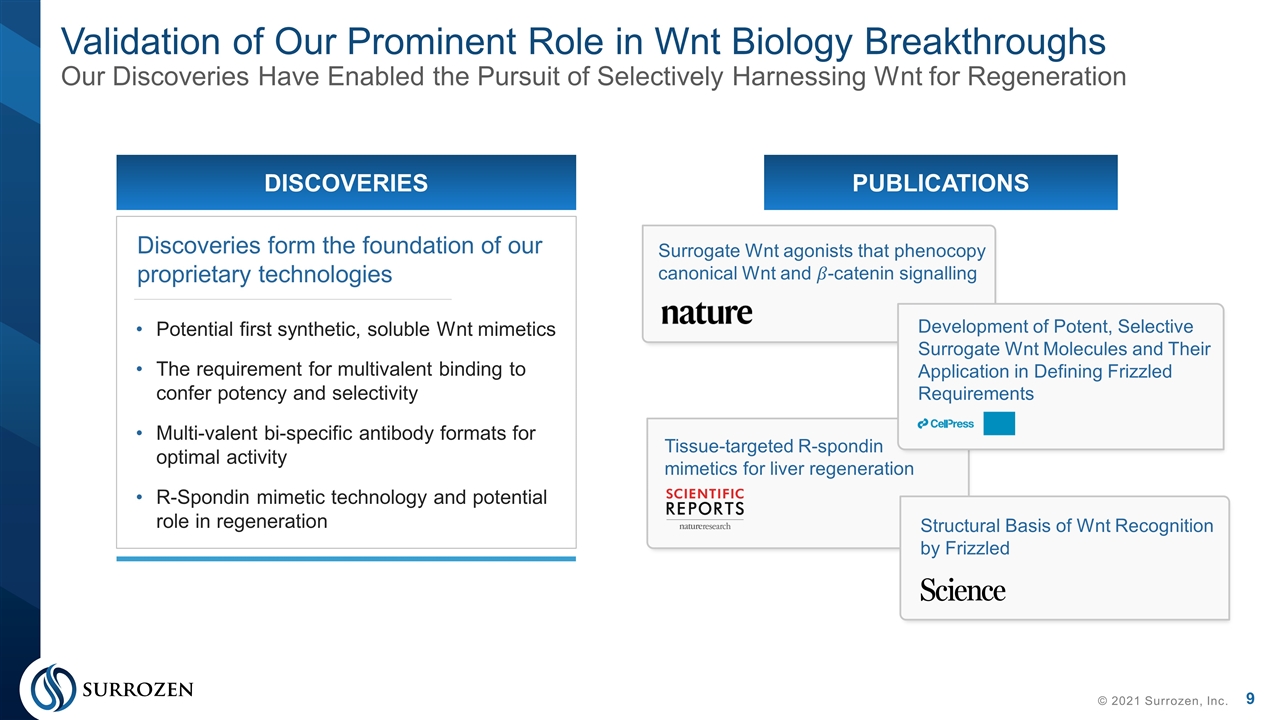
Potential first synthetic, soluble Wnt mimetics The requirement for multivalent binding to confer potency and selectivity Multi-valent bi-specific antibody formats for optimal activity R-Spondin mimetic technology and potential role in regeneration Discoveries form the foundation of our proprietary technologies DISCOVERIES Validation of Our Prominent Role in Wnt Biology Breakthroughs Our Discoveries Have Enabled the Pursuit of Selectively Harnessing Wnt for Regeneration © 2021 Surrozen, Inc. PUBLICATIONS Surrogate Wnt agonists that phenocopy canonical Wnt and ��-catenin signalling Development of Potent, Selective Surrogate Wnt Molecules and Their Application in Defining Frizzled Requirements Tissue-targeted R-spondin mimetics for liver regeneration Structural Basis of Wnt Recognition by Frizzled
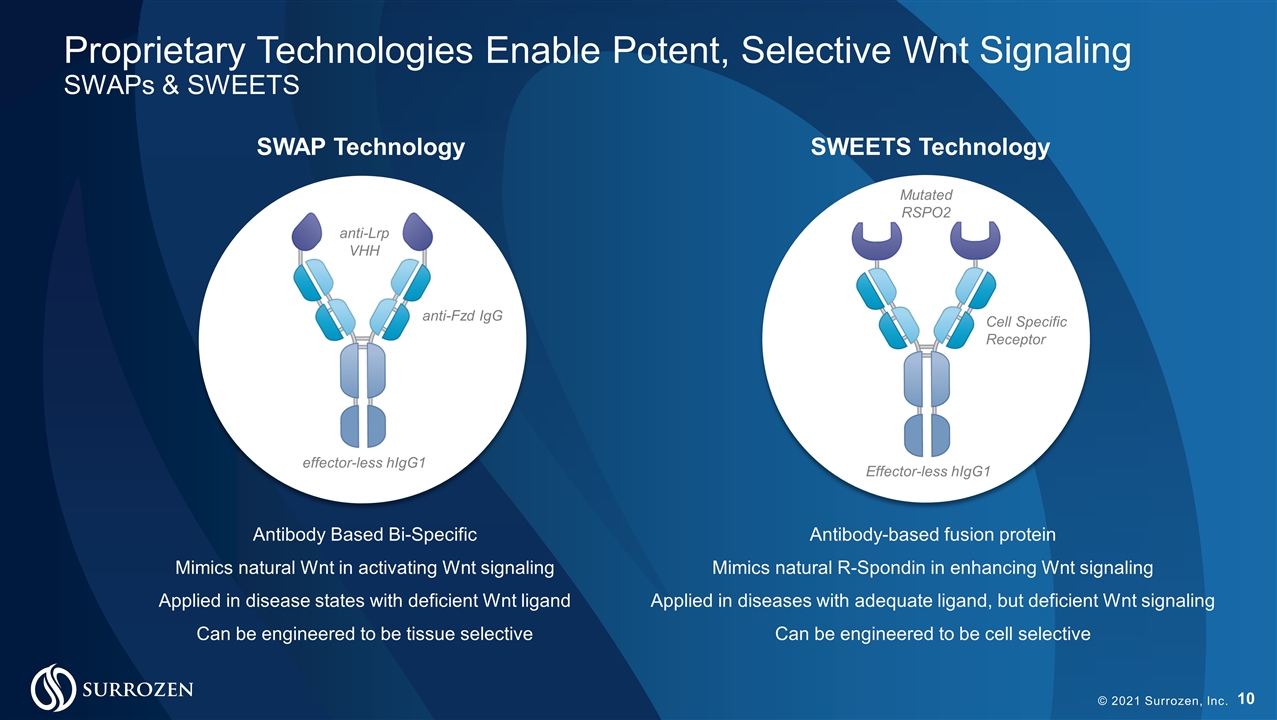
© 2021 Surrozen, Inc. Proprietary Technologies Enable Potent, Selective Wnt Signaling SWAPs & SWEETS SWAP Technology SWEETS Technology anti-Lrp VHH anti-Fzd IgG effector-less hIgG1 Antibody Based Bi-Specific Mimics natural Wnt in activating Wnt signaling Applied in disease states with deficient Wnt ligand Can be engineered to be tissue selective Mutated RSPO2 Cell Specific Receptor Effector-less hIgG1 Antibody-based fusion protein Mimics natural R-Spondin in enhancing Wnt signaling Applied in diseases with adequate ligand, but deficient Wnt signaling Can be engineered to be cell selective
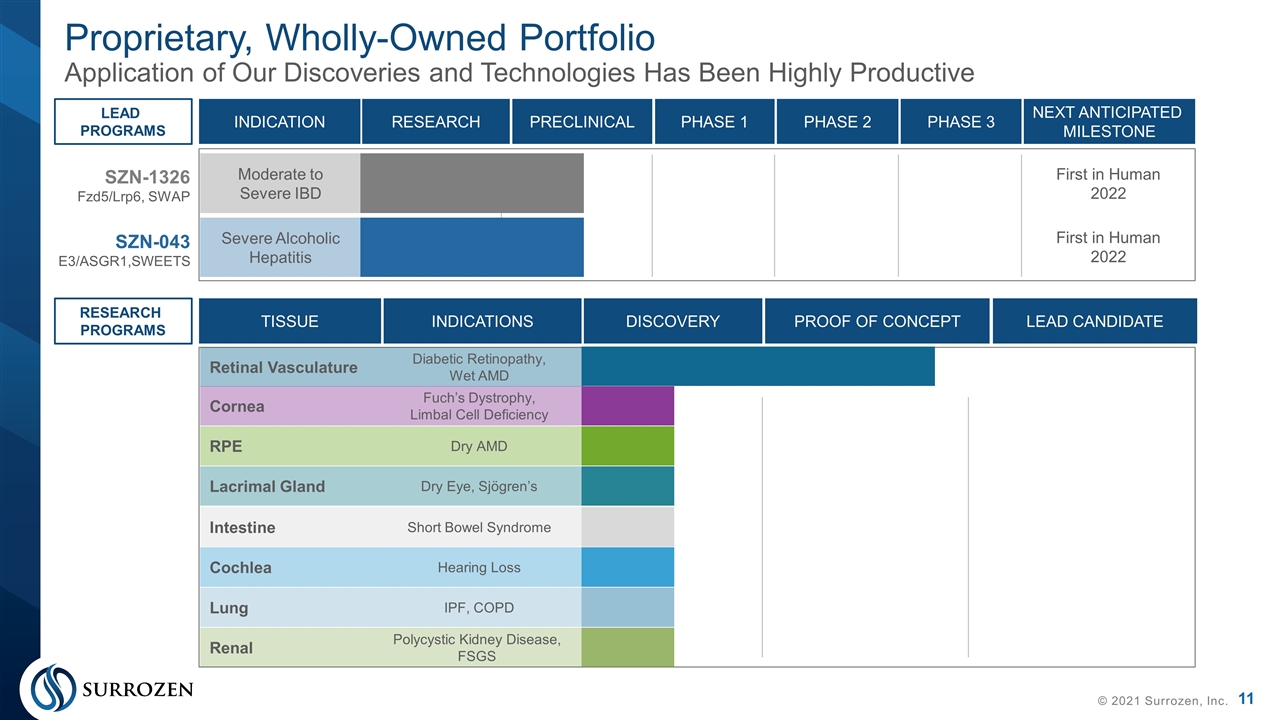
Proprietary, Wholly-Owned Portfolio Application of Our Discoveries and Technologies Has Been Highly Productive © 2021 Surrozen, Inc. Moderate to Severe IBD SZN-1326 Fzd5/Lrp6, SWAP SZN-043 E3/ASGR1,SWEETS First in Human 2022 Severe Alcoholic Hepatitis First in Human 2022 Retinal Vasculature Diabetic Retinopathy, Wet AMD INDICATION RESEARCH PRECLINICAL PHASE 1 NEXT ANTICIPATED MILESTONE PHASE 2 PHASE 3 LEAD PROGRAMS TISSUE INDICATIONS DISCOVERY LEAD CANDIDATE PROOF OF CONCEPT RESEARCH PROGRAMS Cornea Fuch’s Dystrophy, Limbal Cell Deficiency RPE Dry AMD Lacrimal Gland Dry Eye, Sjögren’s Lung IPF, COPD Renal Polycystic Kidney Disease, FSGS Intestine Short Bowel Syndrome Cochlea Hearing Loss
