
From Serendipity to Rational Design Taking Molecular Glue Degraders to New Heights | October 2023

Forward-Looking Statements These materials include express and implied “forward-looking statements,” including forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward looking statements include all statements that are not historical facts, and in some cases, can be identified by terms such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained in herein include, but are not limited to, statements about our product development activities, including our expectations around the potential of molecular glue degraders (“MGDs”), the potential of our herein detailed pipeline of MGDs, our expectations for our collaboration with Roche, including the discovery and development of MGDs therefrom, and our expectations and estimates of potential future payments available under the collaboration, our ongoing pre-clinical and clinical development of our GSPT1 degrader referred to as MRT-2359, including our expectations regarding the potential relevance of certain interim clinical data, our expectations for the nature and timing of any additional clinical data releases for MRT-2359, including any full phase 1 clinical data release, and our expectations for the nature and timing of our ongoing and future clinical development of MRT-2359, including our plan to continue the Phase 1/2 study of MRT-2359 and its anticipated timing and progress, and our ability to enroll the requisite number of patients and dose each dosing cohort in the intended manner, the potential for MRT-2359 to benefit patients living with a variety of difficult-to-treat cancers, our expectations regarding the advancement, and timing thereof, of our pipeline and the various products therein, our ability to advance our development candidates, including MRT-6160 and our expectations for MRT-6160 to enter the clinic in 2024 and its potential applications across multiple autoimmune diseases, our expectations regarding