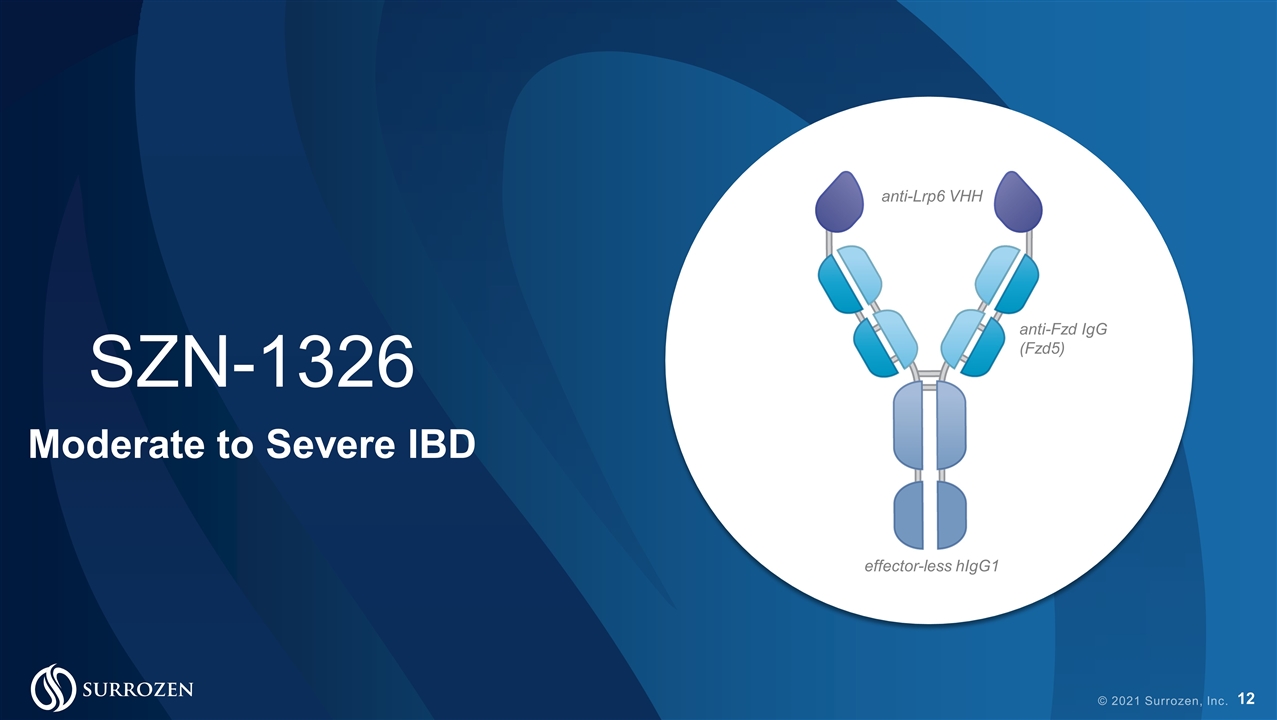
© 2021 Surrozen, Inc. anti-Lrp6 VHH anti-Fzd IgG (Fzd5) effector-less hIgG1 SZN-1326 Moderate to Severe IBD
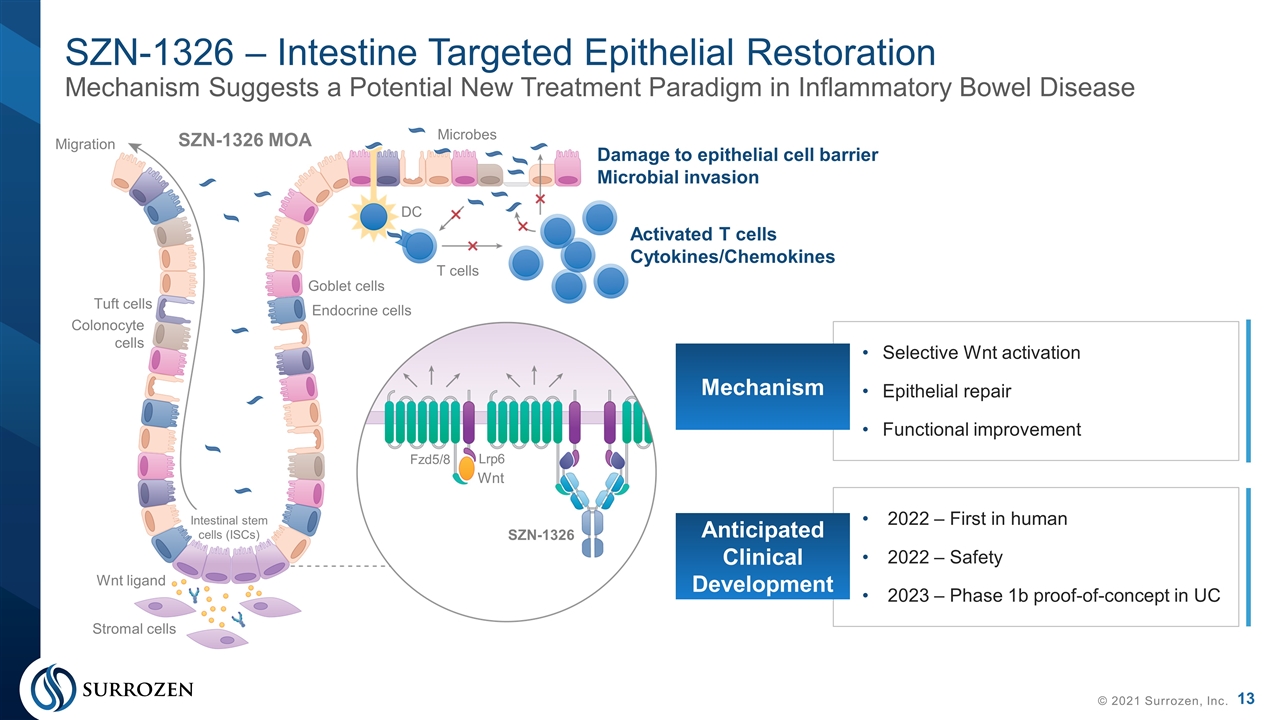
SZN-1326 – Intestine Targeted Epithelial Restoration Mechanism Suggests a Potential New Treatment Paradigm in Inflammatory Bowel Disease © 2021 Surrozen, Inc. Selective Wnt activation Epithelial repair Functional improvement Mechanism 2022 – First in human 2022 – Safety 2023 – Phase 1b proof-of-concept in UC Anticipated Clinical Development Migration Goblet cells Endocrine cells Tuft cells Colonocyte cells Stromal cells Wnt ligand Intestinal stem cells (ISCs) Fzd5/8 Lrp6 Wnt SZN-1326 SZN-1326 MOA DC T cells Microbes Damage to epithelial cell barrier Microbial invasion Activated T cells Cytokines/Chemokines
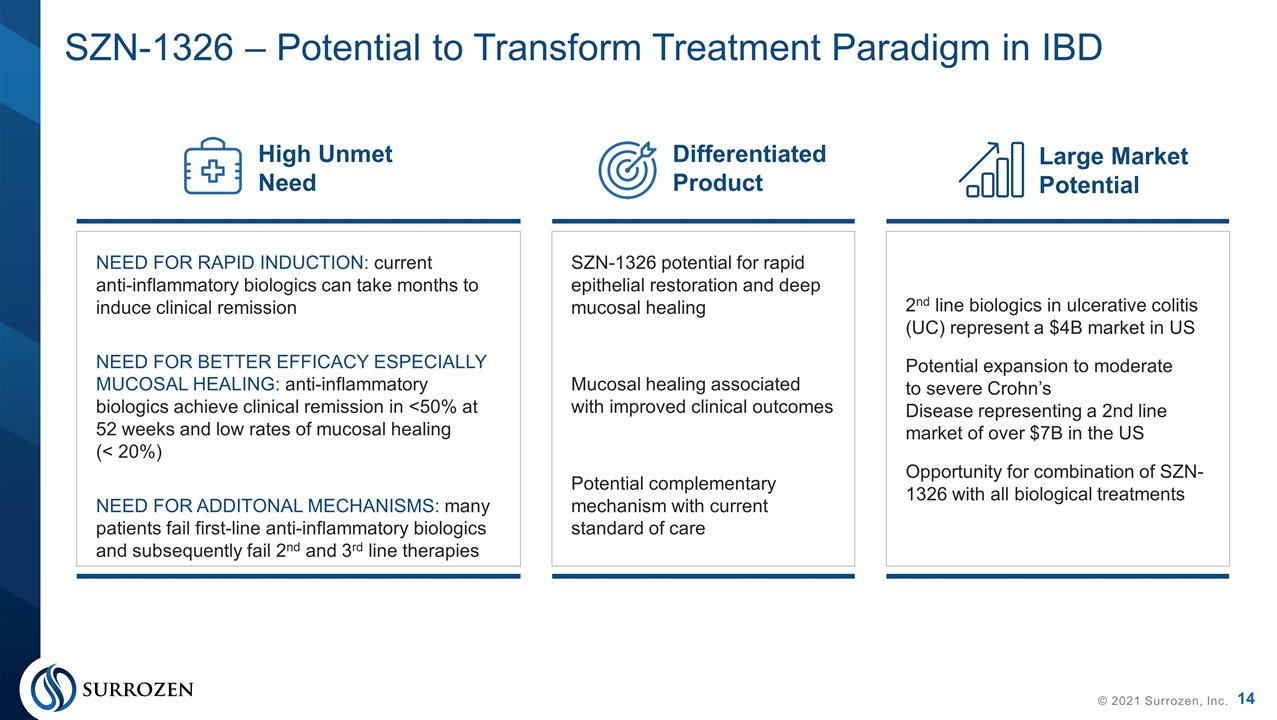
SZN-1326 – Potential to Transform Treatment Paradigm in IBD © 2021 Surrozen, Inc. NEED FOR RAPID INDUCTION: current anti-inflammatory biologics can take months to induce clinical remission NEED FOR BETTER EFFICACY ESPECIALLY MUCOSAL HEALING: anti-inflammatory biologics achieve clinical remission in <50% at 52 weeks and low rates of mucosal healing (< 20%) NEED FOR ADDITONAL MECHANISMS: many patients fail first-line anti-inflammatory biologics and subsequently fail 2nd and 3rd line therapies SZN-1326 potential for rapid epithelial restoration and deep mucosal healing Mucosal healing associated with improved clinical outcomes Potential complementary mechanism with current standard of care 2nd line biologics in ulcerative colitis (UC) represent a $4B market in US Potential expansion to moderate to severe Crohn’s Disease representing a 2nd line market of over $7B in the US Opportunity for combination of SZN-1326 with all biological treatments High Unmet Need Differentiated Product Large Market Potential
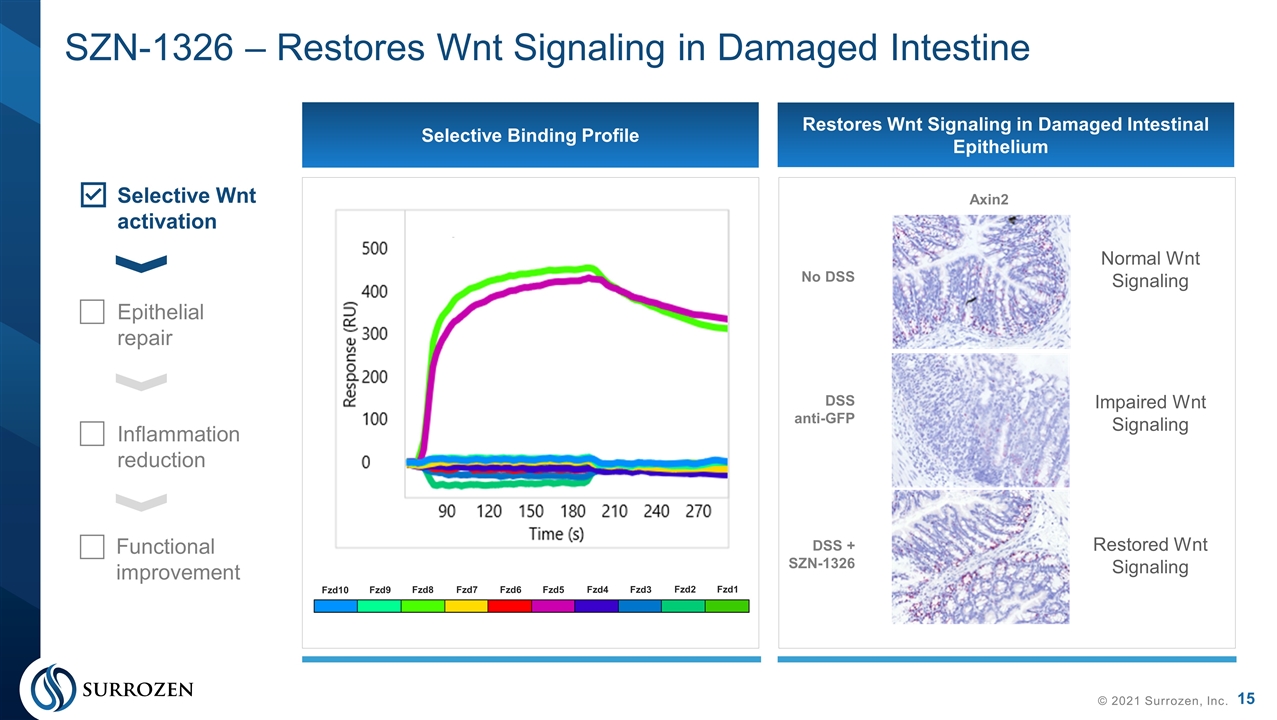
SZN-1326 – Restores Wnt Signaling in Damaged Intestine © 2021 Surrozen, Inc. No DSS DSS anti-GFP DSS + SZN-1326 Axin2 Selective Binding Profile Restores Wnt Signaling in Damaged Intestinal Epithelium Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Fzd10 Fzd9 Fzd8 Fzd7 Fzd6 Fzd5 Fzd4 Fzd3 Fzd2 Fzd1 Normal Wnt Signaling Impaired Wnt Signaling Restored Wnt Signaling
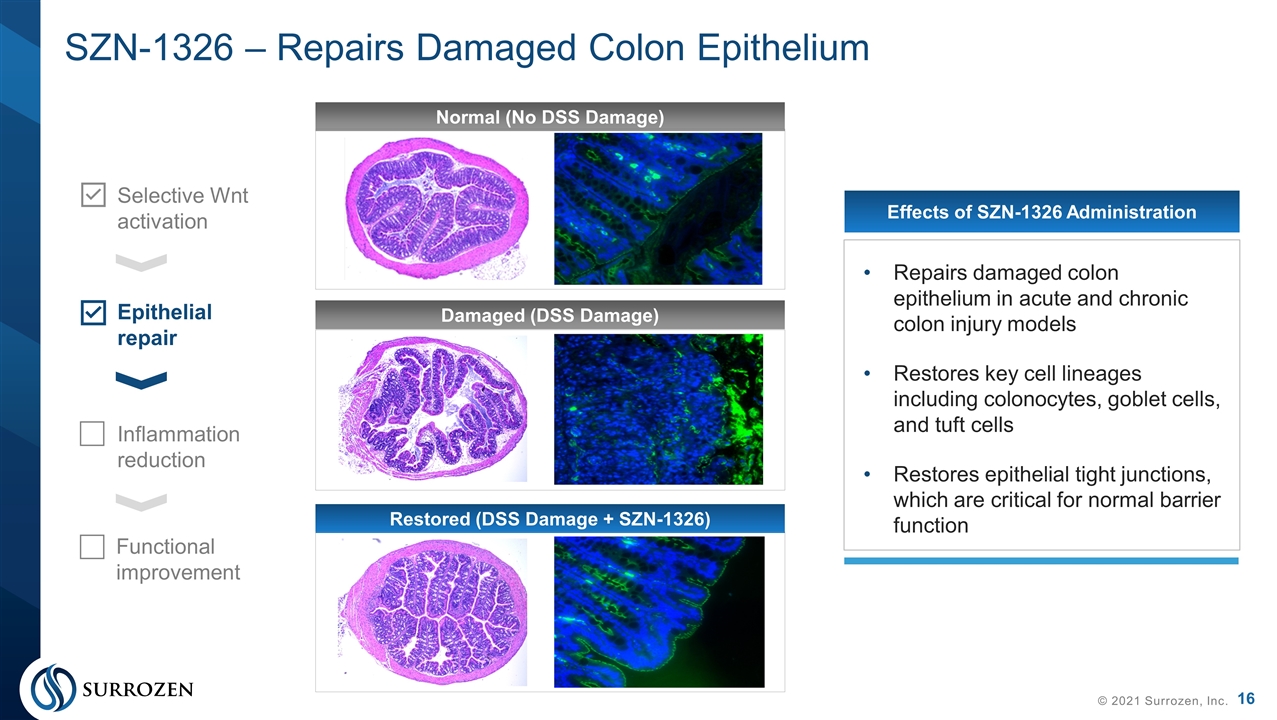
SZN-1326 – Repairs Damaged Colon Epithelium © 2021 Surrozen, Inc. Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Effects of SZN-1326 Administration Repairs damaged colon epithelium in acute and chronic colon injury models Restores key cell lineages including colonocytes, goblet cells, and tuft cells Restores epithelial tight junctions, which are critical for normal barrier function Normal (No DSS Damage) Damaged (DSS Damage) Restored (DSS Damage + SZN-1326)
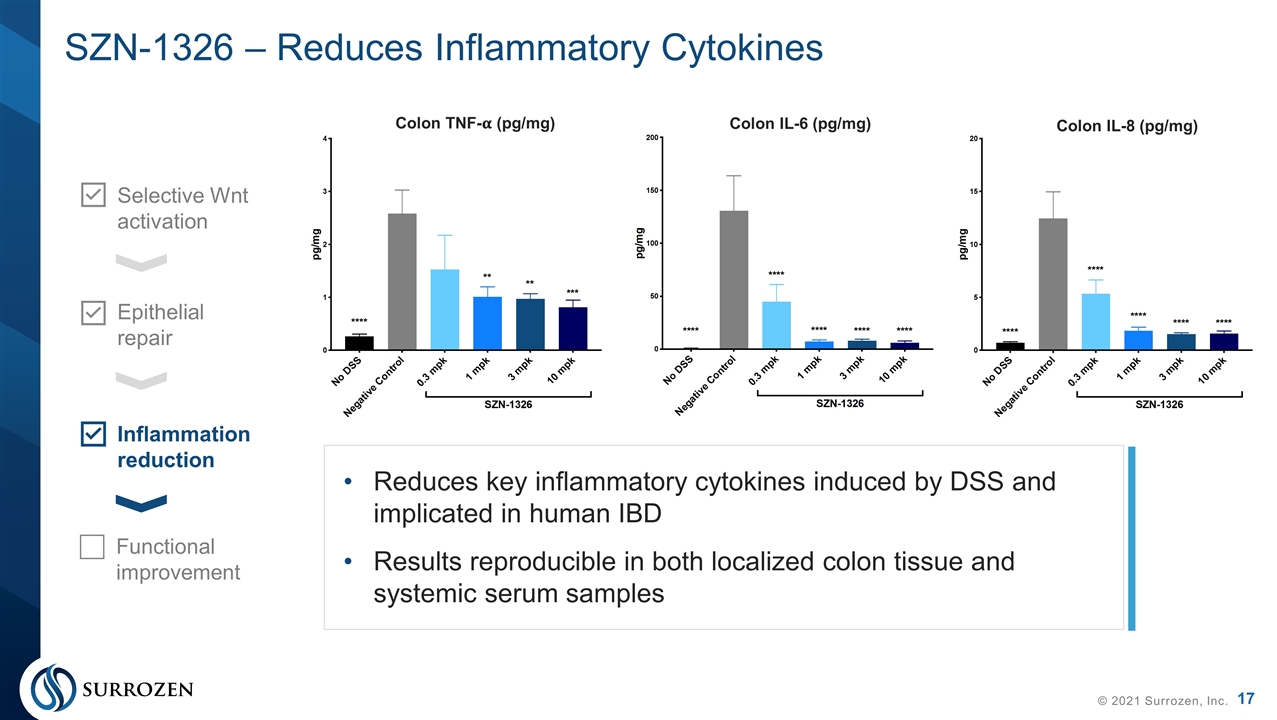
SZN-1326 – Reduces Inflammatory Cytokines © 2021 Surrozen, Inc. Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Reduces key inflammatory cytokines induced by DSS and implicated in human IBD Results reproducible in both localized colon tissue and systemic serum samples Colon TNF-�� (pg/mg) Colon IL-6 (pg/mg) Colon IL-8 (pg/mg)
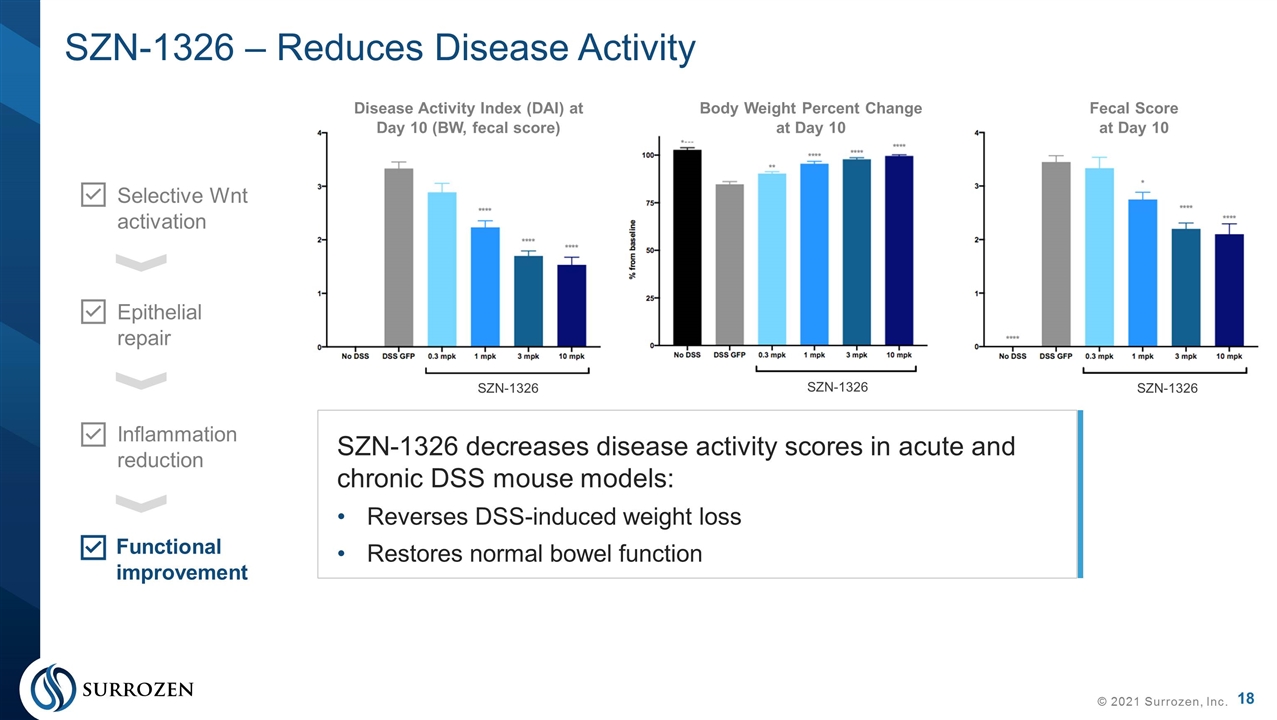
SZN-1326 – Reduces Disease Activity © 2021 Surrozen, Inc. Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Disease Activity Index (DAI) at Day 10 (BW, fecal score) Body Weight Percent Change at Day 10 Fecal Score at Day 10 SZN-1326 SZN-1326 SZN-1326 SZN-1326 decreases disease activity scores in acute and chronic DSS mouse models: Reverses DSS-induced weight loss Restores normal bowel function
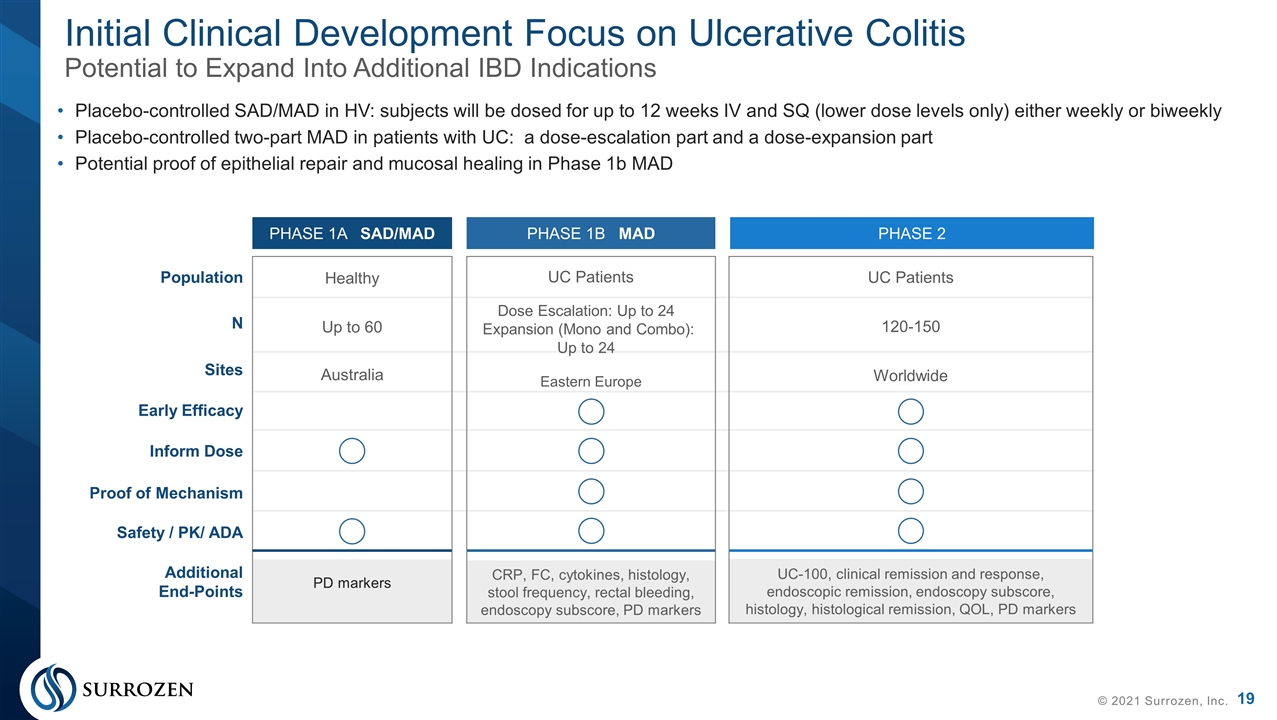