potential therapeutic opportunities for our MGDs, and our clinical development expectations therefor, our expectations regarding patient populations and medical needs for any potential therapeutic opportunities for our MGDs, our expectations regarding our proprietary QuEEN™ platform and its potential to be highly productive and an industry-leading platform for designing MGDs to address hard-to-drug disease targets via degradation, combining experimentation with AI to push the boundaries of what is possible with MGDs, and the strength of our financial position, including our estimates of cash runway, among others. By their nature, these statements are subject to numerous risks and uncertainties, as well as those risks and uncertainties set forth in our Annual Report on Form 10-K for the fourth quarter and full year ended December 31, 2022, filed, with the US Securities and Exchange Commission on March 16, 2023, and any subsequent filings, that could cause actual results, performance or achievement to differ materially and adversely from those anticipated or implied in the statements. You should not rely upon forward looking statements as predictions of future events. Although our management believes that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Recipients are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date such statements are made and should not be construed as statements of fact. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, any future presentations or otherwise, except as required by applicable law. Certain information contained in these materials and any statements made orally during any presentation of these materials that relate to the materials or are based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of these materials, we have not independently verified, and make no representations as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in these materials relating to or based on such internal estimates and research. These materials remain the proprietary intellectual property of Monte Rosa Therapeutics and should not be distributed or reproduced in whole or in part without the prior written consent of Monte Rosa Therapeutics.

Monte Rosa Therapeutics – Call Highlights Updated cash runway into Q3 2025 Interim analysis of data from our Phase I dose escalation study of MRT-2359, providing evidence of optimal PD modulation, clinical activity and a favorable tolerability profile Partnership with Roche to develop MGDs for oncology and neurological conditions – expands platform reach into neuroscience

Monte Rosa Therapeutics – Roche Partnership

Monte Rosa Tx – Roche Partnership: Summary Discovery partnership on targets in oncology and neurological disease Leverages synergies between Monte Rosa Tx – a leading molecular glue degrader company – and Roche – a leading global health care company Monte Rosa Tx performs preclinical drug discovery with Roche leading late preclinical discovery and clinical development Monte Rosa Tx to receive $50M upfront payment and potential preclinical, clinical, regulatory and sales milestones that could exceed $2B* Potential to expand collaboration to additional targets, which triggers potential additional payments including nomination, preclinical, clinical, regulatory and sales milestones Partnership provides additional validation of Monte Rosa Tx’s QuEEN™ discovery engine and its opportunity to go beyond Oncology Monte Rosa Tx’s publicly disclosed pipeline remains unencumbered* * as disclosed in company 8K

GSPT1 Program – Phase I Interim Update
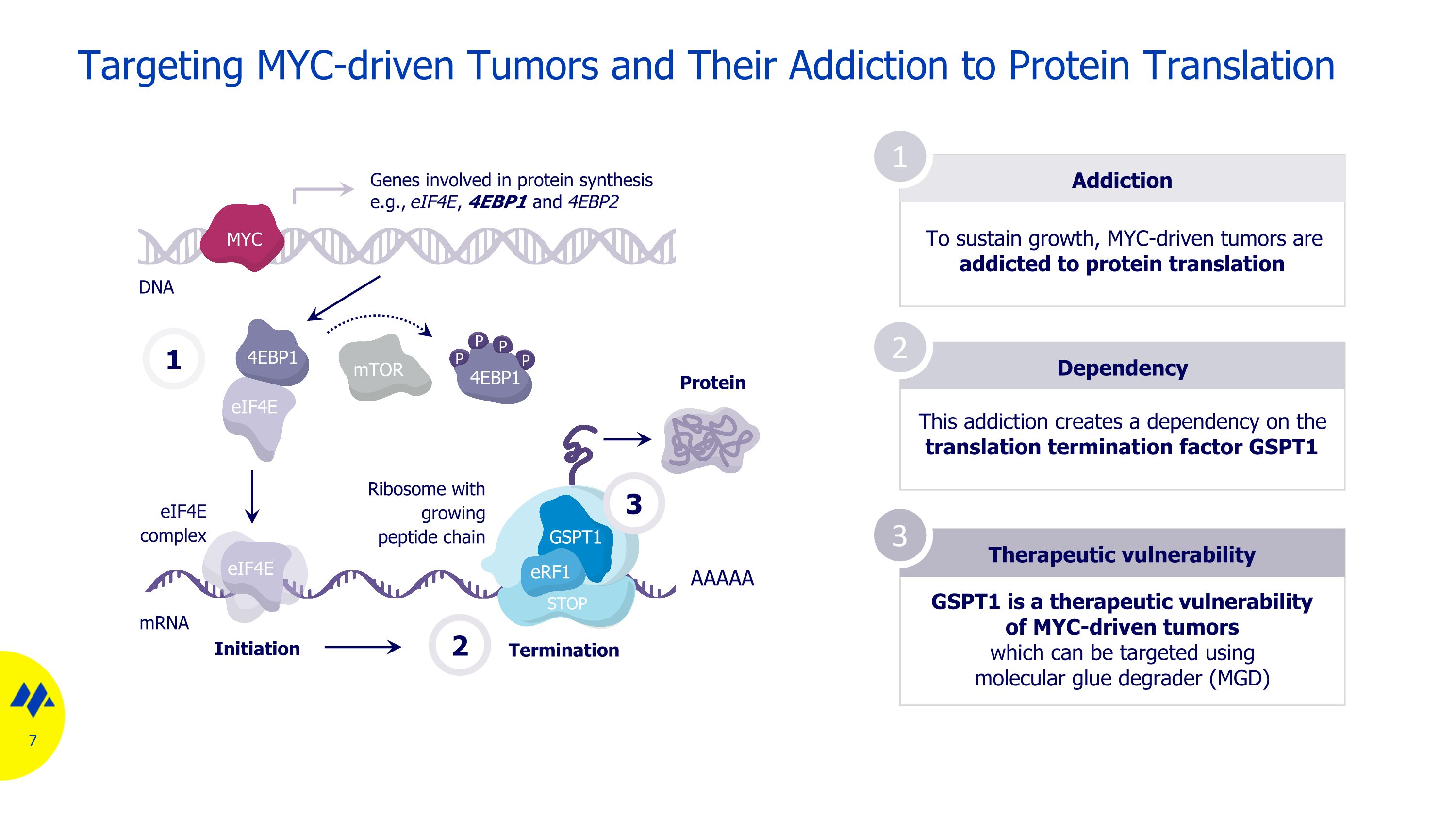
Targeting MYC-driven Tumors and Their Addiction to Protein Translation Addiction To sustain growth, MYC-driven tumors are addicted to protein translation Dependency Therapeutic vulnerability 1 2 3 This addiction creates a dependency on the translation termination factor GSPT1 GSPT1 is a therapeutic vulnerability of MYC-driven tumors which can be targeted using molecular glue degrader (MGD) mRNA DNA 1 mTOR eIF4E 4EBP1 P P P P 4EBP1 eIF4E eIF4E complex Genes involved in protein synthesis e.g., eIF4E, 4EBP1 and 4EBP2 Initiation Termination AAAAA Protein 2 MYC STOP GSPT1 eRF1 Ribosome with growing peptide chain 3