Healthy Up to 60 Australia PD markers Initial Clinical Development Focus on Ulcerative Colitis Potential to Expand Into Additional IBD Indications © 2021 Surrozen, Inc. Placebo-controlled SAD/MAD in HV: subjects will be dosed for up to 12 weeks IV and SQ (lower dose levels only) either weekly or biweekly Placebo-controlled two-part MAD in patients with UC: a dose-escalation part and a dose-expansion part Potential proof of epithelial repair and mucosal healing in Phase 1b MAD PHASE 1A SAD/MAD PHASE 1B MAD PHASE 2 UC Patients Dose Escalation: Up to 24 Expansion (Mono and Combo): Up to 24 Eastern Europe CRP, FC, cytokines, histology, stool frequency, rectal bleeding, endoscopy subscore, PD markers UC Patients 120-150 Worldwide UC-100, clinical remission and response, endoscopic remission, endoscopy subscore, histology, histological remission, QOL, PD markers Population N Sites Early Efficacy Inform Dose Proof of Mechanism Safety / PK/ ADA Additional End-Points
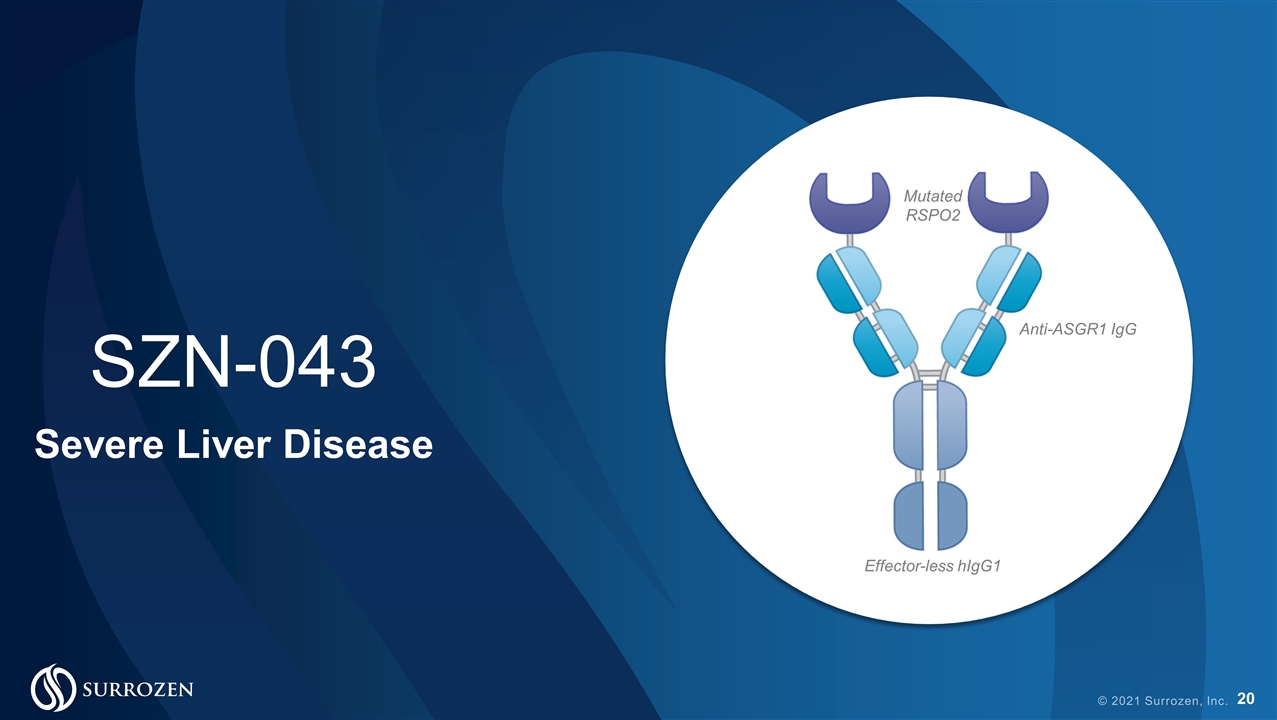
© 2021 Surrozen, Inc. Mutated RSPO2 Anti-ASGR1 IgG Effector-less hIgG1 SZN-043 Severe Liver Disease
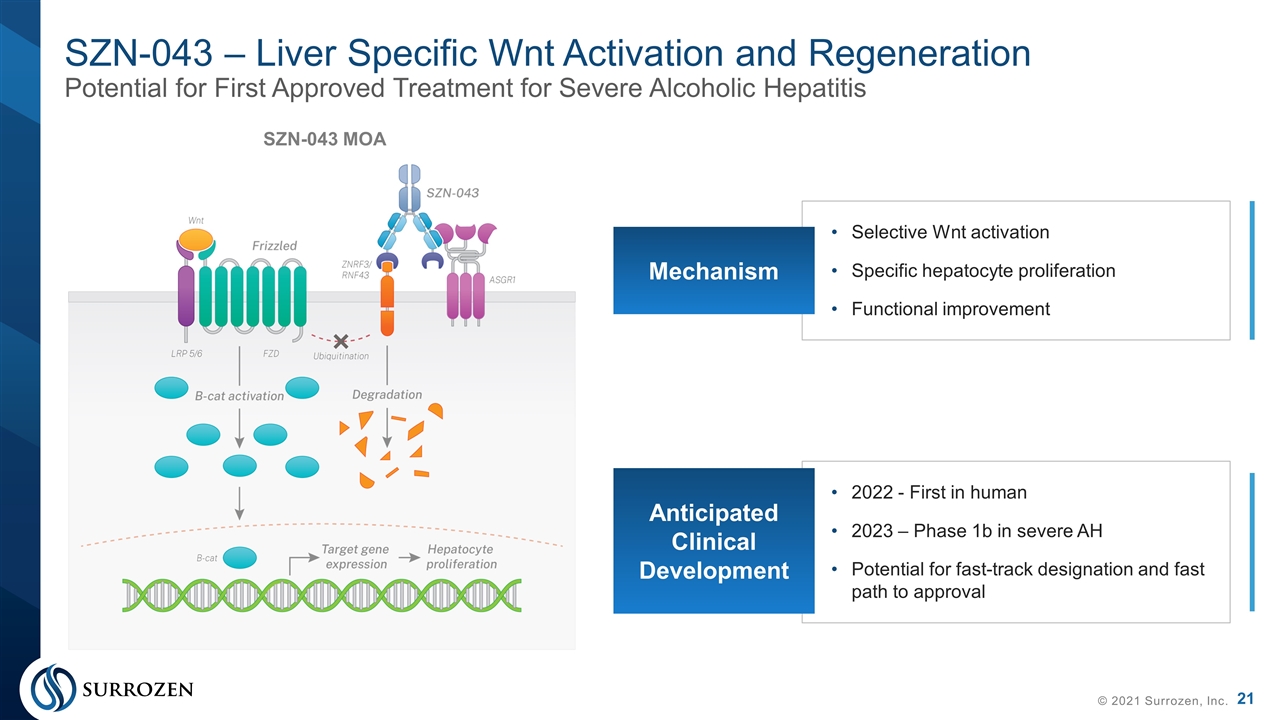
SZN-043 – Liver Specific Wnt Activation and Regeneration Potential for First Approved Treatment for Severe Alcoholic Hepatitis © 2021 Surrozen, Inc. Selective Wnt activation Specific hepatocyte proliferation Functional improvement Mechanism 2022 - First in human 2023 – Phase 1b in severe AH Potential for fast-track designation and fast path to approval Anticipated Clinical Development SZN-043 MOA
SZN-043 – Potential to Significantly Improve Patient Outcomes in Severe Alcoholic Hepatitis © 2021 Surrozen, Inc. NO APPROVED DRUGS: SOC: steroids HIGH MORTALITY: 90-day mortality of 30% due to hepatocyte loss and impaired regeneration leading to liver and organ failure HEPATOCYTE REGENERATION INCREASES SURVIVAL LIVER TRANSPLANTS DENIED: Liver transplants available only in certain centers, dearth of livers, costly, denied due to alcoholism Estimated 100,000 U.S. hospitalizations due to severe AH in 2021 annually; growing with alcohol use Potential for expansion to other severe liver diseases: acute liver failure, end-stage liver disease SZN-043 directly addresses the underlying pathophysiology of severe AH SZN-043 potential for rapid hepatocyte regeneration with short-term IV dosing Rapid induction of hepatocyte proliferation and improved hepatic function in acute and chronic models of hepatocyte destruction and fibrosis Received $3M NIH grant High Unmet Need Differentiated Product Large Market Potential
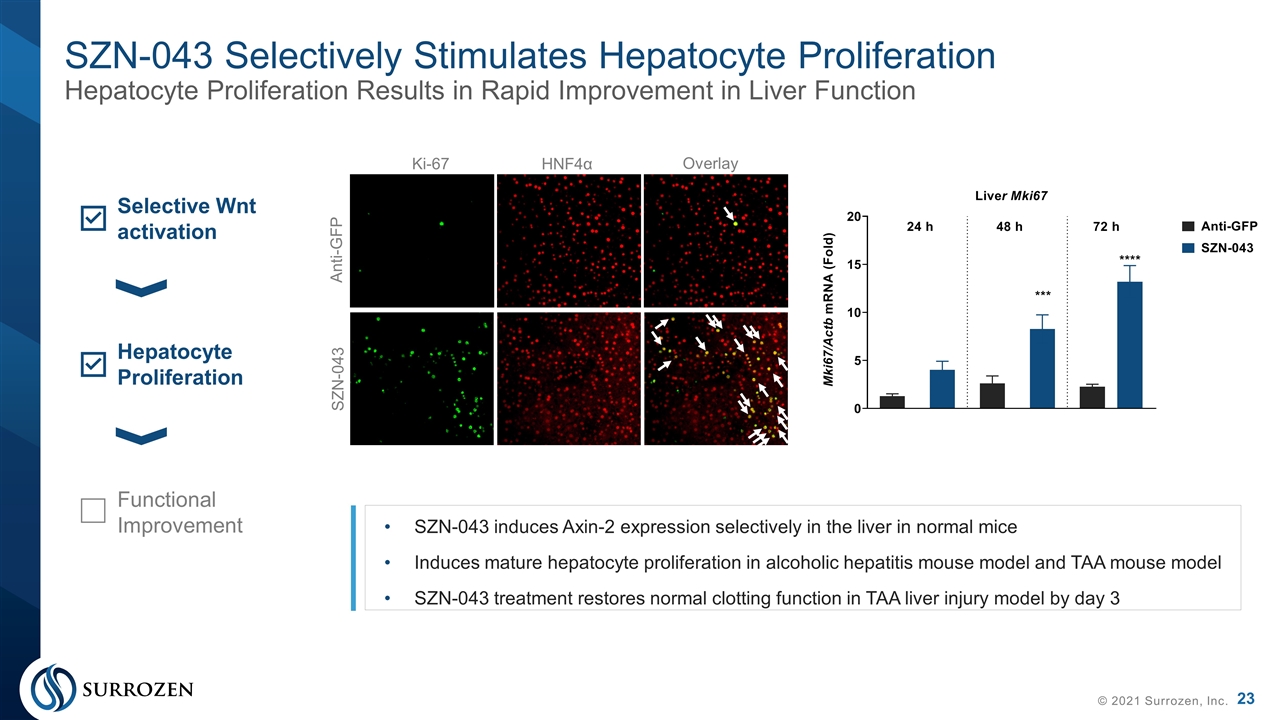
SZN-043 Selectively Stimulates Hepatocyte Proliferation Hepatocyte Proliferation Results in Rapid Improvement in Liver Function © 2021 Surrozen, Inc. Anti-GFP SZN-043 Ki-67 HNF4α Overlay Selective Wnt activation Hepatocyte Proliferation SZN-043 induces Axin-2 expression selectively in the liver in normal mice Induces mature hepatocyte proliferation in alcoholic hepatitis mouse model and TAA mouse model SZN-043 treatment restores normal clotting function in TAA liver injury model by day 3 Functional Improvement
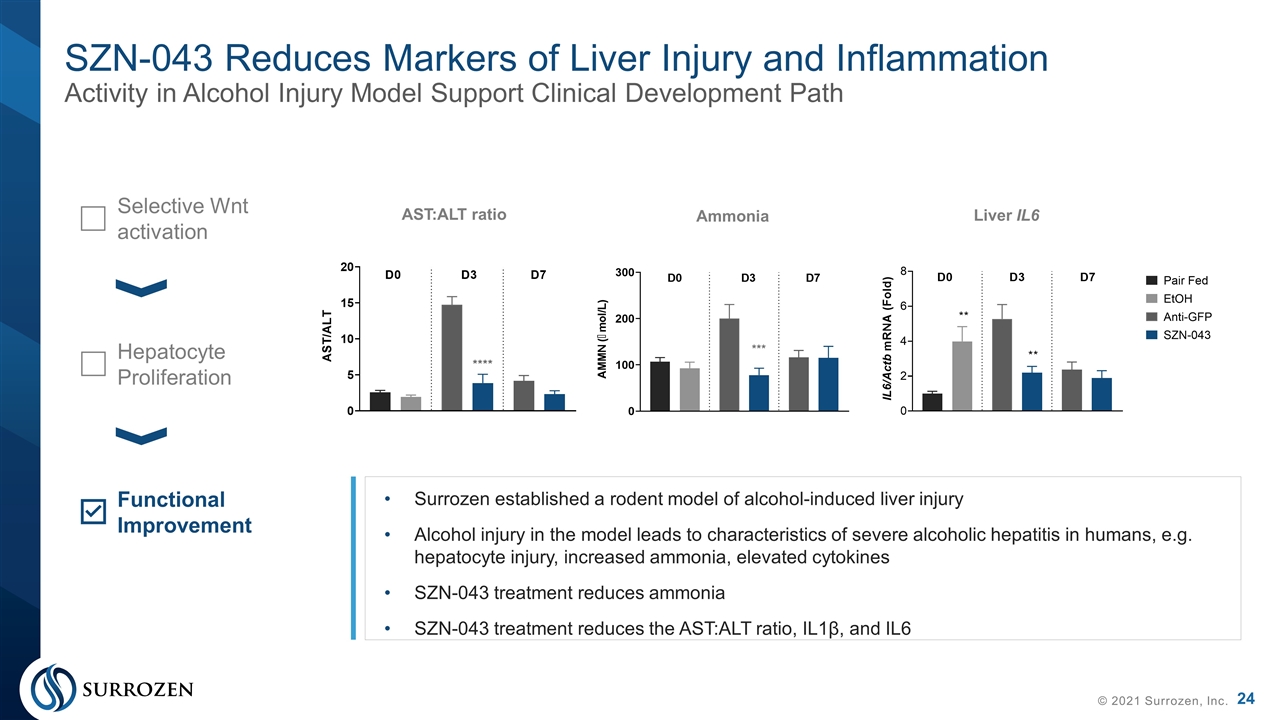
SZN-043 Reduces Markers of Liver Injury and Inflammation Activity in Alcohol Injury Model Support Clinical Development Path © 2021 Surrozen, Inc. AST:ALT ratio Ammonia Liver IL6 Selective Wnt activation Hepatocyte Proliferation Functional Improvement Surrozen established a rodent model of alcohol-induced liver injury Alcohol injury in the model leads to characteristics of severe alcoholic hepatitis in humans, e.g. hepatocyte injury, increased ammonia, elevated cytokines SZN-043 treatment reduces ammonia SZN-043 treatment reduces the AST:ALT ratio, IL1β, and IL6
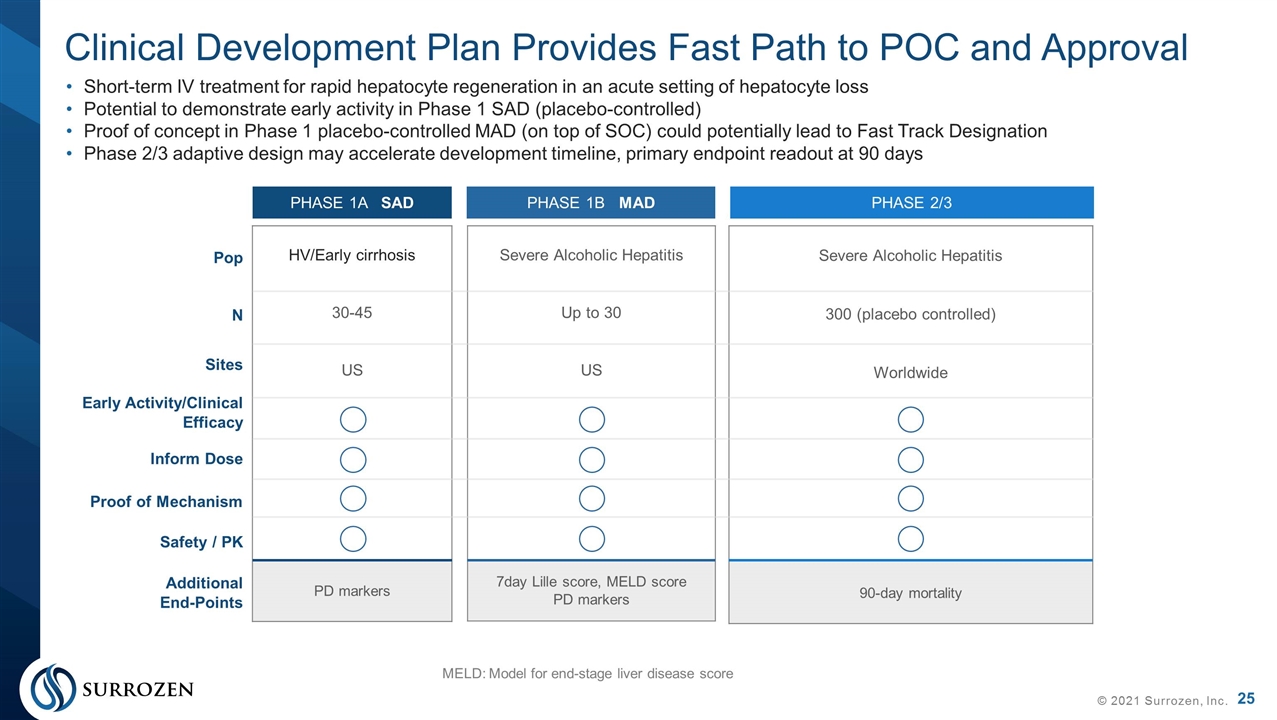
HV/Early cirrhosis 30-45 US PD markers Severe Alcoholic Hepatitis Up to 30 US 7day Lille score, MELD score PD markers Clinical Development Plan Provides Fast Path to POC and Approval © 2021 Surrozen, Inc. Short-term IV treatment for rapid hepatocyte regeneration in an acute setting of hepatocyte loss Potential to demonstrate early activity in Phase 1 SAD (placebo-controlled) Proof