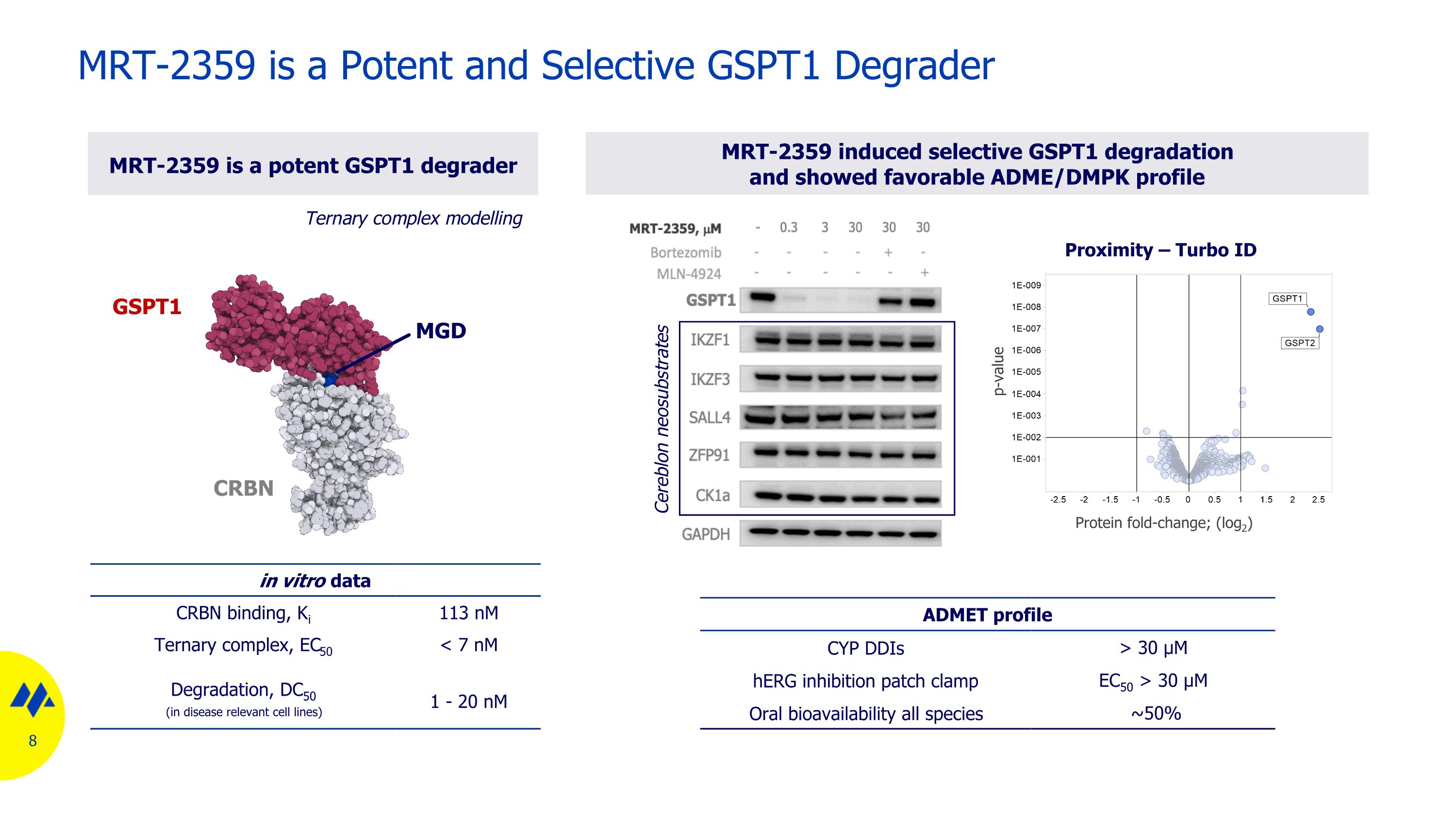
MRT-2359 is a Potent and Selective GSPT1 Degrader in vitro data CRBN binding, Ki 113 nM Ternary complex, EC50 < 7 nM Degradation, DC50 (in disease relevant cell lines) 1 - 20 nM MRT-2359 induced selective GSPT1 degradation and showed favorable ADME/DMPK profile MRT-2359 is a potent GSPT1 degrader ADMET profile CYP DDIs > 30 µM hERG inhibition patch clamp EC50 > 30 µM Oral bioavailability all species ~50% Protein fold-change; (log2) p-value Proximity – Turbo ID Ternary complex modelling Cereblon neosubstrates GSPT1 CRBN MGD
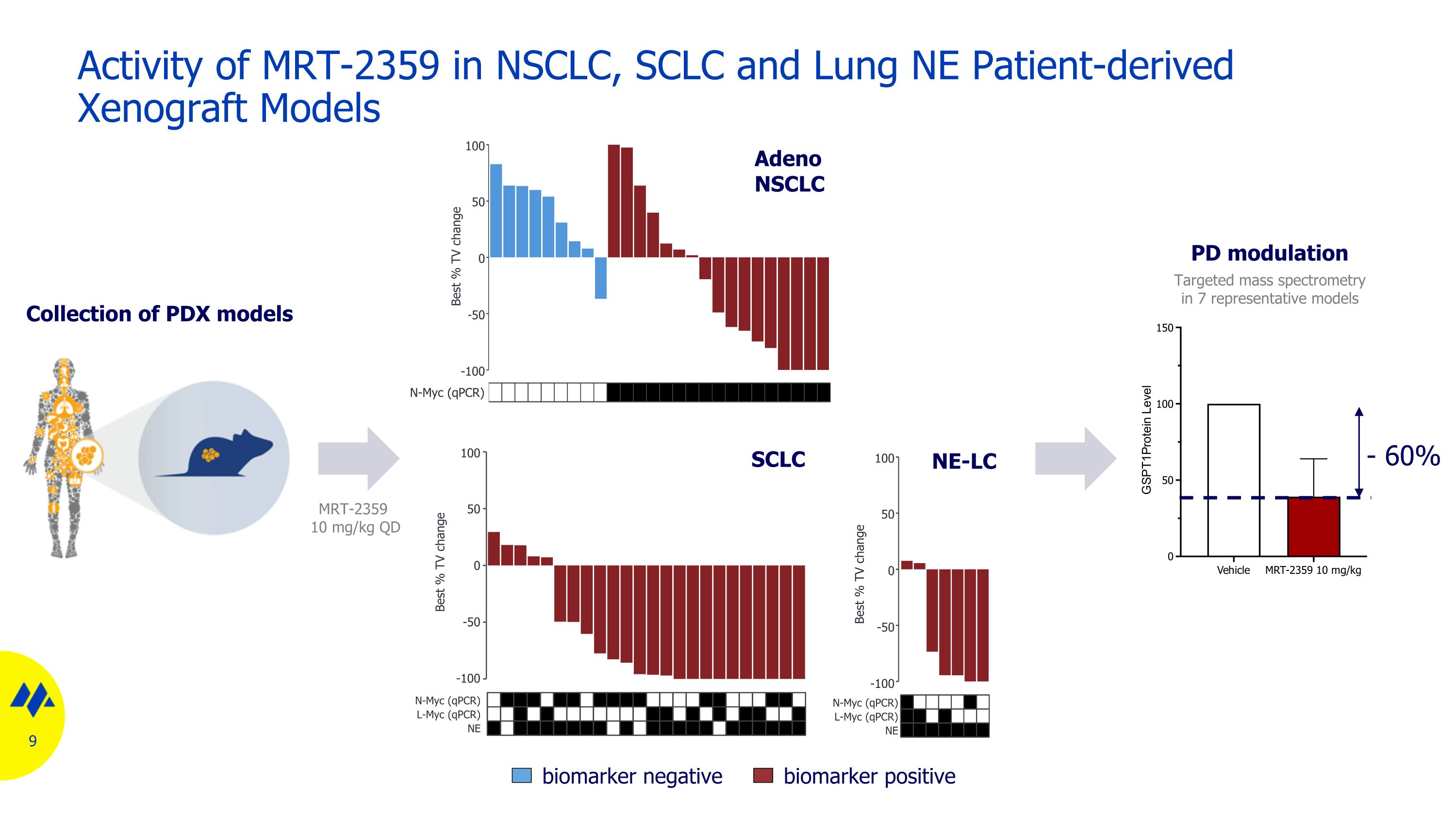
Activity of MRT-2359 in NSCLC, SCLC and Lung NE Patient-derived Xenograft Models Collection of PDX models 9 SCLC Adeno NSCLC NE-LC biomarker negative biomarker positive Targeted mass spectrometry in 7 representative models PD modulation 100 50 0 -50 -100 N-Myc (qPCR) Best % TV change 100 50 0 -50 -100 N-Myc (qPCR) Best % TV change L-Myc (qPCR) NE 100 50 0 -50 -100 N-Myc (qPCR) Best % TV change L-Myc (qPCR) NE MRT-2359 10 mg/kg QD - 60%
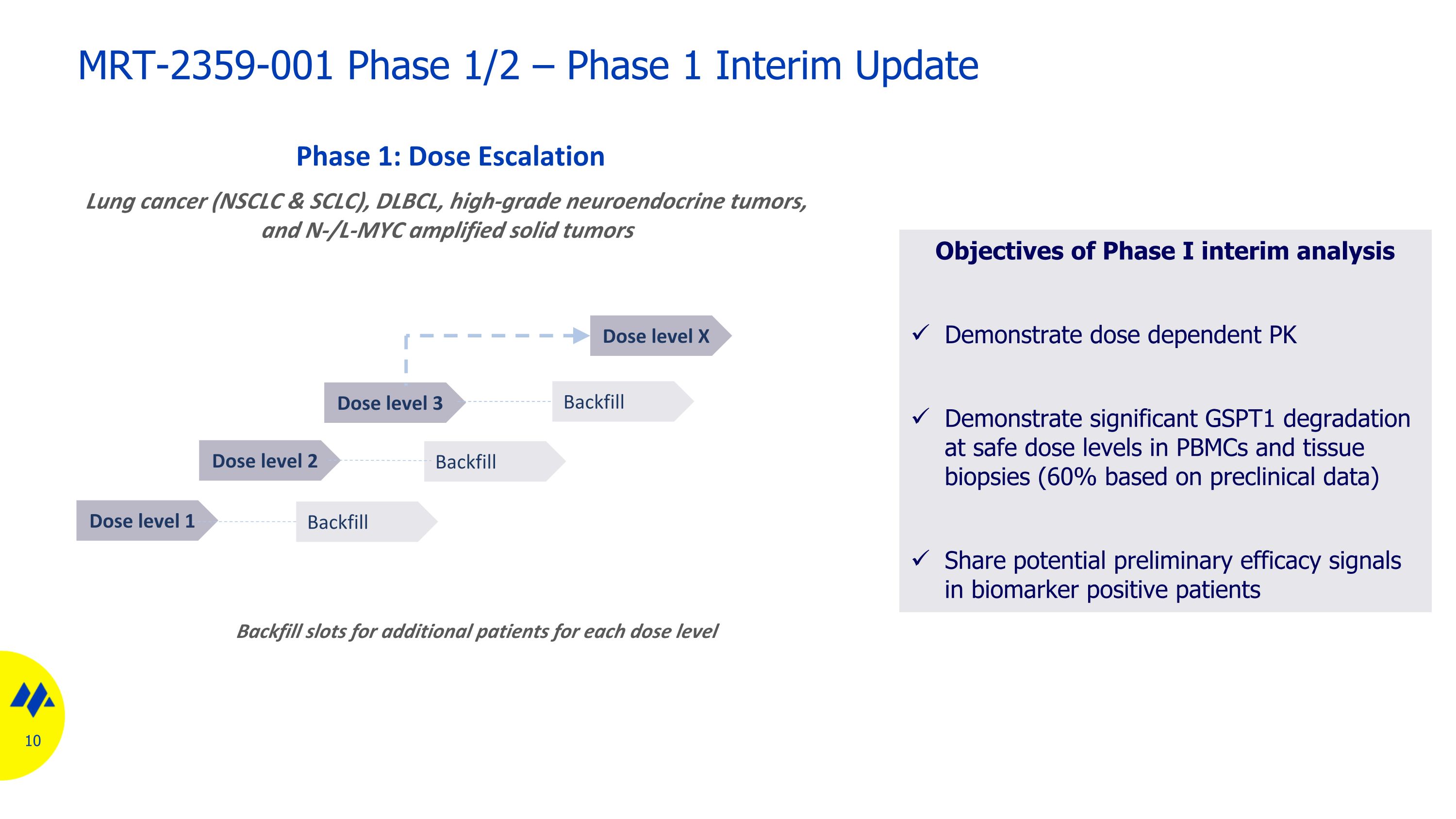
Dose level 1 Phase 1: Dose Escalation Backfill slots for additional patients for each dose level Backfill Dose level 2 Dose level 3 Dose level X Backfill Backfill Lung cancer (NSCLC & SCLC), DLBCL, high-grade neuroendocrine tumors, and N-/L-MYC amplified solid tumors MRT-2359-001 Phase 1/2 – Phase 1 Interim Update Objectives of Phase I interim analysis Demonstrate dose dependent PK Demonstrate significant GSPT1 degradation at safe dose levels in PBMCs and tissue biopsies (60% based on preclinical data) Share potential preliminary efficacy signals in biomarker positive patients