of concept in Phase 1 placebo-controlled MAD (on top of SOC) could potentially lead to Fast Track Designation Phase 2/3 adaptive design may accelerate development timeline, primary endpoint readout at 90 days MELD: Model for end-stage liver disease score PHASE 1A SAD PHASE 1B MAD PHASE 2/3 Severe Alcoholic Hepatitis 300 (placebo controlled) Worldwide 90-day mortality Pop N Sites Early Activity/Clinical Efficacy Inform Dose Proof of Mechanism Safety / PK Additional End-Points
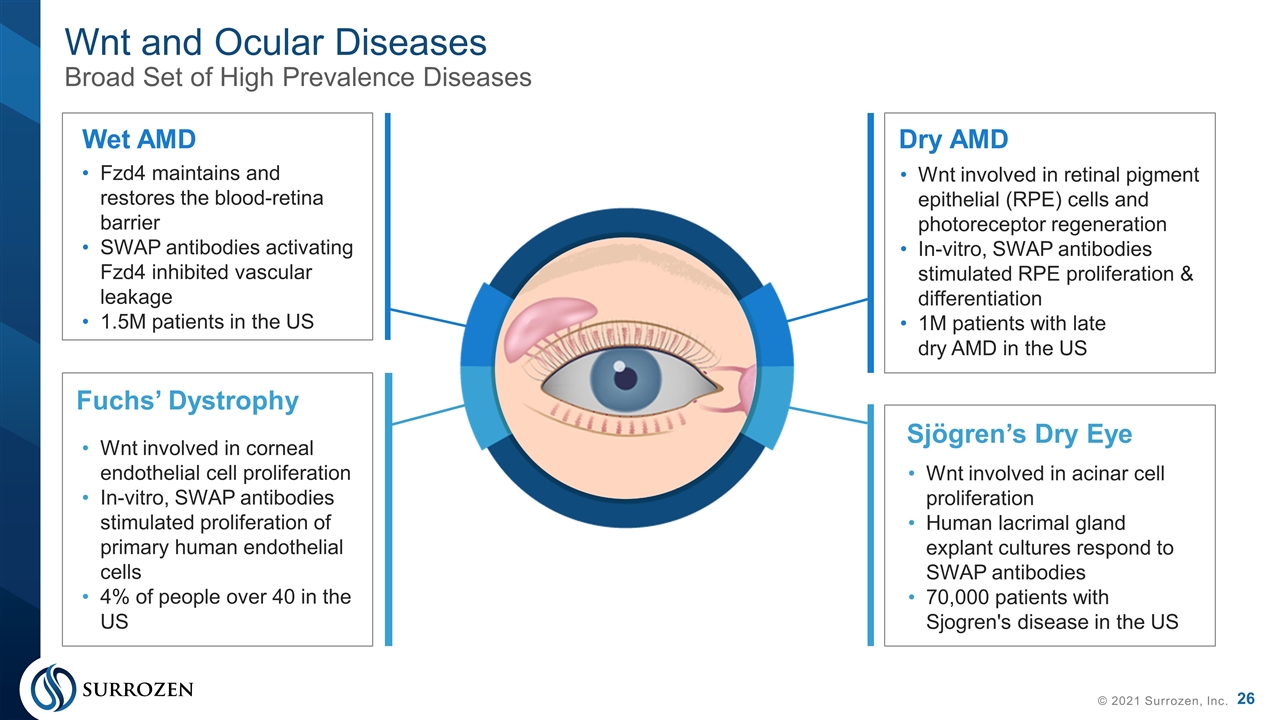
Wnt and Ocular Diseases Broad Set of High Prevalence Diseases © 2021 Surrozen, Inc. Fzd4 maintains and restores the blood-retina barrier SWAP antibodies activating Fzd4 inhibited vascular leakage 1.5M patients in the US Wnt involved in retinal pigment epithelial (RPE) cells and photoreceptor regeneration In-vitro, SWAP antibodies stimulated RPE proliferation & differentiation 1M patients with late dry AMD in the US Wnt involved in acinar cell proliferation Human lacrimal gland explant cultures respond to SWAP antibodies 70,000 patients with Sjogren's disease in the US Wnt involved in corneal endothelial cell proliferation In-vitro, SWAP antibodies stimulated proliferation of primary human endothelial cells 4% of people over 40 in the US Dry AMD Sjögren’s Dry Eye Wet AMD Fuchs’ Dystrophy
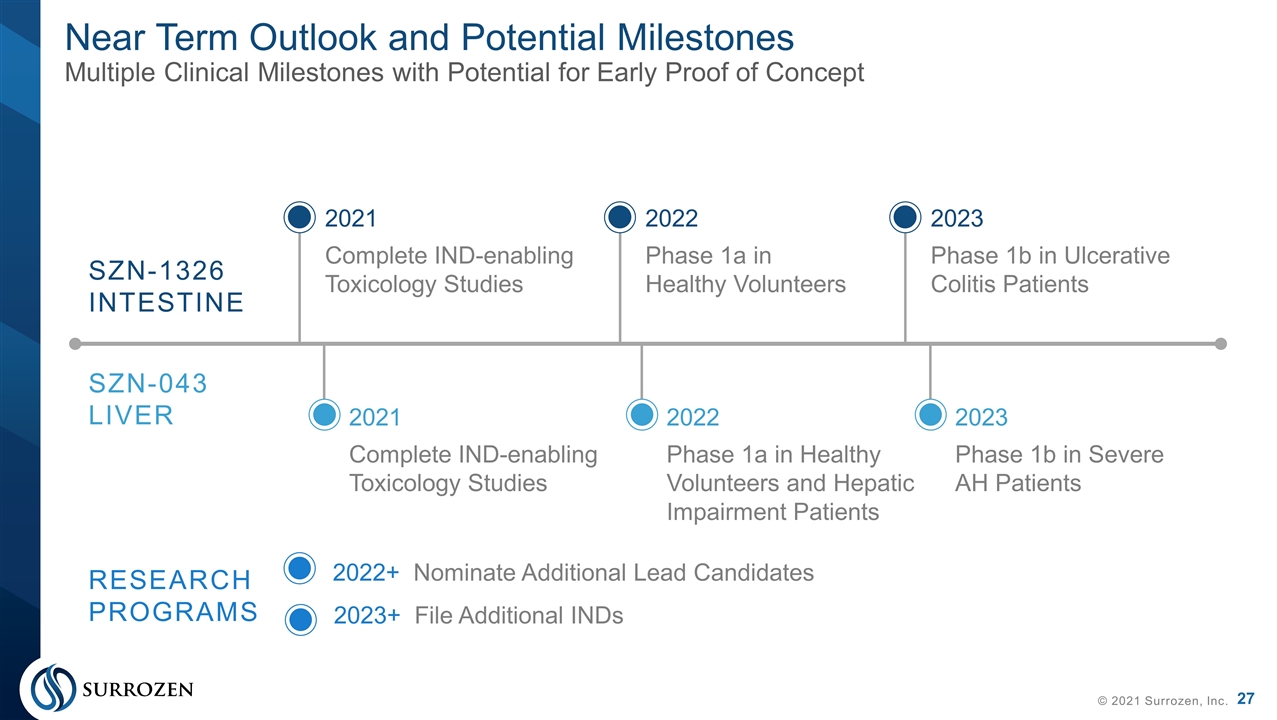
© 2021 Surrozen, Inc. SZN-1326 INTESTINE SZN-043 LIVER RESEARCH PROGRAMS 2021 Complete IND-enabling Toxicology Studies 2021 Complete IND-enabling Toxicology Studies 2022 Phase 1a in Healthy Volunteers 2023 Phase 1b in Ulcerative Colitis Patients 2022 Phase 1a in Healthy Volunteers and Hepatic Impairment Patients 2023 Phase 1b in Severe AH Patients 2022+ Nominate Additional Lead Candidates Near Term Outlook and Potential Milestones Multiple Clinical Milestones with Potential for Early Proof of Concept 2023+ File Additional INDs
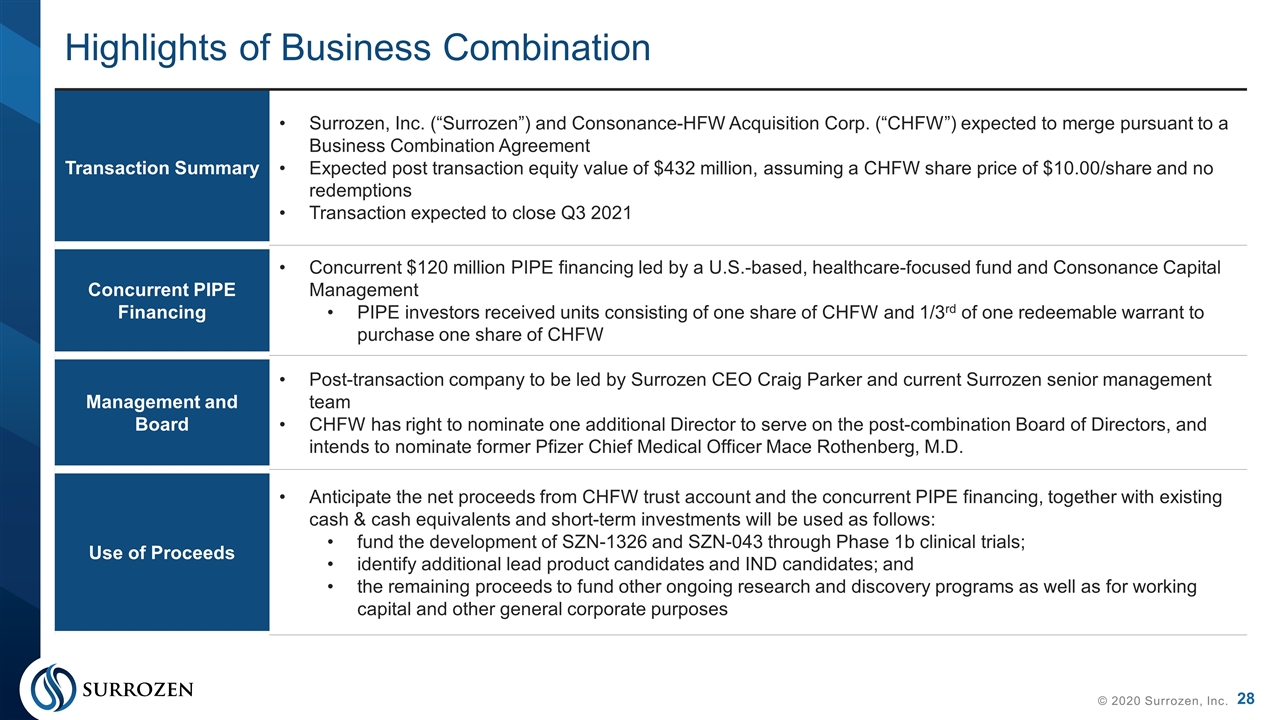
Highlights of Business Combination © 2020 Surrozen, Inc. Transaction Summary Surrozen, Inc. (“Surrozen”) and Consonance-HFW Acquisition Corp. (“CHFW”) expected to merge pursuant to a Business Combination Agreement Expected post transaction equity value of $432 million, assuming a CHFW share price of $10.00/share and no redemptions Transaction expected to close Q3 2021 Concurrent PIPE Financing Concurrent $120 million PIPE financing led by a U.S.-based, healthcare-focused fund and Consonance Capital Management PIPE investors received units consisting of one share of CHFW and 1/3rd of one redeemable warrant to purchase one share of CHFW Management and Board Post-transaction company to be led by Surrozen CEO Craig Parker and current Surrozen senior management team CHFW has right to nominate one additional Director to serve on the post-combination Board of Directors, and intends to nominate former Pfizer Chief Medical Officer Mace Rothenberg, M.D. Use of Proceeds Anticipate the net proceeds from CHFW trust account and the concurrent PIPE financing, together with existing cash & cash equivalents and short-term investments will be used as follows: fund the development of SZN-1326 and SZN-043 through Phase 1b clinical trials; identify additional lead product candidates and IND candidates; and the remaining proceeds to fund other ongoing research and discovery programs as well as for working capital and other general corporate purposes
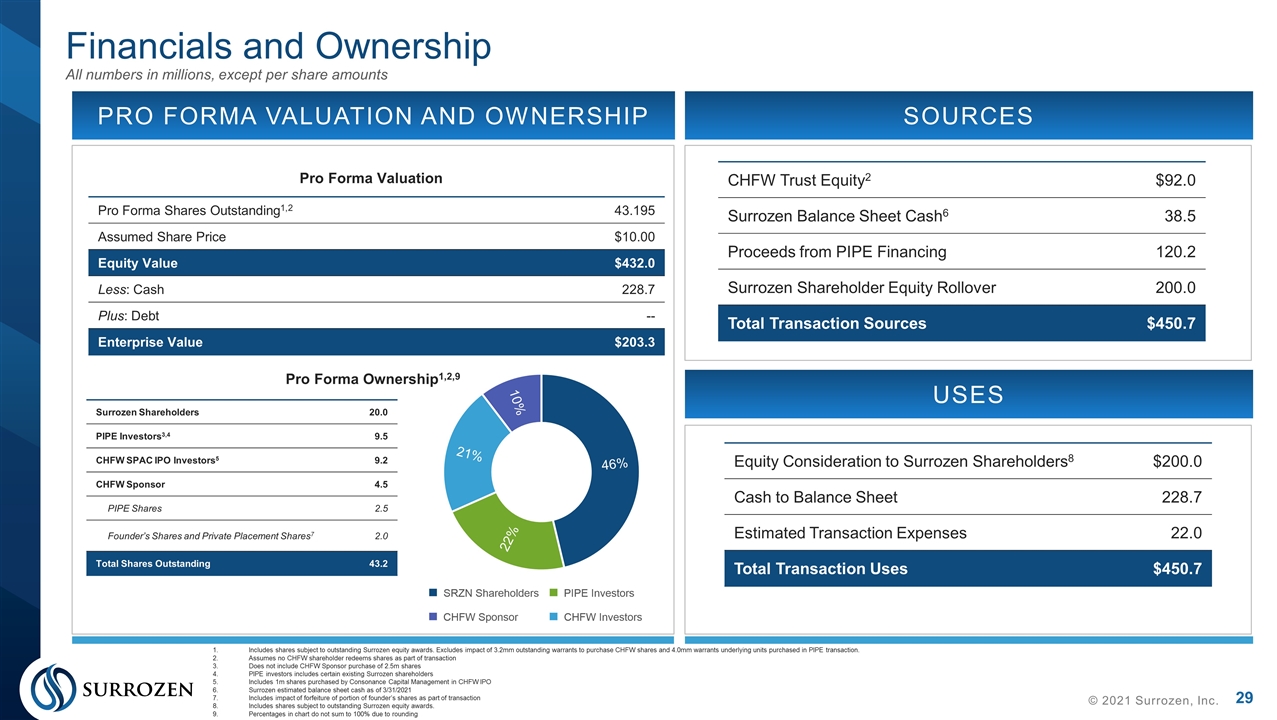
© 2021 Surrozen, Inc. PRO FORMA VALUATION AND OWNERSHIP SOURCES USES CHFW Trust Equity2 $92.0 Surrozen Balance Sheet Cash6 38.5 Proceeds from PIPE Financing 120.2 Surrozen Shareholder Equity Rollover 200.0 Total Transaction Sources $450.7 Includes shares subject to outstanding Surrozen equity awards. Excludes impact of 3.2mm outstanding warrants to purchase CHFW shares and 4.0mm warrants underlying units purchased in PIPE transaction. Assumes no CHFW shareholder redeems shares as part of transaction Does not include CHFW Sponsor purchase of 2.5m shares PIPE investors