Executive Summary As of September 7th, 2023, 3 dose levels (0.5 mg, 1 mg, 2 mg) have been completed with 21 patients enrolled (including backfill patients) Observed dose dependent PK after oral dosing Clinical data support that 0.5 mg starting dose was fully active based on pharmacodynamic (PD) assessment of peripheral blood mononuclear cells (PBMC) and tissue samples from patients, with optimal PD modulation across dose levels tested Encouraging initial clinical activity at all dose levels: 2 partial responses (PRs) (1 confirmed, 1 unconfirmed) and 1 stable disease (SD) in 6 evaluable, heavily pretreated patients identified with biomarker positive tumors; 1 SD in non-small cell lung cancer (NSCLC) with un-evaluable biomarker status Safety profile supports continued development: Favorable adverse event (AE) profile at 0.5 and 1 mg Grade 4 thrombocytopenia identified as dose limiting toxicity (DLT) at 2 mg Previously reported limitations of CC-90009 not observed: No hypotension/cytokine release syndrome or clinically significant hypocalcemia observed at any dose level
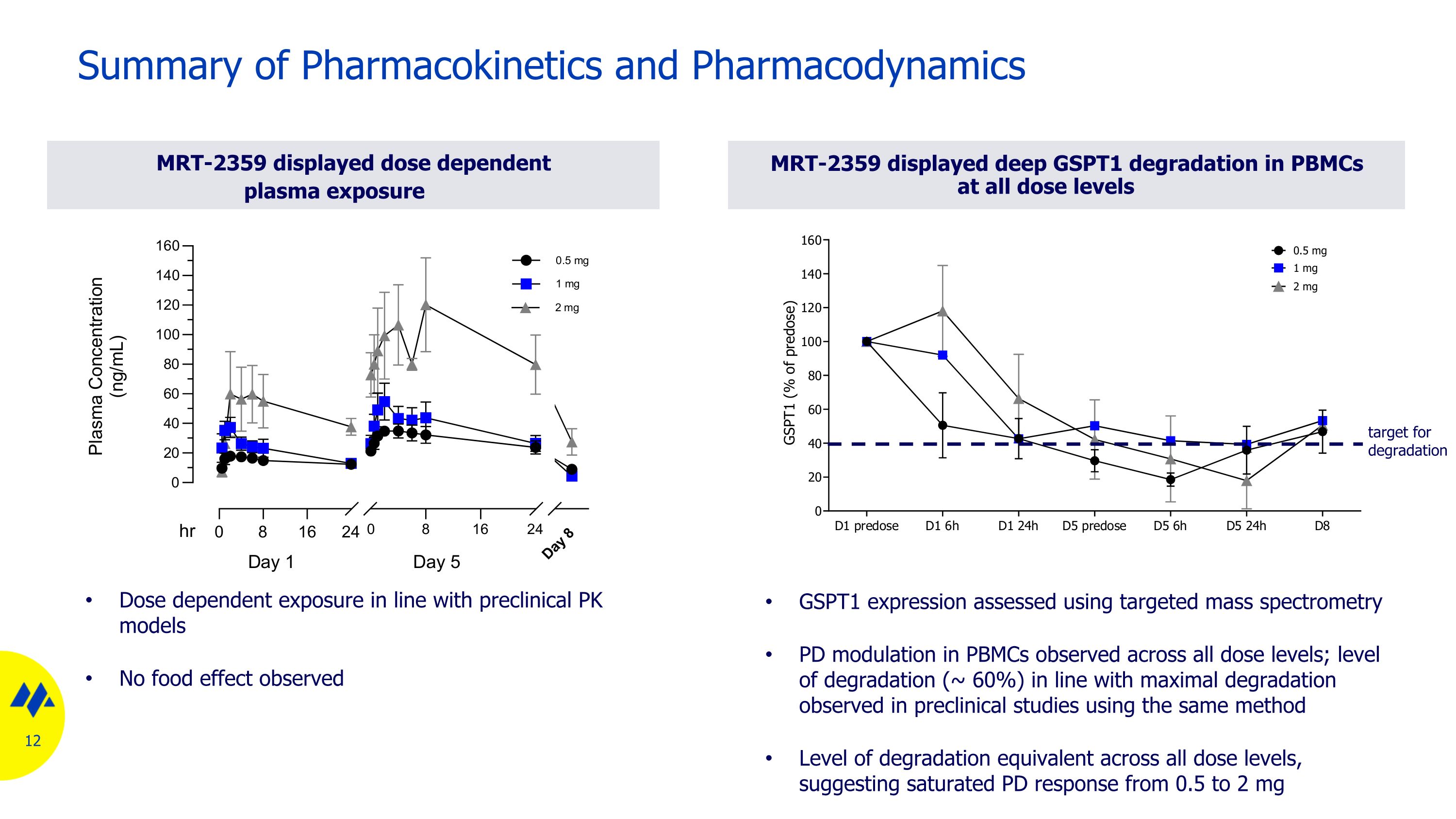
MRT-2359 displayed dose dependent plasma exposure Summary of Pharmacokinetics and Pharmacodynamics MRT-2359 displayed deep GSPT1 degradation in PBMCs at all dose levels GSPT1 expression assessed using targeted mass spectrometry PD modulation in PBMCs observed across all dose levels; level of degradation (~ 60%) in line with maximal degradation observed in preclinical studies using the same method Level of degradation equivalent across all dose levels, suggesting saturated PD response from 0.5 to 2 mg Dose dependent exposure in line with preclinical PK models No food effect observed target for degradation
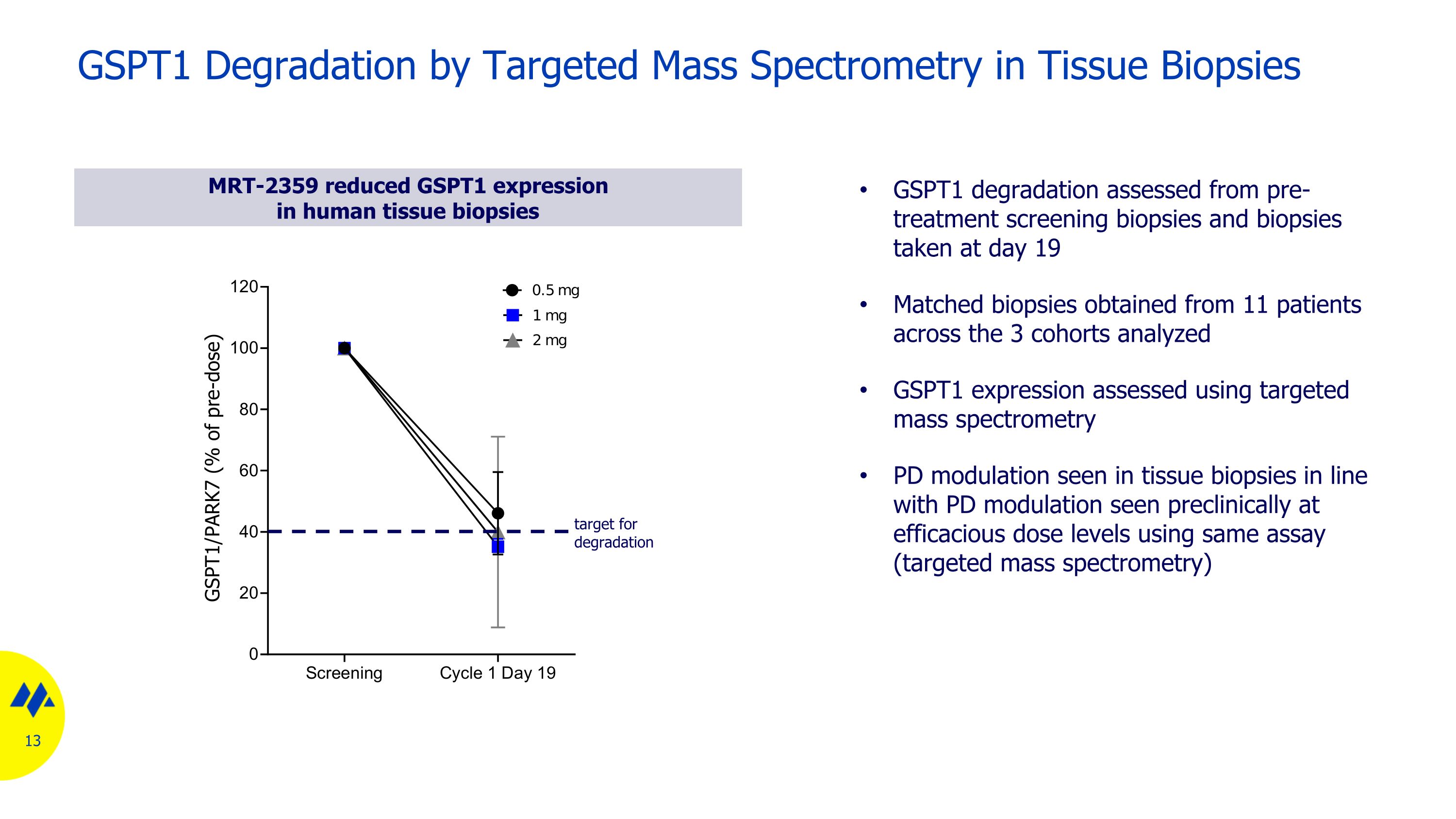
GSPT1 Degradation by Targeted Mass Spectrometry in Tissue Biopsies MRT-2359 reduced GSPT1 expression in human tissue biopsies GSPT1 degradation assessed from pre-treatment screening biopsies and biopsies taken at day 19 Matched biopsies obtained from 11 patients across the 3 cohorts analyzed GSPT1 expression assessed using targeted mass spectrometry PD modulation seen in tissue biopsies in line with PD modulation seen preclinically at efficacious dose levels using same assay (targeted mass spectrometry) target for degradation
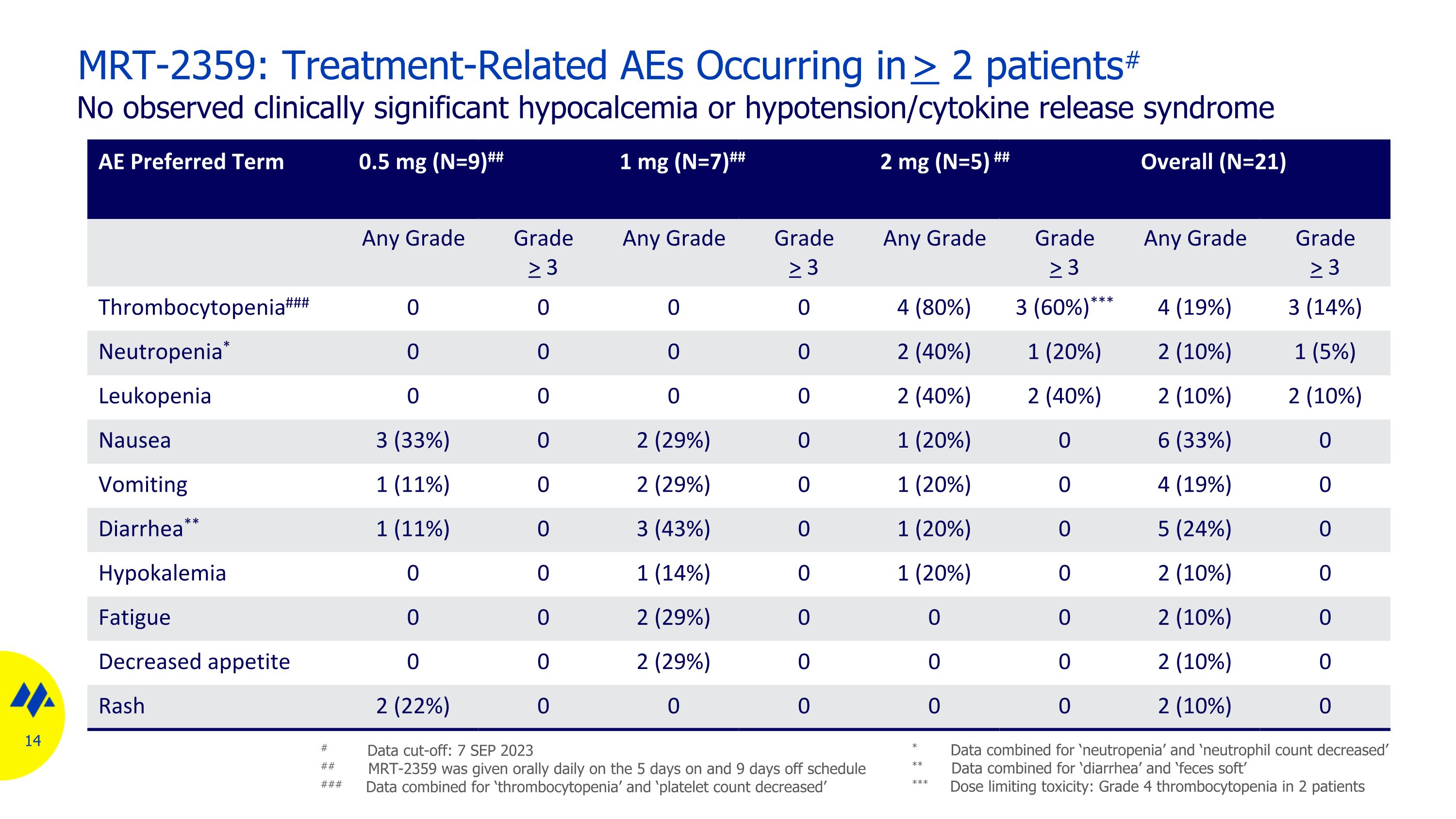
MRT-2359: Treatment-Related AEs Occurring in > 2 patients# No observed clinically significant hypocalcemia or hypotension/cytokine release syndrome AE Preferred Term 0.5 mg (N=9)## 1 mg (N=7)## 2 mg (N=5) ## Overall (N=21) Any Grade Grade > 3 Any Grade Grade > 3 Any Grade Grade > 3 Any Grade Grade > 3 Thrombocytopenia### 0 0 0 0 4 (80%) 3 (60%)*** 4 (19%) 3 (14%) Neutropenia* 0 0 0 0 2 (40%) 1 (20%) 2 (10%) 1 (5%) Leukopenia 0 0 0 0 2 (40%) 2 (40%) 2 (10%) 2 (10%) Nausea 3 (33%) 0 2 (29%) 0 1 (20%) 0 6 (33%) 0 Vomiting 1 (11%) 0 2 (29%) 0 1 (20%) 0 4 (19%) 0 Diarrhea** 1 (11%) 0 3 (43%) 0 1 (20%) 0 5 (24%) 0 Hypokalemia 0 0 1 (14%) 0 1 (20%) 0 2 (10%) 0 Fatigue 0 0 2 (29%) 0 0 0 2 (10%) 0 Decreased appetite 0 0 2 (29%) 0 0 0 2 (10%) 0 Rash 2 (22%) 0 0 0 0 0 2 (10%) 0 # Data cut-off: 7 SEP 2023 ## MRT-2359 was given orally daily on the 5 days on and 9 days off schedule ### Data combined for ‘thrombocytopenia’ and ‘platelet count decreased’ * Data combined for ‘neutropenia’ and ‘neutrophil count decreased’ ** Data combined for ‘diarrhea’ and ‘feces soft’ *** Dose limiting toxicity: Grade 4 thrombocytopenia in 2 patients
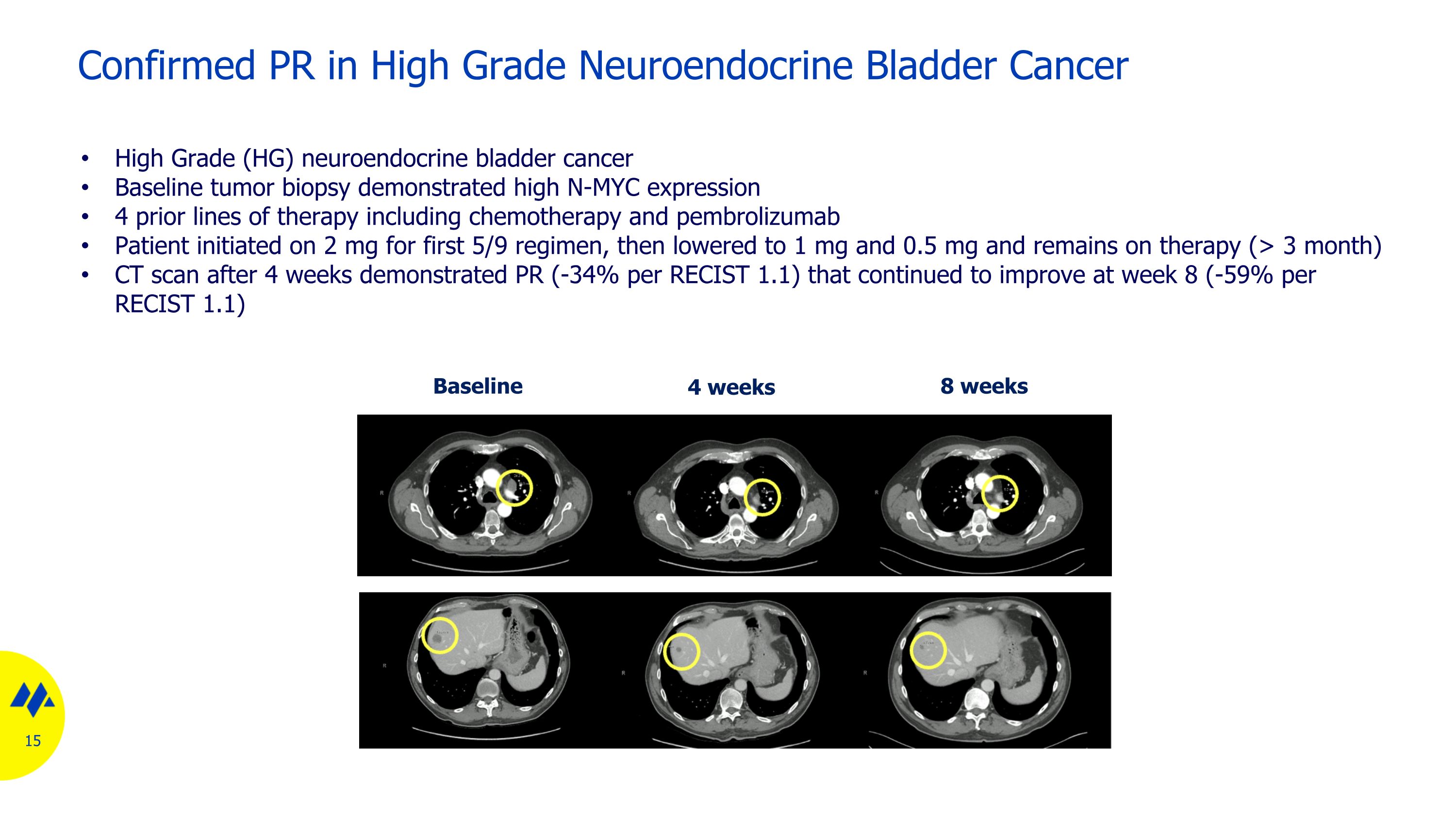
Confirmed PR in High Grade Neuroendocrine Bladder Cancer Baseline 8 weeks 4 weeks High Grade (HG) neuroendocrine bladder cancer Baseline tumor biopsy demonstrated high N-MYC expression 4 prior lines of therapy including chemotherapy and pembrolizumab Patient initiated on 2 mg for first 5/9 regimen, then lowered to 1 mg and 0.5 mg and remains on therapy (> 3 month) CT scan after 4 weeks demonstrated PR (-34% per RECIST 1.1) that continued to improve at week 8 (-59% per RECIST 1.1)
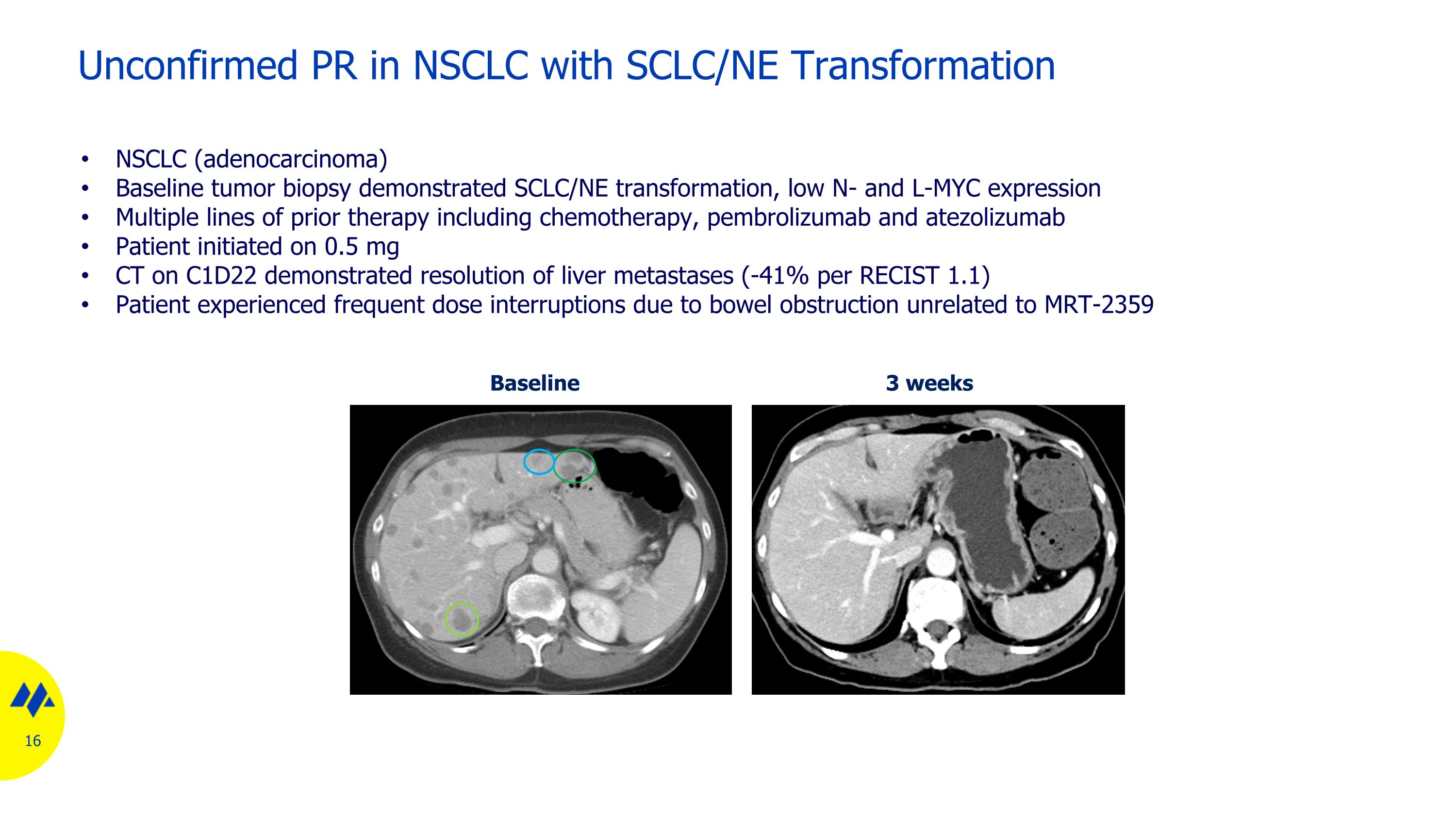