includes certain existing Surrozen shareholders Includes 1m shares purchased by Consonance Capital Management in CHFW IPO Surrozen estimated balance sheet cash as of 3/31/2021 Includes impact of forfeiture of portion of founder’s shares as part of transaction Includes shares subject to outstanding Surrozen equity awards. Percentages in chart do not sum to 100% due to rounding Equity Consideration to Surrozen Shareholders8 $200.0 Cash to Balance Sheet 228.7 Estimated Transaction Expenses 22.0 Total Transaction Uses $450.7 Pro Forma Shares Outstanding1,2 43.195 Assumed Share Price $10.00 Equity Value $432.0 Less: Cash 228.7 Plus: Debt -- Enterprise Value $203.3 Pro Forma Valuation Pro Forma Ownership1,2,9 Surrozen Shareholders 20.0 PIPE Investors3,4 9.5 CHFW SPAC IPO Investors5 9.2 CHFW Sponsor 4.5 PIPE Shares 2.5 Founder’s Shares and Private Placement Shares7 2.0 Total Shares Outstanding 43.2 Financials and Ownership All numbers in millions, except per share amounts
Potential First-in-Class Pioneers in discovering and developing therapeutics that selectively activate the Wnt signaling pathway Highlights © 2021 Surrozen, Inc. Potential for Establishing a New Treatment Paradigm in a Broad Spectrum of Therapeutic Areas Tissue selective regeneration for GI tract, liver, retina, cornea, kidney, lung, and pancreas Two Proprietary Platforms Broad libraries of receptor specific antibodies enable rapid deployment of disease specific candidates Preclinical Proof of Concept Established Cell proliferation, tissue regeneration and functional improvement demonstrated in animal models of multiple diseases Two High-Value Programs Moving Toward the Clinic Inflammatory Bowel Disease (SZN-1326: FIH 2022) and Severe Alcoholic Hepatitis (SZN-043: FIH 2022) Capital Efficient Clinical Development Strategy Both development programs have the potential to provide clinical proof of concept in Phase 1b

The Wnt Company - Powering Regeneration 2021 © 2021 Surrozen, Inc.






