Unconfirmed PR in NSCLC with SCLC/NE Transformation Baseline 3 weeks NSCLC (adenocarcinoma) Baseline tumor biopsy demonstrated SCLC/NE transformation, low N- and L-MYC expression Multiple lines of prior therapy including chemotherapy, pembrolizumab and atezolizumab Patient initiated on 0.5 mg CT on C1D22 demonstrated resolution of liver metastases (-41% per RECIST 1.1) Patient experienced frequent dose interruptions due to bowel obstruction unrelated to MRT-2359
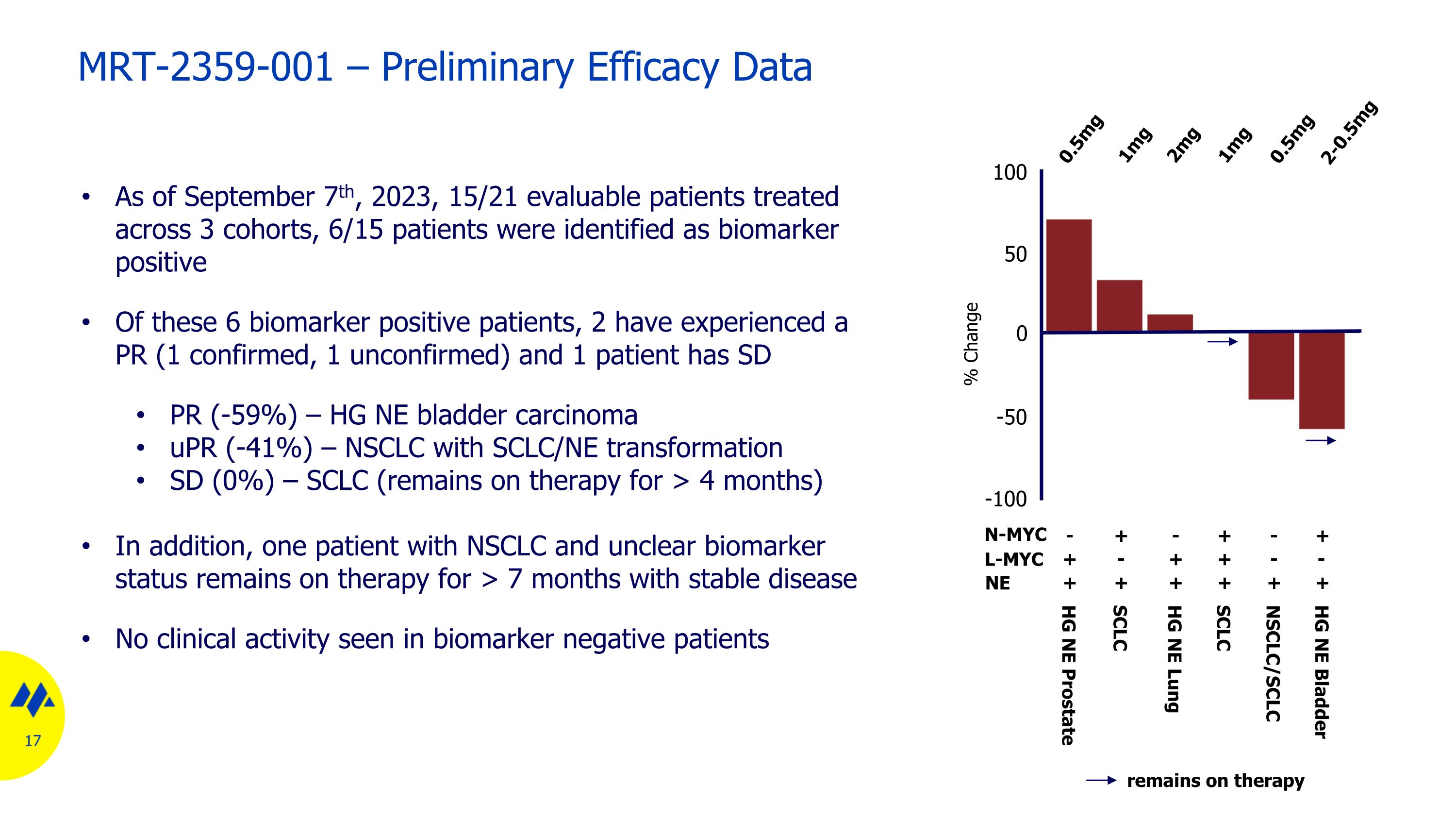
MRT-2359-001 – Preliminary Efficacy Data As of September 7th, 2023, 15/21 evaluable patients treated across 3 cohorts, 6/15 patients were identified as biomarker positive Of these 6 biomarker positive patients, 2 have experienced a PR (1 confirmed, 1 unconfirmed) and 1 patient has SD PR (-59%) – HG NE bladder carcinoma uPR (-41%) – NSCLC with SCLC/NE transformation SD (0%) – SCLC (remains on therapy for > 4 months) In addition, one patient with NSCLC and unclear biomarker status remains on therapy for > 7 months with stable disease No clinical activity seen in biomarker negative patients 100 50 0 -50 -100 0.5mg 0.5mg 2mg 1mg 1mg 2-0.5mg HG NE Prostate SCLC SCLC NSCLC/SCLC HG NE Lung HG NE Bladder % Change remains on therapy N-MYC + + + - - - + + + + + + NE L-MYC + - - + + -

Summary and Next Steps Clinical data support that 0.5 mg starting dose was active based on PD assessment (PBMC and tissue), with optimal PD modulation across dose levels tested Encouraging initial clinical activity at all dose levels: 2 PRs (1 confirmed, 1 unconfirmed) and 1 SD in 6 evaluable, heavily pretreated patients identified with biomarker positive tumors; 1 SD in non-small cell lung cancer (NSCLC) with un-evaluable biomarker status Safety profile support further development: Favorable AE profile at 0.5 and 1 mg Grade 4 thrombocytopenia identified as dose limiting toxicity (DLT) at 2 mg Previously reported limitations of CC-90009 not observed: No hypotension/cytokine release syndrome or clinically significant hypocalcemia observed at any dose level Next Steps Currently dosing 1.5 mg, expected to complete DLT observation period by end of October Based on favorable tox profile, Company initiating intermittent dosing regimen of 21 days on and 7 days off drug (21/7)

Portfolio
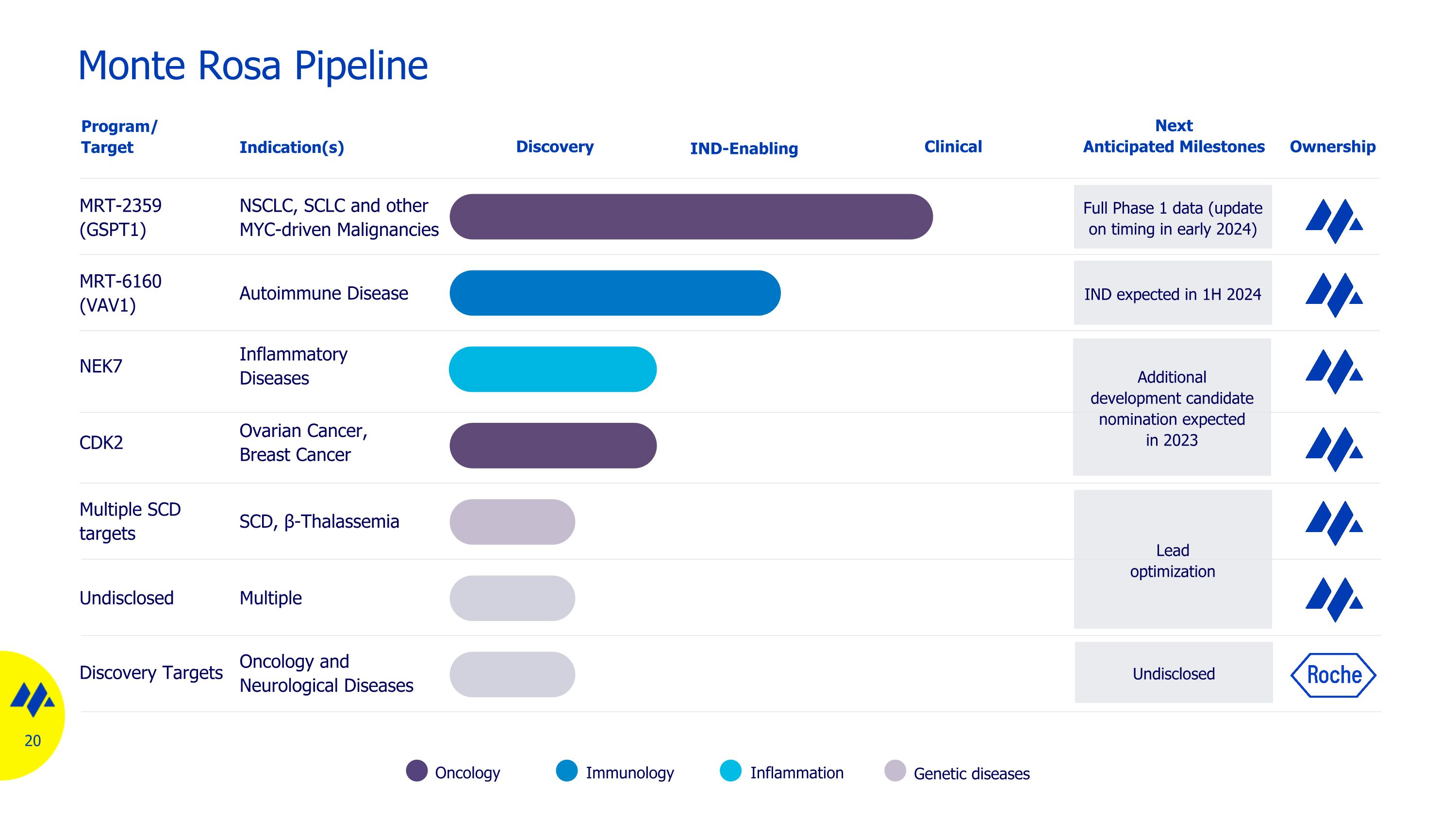
Monte Rosa Pipeline Oncology Inflammation Immunology Genetic diseases MRT-2359 (GSPT1) NSCLC, SCLC and other MYC-driven Malignancies NEK7 Inflammatory Diseases MRT-6160 (VAV1) Autoimmune Disease Discovery Program/ Target Indication(s) Full Phase 1 data (update on timing in early 2024) Multiple SCD targets SCD, β-Thalassemia Next Anticipated Milestones Ownership Undisclosed Multiple IND-Enabling Clinical Lead optimization Additional development candidate nomination expected in 2023 CDK2 Ovarian Cancer, Breast Cancer IND expected in 1H 2024 Discovery Targets Oncology and Neurological Diseases Undisclosed

Monte Rosa Therapeutics - Highlights Taking molecular glue degraders (MGDs) to new heights Rationally designed MGDs with potential to solve many of the limitations of other modalities by selectively degrading therapeutically relevant proteins considered undruggable or inadequately drugged Highly productive, industry-leading platform for designing MGDs to address hard-to-drug disease targets via degradation, combining experimentation with AI to push the boundaries of what is possible with MGDs Five promising, wholly-owned programs spanning oncology, autoimmune, inflammation and other TAs Strong financial position providing cash runway into Q3 2025 Phase 1/2 clinical study ongoing with MRT-2359, with potential to treat difficult-to-drug MYC-driven cancers; optimal pharmacodynamic modulation and early signs of clinical activity observed Partnership with Roche to develop MGDs for oncology and neurological conditions – expands platform reach into neurology MRT-6160, highly selective VAV1-directed MGD, expected to enter clinic in mid-2024, with wide potential applications across autoimmune diseases

Thank You





















